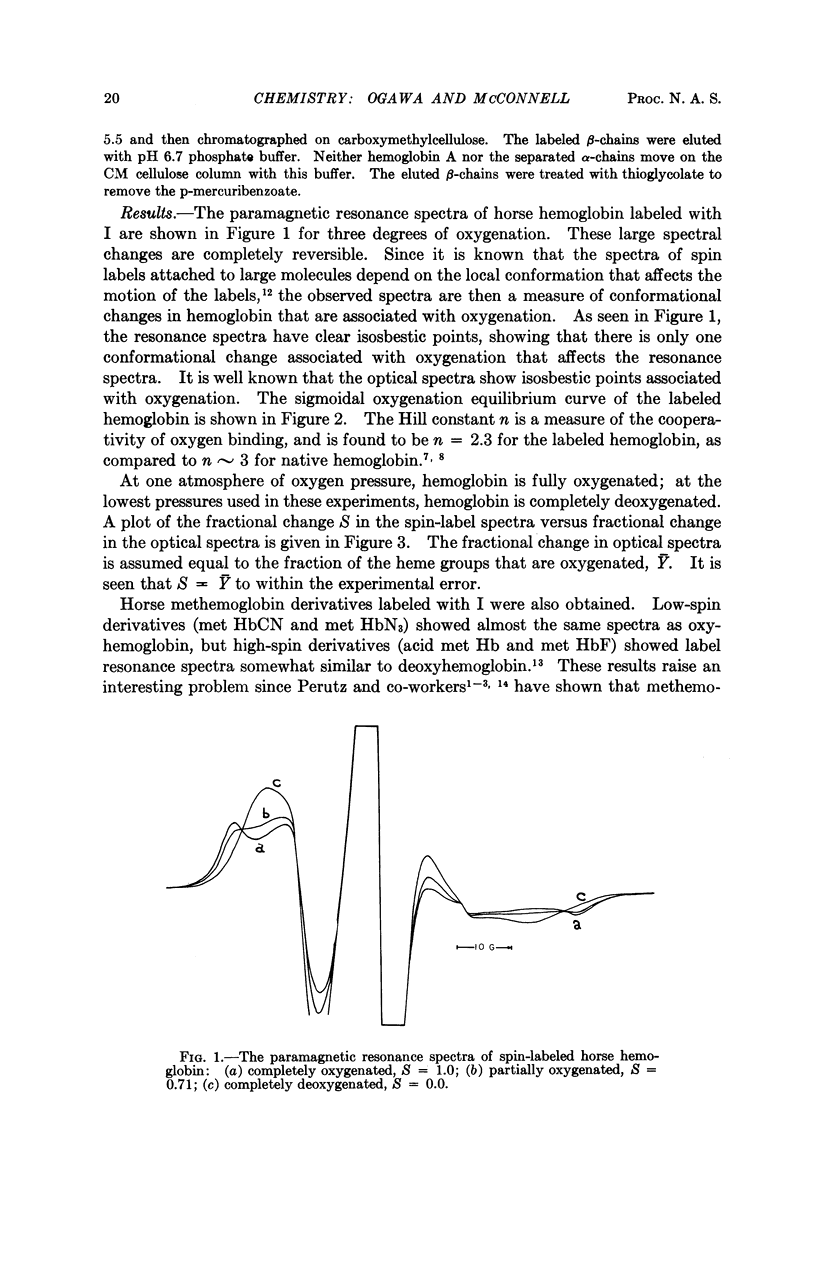
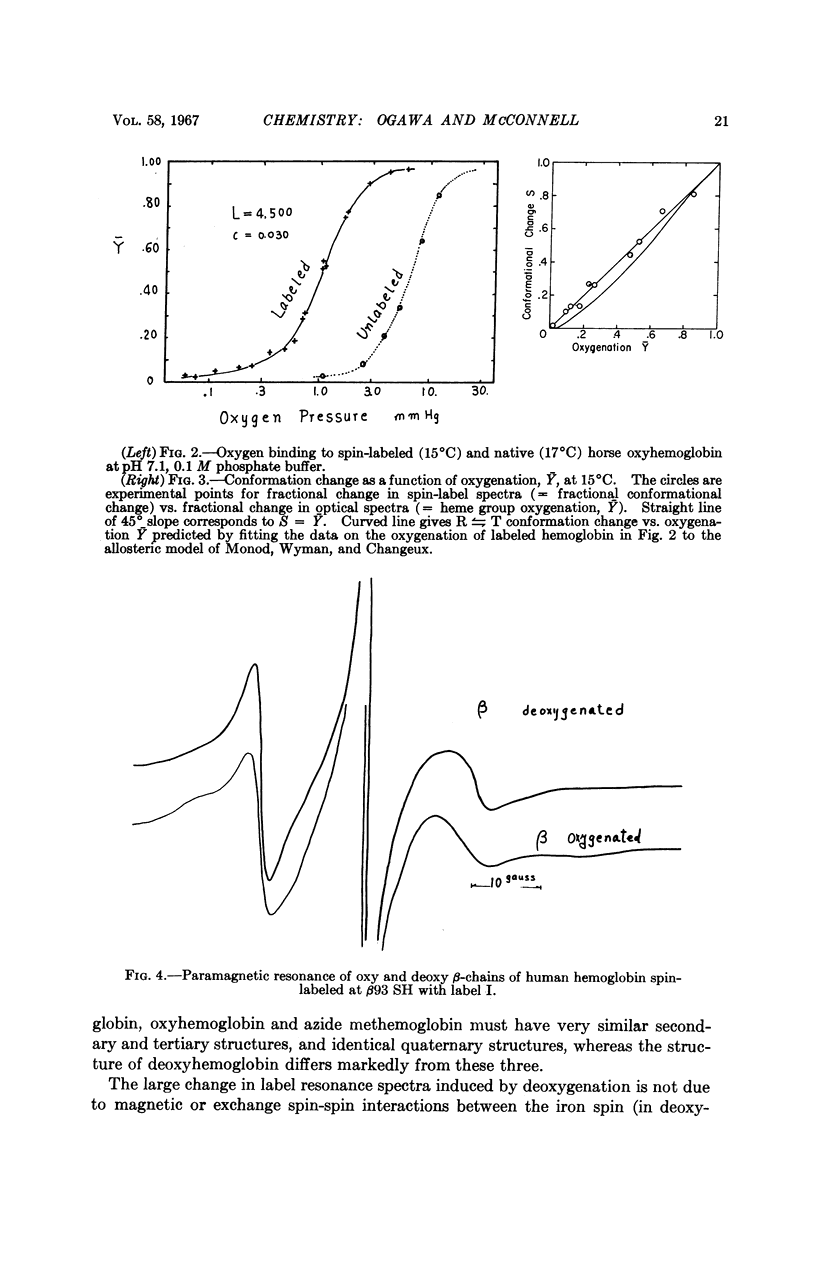
Full text
PDF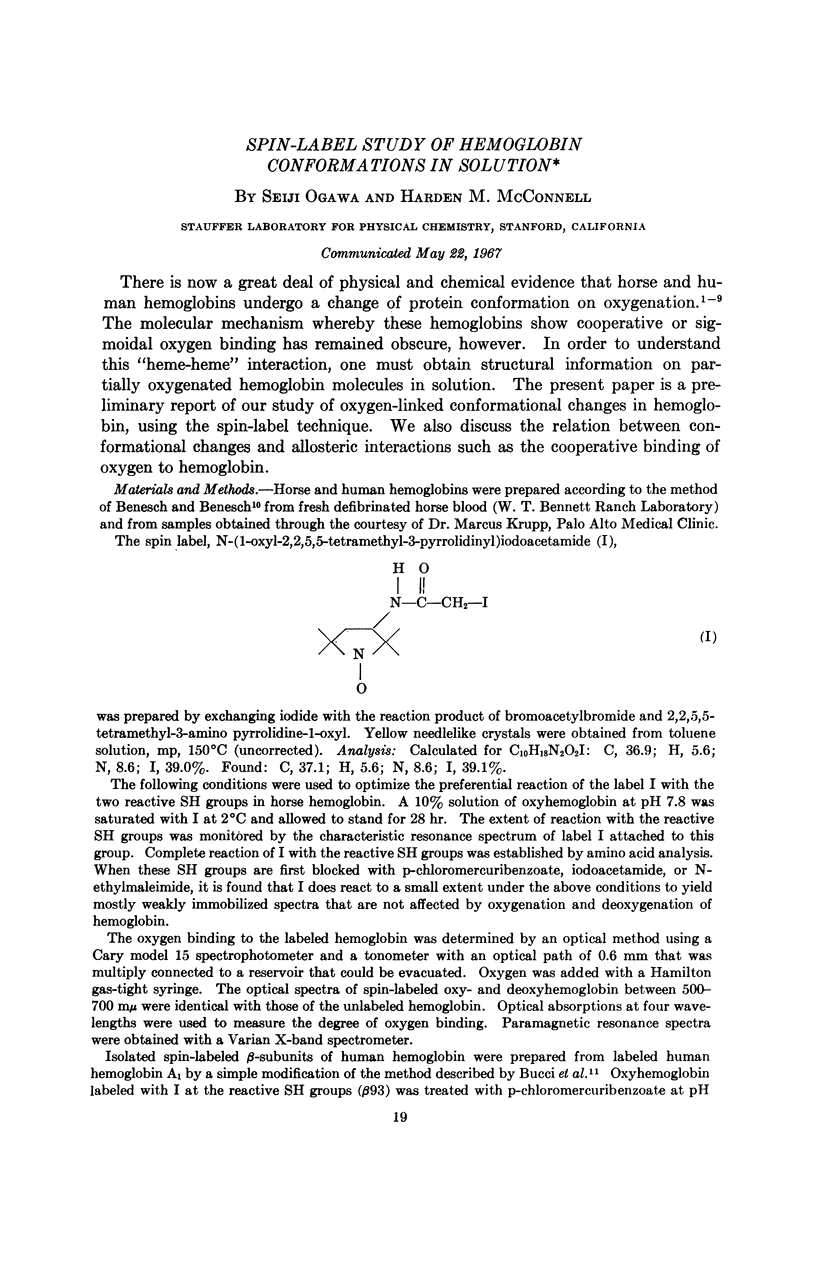





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENESCH R. E., BENESCH R. The influence of oxygenation on the reactivity of the--SH groups of hemoglobin. Biochemistry. 1962 Sep;1:735–738. doi: 10.1021/bi00911a002. [DOI] [PubMed] [Google Scholar]
- BUCCI E., FRONTICELLI C., CHIANCONE E., WYMAN J., ANTONINI E., ROSSI-FANELLI A. THE PROPERTIES AND INTERACTIONS OF THE ISOLATER ALPHA AND BETA CHAINS OF HUMAN HAEMOGLOBIN. I. SEDIMENTATION AND ELECTROPHORETIC BEHAVIOUR. J Mol Biol. 1965 May;12:183–192. doi: 10.1016/s0022-2836(65)80292-3. [DOI] [PubMed] [Google Scholar]
- KENDREW J. C. Myoglobin and the structure of proteins. Science. 1963 Mar 29;139(3561):1259–1266. doi: 10.1126/science.139.3561.1259. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- MUIRHEAD H., PERUTZ M. F. STRUCTURE OF HAEMOGLOBIN. A THREE-DIMENSIONAL FOURIER SYNTHESIS OF REDUCED HUMAN HAEMOGLOBIN AT 5-5 A RESOLUTION. Nature. 1963 Aug 17;199:633–638. doi: 10.1038/199633a0. [DOI] [PubMed] [Google Scholar]
- McConnell H. M., Boeyens J. C. Spin-label determination of enzyme symmetry. J Phys Chem. 1967 Jan;71(1):12–14. doi: 10.1021/j100860a002. [DOI] [PubMed] [Google Scholar]
- Ohnishi S., Boeyens J. C., McConnell H. M. Spin-labeled hemoglobin crystals. Proc Natl Acad Sci U S A. 1966 Sep;56(3):809–813. doi: 10.1073/pnas.56.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERUTZ M. F. Relation between structure and sequence of haemoglobin. Nature. 1962 Jun 9;194:914–917. doi: 10.1038/194914a0. [DOI] [PubMed] [Google Scholar]
- Pauling L., Coryell C. D. The Magnetic Properties and Structure of Hemoglobin, Oxyhemoglobin and Carbonmonoxyhemoglobin. Proc Natl Acad Sci U S A. 1936 Apr;22(4):210–216. doi: 10.1073/pnas.22.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F., Mathews F. S. An x-ray study of azide methaemoglobin. J Mol Biol. 1966 Oct 28;21(1):199–202. doi: 10.1016/0022-2836(66)90088-x. [DOI] [PubMed] [Google Scholar]
- RIGGS A. The binding of N-ethylmaleimide by human hemoglobin and its effect upon the oxygen equilibrium. J Biol Chem. 1961 Jul;236:1948–1954. [PubMed] [Google Scholar]
- ROSSIFANELLI A., ANTONINI E., CAPUTO A. HEMOGLOBIN AND MYOGLOBIN. Adv Protein Chem. 1964;19:73–222. doi: 10.1016/s0065-3233(08)60189-8. [DOI] [PubMed] [Google Scholar]
- Rosemeyer M. A., Huehns E. R. On the mechanism of the dissociation of haemoglobin. J Mol Biol. 1967 Apr 28;25(2):253–273. doi: 10.1016/0022-2836(67)90141-6. [DOI] [PubMed] [Google Scholar]
- Stone T. J., Buckman T., Nordio P. L., McConnell H. M. Spin-labeled biomolecules. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1010–1017. doi: 10.1073/pnas.54.4.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts-Tobin R. J. The oxidation of haemoglobin. J Mol Biol. 1967 Feb 14;23(3):305–322. doi: 10.1016/s0022-2836(67)80107-4. [DOI] [PubMed] [Google Scholar]


