Abstract
Objectives
To evaluate the associations between visual field loss and non-spine fractures.
Design
Prospective cohort study.
Setting and Patients
We investigated binocular visual field (BVF) loss in a cohort of 4,773 community-dwelling white and African-American women who were 65 years of age or older and had no previous history of hip fracture at the time of recruitment.
Measurements
Radiographically confirmed hip and non-spine/non-hip fractures identified from September, 1997 to April, 2008. Visual field loss was measured using a Humphrey Field Analyzer suprathreshold screening test of the peripheral and central vision of each eye and was classified into an ordinal rating of no/mild/moderate/severe BVF loss.
Results
For both hip and non-spine/non-hip fractures, and in both unadjusted and covariate-adjusted analyses, the highest incidence (hazard rate) of fractures was seen in women with the most severe BVF loss. In covariate-adjusted analysis, compared to women without BVF loss, women with mild, moderate and severe BVF loss had a 49% (HR [hazards ratio]=1.49; 95% CI: 1.18-1.88), 25% (HR=1.25; 95% CI: 0.87-1.80), and 66% (HR=1.66; 95% CI: 1.19-2.32) greater risk for hip fractures. Similarly, women with mild visual field loss had a 12% (HR=0.88; 95% CI: 0.75-1.04) reduced risk for non-spine/non-hip fractures while women with moderate and severe visual field loss had a 18% (HR=1.18; 95% CI: 0.92-1.52) and 59% (HR=1.59; 95% CI: 1.24-2.03) greater risk of non-spine/non-hip fractures compared to women without BVF loss.
Conclusion
BVF loss is independently associated with hip and non-spine/non-hip fractures in older volunteer women.
Keywords: fractures, visual field loss, visual acuity
INTRODUCTION
Poor vision has long been recognized as a potential risk factor for fractures in older people.1-4 Previously, we reported on findings from the Study of Osteoporotic Fractures (SOF) that visual impairment as measured by depth perception and contrast sensitivity was associated with an elevated risk of hip fractures5 and that decreased visual acuity was associated with an elevated risk of wrist fractures in older women.6 Another readily available clinical measure is the evaluation of central and peripheral vision. Such vision is measured by testing for visual field deficits. In visual field testing, patients indicate when they perceive objects or points of light presented in different locations associated with central and peripheral vision. In automated visual field testing, this information is translated into pixels showing a map of the areas in the eye where perception occurs. Visual field loss is a measure of decreased or no perception of light across one or more pixels. Visual field measurement is a standard diagnostic tool for disorders within the visual pathway from the photoreceptor cells in the retina to the occipital lobe. Studies have reported a prevalence of visual field loss ranging from 5.6% to 17% in adults 40 years and older.7,8
Recently, we reported that binocular visual field (BVF) loss was associated with an increased risk of frequent falls in older white women.9 In the limited research conducted, longitudinal studies have reported conflicting results on the link between visual field loss and the risk of hip fractures.8,10 One study reported that older whites with visual field loss did not have a higher risk of hip fracture,8 while another investigation found that the relationship between visual field loss and hip fractures in whites waned with increasing follow-up time since the clinical exam.10 Another prior study failed to find an association between visual field loss and wrist fractures in whites.11
To test the hypothesis that BVF loss is an independent risk factor for fracture, we performed a comprehensive eye examination including visual field testing in a cohort of 4773 older women enrolled in SOF12-14 and followed them prospectively for eight years.
METHODS
Subjects
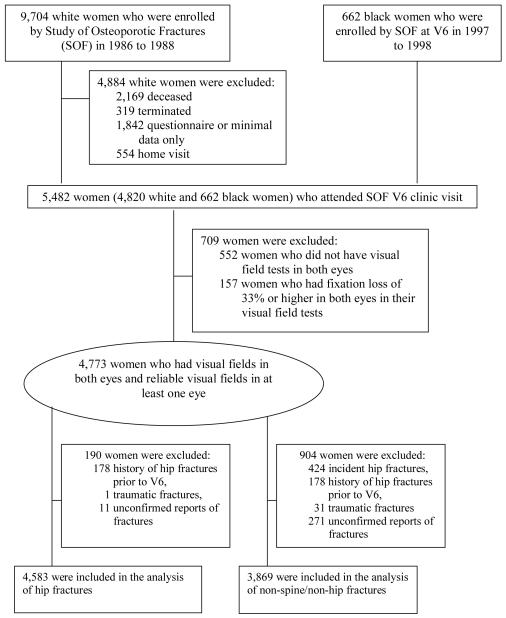
From 1986 to 1988, 9,704 ambulatory white female volunteers who were 65 years or older, ambulatory with no history of bilateral hip replacement, were enrolled in SOF, a multi-center, prospective longitudinal cohort study for identifying potential risk factors of osteoporotic fractures, which has been described previously12-14. Beginning in January 1997, and continuing through September, 1998, all surviving participants were invited to participate in a follow-up clinical examination (6th clinic visit, or V6), which included a comprehensive eye examination. In addition, a cohort of 662 women who identified themselves as African-Americans were recruited from population listings at each of the four clinic centers to participate in SOF and the eye examination. The same recruitment strategy was used to recruit both African-Americans and whites. The two cohorts have been pooled in a prior SOF manuscript.12 All individuals in the study gave informed consent to participate. Institutional Review Board approvals were obtained from all participating institutions. A total of 5,482 women, including 662 African-American and 4,820 white participants, attended the 6th clinic visit (V6). This sample of white women represented 63% of the surviving cohort. Our analyses excluded 552 women who did not have visual field tests in both eyes. They also excluded 157 women who had unreliable visual field tests (i.e. tests with fixation losses of 33% or higher in both eyes), leaving a final sample of 4,773 women (87% of those with a clinic visit permitting visual field testing) (Figure 1). Visual field testing could not be carried out in participants who were unable to attend the clinic-based examinations.
Figure 1.
Study Participant Flow Chart.
Ascertainment of Fractures
All participants were contacted by postcard or telephone approximately every four months from September 1997 to April 2008 to determine whether they had a new fracture after V6. There has been a cumulative completion rate of 98% for these contacts since the inception of SOF.14 To distinguish clearly between fracture and non-fracture cases, we excluded cases with reports of fractures that were not confirmed by x-ray records. Separate analyses were conducted for incident hip fractures and incident non-spine/non-hip fractures. A total of 4,583 women were included in the analysis of hip fractures, and after separating out hip and spine fractures, 3,869 women were included in the non-spine/non-hip fractures analysis (Figure 1).
Assessment of Vision
Visual field tests were performed on each eye of a participant with the Humphrey Field Analyzer suprathreshold 76-point 30 ° visual field program (Humphrey Field Analyzer; Zeiss, Oberkochen, Germany).15,16 The suprathreshold 76-point program is a screening visual field test measuring whether the eye’s visual pathway detects a light 6 dB brighter than that which could be detected by an eye of a healthy subject who is the same age. A total of 76 points of light are presented in the central and peripheral fields of each eye. Examiners were trained for a half day in the use of the Humphrey Field Analyzer. The training session covered calibration of the perimeter, the choice of the corrective lenses used for the test, and the explanation of the test to subjects. Examiners performed screening visual field tests on volunteers until they could be certified as proficient by the study coordinator or investigators.
A BVF for each participant was created by overlapping two 76-screening visual fields for each eye, using a method adapted from Esterman’s BVF functional scoring algorithm.9,16 The total number of points missed out of the 96 possible points in the BVF was recorded. BVF loss was categorized into four groups: no loss (0 points missed), mild loss (1 to 9 points missed), moderate loss (10 to 19 points missed), and severe loss (20 or more points missed).9
Distance visual acuity was measured in each eye separately with habitual correction under standard illumination using Bailey-Lovie charts,17 which feature geometric progression in letter size from line to line. The number of letters seen correctly was recorded. Contrast sensitivity was also measured in each eye with habitual correction under standard illumination using the VCTS 6500 charts (Vistech Consultants, Inc., Dayton, Ohio),18 which present a series of sine-wave gratings at calibrated levels of contrast at specific spatial frequencies (cycles per degree). The number of gratings seen correctly was recorded and converted to a contrast sensitivity score at each spatial frequency according to the manufacturer’s manual.
Other measurements
We sought to incorporate in our analyses both vision-related and other clinical characteristics reported to be risk factors or confounders for fractures. As described in prior SOF publications,5, 6, 9, 12-14 all risk factors and confounders were collected at V6 except for history of any fractures since age 50 which was obtained at Visit 1 on the original cohort and at V6 in the black cohort at V6 because V6 is their first (baseline) visit.
Statistical Analysis
The primary objective of the analysis was to determine the extent to which visual field loss was associated with risk of incident hip and non-spine/non-hip fractures in older white and African-American women. Visual acuity in the better-seeing eye was dichotomized into Snellen visual acuity levels of 20/40 or better versus worse than 20/40. Contrast sensitivity (CS) in the better-seeing eye at low spatial frequency (1.5 cycles/degree) was categorized into CS score of less than 25 versus 25 or greater. When information was not available for at least one eye at 1.5 cycles/degree, we used the contrast sensitivity values for 3 cycles/degree. Because certain distributions were skewed, Spearman correlation coefficients were used to characterize associations among the continuous vision variables: BVF loss, visual acuity, and contrast sensitivity.
We used Cox proportional-hazards analysis to determine whether BVF loss was a risk factor for time until the occurrence of a fracture. We considered the following potential confounding variables: age, study site, race, self-rated health status, current smoker status, alcohol use, self-reported diabetes, self-reported hyperthyroidism, self-reported osteoporosis, current use of anticonvulsant drugs, current use of long-acting benzodiazepines, average grip strength, use arms to stand up, body mass index, depression, cognitive function, walking speed, falls in the past year (one or more falls within 12 months prior to the examination), hip bone mineral density, and history of any fractures since age 50. Separate models considered different subsets of potential confounders as described in further detail in the Results section.
We also evaluated interactions between BVF loss and age, as well as interactions between BVF loss and race. We calculated the percent attributable risk (i.e. the proportion of fractures among women who had BVF loss that is attributable to this loss, calculated using the formula 100 × (RR-1)/RR, where RR is the risk ratio estimate) and the population attributable risk percentage (i.e. the proportion of fractures among the total population that is attributable to BVF loss, calculated using the formula 100 × P(e) × (RR-1)/(1+P(e) × (RR-1)), where P(e) is the prevalence of severe BVF loss in the population).19 To evaluate predictors of fractures, we used Akaike’s information criterion (AIC)20 to compare different models. Larger AIC values indicate the model with the better fit.
In secondary sensitivity analyses, BVF loss, visual acuity in the better eye, and CS at low spatial frequency in the better eye were analyzed as continuous variables in regression models adjusted for the same covariates as above. To control for skewness and extreme values, the continuous BVF loss variable was analyzed as the number of points lost up to 40 points, with any greater value fixed at 40 to improve linearity in a manner similar to rank transformations. The continuous visual acuity variables were analyzed as the number of letters read correctly, which is a logarithmic transformation of Snellen visual acuity. The continuous CS variables were analyzed as a logarithmic transformation of CS score at low frequency. All continuous vision variables were then standardized by dividing by their respective standard deviations (SD) after transformation, and the results were presented as risk ratios of incident fractures per one SD change in specific vision parameter.
All statistical analyses were performed using SAS version 9.1 software (SAS Institute Inc., Cary, NC). A p-value of < .05 was considered statistically significant.
RESULTS
The 4773 volunteers included in this analysis were slightly younger and had better self-rated health status, experienced less depression, and had better visual acuity in the better eye than the 709 women who were excluded. A total of 1,773 women (37%) had no BVF loss, 2,015 (42%) had mild BVF loss, 485 (10%) had moderate BVF loss, and 500 (11%) had severe BVF loss. The range of points lost across both eyes was from 0 to 87 with a mean of 6.4 (standard deviation [SD]=11.7). The Spearman correlation coefficients were -0.17 (p<0.0001) between BVF loss and visual acuity, -0.17 (p<0.0001) between BVF loss and contrast sensitivity, and 0.38 (p<0.0001) between visual acuity and contrast sensitivity. Table 1 summarizes characteristics of the study sample by the status of BVF loss.
Table 1.
Baseline (V6) characteristics of women who participated the SOF clinic visit by the status of binocular visual field loss (N=4,773)
| Characteristics | Binocular Visual Field loss | |||
|---|---|---|---|---|
| None (0) (N=1,773) |
Mild (1-9) (N=2,015) |
Moderate (10-19) (N=485) |
Severe (20+) (N=500) |
|
| Study sites | ||||
| Site 1 | 440 (25%) | 489 (24%) | 114 (24%) | 99 (20%) |
| Site 2 | 625 (35%) | 582 (29%) | 129 (27%) | 98 (20%) |
| Site 3 | 412 (23%) | 517 (26%) | 121 (25%) | 122 (24%) |
| Site 4 | 296 (17%) | 427 (21%) | 121 (25%) | 181 (36%) |
| Age (year) | ||||
| Mean ± SD | 78.2 ± 3.8 | 79.5 ± 4.3 | 80.4 ± 4.7 | 81.4 ± 4.9 |
| ≤ 79 | 1,210 (68%) | 1,115 (55%) | 232 (48%) | 192 (38%) |
| 80 – 84 | 460 (26%) | 645 (32%) | 161 (33%) | 176 (35%) |
| ≥ 85 | 103 (6%) | 255 (13%) | 92 (19%) | 132 (26%) |
| Race | ||||
| White | 1,579 (89%) | 1,792 (89%) | 423 (87%) | 422 (84%) |
| Black | 194 (11%) | 223 (11%) | 62 (13%) | 78 (16%) |
| Habitual visual acuity in the better eye (number of letters, 0 to 70); n=4,769 |
||||
| Mean ± SD | 47.5 ± 6.6 | 46.5 ± 7.0 | 44.9 ± 8.0 | 42.9 ± 9.6 |
| Worse than 20/40 | 352 (20%) | 467 (23%) | 161 (33%) | 185 (37%) |
| Low frequency (1.5 or 3 cycles/degree) CS in the better eye (CS score, 0 to 220); n=4,755 |
||||
| Mean ± SD | 37.9 ± 20.7 | 34.9 ± 19.9 | 31.1 ± 17.5 | 29.4 ± 16.4 |
| Less than 25 | 545 (31%) | 743 (37%) | 242 (50%) | 256 (52%) |
| MMSE score (6 to 30); n=4,708 |
||||
| Mean ± SD | 28.3 ± 1.8 | 27.8 ± 2.1 | 27.4 ± 2.4 | 26.7 ± 2.7 |
| Current smoker; n=4,768 | 63 (4%) | 92 (5%) | 26 (5%) | 30 (9%) |
| Current alcohol use; n=4,766 |
877 (49%) | 867 (43%) | 176 (36%) | 152 (30%) |
| Self-rated health status; n=4,768 |
||||
| Fair/Poor/Very Poor | 273 (15%) | 409 (20%) | 105 (22%) | 137 (27%) |
| Excellent/Good | 1,499 (85%) | 1,603 (80%) | 379 (78%) | 363 (73%) |
| Self-reported diabetes; n=4,766 |
100 (6%) | 119 (6%) | 32 (7%) | 43 (9%) |
| Self-reported hyperthyroidism; n=4,768 |
62 (4%) | 89 (4%) | 13 (3%) | 23 (5%) |
| Self-reported osteoporosis; n=4,767 |
336 (19%) | 391 (19%) | 90 (19%) | 92 (18%) |
| Depression (GDS≥6) ; n=4,767 |
113 (6%) | 196 (10%) | 60 (12%) | 73 (15%) |
| Current use of anticonvulsant drugs; n=4,768 |
27 (2%) | 32 (2%) | 9 (2%) | 16 (3%) |
| Current use of long-acting benzodiazepines; n=4,768 |
36 (2%) | 43 (2%) | 15 (3%) | 18 (4%) |
| BMI (kg/m2); n=4,726 | ||||
| Mean ± SD | 26.9 ± 5.0 | 27.1 ± 5.2 | 26.7 ± 5.1 | 26.3 ± 4.7 |
| Walking speed (meter/second); n=4,734 |
||||
| Mean ± SD | 0.96 ± 0.20 | 0.90 ± 0.20 | 0.84 ± 0.20 | 0.79 ± 0.22 |
| Average grip strength (kg); n=4,687 |
||||
| Mean ± SD | 18.2 ± 4.2 | 17.2 ± 4.3 | 16.5 ± 4.1 | 16.0 ± 4.3 |
| Use arms to stand up; n=4,768 |
177 (10%) | 283 (14%) | 97 (20%) | 121 (24%) |
| Self-reported at least one fall in last year; n=4,765 |
503 (28%) | 583 (29%) | 172 (36%) | 177 (35%) |
| History of any fractures; n=4,751 |
827 (47%) | 966 (48%) | 244 (50%) | 261 (53%) |
| Hip BMD (g/cm2); n=4,653 | ||||
| Mean ± SD | 0.75 ± 0.14 | 0.74 ± 0.14 | 0.74 ± 0.15 | 0.70 ± 0.13 |
| Self-reported glaucoma in at least one eye |
168 (9%) | 228 (11%) | 80 (16%) | 104 (21%) |
| Self-reported treatment for glaucoma |
157 (9%) | 221 (11%) | 79 (16%) | 102 (20%) |
| Self-reported AMD in at least one eye |
119 (7%) | 197 (10%) | 51 (11%) | 70 (14%) |
| Self-reported treatment for AMD |
4 (0.2%) | 6 (0.3%) | 2 (0.4%) | 1 (0.2%) |
| Self-reported cataract in at least one eye |
1,179 (67%) | 1,457 (72%) | 372 (77%) | 378 (76%) |
| Self-reported cataract surgery in at least one eye |
539 (30%) | 824 (41%) | 236 (49%) | 260 (52%) |
SD = Standard deviation, CS = Contrast sensitivity, GDS = Geriatric depression scale, BMI = Body mass index, MMSE = Mini Mental State Examination, BMD = Bone mineral density, AMD = Age-related macular degeneration.
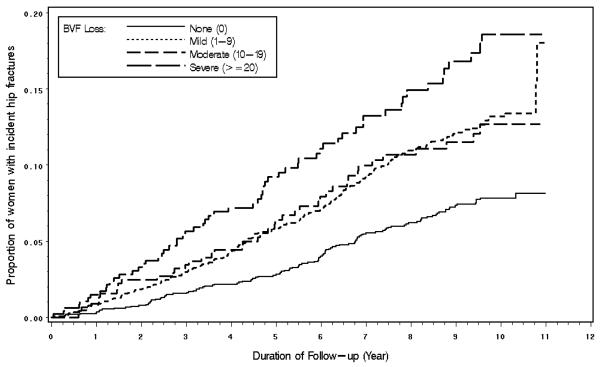
During a mean of 8.1 years of follow-up (SD = 2.70), 424 of 4,583 (9.3%) women suffered a first hip fracture (incidence rate of 11.4 per 1000 person-years; 95% confidence interval [CI]: 10.4-12.6). 1,720 women without BVF loss had 115 (6.7%) hip fractures (incidence=7.7 per 1000 person-years; 95% CI: 6.4-9.3 per 1000 person-years); 1938 women with mild had 205 (10.6%) hip fractures (incidence=13.2 per 1000 person-years; 95% CI:11.5–15.1 per 1000 person-years), 455 women with moderate BVF loss had 43 (9.5%) hip fractures (incidence=12.8 per 1000 person-years; 95% CI:9.5-17.3 per 1000 person-years); and 470 women with severe BVF loss had 61 (13.0%) hip fractures (incidence=18.4 per 1000 person-years; 95% CI: 14.3-23.7 per 1000 person-years). Women with a first hip fracture missed a mean of 8.3 points (SD=13.5) on the BVF test, while women who did not have any fractures missed a mean of 6.1 points (SD=11.3) (p<0.0001). Figure 2 shows the Kaplan-Meier curves of time to hip fracture by the status of BVF loss. In analyses adjusted for age, race, study site, and cognitive function, women with severe BVF loss had a risk of hip fracture estimated to be 66% greater than that of women with no visual field loss (HR [hazards ratio]=1.66; 95% CI: 1.19-2.32) (Table 2). The association between severe visual field loss and the risk of hip fracture was borderline significant in the fully-adjusted model (HR=1.37; 95% CI: 0.97-1.95) (Table 2) and was not significant when hip bone mineral density was included in the fully-adjusted model (HR=1.24; 95% CI: 0.86-1.77).
Figure 2.
Estimated Incidence of Hip Fractures by Binocular Visual Field Loss from Kaplan-Meier Analysis.
BVF=Binocular visual field
Table 2.
Associations between the risk of hip fractures and vision risk factors. (N=4,583)
| Vision Risk Factors* |
Number of women who had incident hip fractures out of the number of subjects in the study population (%) |
Crude Incidence 95% CI (per 1000 person years) |
Model adjusted for age, race, study site, and cognitive function HR; 95% CI P-value |
Fully-adjusted model† HR; 95% CI P-value |
|---|---|---|---|---|
|
Binocular VF loss: None (0) |
115/1,720 (7%) | 7.7 (6.4 – 9.3) | 1.00; referent - |
1.00; referent - |
| Mild (1-9) | 205/1,938 (11%) | 13.2 (11.5 – 15.1) |
1.49; (1.18-1.88) 0.0008 |
1.40; (1.11-1.78) 0.006 |
| Moderate (10- 19) |
43/455 (9%) | 12.8 (9.5 – 17.3) |
1.25; (0.87-1.80) 0.23 |
1.11; (0.77-1.62) 0.57 |
| Severe (20+) | 61/470 (13%) | 18.4 (14.3 – 23.7) |
1.66; (1.19-2.32) 0.003 |
1.37; (0.97-1.95) 0.08 |
| P-value for trend | 0.052 | 0.44 | ||
|
VA: 20/40 or better |
306/3,481 (9%) | 10.6 (9.5 – 11.9) |
1.00; referent - |
1.00; referent - |
| Worse than 20/40 |
116/1,098 (11%) | 14.1 (11.8 – 16.9) |
1.12; (0.90-1.41) 0.31 |
1.01; (0.80-1.27) 0.96 |
| CS: ≥25 CS score | 254/2,868 (9%) | 10.6 (9.4 – 12.0) |
1.00; referent - |
1.00; referent - |
| <25 CS score | 165/1,698 (10%) | 12.7 (10.9 – 14.8) |
1.11; (0.90-1.36) 0.34 |
1.09; (0.88-1.35) 0.42 |
HR = Hazards ratio, CI = Confidence interval, VF = Visual field, VA = Visual acuity, CS = Contrast sensitivity.
Each vision risk factor was examined in a separate model.
Adjusted for age, race (black vs. white), study sites (four sites), cognitive function (MMSE), current smoker (yes vs. no), alcohol use (yes vs. no), self-reported health status (good/excellent vs. poor/fair), self-reported diabetes (yes vs. no), self-reported hyperthyroidism (yes vs. no), self-reported osteoporosis (yes vs. no), depression (yes vs. no), current use of anticonvulsant drugs (yes vs. no), current use of long-acting benzodiazepines (yes vs. no), body mass index, walking speed, average grip strength, use arms to stand up (yes vs. no), at least one fall in last year (yes vs. no), history of any fractures (yes vs. no).
Among the 3,869 women included in the analysis examining the association between visual field loss and risk of non-hip/non-spine fracture, 770 (19.9%) women experienced a non-spine/non-hip fracture during a mean of 8.0 years (SD=2.8) of follow-up. 1494 women without BVF loss had 307 (20.5%) non-spine/non-hip fractures (incidence=23.9 per 1000 person-years; 95% CI:21.3-26.7 per 1000 person-years); 1615 women with mild visual field loss had 285 (17.6%) non-spine/non-hip fractures (incidence=22.1 per 1000 person-years; 95% CI: 19.7-24.9 per 1000 person-years); 378 women with moderate visual field loss had 80 (21.2%) non-spine/non-hip fractures (incidence=29.0 per 1000 person-years; 95% CI: 23.3-36.1 per 1000 person-years); 382 with severe visual field loss had 98 (25.7%) non-spine/non-hip fractures (incidence=37.5 per 1000 person-years; 95% CI:30.7-45.7 per 1000 person-years). Women with at least one incident non-spine/non-hip fracture missed a mean of 7.3 points (SD=13.4) on the BVF test, while women who did not have any fractures missed a mean of 5.9 points (SD=10.8) (p=0.30). Women with severe BVF loss had roughly a 1.6-fold risk of a non-spine/non-hip fractures compared to women without any visual field loss in analyses adjusted for age, race, study site, and cognitive function (HR=1.59; 95% CI: 1.24-2.03) (Table 3). This elevated risk remained significant in the fully-adjusted model (HR=1.46; 95% CI: 1.13-1.89) (Table 3) and when hip bone mineral density (HR=1.44; 95% CI: 1.11-1.86) was included in the fully-adjusted model.
Table 3.
Associations between the risk of non-spine/non-hip fractures and vision risk factors. (N=3,869)
| Vision Risk Factors* |
Number of women who had incident non- spine/non-hip fractures out of the number of subjects in the study population (%) |
Crude Incidence 95% CI (per 1000 person years) |
Model adjusted for age, race, study site, and cognitive function HR; 95% CI P-value |
Fully-adjusted model† HR; 95% CI P-value |
|---|---|---|---|---|
|
Binocular VF loss: None (0) |
307/1,494 (21%) | 23.9 (21.3 – 26.7) |
1.00; referent - |
1.00; referent - |
| Mild (1-9) | 285/1,615 (18%) | 22.1 (19.7 – 24.9) |
0.88; (0.75- 1.04) 0.12 |
0.89; (0.75- 1.05) 0.16 |
| Moderate (10-19) | 80/378 (21%) | 29.0 (23.3 – 36.1) |
1.18; (0.92- 1.52) 0.19 |
1.13; (0.87- 1.47) 0.35 |
| Severe (20+) | 98/382 (26%) | 37.5 (30.7 – 45.7) |
1.59; (1.24- 2.03) 0.0002 |
1.46; (1.13- 1.89) 0.004 |
| P for trend | <0.0001 | 0.0004 | ||
| VA: 20/40 or better | 578/2,949 (20%) | 23.8 (21.9 – 25.8) |
1.00; referent - |
1.00; referent - |
| Worse than 20/40 | 192/918 (21%) | 28.4 (24.6 – 32.7) |
1.19; (1.00- 1.41) 0.046 |
1.12; (0.94- 1.33) 0.21 |
| CS: ≥25 CS score | 465/2,420 (19%) | 23.0 (21.0 – 25.2) |
1.00; referent - |
1.00; referent - |
| <25 CS score | 304/1,437 (21%) | 28.0 (25.0 – 31.3) |
1.28; (1.10- 1.49) 0.001 |
1.28; (1.09- 1.50) 0.002 |
HR = Hazards ratio, CI = Confidence interval, VF = Visual field, VA = Visual acuity, CS = Contrast sensitivity.
Each vision risk factor was examined in a separate model.
Adjusted for age, race (black vs. white), study sites (four sites), cognitive function (MMSE), current smoker (yes vs. no), alcohol use (yes vs. no), self-reported health status (good/excellent vs. poor/fair), self-reported diabetes (yes vs. no), self-reported hyperthyroidism (yes vs. no), self-reported osteoporosis (yes vs. no), depression (yes vs. no), current use of anticonvulsant drugs (yes vs. no), current use of long-acting benzodiazepines (yes vs. no), body mass index, walking speed, average grip strength, use
The number of incident hip fracture was not large enough for examining reliably the interaction effects in the hip fracture models, and we found no evidence of an interaction between age and visual field loss (p=0.48) and between race and visual field loss (p=0.15) in the non-spine/non-hip fracture models.
Visual acuity worse than 20/40 was not significantly associated with risk of hip fractures (Table 2) but was associated with a 19% increased risk (HR=1.19; 95% CI: 1.00-1.41) of non-spine/non-hip fractures when the model was adjusted for age, race, study site, and cognitive function (Table 3). Women with poor contrast sensitivity (<25 CS score) had a higher risk of non-spine/non-hip fracture in the fully-adjusted model (Table 3) which remained present when hip bone mineral density was included in the model (HR=1.27; 95% CI: 1.09-1.49).
Using the estimate of 1.37 in the fully adjusted model as the relative risk of hip fracture for severe BVF loss compared to no loss, the percent attributable risk for severe BVF loss was 27%. Assuming that 10% of older women had severe loss similar to the SOF population, the population attributable risk percentage for severe BVF loss for hip fracture was 3.7%. Using the estimates of 1.46 for severe BVF loss in the fully adjusted models, for non-spine/non-hip fractures, the percent attributable risk for severe BVF loss was 31.5%, and the population attributable risk percentage was 4.6% (assuming that 10% of older women have severe BVF loss).
In secondary sensitivity analyses where continuous vision variables were used, women who missed more points on the BVF had a higher risk of hip fractures (HR=1.11 per SD; 95% CI: 1.01-1.21) and non-spine/non-hip fractures (HR=1.18 per SD; 95% CI: 1.10-1.27). Women who recognized more letters (visual acuity was better) with the better-seeing eye had less risk of hip (HR=0.90 per SD; 95% CI: 0.82-0.99) and non-spine/non-hip fractures (HR=0.87 per SD; 95% CI: 0.81-0.94). Women with higher contrast sensitivity scores in the better eye had less risk of non-spine/non-hip fractures (HR=0.87 per SD; 95% CI: 0.80-0.93) and no risk of hip fractures (HR=0.98 per SD; 95% CI: 0.89-1.09).
The test for trend where risk was posited to increase linearly across severity categories of BVF loss was statistically significant for non-spine/non-hip fractures (trend p<0.0001) and was of borderline significance for hip fractures (trend p= 0.052), after adjustment for age, site, race, and cognitive function. Using AIC values, the inclusion of BVF loss in the fully-adjusted models for hip and non-spine/non-hip fractures improved model fit more than the inclusion of visual acuity or contrast sensitivity. The inclusion of hip bone mineral density in the fully-adjusted models decreased the AIC scores of all the models, suggesting poorer predictive ability. The highest AIC values were obtained in the models adjusted for age, race, study site, and cognitive function.
DISCUSSION
Older women with BVF loss have an increase in the risk of subsequent hip and non-spine/non-hip fractures. The association between visual field loss and risk of fracture was graded in nature. We estimated that 3.7% of hip fractures and 4.6% of non-spine/non-hip fractures were attributable to severe visual field loss using standard epidemiological estimation procedures.
Visual field loss can be induced by ocular diseases, such as glaucoma, cataracts and retinal disease,21 by a tumor22 or vascular occlusion8 along the cerebral visual pathway, or by cognitive factors such as inattention during the visual field test.21 The leading cause of visual field loss in persons 55 years or older is glaucoma,8 which is the second leading cause of preventable blindness worldwide.23 Since vision loss from glaucoma can be prevented or slowed down with treatment24 and half or more of individuals with glaucoma in the population are undiagnosed,25,26 screening tests can identify not only patients at increased risk for subsequent fractures but also patients who have potentially blinding yet treatable ocular diseases.
Technicians or nurses in the offices of clinicians can screen for visual field loss with a suprathreshold screening test as was used in this study. The test takes 4 minutes per subject and is straightforward to administer. Fewer than 13% of subjects visiting the clinic were unable to complete the visual field testing in our study. The sensitivity and specificity of suprathreshold testing was 87% and 89%, respectively, in a study evaluating the use of suprathreshold testing in screening for neuro-ophthalmic diseases such as glaucoma.27 Once visual field loss is detected on a screening test, subjects should be referred for an eye evaluation by an eye doctor if the visual field loss is present on repeat visual field testing.21
Prior studies have not evaluated whether BVF loss is associated with an increased risk of fractures in older women, but have evaluated the association between visual field loss in at least one eye and fractures among younger white subjects.8,10,11 Both studies had younger cohorts and did not have as many incident fractures as SOF. Their findings contrast with those of our study that suggest that the greater risk of hip and non-spine/non-hip fractures in older women secondary to visual field loss persists for at least 8 years after visual field testing.
In a prior SOF manuscript,5 we have reported that there is increased risk of hip fractures in women with decreased contrast sensitivity while reductions of visual acuity were not associated with increased risk of fractures. In this study we report that contrast sensitivity is not as strong of a predictor for hip fractures as BVF loss. These results are not surprising since cataracts, 28-30 glaucoma,29,31 and AMD29,32 may cause both decreased contrast sensitivity and visual field loss.
Strengths of our study include a large sample size, a long follow-up period, a well-defined cohort, radiographically adjudicated fractures, assessment of different vision components, and the utilization of standardized protocols for all measurements. A potential limitation of this study is that we calculated the BVF loss using the Esterman’s BVF scoring algorithm rather than directly measuring visual fields with both eyes. This method of assessing BVFs is accepted in vision research and is consistent with the results found when an Esterman BVF test is performed.33-35
Our study population was comprised of older volunteer women initially living in communities; therefore, our results might not apply to other populations such as men, younger women, or those with poorer health or living in institutions. It is possible that in a less healthy population, women may have even greater risk of fractures than we reported, especially since the women in our study are survivors and most likely are healthier than women who did not continue in SOF because of death or illness. In addition, the women in this study were highly motivated. Fewer than 13% of the women in SOF were excluded from this analysis because of either inability to perform visual fields or unreliable visual fields. We excluded women who participated only in home visits from our analyses. Their visual field loss could not be assessed since visual field testing cannot be performed in the home. Women participating only in home visits were slightly older and had worse self-rated health status. The women excluded from the analysis may be more likely to be immobile and thus may have lower risks of fracture; alternatively, excluded women may be at greater risk of fracture because they are likely to have worse vision than the women whose visual fields were assessed.
A limitation of our study is that we did not assess spine fractures at V6, and to diagnose radiographic spine fractures both baseline and follow-up spine films are needed. In addition, while we adjusted for cognitive impairment using the Mini Mental State Examination (MMSE), the MMSE may not detect all types of dementia that may be associated with the presence of visual field loss. Another limitation of this study is that all risk factors and confounders were assessed at V6 and may have changed over time. This is especially true for the assessment of BVF loss, which was only done at V6. Continued visual field loss may be detected a mean of 7.5 years after the first glaucomatous visual field loss is detected.36
Since visual field loss is an independent risk factor for subsequent fractures, clinicians are advised to evaluate patients’ vision when they report recurrent falling.37 This recommendation, however, does not specify a method for evaluating vision among the multitude of tests that could be performed. Our data suggest that future guidelines for the care of older patients should consider recommending evaluations of the visual fields in patients at heightened risk of fractures by recommending that clinicians either refer their patients who are recurrent fallers for visual field loss assessment or screen for visual field loss themselves using suprathreshold tests such as was done in SOF. Potential areas for future research include study of interventions to prevent fractures by managing visual field loss or through mobility training as well as further research on the impact of visual field loss on ability to function and quality of life.
ACKNOWLEDGMENTS
Supported by an unrestricted grant to the UCLA Jules Stein Eye Institute from Research to Prevent Blindness and Center for Eye Epidemiology at the UCLA Jules Stein Eye Institute. The Study of Osteoporotic Fractures (SOF) is supported by Public Health Service research grants from the National Institutes of Health (AR35582, AG05394, AR35584, AR35583, R01 AG005407, R01 AG027576-22, 2 R01 AG005394-22A1, 2 R01 AG027574-22A1).
Footnotes
Conflict of Interest: None of the authors has a proprietary interest in any of the products mentioned in this manuscript. No author has any financial conflict.
Anne L. Coleman, MD, PhD, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Contributions of each author: AL Coleman: concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, obtaining funding, administrative, technical and material support, supervision SR Cummings: concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, obtaining funding, administrative, technical and material support, supervision KE Ensrud: concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, obtaining funding, administrative, technical and material support, supervision F Yu: analysis and interpretation of data, critical revision of the manuscript for important intellectual content, statistical analysis P Gutierrez: acquisition of data, critical revision of the manuscript for important intellectual content, administrative, technical and material support, KL Stone: concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, obtaining funding, administrative, technical and material support, supervision JA Cauley: concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, obtaining funding, administrative, technical and material support, supervision KL Pedula:, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, technical support, MC Hochberg: concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, obtaining funding, administrative, technical and material support, supervisionCM Mangione: concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, obtaining funding, administrative, technical and material support, supervision Sponsor’s Role: The funding agencies were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Presented in part at the Annual Meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, April 26, 2004.
REFERENCES
- 1.Grisso JA, Kelsey JL, Strom BL, et al. Risk factors for falls as a cause of hip fracture in women. N Engl J Med. 1991;324:1326–1331. doi: 10.1056/NEJM199105093241905. [DOI] [PubMed] [Google Scholar]
- 2.Anastastasopoulos E, Yu F, Coleman AL. Age-related macular degeneration is associated with an increased risk of hip fractures in the Medicare database. Am J Ophthalmol. 2006;142:1081–1083. doi: 10.1016/j.ajo.2006.06.058. [DOI] [PubMed] [Google Scholar]
- 3.Dargent-Molina P, Favier F, Grandjean H, et al. Fall-related factors and risk of hip fracture: The EPIDOS prospective study. Lancet. 1996;348:145–149. doi: 10.1016/s0140-6736(96)01440-7. [DOI] [PubMed] [Google Scholar]
- 4.Klein BE, Klein R, Lee KE, et al. Performance-based and self-assessed measures of visual function as related to history of falls, hip fractures, and measured gait time. The Beaver Dam Eye Study. Ophthalmology. 1998;105:160–164. doi: 10.1016/s0161-6420(98)91911-x. [DOI] [PubMed] [Google Scholar]
- 5.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 6.Kelsey JL, Browner WS, Seeley DG, et al. Risk Factors for Fractures of the Distal Forearm and Proximal Humerus. Am J Epidemiol. 1992;135:477–489. doi: 10.1093/oxfordjournals.aje.a116314. [DOI] [PubMed] [Google Scholar]
- 7.Taylor HR, Livingston PM, Stanislavsky YL, et al. Visual impairment in Australia: distance visual acuity, near vision, and visual field findings of the Melbourne Visual Impairment Project. Am J Ophthalmol. 1997;123:328–337. doi: 10.1016/s0002-9394(14)70128-x. [DOI] [PubMed] [Google Scholar]
- 8.Ramrattan RS, Wolfs RCW, Panda-Jonas S, et al. PTVM. Prevalence and causes of visual field loss in the elderly and associations with impairment in daily functioning. Arch Ophthalmol. 2001;119:1788–1794. doi: 10.1001/archopht.119.12.1788. [DOI] [PubMed] [Google Scholar]
- 9.Coleman AL, Cummings SR, Yu F, et al. Binocular visual-field loss increases the risk of future falls in older White women. J Am Geriatr Soc. 2007;55:357–364. doi: 10.1111/j.1532-5415.2007.01094.x. [DOI] [PubMed] [Google Scholar]
- 10.Ivers RQ, Optom B, Cummings RG, et al. Visual Risk Factors for Hip Fracture in Older People. J Am Geriatr Soc. 2003;51:356–363. doi: 10.1046/j.1532-5415.2003.51109.x. [DOI] [PubMed] [Google Scholar]
- 11.Ivers RQ, Cummings RG, Mitchell P, et al. Risk Factors for Fractures of the Wrist, Shoulder and Ankle: The Blue Mountain Eye Study. Osteoporosis International. 2002;13:513–518. doi: 10.1007/s001980200063. [DOI] [PubMed] [Google Scholar]
- 12.Cauley JA, Lui LY, Ensrud KE, et al. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA. 2005;293:2102–2108. doi: 10.1001/jama.293.17.2102. [DOI] [PubMed] [Google Scholar]
- 13.Cummings SR, Black DM, Nevitt MC, et al. Appendicular bone density and age predict hip fracture in women. JAMA. 1990;263:665–668. [PubMed] [Google Scholar]
- 14.Nevitt MC, Cummings SR, Browner WS, et al. The accuracy of self-report of fractures in elderly women: Evidence from a prospective study. Am J Epidemiol. 1992;135:490–499. doi: 10.1093/oxfordjournals.aje.a116315. [DOI] [PubMed] [Google Scholar]
- 15.Topouzis F, Coleman AL, Yu F, et al. Sensitivity and Specificity of the 76-Suprathreshold Visual Field Test to Detect Eyes with Visual Field Defect by Humphrey Threshold Testing in a Population-based Setting: Thessaloniki Eye Study. Am J Ophthalmol. 2004;137:420–425. doi: 10.1016/j.ajo.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 16.Esterman B. Functional scoring of the binocular field. Ophthalmol. 1982;89:1226–1234. doi: 10.1016/s0161-6420(82)34647-3. [DOI] [PubMed] [Google Scholar]
- 17.Bailey I, Lovie J. New design principles for visual acuity letter charts. Am J Optom Physiol Opt. 1976;53:740–745. doi: 10.1097/00006324-197611000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Vistech Vision Contrast Test System Instructions . Vistech Consultants Inc.; [Accessed May 10, 2009]. 1988. (online). http://www.agingeye.net/cataract/Vistech2.pdf. [Google Scholar]
- 19.Sahai H, Khurshid A. Methods, Techniques, and Applications. CRC Press, Inc; 1996. Statistics in epidemiology; pp. 203–211. [Google Scholar]
- 20.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- 21.Choplin NT, Edwards RP. Visual Field Testing with the Humphrey Field Analyzer. SLACK Incorporated; Thorofare, NJ: 1995. [Google Scholar]
- 22.Kerrison JB, Lynn MJ, Baer CA, et al. Stages of improvement in visual fields after pituitary tumor resection. Am J Ophthalmol. 2000;130:813–820. doi: 10.1016/s0002-9394(00)00539-0. [DOI] [PubMed] [Google Scholar]
- 23.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman AL. Glaucoma. Lancet. 1999;354:1803–1810. doi: 10.1016/S0140-6736(99)04240-3. [DOI] [PubMed] [Google Scholar]
- 25.Tielsch JM, Sommer A, Katz J, et al. Racial variations in the prevalence of primary open angle glaucoma. The Baltimore Eye Survey. JAMA. 1991;266:269–274. [PubMed] [Google Scholar]
- 26.Varma R, Ying-Lai M, Francis BM, et al. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: The Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1439–1448. doi: 10.1016/j.ophtha.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 27.Siatkowski RM, Lam BL, Anderson DR, et al. Automated suprathreshold static perimetry screening for detecting neuro-ophthalmologic disease. Ophthalmology. 1996;103:907–917. doi: 10.1016/s0161-6420(96)30588-5. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi K, Hayashi H, Nakao F, et al. Influence of cataract surgery on automated perimetry in patients with glaucoma. Am J Ophthalmol. 2001;132:41–46. doi: 10.1016/s0002-9394(01)00920-5. [DOI] [PubMed] [Google Scholar]
- 29.Coleman AL. Sources of binocular suprathreshold visual field loss in a cohort of older women being followed for risk of falls (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2007;105:312–329. [PMC free article] [PubMed] [Google Scholar]
- 30.Rubin GS, Adamsons IA, Stark WJ. Comparison of acuity, contrast sensitivity, and disability glare before and after cataract surgery. Arch Ophthalmol. 1993;111:56–61. doi: 10.1001/archopht.1993.01090010060027. [DOI] [PubMed] [Google Scholar]
- 31.Hawkins AS, Szlyk JP, Ardickas Z, et al. Comparison of Contrast Sensitivity, Visual Acuity, and Humphrey Visual Field Testing in Patients with Glaucoma. J Glaucoma. 2003;12:134–138. [PubMed] [Google Scholar]
- 32.Midena E, Angeli CD, Blarzino MC, et al. Macular function impairment in eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 1997;38:469–477. [PubMed] [Google Scholar]
- 33.Mills RP, Drance SM. Esterman disability rating in severe glaucoma. Ophthalmology. 1986;93:371–378. doi: 10.1016/s0161-6420(86)33732-1. [DOI] [PubMed] [Google Scholar]
- 34.Crabb DP, Viswanathan AC. Integrated visual fields: a new approach to measuring binocular visual field of view and visual disability. Graefes Arch Clin Exp Ophthalmol. 2005;243:210–216. doi: 10.1007/s00417-004-0984-x. [DOI] [PubMed] [Google Scholar]
- 35.Crabb DP, Fitzke FW, Hitchings RA, et al. A practical approach to measuring visual field component of fitness to drive. Br J Ophthalmol. 2004;88:1191–1196. doi: 10.1136/bjo.2003.035949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eid TM, Spaeth GL, Bitterman A, et al. Rate and amount of visual loss in 102 patients with Open-Angle glaucoma followed up for at least 15 years. Ophthalmology. 2003;110:900–907. doi: 10.1016/S0161-6420(03)00076-9. [DOI] [PubMed] [Google Scholar]
- 37.Summary of the Guideline. American Geriatrics Society; [Accessed May 1, 2008]. 2006. Update of the guideline for prevention of falls in older persons. (online). http://www.americangeriatrics.org/education/2006Falls_summary120105.shtml. [Google Scholar]