Abstract
Trichloroethylene (TCE) is a volatile chlorinated organic compound that is commonly used as a solvent for lipophilic compounds. Although recognized as an animal carcinogen, TCE’s carcinogenic potential in humans is still uncertain. We have carried out a cross-sectional study of 80 workers exposed to TCE and 96 unexposed controls matched on age and sex in Guangdong, China to study TCE’s early biologic effects. We previously reported that the total lymphocyte count and each of the major lymphocyte subsets (i.e., CD4+ T cells, CD8+ T cells, natural killer cells, and B cells) were decreased in TCE-exposed workers compared to controls, suggesting a selective effect on lymphoid progenitors, and/or lymphocyte survival. To explore which T lymphocyte subsets are affected in the same study population, we investigated the effect of TCE exposure on the numbers of CD4+ naïve and memory T cells, CD8+ naïve and memory T cells, and regulatory T cells by FACS analysis. Linear regression of each subset was used to test for differences between exposed workers and controls adjusting for potential confounders. We observed that CD4+ and CD8+ naïve T cell counts were about 8% (p = 0.056) and 17% (p = 0.0002) lower, respectively, among exposed workers. CD4+ effector memory T cell counts were decreased by about 20% among TCE-exposed workers compared to controls (p = 0.001). The selective targeting of TCE on CD8+ naive and possibly CD4+ naive T cells, and CD4+ effector memory T cells, provide further insights into the immunosuppression-related response of human immune cells upon TCE exposure.
Keywords: trichloroethylene, lymphocyte, CD4, CD8, naïve, memory
Introduction
Trichloroethylene (TCE) is a volatile chlorinated organic compound that is used as a degreaser for metal parts and a general-purpose solvent for lipophilic compounds. With the widespread workplace and environmental exposures in the United States, it is not surprising that about 10% of the population has been reported to have detectable levels of TCE in their blood (Gist and Burg, 1995; Ashley et al., 1996; Wu and Schaum, 2000). While TCE has been deemed an animal carcinogen, its carcinogenicity potential in humans has not been fully characterized (IARC, 1995). Epidemiological studies have found associations between TCE exposure and cancers of the kidney and liver, and non-Hodgkin lymphoma (NHL; Raaschou-Nielsen et al., 2003; Scott and Chiu, 2006; Alexander et al., 2007; Kelsh et al., 2010; Purdue et al., 2010).
To better understand the human health effects associated with TCE, we carried out a cross-sectional study to evaluate the biologic plausibility of NHL associated with occupational exposure to TCE in Guangdong, China (Lan et al., 2010). The primary goal of this study was to follow up experimental studies indicating TCE’s potential to alter key lymphocyte subset levels, such as CD4+ T cells (Blossom et al., 2004; Chen et al., 2006). Specifically, we reported that the total lymphocyte count (but not the granulocyte count) and each of the major lymphocyte subsets including CD4+ T cells, CD8+ T cells, natural killer (NK) cells, and B cells were decreased among the TCE-exposed workers compared to controls, suggesting that TCE exposure has an effect on lymphoid progenitors or on lymphocyte maturation (Lan et al., 2010). These findings are of interest clinically since decreases in lymphocyte subsets may suggest reduced immune capacity (Sallusto and Lanzavecchia, 2000).
To further explore the effects that TCE exposure may potentially exert on specific lymphomagensis processes, we measured T cell lymphocyte subsets including CD4+ naïve and memory T cells, CD8+ naïve and memory T cells, and regulatory T cells in the same population enrolled in our cross-sectional study of 80 workers exposed to TCE and 96 unexposed controls matched on age and sex in Guangdong, China.
Materials and Methods
The study design, participants, environmental exposure assessment, and biological sample collection of this cross-sectional molecular epidemiological study have been previously described (Lan et al., 2010). Briefly, a cross-sectional study of 80 workers currently exposed to TCE in six study factories with TCE cleaning operations and 96 unexposed controls were enrolled in June and July of 2006. Controls, frequency-matched by sex, and age (±5 years), were enrolled from two clothes manufacturing factories, one food production factory, and a hospital that did not use TCE, and were in the same geographic region as the factories that used TCE. For all potentially exposed workers and unexposed controls, subjects were excluded if they had a history of cancer, chemotherapy, and radiotherapy, as well as previous occupations with notable exposure to benzene, butadiene, styrene, and/or ionizing radiation. The study was approved by Institutional Review Boards at the U.S., National Cancer Institute and the Guangdong Poison Control Center in China. Participation was voluntary and all subjects gave written informed consent.
Personal air exposure measurements were conducted for all study subjects. Full-shift personal air exposure measurements were taken in the factories using 3 M organic vapor monitoring (OVM) badges before blood-collection. All samples were analyzed for TCE and a subset (48 from TCE-exposed workers) was analyzed for a panel of organic hydrocarbons including benzene, methylene chloride, perchloroethylene, and epichlorohydrin. OVM samples were also obtained on a subgroup of control workers. Subjects were categorized into three groups by mean TCE levels measured during the month before phlebotomy (unexposed workers, workers with <12 ppm TCE exposure, and workers with ≥12 ppm TCE exposure). A questionnaire-based interview, assessing demographics, lifestyle characteristics, and occupational history, was administered to all subjects.
After phlebotomy, lymphocyte subsets were analyzed on the same day that a peripheral blood sample was collected (Sallusto et al., 1999). For the T lymphocyte subsets, peripheral white blood cells were preincubated with Fc block for 15 min at 4°C and immunostained with Abs recognizing the following: CD3 (UCHT1), CD4 (RPA-T4 or SK3), CD8 (SK1), CD45 (HI30), CD45RA (HI100), CCR7 (150503, R&D systems; all from BD Biosciences unless otherwise specified). Mouse IgG1 κ (MOPC-21) served as an isotype control. FCM data were acquired using a flow cytometer. For intracellular FoxP3 staining, peripheral white blood cells were first surface-stained with the following fluorescent Abs against CD4 (RPA-T4) and CD25 (M-A251). The cells were then fixed, permeabilized, and stained with anti-FoxP3 antibody using the Anti-Human Foxp3 Staining Set kit according to the manufacturer’s instructions (eBiosciences). Cells were then acquired and analyzed by FCM as described above. Measurements from blinded quality control replicates interspersed among the samples did not identify outlier batches. Assay CVs were <10% for each T cell lymphocyte subset analysis shown in Table 2, except for CD8+ central memory T cells which was 23%.
Table 2.
T cell subset counts mean ± SD among trichloroethylene (TCE) exposed workers and unexposed controls.
| Low exposed workers |
High exposed workers |
Ptrend††† | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls† |
TCE-exposed Workers† |
(<12ppm)† |
(≥12ppm)† |
|||||||||||||
| n | Mean | SD | n | Mean | SD | p Value†† | n | Mean | SD | p Value†† | n | Mean | SD | p Value†† | ||
| CD3+CD4+ | ||||||||||||||||
| CD4 naïve (CD45RA+CCR7+)¥ | 86 | 283 | 126 | 70 | 262 | 130 | 0.056 | 32 | 293 | 142 | 0.58 | 38 | 236 | 113 | 0.017 | 0.02 |
| CD4 MEMORY (CD45RA−) | ||||||||||||||||
| Effector memory (CD45RA−CCR7−)‡ | 86 | 225 | 93 | 70 | 183 | 75 | 0.001 | 32 | 183 | 55 | 0.014 | 38 | 184 | 89 | 0.0034 | 0.001 |
| Central memory (CD45RA−CCR7+) | 86 | 169 | 71 | 70 | 175 | 68 | 0.21 | 32 | 183 | 71 | 0.024 | 38 | 169 | 66 | 0.86 | 0.59 |
| T REGULATION SUBSET | ||||||||||||||||
| CD4+CD25+ | 96 | 69 | 35 | 80 | 71 | 36 | 0.92 | 39 | 79 | 35 | 0.16 | 41 | 64 | 36 | 0.18 | 0.35 |
| CD4+FoxP3+ | 96 | 52 | 21 | 80 | 50 | 26 | 0.24 | 39 | 51 | 28 | 0.22 | 41 | 48 | 24 | 0.41 | 0.34 |
| CD25+FoxP3+ | 96 | 53 | 21 | 80 | 50 | 26 | 0.13 | 39 | 50 | 26 | 0.15 | 41 | 49 | 25 | 0.23 | 0.19 |
| CD3+CD8+ | ||||||||||||||||
| CD8 naïve (CD45RA+CCR7+) | 86 | 216 | 117 | 70 | 179 | 101 | 0.0002 | 32 | 212 | 101 | 0.22 | 38 | 152 | 93 | <0.0001 | <0.0001 |
| CD8 MEMORY (CD45RA−) | ||||||||||||||||
| Effector memory (CD45RA−CCR7−)* | 86 | 150 | 72 | 70 | 132 | 62 | 0.25 | 32 | 143 | 68 | 0.82 | 38 | 123 | 55 | 0.057 | 0.09 |
| Central memory (CD45RA−CCR7+) | 86 | 9 | 7 | 70 | 8 | 9 | 0.72 | 32 | 8 | 5 | 0.21 | 38 | 9 | 12 | 0.80 | 0.97 |
† Unadjusted mean (±SD) cells/μl blood; ††p value compares exposed workers, low exposed workers, and high exposed workers to controls, adjusted forage and sex; †††Ptrend using category of TCE levels (controls, <12, ≥12 ppm) as a continuous variable. Twelve ppm was the median TCE concentration of the exposed subjects. Ptrend adjusted forage and sex; ‡ adjusted for age, sex, and smoking; ¥ adjusted for age, sex, and infection; * adjusted for age, sex, and BMI.
Unadjusted means and standard deviations were determined for each cell count subset. Linear regression using the natural logarithm (ln) of each subset was used to test for differences between unexposed and exposed workers, and to evaluate for a dose–response across exposure groups (unexposed workers, workers exposed to <12 ppm TCE, and workers exposed to ≥12 ppm TCE). All statistical models were adjusted for age (as a continuous variable) and sex. Potential confounders, including current cigarette smoking status (yes/no), current alcohol consumption (yes/no), recent infections (flu or respiratory infections in the previous month), and body mass index (BMI), were also included in a model for a specific subset if the regression coefficient was altered by ±15%. The total lymphocyte percent from the CBC was used to calculate lymphocyte subsets, and an additional calculation was carried out using the lymphocyte percent obtained by flow cytometry.
Results
Table 1 includes the demographic characteristics and TCE exposure levels of our study population. Age, sex, and current smoking status were comparable among the workers not exposed to TCE and workers with low and high TCE exposure (Table 1). Measured air levels of TCE exposure were dramatically higher for the exposed groups compared to the unexposed workers.
Table 1.
Demographic characteristics by trichloroethylene (TCE) exposure level of study subjects from Guangdong, China.
| Controls (n = 96) | TCE-exposed workers |
|||
|---|---|---|---|---|
| All (n = 80) | <12 ppm (n = 39) | ≥12 ppm (n = 41) | ||
| Age, mean (SD) | 27 (7) | 25 (7) | 24 (5) | 27 (8) |
| BMI, mean (SD) | 22 (3) | 21 (3) | 21 (2) | 22 (3) |
| SEX | ||||
| Female n (%) | 23 (24) | 23 (29) | 15 (38) | 8 (20) |
| Male n (%) | 73 (76) | 57 (71) | 24 (62) | 33 (80) |
| SMOKE | ||||
| No n (%) | 58 (60) | 46 (58) | 22 (56) | 24 (59) |
| Yes n (%) | 38 (40) | 34 (42) | 17 (44) | 17 (41) |
| INFECTION | ||||
| No n (%) | 75 (78) | 65 (81) | 31 (79) | 34 (83) |
| Yes n (%) | 21 (22) | 15 (19) | 8 (21) | 7 (17) |
| TCE EXPOSURE | ||||
| TCE air level (ppm)1 mean (SD) | <0.03 | 22.19 (35.94) | 5.19 (3.47) | 38.36 (44.61) |
1TCE air level is the arithmetic mean (±SD) of an average of two measurements per subject collected during the month before phlebotomy.
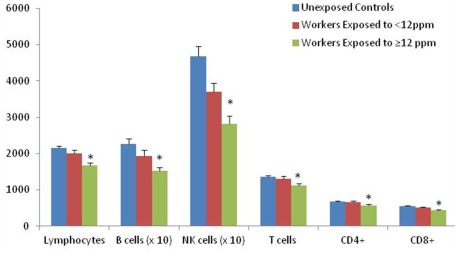
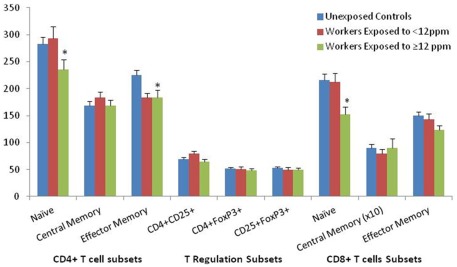
To expand on our previous report (Lan et al., 2010), which found lymphocytes, T cells, and CD4+ T cells to be decreased among exposed workers compared to unexposed controls (Figure 1), we analyzed three subsets of CD4+ T cells, including CD4+ naïve T cells, CD4+ memory T cells, and the T regulation subset. The associations between lymphocyte subsets are provided in Table 2 comparing the unexposed workers to all exposed workers, as well as to low exposed workers and high exposed workers. Of note, we observed that CD4+ naïve (CD45RA+CCR7+) T cell counts were about 8% (p = 0.056) lower among exposed workers compared to unexposed controls (Figure 2), which was restricted to workers exposed to ≥12 ppm of TCE (p = 0.02; p = 0.58 for workers exposed to <12 ppm; Table 2). Furthermore, CD4+ effector memory (CD45RA−CCR7−) T cell counts were decreased by about 20% among TCE-exposed workers compared to controls (p = 0.001) whereas the CD4+ central memory (CD45RA−CCR7+) T cell population was not significantly changed (Table 2). The decreased counts for the CD4+ effector memory T cells were observed in workers with <12 ppm (p = 0.014) and workers with ≥12 ppm of exposure (p = 0.0034), in a dose-dependent manner (Ptrend = 0.001; Figure 2).
Figure 1.
Lymphocyte and lymphocyte subset counts (mean ± SE) among trichloroethylene (TCE) exposed workers and unexposed controls. Results previously reported in (Lan et al., 2010). Lymphocytes and lymphocyte subset counts in relation to TCE exposure level. Ptrend using category of TCE levels (controls, <12 and ≥12 ppm) as a continuous variable (*Ptrend < 0.05). The median TCE concentration of all exposed subjects was 12 ppm. Differences in cell counts were tested by linear regression analysis of ln-transformed end point, adjusting for relevant covariates [lymphocytes: adjusted for age and sex; CD4+, CD8+, and NK T cells: adjusted for age and sex, three subjects (two controls and one exposed) were deleted due to inconsistent cell counts using complete blood count data versus flow cytometry to calculate % lymphocytes; B cell: adjusted for age, sex, and smoking status].
Figure 2.
T cell subset counts (mean ± SE) among trichloroethylene (TCE) exposed workers and unexposed controls. T cell lymphocyte subset counts in relation to TCE exposure level. Ptrend using category of TCE levels (controls, <12 and ≥12 ppm) as a continuous variable (*Ptrend < 0.05). The median TCE concentration of all exposed subjects was 12 ppm. Differences in cell counts were tested by linear regression analysis of ln-transformed end point, adjusting for relevant covariates [CD4+ central memory, T regulation subsets, CD8+ naïve, CD8+ central memory cells: adjusted for age, sex; CD4+ naïve cells: adjusted for age, sex, infection; CD4+ effector memory: adjusted for age, sex, smoking status; CD8+ effector memory: adjusted for age, sex, BMI]; *Ptrend < 0.05.
Of the CD8+ T cell subsets, we found that CD8+ naïve (CD45RA+CCR7+) T cell counts were about 17% (p = 0.0002) less in the exposed versus controls whereas the numbers of CD8+ effector memory (CD45RA−CCR7−) were slightly decreased and central memory (CD45RA−CCR7+) T cells were comparable (Figure 2; Table 2). Similar to the effects observed in for CD4+ naïve T cells, the decreased counts for CD8+ naïve T cells for were restricted to workers exposed to ≥12 ppm of TCE (p < 0.0001; p = 0.22 for workers exposed to <12 ppm; Table 2).
The regulatory T cells were not significantly different between the controls and exposed workers (CD4+CD25+, p = 0.92; CD4+FoxP3+, p = 0.24; CD25+FoxP3+, p = 0.13; Figure 2; Table 2).
Discussion
To the best of our knowledge, this is the first epidemiological investigation of the impact that occupational exposure to TCE has on T cell lymphocyte subsets, such as CD4+ naïve and memory T cells, CD8+ naïve and memory T cells, and regulatory T cells. While no differences were observed between the regulatory T cells among controls and exposed workers, we did observe that occupational exposure to TCE decreased CD4+ effector memory T cell, CD4+ naïve T cell, and CD8+ naïve T cell counts.
T cells function in response to immune related functions, such as infectious agents and transplantation antigens, and are up- or down-regulated accordingly. Within the human immune system, T cells differentiate into two major subsets, CD4+ and CD8+ T cells. When the body is presented with an antigenic stimulation, mature CD4+, and CD8+ naïve T cells are found to undergo clonal expansion and differentiate into effector cells, which either undergo apoptosis or develop into effector–memory cells (Marrack and Kappler, 2004; Stockinger et al., 2004). The resulting CD4+ effector memory and CD8+ effector memory T cells that lack CCR7 expression, do not recirculate to the lymph nodes but instead circulate to inflamed tissues where the effector cells can react to antigens (Sallusto and Lanzavecchia, 2000). Therefore, a decrease in CD4+ and CD8+ effector memory T cells may lead to a decreased capacity of the body to respond to antigenic-related inflammation. Through this mechanism, our observed decrease in CD4+ effector memory T cells among TCE-exposed workers compared to unexposed controls suggests that TCE exposure may result in immunosuppression resulting in a reduced capacity for individuals to respond to antigens and antigenic-related inflammation.
In support of the biological plausibility of occupational TCE exposure leading to decreased immune functions, TCE exposure has been associated with immunosuppression in animals, and to a limited extent in humans (Wartenberg et al., 2000; Iavicoli et al., 2005; Cooper et al., 2009). Given that altered immunity, including immunosuppression, is an established risk factor for NHL (Ducloux et al., 2002), it is conceivable that TCE exposure may be mechanistically associated with NHL through the reduced capacity to respond to antigenic-related inflammation. This would be consistent with a number of studies that have linked occupational exposure to TCE and NHL (Raaschou-Nielsen et al., 2003; Purdue et al., 2010). Therefore, our findings add to the biologic plausibility that TCE is a lymphomagen.
In conclusion, the selective targeting of TCE exposure on CD4+ naïve and CD4+ effector memory T cells, and CD8+ naïve T cells observed in our study, provide further insights into the biologic response of human immune cells upon exposure to TCE, which may include immunosuppression through reduced capacity to respond to antigens. These results show that TCE exposure specifically affects CD4+ and CD8+ T cell subsets and provide further support for the biologic plausibility that TCE is associated with risk of NHL; however, caution should be used when interpreting these findings until confirmed in additional studies given the small sample size of our study and the potential confounding from additional factors that may influence lymphocyte cell counts.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by intramural funds from the National Cancer Institute, and grants from the National Institute of Environmental Health Sciences (P42ES04705 and P30ES01896), the Northern California Center for Occupational and Environmental Health, and a grant from the Department of Science and Technology of Guangdong Province, PR China (2007A050100004 to Xiaojiang Tang).
References
- Alexander D. D., Kelsh M. A., Mink P. J., Mandel J. H., Basu R., Weingart M. (2007). A meta-analysis of occupational trichloroethylene exposure and liver cancer. Int. Arch. Occup. Environ. Health 81, 127–143 10.1007/s00420-007-0201-4 [DOI] [PubMed] [Google Scholar]
- Ashley D. L., Bonin M. A., Cardinali F. L., McCraw J. M., Wooten J. V. (1996). Measurement of volatile organic compounds in human blood. Environ. Health Perspect. 104(Suppl. 5), 871–877 10.2307/3433004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blossom S. J., Pumford N. R., Gilbert K. M. (2004). Activation and attenuation of apoptosis of CD4+ T cells following in vivo exposure to two common environmental toxicants, trichloroacetaldehyde hydrate and trichloroacetic acid. J. Autoimmun. 23, 211–220 10.1016/j.jaut.2004.06.007 [DOI] [PubMed] [Google Scholar]
- Chen X. Y., Zhuang Z. X., Wang X. H., Zhang J. Z. (2006) Immune responses to trichloroethylene and skin gene expression profiles in Sprague Dawley rats. Biomed. Environ. Sci. 19, 346–352 [PubMed] [Google Scholar]
- Cooper G. S., Makris S. L., Nietert P. J., Jinot J. (2009). Evidence of autoimmune-related effects of trichloroethylene exposure from studies in mice and humans. Environ. Health Perspect. 117, 696–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducloux D., Carron P. L., Motte G., Ab A., Rebibou J. M., Bresson-Vautrin C., Tiberghien P., Saint-Hillier Y., Chalopin J. M. (2002). Lymphocyte subsets and assessment of cancer risk in renal transplant recipients. Transpl. Int. 15, 393–396 10.1111/j.1432-2277.2002.tb00187.x [DOI] [PubMed] [Google Scholar]
- Gist G. L., Burg J. R. (1995). Trichloroethylene–a review of the literature from a health effects perspective. Toxicol. Ind. Health 11, 253–307 [DOI] [PubMed] [Google Scholar]
- IARC. (1995). Dry Cleaning, Some Chlorinated Solvents, and Other Industrial Chemicals. Lyon: International Agency for Research on Cancer; [PMC free article] [PubMed] [Google Scholar]
- Iavicoli I., Marinaccio A., Carelli G. (2005) Effects of occupational trichloroethylene exposure on cytokine levels in workers. J. Occup. Environ. Med. 47, 453–457 10.1097/01.jom.0000161728.23285.66 [DOI] [PubMed] [Google Scholar]
- Kelsh M. A., Alexander D. D., Mink P. J., Mandel J. H. (2010). Occupational trichloroethylene exposure and kidney cancer: a meta-analysis. Epidemiology 21, 95–102 10.1097/EDE.0b013e3181c30e92 [DOI] [PubMed] [Google Scholar]
- Lan Q., Zhang L., Tang X., Shen M., Smith M. T., Qiu C., Ge Y., Ji Z., Xiong J., He J., Reiss B., Hao Z., Liu S., Xie Y., Guo W., Purdue M. P., Galvan N., Xin K. X., Hu W., Beane Freeman L. E., Blair A. E., Li L., Rothman N., Vermeulen R., Huang H. (2010). Occupational exposure to trichloroethylene is associated with a decline in lymphocyte subsets and soluble CD27 and CD30 markers. Carcinogenesis 31, 1592–1596 10.1093/carcin/bgq121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. (2004). Control of T cell viability. Annu. Rev. Immunol. 22, 765–787 10.1146/annurev.immunol.22.012703.104554 [DOI] [PubMed] [Google Scholar]
- Purdue M. P., Bakke B., Stewart P., De Roos A. J., Schenk M., Lynch C. F., Bernstein L., Morton L. M., Cerhan J. R., Severson R. K., Cozen W., Davis S., Rothman N., Hartge P., Colt J. S. (2010). A case-control study of occupational exposure to trichloroethylene and non-hodgkin lymphoma. Environ. Health Perspect. 119, 232–238 10.1289/ehp.1002106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaschou-Nielsen O., Hansen J., McLaughlin J. K., Kolstad H., Christensen J. M., Tarone R. E., Olsen J. H. (2003). Cancer risk among workers at Danish companies using trichloroethylene: a cohort study. Am. J. Epidemiol. 158, 1182–1192 10.1093/aje/kwg282 [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. (2000). Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol. Rev. 177, 134–140 10.1034/j.1600-065X.2000.17717.x [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lenig D., Forster R., Lipp M., Lanzavecchia A. (1999). Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 10.1038/44385 [DOI] [PubMed] [Google Scholar]
- Scott C. S., Chiu W. A. (2006). Trichloroethylene cancer epidemiology: a consideration of select issues. Environ. Health Perspect. 114, 1471–1478 10.1289/ehp.8949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B., Kassiotis G., Bourgeois C. (2004). CD4 T-cell memory. Semin. Immunol. 16, 295–303 10.1016/j.smim.2004.08.010 [DOI] [PubMed] [Google Scholar]
- Wartenberg D., Reyner D., Scott C. S. (2000). Trichloroethylene and cancer: epidemiologic evidence. Environ. Health Perspect. 108(Suppl. 2), 161–176 10.1289/ehp.00108s2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Schaum J. (2000). Exposure assessment of trichloroethylene. Environ. Health Perspect. 108(Suppl. 2), 359–363 10.1289/ehp.00108s2359 [DOI] [PMC free article] [PubMed] [Google Scholar]