Abstract
BACKGROUND
The ISEL (Iressa Survival Evaluation in Lung Cancer) clinical trial evaluated the efficacy of gefitinib versus placebo in pretreated nonsmall-cell lung cancer patients. Two different antibodies, scoring systems, and cutoff points of epidermal growth factor receptor (EGFR) protein expression were compared to predict response and survival of enrolled patients.
METHODS
EGFR expression was assessed in tumor samples by immunohistochemistry using the Dako EGFR pharmDx kit (scoring percent of tumor cells with positive staining) and Zymed monoclonal antibody clone 31G7 (scoring staining index derived from proportion of positive cells times staining intensity).
RESULTS
Data for EGFR expression were available for 379 patients for Dako and 357 patients for Zymed antibody (22% and 21%, respectively, of trial population). Objective response rates in gefitinib-treated EGFR-positive patients defined with various cutpoints with Dako antibody varied between 8% and 12%, and with Zymed antibody between 10% and 13%. Lower cutoff points with Dako antibody provided the best discrimination between EGFR-positive and EGFR-negative patients for survival hazard ratios comparing gefitinib to placebo, with a significant treatment/cutoff point interaction for 10% cutoff point (P = .049). A similar but less apparent trend was noted for Zymed antibody, although the discrimination between hazard ratios was not significant for any cutoff point analyzed.
CONCLUSIONS
Assessment with the Dako PharmDx kit and percentage of cells with positive staining may provide more accurate prediction of differential effect on survival with gefitinib than assessment with Zymed antibody and staining index. Using higher cutpoints to define positivity does not improve test discrimination.
Keywords: nonsmall-cell lung cancer, epidermal growth factor receptor, immunohistochemistry, phase 3 trial, cutoff point
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs, gefitinib and erlotinib) are active in a subset of nonsmall-cell lung cancer (NSCLC) patients. The response rates and disease control rates reported in clinical trials of EGFR TKIs in advanced pretreated NSCLC patients in Western populations are 10% to 20% and 40%, respectively,1–3 indicating that a proportion of NSCLC patients do not derive any benefit from EGFR TKIs. A major research effort over the last decade focused on the identification of predictive biomarkers for response and survival benefit to EGFR TKIs, and many of these studies analyzed EGFR protein expression by immunohistochemistry as the most applicable method to assess the presence of molecular target in the tumor.
Results of the ISEL (Iressa Survival Evaluation in Lung cancer) phase 3 clinical trial in advanced NSCLC patients who were refractory to or intolerant of their latest chemotherapy regimen showed some improvement in survival with gefitinib (plus best supportive care), which failed to reach statistical significance compared with placebo (plus best supportive care), in the overall population and in patients with adenocarcinoma.4 Preplanned subgroup analysis of the ISEL demonstrated a statistically significant increase in survival with gefitinib in patients of Asian ethnicity and in patients who had never smoked.
A biomarker analysis of this study demonstrated a nonsignificant 23% reduction in the risk of death for gefitinib-treated patients who expressed EGFR protein as assessed by the EGFR Dako PharmDx kit with a cutoff point of 10% of cells exhibiting the staining of at least slight intensity.5 No benefit was observed in the subset of patients who were classified as EGFR protein-negative. The National Cancer Institute of Canada BR.21 clinical trial that demonstrated a significant improvement in survival of erlotinib versus placebo-treated advanced NSCLC patients who failed at least 1 chemotherapy regimen6 showed a 32% reduction in the risk of death for patients with EGFR protein-positive tumor samples.7 No survival advantage was seen among patients with EGFR protein-negative tumor samples. This study also used a cutoff point of 10% stained cells and the Dako PharmDx kit. In a retrospective evaluation of gefitinib-treated NSCLC patients, Cappuzzo et al.8 used Zymed anti-EGFR monoclonal antibody and a staining index that takes into account the percent of positive cells and staining intensity, scored from 0 to 4. Using a cutpoint of 200 on the scale from 0 to 400, superior survival was demonstrated in EGFR protein-positive versus negative patients (P = .01). However, other studies performed on tumor samples from phase 3 clinical trials investigating the combination of gefitinib or erlotinib with chemotherapy failed to show any predictive value of EGFR protein expression for either clinical response or survival.9,10 Also, there was no association with EGFR protein expression and survival for NSCLC patients who received gefitinib monotherapy in the phase 2 clinical studies IDEAL1 and 2 (Iressa Dose Evaluation in Advanced Lung cancer).11
Clinical trials of cetuximab, a monoclonal antibody targeted against the EGFR in both lung and colorectal cancer required EGFR protein expression in tumor samples for study entry in most trials. EGFR protein expression was evaluated by the EGFR PharmDx kit with cutoff points of at least 1+ (at least 1% or at least 10% of cells with weak staining according to individual study). More than 90% of screened patients were scored as EGFR protein-positive in phase 2 clinical studies with cetuximab in lung cancer12–14 and >75% in phase 2 or 3 colorectal cancer trials.15,16 Because these trials were performed largely in EGFR protein-positive patients, the efficacy of cetuximab in EGFR-negative patients remains unknown, although 1 report suggests that EGFR protein-negative colorectal cancer patients may respond to cetuximab.17 In a pivotal phase 3 clinical trial comparing cetuximab versus cetuximab and irinotecan in metastatic colorectal cancer that was refractory to treatment with irinotecan, the degree of EGFR staining did not associate with response rates in either study groups.15
The presence of mutations in the EGFR gene has also been investigated in tumor samples and is linked with increased responsiveness to EGFR TKIs in numerous NSCLC studies.5,18–20 An additional approach of measuring the EGFR gene copy number in tumor samples has also demonstrated a survival advantage for patients with a high copy number in prospective placebo-controlled clinical trials.5,7,21 These 2 gene-based biomarkers appear to outperform EGFR protein evaluation in predicting the benefit from EGFR TKIs, but, particularly for EGFR mutations, prospective placebo-controlled clinical studies are lacking. In contrast to the above evaluations of EGFR gene mutations and copy number, EGFR immunohistochemistry is a widely applicable and inexpensive test to conduct. HER-2 protein expression evaluation by immunohistochemistry, in conjunction with HER-2 FISH assay, is used for the selection of breast cancer patients most likely to benefit from trastuzumab therapy,22 and HER-2 gene copy number has been closely associated with HER-2 protein expression.23
This biomarker study of the placebo-controlled ISEL trial has provided us the opportunity to compare 2 antibodies (Dako and Zymed), which have previously been associated with clinical outcome for NSCLC patients treated with gefitinib, and evaluate whether different cutoff levels of protein expression could improve the prediction of response and survival benefit from gefitinib.
MATERIALS AND METHODS
Clinical Study Design
The results of the ISEL study were previously published.4 This was a randomized, double-blind, phase 3 clinical trial comparing the efficacy of gefitinib 250 mg/day (plus best supportive care) versus placebo (plus best supportive care) in 1692 patients with advanced NSCLC who were refractory to or intolerant of their latest chemotherapy regimen. The associations between selected biomarkers (EGFR protein expression, EGFR gene copy number, EGFR, K-ras and B-raf gene mutations) and treatment outcome in the ISEL were also reported.5 Of the 379 patients who were evaluable for EGFR protein expression by the Dako PharmDx kit in this biomarker analysis, 177 patients were also evaluable for EGFR gene copy number and EGFR mutation, and some overlap was seen between those patients who were positive for these biomarkers.5
EGFR Protein Expression
EGFR protein expression was assessed by immunohistochemistry using the Dako EGFR PharmDx kit (Dako, Glostrup, Denmark) and Zymed mouse anti-human EGFR monoclonal antibody clone 31G7 (Zymed Laboratories, San Francisco, Calif). The staining procedures were performed according to the antibody manufacturer’s recommendations as previously reported.8,24 For scoring of samples stained with the Dako antibody, the percentage of tumor cells showing membranous staining was recorded, and a predefined cutoff point of ≥10% cells with at least weak staining intensity was applied to define protein positivity. Scoring was performed by a trained individual (D.V.P.) who was blinded to the clinical outcome data. For scoring of samples stained with the Zymed antibody, a staining index calculated as percent of stained tumor cells × average staining intensity graded from 0 to 4 was used, resulting in an index value between 0 and 400. Consistent with our previous reports,8,24 samples with a staining index of 200 or higher were predefined as EGFR protein-positive. The scoring was performed by 2 pathologists (F.R.H., W.A.F.) who were blinded to the clinical outcome data. Consensus scoring was performed for all specimens with staining index difference of 50 or more. Otherwise, the mean of the 2 readings was reported.
Statistical Analyses
Summary tables and scatterplots were obtained to explore the relation between percentage of tumor cells stained with the Dako antibody and the staining index with the Zymed antibody.
An evaluation of different cutoff points of EGFR expression with Dako to predict overall survival was performed using a Cox Proportional Hazards regression model. Separate models were fitted for the different cutoff points used to define EGFR protein-positive. The fitted models allowed for the effect of treatment and included terms for histology, sex, smoking history, reason for prior chemotherapy failure, number of prior chemotherapy regimens, and performance status. From the fitted models a hazard ratio and 95% confidence interval, for gefitinib versus placebo were calculated for patients with EGFR-positive and -negative tumor samples for each of the different cutoff points analyzed. A treatment by EGFR expression interaction was assessed to determine whether the treatment effect is consistent across the EGFR protein-positive and -negative tumor samples as defined by different cutoff points. This therefore reflects the degree of discrimination between positive and negative tumor samples for each cutoff point. As the analysis performed was exploratory in nature no adjustments for multiple testing were made.
The number of patients, deaths, response rate, and disease control rate for gefitinib and placebo were also calculated for EGFR-positive and -negative tumor samples, as defined by different cutoff points.
The evaluation of different cutoff points of EGFR expression with Dako was repeated to evaluate different cutoff points of EGFR expression with Zymed antibody scoring index.
A correlation coefficient between the staining index with the Zymed antibody as assessed by 2 pathologists (F.R.H., W.A.F.) was calculated. In order to estimate the inter- and intrareader components of variability a random effects model was fitted to the data with reader effects taken as random and subject effects as fixed.
RESULTS
Patient Characteristics
EGFR protein expression was assessed by the Dako PharmDx kit in 379 patients (22.4% of all study subjects) and using Zymed clone 31G7 antibody in 357 patients (21.0% of all study subjects). Data for both antibodies were available for 296 patients (17.5%). Demographic and baseline characteristics of patients evaluable for expression of EGFR with the Dako or Zymed antibody compared with overall study population are presented in Table 1. The subset of patients analyzed for protein expression was comparable to overall study population with the exception of never-smokers and patients of Asian origin, who are underrepresented in our analysis.
TABLE 1.
Demographics and Clinical Outcome With Gefitinib for Patients With Evaluable Tissue Samples for EGFR Protein Expression Studies Versus the Overall Study Population
| Characteristic | EGFR protein expression by dako pharm Dx kit, n = 379 No. (%) |
EGFR protein expression by Zymed clone 31G7 antibody, n = 357 No. (%) |
Overall study population, n = 1692 No. (%) |
|---|---|---|---|
| Adenocarcinoma | 168 (44.3) | 150 (42.0) | 812 (48.0) |
| Female | 122 (32.2) | 111 (31.1) | 553 (32.7) |
| WHO PS 0 or 1 | 233 (61.5) | 218 (61.1) | 1126 (66.5) |
| Never smoker | 51 (13.5) | 49 (13.7) | 375 (22.2) |
| Second-line* | 177 (46.7) | 175 (49.0) | 823 (48.6) |
| Asian origin† | 21 (5.5) | 13 (3.6) | 342 (20.2) |
| Refractory‡ | 335 (88.4) | 314 (88.0) | 1523 (90.0) |
| HR for survival [95% CI]§ | 0.94 [0.71. 1.25] | 0.91 [0.68. 1.23] | 0.86 [0.76. 0.99] |
| Response rate on gefitinib | (6.2) | (8.9) | (8.0) |
EGFR indicates epidermal growth factor receptor; WHO, world health organization; HR, hazard ratio; CI, confidence interval.
Second-line refers to patients who received 1 previous line of chemotherapy.
The definition of Asian racial origin excludes those of Indian origin and refers to the racial origin of a patient group and not necessarily their place of birth.
Refractory defined as recurrent or progressive disease (clinical or radiological) while receiving or within 90 days of last dose of chemotherapy.
From Cox regression analysis with stratification factors; HR<1 are in favor of gefitinib.
Comparison of Antibodies for Protein Expression Evaluation
A correlation coefficient between the staining index with the Zymed antibody as assessed by 2 investigators was calculated as 0.96 (P < .001).
The resulting components of variation for the interreader and intrareader (interpatient) variation in determining the staining index with the Zymed antibody were 5.4 and 876.7 respectively. The intrareader variability component is very small compared with the variability between patients, indicating good consistency between readers.
Using predefined criteria for protein positivity (≥10% of cells with any positive staining for Dako and staining index ≥200 for Zymed antibody), 70% (264 of 379) of tumor samples were scored as positive with Dako and 68% (244 of 357) were scored as positive with Zymed antibody. For the samples with available data for both antibodies the agreement between assessments was 76% (Table 2). The proportion of tumor samples showing no staining of any intensity with the Dako antibody was 26% as compared with 4% for the Zymed antibody. The relation between both indices appears to be cubic, and the majority of the best fit cubic line is in the region where both scores are positive or both are negative (data not shown).
TABLE 2.
Agreement Between EGFR Assessments With Dako and Zymed Antibodies Using Predefined Criteria for Protein Expression Positivity
| Zymed+ No. (%) | Zymed− No. (%) | Total No. (%) | |
|---|---|---|---|
| Dako+N (%) | 173 (58) | 39 (13) | 212 (72) |
| Dako−N (%) | 32 (11) | 52 (18) | 84 (28) |
| Total N (%) | 205 (69) | 91 (31) | 296 (100) |
EGFR indicates epidermal growth factor receptor.
Assessment With Dako PharmDx Kit, Zymed Antibody, and Clinical Outcome With Gefitinib
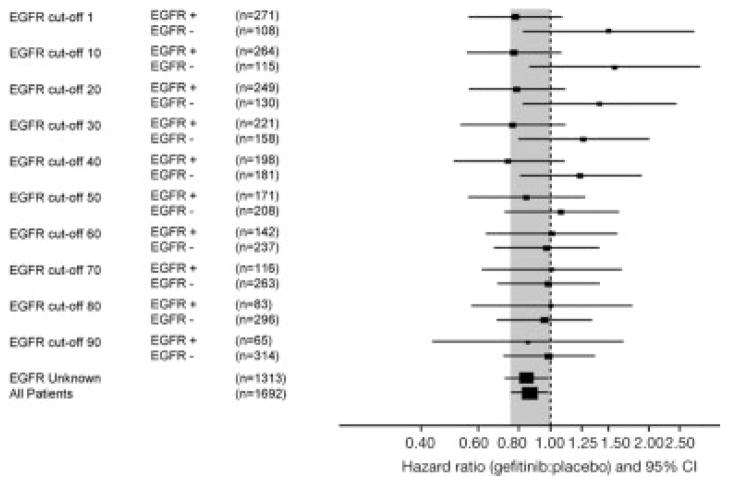
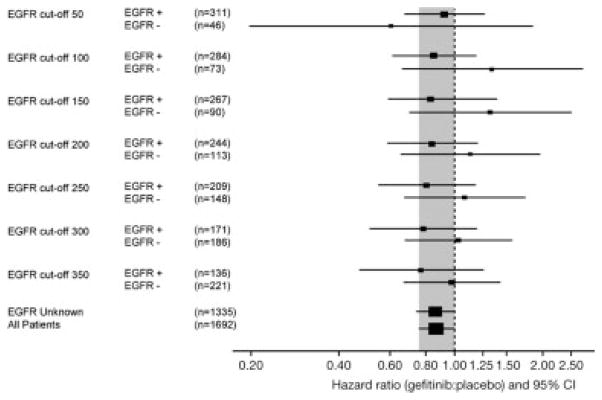
Objective response rates to gefitinib according to various cutoff points of EGFR immunostaining with Dako and Zymed antibody are shown in Tables 3 and 4. For the Dako antibody the objective response rates for EGFR protein-positive/negative patients treated with gefitinib were 8.0%/1.6% with a cutoff point of ≥1% of positive cells and 10.0%/5.3% with a cutoff point ≥90% positive cells, respectively. For the Zymed antibody the objective response rates with gefitinib were 9.9%/3.0% with the cutoff staining index ≥50 and 12.8%/6.3% with the cutoff staining index ≥350, respectively. Disease control rates were similar for patients with EGFR-positive tumors across all cutoff points analyzed for both antibodies. The survival hazard ratios (HRs) for gefitinib versus placebo according to the cutoff points are presented in Tables 3 and 4 and Figures 1 and 2. For the Dako PharmDx kit, and percent of positive staining, the largest differences between EGFR protein-positive and -negative HRs for survival were with low cutoff levels, indicating better discrimination between positive and negative subgroups at low cutoff levels. The originally predefined cutoff point ≥10% was the only cutoff with a P-value of less than 5% (P = .049) in the interaction test. Similar tendency was noted for the Zymed antibody, although the differences between survival HRs appeared smaller and were nonsignificant in the interaction test for any cutoff point analyzed.
TABLE 3.
Objective Response Rates, Disease Control Rates, and Survival Hazard Ratios (HRs) With Gefitinib Versus Placebo for EGFR Protein Expression (Percent of Positive Staining) With Dako PharmDx Kit
| EGFR staining cutoff level | EGFR subgroup | No. of patients | No. of deaths | Survival HR (95% CI)* | Interaction test P | Gefitinib responders No. (%) | Placebo responders No. (%) | Gefitinib disease control rate No. (%) | Placebo disease control rate No. (%) |
|---|---|---|---|---|---|---|---|---|---|
| EGFR cutoff 1 | EGFR+ | 271 | 167 | 0.78 (0.56–1.08) | .067 | 13 (8) | 1 (1.5) | 60 (36.8) | 26 (38.8) |
| EGFR cutoff 1 | EGFR− | 108 | 65 | 1.51 (0.82–2.77) | 1 (1.6) | 0 (0) | 18 (28.1) | 10 (34.5) | |
| EGFR cutoff 10 | EGFR+ | 264 | 166 | 0.77 (0.56–1.08) | .049 | 13 (8.2) | 1 (1.5) | 57 (36.1) | 25 (37.9) |
| EGFR cutoff 10 | EGFR− | 115 | 66 | 1.57 (0.86–2.87) | 1 (1.4) | 0 (0) | 21 (30.4) | 11 (36.7) | |
| EGFR cutoff 20 | EGFR+ | 249 | 156 | 0.79 (0.56–1.11) | .114 | 13 (8.7) | 1 (1.7) | 55 (36.7) | 21 (35) |
| EGFR cutoff 20 | EGFR− | 130 | 76 | 1.42 (0.82–2.44) | 1 (1.3) | 0 (0) | 23 (29.9) | 15 (41.7) | |
| EGFR cutoff 30 | EGFR+ | 221 | 138 | 0.77 (0.53–1.11) | .129 | 13 (9.4) | 1 (2) | 54 (38.8) | 16 (31.4) |
| EGFR cutoff 30 | EGFR− | 158 | 94 | 1.27 (0.8–2) | 1 (1.1) | 0 (0) | 24 (27.3) | 20 (44.4) | |
| EGFR cutoff 40 | EGFR+ | 198 | 124 | 0.75 (0.51–1.1) | .135 | 12 (9.6) | 1 (2.2) | 53 (42.4) | 14 (30.4) |
| EGFR cutoff 40 | EGFR− | 181 | 108 | 1.24 (0.81–1.9) | 2 (2) | 0 (0) | 25 (24.5) | 22 (44) | |
| EGFR cutoff 50 | EGFR+ | 171 | 111 | 0.84 (0.56–1.28) | .555 | 10 (9.3) | 1 (2.4) | 43 (39.8) | 14 (33.3) |
| EGFR cutoff 50 | EGFR− | 208 | 121 | 1.08 (0.72–1.63) | 4 (3.4) | 0 (0) | 35 (29.4) | 22 (40.7) | |
| EGFR cutoff 60 | EGFR+ | 142 | 94 | 1.01 (0.63–1.61) | .756 | 8 (8.7) | 1 (3.1) | 37 (40.2) | 10 (31.3) |
| EGFR cutoff 60 | EGFR− | 237 | 138 | 0.97 (0.67–1.41) | 6 (4.4) | 0 (0) | 41 (30.4) | 26 (40.6) | |
| EGFR cutoff 70 | EGFR+ | 116 | 81 | 1.01 (0.62–1.65) | .802 | 7 (9.5) | 1 (3.4) | 30 (40.5) | 9 (31) |
| EGFR cutoff 70 | EGFR− | 263 | 151 | 0.99 (0.69–1.41) | 7 (4.6) | 0 (0) | 48 (31.4) | 27 (40.3) | |
| EGFR cutoff 80 | EGFR+ | 83 | 60 | 1.01 (0.57–1.78) | .857 | 6 (11.5) | 0 (0) | 20 (38.5) | 4 (21.1) |
| EGFR cutoff 80 | EGFR− | 296 | 172 | 0.96 (0.69–1.34) | 8 (4.6) | 1 (1.3) | 58 (33.1) | 32 (41.6) | |
| EGFR cutoff 90 | EGFR+ | 65 | 44 | 0.85 (0.43–1.68) | .760 | 4 (10) | 0 (0) | 17 (42.5) | 1 (7.1) |
| EGFR cutoff 90 | EGFR− | 314 | 188 | 0.99 (0.72–1.37) | 10 (5.3) | 1 (1.2) | 61 (32.6) | 35 (42.7) | |
| EGFR unknown | — | 1313 | 744 | 0.84 (0.73–0.98) | — | 63 (8.6) | 5 (1.3) | 303 (41.4) | 118 (30.7) |
| All patients | — | 1692 | 976 | 0.86 (0.76–0.99) | — | 77 (8) | 6 (1.3) | 381 (39.7) | 154 (32.1) |
EGFR indicates epidermal growth factor receptor; CI, confidence interval.
From Cox regression analysis with stratification factors; HR < 1 are in favor of gefitinib.
TABLE 4.
Objective Response Rates, Disease Control Rates, and Survival Hazard Ratios (HRs) With Gefitinib Versus Placebo for EGFR Protein Expression (Staining Index) With Zymed Clone 31G7 Antibody
| EGFR staining cutoff level | EGFR subgroup | No. of patients | No. of deaths | Survival HR (95% CI)* | Interaction test P | Gefitinib responders No. (%) of patients | Placebo responders No. (%) of patients | Gefitinib disease control rate No. (%) of patients | Placebo disease control rate No. (%) of patients |
|---|---|---|---|---|---|---|---|---|---|
| EGFR cutoff 50 | EGFR+ | 311 | 182 | 0.92 (0.67–1.26) | .715 | 18 (9.9) | 1 (1.6) | 72 (39.8) | 28 (32.6) |
| EGFR cutoff 50 | EGFR− | 46 | 25 | 0.61 (0.2–1.85) | 1 (3) | 0 (0) | 15 (45.5) | 3 (37.5) | |
| EGFR cutoff 100 | EGFR+ | 284 | 164 | 0.85 (0.61–1.18) | .483 | 18 (10.8) | 1 (1.9) | 68 (40.7) | 24 (31.6) |
| EGFR cutoff 100 | EGFR− | 73 | 43 | 1.34 (0.66–2.73) | 1 (2.1) | 0 (0) | 19 (40.4) | 7 (38.9) | |
| EGFR cutoff 150 | EGFR+ | 267 | 157 | 0.83 (0.59–1.16) | .310 | 17 (10.8) | 1 (2.5) | 64 (40.5) | 24 (34.3) |
| EGFR cutoff 150 | EGFR− | 90 | 50 | 1.32 (0.7–2.5) | 2 (3.6) | 0 (0) | 23 (41.1) | 7 (29.2) | |
| EGFR cutoff 200 | EGFR+ | 244 | 144 | 0.84 (0.59–1.2) | .502 | 16 (11) | 1 (3.1) | 60 (41.1) | 22 (34.4) |
| EGFR cutoff 200 | EGFR− | 113 | 63 | 1.13 (0.66–1.96) | 3 (4.4) | 0 (0) | 27 (39.7) | 9 (30) | |
| EGFR cutoff 250 | EGFR+ | 209 | 123 | 0.8 (0.55–1.17) | .483 | 16 (12.6) | 5 (1.3) | 53 (41.7) | 16 (29.6) |
| EGFR cutoff 250 | EGFR− | 148 | 84 | 1.09 (0.68–1.75) | 3 (3.4%) | 6 (1.3) | 34 (39.1) | 15 (37.5%) | |
| EGFR cutoff 300 | EGFR+ | 171 | 103 | 0.78 (0.51–1.19) | .582 | 13 (12.1) | 0 (0) | 46 (43) | 13 (32.5) |
| EGFR cutoff 300 | EGFR− | 186 | 104 | 1.03 (0.68–1.57) | 6 (5.6%) | 0 (0) | 41 (38.3) | 18 (33.3) | |
| EGFR cutoff 350 | EGFR+ | 136 | 81 | 0.77 (0.47–1.26) | .659 | 11 (12.8) | 0 (0) | 37 (43) | 10 (31.3) |
| EGFR cutoff 350 | EGFR− | 221 | 126 | 0.98 (0.67–1.43) | 8 (6.3) | 0 (0) | 50 (39.1) | 21 (33.9) | |
| EGFR unknown | — | 1,335 | 769 | 0.86 (0.74–1) | — | 58 (7.8) | 0 (0) | 294 (39.5) | 123 (31.9) |
| All patients | — | 1,692 | 976 | 0.86 (0.76–0.99) | — | 77 (8) | 0 (0) | 381 (39.7) | 154 (32.1) |
EGFR indicates epidermal growth factor receptor; CI, confidence interval.
From Cox regression analysis with stratification factors; HR < 1 are in favor of gefitinib.
FIGURE 1.
Comparison of hazard ratios for gefitinib versus placebo in patients with epidermal growth factor receptor (EGFR)-positive versus -negative tumor samples with various cutoff points to define positivity with the Dako EGFR PharmDx kit and percentage of cells with positive staining.
FIGURE 2.
Comparison of hazard ratios for gefitinib versus placebo in patients with epidermal growth factor receptor (EGFR)-positive versus -negative tumor samples with various cutoff points to define positivity with the Zymed antibody and scoring index.
DISCUSSION
In this study we compared immunohistochemical assessments of EGFR protein expression with 2 different procedures, the Dako PharmDx kit and the Zymed clone 31G7 antibody, which are most frequently used in evaluation of NSCLC patients treated with EGFR tyrosine kinase inhibitors. We also assessed the predictive significance of using different cutoff points to define EGFR positivity for outcome of gefitinib therapy and we found that lower cutoff points are better predictors of survival outcomes than higher cutoff points for both antibodies studied.
To our knowledge, a comparison of EGFR immunostaining with Dako and Zymed antibodies in NSCLC tumor samples has not previously been published. Both antibodies are being used in ongoing and planned studies of EGFR inhibitors in NSCLC. The setting of the ISEL placebo-controlled randomized phase 3 clinical study enabled us to compare the procedures of EGFR assessment (with different antibodies but also different staining protocols and scoring criteria). Thus, this comparison reflects not only the properties of individual antibodies but also the predictive power of the procedures to distinguish the subsets of patients with different outcomes after gefitinib therapy.
According to previously established criteria of EGFR positivity for the Dako and Zymed antibodies,7,24 the proportions of patients with results for both antibodies that scored as positive is almost identical (69% and 72%, respectively). The agreement in identifying negative and positive samples is 76%, indicating that these 2 procedures identify slightly different subsets of patients. The number of patients with no staining of any intensity was higher with the Dako PharmDx kit as compared with Zymed (26% vs 4%), indicating that staining with Zymed is more sensitive compared with the Dako PharmDx kit. Similar results were reported by Penault-Llorca et al.25 in colorectal cancer specimens. The authors compared the FDA-approved Dako PharmDx kit, Zymed EGFR kit with clone 31G7 antibody, Ventana EGFR 3C6 antibody, and concluded that the Dako antibody is less sensitive than other antibodies for all cutoff points analyzed. In another study in normal colon, adenoma, and colon carcinoma samples, similar sensitivity of the EGFR PharmDx Kit and Zymed Clone 31G7 antibody was found according to manual and automatic scoring systems.26
The range of gefitinib response rates in patients with EGFR-positive were similar for both the Zymed and Dako antibodies (10%–13% and 8%–12% gefitinib responders with any cutoff level, respectively; Tables 3 and 4). Disease control rates appeared to be similar for patients with EGFR-positive across all cutoff levels analyzed for both antibodies. The Zymed antibody had a higher sensitivity to detect samples with any staining. The Dako antibody appeared to better predict the differential survival outcome with gefitinib, as indicated by a greater difference between EGFR protein-positive and -negative HRs. Although these HRs are overlapping over 1.00 for any cutoff point analyzed, it seems unlikely that patients that are EGFR-negative, defined as staining <10%, benefit from gefitinib. It should be stressed that our study did not directly compare the antibodies, for which the same methodology of staining procedure and the same scoring system should have been performed. We sought to compare different antibodies together with the staining procedures and scoring systems originally reported in large clinical studies.5–7 Applying the standard Dako cutoff of 10% to both antibodies gives 72% EGFR-positive for Dako but 94% EGFR-positive for Zymed. Applying the standard Zymed 200 cutoff to both antibodies gives 69% EGFR-positive for Zymed but 15% EGFR-positive for Dako. Therefore, the scoring systems appear unique to the antibody.
Similar observation of better prediction of survival benefit from erlotinib with lower cutoff points to define positive EGFR immunostaining was published by Clark et al.27 In their study, based on the results of the BR.21 trial and EGFR immunohistochemical staining of tumor samples with the Dako PharmDx kit, the largest differences between EGFR protein-positive and -negative HRs for survival were observed with low cutoff points of percent of positive cells, staining intensity, and staining index. These data are in agreement with the present study and do not support the selection of patients to EGFR tyrosine kinase inhibitors based on the higher cutoff points to define EGFR positivity.
Lack of association between the degree of EGFR protein expression and the effectiveness of EGFR inhibitors was demonstrated in colorectal cancer studies.15,16 Based on a specific ligand binding assay (Scatchard analysis), the presence of low- and high-affinity EGFRs was identified in tumor samples from colorectal cancer patients.28 In this study, high-affinity receptors represented a smaller fraction of the total receptor pool on tumor cells in most samples. Another report suggested that a subclass of high-affinity receptors is primarily activated after EGFR binding and is responsible for signal transduction downstream of EGFR.29 The distinction between low-and high-affinity receptors is not possible by immunohistochemistry with most anti-EGFR antibodies, including both the Dako and Zymed antibodies, which were generated from mice immunized with the purified EGFR antigen from the A-431 cell line containing both low- and high-affinity EGFRs.28–30 Thus, it is possible that the results of immunohistochemical staining may not adequately reveal the pool of biologically active EGFRs.
The type of biopsy (eg, lung biopsy vs resection specimen) may influence the results of EGFR protein expression analysis, as demonstrated recently by Taillade et al.31 In this study the authors used anti-EGFR 3C6 primary antibody from Ventana Medical Systems (Illkirch, France) and scoring based on percentage of positive staining. The concordance between EGFR protein expression in lung biopsies versus surgical specimens was poor (correlation coefficient r = 0.24, P = .17, n = 41).
In summary, we have demonstrated that EGFR immunostaining with the Dako PharmDx kit according to percent of cells with positive staining appears to better predict for survival outcome with gefitinib than Zymed antibody according to staining index. Further analysis using similar methods to our study with samples from other large randomized phase 3 studies would be useful to provide additional evidence of whether 1 immunohistochemical antibody/methodology is superior. If EGFR immunohistochemistry is to be applied for patient selection, low cutoff levels to define protein positivity should be used.
Acknowledgments
Fred R. Hirsch has participated on advisory boards and received honoraria from AstraZeneca. Helen Mann owns stock in AstraZeneca and has received honoraria and research support from AstraZeneca, Roche, Lily, and Sanofi-Aventis. Claire Watkins, Georgina Speake, and Brian Holloway own stock in AstraZeneca.
Supported by Specialized Program of Research Excellence P01-CA58187 and by AstraZeneca. R. Dziadziuszko is on leave from the Medical University of Gdansk, Poland, and is supported by a fellowship from the International Association for the Study of Lung Cancer.
We thank Nick Botwood from AstraZeneca for input to study design and critical review of the article, Emma Boning from Complete Medical Communications, who provided editorial assistance funded by AstraZeneca, and Barbara Murray from AstraZeneca for helpful comments on the article.
References
- 1.Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (the IDEAL 1 Trial) J Clin Oncol. 2003;21:2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 2.Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Soler R. Phase II clinical trial data with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib (OSI-774) in non-small-cell lung cancer. Clin Lung Cancer. 2004;6(suppl 1):S20–S23. doi: 10.3816/clc.2004.s.010. [DOI] [PubMed] [Google Scholar]
- 4.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch FR, Varella-Garcia M, Bunn PA, Jr, et al. Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:5034–5042. doi: 10.1200/JCO.2006.06.3958. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd FA, Rodrigues PJ, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 7.Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 8.Cappuzzo F, Hirsch FR, Rossi E, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005;97:643–655. doi: 10.1093/jnci/dji112. [DOI] [PubMed] [Google Scholar]
- 9.Bailey LR, Janas MSK, Bindlsev N, et al. Evaluation of epidermal growth factor receptor (EGFR) as a predictive marker in patients with non-small-cell lung cancer (NSCLC) receiving first-line gefitinib combined with platinum-based chemotherapy [abstract] J Clin Oncol. 2004;22:7013. [Google Scholar]
- 10.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 11.Bailey LR, Kris MG, Wolf M, et al. Tumor EGFR membrane staining is not clinically relevant for predicting response in patients receiving gefitinib (‘Iressa’, ZD1939) monotherapy for pretreated advanced non-small-cell lung cancer: IDEAL1 and 2 [abstract] Proc Am Assoc Cancer Res. 2003;44:LB–212. [Google Scholar]
- 12.Hanna N, Lilenbaum R, Ansari R, et al. Phase II trial of cetuximab in patients with previously treated non-small-cell lung cancer. J Clin Oncol. 2006;24:5253–5258. doi: 10.1200/JCO.2006.08.2263. [DOI] [PubMed] [Google Scholar]
- 13.Rosell R, Daniel C, Ramlau R, et al. Randomized phase II study of cetuximab in combination with cisplatin (C) and vinorelbine (V) vs. CV alone in the first-line treatment of patients (pts) with epidermal growth factor receptor (EGFR)-expressing advanced non-small-cell lung cancer (NSCLC) [abstract] J Clin Oncol. 2004;22(suppl 14S):7012. [Google Scholar]
- 14.Thienelt CD, Bunn PA, Jr, Hanna N, et al. Multicenter phase I/II study of cetuximab with paclitaxel and carboplatin in untreated patients with stage IV non-small-cell lung cancer. J Clin Oncol. 2005;23:8786–8793. doi: 10.1200/JCO.2005.03.1997. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 16.Saltz LB, Meropol NJ, Loehrer PJ, Sr, et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–1208. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 17.Chung KY, Shia J, Kemeny NE, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–1810. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 18.Mitsudomi T, Kosaka T, Endoh H, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005;23:2513–2520. doi: 10.1200/JCO.2005.00.992. [DOI] [PubMed] [Google Scholar]
- 19.Takano T, Ohe Y, Sakamoto H, et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23:6829–6837. doi: 10.1200/JCO.2005.01.0793. [DOI] [PubMed] [Google Scholar]
- 20.Taron M, Ichinose Y, Rosell R, et al. Activating mutations in the tyrosine kinase domain of the epidermal growth factor receptor are associated with improved survival in gefitinib-treated chemorefractory lung adenocarcinomas. Clin Cancer Res. 2005;11:5878–5885. doi: 10.1158/1078-0432.CCR-04-2618. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch FR, Varella-Garcia M, McCoy J, et al. Increased epidermal growth factor receptor gene copy number detected by fluorescence in situ hybridization associates with increased sensitivity to gefitinib in patients with bronchioloalveolar carcinoma subtypes: a Southwest Oncology Group Study. J Clin Oncol. 2005;23:6838–6845. doi: 10.1200/JCO.2005.01.2823. [DOI] [PubMed] [Google Scholar]
- 22.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 23.Dybdal N, Leiberman G, Anderson S, et al. Determination of HER2 gene amplification by fluorescence in situ hybridization and concordance with the clinical trials immunohistochemical assay in women with metastatic breast cancer evaluated for treatment with trastuzumab. Breast Cancer Res Treat. 2005;93:3–11. doi: 10.1007/s10549-004-6275-8. [DOI] [PubMed] [Google Scholar]
- 24.Hirsch FR, Varella-Garcia M, Bunn PA, Jr, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003;21:3798–3807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 25.Penault-Llorca F, Cayre A, Arnould L, et al. Is there an immunohistochemical technique definitively valid in epidermal growth factor receptor assessment? Oncol Rep. 2006;16:1173–1179. [PubMed] [Google Scholar]
- 26.Bhargava R, Chen B, Klimstra DS, et al. Comparison of 2 antibodies for immunohistochemical evaluation of epidermal growth factor receptor expression in colorectal carcinomas, adenomas, and normal mucosa. Cancer. 2006;106:1857–1862. doi: 10.1002/cncr.21782. [DOI] [PubMed] [Google Scholar]
- 27.Clark GM, Zborowski DM, Culbertson JL, et al. Clinical utility of epidermal growth factor receptor expression for selecting patients with advanced non-small cell lung cancer for treatment with erlotinib. J Thorac Oncol. 2006;1:837–846. [PubMed] [Google Scholar]
- 28.Francoual M, Etienne-Grimaldi MC, Formento JL, et al. EGFR in colorectal cancer: more than a simple receptor. Ann Oncol. 2006;17:962–967. doi: 10.1093/annonc/mdl037. [DOI] [PubMed] [Google Scholar]
- 29.Defize LH, Boonstra J, Meisenhelder J, et al. Signal transduction by epidermal growth factor occurs through the subclass of high affinity receptors. J Cell Biol. 1989;109:2495–2507. doi: 10.1083/jcb.109.5.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Defize LH, Arndt-Jovin DJ, Jovin TM, et al. A431 cell variants lacking the blood group A antigen display increased high affinity epidermal growth factor-receptor number, protein-tyrosine kinase activity, and receptor turnover. J Cell Biol. 1988;107:939–949. doi: 10.1083/jcb.107.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taillade L, Penault-Llorca F, Boulet T, et al. Immunohistochemical expression of biomarkers: a comparative study between diagnostic bronchial biopsies and surgical specimens of non-small-cell lung cancer. Ann Oncol. 2007;18:1043–1050. doi: 10.1093/annonc/mdm072. [DOI] [PubMed] [Google Scholar]