Abstract
An increasing number of studies suggest that distinct pools of synaptic vesicles drive specific forms of neurotransmission. Interspersed with these functional studies are analyses of the synaptic vesicle proteome which have consistently detected the presence of so-called “non-canonical” SNAREs that typically function in fusion and trafficking of other subcellular structures within the neuron. The recent identification of certain non-canonical vesicular SNAREs driving spontaneous (e.g., VAMP7 and vti1a) or evoked asynchronous (e.g., VAMP4) release integrates and corroborates existing data from functional and proteomic studies and implies that at least some complement of non-canonical SNAREs resident on synaptic vesicles function in neurotransmission. Here, we discuss the specific roles in neurotransmission of proteins homologous to each member of the classical neuronal SNARE complex consisting of synaptobrevin2, syntaxin-1 and SNAP-25.
Keywords: asynchronous neurotransmitter release, SNAREs, spontaneous neurotransmitter release, synaptic vesicle recycling, synaptobrevin, VAMP4, VAMP7, vti1a
Introduction
The classical neuronal SNARE complex, comprised of the vesicular protein synaptobrevin2 (syb2) and the plasma membrane-associated proteins syntaxin-1 and SNAP-25 mediates synaptic vesicle exocytosis.1 Studies of synaptic transmission in animals lacking individual components of the synaptic vesicle exocytic SNARE complex showed that SNAP-25, syntaxin-1 and syb2 are required for normal synaptic transmission, but some types of transmission are less dependent on these proteins. Deletion of the mouse syb2 or SNAP-25 genes leads to lethality at birth, and stimulus evoked neurotransmitter secretion is severely impaired. In contrast, forms of neurotransmission such as spontaneous neurotransmitter release and hypertonic sucrose evoked responses, where calcium does not play an instructive role, are relatively less affected by the lack of syb22 or SNAP-25.3-6 A similar phenotype was observed in flies lacking syb2.7,8 Although syb2 and SNAP-25 appear to function in concert to promote fusion, a specific role of syb2 in fast synaptic vesicle endocytosis after fusion has been described9 that is not shared by SNAP-25.3 These results indicate specific functions of related SNARE proteins during synaptic vesicle exo-endocytosis coupling, and suggest the existence of additional, non-canonical SNARE proteins involved in synaptic vesicle fusion that may preferentially support spontaneous or other forms of neurotransmission. In agreement with this notion, the secretagogue α-latrotoxin can increase the rate of spontaneous vesicle fusion without relying on the canonical SNARE machinery components, implying that a separate complement of molecules may support spontaneous transmission.10 Further support for this proposal comes from recent proteomic analyses of purified synaptic vesicles which have consistently identified many proteins homologous to those forming the classic neuronal SNARE complex that typically reside in other subcellular organelles11-15 (reviewed in ref. 16). Furthermore, a recent study using a single-molecule quantification approach to assess the intervesicle variability of several synaptic vesicle proteins found that syb2 copy number varies significantly among individual vesicles, again consistent with a role of non-canonical v-SNAREs in synaptic vesicle fusion.17
In addition to these molecular studies, a growing number of observations suggest that spontaneous and evoked transmission arise from separate synaptic vesicle pools.18,19 The spontaneously and activity-dependent recycling pools are differentially sensitive to phorbol ester regulation20,21 as well as dynamin inhibition.22 Divergence in the vesicle populations released at rest or with stimulation has also been observed in GABAergic terminals23 as well as throughout neuronal development and synaptic maturation.24,25 Furthermore, vesicles released under different forms of neurotransmission may undergo mechanistically different fusion reactions.26,27 Nevertheless, this proposal remains controversial as a number of studies have concluded that spontaneous and evoked release are dependent on the same vesicle pool.28-30 However, in light of the earlier genetic knockout studies, from a molecular perspective it is plausible that at least some fraction of vesicles driving spontaneous and evoked synaptic vesicle fusion are likely to diverge from the classical SNARE composition and thus constitute separate pools. Indeed, such molecular tags for vesicles released during specific modes of neurotransmission have recently been identified for spontaneous31,32 and evoked asynchronous33 neurotransmitter release. Below we discuss these and a number of other non-canonical synaptic SNARE proteins that have been characterized with regard to their potential role(s) in neurotransmission.
Vesicular SNAREs
Assembly of SNARE complexes catalyzes fusion by forcing membranes into close proximity.34 Although SNARE complex formation is primarily driven by hydrophobic interactions, SNARE complexes contain a central ‘zero layer’ composed of a hydrophilic electrostatic interaction mediated by three glutamine residues and one arginine residue.35 As a result, SNARE proteins are designated as Q-SNAREs (such as syntaxin-1 and SNAP-25) or R-SNAREs (such as syb2) based on their SNARE motif sequences.36 All R-SNAREs contain similar domain structures and minimally contain a SNARE motif followed by a transmembrane anchor. Subgroups of R-SNAREs contain either a short N-terminal region ahead of the SNARE motif, known as the brevins, or an extended N-terminal region of 120–140 amino acids, known as the longins.37-39 The major synaptic vesicle R-SNARE, syb2/VAMP2, exemplifies the brevin subclass of vesicular (v-) SNAREs, whereas the prototypical longin is VAMP7,38 also present in some synaptic terminals.40,41 Several R-SNAREs in addition to syb2 and VAMP7 have been detected in synaptic vesicles by mass spectrometry, including syb1/VAMP1, cellubrevin/VAMP3, VAMP4 and the structurally homologous Q-SNARE Vti1a.11,12,14,15 Many of these synaptic vesicle SNAREs have recently been shown to support membrane fusion in an in vitro assay at levels comparable to the canonical syb2,42 and accordingly each has specific roles in neurotransmitter release as outlined below.
Brevins: synaptobrevin1 and cellubrevin
Other brevins that reside on synaptic vesicles include syb1 and cellubrevin (also known as VAMP3).11,12,14,15 Similar to syb2, syb1 is predominantly expressed in the nervous system; however, while syb2 is the major isoform in the brain, syb1 is more highly expressed in the spinal cord.43,44 Syb1 and syb2 are both present in excitatory and inhibitory central nerve terminals12,45 but an investigation of the relative contributions of syb1 and syb2 to central synaptic transmission has not yet been performed. Syb1 was recently shown to mediate neuromuscular transmission.46 Interestingly, the absence of syb1 produced similar deficits in both evoked and spontaneous release at the neuromuscular junction as did the absence of syb2 in central synapses,2 though the effects were somewhat less pronounced, perhaps due to compensation by endogenous syb2 at the motor terminal.46
Cellubrevin is ubiquitously expressed and was originally described as having a function in receptor-mediated endocytosis.47 Our group has previously tested the ability of cellubrevin to rescue synaptic transmission in the syb2 knockout. Cellubrevin was able to rescue both spontaneous and evoked vesicle fusion in the syb2 knockout, but a double knockout of syb2 and cellubrevin showed no greater loss of transmission than the single knockout of syb2.26 These data demonstrated that although syb2 and cellubrevin are potentially functionally interchangeable, the remaining fusion in syb2-deficient central synapses is not mediated by cellubrevin. While cellubrevin may in fact exhibit functional redundancy in both neurons and non-neuronal tissues, recent studies implicate this protein in regulated secretion in both astrocytes48 and endothelial cells.49
Longins and related proteins: VAMP7, Vti1a and VAMP4
The longin family of R-SNAREs has a similar domain organization to that of the brevins, but these proteins also possess conserved extended N-terminal regions. VAMP7 (also known as tetanus-insensitive or TI-VAMP) is the founding member of this subfamily.38 Although vti1a and VAMP7 have similarly extended N-termini and are both vesicular SNAREs, vti1a is not technically a member of the longin family due to its designation as a Q-SNARE. The N-terminus of VAMP4 is intermediate in length between the brevins and the true longins. In both neurons and non-neuronal cells, these proteins function in fusion reactions of the endocytic pathway and reside predominantly in the Golgi apparatus, endosomes and, in the case of VAMP7, lysosomes.50-52 VAMP7 is also well-known to regulate neurite outgrowth,53-55 and a second, rapid form of neurite outgrowth has been recently described that depends on VAMP4-mediated fusion of specialized exocytic organelles called enlargeosomes.56,57
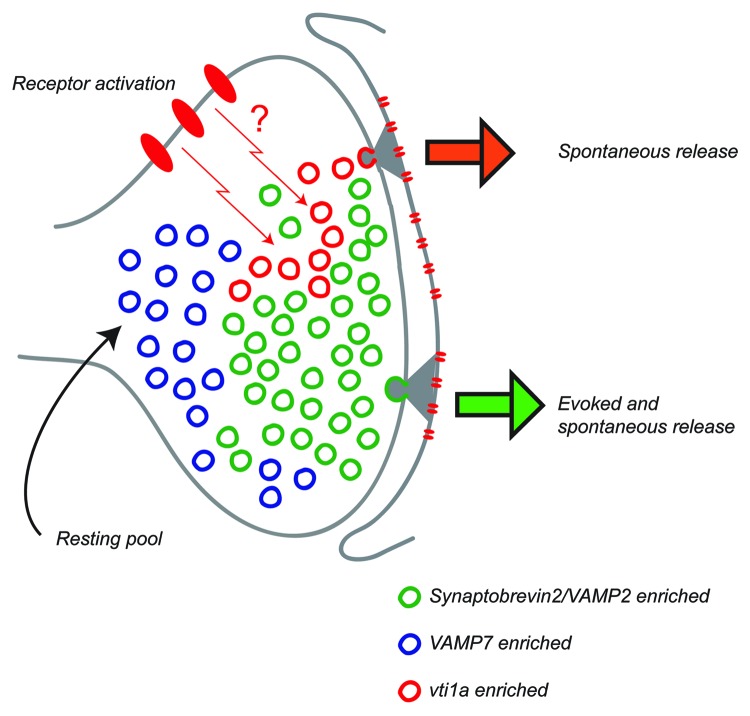
The functions of VAMP7, Vti1a and VAMP4 in neurotransmission are still emerging, but recent work has illuminated essential roles in specific types of neurotransmission for each of these proteins. VAMP7 is expressed throughout the adult brain, typically in somatodendritic compartments, but is found in presynaptic terminals in a subset of brain regions, most notably in the granule cells of the hippocampal dentate gyrus,40 where it appears to mediate a form of asynchronous neurotransmitter release at the mossy fiber terminals dependent on its proper presynaptic targeting by the adaptor complex AP-3.41 VAMP7 was recently identified as a specific marker of the resting vesicle pool which is generally unresponsive to stimulation (Fig. 1).31 VAMP7 is clearly less responsive to stimulation than a broad glutamatergic vesicle marker, VGLUT1, though some vesicles containing VAMP7 do exhibit stimulation-dependent exocytosis. A larger fraction of VAMP7-pHluorin tagged vesicles resides in the resting pool than does VGLUT1, as suggested by the observation that VAMP7 release at rest proceeded at a faster rate than that of VGLUT1, but slower than that of syb2. However, VAMP7-pHluorin appears to undergo both stimulus-evoked and spontaneous release. In agreement with this study, further analysis of the trafficking of VAMP7-pHluorin demonstrated measureable amounts of both spontaneous and evoked release of vesicles containing this protein, although both parameters were decreased relative to that of syb2 measured concurrently in the same synapses.32
Figure 1. This cartoon depicts an emerging model on the distributions of vesicular SNAREs syb2, vti1a and VAMP7 among synaptic vesicle pools. At central synapses, syb2 is the predominant vesicular SNARE that ensures rapid execution of synaptic vesicle fusion. However, loss-of-function studies of syb2 suggest that a parallel pathway involving non-canonical SNAREs may mediate fusion and recycling of a subset of vesicles. Recent studies revealed that both vti1a and VAMP7 could fulfill this role and specifically traffic at rest. Vti1a possesses a more prominent intracellular pool and more robust trafficking in the absence of activity compared with VAMP7. On the other hand, vesicles containing vti1a or VAMP7 show relatively reluctant responses to action potential evoked stimulation compared with swift mobilization of syb2-containing vesicles during evoked neurotransmission. Given their relative reluctance for mobilization VAMP7 containing vesicles could constitute at least a fraction of the vesicles within the resting pool. The co-existence of molecularly distinct synaptic vesicle populations with different fusion properties may allow certain regulatory pathways to impact a particular type of neurotransmission selectively, thereby triggering a specific cellular response. In this way, the nature of presynaptic activity can determine the impact of downstream postsynaptic signaling events.
Vti1a is another synaptic vesicle SNARE that appears to specifically mediate spontaneous neurotransmission.32 Optical imaging experiments demonstrated little mobilization of vesicles containing pHluorin-tagged vti1a during stimulation, but robust release of these vesicles at rest. The effect was confirmed electrophysiologically in both inhibitory and excitatory synapses, where loss- or gain-of function of vti1a produced bidirectional effects on spontaneous event frequency but no effect on evoked neurotransmission. These results are in agreement with a recent proteomic study comparing the protein composition of glutamatergic and GABAergic synaptic vesicles which confirmed the presence of vti1a on synaptic vesicles but found no differential expression of this protein between excitatory and inhibitory synaptic vesicles.12 Furthermore, the ability of vti1a to support spontaneous neurotransmission does not require the canonical v-SNARE, syb2, as shown by similar effects of vti1a loss or gain-of-function in neurons cultured from syb2 knockout embryos. This result, taken together with optical demonstrations of concurrent evoked release of syb2 with little response of vti1a in the same boutons, supports the segregation of vesicle pools driving spontaneous and evoked neurotransmitter release (Fig. 1). It is not surprising that two distinct non-canonical v-SNAREs (VAMP7 and vti1a) can mediate spontaneous release; indeed, as shown in syb2 knockout neurons, syb2 is quantitatively the most important v-SNARE for neurotransmitter release in all its forms, including spontaneous and stimulus-evoked synchronous and asynchronous release.2,9 From the existing data, it appears that vti1a has a more restricted localization to spontaneously releasing vesicles than does VAMP7, although electrophysiological analysis of the VAMP7 loss- or gain-of-function on spontaneous and evoked neurotransmission has not yet been performed. Even though VAMP7 and vti1a can both undergo spontaneous release, it is unlikely that these proteins are participating in the same SNARE complex to mediate vesicle fusion due to their differences in kinetics and absolute magnitudes of release.32 However, it is important to note that a SNARE complex containing VAMP7 and vti1a has been reported to function in a novel constitutive trafficking pathway in neurons.58
Recent work has identified VAMP4 as a v-SNARE specifically driving evoked asynchronous release.33 VAMP4 localization was confirmed at hippocampal synapses and VAMP4 expression was shown to rescue both evoked asynchronous release and some spontaneous release in the absence of syb2. Furthermore, up- or downregulating VAMP4 levels could directly regulate asynchronicity of the evoked responses. Finally, optical imaging experiments identified a unique trafficking pathway of this protein, whereby VAMP4-enriched vesicles are generated by endocytosis from the plasma membrane and are subsequently utilized to maintain asynchronous release during periods of intense neuronal activity (Fig. 2). It is interesting to note that a SNARE complex consisting of syntaxin-6, syntaxin-13, vti1a and VAMP4 is well-documented in the endosomal system of neurons and non-neuronal cells and functions in the retrograde transport of endosomes to the Golgi apparatus59-61 and in the homotypic fusion of early endosomes.62 Thus, in light of the ability of VAMP4 to support a small amount of spontaneous transmission in the absence of syb2, VAMP4 and vti1a may have some overlapping functions in neurotransmission. Together with the recent work described above identifying VAMP7 as a resident of the resting vesicular pool31 and vti1a as a specific mediator of spontaneous neurotransmitter release,32 it seems clear that the particular complement of v-SNAREs on synaptic vesicles can directly influence their fusion behavior.
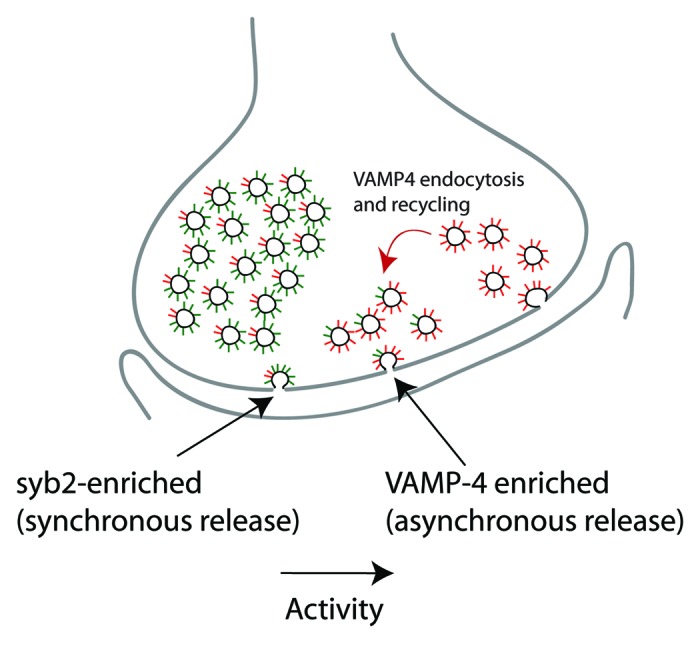
Figure 2. Recent work supports a model where the vesicle-associated SNARE VAMP4 functionally diverges from the key vesicular SNARE syb2 and predominantly maintains asynchronous release. Experiments using a combination of electrophysiology and optical imaging indicate that a small but significant population of vesicles appears to be enriched in VAMP4, follows a distinct route of stimulation-dependent trafficking facilitated by VAMP4's N-terminal di-leucine motif and selectively supports asynchronous release. According to this model, sustained activity can generate a synaptic vesicle population enriched in VAMP4. A VAMP4-dependent SNARE complex formed after recruitment of these vesicles provides a substrate upon which a Ca2+ sensor acts to drive asynchronous release.
Negative regulation of SNARE complex formation by intramolecular binding of the longin domain with the SNARE motif has been demonstrated for the longins Ykt6 and Sec22,63,64 and suggested for VAMP7.65 Indeed, exogenous expression of the longin domain of VAMP7 in PC12 cells has a dominant negative effect on neurite outgrowth, whereas overexpression of a mutant VAMP7 lacking the longin domain enhances this process.55 Furthermore, VAMP7-pHluorin lacking the longin domain facilitated both spontaneous and evoked release of VAMP7- and syb2-pHluorin and has an increased rate of spontaneous exocytosis compared with full-length VAMP7.31 These results are consistent with the increase in spontaneous excitatory release at the mossy fiber synapses in brain slices from mocha mice which are deficient in functional AP-3.41 Consistent with the effects of deleting the VAMP7 N-terminus described above, expression of truncated vti1a triggered a prominent augmentation of baseline levels of spontaneous release detected electrophysiologically, suggesting the existence of a mechanism that may relieve potential autoinhibition of vti1a.32 Autoinhibition of SNARE complex formation is a well-known feature of some syntaxins, which also possess an elongated N-terminus, although their fold is markedly different from that of the longins.37,39,66 Therefore, it is not unexpected that the extended N-termini of vti1a and VAMP7 regulate their abilities to support synaptic vesicle fusion.
Syntaxins 6, 7, 12/13 and 16
Alternative syntaxin isoforms typically involved in fusion reactions throughout the endocytic pathway have been recently detected in purified synaptic vesicle fractions by several proteomic analyses. These include syntaxin-6, syntaxin-7, syntaxin-12/13 and syntaxin-16.11,12,14,15 Each of these proteins is highly expressed in brain, but also widely distributed in other tissues.67-70 The detection of these particular endosomal syntaxins by multiple proteomic approaches in which synaptic vesicles were isolated by different methods is striking. These results suggest that they may have additional functions specific to synaptic vesicle fusion or recycling. However, none of these proteins has a documented role in neurotransmission, with the possible exception of syntaxin-13. Recent work provided evidence that synaptic vesicles belonging to the readily releasable pool (RRP) are sorted through an endosomal intermediate, and showed significant reduction of the RRP size upon expression of a dominant-negative syntaxin-13 soluble fragment.71 Thus, the endosomal syntaxins may influence neurotransmission in an indirect manner by regulating synaptic vesicle recycling. Indeed, inhibition of presynaptic endosomal recycling via the dominant-negative syntaxin-13 fragment was also reported to increase the spontaneously released vesicle pool.71 Real-time measurements of spontaneous synaptic vesicle fusion using pHluorin-tagged syntaxin-6 (presumably operating in the same SNARE complex as syntaxin-13) failed to detect any spontaneous fusion of structures containing this protein, though a small amount of trafficking was observed upon supraphysiological high potassium stimulation,32 consistent with the notion that endosomal proteins may be enriched in a subpopulation of synaptic vesicles (such as the RRP).71
SNAP-25-Related Proteins
Three proteins related to the canonical plasma membrane-anchored SNARE protein SNAP-25 have been identified, including SNAP-23, SNAP-29, and SNAP-47. Putative functions of these Qbc SNAREs in neurotransmission have not been extensively characterized but are summarized below.
SNAP-23
SNAP-23 is ubiquitously expressed,72 preferentially but promiscuously binds a number of plasma membrane-associated syntaxins,73 and assumes the essential functions of SNAP-25 in a variety of exocytic reactions in non-neuronal cells.74-76 A recent study revealed an essential role of SNAP-23 in embryogenesis, as Snap23 null mouse embryos died prior to implantation at E3.5.77 Although SNAP-23 appears to function primarily in non-neuronal cells, it has been detected in cortical neurons78 and in purified synaptic vesicles.14 SNAP-23 has been shown to support the evoked4 and basal79 release of granules in neuroendocrine cells, as well as both spontaneous and evoked asynchronous synaptic vesicle release in Snap25 null neurons,80 indicating a possible role for the endogenous protein in neurotransmission. Interestingly, the evoked asynchronous release mediated by SNAP-23 in the absence of SNAP-25 appears strikingly similar to that observed in the absence of synaptotagmin1, the fast calcium sensor.81 While the canonical neuronal SNARE complex including SNAP-25 is well known to utilize syt1 as the calcium sensor in the presence of elevated intraterminal calcium, SNAP-23 is proposed to bind another synaptotagmin isoform, syt7, which binds calcium with ~10-fold higher affinity82,83 and allows SNAP-23 to mediate granule docking and fusion at resting calcium levels.79 Thus, SNAP-23 can support synaptic vesicle fusion in the absence of SNAP-25 and may function in a SNARE complex driving asynchronous and/or spontaneous neurotransmitter release. A postsynaptic role in NMDA receptor trafficking has also been recently ascribed to SNAP-23.84 Taken together these results point to a rather ubiquitous role for SNAP-23 beyond synaptic vesicle fusion.
SNAP-29
SNAP-29 is ubiquitously expressed and localizes to multiple intracellular organelles, including the endosome, lyosome and Golgi apparatus, where it binds plasma membrane and intracellular syntaxins equally well.73,85 Due to these characteristics, SNAP-29 was proposed to be involved in general membrane trafficking reactions and was recently identified in a screen of genes required for constitutive secretion in mammalian cells.86 However, synaptic SNAP-29 has been proposed to function not in exocytosis but rather as a negative regulator of SNARE complex disassembly after fusion. This action is mediated by SNAP-29 binding to the assembled SNARE complex and competitively preventing the binding of α-SNAP, thus slowing synaptic vesicle recycling and inhibiting efficient transmission under repetitive stimulation.87,88
SNAP-47
SNAP-47 is the most recently identified protein in this subfamily, and is ubiquitously expressed but found at particularly high levels in the brain.89 It is also enriched in crude synaptic vesicle preparations as detected by immunoblotting89 and was detected in purified synaptic vesicles by mass spectrometry.14 SNAP-47 reportedly can functionally substitute for SNAP-25 by forming SNARE complexes with syntaxin1 and synaptobrevin2 in vitro and in a liposome fusion assay, although with reduced efficiency compared with SNAP-25.89 These results imply that endogenous SNAP-47 may have a role in synaptic vesicle fusion but so far none has been identified. However, regulated exocytosis in adrenal chromaffin cells lacking SNAP-25 cannot be rescued by SNAP-47 expression.89
Conclusion
The recent identification of VAMP7,31 vti1a32 and VAMP433 as molecular tags for independently functioning synaptic vesicle populations strongly supports the divergence of the synaptic vesicle pools that drive spontaneous and evoked neurotransmission (reviewed in ref. 90). These studies molecularly dissect vesicle populations within individual synapses via identification of synaptic vesicle pool-specific integral membrane proteins. This notion also extends to the postsynaptic side of the synapse, where emerging evidence points to spatially segregated receptor activation91,92 triggering independent signaling pathways downstream of spontaneous or evoked release.93,94 Therefore, it is plausible that one could target the vesicular proteins vti1a, VAMP4 or VAMP7, eliciting selective regulation of spontaneous or asynchronous neurotransmitter release without significantly altering fast synchronous neurotransmitter release. These manipulations may nevertheless trigger specific behavioral responses, as shown by a recent report of increased anxiety in mice lacking VAMP7.95 As fast synchronous release is critical for information coding and processing in the brain, any manipulation sparing this type of synaptic transmission would be expected to have limited side effects compared with more global regulation of neurotransmission. This approach has important implications for the development of novel treatment strategies targeted against neuropsychiatric disorders, as suggested by recent work describing and essential role of spontaneous neurotransmission in mediating the fast anti-depressant effects of NMDA receptor antagonists.96
Acknowledgments
This work is supported by grants from the NIMH (R01MH066198) to E.T.K and (F32MH093109) to D.M.O.R. We thank Austin Reese and other members of the Kavalali laboratory for their critical input. We also thank Dr Lisa Monteggia for her support and insightful discussions.
References
- 1.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–47. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 2.Schoch S, De´k F, Königstorfer A, Mozhayeva M, Sara Y, Südhof TC, et al. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–22. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- 3.Bronk P, De´k F, Wilson MC, Liu X, Südhof TC, Kavalali ET. Differential effects of SNAP-25 deletion on Ca2+ -dependent and Ca2+ -independent neurotransmission. J Neurophysiol. 2007;98:794–806. doi: 10.1152/jn.00226.2007. [DOI] [PubMed] [Google Scholar]
- 4.Sørensen JB, Nagy G, Varoqueaux F, Nehring RB, Brose N, Wilson MC, et al. Differential control of the releasable vesicle pools by SNAP-25 splice variants and SNAP-23. Cell. 2003;114:75–86. doi: 10.1016/S0092-8674(03)00477-X. [DOI] [PubMed] [Google Scholar]
- 5.Tafoya LC, Mameli M, Miyashita T, Guzowski JF, Valenzuela CF, Wilson MC. Expression and function of SNAP-25 as a universal SNARE component in GABAergic neurons. J Neurosci. 2006;26:7826–38. doi: 10.1523/JNEUROSCI.1866-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Benditó G, et al. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci. 2002;5:19–26. doi: 10.1038/nn783. [DOI] [PubMed] [Google Scholar]
- 7.Broadie K, Prokop A, Bellen HJ, O’Kane CJ, Schulze KL, Sweeney ST. Syntaxin and synaptobrevin function downstream of vesicle docking in Drosophila. Neuron. 1995;15:663–73. doi: 10.1016/0896-6273(95)90154-X. [DOI] [PubMed] [Google Scholar]
- 8.Deitcher DL, Ueda A, Stewart BA, Burgess RW, Kidokoro Y, Schwarz TL. Distinct requirements for evoked and spontaneous release of neurotransmitter are revealed by mutations in the Drosophila gene neuronal-synaptobrevin. J Neurosci. 1998;18:2028–39. doi: 10.1523/JNEUROSCI.18-06-02028.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De´k F, Schoch S, Liu X, Südhof TC, Kavalali ET. Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nat Cell Biol. 2004;6:1102–8. doi: 10.1038/ncb1185. [DOI] [PubMed] [Google Scholar]
- 10.De´k F, Liu X, Khvotchev M, Li G, Kavalali ET, Sugita S, et al. Alpha-latrotoxin stimulates a novel pathway of Ca2+-dependent synaptic exocytosis independent of the classical synaptic fusion machinery. J Neurosci. 2009;29:8639–48. doi: 10.1523/JNEUROSCI.0898-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burre´ J, Beckhaus T, Schägger H, Corvey C, Hofmann S, Karas M, et al. Analysis of the synaptic vesicle proteome using three gel-based protein separation techniques. Proteomics. 2006;6:6250–62. doi: 10.1002/pmic.200600357. [DOI] [PubMed] [Google Scholar]
- 12.Grønborg M, Pavlos NJ, Brunk I, Chua JJ, Münster-Wandowski A, Riedel D, et al. Quantitative comparison of glutamatergic and GABAergic synaptic vesicles unveils selectivity for few proteins including MAL2, a novel synaptic vesicle protein. J Neurosci. 2010;30:2–12. doi: 10.1523/JNEUROSCI.4074-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morciano M, Burre´ J, Corvey C, Karas M, Zimmermann H, Volknandt W. Immunoisolation of two synaptic vesicle pools from synaptosomes: a proteomics analysis. J Neurochem. 2005;95:1732–45. doi: 10.1111/j.1471-4159.2005.03506.x. [DOI] [PubMed] [Google Scholar]
- 14.Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–46. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Blondeau F, Ritter B, Allaire PD, Wasiak S, Girard M, Hussain NK, et al. Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proc Natl Acad Sci U S A. 2004;101:3833–8. doi: 10.1073/pnas.0308186101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burre´ J, Volknandt W. The synaptic vesicle proteome. J Neurochem. 2007;101:1448–62. doi: 10.1111/j.1471-4159.2007.04453.x. [DOI] [PubMed] [Google Scholar]
- 17.Mutch SA, Kensel-Hammes P, Gadd JC, Fujimoto BS, Allen RW, Schiro PG, et al. Protein quantification at the single vesicle level reveals that a subset of synaptic vesicle proteins are trafficked with high precision. J Neurosci. 2011;31:1461–70. doi: 10.1523/JNEUROSCI.3805-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fredj NB, Burrone J. A resting pool of vesicles is responsible for spontaneous vesicle fusion at the synapse. Nat Neurosci. 2009;12:751–8. doi: 10.1038/nn.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sara Y, Virmani T, De´k F, Liu X, Kavalali ET. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron. 2005;45:563–73. doi: 10.1016/j.neuron.2004.12.056. [DOI] [PubMed] [Google Scholar]
- 20.Virmani T, Ertunc M, Sara Y, Mozhayeva M, Kavalali ET. Phorbol esters target the activity-dependent recycling pool and spare spontaneous vesicle recycling. J Neurosci. 2005;25:10922–9. doi: 10.1523/JNEUROSCI.3766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waters J, Smith SJ. Phorbol esters potentiate evoked and spontaneous release by different presynaptic mechanisms. J Neurosci. 2000;20:7863–70. doi: 10.1523/JNEUROSCI.20-21-07863.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung C, Barylko B, Leitz J, Liu X, Kavalali ET. Acute dynamin inhibition dissects synaptic vesicle recycling pathways that drive spontaneous and evoked neurotransmission. J Neurosci. 2010;30:1363–76. doi: 10.1523/JNEUROSCI.3427-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathew SS, Pozzo-Miller L, Hablitz JJ. Kainate modulates presynaptic GABA release from two vesicle pools. J Neurosci. 2008;28:725–31. doi: 10.1523/JNEUROSCI.3625-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andreae LC, Fredj NB, Burrone J. Independent vesicle pools underlie different modes of release during neuronal development. J Neurosci. 2012;32:1867–74. doi: 10.1523/JNEUROSCI.5181-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mozhayeva MG, Sara Y, Liu X, Kavalali ET. Development of vesicle pools during maturation of hippocampal synapses. J Neurosci. 2002;22:654–65. doi: 10.1523/JNEUROSCI.22-03-00654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De´k F, Shin OH, Kavalali ET, Südhof TC. Structural determinants of synaptobrevin 2 function in synaptic vesicle fusion. J Neurosci. 2006;26:6668–76. doi: 10.1523/JNEUROSCI.5272-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber JP, Reim K, Sørensen JB. Opposing functions of two sub-domains of the SNARE-complex in neurotransmission. EMBO J. 2010;29:2477–90. doi: 10.1038/emboj.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groemer TW, Klingauf J. Synaptic vesicles recycling spontaneously and during activity belong to the same vesicle pool. Nat Neurosci. 2007;10:145–7. doi: 10.1038/nn1831. [DOI] [PubMed] [Google Scholar]
- 29.Hua Y, Sinha R, Martineau M, Kahms M, Klingauf J. A common origin of synaptic vesicles undergoing evoked and spontaneous fusion. Nat Neurosci. 2010;13:1451–3. doi: 10.1038/nn.2695. [DOI] [PubMed] [Google Scholar]
- 30.Wilhelm BG, Groemer TW, Rizzoli SO. The same synaptic vesicles drive active and spontaneous release. Nat Neurosci. 2010;13:1454–6. doi: 10.1038/nn.2690. [DOI] [PubMed] [Google Scholar]
- 31.Hua Z, Leal-Ortiz S, Foss SM, Waites CL, Garner CC, Voglmaier SM, et al. v-SNARE composition distinguishes synaptic vesicle pools. Neuron. 2011;71:474–87. doi: 10.1016/j.neuron.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramirez DM, Khvotchev M, Trauterman B, Kavalali ET. Vti1a identifies a vesicle pool that preferentially recycles at rest and maintains spontaneous neurotransmission. Neuron. 2012;73:121–34. doi: 10.1016/j.neuron.2011.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raingo J, Khvotchev M, Liu P, Darios F, Li YC, Ramirez DM, et al. VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission. Nat Neurosci. 2012 doi: 10.1038/nn.3067. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, et al. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–72. doi: 10.1016/S0092-8674(00)81404-X. [DOI] [PubMed] [Google Scholar]
- 35.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–53. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 36.Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci U S A. 1998;95:15781–6. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dietrich LE, Boeddinghaus C, LaGrassa TJ, Ungermann C. Control of eukaryotic membrane fusion by N-terminal domains of SNARE proteins. Biochim Biophys Acta. 2003;1641:111–9. doi: 10.1016/S0167-4889(03)00094-6. [DOI] [PubMed] [Google Scholar]
- 38.Filippini F, Rossi V, Galli T, Budillon A, D’Urso M, D’Esposito M. Longins: a new evolutionary conserved VAMP family sharing a novel SNARE domain. Trends Biochem Sci. 2001;26:407–9. doi: 10.1016/S0968-0004(01)01861-8. [DOI] [PubMed] [Google Scholar]
- 39.Rossi V, Banfield DK, Vacca M, Dietrich LE, Ungermann C, D’Esposito M, et al. Longins and their longin domains: regulated SNAREs and multifunctional SNARE regulators. Trends Biochem Sci. 2004;29:682–8. doi: 10.1016/j.tibs.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Muzerelle A, Alberts P, Martinez-Arca S, Jeannequin O, Lafaye P, Mazie´ JC, et al. Tetanus neurotoxin-insensitive vesicle-associated membrane protein localizes to a presynaptic membrane compartment in selected terminal subsets of the rat brain. Neuroscience. 2003;122:59–75. doi: 10.1016/S0306-4522(03)00567-0. [DOI] [PubMed] [Google Scholar]
- 41.Scheuber A, Rudge R, Danglot L, Raposo G, Binz T, Poncer JC, et al. Loss of AP-3 function affects spontaneous and evoked release at hippocampal mossy fiber synapses. Proc Natl Acad Sci U S A. 2006;103:16562–7. doi: 10.1073/pnas.0603511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasan N, Corbin D, Hu C. Fusogenic pairings of vesicle-associated membrane proteins (VAMPs) and plasma membrane t-SNAREs--VAMP5 as the exception. PLoS One. 2010;5:e14238. doi: 10.1371/journal.pone.0014238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elferink LA, Trimble WS, Scheller RH. Two vesicle-associated membrane protein genes are differentially expressed in the rat central nervous system. J Biol Chem. 1989;264:11061–4. [PubMed] [Google Scholar]
- 44.Jacobsson G, Piehl F, Meister B. VAMP-1 and VAMP-2 gene expression in rat spinal motoneurones: differential regulation after neuronal injury. Eur J Neurosci. 1998;10:301–16. doi: 10.1046/j.1460-9568.1998.00050.x. [DOI] [PubMed] [Google Scholar]
- 45.Bragina L, Giovedì S, Barbaresi P, Benfenati F, Conti F. Heterogeneity of glutamatergic and GABAergic release machinery in cerebral cortex: analysis of synaptogyrin, vesicle-associated membrane protein, and syntaxin. Neuroscience. 2010;165:934–43. doi: 10.1016/j.neuroscience.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Sugiura Y, Lin W. The role of synaptobrevin1/VAMP1 in Ca2+-triggered neurotransmitter release at the mouse neuromuscular junction. J Physiol. 2011;589:1603–18. doi: 10.1113/jphysiol.2010.201939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McMahon HT, Ushkaryov YA, Edelmann L, Link E, Binz T, Niemann H, et al. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature. 1993;364:346–9. doi: 10.1038/364346a0. [DOI] [PubMed] [Google Scholar]
- 48.Schubert V, Bouvier D, Volterra A. SNARE protein expression in synaptic terminals and astrocytes in the adult hippocampus: a comparative analysis. Glia. 2011;59:1472–88. doi: 10.1002/glia.21190. [DOI] [PubMed] [Google Scholar]
- 49.Pulido IR, Jahn R, Gerke V. VAMP3 is associated with endothelial weibel-palade bodies and participates in their Ca(2+)-dependent exocytosis. Biochim Biophys Acta. 2011;1813:1038–44. doi: 10.1016/j.bbamcr.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 50.Advani RJ, Bae HR, Bock JB, Chao DS, Doung YC, Prekeris R, et al. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J Biol Chem. 1998;273:10317–24. doi: 10.1074/jbc.273.17.10317. [DOI] [PubMed] [Google Scholar]
- 51.Chaineau M, Danglot L, Galli T. Multiple roles of the vesicular-SNARE TI-VAMP in post-Golgi and endosomal trafficking. FEBS Lett. 2009;583:3817–26. doi: 10.1016/j.febslet.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 52.Steegmaier M, Klumperman J, Foletti DL, Yoo JS, Scheller RH. Vesicle-associated membrane protein 4 is implicated in trans-Golgi network vesicle trafficking. Mol Biol Cell. 1999;10:1957–72. doi: 10.1091/mbc.10.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alberts P, Rudge R, Hinners I, Muzerelle A, Martinez-Arca S, Irinopoulou T, et al. Cross talk between tetanus neurotoxin-insensitive vesicle-associated membrane protein-mediated transport and L1-mediated adhesion. Mol Biol Cell. 2003;14:4207–20. doi: 10.1091/mbc.E03-03-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alberts P, Rudge R, Irinopoulou T, Danglot L, Gauthier-Rouvière C, Galli T. Cdc42 and actin control polarized expression of TI-VAMP vesicles to neuronal growth cones and their fusion with the plasma membrane. Mol Biol Cell. 2006;17:1194–203. doi: 10.1091/mbc.E05-07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinez-Arca S, Alberts P, Zahraoui A, Louvard D, Galli T. Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth. J Cell Biol. 2000;149:889–900. doi: 10.1083/jcb.149.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cocucci E, Racchetti G, Rupnik M, Meldolesi J. The regulated exocytosis of enlargeosomes is mediated by a SNARE machinery that includes VAMP4. J Cell Sci. 2008;121:2983–91. doi: 10.1242/jcs.032029. [DOI] [PubMed] [Google Scholar]
- 57.D’Alessandro R, Racchetti G, Meldolesi J. Outgrowth of neurites is a dual process. Commun Integr Biol. 2010;3:576–8. doi: 10.4161/cib.3.6.13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Flowerdew SE, Burgoyne RDA. A VAMP7/Vti1a SNARE complex distinguishes a non-conventional traffic route to the cell surface used by KChIP1 and Kv4 potassium channels. Biochem J. 2009;418:529–40. doi: 10.1042/BJ20081736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kreykenbohm V, Wenzel D, Antonin W, Atlachkine V, von Mollard GF. The SNAREs vti1a and vti1b have distinct localization and SNARE complex partners. Eur J Cell Biol. 2002;81:273–80. doi: 10.1078/0171-9335-00247. [DOI] [PubMed] [Google Scholar]
- 60.Laufman O, Hong W, Lev S. The COG complex interacts directly with Syntaxin 6 and positively regulates endosome-to-TGN retrograde transport. J Cell Biol. 2011;194:459–72. doi: 10.1083/jcb.201102045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mallard F, Tang BL, Galli T, Tenza D, Saint-Pol A, Yue X, et al. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J Cell Biol. 2002;156:653–64. doi: 10.1083/jcb.200110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brandhorst D, Zwilling D, Rizzoli SO, Lippert U, Lang T, Jahn R. Homotypic fusion of early endosomes: SNAREs do not determine fusion specificity. Proc Natl Acad Sci U S A. 2006;103:2701–6. doi: 10.1073/pnas.0511138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mancias JD, Goldberg J. The transport signal on Sec22 for packaging into COPII-coated vesicles is a conformational epitope. Mol Cell. 2007;26:403–14. doi: 10.1016/j.molcel.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 64.Tochio H, Tsui MM, Banfield DK, Zhang M. An autoinhibitory mechanism for nonsyntaxin SNARE proteins revealed by the structure of Ykt6p. Science. 2001;293:698–702. doi: 10.1126/science.1062950. [DOI] [PubMed] [Google Scholar]
- 65.Pryor PR, Jackson L, Gray SR, Edeling MA, Thompson A, Sanderson CM, et al. Molecular basis for the sorting of the SNARE VAMP7 into endocytic clathrin-coated vesicles by the ArfGAP Hrb. Cell. 2008;134:817–27. doi: 10.1016/j.cell.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.MacDonald C, Munson M, Bryant NJ. Autoinhibition of SNARE complex assembly by a conformational switch represents a conserved feature of syntaxins. Biochem Soc Trans. 2010;38:209–12. doi: 10.1042/BST0380209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bock JB, Lin RC, Scheller RH. A new syntaxin family member implicated in targeting of intracellular transport vesicles. J Biol Chem. 1996;271:17961–5. doi: 10.1074/jbc.271.30.17961. [DOI] [PubMed] [Google Scholar]
- 68.Tang BL, Low DY, Lee SS, Tan AE, Hong W. Molecular cloning and localization of human syntaxin 16, a member of the syntaxin family of SNARE proteins. Biochem Biophys Res Commun. 1998;242:673–9. doi: 10.1006/bbrc.1997.8029. [DOI] [PubMed] [Google Scholar]
- 69.Tang BL, Tan AE, Lim LK, Lee SS, Low DY, Hong W. Syntaxin 12, a member of the syntaxin family localized to the endosome. J Biol Chem. 1998;273:6944–50. doi: 10.1074/jbc.273.12.6944. [DOI] [PubMed] [Google Scholar]
- 70.Wang H, Frelin L, Pevsner J. Human syntaxin 7: a Pep12p/Vps6p homologue implicated in vesicle trafficking to lysosomes. Gene. 1997;199:39–48. doi: 10.1016/S0378-1119(97)00343-0. [DOI] [PubMed] [Google Scholar]
- 71.Hoopmann P, Punge A, Barysch SV, Westphal V, Bückers J, Opazo F, et al. Endosomal sorting of readily releasable synaptic vesicles. Proc Natl Acad Sci U S A. 2010;107:19055–60. doi: 10.1073/pnas.1007037107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ravichandran V, Chawla A, Roche PA. Identification of a novel syntaxin- and synaptobrevin/VAMP-binding protein, SNAP-23, expressed in non-neuronal tissues. J Biol Chem. 1996;271:13300–3. doi: 10.1074/jbc.271.23.13300. [DOI] [PubMed] [Google Scholar]
- 73.Hohenstein AC, Roche PA. SNAP-29 is a promiscuous syntaxin-binding SNARE. Biochem Biophys Res Commun. 2001;285:167–71. doi: 10.1006/bbrc.2001.5141. [DOI] [PubMed] [Google Scholar]
- 74.Chen D, Bernstein AM, Lemons PP, Whiteheart SW. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 in dense core granule release. Blood. 2000;95:921–9. [PubMed] [Google Scholar]
- 75.Hepp R, Puri N, Hohenstein AC, Crawford GL, Whiteheart SW, Roche PA. Phosphorylation of SNAP-23 regulates exocytosis from mast cells. J Biol Chem. 2005;280:6610–20. doi: 10.1074/jbc.M412126200. [DOI] [PubMed] [Google Scholar]
- 76.Rea S, Martin LB, McIntosh S, Macaulay SL, Ramsdale T, Baldini G, et al. Syndet, an adipocyte target SNARE involved in the insulin-induced translocation of GLUT4 to the cell surface. J Biol Chem. 1998;273:18784–92. doi: 10.1074/jbc.273.30.18784. [DOI] [PubMed] [Google Scholar]
- 77.Suh YH, Yoshimoto-Furusawa A, Weih KA, Tessarollo L, Roche KW, Mackem S, et al. Deletion of SNAP-23 results in pre-implantation embryonic lethality in mice. PLoS One. 2011;6:e18444. doi: 10.1371/journal.pone.0018444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bragina L, Candiracci C, Barbaresi P, Giovedì S, Benfenati F, Conti F. Heterogeneity of glutamatergic and GABAergic release machinery in cerebral cortex. Neuroscience. 2007;146:1829–40. doi: 10.1016/j.neuroscience.2007.02.060. [DOI] [PubMed] [Google Scholar]
- 79.Chieregatti E, Chicka MC, Chapman ER, Baldini G. SNAP-23 functions in docking/fusion of granules at low Ca2+ Mol Biol Cell. 2004;15:1918–30. doi: 10.1091/mbc.E03-09-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Delgado-Marti´nez I, Nehring RB, Sørensen JB. Differential abilities of SNAP-25 homologs to support neuronal function. J Neurosci. 2007;27:9380–91. doi: 10.1523/JNEUROSCI.5092-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, et al. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–27. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 82.Shin OH, Rizo J, Südhof TC. Synaptotagmin function in dense core vesicle exocytosis studied in cracked PC12 cells. Nat Neurosci. 2002;5:649–56. doi: 10.1038/nn869. [DOI] [PubMed] [Google Scholar]
- 83.Sugita S, Shin OH, Han W, Lao Y, Südhof TC. Synaptotagmins form a hierarchy of exocytotic Ca(2+) sensors with distinct Ca(2+) affinities. EMBO J. 2002;21:270–80. doi: 10.1093/emboj/21.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suh YH, Terashima A, Petralia RS, Wenthold RJ, Isaac JT, Roche KW, et al. A neuronal role for SNAP-23 in postsynaptic glutamate receptor trafficking. Nat Neurosci. 2010;13:338–43. doi: 10.1038/nn.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Steegmaier M, Yang B, Yoo JS, Huang B, Shen M, Yu S, et al. Three novel proteins of the syntaxin/SNAP-25 family. J Biol Chem. 1998;273:34171–9. doi: 10.1074/jbc.273.51.34171. [DOI] [PubMed] [Google Scholar]
- 86.Gordon DE, Bond LM, Sahlender DA, Peden AA. A targeted siRNA screen to identify SNAREs required for constitutive secretion in mammalian cells. Traffic. 2010;11:1191–204. doi: 10.1111/j.1600-0854.2010.01087.x. [DOI] [PubMed] [Google Scholar]
- 87.Pan PY, Cai Q, Lin L, Lu PH, Duan S, Sheng ZH. SNAP-29-mediated modulation of synaptic transmission in cultured hippocampal neurons. J Biol Chem. 2005;280:25769–79. doi: 10.1074/jbc.M502356200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Su Q, Mochida S, Tian JH, Mehta R, Sheng ZH. SNAP-29: a general SNARE protein that inhibits SNARE disassembly and is implicated in synaptic transmission. Proc Natl Acad Sci U S A. 2001;98:14038–43. doi: 10.1073/pnas.251532398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Holt M, Varoqueaux F, Wiederhold K, Takamori S, Urlaub H, Fasshauer D, et al. Identification of SNAP-47, a novel Qbc-SNARE with ubiquitous expression. J Biol Chem. 2006;281:17076–83. doi: 10.1074/jbc.M513838200. [DOI] [PubMed] [Google Scholar]
- 90.Ramirez DM, Kavalali ET. Differential regulation of spontaneous and evoked neurotransmitter release at central synapses. Curr Opin Neurobiol. 2011;21:275–82. doi: 10.1016/j.conb.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Atasoy D, Ertunc M, Moulder KL, Blackwell J, Chung C, Su J, et al. Spontaneous and evoked glutamate release activates two populations of NMDA receptors with limited overlap. J Neurosci. 2008;28:10151–66. doi: 10.1523/JNEUROSCI.2432-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zenisek D. Vesicle association and exocytosis at ribbon and extraribbon sites in retinal bipolar cell presynaptic terminals. Proc Natl Acad Sci U S A. 2008;105:4922–7. doi: 10.1073/pnas.0709067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jakawich SK, Nasser HB, Strong MJ, McCartney AJ, Perez AS, Rakesh N, et al. Local presynaptic activity gates homeostatic changes in presynaptic function driven by dendritic BDNF synthesis. Neuron. 2010;68:1143–58. doi: 10.1016/j.neuron.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55:648–61. doi: 10.1016/j.neuron.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 95.Danglot L, Zylbersztejn K, Petkovic M, Gauberti M, Meziane H, Combe R, et al. Absence of TI-VAMP/Vamp7 leads to increased anxiety in mice. J Neurosci. 2012;32:1962–8. doi: 10.1523/JNEUROSCI.4436-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–5. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]