Abstract
An exciting synergistic interaction occurs among researchers working at the interface of reproductive biology and energy homeostasis. Reproductive biologists benefit from the theories, experimental designs, and methodologies used by experts on energy homeostasis while they bring context and meaning to the study of energy homeostasis. There is a growing recognition that identification of candidate genes for obesity is little more than meaningless reductionism unless those genes and their expression are placed in a developmental, environmental, and evolutionary context. Reproductive biology provides this context because metabolic energy is the most important factor that controls reproductive success and gonadal hormones affect energy intake, storage, and expenditure. Reproductive hormone secretion changes during development, and reproductive success is key to evolutionary adaptation, the process that most likely molded the mechanisms that control energy balance. It is likely that by viewing energy intake, storage, and expenditure in the context of reproductive success, we will gain insight into human obesity, eating disorders, diabetes, and other pathologies related to fuel homeostasis. This review emphasizes the metabolic hypothesis: a sensory system monitors the availability of oxidizable metabolic fuels and orchestrates behavioral motivation to optimize reproductive success in environments where energy availability fluctuates or is unpredictable.
Keywords: appetitive behavior, hoarding, metabolic hypothesis, motivation, nutritional infertility, sex behavior, vaginal scent marking
Research at the interface of energy balance and reproduction is gaining momentum as investigators in many different fields recognize the relevance of this topic to basic biology and clinical practice. According to a recent PubMed search (using the search keywords energy balance and reproduction) only 135 journal articles were published in the decade between 1980 and 1990, whereas 609 articles were published in the last decade. Eighty of the 135 articles published between 1980 and 1990 were concerned with lactation in dairy animals, whereas the 600 or more published in the last decade covered a broad range of topics including the many orexigenic and anorectic peptides that influence reproduction in a wide variety of organisms, including invertebrates and vertebrates, human and non-human primates, and males and females (Table 1). The recent momentum reflects an exciting synergy that arises from melding reproductive biology with neuroendocrinology of ingestive behavior. Reproductive biologists benefit from the theories, experimental designs, and methodologies used by experts in ingestive behavior and energy homeostasis. Reproductive biology and physiological ecology bring context and meaning to the study of ingestive behavior and energy metabolism. This current special issue of Frontiers in Translational Endocrinology illustrates that metabolic control of reproduction is now, on its own, an established field of basic biological research with the most exciting discoveries just around the corner. New investigators are beginning to recognize that by viewing energy metabolism in the context of reproductive success, we open a window into human obesity, eating disorders, diabetes, and other pathologies related to fuel homeostasis.
Table 1.
Orexigenic and anorectic peptides that influence reproduction.
| Central peptides | Ingestive behavior effect | Reproductive effects |
|---|---|---|
| Agouti-related protein (AgRP) | Increases food intake and hoarding (Rossi et al., 1998; Day and Bartness, 2004) | Inhibits LH in the presence of E (Schioth et al., 2001), stimulates LH in males (Stanley et al., 1999) |
| α-melanocyte stimulating hormone (α-MSH), MTII | Decreases food intake and food hoarding (Shimizu et al., 1989; Keen-Rhinehart and Bartness, 2007) | Stimulates LH secretion (Alde and Celis, 1980) |
| Bombesin-like peptides | Decrease food intake (Gibbs and Smith, 1988) | Stimulate LH secretion (Babu and Vijayan, 1983) |
| β-endorphin | Increases food intake (McKay et al., 1981) | Inhibits LH secretion and sexual libido (Sirinathsinghji et al., 1983) |
| Cocaine and amphetamine-regulated transcript (CART) | Decreases food intake (Kristensen et al., 1998) | Stimulates GnRH secretion (Lebrethon et al., 2000; Parent et al., 2000) |
| Cholecystokinin (CCK) | Decreases food intake and food hoarding (Bailey and Dela-Fera, 1995; Teubner and Bartness, 2010) | Simulates LH secretion (Kimura et al., 1983; Perera et al., 1993) |
| Corticotropin releasing hormone (CRH) | Decreases food intake (Levine et al., 1983; Heinrichs and Richard, 1999) and food hoarding in rats (Cabanac and Richard, 1995) | Inhibits LH secretion and lordosis (Olster and Ferin, 1987) and sex behavior (Jones et al., 2002) |
| Dopamine (DA) | Decreases food intake (Heffner et al., 1977), increases food hoarding (Kelley and Stinus, 1985; Borker and Mascarenhas, 1991), and reward (Wise, 2004) | Stimulates sexual arousal, motivation and reward (Meisel and Mullins, 2006) |
| Galanin | Increases food intake (Kyrkouli et al., 1986; Krasnow et al., 2003) | Inhibits LH secretion (Sahu et al., 1987) |
| Galanin-like peptides | Increases food intake (Krasnow et al., 2003; Van Der Kolk et al., 2010) | Inhibits LH secretion (Gundlach, 2002; Krasnow et al., 2003; Van Der Kolk et al., 2010) |
| Glucagon-like peptide (GLP-I) | Decreases food intake (Turton et al., 1996) | Stimulates LH secretion (Beak et al., 1998) |
| Gonadotropin releasing hormone (GnRH I or II) | Decreases food intake (Kauffman and Rissman, 2004b) | Stimulates LH secretion and sex behavior (Moss and McCann, 1975; Clarke and Cummins, 1982; Temple et al., 2003) |
| Kisspeptin | Decreases food intake (Stengel et al., 2011) | Stimulates GnRH and LH secretion (Gottsch et al., 2004; Irwig et al., 2004) |
| Melanin concentrating hormone (MCH) | Increases food intake (Presse et al., 1996) | Inhibits LH secretion (Tsukamura et al., 2000a) |
| Motilin (peripheral) delete | Increases food intake in fasted rats (Garthwaite, 1985) | Inhibits LH secretion (Tsukamura et al., 2000b) |
| Neuropeptide Y (NPY) | Increases food intake (Stanley and Leibowitz, 1984) and food hoarding (Dailey and Bartness, 2009) | Inhibits LH in the absence of, stimulates LH in the presence of estradiol (Crowley et al., 1985; Sahu et al., 1987), inhibits sex behavior (Ammar et al., 2000), |
| Orexin/hypocretin | Increases food intake (Sakurai et al., 1998) | Inhibits LH in the absence of, stimulates in the presence of estradiol (Pu et al., 1998) |
| Oxytocin | Decreases food intake (Olson et al., 1991) | Stimulates sex behavior (Whitman and Albers, 1995) |
| RFamide-related peptide-3 = Gonadotropin inhibiting hormone | Increases food intake (Tachibana et al., 2005; Johnson et al., 2007) | Inhibits GnRH and LH secretion and sex behavior (Bentley et al., 2006; Kriegsfeld et al., 2006; Smith et al., 2008) |
| Secretin (move to VIP) | Decreases food intake (Cheng et al., 2011) | Stimulates LH secretion (Babu and Vijayan, 1983) |
| Serotonin (5HT) | Decreases food intake (Blundell, 1977) | Stimulates LH in the presence of estradiol (Coen and MacKinnon, 1979) Inhibits LH secretion in the absence of estradiol (Coen et al., 1980; Koh et al., 1984) |
| Thyroptropin releasing hormone | Decreases food intake (Vijayan and McCann, 1977) | Stimulates LH secretion in pituitary in vitro not in vivo (Fujihara and Shiino, 1983), and indirectly by effects on thyroid hormones (Barrett et al., 2007) |
| Urocortin | Decreases food intake (Spina et al., 1996) | Stimulates LH secretion in ewes (Holmberg et al., 2001), inhibits LH secretion (potentially; Li et al., 2005; Nemoto et al., 2010), directly inhibits Leydig cell function (Rivier, 2008) |
| Vasopressin | Decreases food intake (Meyer et al., 1989) | Inhibits LH secretion (Heisler et al., 1994) |
With so many new researchers entering the field, it might be useful to provide sign posts to the most fruitful avenues of research, as well as to potential hazards, wrong turns, and dead ends. In particular, new investigators need to know which hypotheses and assumptions are supported by a preponderance of evidence and which are largely unsupported. Unfortunately, some of the most often repeated ideas in this field happen to be as untestable as they are seductive. Future research will be centered on molecular mechanisms at the level of the gene, but will be meaningless without attention to the developmental, epigenetic, environmental, and evolutionary context.
This review will focus on the five ideas that are likely to facilitate progress in research at the interface of reproduction and energy homeostasis.
-
(1)
We will emphasize experimental designs that incorporate varying degrees of metabolic challenge and behavioral options relevant to the natural habitats in which those behaviors evolved, i.e., habitats in which food is not available ad libitum and the experimental subjects have options to choose between reproductive and ingestive behaviors.
-
(2)
We will illustrate the importance of measuring behavioral motivation by quantifying appetitive sex and ingestive behaviors, i.e., not only eating and copulation, but also behaviors that bring individuals in contact with food or potential mating partners, such as food hoarding and courtship.
-
(3)
We will emphasize the metabolic hypothesis, the idea that neuroendocrine systems function to maintain fuel (not body fat or food intake) homeostasis.
-
(4)
We will urge investigators to focus specifically on mechanisms that promote opportunistic overeating or hoarding and fuel storage in anticipation of the high energetic demands of reproduction.
-
(5)
All of the above concepts will be discussed within the context of a distributed neural network with multiple, redundant function. We will argue that metabolic control of reproduction must include the hindbrain, midbrain and forebrain including the ventral premammillary nucleus. This is in sharp contrast to the typical focus on the arcuate nucleus of the hypothalamus (Arc).
The central unifying hypothesis is that neuroendocrine systems that control energy homeostasis optimize reproductive success in environments where energy fluctuates or is unpredictable. The testable predictions that emerge from this hypothesis are fundamentally different from those that follow from the idea that neuroendocrine systems exist to maintain body weight or adiposity at some particular level.
An Evolutionary Context and the Relevance of a Semi-Natural Environment
Metabolic control of reproduction is obscured in the laboratory when females are housed in isolation, in small cages, where locomotion is restricted, temperature is controlled, and where food availability is unlimited (Bronson, 1998). Under these conditions, there is more than enough energy available for all of the cellular and systemic processes including reproduction. Likewise, women in modern, westernized, industrial societies live surrounded by “foraging” opportunities that require very little energy expenditure. It is under these conditions of unlimited food availability that misconceptions developed about hormone effects on behavior. Two such misconceptions, for example, are that female sexual motivation in primates is emancipated from the effects of hormones, and that the main function of “satiety” peptides is to maintain stability in body weight. These naïve notions are shattered when animals are housed in semi-natural environments that mimic important aspects of their natural habitats that include a limited food supply, high energy demands, and the ability to choose between sex and ingestive behavior. When animals are studied under environmental conditions that approximate those in which the traits evolved, presumed “satiety” peptides increase sexual motivation and presumed “orexigenic” peptides promote vigilant foraging and food hoarding and opportunistic overeating. These effects are masked under conditions of ad libitum food intake and low energetic demand. Under these conditions, sex drive is already elevated, obscuring increases that might otherwise be induced by the release of anorectic peptides. Laboratory animals are perhaps in some ways more like modern humans in westernized societies in that, in both cases, the link between hormones, behavior, and environmental energy availability is obscured. An important function of these peptides, to orchestrate the appetites for food and sex, is revealed by studying laboratory animals under the energetically challenging conditions in which they evolved. As will be described in this review, laboratory rodents can be housed in a semi-natural burrow system that 1) requires that individuals expend energy on locomotion in order to gain access to food, and 2) provides opportunities to encounter potential mating partners during foraging expeditions. Under these conditions, the effects of hormones on motivation are revealed in sharp relief. Furthermore, fluctuation, rather than stability in ingestive behavior is the norm.
In contrast to the typical laboratory situation, food supplies in the natural habitats of most wild animals are not unlimited. Rather, in nature, food availability often fluctuates seasonally, with a nadir in the winter, dry, or rainy season depending on the geographic area. In addition, food availability varies unpredictably due to myriad factors including population density, inter and intraspecies competition, famine, drought, storms, hurricane, tornadoes, floods, fires, global climate change, and diseases that destroy edible plants, crops, prey animals, and livestock (Bronson, 1989, 1998). Given the importance of environmental energy availability on reproductive function in members of every mammalian order, it is reasonable to hypothesize the mechanisms that control energy intake, storage, and expenditure serve to optimize reproductive success in environments where energy fluctuates (Bronson, 1989; Wade and Schneider, 1992; Schneider, 2006; Schneider et al., 2007). One prediction from this hypothesis is that the effects of ovarian hormones on ingestive and sex behavior will vary with the degree of energetic challenge, that is, the balance of energy supply and expenditure. This idea is inspired by the book, Mammalian Reproduction, which provides evidence for metabolic control of reproduction in females from representative species of every mammalian order and for the conclusion that energy availability is the most important factor that controls reproduction in female mammals (Bronson, 1989).
There are three important features to this perspective.
First, females anticipate the energetic demands of reproduction by eating more than their immediate energetic needs and storing the extra energy as body fat or as a food cache. All ingested macronutrients derived from food, including carbohydrates and fats, can be stored as triglycerides in adipocytes, so that later, these triglycerides can be hydrolyzed to release glycerol and free fatty acids for oxidation. During pregnancy and lactation, these fuels are mobilized for the growing conceptus and to produce milk for the offspring. In rats, progesterone (elevated during the luteal phase of the ovulatory cycle and pregnancy), in the presence of estradiol, promotes increases in food intake, and body fat storage, at least in the early phases of pregnancy (Wade, 1975; Trujillo et al., 2011). Models of mechanisms that affect ingestive behavior and body weight must incorporate the ability to anticipate future energy shortages.
Second, females anticipate opportunities for fertile matings by virtue of the fact that the same hormones that induce ovulation also stimulate sexual appetite while reducing the appetite for food. Neuroendocrine mechanisms stimulate sexual (rather than ingestive) motivation during the times of highest fertility, when mate searching, courtship, and copulatory activities are most likely to pay off in terms of genetic contributions to the next generation. The sequence of hormones necessary for ovulation is permissive for copulatory behavior and these same neuroendocrine mechanisms inhibit foraging, hoarding, and eating during the most fertile part of the cycle (Zucker, 1969, 1972; Wade and Zucker, 1970; Zucker et al., 1972; Wade and Gray, 1979; Wade and Schneider, 1992; Asarian and Geary, 2006; Klingerman et al., 2010; Michopoulos and Wilson, 2011). Thus, models of ingestive behavior and physiology must include the choice between food and sex, fluctuations in ovarian hormones, and sex differences in ingestive and sex behavior responses to ovarian steroids.
Third, females have mechanisms that delay reproductive processes during energetic challenges and re-initiate reproductive activities when the energetic conditions improve. In species from rodents to primates, sexual motivation, sexual performance, puberty, birth intervals, hypothalamic gonadotropin releasing hormone (GnRH) secretion, luteinizing hormone (LH) secretion, and ovarian steroid synthesis and secretion are inhibited by energetic challenges (McClure, 1962; Kennedy and Mitra, 1963; Morin, 1975; Ronnekleiv et al., 1978; Bronson and Marsteller, 1985; Cameron et al., 1985; Foster and Olster, 1985; Armstrong and Britt, 1987; Bronson, 1987, 1988; Lively and Piacsek, 1988; Sprangers and Piacsek, 1988; Schneider and Wade, 1989; de Ridder et al., 1990; Thomas et al., 1990; Cameron, 1996; Shahab et al., 1997, 2006; Ellison, 2001; Temple et al., 2002; Terry et al., 2005). When energetic challenges are so severe that they induce anestrous or inhibit the menstrual cycle, the primary locus of effect is the GnRH pulse generator, a diffusely located cell group in the medial basal hypothalamus. In support of this idea, pulsatile LH secretion, follicle development, and ovulation can be reinstated by treatment with pulses of GnRH at species-specific frequency and amplitude in food-deprived or -restricted rats, sheep, pigs, cows, monkeys, and women (Nillius et al., 1975; Foster and Olster, 1985; Bronson, 1986; Day et al., 1986; Armstrong and Britt, 1987; Cameron and Nosbisch, 1991; Kile et al., 1991; Manning and Bronson, 1991). Severe metabolic challenges can have effects at many levels (the gonad, pituitary, or hypothalamic GnRH generator, or the neural substrates that control sex behavior). Metabolic control of the GnRH pulse generator is the most widely studied. What about less severe metabolic challenges?
Fourth, and most recently, mild energetic challenges can have significant effects on reproductive and ingestive behavior long before there are effects on the mechanisms that govern steroid synthesis and secretion (Schneider, 2004; Schneider et al., 2007; Klingerman et al., 2010). Furthermore, in order to observe the effects of mild energetic challenges on the reproductive system, it is necessary to house animals in semi-natural environments in which energy expenditure is high relative to energy supply and both food and mates are available simultaneously (Schneider et al., 2007; Klingerman et al., 2010).
Attention to appetitive and consummatory aspects of behavior might shed light on so-called “feeding” hormones and neuropeptides which tend to stimulate ingestive behavior and inhibit aspects of the reproductive system, e.g., ghrelin, neuropeptide Y (NPY), and RF amide-peptide-3 (RFRP-3), also known as gonadotropin inhibiting hormone (GnIH), which tend to stimulate ingestive behavior and inhibit various aspects of the reproductive system (Table 1) (Clark et al., 1985; Guy et al., 1988; Kalra et al., 1988; Wren et al., 2000; Furuta et al., 2001; Johnson et al., 2007; Kriegsfeld et al., 2010; Shah and Nyby, 2010). It might also illuminate the functional significance of leptin, α-melanocortin stimulating hormone (α-MSH), kisspeptins, glucagon-like peptide, cholecystokinin, and GnRH, which tend to decrease ingestive behavior and stimulate reproductive behavior and physiology (Table 1) (Gonzalez et al., 1993; Wade et al., 1997; Schneider et al., 1998; Ammar et al., 2000; Cragnolini et al., 2000; Kauffman and Rissman, 2004a; Castellano et al., 2005, 2010; Kauffman et al., 2005; Fernandez-Fernandez et al., 2006; Crown et al., 2007; Millington, 2007). Given that many of these hormone–behavior systems were molded by natural selection in response to environmental energy availability, and that natural selection works via differential reproductive success, progress can be facilitated by attention to the influence of these hormone–behavior systems on reproductive success.
The hypothesis that putative anorectic and orexigenic peptides function to optimize reproductive success in environments where energy fluctuates or is unpredictable is relevant even for our own species. A look at modern, non-contracepting, non-industrialized societies shows that seasonal fluctuations and unpredictable scarcity in food availability have profound, measurable effects on reproductive success (Ellison, 2001). The link between reproduction and environmental energy is obvious in the !Kung, non-contracepting bush people who live in the Kalahari Desert where rainfall and hence food availability fluctuates dramatically within a year. Among the !Kung, the fluctuating food supply is coupled with a continuous need to expend energy because the workload is high throughout the year. !Kung women, for example, engage in miles of walking, carrying water, gathering firewood, harvesting, and cooking food, all while toting infants and toddlers. The !Kung show a dramatic loss of body weight during the “hungry season” and a dramatic drop in birth rate 9 months following the hungry season, suggesting that they only rarely ovulate during the this time of low food production (van der Walt et al., 1978). Given that evolutionary change occurs via differential reproductive success, plus the clear effect of seasonal fluctuation in food availability on reproductive success in extant populations of humans, it is likely that fluctuations in food availability molded ingestive and reproductive traits in our own species during human history.
Furthermore, the effects of environmental energy availability on human reproductive function are not limited to rural, indigenous, tribal peoples. Starvation and food insecurity has impact on reproductive function today in many, if not all societies. In fact, most societies have members who expend more energy than they can acquire, and nutritional amenorrhea occurs in these subpopulations, either because they experience starvation in relation to poverty or because they voluntarily engage in exercise and limit their food intake (Loucks, 2003a,b; Loucks and Thuma, 2003; Rosetta and Mascie-Taylor, 2009). For example, a high incidence of delayed menarche, adult amenorrhea, decreased birth rates, and high infant mortality result from low food availability in non-contracepting populations in India and Bangladesh (Gopalon and Naidu, 1972; Chen et al., 1974). Birth intervals are longer and birth rates plummet along with low energy balance (energy intake and storage minus expenditure) in women from extant, diverse, subsistence gardening/farming cultures, including the Lese of the Congo’s Ituri Forest, the Tamang in the foothills of the Himalaya of central Nepal, and women who live in mountain valleys in rural Poland (Ellison, 2001). Not only are energetic effects on fertility present in the economically challenged people in every society, but these effects are seen in all strata of every society during famine (Chakravarty et al., 1982). Thus, examples of the link between energy availability and reproductive success in our own species come from extant, modern populations as well as populations descended from subsistence agricultural societies. Thus, when we hypothesize that the mechanisms that control energy balance serve to optimize reproductive success in environments where energy fluctuates, this likely applies to our ancestors and to modern human beings.
The idea that the energy balancing system optimizes reproductive success is, in some ways, similar to the so-called “thrifty gene” hypothesis (Neel, 1962, 1999), with important differences. According to Neel, fluctuations in energy availability during the early evolution of Homo sapiens have favored genotypes that code for metabolic efficiency, the ability to overeat and store excess energy in adipose tissue that would increase the chances of survival during famine (Neel, 1962, 1999). The idea is often invoked to explain the so-called obesity epidemic. Individuals predisposed toward body fat storage had selective advantage in Paleolithic times, whereas in the presence of modern food abundance, the same individuals become obese. Various reiterations of Neel’s ideas tend to be incomplete, and thus, in this review we emphasize three specific modifications. First, ingestive behavior, body weight, and body fat content are polygenic; many genes contribute to metabolic efficiency, body fat storage, and hunger, not just one “thrifty” gene. Second, survival during famine was not the sole function of the energy balancing system. The critical function was to modulate reproductive output according to environmental energy availability. Finally, overeating and obesity are not the inevitable outcome of one “thrifty” gene in an energy rich environment, but rather, the interaction of many genes with reproductive hormones, epigenetic factors, and environmental energy availability.
Appetitive and Consummatory Behaviors
Reproductive behavior is far more complex than the simple act of copulation, ingestive behavior is more than the act of eating food, and these complexities are important. The hormonal effects on reproduction are not limited to the hypothalamic–pituitary–gonadal (HPG) system, but extend to the brain mechanisms that control the hunger for food and sex. Survival and reproduction involves appetitive behaviors that bring animals in contact with food or mating partners (Sherrington, 1906; Craig, 1917). Appetitive sex behaviors, however, occur separated in time from lordosis and reflect sexual motivation but not necessarily the ability to perform the sex act (Sherrington, 1906; Craig, 1917; Lorenz, 1950; Johnston, 1974, 1977; Lisk et al., 1983; Everitt, 1990). These appetitive behaviors might include mate searching and assessment, competition, courtship, and the ability to attract a mate. Similarly, ingestive behavior is more than the amount of calories ingested or meal size, it is a multifaceted array of interrelated behaviors and physiological traits that include foraging, food hoarding, food defense, and diet preference.
The history of behavioral endocrinology contains hints that we have missed something important in our narrow focus on food intake and copulation. Primate research based on laboratory studies led to erroneous conclusions about hormonal effects on primate sex behavior. These conclusions were overturned when researchers such as Kim Wallen began studying primates in semi-natural environments wherein females were able to exercise volition in their sexual interactions. Contrary to the commonly held idea that female primates are emancipated from the effects of hormones on behavior, appetitive sex behaviors, such as male-female grooming and proximity to mating partners, are correlated with peri-ovulatory increases in circulating estradiol when primates are studied in a semi-natural habitat where they experience social risks and can exercise volition with regard to sex behavior. These effects on sex behavior in a semi-natural environment have been traced to circulating levels of estradiol (Wallen, 2000, 2001). Similar misconceptions about hormonal effects on human behavior result from the narrow focus on copulation of the pair, rather than on underlying sexual motivation of the individuals in question. The idea that women are somehow emancipated from the effects of ovarian hormones on sex behavior is supported only by the difficulty in showing repeatable, statistically significant correlations between menstrual fluctuations in ovarian hormones and the incidence of sexual intercourse in married women from modern, industrialized societies (with unlimited food intake and low energetic demands). In contrast, when the motivation of individual women is assessed, there are statistically significant associations between these measures of appetitive behavior and phases of the menstrual cycle in a growing number of studies (Stanislaw and Rice, 1988; Meuwissen and Over, 1992; Gangestad et al., 2002; Gangestad and Thornhill, 2008; Durante and Li, 2009). These examples from sexual behavior in primates all suggest that it is important to create a relevant context in the study of behavioral endocrinology.
A similar problem occurs in the study of human ingestive behavior. Researchers in industry and academia alike commonly operate under the assumption that women are emancipated from effects of their ovarian hormones on ingestive behaviors. The effect of phases of the menstrual cycle on food intake in women, i.e., a periovulatory nadir in food intake, is subtle and is statistically significant in most, but not all studies (Fessler, 2003). The periovulatory increase in locomotor activity is even more elusive in women (Fessler, 2003).
What would we find if sexual and ingestive motivation (not just food intake and the incidence of sexual intercourse) were measured in females with limited food availability and high energetic demands? Inspired by these questions, we have initiated a new line of research that manipulates energy availability and examines the effects of ovarian steroids on behavioral motivation (Klingerman et al., 2010, 2011a), and a recent example of this work is illustrated in the article by Klingerman et al. (2011b) in this issue.
The idea that any of these particular chemical messengers evolved to optimize reproductive success in environments where energy availability fluctuates is testable (i.e., it constitutes a hypotheses in which there is a realistic outcome that will refute the hypothesis). This hypothesis leads to the following testable predictions:
-
(a)
The effects of the chemical messenger in question will vary when energy availability is manipulated. The effects of so-called orexigenic and anorectic peptides will be amplified in testing environments that mimic the habitats in which these neuroendocrine systems evolved, including the choice between food and sex, and the need to expend energy in order to acquire energy. Specifically, the greater the energetic challenges, the more putative orexigenic peptides inhibit sexual motivation, and directly or indirectly promote vigilant foraging, hoarding, and eating. The choice between food and sex is a prerequisite for observation of the phenomenon.
-
(b)
The “orchestration of motivation” implies effect on the choice between food and sex, because in natural environments animals do not live and forage in the absence of conspecifics and do not engage in sex in a separate enclosed space precluded from eating and foraging. Thus, sexual motivation, i.e., the appetitive aspects of behavioral choice, will be more sensitive to energetic challenges than the consummatory aspects of behavior. Food hoarding and the preference for spending time with males vs. food will be significantly affected by food restriction prior to significant changes in follicle development and estradiol secretion.
-
(c)
Periovulatory increases in estradiol disinhibit mechanisms that control sexual motivation and behavior, thereby switching behavioral priorities from ingestive to sexual.
These hypotheses have been examined in female Syrian hamsters, rodents in which motivation (appetitive behavior) and ability (consummatory behavior) are easily measured, with respect to both ingestive and sex behavior. Sexual performance of the lordosis posture is a reflex triggered by male conspecific odors and tactile stimulation. These sensory cues are integrated in neural structures only when those neural structures are stimulated by periovulatory levels of estradiol and progesterone, which bind to their receptors, estradiol receptor-alpha (ER-α), and progestin receptor (PR). Lordosis reflects an unknown combination of both motivation and ability and consistently occurs on day 4 of the 4-day estrous cycle, known as proestrous in rats (Ciaccio and Lisk, 1971; Lisk et al., 1972; Steel, 1981). Motivation, in contrast to performance, can be measured in this species by counting the number of vaginal scent marks, or by measurement of the preference for sex vs. food. Vaginal scent marking increases gradually over days 1–3 of the Syrian hamster estrous cycle (known as diestrous 1 and 2 in rats) as circulating estradiol (but not progesterone) is rising. In addition, female hamsters decrease levels of agonist behavior toward males, and spend progressively more time in closer proximity to males as circulating levels of estradiol increase (Johnston, 1974, 1975). Hamster appetitive sex behaviors increase linearly with increasing levels of estradiol alone and are inhibited by progesterone (Ciaccio et al., 1979; Steel, 1981).
Hamsters are prodigious hoarders in the wild and in the laboratory. Metabolic challenges, such as food deprivation increase the appetitive ingestive behavior, food hoarding, but not the consummatory measure, food intake (Silverman and Zucker, 1976; Rowland, 1982). In their natural habitat, hamsters emerge from underground burrows for only 90 min per day and spend virtually every minute of this time hoarding food (Gattermann et al., 2008). These ecological observations suggest that the choice between hoarding and courtship has important consequences for reproductive success and evolution by natural selection. Thus, female hamsters were acclimated to a burrow system that included vertical tubes in a t-configuration leading in one direction to food and in the opposite direction to an adult male hamster. For 8 days prior to testing, they were either fed ad libitum or food-restricted to 75% of their ad libitum intake. At the onset of the dark period of the light–dark cycle, they were tested every day of the estrous cycle for their preference for males vs. food, food hoarding, and food intake (Klingerman et al., 2010).
According to our first hypothesis, we predicted that the effects of the anorectic hormone estradiol would differ according to the availability of metabolic energy, and that appetitive behaviors would be more sensitive than consummatory behaviors to energetic challenges. We first tested this by measuring ingestive and sexual motivation over the estrous cycle under two different energetic conditions: limited and unlimited food availability. In two other experiments we manipulated the available energy by increasing the demand for energy expenditure. We used two conditions in which hamsters must increase their energy expenditure: housing at cold ambient temperature (5°C for at least 7 days before testing), and access to voluntary exercise (the hamsters were housed with running wheels). In all types of energetically challenged females, whether food-limited, cold-housed, or exercising), we predicted that there would be clear fluctuations in appetitive behaviors over the estrous cycle, with food preference at its nadir and sexual motivation at its peak on estrous cycle days 3 and 4. If our first hypothesis were correct, only energetically challenged females would fluctuate over the cycle in food hoarding and the preference for males vs. food, whereas females with unlimited energy availability would not fluctuate to the same degree. Our hypothesis would be refuted if females with unlimited energy availability did not differ from those with limited intake or increased energetic demands. If our second hypothesis were correct, energetically challenged females would differ from energy unchallenged females in appetitive (food hoarding and preference for spending time with males), but not consummatory behavior (food intake and the incidence of lordosis).
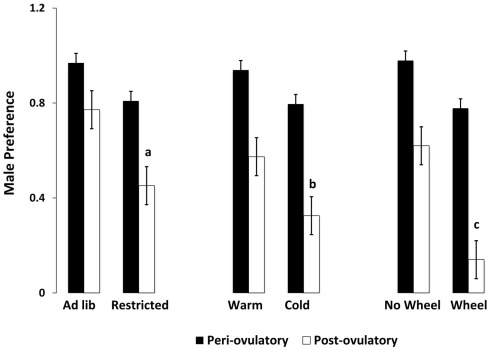
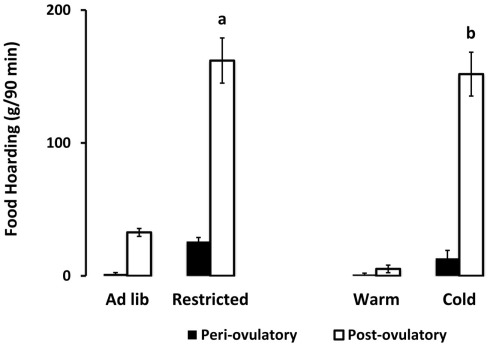
As hypothesized, only the food-limited, cold-housed, and wheel-running females varied significantly over the estrous cycle in appetitive sex behaviors. Those with unlimited energy availability showed consistently high preference for sex throughout the estrous cycle. For example, food-limited, cold-housed, and wheel-running females showed significant fluctuations in male preference [(time spent with males – time spent with food) divided by total time; Figure 1; Klingerman et al., 2010]. Females with unlimited food, housed at room temperature without access to running wheels varied little if any over the estrous cycle in male preference (i.e., they preferred to visit the males more than 75% of the time of the time; Figure 1). Similarly, the food-restricted and cold-housed females varied significantly over the estrous cycle in food hoarding (Figure 2). Food-limited and cold-housed females showed low levels of food hoarding and high levels of male preference on the night of high circulating concentrations of estradiol and ovulation, and high levels of hoarding and low levels of male preference on all other estrous cycle day (Figure 2), and yet the same females showed little fluctuation in food intake over the estrous cycle (Figure 3). Females with unlimited food supply, housed at warm temperatures and without access to running wheels showed little variation in food hoarding over the estrous cycle, and none of the groups showed dramatic changes in food intake. With regard to sex behavior, the response to energy deficit was similar, whether the deficit was due to limited food availability, cold ambient temperature, or wheel running. Food hoarding, however, was increased by limited food availability and cold temperatures, but not by increases in voluntary wheel running. Females with wheels decreased their preference for males and yet hoarded very little food, perhaps providing a window into brain areas that partition energy for either sexual motivation or hunger for food.
Figure 1.
Mean and SEM of male preference, calculated as the (time spent with the male minus the time spent with food) divided by the total time. Male preference is shown for females on either the periovulatory day (when circulating estradiol is high, black bars) or the postovulatory day when (circulating estradiol is low, white bars), in three experiments. Experiment 1 compared food-limited to food-unlimited females at 22°C without access to wheels. Experiment 2 compared cold-housed (5°C) to warm housed (22°C) in females with unlimited food availability. Experiment 3 compared females housed with running wheels to those without running wheels at 22°C with levels of food equal to those fed ad libitum without wheels. a = significantly different from ad libitum-fed at P < 0.01, b = significantly different from warm housed at P < 0.01, c = significantly different from no-wheel group at P < 0.001. Food-limited and unlimited groups adapted from Klingerman et al. (2010).
Figure 2.
Mean and SEM of food hoarding shown for females on either the periovulatory day (when circulating estradiol is high, black bars) or the postovulatory day when (circulating estradiol is low, white bars), in two experiments. Experiment 1 compared food-limited to food-unlimited females at 22°C. Experiment 2 compared cold-housed (5°C) to warm housed (22°C) in females with unlimited food availability. a = significantly different from ad libitum-fed at P < 0.001, b = significantly different from warm housed at P < 0.001. Food-limited and unlimited groups adapted from Klingerman et al. (2010).
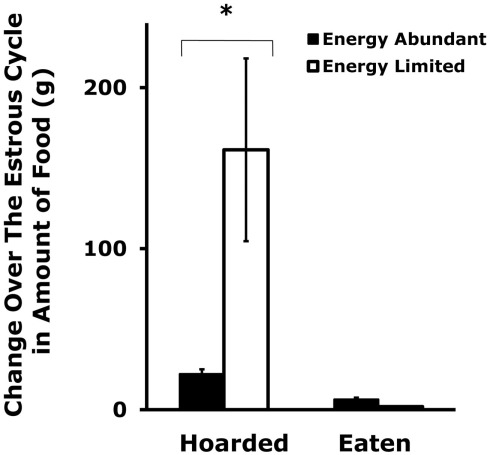
Figure 3.
Mean and SE of the change in food hoarding that is seen over the estrous cycle in food-limited female Syrian hamsters compared to those with unlimited food. * = significantly different from females with unlimited food. After Klingerman et al. (2010).
These experiments suggest that changes in energy intake and expenditure change the responsiveness or sensitivity to ovarian steroids. A follow-up experiment showed the same effects of limited energy availability when ovariectomized (Ovx) females were compared to females treated with the hormonal regimen typically used to induce lordosis in this species (estradiol 48 h and progesterone 6 h prior to testing). Four days of food restriction increased food hoarding and decreased male preference in Ovx + vehicle females relative to Ovx + estradiol and progesterone treated females (Klingerman et al., 2010). The effects are not attributable to changes in circulating levels of ovarian steroids, but to the response to those steroids (Klingerman et al., 2010). In other words, ovarian steroids had obvious measurable effects on appetitive behaviors in females with limited food availability, but these were attenuated in females fed ad libitum. Furthermore, at these short durations of mild food restriction, steroid-energy availability interaction was apparent only in appetitive, not consummatory behaviors, and they occurred in response to exogenous steroid treatment.
Together, these experiments are consistent with the idea that an important role of estradiol is to orchestrate the choice between ingestive and sex behavior under conditions where energy availability fluctuates and that this decision occurs at the level of behavioral motivation rather than performance. Furthermore these experiments show that the effects are not limited to food deprivation, but they extend to any situation in which overall availability of energy is limited, for example, when the need for energy expenditure is elevated relative to energy intake.
These results lead to additional testable hypothesis about the mechanisms by which estradiol orchestrates behavioral choice. For example, we hypothesize that during the early follicular phase of the estrous cycle when circulating estradiol levels are low, sexual motivation is tonically inhibited by one or a number of putative “orexigenic” peptides, such as ghrelin, GnIH, NPY, agouti-related protein (AgRP), endocannabinoids, or some combination of these. Furthermore, we hypothesize that periovulatory levels of estradiol disinhibit the effects of ghrelin, and/or other neuropeptides in order to couple reproductive motivation with fertility and inhibit food hoarding. These hypotheses lead to testable predictions about the effects of estradiol on neural activation in identified neurons, e.g., those that contain ghrelin or endocannabinoid receptors and secrete GnIH, NPY, or AgRP. Consistent with this idea, changes in neural activation in GnIH cells are more closely associated with appetitive than consummatory sex and ingestive behaviors (Klingerman et al., 2011b).
These studies illustrate that, when studying the effects of energy availability, it is imperative to differentiate appetitive from consummatory behaviors. Appetitive behaviors are more sensitive, and occur prior to effects on gonadal steroids. By exclusive focus on food intake estrous cyclicity and pulsatile gonadotropin secretion, the effects of energy availability will be missed. Furthermore, it is well known that appetitive aspects of behavior often involve different brain areas and different hormonal stimulation (Ball and Balthazart, 2008), and these are often more relevant to human behavior. Most relevant to metabolic control of reproduction and ingestive behavior, cellular activation that corresponds with NPY/AgRP effects on food hoarding occurs in the subzona incerta and central nucleus of the amygdala, whereas cellular activation that corresponds to effects on food intake involve the typical activation of paraventricular nucleus of the hypothalamus (PVH), and other areas, but not the subzona incerta or central nucleus of the amygdala (Teubner et al., 2011). Food hoarding species, such as Siberian and Syrian hamsters should receive a great deal more attention in the future, now that it has been documented that our own species is more like hamsters than rats in that they do not show postfast hyperphagia to the same degree as laboratory rats and mice (Hetherington et al., 2000; Al-Hourani and Atoum, 2007; Levitsky and DeRosimo, 2010), but instead show significant changes in grocery shopping, i.e., carrying food from a source outside the home and storing it in their home prior to consumption (Dodd et al., 1977; Tom, 1983; Beneke and Davis, 1985; Beneke et al., 1988; Mela et al., 1996). These ideas are extensively covered in a recent, lucid review of the appetitive behavior, food hoarding, by Bartness et al. (2011).
Sense and Nonsense, the Lipostat Hypothesis
The above-mentioned results suggest that not all hormones and neuropeptides function to keep body weight within limits that we imagine to be healthy and fashionable. Rather, they function to respond to changes in environmental energy availability by modulation of reproductive and ingestive behaviors. In nature, energetic demands differ in males and females, within groups of males and females, over seasons, and over the entire lifespan.
This is important because translational research programs are often built upon the idea that “normal” individuals have a healthy “set point” for body weight and adiposity, whereas overweight and obese individuals do not. The “lipostat” hypothesis purports that factors secreted from adipose tissue dictate the level of food intake in service of maintaining this set point in adiposity. A modern version of the lipostat hypothesis purports that factors such as leptin are secreted from fat cells in proportion to overall body adiposity, induce satiety, and therefore decrease meal size. When extended to reproduction, the lipostat hypothesis is called the “critical body fat” hypothesis, which suggests that puberty is delayed or adult reproduction is inhibited when body fat content and levels of the lipostatic hormone falls below a particular threshold.
The lipostat, set point, and the critical body fat concepts are intuitively satisfying descriptions that are seldom tested directly but are often reinforced with circular reasoning. In contrast, science progresses by testing hypotheses that can conceivably be ruled out by a realistic experimental outcome (Popper, 1963). The set point model is refuted whenever an experimental group fails to defend a set point for body weight (e.g., due to a change in diet, reproductive cycle, photoperiod, or ambient temperature). Instead, any significant increase in body weight is interpreted as evidence for a “resetting” or “sliding” set point. Like the existence of God, the sliding set point hypothesis cannot be refuted. It is difficult to imagine how natural selection (based on individual survival and superior reproductive success) would favor defense of a set point in environments where food availability fluctuates or is unpredictable. When food is scarce and energy demands are high, the maintenance of a particular set point for body weight or adiposity should receive low priority compared to the break down of lipids in adipose tissue to usable metabolic fuels necessary for survival. Conversely, if females encounter a windfall of energy rich food, why abstain from overeating in order to preserve a putative set point for body weight when pregnancy and lactation are so energetically demanding and the food supply so unreliable? Why not eat heartily and store the excess energy as lipids in adipose tissue depots especially designed to provide fuels for milk production that will feed your genetic contribution to the next generation? Females might develop obesity but would be more likely to get their genes into the next generation, particularly if the unhealthy consequences of obesity accrue after the reproductive years.
The set point is a seductively satisfying label or analogy that has been criticized because it terminates, rather than stimulates further investigation. To name a phenomenon is not, in and of itself, progress in understanding the phenomenon. The set point is used as an analogy, but that does not translate into a testable hypothesis. The lipostat and the set point idea are inspired by the engineers’ design of the mechanical thermostat. A homeowner’s thermostat contains a physical object, a thermometer, that can measure temperature and can be calibrated and set by the homeowner. The room temperature varies above or below the set point, and when it deviates too far from set point, the heating or air conditioning corrects the error. In animals, there is no physical object that corresponds to the thermostat. We have not identified the location and biochemical nature of the detectors for metabolic fuel availability, and we have no idea how such a set point for fuel availability or for body fat content might be set or calibrated. The set point theory has been repeatedly refuted on both empirical and theoretical grounds in numerous excellent reviews that are highly recommended (Wirtshafter and Davis, 1977; Van Itallie and Kissileff, 1990; Bronson and Manning, 1991; Bronson, 1998). Due to its intuitive and seductive nature, however, it is bound to come up whenever a new investigator enters the field of metabolic control of reproduction.
In contrast to the lipostat idea, the hypothesis that putative orexigenic and anorectic hormones function to optimize reproductive success in environments where energy availability fluctuates leads to testable hypotheses. It is in line with data showing that putative satiety peptides ensure overeating when those peptides are low, but are often ineffective in curbing appetite when they are high (Ahima et al., 1996; Flier, 1998). The hypotheses are reminiscent of Optimal Foraging Theory, which has its origins in ecology and is based on the ubiquitous observation among wild animals that food intake decreases with increasing cost in terms of time and energy expenditure. The corollary is that energy intake increases when food is cheap, plentiful, and requires little energy to obtain and digest (Emlen, 1966; MacArthur, 1966). When animals encounter diets of different caloric density that can be consumed at a particular energetic cost, intake increases as cost decreases and as net calories increase, and this choice ultimately results in accumulation of body fat (Collier et al., 1972; Houston and McNamara, 1989). This effect is linear, and thus, any lipostatic explanation would have to include a new set point reached for every excess calorie consumed. Instead of stability, body weight displays remarkable plasticity that allows anticipation of future metabolic challenges and permits the learning and memory formation that occurs when postingestional cues reinforce sensory cues from food. It is important to embrace this fact of life, and study the mechanisms whereby cheap and calorically dense food elicits changes in metabolism that allow excess storage, rather than search for an elusive and possibly non-existent mechanism that is suppose to maintain one set point for body weight throughout adult lifespan.
Like body fat content, food intake is not held at a set point. For example, when laboratory chow is diluted with non-nutritive bulk, laboratory rodents do not maintain their food intake at a set point. Quite the contrary, they show a controlled increase in food intake in proportion to the caloric dilution, and reproductive function is related to a threshold of usable fuels, not a particular level of bulk intake (Adolph, 1947; Peterson and Baumgardt, 1971; Nance and Gorski, 1977; Louis-Sylvestre, 1987; Szymanski et al., 2009). In contrast, changing the vitamin, mineral or essential fatty acid content of food while keeping calories constant elicits little or no change in food intake (Adolph, 1947). Furthermore, when animals must exercise or expend energy to obtain food, or when animals must expend more energy to keep warm at cold ambient temperatures, animals increase their food intake to compensate for the extra energy expended (Kraly and Blass, 1976; Browne and Borer, 1978; Tsai et al., 1982; Bart ness et al., 1984; Rowland, 1984; Louis-Sylvestre, 1987; Manning and Bronson, 1990; Schneider and Wade, 1990b). Females of many species will more than double their food intake during lactation to meet the energetic demands of milk production (Cripps and Williams, 1975; Fleming, 1976a,b, 1978; McLaughlin et al., 1983; Louis-Sylvestre, 1987; Woodside et al., 2000). Lactating female mice, Peromyscus leucopus, increase their food intake 230% when housed in cold ambient temperature compared to their prepregnant food intake at laboratory temperatures (Perrigo, 1987).
A particularly convincing argument for metabolic, rather than lipostatic control of food hoarding, comes from studies of Siberian hamsters fed a diet diluted with non-nutritive cellulose. Hamsters increase their food hoarding (but not their food intake) in proportion to the dilution and decrease their food hoarding in proportion to increases in caloric density. The change in food hoarding is immediate, and thus cannot be mediated by changes in body fat content, but is more likely controlled by postingestive cues that occur when the hamsters taste the diet just prior to hoarding (Wood and Bartness, 1996). Like internally stored energy, externally stored energy is flexible and varies with the energetic demands of the individual, its life history stage, and the environment. Rather than maintain a set point, food intake, food hoarding, body weight, and adiposity change dramatically in response to environmental cues (energy, ambient temperature, and photoperiod) in order to maintain the availability of oxidizable metabolic fuels for survival and reproduction (Friedman, 2008).
Contrary to commonly held dogma, stability in body weight is not a consistent feature in ad libitum-fed mammals, especially female mammals. The myth of stability in body weight is based on longitudinal studies, but is not supported by studies in which the same individuals are studied over many weeks. Far from the stability of body weight predicted by the lipostat hypothesis, body weight in animals with unlimited access to food increases steadily over time (Ahren et al., 1997). When genetically heterogeneous (outbred) strains of rats are singly housed with unlimited access to standard, laboratory chow, and monitored for more than 100 weeks, they fail to maintain “body weight homeostasis.” Instead they not only gain significantly more body weight and adiposity than food-limited rats, but they increase their body fat content from 6–7% at 6–7 weeks of age, to 25% at 14 weeks, to 36–42% at 106 weeks of age. They also develop significant hypertriglyceridemia and hypercholesterolemia relative to food-limited rats (Keenan et al., 2005). Male rats show the largest increase in body weight during the first year; females show the largest body weight gain in the second year after cessation of their estrous cycles. The study by Keenan et al. (2005) illustrates two points. First, rats in isolation and in a confined space do not self-regulate their intake, and do not avoid the negative consequences of obesity. Second, reproductive factors create differences in the propensity to store body fat. In females, the body weight gain was exaggerated after the postmenopausal decrease in ovarian steroid secretion, whereas in males, body weight gain occurred early in the life history cycle (Keenan et al., 2005). Thus, in addition to mechanisms that maintain fuel homeostasis for individual survival, there lies another layer of control that promotes internal fuel storage as body fat to anticipate the need to forego ingestive behavior in favor of reproductive behavior necessary for Darwinian fitness.
This brings us to the lipostatic hypothesis of reproduction popularized by Rose Frisch (Frisch and McArthur, 1974; Frisch, 1990), which purports that there is a critical level of body fat necessary for the initiation of puberty and menarche, the maintenance of adult menstrual cycles and fertility and for the restoration of reproductive function in animals that have become anestrous or hypogonadotropic after food restriction or deprivation. Little evidence actually supports this hypothesis beyond the obvious correlation between body fat content and reproductive function; but correlation is not causation. Both body fat content and reproductive competence depend upon a third factor, the availability of oxidizable metabolic fuels, thus, body fat content and reproduction are correlated. When strong inference hypothesis testing is used as a direct test of the “critical body fat” hypothesis, the critical body fat hypothesis is refuted. For example, when females are rendered hypogonadotropic by food deprivation and are then re-fed, the restoration of estrous cycles and pulsatile LH secretion occurs more rapidly than the recovery of body fat levels, and without significant increases in plasma leptin concentrations in sheep and hamsters (Schneider et al., 2000a; Jones and Lubbers, 2001; Szymanski et al., 2007). Finally, most animals respond rapidly to changes in fuel availability, too rapidly for the changes in reproduction to be due to the slow process of lipid accumulation (Bronson, 1986, 1998, 2000; Armstrong and Britt, 1987). Thus, contrary to the critical body fat hypothesis, LH pulses can be restored whenever overall metabolic fuel availability increases, even when body fat levels and levels of plasma leptin concentrations lag behind and remain at the same level as hypogonadal food-restricted females.
Sense and Nonsense, the Metabolic Hypothesis
“The simplest way in which this lipostasis could be achieved is by sensitivity to the concentration of circulating metabolites.”
(Kennedy, 1953)
Gerald Kennedy is often credited with coining the word lipostat, which, over time, became associated with the set point hypothesis. It is important to note, however, that Kennedy’s idea of a fat-derived signal came not from circulating hormones or neural signals from adipose tissue, but rather from circulating metabolites associated with either lipolysis or lipogenesis (Kennedy, 1953). Furthermore, he proposed that the ability to sense metabolic fuel availability was critical for the control of food intake and reproductive development. Kennedy’s lipostat was more in line with the large body of data showing that the productive system is responsive to the availability of oxidizable metabolic fuels (Schneider and Wade, 1989; Wade and Schneider, 1992; Foster et al., 1995; Nagatani et al., 1995; Murahashi et al., 1996). Gerald Kennedy was perhaps the first to speculate that if we understood the mechanisms that switched animals from lipolysis and free fatty acid oxidation to lipogenesis and fat storage we would understand the onset of puberty and the control of food intake (Kennedy, 1953; Kennedy and Mitra, 1963).
Metabolic control of reproduction has a long history spearheaded by ingestive behavior researchers and reproductive endocrinologists working together. Inspired by the original papers by Kennedy, M. I. Friedman, S. Ritter, and others (Ritter, 1986; Friedman, 1989), Schneider and Wade (1989) used pharmacological inhibitors of glucose or free fatty acid oxidation to differentiate between the effects of fuels vs. the effects of body fat content on estrous cyclicity. They studied Syrian hamsters because a mere 48 h period of food deprivation induces anestrous in this species (Morin, 1986). Schneider and Wade (1990a) compared the effects of 48 h deprivation in fat vs. lean females and demonstrated that body fat content could buffer against food deprivation-induced anestrous. However, as Kennedy predicted, fat hamsters were protected not by their body fat content per se, but by the metabolic fuels hydrolyzed and mobilized from lipids in adipocytes, i.e., free fatty acids. This was demonstrated when 48 h of food deprivation-induced anestrous in lean, but not fat female hamsters, and the protective effects of fat were blocked by treating fat hamsters with the inhibitor of fatty acid oxidation, methyl palmoxirate (MP; Schneider and Wade, 1989). Food-deprived hamsters treated with MP became anestrus even though they did not differ in body fat content from the estrous-cycling, food-deprived hamsters treated with vehicle. Thus, the HPG system is not controlled directly by body fat content, but rather, by the peripheral free fatty acids released from adipocytes, although the effect of those peripheral fatty acids might be to spare glucose for the brain. Since the publication of Schneider and Wade (1989), control of reproductive processes by the availability of oxidizable metabolic fuels has been documented in other model systems (Dickerman et al., 1990; Berriman et al., 1992; Bucholtz et al., 1996; Murahashi et al., 1996; Nagatani et al., 1996; Medina et al., 1998; Temple et al., 2002; I’Anson et al., 2003a; Moriyama et al., 2003; Shahab et al., 2006).
The above-mentioned research was focused on changes in the periphery. More recent research is focused on CNS fatty acid oxidation and synthesis, and the bulk of this work is concerned with ingestive behavior. Prior to diving into this field of research, it is important to examine the role of glucose sensing, as well as the idea that food intake and reproduction are controlled by the availability of ATP, or the ratio of ATP:AMP (Friedman, 2008). Glucose and fatty acid oxidation do not occur independently, but rather they are interrelated. The availability of glucose and the ratio of ATP to AMP and ADP, for example, determine the extent to which cells engage in fatty acid oxidation. This is important because translational research aimed at one metabolic pathway will have to account for compensatory coordinated changes in the other, and detection of these different fuels may take place in different parts of the brain.
Cellular detectors of glucose availability important for control of estrous cycles have been localized to the brainstem, just as those for food intake, adrenal glucocorticoid secretion, and counterregulatory responses to glucoprivation are localized in the brainstem (Ritter et al., 2011). There are “glucose-sensitive” cells in many brain areas involved in other diverse functions (including neuroprotection, circadian rhythms, and reward, to name a few; Levin et al., 2004), but those outside the hindbrain require more than 10-fold higher concentrations and 100-fold higher volume of inhibitors of glucose oxidation for significant effects on ingestive behavior (Ritter et al., 2011). Ingestive behavior is stimulated (Smith and Epstein, 1969; Ritter, 1986), and reproductive processes are inhibited by treatments that block glucose oxidation given systemically (Schneider and Wade, 1989) or into the third or fourth ventricle (Ritter et al., 1981; Murahashi et al., 1996), and the effects of glucoprivation are prevented by lesions of the AP in the caudal most part of the hindbrain (Ritter and Taylor, 1990; Schneider and Zhu, 1994; Panicker et al., 1998). For both ingestive behavior and reproduction, catecholaminergic projections from hindbrain to the PVH are necessary for the effects of glucoprivation (Ritter and Calingasan, 1994; I’Anson et al., 2003b; Bugarith et al., 2005).
For the HPG system and sex behavior, we are likely to find that brainstem structures are far more sensitive to changes in glucose availability than are forebrain structures. This is foreshadowed by work on ingestive behavior. Microinjections of an inhibitor of glucose utilization into hundreds of brainstem areas in the NTS increased food intake, whereas only a few did so when administered to hypothalamic areas (reviewed by Ritter et al., 2011). Small (200 nl volumes of 12–24 g per animal) of glucoprivic agents microinjected unilaterally into discrete hindbrain regions increase food intake and initiated counterregulatory responses. The same doses and even higher doses are not effective when injected into hypothalamic sites or even into the fourth ventricle (Ritter et al., 2000). Furthermore, small microinjections of glucoprivic agents that increase food intake also increase mRNA for the orexigenic peptide NPY. The hyperphagia and increase in NPY mRNA are significantly decreased by immunotoxic destruction of the catecholaminergic/NPYergic cells that originate in the hindbrain. This is not true in the hypothalamus. In contrast to brainstem lesions, NPY-saporin-induced lesions of Arc NPY neurons do not impair glucoprivic feeding or hyperglycemic responses (Ritter et al., 2006). Careful mapping of metabolic stimuli that affect food intake strongly suggests that the important signals are detected in the caudal brainstem, and this type of mapping should be done for metabolic control of reproduction. These brainstem structures and projections to the PVH are not required for the effects of food deprivation on food intake and reproduction, only for changes elicited by glucoprivation. Thus, glucoprivic control cannot explain all effects of natural energetic challenges on food intake and reproduction. However, the parallels between glucoprivic control of food intake and glucoprivic control of estrous cycles are striking and worth remembering when trying to unravel the effects of intracellular fuel metabolism on reproduction.
Another important line of research showed that food intake is not controlled by either glucose or free fatty acid availability, per se, but by the general availability of oxidizable fuels or a metabolic event in the final common pathway to ATP production, perhaps ATP content itself. This idea is supported by the results of experiments in which pharmacological agents that reduce hepatic (liver) ATP increase food intake, and treatments that prevent depletion of hepatic ATP content also preclude increases in food intake (Rawson and Friedman, 1994; Rawson et al., 1994; Ji and Friedman, 1999; Ji et al., 2000). There is evidence that detectors of fatty acid oxidation and hepatic ATP content are in the periphery, most likely in the liver, because effects of fatty acids and of hepatic ATP content on food intake are blocked by treatments that disconnect the communication between the liver and brain via the vagus nerve (Ritter and Taylor, 1990; Tordoff et al., 1991). Metabolic inhibitors that decrease hepatic ATP status increase intracellular sodium and calcium concentrations in hepatocytes in vitro (Friedman et al., 2003; Rawson et al., 2003). Future research on metabolic control of food intake and reproduction will have to contend with the possibility that changes in the brain have effects on these peripheral ATP detectors.
The metabolic hypothesis was diluted in the literature after the discovery that leptin, the protein product of the ob gene, decreased food intake and restored reproductive capabilities in obese, hyperphagic, infertile ob/ob mice (mice homozygous for a mutation in the ob gene; Campfield et al., 1995; Halaas et al., 1995; Pelleymounter et al., 1995). Most of the literature in the decade from 1995 to 2005 portrays leptin action as nothing more than endocrine signaling, with less attention to leptin’s effects on intracellular fuel oxidation or to the metabolic events that control leptin synthesis and secretion. However, some investigators gathered evidence from diverse sources, which together suggested that peripheral hormones like insulin and leptin act via intracellular fuel oxidation. M. I. Friedman pointed out that effects of insulin and leptin on ingestive behavior…
“… is an indirect response to a shift in fuels from storage to oxidation, not a direct response to a satiety signal associated with the level of adiposity. Many experimental treatments that affect eating behavior, whether restricted to the central nervous system or not, alter peripheral metabolism. Because changes in fuel partitioning can affect food intake, knowing the metabolic consequences of such experimental manipulations may be necessary to understand their effect on eating behavior.”
(Friedman, 1998)
Inspired by conversations with Friedman, in the year 2000, J. E. Schneider et al. pointed out that…
“There are at least two possible ways that leptin might interact with the metabolic sensory system that controls reproduction. First, leptin synthesis and secretion in various tissues might be sensitive to metabolic fuel availability… as a mediator of the metabolic signal. Second, leptin might affect reproduction indirectly by way of modulating the metabolic signal that is known to influence reproduction. Leptin’s effects on reproduction coupled with its unique effects of fuel metabolism, i.e., it’s ability to promote in situ fuel oxidation without increasing the general availability of metabolic fuels in circulation, might provide new clues to the nature of the metabolic stimulus that controls reproduction.”
(Schneider et al., 2000b)
Leptin decreases food intake and stimulates reproductive process in a wide variety of species (reviewed by Schneider, 2000), but contrary to the lipostatic hypothesis, leptin acts on estrous cycles by modulating the intracellular availability of oxidizable fuels. The first evidence for the notion that leptin modulates intracellular fuel availability was demonstrated in vitro by the laboratories of Ungar and Rossetti (Rossetti et al., 1997; Shimabukuro et al., 1997; Wang et al., 1998). Does leptin modulate ingestive behavior and/or reproduction by modulating the intracellular availability of glucose or free fatty acids?
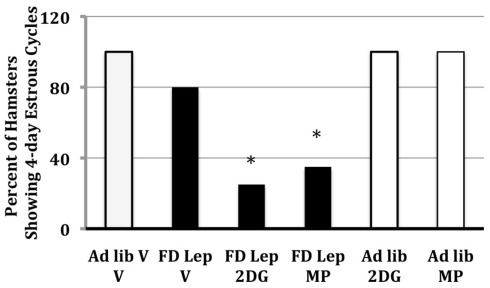
Treatment with leptin prevents food deprivation-induced anestrus in Syrian hamsters, and, thus, follow-up experiments were designed to ask whether follow-up experiments show that leptin’s ability to prevent food deprivation-induced anestrous is dependent upon the ability to increase glucose and/or fatty acid oxidation (Schneider et al., 1998). Syrian hamsters were either food deprived or fed ad libitum and treated with doses of MP, the inhibitor of free fatty acid oxidation, or 2-deoxy-d-glucose (2DG), an inhibitor of glucose utilization. Leptin was given either systemically or intracerebroventricularly. Doses of MP and 2DG were used that do not inhibit estrous cycles in ad libitum-fed females. The stimulatory effects of systemic and intracerebroventricular leptin on estrous cycles are blocked by treatments that blocked intracellular glucose or fatty acid oxidation (Figure 4; Schneider et al., 1998; Schneider and Zhou, 1999). This, to the best of our knowledge, was the first experiment to demonstrate the interaction between leptin and intracellular fuel oxidation on reproduction.
Figure 4.
The frequency of hamsters that showed regular, 4-day estrous cycles after 48 h of food deprivation on days 1 and 2 of the estrous cycle and treatment with either leptin (intraperitoneal injection) or vehicle. Half of each group was treated with 2-deoxy-d-glucose (2DG), methyl palmoxirate (MP), or the vehicles of these inhibitors of glucose or fatty acid oxidation respectively. * = significantly different from ad libitum vehicle at P < 0.05. After Schneider et al. (1998).
Whereas the above-mentioned experiments examined the effects of fatty acid oxidation, later work examined the effects of fatty acid synthesis. Generally, fatty acid synthesis is stimulated by excess fuel availability, i.e., when there is more than ample substrate availability for fuel oxidation and the formation of new ATP. Fatty acid oxidation occurs is predominant during fasting when the primary fuel available in the periphery is in the form of free fatty acids released from triacylglycerides in adipose tissue. If inhibition of fatty acid oxidation increases food intake and inhibits reproduction, does inhibition of fatty acid synthesis have the opposite effects? Inhibition of fatty acid synthase (FAS) in the brain and periphery decreases food intake (Loftus et al., 2000). FAS is the multienzyme protein that catalyzes the synthesis of fatty acids from the substrate malonyl-CoA under conditions of excess fuel availability. The discovery that food intake is inhibited by agents that inhibit the activity of FAS created a renewed interest in metabolic control of ingestive behavior focused on the effects of metabolic challenges (such as starvation and diabetes) and peripheral hormones such as leptin and ghrelin on enzymes and substrates involved in fatty acid synthesis in the brain.
The inhibitory effects of centrally administered FAS inhibitors on food intake was a surprise to many neuroscientists, given that glucose is the primary substrate oxidized in the CNS. It turns out that free fatty acids, and some particular amino acids also reach the brain. The same metabolic pathway for synthesis of fatty acids that functions in peripheral cells also exists in the CNS.
To review some of these basic pathways, fatty acids (i.e., LCFacyl-CoA, used in formation of triacylglycerides) are synthesized from malonyl-CoA (the reaction catalyzed by FAS). Malonyl-CoA is synthesized from acetyl-CoA [catalyzed by acetyl-CoA-carboxylase (ACC)]. ACC is inhibited by 5′-adenosine monophosphate-activated protein kinase (AMPK) as well as palmitate. AMPK is sensitive to energy availability, specifically the ratio of ATP to AMP. The formation of fatty acids (LCFacyl-CoA) for storage as triacylglycerides is an ATP-using process. Thus, stimulation of this pathway is appropriate under conditions of high energy availability.
There is a very large and confusing body of literature on the effects of fatty acid synthesis intermediates on control of food intake. Understanding these data requires that we step back and remember that these cerebroventricularly applied metabolic inhibitors might not mimic the endogenous events that control normal ingestion. Furthermore, these artificial CNS manipulations are likely to have effects on peripheral fuel metabolism that could, in turn, affect the behaviors in questions (Cha et al., 2005; Lam et al., 2005; Bartness et al., 2010; Bachman et al., 2002). Keeping this in mind, the general outcome of these studies is that most factors that decrease food intake tend to decrease the CNS activity of AMPK, the nutrient-sensitive kinase that inhibits ACC. For example, ICV leptin, insulin, and GLP-I decrease the CNS activity of CNS AMPK, which would be expected to increase the synthesis of malonyl-CoA and cause the accumulation of newly synthesized fatty acids. In line with this idea, factors that decrease food intake also increase the activity of CNS ACC, the key enzyme that catalyzes the rate-limiting step in malonyl-CoA synthesis. Factors that decrease food intake inhibit a brain-specific CPT-I, the enzyme that transports free fatty acids into mitochondria for oxidation in the periphery but is hypothesized to have a nutrient sensing function in brain. Factors that decrease food intake also increase mammalian target of rapamycin (mTOR), another nutrient-sensitive kinase involved in regulation of protein synthesis and energy balance. Conversely, factors that increase food intake tend have the opposite effects in hypothalamic fatty acid synthesis and oxidation, i.e., activation of AMPK and CPT-I, and inhibition of ACC, malonyl-CoA, and mTOR (Minokoshi et al., 2002, 2008; Cota et al., 2006, 2008; Pocai et al., 2006; Proulx et al., 2008; Wolfgang et al., 2008).
The relative importance of each of these intermediates, the mechanisms involved, and their importance for ingestive behavior in brain vs. periphery, hypothalamus vs. brainstem is still in question. For example, agents that inhibit (e.g., leptin, Compound C) and stimulate (ghrelin, AICAR) the activity of AMPK in hypothalamus decrease and increase food intake respectively. However, these exogenously applied agents are likely to have myriad side effects. For example, transgenic knockout of AMPKα2 exclusively in NPY neurons results in a lean phenotype, whereas knockout of AMPKα2 in POMC neurons results in an obese phenotype, and both types of neurons with these knockouts show normal electrophysiological responses to leptin but are insensitive to glucose (Claret et al., 2007). The effects of energetic challenges and ghrelin on AMPK in liver and adipocytes are the opposite of that in brain, and thus, hypotheses about the role of AMPK and other intermediates in control of ingestive behavior must consider the whole organism. AMPK is an ancient and ubiquitous enzyme. Some specificity might be added by consideration of other proteins involved in fatty acid oxidation and mitochondrial respiration, such as uncoupling protein-2 (UCP-2). For example, studies that compare knockouts for UCP-2 with wild type mice show that effects of leptin and ghrelin depend upon a functional gene for UCP-2, whereas substrate-mediated effects of AMPK occur with or without a functional gene for UCP-2 (Andrews, 2011; Diano and Horvath, 2012).
The importance of taking a broad perspective that includes the whole organism (brain and periphery) is exemplified by the exaggerated diet-induced obesity that occurs in knockout mice that lack the functional gene for CPT-Ic (Wolfgang et al., 2008). This is not predicted by the theory that inhibition of CPT-Ic in brain decreases appetite, but is instead consistent with the idea that decreased fatty acid oxidation produces a deficit in fuels for oxidation that leads to peripheral mechanisms that conserve energy by inhibition of energy expenditure and promote fuel storage.
Given that food restriction tends to increase food intake and inhibit the HPG system and sex behavior, it might be expected that central inhibition of fatty acid oxidation might inhibit the reproductive system. Investigators have begun to explore the potential role of AMPK, mTOR, and glucokinase (GK) in metabolic control of reproduction. Central treatment with inhibitors of free fatty acid oxidation inhibit the HPG system (Sajapitak et al., 2008). Pulsatile LH secretion is suppressed in a dose-dependent manner by fourth ventricular treatment with an inhibitor of fatty acid oxidation in ovariectomized female rats that were either treated with estradiol or vehicle. These results suggest that central inhibition of free fatty acid oxidation inhibits the HPG system. In other studies, activation of hypothalamic mTOR reverses food restriction-induced inhibition of LH secretion (i.e., stimulated LH secretion). Furthermore, blockade of mTOR delayed reproductive maturation, prevented the restorative effects of leptin on puberty, and suppressed KiSS1 mRNA levels in the Arc (Roa et al., 2009). Still other lines of research have focused on intermediate metabolism in GnRH neurons (in slice preparation). GnRH neurons are differentially responsive to glucose according to the steroid milieu, and these effects are mediated by AMPK (Roland and Moenter, 2011a,b,c). These direct effects of glucose availability on GnRH neurons are particularly interesting in light of evidence that GnRH neurons are privy to circulating metabolites from the periphery (Herde et al., 2011). These results must be reconciled with the fact that glucoprivic control of reproduction requires and intact AP. Why, if GnRH secretion is influenced directly by glucose availability, are the effects of systemic inhibition of glucose oxidation on estrous cycles and LH secretion prevented by lesions of the AP/NTS or by selective immunotoxic destruction of NE/NPY neurons from the brainstem to the PVH? In other words, if there are multiple sites of glucose detection in brain and periphery: which are the most relevant when metabolic challenges orchestrate ingestive and reproductive choices?
If these mechanisms function to maintain fuel homeostasis, how can animals overeat, store fat, and survive winter, migration, famine, and lactation? Enzymes such as AMPK, mTOR, and GK are linked to ATP formation, making them critical for maintaining intracellular fuel homeostasis, but what about the need to anticipate future increases in energy expenditure for reproduction? When ATP content is sufficient for energetic needs, the biochemical pathways leading to formation of ATP are halted, thus, it might be predicted that this would blind AMPK to the availability of oxidizable fuels. Thus, another type of energy sensor that is uncoupled from ATP formation might be required to enable animals to anticipate future food shortages and increase energy demands of reproduction by storing energy as adipose tissue. These sensors might also play a role in moment-to-moment decisions about the choice between conflicting behaviors (whether to forage for food or for mates, or whether to risk predation in order to consume or hoard food). The sodium–glucose cotransporter (SGLT) might fill this role because it appears to function independently of energy metabolism. SGLTs are transmembrane proteins that transport glucose and other sugars by coupling the uptake of a sugar molecule to the influx of one or two Na+ions (Wright, 2001). Because glucose is electroneutral, SGLT activity generates a net inward current that causes direct membrane depolarization and increased electrical activity without the need for glucose metabolism (Gribble et al., 2003). It has been known for more than 30 years that phloridzin, a SGLT antagonist, increases food intake in rats when given intracerebroventricularly (Glick and Mayer, 1968). Furthermore, SGLT is expressed in peripheral glucose-sensitive cholinergic neurons (Diez-Sampedro et al., 2003). Most glucose-sensitive neurons that are depolarized in response to non-metabolizable glucose analogs (such as α-MDG) are SGLTs (O’Malley et al., 2006), and furthermore, the effects of both glucose and α-MDG are abolished by phloridzin or by the removal of extracellular Na+. Together these results are consistent with the possibility that generation of ATP is not an essential prerequisite for sugar sensing in glucose-sensitive neurons. Thus, it is plausible that the SGLT and other similar metabolic sensors might allow orchestration of the energy economy under conditions of excess fuel availability. SGLT has not been explored with regard to reproduction, to the best of our knowledge.
Despite the excellent work in this field, the role of these intermediates in control of ingestive behavior is not understood. With regard to reproduction, the initial probes have been launched only recently. Virtually nothing is known about the role of these intermediates and metabolic substrates in the control of appetitive behavior and underlying motivation. These metabolic events might be important pivot points for decisions about whether to eat food or engage in reproductive activities.
Beyond the Hypothalamus
A final warning to new investigators would be to double check all assumptions about the importance of a neuropeptide, hormone, or brain area based on the number of published articles concerned with that topic. The vast majority of research on metabolic control of reproduction examines projections to and from the hypothalamus, and of those, most concern Arc NPY/AgRP, POMC, and more recently the Kisspeptin/GnIH system that includes the Arc, PVH, preoptic area, dorsomedial hypothalamus (DMH), and AVPV (Estrada et al., 2006; Franceschini et al., 2006; Kriegsfeld et al., 2006; Roa et al., 2006; Smith et al., 2008; Yeo and Herbison, 2011). In fact, a large body of elegant work in metabolic control of reproduction and ingestive behavior supports a widely distributed neural network that includes the caudal brain stem, midbrain, and forebrain (Schneider et al., 1993, 1995; Horn et al., 1999, 2001; Ritter et al., 2001; I’Anson et al., 2003b; la Fleur et al., 2003; Hudson and Ritter, 2004; Bugarith et al., 2005; Hayes et al., 2009; Skibicka and Grill, 2009). This work has been the subject of thoughtful and scholarly reviews (Grill and Kaplan, 1990, 2002; Grill, 2006; Friedman, 2008; Grill and Hayes, 2009). In the 1980s and 1990s, neuroanatomical characterization of POMC NPY cells located these molecules and their receptors in the brain stem as well as in other areas, and the notion of distributed neuroanatomical control of energy balance was well accepted (Sawchenko et al., 1985; Bronstein et al., 1992).
Agents that increase or decrease voluntary food intake do so when microinjected into the hindbrain, and, in many cases, these agents can have the same effects on passive intake in decerebrate animals (reviewed by Grill and Hayes, 2009). This applies to leptin (Grill et al., 2002; Harris et al., 2007). Furthermore, these hindbrain effects on food intake involve the activity of AMPK, and fail to occur when leptin-induced inhibition of AMPK is prevented (Hayes et al., 2009). Furthermore, the stimulatory effects of 2DG on food intake activate AMPK, and 2DG-induced hyperphagia does not occur without AMPK activation (Li et al., 2011). The main point for reproductive endocrinologists is that it would be a mistake to imagine that metabolic control of ingestive and reproductive behavior is restricted to the Arc or even the entire hypothalamus.
Both food intake and energy expenditure, and presumably energy expenditure for reproduction, are under the influence of the peptide systems in these widely distributed interconnected nuclei. For example, Skibicka and Grill (2009) measured food intake, core body temperature, heart rate, and spontaneous activity in rats that received two different picomolar doses of an MC4R agonist (MTII) into six different brain subnuclei distributed along the neuraxis. The MTII doses were titrated to be well below the threshold dose known to be effective when infused intracerebroventricularly. The results were unequivocal. In each case when the MTII infusion was demonstrated histologically to hit its intended target, five of the six brain areas, including the NTS and midbrain, showed increases in body temperature and heart rate as well as decreased food intake. The data confirm a growing body of data demonstrating that melanocortinergic effects on food intake and energy expenditure occur in the caudal brain stem (Grill et al., 1998; Williams et al., 2000, 2003; Grill and Kaplan, 2001, 2002).
The important implication for researchers interested in metabolic control of reproduction is that the brain is organized into a network of areas that display a great deal of redundancy of function, most of which impact aspects of energy expenditure as well as food intake. At this point, it should be obvious that a significant portion of total energy expenditure is allocated for reproduction. Investigators new to this field of research will no doubt come across published articles that employ “expression of a particular neuropeptide in the Arc” as evidence for the function of that peptide in control of ingestion. After reading this review, it should be obvious that an equally probably function is control of reproduction, as well as general activity, thermogenesis, body temperature, heart rate, and oxygen consumption.
Summary
This review is intended to provide a foundation for future research at the interface of ingestive behavior and reproduction. At the interface of ingestive behavior and reproduction lies a metabolic sensory system that detects changes in fuel availability and initiates changes in motivation, appetitive sex, and ingestive behaviors, in addition to changes in the HPG system. The observed changes in behavior, hormones, and metabolic fuel partitioning are best understood within the metabolic hypothesis:
A sensory system monitors the availability of oxidizable metabolic fuels and orchestrates behavioral motivation to optimize reproductive success in environments where energy availability fluctuates or is unpredictable.
This hypothesis leads to testable predictions. For example, it predicts that orexigenic and anorectic hormones and neuropeptides will have different effects on ingestive and sex behavior depending on the energetic challenges faced by the animals. This prediction was realized with regard to the effects of estradiol on food hoarding and courtship in Syrian hamsters (Figures 1 and 2). It also predicts that animals housed alone, with unlimited food, with limited behavioral options are not the control group, but the experimental group. It predicts that behavioral motivation will be more sensitive than food intake or copulation (Figures 1–3), and that mechanisms that function to in short term choices between food and sex might not be capable of maintenance of long term stability in body fat content in environments where energy availability and energetic demands fluctuate.
There is now recognition that so-called lipostatic hormones, once thought to maintain a set point in body fat content, are actually modulators of metabolic fuel availability, more specifically, fuel oxidation, and synthesis. The challenge is to incorporate what is known about these mechanisms in fuel homeostasis to the demonstrated ability to engage in opportunistic overeating in anticipation of the high energetic demands of reproduction (as well as seasonal and unpredictable changes in energy availability).
Finally, all of the above concepts must be studied in the context of a distributed neural network with multiple, redundant function. More specifically, metabolic control of reproduction must include the hindbrain (AP/NTS), midbrain (e.g., the lateral parabrachial nucleus and VTA), and forebrain (e.g., hypothalamus and striatum), as well as areas such as the ventral premammillary nucleus (Donato et al., 2009) in sharp contrast to the more common exclusive focus on Arc. In fact, the PMV and medial amygdala are good candidates for mediation of metabolic control of motivation for ingestive and sex behavior (Donato et al., 2010).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Adolph E. F. (1947). Urges to eat and drink in rats. Am. J. Physiol. 151, 110–125 [DOI] [PubMed] [Google Scholar]
- Ahima R. S., Prabakaran D., Mantzoros C., Qu D., Lowell B., Maratos-Flier E., Flier J. S. (1996). Role of leptin in the neuroendocrine response to fasting. Nature 382, 250–252 10.1038/382250a0 [DOI] [PubMed] [Google Scholar]
- Ahren B., Mansson S., Gingerich R. L., Havel P. J. (1997). Regulation of plasma leptin in mice: influence of age, high-fat diet, and fasting. Am. J. Physiol. 273, R113–R120 [DOI] [PubMed] [Google Scholar]
- Alde S., Celis M. E. (1980). Influence of alpha-melanotropin on LH release in the rat. Neuroendocrinology 31, 116–120 10.1159/000123061 [DOI] [PubMed] [Google Scholar]
- Al-Hourani H. M., Atoum M. F. (2007). Body composition, nutrient intake and physical activity patterns in young women during Ramadan. Singapore Med. J. 48, 906–910 [PubMed] [Google Scholar]
- Ammar A. A., Sederholm F., Saito T. R., Scheurink A. J., Johnson A. E., Sodersten P. (2000). NPY-leptin: opposing effects on appetitive and consummatory ingestive behavior and sexual behavior. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R1627–R1633 [DOI] [PubMed] [Google Scholar]
- Andrews Z. B. (2011). Central mechanisms involved in the orexigenic actions of ghrelin. Peptides. [DOI] [PubMed] [Google Scholar]
- Armstrong J. D., Britt J. H. (1987). Nutritionally-induced anestrus in gilts: metabolic and endocrine changes associated with cessation and resumption of estrous cycles. J. Anim. Sci. 65, 508–523 [DOI] [PubMed] [Google Scholar]
- Asarian L., Geary N. (2006). Modulation of appetite by gonadal steroid hormones. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1251–1263 10.1098/rstb.2006.1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu G. N., Vijayan E. (1983). Plasma gonadotropin, prolactin levels and hypothalamic tyrosine hydroxylase activity following intraventricular bombesin and secretin in ovariectomized conscious rats. Brain Res. Bull. 11, 25–29 10.1016/0361-9230(83)90053-9 [DOI] [PubMed] [Google Scholar]
- Bachman E. S., Dhillon H., Zhang C. Y., Cinti S., Bianco A. C., Kobilka B. K., Lowell B. B. (2002). betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297, 843–845 10.1126/science.1073160 [DOI] [PubMed] [Google Scholar]
- Bailey C. A., Dela-Fera M. A. (1995). Central nervous system cholecystokinin and the control of feeding. Ann. N. Y. Acad. Sci. 448, 417–423 [DOI] [PubMed] [Google Scholar]
- Ball G. F., Balthazart J. (2008). How useful is the appetitive and consummatory distinction for our understanding of the neuroendocrine control of sexual behavior? Horm. Behav. 53, 307–311 10.1016/j.yhbeh.2007.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett P., Ebling F. J., Schuhler S., Wilson D., Ross A. W., Warner A., Jethwa P., Boelen A., Visser T. J., Ozanne D. M., Archer Z. A., Mercer J. G., Morgan P. J. (2007). Hypothalamic thyroid hormone catabolism acts as a gatekeeper for the seasonal control of body weight and reproduction. Endocrinology 148, 3608–3617 10.1210/en.2007-0316 [DOI] [PubMed] [Google Scholar]
- Bartness T. J., Shrestha Y. B., Vaughan C. H., Schwartz G. J., Song C. K. (2010). Sensory and sympathetic nervous system control of white adiose tissue lipolysis. Mol. Cell. Endocrinol. 318, 34–43 10.1016/j.mce.2009.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness T. J., Keen-Rhinehart E., Dailey M. J., Teubner B. J. (2011). Neural and hormonal control of food hoarding. Am. J. Physiol. 301, R641–R655 10.1152/ajprenal.00120.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness T. J., Ruby N. F., Wade G. N. (1984). Dietary obesity in exercising or cold-exposed Syrian hamsters. Physiol. Behav. 32, 85–90 10.1016/0031-9384(84)90075-1 [DOI] [PubMed] [Google Scholar]
- Beak S. A., Heath M. M., Small C. J., Morgan D. G., Ghatei M. A., Taylor A. D., Buckingham J. C., Bloom S. R., Smith D. M. (1998). Glucagon-like peptide-1 stimulates luteinizing hormone-releasing hormone secretion in a rodent hypothalamic neuronal cell line. J. Clin. Invest. 101, 1334–1341 10.1172/JCI610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneke W. M., Davis C. H. (1985). Relationship of hunger, use of a shopping list and obesity to food purchases. Int. J. Obes. 9, 391–399 [PubMed] [Google Scholar]
- Beneke W. M., Davis C. H., Vander Tuig J. G. (1988). Effects of a behavioral weight-loss program food purchases: instructions to shop with a list. Int. J. Obes. 12, 335–342 [PubMed] [Google Scholar]
- Bentley G. E., Jensen J. P., Kaur G. J., Wacker D. W., Tsutsui K., Wingfield J. C. (2006). Rapid inhibition of female sexual behavior by gonadotropin-inhibitory hormone (GnIH). Horm. Behav. 49, 550–555 10.1016/j.yhbeh.2005.12.005 [DOI] [PubMed] [Google Scholar]
- Berriman S. J., Wade G. N., Blaustein J. D. (1992). Expression of Fos-like proteins in gonadotropin-releasing hormone neurons of Syrian hamsters: effects of estrous cycles and metabolic fuels. Endocrinology 131, 2222–2228 10.1210/en.131.5.2222 [DOI] [PubMed] [Google Scholar]
- Blundell J. E. (1977). Is there a role for serotonin (5-hydroxytryptamine) in feeding? Int. J. Obes. 1, 15–42 [PubMed] [Google Scholar]
- Borker A. S., Mascarenhas J. F. (1991). Role of acetylcholine and dopamine in dorsal hippocampus on hoarding behavior in rats. Indian J. Physiol. Pharmacol. 35, 71–73 [PubMed] [Google Scholar]
- Bronson F. H. (1986). Food-restricted, prepubertal, female rats: rapid recovery of luteinizing hormone pulsing with excess food, and full recovery of pubertal development with gonadotropin-releasing hormone. Endocrinology 118, 2483–2487 10.1210/endo-118-6-2483 [DOI] [PubMed] [Google Scholar]
- Bronson F. H. (1987). Puberty in female rats: relative effect of exercise and food restriction. Am. J. Physiol. 252, R140–R144 [DOI] [PubMed] [Google Scholar]
- Bronson F. H. (1988). Effect of food manipulation on the GnRH-LH-estradiol axis of young female rats. Am. J. Physiol. 254, R616–R621 [DOI] [PubMed] [Google Scholar]
- Bronson F. H. (1989). Mammalian Reproductive Biology. Chicago: The University of Chicago Press [Google Scholar]
- Bronson F. H. (1998). Energy balance and ovulation: small cages versus natural habitats. Reprod. Fertil. Dev. 10, 127–137 10.1071/R97075 [DOI] [PubMed] [Google Scholar]
- Bronson F. H. (2000). “Puberty and energy reserves: a walk on the wild side,” in Reproduction in Context: Social and Environmental Influences on Reproduction, eds Wallen K., Schneider J. E. (Cambridge, MA: MIT Press; ), 15–33 [Google Scholar]
- Bronson F. H., Manning J. M. (1991). The energetic regulation of ovulation: a realistic role for body fat. Biol. Reprod. 44, 945–950 10.1095/biolreprod44.6.945 [DOI] [PubMed] [Google Scholar]
- Bronson F. H., Marsteller F. A. (1985). Effect of short-term food deprivation on reproduction in female mice. Biol. Reprod. 33, 660–667 10.1095/biolreprod33.3.660 [DOI] [PubMed] [Google Scholar]
- Bronstein D. M., Schafer M. K., Watson S. J., Akil H. (1992). Evidence that beta-endorphin is synthesized in cells in the nucleus tractus solitarius: detection of POMC mRNA. Brain Res. 587, 269–275 10.1016/0006-8993(92)91007-2 [DOI] [PubMed] [Google Scholar]
- Browne S. A. H., Borer K. T. (1978). The basis for the exercise-induced hyperphagia in adult hamster. Physiol. Behav. 20, 553–557 10.1016/0031-9384(78)90246-9 [DOI] [PubMed] [Google Scholar]
- Bucholtz D. C., Vidwans N. M., Herbosa C. G., Schillo K. K., Foster D. L. (1996). Metabolic interfaces between growth and reproduction. V. Pulsatile luteinizing hormone secretion is dependent on glucose availability. Endocrinology 137, 601–607 10.1210/en.137.2.601 [DOI] [PubMed] [Google Scholar]
- Bugarith K., Dinh T. T., Li A. J., Speth R. C., Ritter S. (2005). Basomedial hypothalamic injections of neuropeptide Y conjugated to saporin selectively disrupt hypothalamic controls of food intake. Endocrinology 146, 1179–1191 10.1210/en.2004-1166 [DOI] [PubMed] [Google Scholar]
- Cabanac M., Richard D. (1995). Acute intraventricular CRF lowers the hoarding threshold in male rats. Physiol. Behav. 57, 705–710 10.1016/0031-9384(94)00322-X [DOI] [PubMed] [Google Scholar]
- Cameron J. L. (1996). Regulation of reproductive hormone secretion in primates by short-term changes in nutrition. Rev. Reprod. 1, 117–126 10.1530/ror.0.0010117 [DOI] [PubMed] [Google Scholar]
- Cameron J. L., Hansen P. D., Mcneill T. H., Koerker D. J., Clifton D. K., Rogers K. V., Bremner W. J., Steiner R. A. (1985). “Metabolic cues for the onset of puberty in primate species,” in Adolescence in Females, eds Flamigni C., Venturali S., Givens J. R. (Chicago: Yearbook Medical Publishers; ), 59–78 [Google Scholar]
- Cameron J. L., Nosbisch C. (1991). Suppression of pulsatile luteinizing hormone and testosterone secretion during short term food restriction in the adult male rhesus monkey (Macaca mulatta). Endocrinology 128, 1532–1540 10.1210/endo-128-3-1532 [DOI] [PubMed] [Google Scholar]
- Campfield L. A., Smith F. J., Guisez Y., Devos R., Burn P. (1995). Recombinant mouse ob protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269, 546–549 10.1126/science.7624778 [DOI] [PubMed] [Google Scholar]
- Castellano J. M., Bentsen A. H., Mikkelsen J. D., Tena-Sempere M. (2010). Kisspeptins: bridging energy homeostasis and reproduction. Brain Res. 1364, 129–138 10.1016/j.brainres.2010.08.057 [DOI] [PubMed] [Google Scholar]
- Castellano J. M., Navarro V. M., Fernandez-Fernandez R., Nogueiras R., Tovar S., Roa J., Vazquez M. J., Vigo E., Casanueva F. F., Aguilar E., Pinilla L., Dieguez C., Tena-Sempere M. (2005). Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology 146, 3917–3925 10.1210/en.2005-0337 [DOI] [PubMed] [Google Scholar]
- Cha S. H., Hu Z., Chohnan S., Lane M. D. (2005). Inhibition of hypothalamic fatty acid synthase triggers rapid activation of fatty acid oxidation in skeletal muscle. Proc. Natl. Acad. Sci. U. S. A. 102, 14557–14562 10.1073/pnas.0500346102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty I., Sreedhar R., Gosh K. K., Bulusu S. (1982). Circulating gonadotropin profiles in severe cases of protein-calorie malnutrition. Fertil. Steril. 37, 650–654 [DOI] [PubMed] [Google Scholar]
- Chen L. C., Ahmed S., Gesche M., Mosely W. H. (1974). A prospective study of birth interval dynamics in rural Bangladesh. Popul. Stud. (Camb.) 28, 277–297 10.2307/2173959 [DOI] [PubMed] [Google Scholar]
- Cheng C. Y., Chu J. Y., Chow B. K. (2011). Central and peripheral administration of secretin inhibits food intake in mice through the activation of the melanocortin system. Neuropsychopharmacology 36, 459–471 10.1038/npp.2010.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaccio L. A., Lisk R. D. (1971). Hormonal control of cyclic estrus in the female hamster. Am. J. Physiol. 221, 936–942 [DOI] [PubMed] [Google Scholar]
- Ciaccio L. A., Lisk R. D., Reuter L. A. (1979). Prelordotic behavior in the hamster: a hormonally modulated transition from aggression to sexual receptivity. J. Comp. Physiol. Psychol. 93, 771–780 10.1037/h0077604 [DOI] [PubMed] [Google Scholar]
- Claret M., Smith M. A., Batterham R. L., Selman C., Choudhury A. I., Fryer L. G., Clements M., Al-Qassab H., Heffron H., Xu A. W., Speakman J. R., Barsh G. S., Viollet B., Vaulont S., Ashford M. L., Carling D., Withers D. J. (2007). AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J. Clin. Invest. 117, 2325–2336 10.1172/JCI31516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. T., Kalra P. S., Kalra S. P. (1985). Neuropeptide Y stimulates feeding but inhibits sexual behavior in rats. Endocrinology 117, 2435–2442 10.1210/endo-117-6-2435 [DOI] [PubMed] [Google Scholar]
- Clarke I. J., Cummins J. T. (1982). The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology 111, 1737–1739 10.1210/endo-111-5-1737 [DOI] [PubMed] [Google Scholar]
- Coen C. W., Franklin M., Laynes R. W., Mackinnon P. C. (1980). Effects of manipulating serotonin on the incidence of ovulation in the rat. J. Endocrinol. 87, 195–201 10.1677/joe.0.0870195 [DOI] [PubMed] [Google Scholar]
- Coen C. W., MacKinnon P. C. (1979). Serotonin involvement in the control of phasic luteinizing hormone release in the rat: evidence for a critical period. J. Endocrinol. 82, 105–113 10.1677/joe.0.0820105 [DOI] [PubMed] [Google Scholar]
- Collier G., Hirsch E., Hamlin P. H. (1972). The ecological determinants of reinforcement in the rat. Physiol. Behav. 9, 705–716 10.1016/0031-9384(72)90038-8 [DOI] [PubMed] [Google Scholar]
- Cota D., Matter E. K., Woods S. C., Seeley R. J. (2008). The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J. Neurosci. 28, 7202–7208 10.1523/JNEUROSCI.1389-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D., Proulx K., Smith K. A., Kozma S. C., Thomas G., Woods S. C., Seeley R. J. (2006). Hypothalamic mTOR signaling regulates food intake. Science 312, 927–930 10.1126/science.1124147 [DOI] [PubMed] [Google Scholar]
- Cragnolini A., Scimonelli T., Celis M. E., Schioth H. B. (2000). The role of melanocortin receptors in sexual behavior in female rats. Neuropeptides 34, 211–215 10.1054/npep.2000.0815 [DOI] [PubMed] [Google Scholar]
- Craig W. (1917). Appetites and aversions as constituents of instinct. Proc. Natl. Acad. Sci. U.S.A. 3, 685–688 10.1073/pnas.3.12.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps A. W., Williams V. J. (1975). The effect of pregnancy and lactation on food intake, gastrointestinal anatomy and the absorptive capacity of the small intestine in the albino rat. Br. J. Nutr. 33, 17–32 10.1079/BJN19750005 [DOI] [PubMed] [Google Scholar]
- Crowley W. R., Tessel R. E., O’Donohue T. L., Adler B. A., Kalra S. P. (1985). Effects of ovarian hormones on the concentrations of immunoreactive neuropeptide Y in discrete brain regions of the female rat: correlation with serum luteinizing hormone (LH) and median eminence LH-releasing hormone. Endocrinology 117, 1151–1155 10.1210/endo-117-3-1151 [DOI] [PubMed] [Google Scholar]
- Crown A., Clifton D. K., Steiner R. A. (2007). Neuropeptide signaling in the integration of metabolism and reproduction. Neuroendocrinology 86, 175–182 10.1159/000109095 [DOI] [PubMed] [Google Scholar]
- Dailey M. J., Bartness T. J. (2009). Appetitive and consummatory ingestive behaviors stimulated by PVH and perifornical area NPY injections. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R877–R892 10.1152/ajpregu.90568.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. E., Bartness T. J. (2004). Agouti-related protein increases food hoarding more than food intake in Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R38–R45 10.1152/ajpregu.00284.2003 [DOI] [PubMed] [Google Scholar]
- Day M. L., Imakawa K., Zalesky D. D., Kittok R. J., Kinder J. E. (1986). Effects of restriction of dietary energy intake during the prepubertal period on secretion of luteinizing hormone and responsiveness of the pituitary to luteinizing hormone-releasing hormone in heifers. J. Anim. Sci. 62, 1641–1647 [DOI] [PubMed] [Google Scholar]
- de Ridder C. M., Bruning P. F., Zonderland M. L., Thijssen J. H., Bonfrer J. M., Blankenstein M. A., Huisveld I. A., Erich W. B. (1990). Body fat mass, body fat distribution, and plasma hormones in early puberty in females. J. Clin. Endocrinol. Metab. 70, 888–893 10.1210/jcem-70-4-888 [DOI] [PubMed] [Google Scholar]
- Diano S., Horvath T. L. (2012). Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism. Trends Mol. Med. 18, 52–58 10.1016/j.molmed.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Dickerman R. W., Schneider J. E., Wade G. N. (1990). Decreased availability of metabolic fuels attenuates the preovulatory LH surge in Syrian hamsters. Soc. Neurosci. Abstr. 16, 399 [Google Scholar]
- Diez-Sampedro A., Hirayama B. A., Osswald C., Gorboulev V., Baumgarten K., Volk C., Wright E. M., Koepsell H. (2003). A glucose sensor hiding in a family of transporters. Proc. Natl. Acad. Sci. U. S. A. 100, 11753–11758 10.1073/pnas.1733027100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd D. K., Stalling R. B., Bedell J. (1977). Grocery purchases as a function of obesity and assumed food deprivation. Int. J. Obes. 1, 43–47 [PubMed] [Google Scholar]
- Donato J., Jr., Cavalcante J. C., Silva R. J., Teixeira A. S., Bittencourt J. C., Elias C. F. (2010). Male and female odors induce Fos expression in chemically defined neuronal population. Physiol. Behav. 99, 67–77 10.1016/j.physbeh.2009.10.012 [DOI] [PubMed] [Google Scholar]
- Donato J., Jr., Silva R. J., Sita L. V., Lee S., Lee C., Lacchini S., Bittencourt J. C., Franci C. R., Canteras N. S., Elias C. F. (2009). The ventral premammillary nucleus links fasting-induced changes in leptin levels and coordinated luteinizing hormone secretion. J. Neurosci. 29, 5240–5250 10.1523/JNEUROSCI.0405-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante K. M., Li N. P. (2009). Oestradiol level and opportunistic mating in women. Biol. Lett. 5, 179–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison P. T. (2001). On Fertile Ground: A Natural History of Human Reproduction. Cambridge, MA: Harvard University Press [Google Scholar]
- Emlen J. (1966). The role of time and energy in food preference. Am. Nat. 100, 611–617 10.1086/282455 [DOI] [Google Scholar]
- Estrada K. M., Clay C. M., Pompolo S., Smith J. T., Clarke I. J. (2006). Elevated KiSS-1 expression in the arcuate nucleus prior to the cyclic preovulatory gonadotropin-releasing hormone/lutenising hormone surge in the ewe suggests a stimulatory role for kisspeptin in oestrogen-positive feedback. J. Neuroendocrinol. 18, 806–809 10.1111/j.1365-2826.2006.01485.x [DOI] [PubMed] [Google Scholar]
- Everitt B. J. (1990). Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci. Biobehav. Rev. 14, 217–232 10.1016/S0149-7634(05)80222-2 [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez R., Martini A. C., Navarro V. M., Castellano J. M., Dieguez C., Aguilar E., Pinilla L., Tena-Sempere M. (2006). Novel signals for the integration of energy balance and reproduction. Mol. Cell. Endocrinol. 254–255, 127–132. 10.1016/j.mce.2006.04.026 [DOI] [PubMed] [Google Scholar]
- Fessler D. M. T. (2003). No time to eat: an adaptationist account of periovulatory behavioral changes. Q. Rev. Biol. 78, 3–21 10.1086/367579 [DOI] [PubMed] [Google Scholar]
- Fleming A. S. (1976a). Control of food intake in the lactating rat: role of suckling and hormones. Physiol. Behav. 17, 841–848 10.1016/0031-9384(76)90051-2 [DOI] [PubMed] [Google Scholar]
- Fleming A. S. (1976b). Ovarian influences on food intake and body weight regulation in lactating rats. Physiol. Behav. 17, 969–978 10.1016/0031-9384(76)90051-2 [DOI] [PubMed] [Google Scholar]
- Fleming A. S. (1978). Food intake and body weight regulation during the reproductive cycle of the golden hamster (Mesocricetus auratus). Behav. Biol. 24, 291–306 10.1016/S0091-6773(79)90145-7 [DOI] [PubMed] [Google Scholar]
- Flier J. S. (1998). Clinical review 94: What’s in a name? In search of leptin’s physiologic role. J. Clin. Endocrinol. Metab. 83, 1407–1413 10.1210/jc.83.5.1407 [DOI] [PubMed] [Google Scholar]
- Foster D. L., Bucholtz D. C., Herbosa C. G. (1995). “Metabolic signals and the timing of puberty in sheep,” in The Neurobiology of Puberty, eds Plant T. M., Lee P. A. (Bristol: Journal of Endocrinology Ltd.), 243–257 [Google Scholar]
- Foster D. L., Olster D. H. (1985). Effect of restricted nutrition on puberty in the lamb: patterns of tonic luteinizing hormone (LH) secretion and competency of the LH surge system. Endocrinology 116, 375–381 10.1210/endo-116-1-375 [DOI] [PubMed] [Google Scholar]
- Franceschini I., Lomet D., Cateau M., Delsol G., Tillet Y., Caraty A. (2006). Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci. Lett. 10.1016/j.neulet.2006.03.039 [DOI] [PubMed] [Google Scholar]
- Friedman M. I. (1989). Metabolic control of food intake. Bol. Asoc. Med. P. R. 81, 111–113 [PubMed] [Google Scholar]
- Friedman M. I. (1998). Fuel partitioning and food intake. Am. J. Clin. Nutr. 67, 513S–518S [DOI] [PubMed] [Google Scholar]
- Friedman M. I. (2008). “Food intake: control, regulation and the illusion of dysregulation,” in Appetite and Food Intake: Behavioral and Physiological Considerations, eds Harris R., Mattes R. (Boca Raton, FL: CRC Press; ), 1–19 [Google Scholar]
- Friedman M. I., Graczyk-Millbrandt G., Ji H., Rawson N. E., Osbakken M. D. (2003). 2,5-Anhydro-D-mannitol increases hepatocyte sodium: transduction of a hepatic hunger stimulus? Biochim. Biophys. Acta 1642, 53–58 10.1016/S0167-4889(03)00098-3 [DOI] [PubMed] [Google Scholar]
- Frisch R. E. (1990). “Body fat, menarche, fitness and fertility,” in Adipose Tissue and Reproduction, ed. Frisch R. E. (Basel: Karger; ), 1–26 [Google Scholar]
- Frisch R. E., McArthur J. (1974). Menstrual cycles: fatness as a determinant of minimum weight for height necessary for their maintenance or onset. Science 185, 949–951 10.1126/science.185.4155.949 [DOI] [PubMed] [Google Scholar]
- Fujihara N., Shiino M. (1983). Stimulatory effect of thyrotrophin-releasing hormone (TRH) on the release of luteinizing hormone (LH) from rat anterior pituitary cells in vitro. Can. J. Physiol. Pharmacol. 61, 186–189 10.1139/y83-029 [DOI] [PubMed] [Google Scholar]
- Furuta M., Funabashi T., Kimura F. (2001). Intracerebroventricular administration of ghrelin rapidly suppresses pulsatile luteinizing hormone secretion in ovariectomized rats. Biochem. Biophys. Res. Commun. 288, 780–785 10.1006/bbrc.2001.5854 [DOI] [PubMed] [Google Scholar]
- Gangestad S. W., Thornhill R. (2008). Human oestrus. Proc. Biol. Sci. 275, 991–1000 10.1098/rspb.2007.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangestad S. W., Thornhill R., Garver C. E. (2002). Changes in women’s sexual interests and their partners’ mate-retention tactics across the menstrual cycle: evidence for shifting conflicts of interest. Proc. Biol. Sci. 269, 975–982 10.1098/rspb.2001.1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite T. L. (1985). Peripheral motilin administration stimulates feeding in fasted rats. Peptides 6, 41–44 10.1016/0196-9781(85)90133-0 [DOI] [PubMed] [Google Scholar]
- Gattermann R., Johnston R. E., Yigit N., Fritzsche P., Larimer S., Ozkurt S., Neumann K., Song Z., Colak E., Johnston J., Mcphee M. E. (2008). Golden hamsters are nocturnal in captivity but diurnal in nature. Biol. Lett. 4, 253–255 10.1098/rsbl.2008.0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J., Smith G. P. (1988). The actions of bombes in-like peptides on food intake. Ann. N. Y. Acad. Sci. 547, 210–216 10.1111/j.1749-6632.1988.tb23889.x [DOI] [PubMed] [Google Scholar]
- Glick Z., Mayer J. (1968). Hyperphagia caused by cerebral ventricular infusion of phloridzin. Nature 219, 1374. 10.1038/2191374a0 [DOI] [PubMed] [Google Scholar]
- Gonzalez M. I., Celis M. E., Hole D. R., Wilson C. A. (1993). Interaction of oestradiol, alpha-melanotrophin and noradrenaline within the ventromedial nucleus in the control of female sexual behaviour. Neuroendocrinology 58, 218–226 10.1159/000126536 [DOI] [PubMed] [Google Scholar]
- Gopalon C., Naidu A. N. (1972). Nutrition and fertility. Lancet ii, 1077–1079 10.1016/S0140-6736(72)92355-0 [DOI] [PubMed] [Google Scholar]
- Gottsch M. L., Cunningham M. J., Smith J. T., Popa S. M., Acohido B. V., Crowley W. F., Seminara S., Clifton D. K., Steiner R. A. (2004). A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145, 4073–4077 10.1210/en.2004-0431 [DOI] [PubMed] [Google Scholar]
- Gribble F. M., Williams L., Simpson A. K., Reimann F. (2003). A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 52, 1147–1154 10.2337/diabetes.52.5.1147 [DOI] [PubMed] [Google Scholar]
- Grill H. J. (2006). Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity (Silver Spring) 14(Suppl. 5), 216S–221S 10.1038/oby.2006.312 [DOI] [PubMed] [Google Scholar]
- Grill H. J., Ginsberg A. B., Seeley R. J., Kaplan J. M. (1998). Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J. Neurosci. 18, 10128–10135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill H. J., Hayes M. R. (2009). The nucleus tractus solitarius: a portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. Int. J. Obes. (Lond.) 33(Suppl. 1), S11–S15 10.1038/ijo.2009.10 [DOI] [PubMed] [Google Scholar]
- Grill H. J., Kaplan J. M. (1990). “Caudal brainstem participates in the distributed neural control of feeding,” in Handbook of Behavioral Neurobiology: Neurobiology of Food and Fluid Intake, ed. Stricker E. M. (New York: Plenum Press; ), 125–150 [Google Scholar]
- Grill H. J., Kaplan J. M. (2001). Interoceptive and integrative contributions of forebrain and brainstem to energy balance control. Int. J. Obes. Relat. Metab. Disord. 25(Suppl. 5), S73–S77 10.1038/sj.ijo.0801917 [DOI] [PubMed] [Google Scholar]
- Grill H. J., Kaplan J. M. (2002). The neuroanatomical axis for control of energy balance. Front. Neuroendocrinol. 23, 2–40 10.1006/frne.2001.0224 [DOI] [PubMed] [Google Scholar]
- Grill H. J., Schwartz M. W., Kaplan J. M., Foxhall J. S., Breininger J., Baskin D. G. (2002). Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143, 239–246 10.1210/en.143.1.239 [DOI] [PubMed] [Google Scholar]
- Gundlach A. L. (2002). Galanin/GALP and galanin receptors: role in central control of feeding, body weight/obesity and reproduction? Eur. J. Pharmacol. 440, 255–268 10.1016/S0014-2999(02)01433-4 [DOI] [PubMed] [Google Scholar]
- Guy J., Li S., Pelletier G. (1988). Studies on the physiological role and mechanism of action of neuropeptide Y in the regulation of luteinizing hormone secretion in the rat. Regul. Pept. 23, 209–216 10.1016/0167-0115(88)90028-6 [DOI] [PubMed] [Google Scholar]
- Halaas J. L., Gajiwala K. S., Maffei M., Cohen S. L., Chait B. T., Rabinowitz D., Lallone R. L., Burley S. K., Friedman J. M. (1995). Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546 10.1126/science.7624777 [DOI] [PubMed] [Google Scholar]
- Harris R. B., Bartness T. J., Grill H. J. (2007). Leptin responsiveness in chronically decerebrate rats. Endocrinology 148, 4623–4633 10.1210/en.2006-1565 [DOI] [PubMed] [Google Scholar]
- Hayes M. R., Skibicka K. P., Bence K. K., Grill H. J. (2009). Dorsal hindbrain 5’-adenosine monophosphate-activated protein kinase as an intracellular mediator of energy balance. Endocrinology 150, 2175–2182 10.1210/en.2008-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner T. G., Zigmond M. J., Stricker E. M. (1977). Effects of dopaminergic agonists and antagonists of feeding in intact and 6-hydroxydopamine-treated rats. J. Pharmacol. Exp. Ther. 201, 386–399 [PubMed] [Google Scholar]
- Heinrichs S. C., Richard D. (1999). The role of corticotropin-releasing factor and urocortin in the modulation of ingestive behavior. Neuropeptides 33, 350–359 10.1054/npep.1999.0047 [DOI] [PubMed] [Google Scholar]
- Heisler L. E., Tumber A. J., Reid R. L., Van Vugt D. A. (1994). Vasopressin mediates hypoglycemia-induced inhibition of luteinizing hormone secretion in the ovariectomized rhesus monkey. Neuroendocrinology 60, 297–304 10.1159/000126762 [DOI] [PubMed] [Google Scholar]
- Herde M. K., Geist K., Campbell R. E., Herbison A. E. (2011). Gonadotropin-releasing hormone neurons extend complex highly branched dendritic trees outside the blood-brain barrier. Endocrinology 152, 3832–3841 10.1210/en.2011-1228 [DOI] [PubMed] [Google Scholar]
- Hetherington M. M., Stoner S. A., Andersen A. E., Rolls B. J. (2000). Effects of acute food deprivation on eating behavior in eating disorders. Int. J. Eat. Disord. 28, 272–283 [DOI] [PubMed] [Google Scholar]
- Holmberg B. J., Morrison C. D., Keisler D. H. (2001). Endocrine responses of ovariectomized ewes to i.c.v. infusion of urocortin. J. Endocrinol. 171, 517–524 10.1677/joe.0.1710517 [DOI] [PubMed] [Google Scholar]
- Horn C. C., Addis A., Friedman M. I. (1999). Neural substrate for an integrated metabolic control of feeding behavior. Am. J. Physiol. 276, R113–R119 [DOI] [PubMed] [Google Scholar]
- Horn C. C., Tordoff M. G., Friedman M. I. (2001). Role of vagal afferent innervation in feeding and brain Fos expression produced by metabolic inhibitors. Brain Res. 919, 198–206 10.1016/S0006-8993(01)02963-8 [DOI] [PubMed] [Google Scholar]
- Houston A. I., McNamara J. M. (1989). The value of food: effects of open and closed economies. Anim. Behav. 37, 546–562 10.1016/0003-3472(89)90034-1 [DOI] [Google Scholar]
- Hudson B., Ritter S. (2004). Hindbrain catecholamine neurons mediate consummatory responses to glucoprivation. Physiol. Behav. 82, 241–250 10.1016/j.physbeh.2004.03.032 [DOI] [PubMed] [Google Scholar]
- I’Anson H., Starer C. A., Bonnema K. R. (2003a). Glucoprivic regulation of estrous cycles in the rat. Horm. Behav. 43, 388–393 10.1016/S0018-506X(03)00007-2 [DOI] [PubMed] [Google Scholar]
- I’Anson H., Sundling L. A., Roland S. M., Ritter S. (2003b). Immunotoxic destruction of distinct catecholaminergic neuron populations disrupts the reproductive response to glucoprivation in female rats. Endocrinology 144, 4325–4331 10.1210/en.2003-0258 [DOI] [PubMed] [Google Scholar]
- Irwig M. S., Fraley G. S., Smith J. T., Acohido B. V., Popa S. M., Cunningham M. J., Gottsch M. L., Clifton D. K., Steiner R. A. (2004). Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80, 264–272 10.1159/000083140 [DOI] [PubMed] [Google Scholar]
- Ji H., Friedman M. I. (1999). Compensatory hyperphagia after fasting tracks recovery of liver energy status. Physiol. Behav. 68, 181–186 10.1016/S0031-9384(99)00173-0 [DOI] [PubMed] [Google Scholar]
- Ji H., Graczyk-Milbrandt G., Friedman M. I. (2000). Metabolic inhibitors synergistically decrease hepatic energy status and increase food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R1579–R1582 [DOI] [PubMed] [Google Scholar]
- Johnson M. A., Tsutsui K., Fraley G. S. (2007). Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm. Behav. 51, 171–180 10.1016/j.yhbeh.2006.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. E. (1974). Sexual attraction function of golden hamster vaginal secretion. Behav. Biol. 12, 111–117 10.1016/S0091-6773(74)91101-8 [DOI] [PubMed] [Google Scholar]
- Johnston R. E. (1975). Scent marking by male golden hamsters (Mesocricetus auratus) III. Behavior in a seminatural environment. Z. Tierpsychol. 37, 213–221 [PubMed] [Google Scholar]
- Johnston R. E. (1977). The causation of two scent-marking behavior patterns in female hamsters (Mesocricentus auratus). Anim. Behav. 25, 317–327 10.1016/0003-3472(77)90007-0 [DOI] [PubMed] [Google Scholar]
- Jones J. E., Lubbers L. S. (2001). Suppression and recovery of estrous behavior in Syrian hamsters after changes in metabolic fuel availability. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R1393–R1398 [DOI] [PubMed] [Google Scholar]
- Jones J. E., Pick R. R., Davenport M. D., Keene A. C., Corp E. S., Wade G. N. (2002). Disinhibition of female sexual behavior by a CRH receptor antagonist in Syrian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R591–R597 [DOI] [PubMed] [Google Scholar]
- Kalra S. P., Clark J. T., Sahu A., Dube M. G., Kalra P. S. (1988). Control of feeding and sexual behaviors by neuropeptide Y: physiological implications. Synapse 2, 254–257 10.1002/syn.890020313 [DOI] [PubMed] [Google Scholar]
- Kauffman A. S., Rissman E. F. (2004a). A critical role for the evolutionarily conserved gonadotropin-releasing hormone II: mediation of energy status and female sexual behavior. Endocrinology 145, 3639–3646 10.1210/en.2004-0148 [DOI] [PubMed] [Google Scholar]
- Kauffman A. S., Rissman E. F. (2004b). The evolutionarily conserved gonadotropin-releasing hormone II modifies food intake. Endocrinology 145, 686–691 10.1210/en.2003-1150 [DOI] [PubMed] [Google Scholar]
- Kauffman A. S., Wills A., Millar R. P., Rissman E. F. (2005). Evidence that the type-2 gonadotropin-releasing hormone (GnRH) receptor mediates the behavioural effects of GnRH-II on feeding and reproduction in musk shrews. J. Neuroendocrinol. 17, 489–497 10.1111/j.1365-2826.2005.01334.x [DOI] [PubMed] [Google Scholar]
- Keenan K. P., Hoe C. M., Mixson L., Mccoy C. L., Coleman J. B., Mattson B. A., Ballam G. A., Gumprecht L. A., Soper K. A. (2005). Diabesity: a polygenic model of dietary-induced obesity from ad libitum overfeeding of Sprague-Dawley rats and its modulation by moderate and marked dietary restriction. Toxicol. Pathol. 33, 650–674 10.1080/01926230500311222 [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E., Bartness T. J. (2007). MTII attenuates ghrelin- and food deprivation-induced increases in food hoarding and food intake. Horm. Behav. 52, 612–620 10.1016/j.yhbeh.2007.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley A. E., Stinus L. (1985). Disappearance of hoarding behavior after 6-hydroxydopamine lesions of the mesolimbic dopamine neurons and its reinstatement with L-dopa. Behav. Neurosci. 99, 531–545 10.1037/0735-7044.99.3.531 [DOI] [PubMed] [Google Scholar]
- Kennedy G. C. (1953). The role of depot fat in the hypothalamic control of food intake in the rat. Proc. R. Soc. Lond. B Biol. Sci. 140, 578–592 10.1098/rspb.1953.0009 [DOI] [PubMed] [Google Scholar]
- Kennedy G. C., Mitra J. (1963). Body weight and food intake as initiating factors for puberty in the rat. J. Physiol. 166, 408–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile J. P., Alexander B. M., Moss G. E., Hallford D. M., Nett T. M. (1991). Gonadotropin-releasing hormone overrides the negative effect of reduced dietary energy on gonadotropin synthesis and secretion in ewes. Endocrinology 128, 843–849 10.1210/endo-128-2-843 [DOI] [PubMed] [Google Scholar]
- Kimura F., Hashimoto R., Kawakami M. (1983). The stimulatory effect of cholecystokinin implanted in the medial preoptic area on luteinizing hormone secretion in the ovariectomized estrogen-primed rat. Endocrinol. Jpn. 30, 305–309 10.1507/endocrj1954.30.297 [DOI] [PubMed] [Google Scholar]
- Klingerman C. M., Krishnamoorthy K., Patel K., Spiro A. B., Struby C., Patel A., Schneider J. E. (2010). Energetic challenges unmask the role of ovarian hormones in orchestrating ingestive and sex behaviors. Horm. Behav. 58, 563–574 10.1016/j.yhbeh.2010.06.019 [DOI] [PubMed] [Google Scholar]
- Klingerman C. M., Patel A., Hedges V. L., Meisel R. L., Schneider J. E. (2011a). Food restriction dissociates sexual motivation, sexual performance, and the rewarding consequences of copulation in female Syrian hamsters. Behav. Brain Res. 223, 356–370 10.1016/j.bbr.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingerman C. M., Willimas W. P., Prasad A., Brahme N., Simberlund J., Schneider J. E., Kriegsfeld L. J. (2011b). Food restriction-induced changes in gonadotropin-inhibiting hormone cells are associated with changes in sexual motivation and food hoarding, but not sexual performance and food intake. Front. Endocrinol. 2:101. 10.3389/fendo.2011.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh T., Nakai Y., Kinoshita F., Tsukada T., Tsujii S., Imura H., Maeda K. (1984). Serotonin involvement in the inhibition of luteinizing hormone (LH) release during immobilization in castrated male rats. Life Sci. 34, 1635–1641 10.1016/0024-3205(84)90634-9 [DOI] [PubMed] [Google Scholar]
- Kraly F. S., Blass E. M. (1976). Mechanisms for enhanced feeding in the cold in rats. J. Comp. Physiol. Psychol. 90, 714–726 10.1037/h0077225 [DOI] [PubMed] [Google Scholar]
- Krasnow S. M., Fraley G. S., Schuh S. M., Baumgartner J. W., Clifton D. K., Steiner R. A. (2003). A role for galanin-like peptide in the integration of feeding, body weight regulation, and reproduction in the mouse. Endocrinology 144, 813–822 10.1210/en.2002-220982 [DOI] [PubMed] [Google Scholar]
- Kriegsfeld L. J., Gibson E. M., Williams W. P., III, Zhao S., Mason A. O., Bentley G. E., Tsutsui K. (2010). The roles of RFamide-related peptide-3 in mammalian reproductive function and behaviour. J. Neuroendocrinol. 22, 692–700 10.1111/j.1365-2826.2010.02031.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld L. J., Mei D. F., Bentley G. E., Ubuka T., Mason A. O., Inoue K., Ukena K., Tsutsui K., Silver R. (2006). Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc. Natl. Acad. Sci. U.S.A. 103, 2410–2415 10.1073/pnas.0511003103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen P., Judge M. E., Thim L., Ribel U., Christjansen K. N., Wulff B. S., Clausen J. T., Jensen P. B., Madsen O. D. (1998). Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 72–76 10.1038/29993 [DOI] [PubMed] [Google Scholar]
- Kyrkouli S. E., Stanley B. G., Leibowitz S. F. (1986). Galanin: stimulation of feeding induced by medial hypothalamic injection of this novel peptide. Eur. J. Pharmacol. 122, 159–160 10.1016/0014-2999(86)90175-5 [DOI] [PubMed] [Google Scholar]
- Lam T. K., Pocai A., Gutierrez-Juarez R., Obici S., Bryan J., Aguilar-Bryan L., Schwartz G. J., Rosetti L. (2005). Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat. Med. 11, 320–327 10.1038/nm1201 [DOI] [PubMed] [Google Scholar]
- la Fleur S. E., Ji H., Manalo S. L., Friedman M. I., Dallman M. F. (2003). The hepatic vagus mediates fat-induced inhibition of diabetic hyperphagia. Diabetes 52, 2321–2330 10.2337/diabetes.52.9.2321 [DOI] [PubMed] [Google Scholar]
- Lebrethon M. C., Vandersmissen E., Gerard A., Parent A. S., Bourguignon J. P. (2000). Cocaine and amphetamine-regulated-transcript peptide mediation of leptin stimulatory effect on the rat gonadotropin-releasing hormone pulse generator in vitro. J. Neuroendocrinol. 12, 383–385 10.1046/j.1365-2826.2000.00497.x [DOI] [PubMed] [Google Scholar]
- Levin B. E., Routh V. H., Kang L., Sanders N. M., Dunn-Meynell A. A. (2004). Neuronal glucosensing: what do we know after 50 years? Diabetes 53, 2521–2528 10.2337/diabetes.53.10.2521 [DOI] [PubMed] [Google Scholar]
- Levine A. S., Rogers B., Kneip J., Grace M., Morley J. E. (1983). Effect of centrally administered corticotropin releasing factor (CRF) on multiple feeding paradigms. Neuropharmacology 22, 337–339 10.1016/0028-3908(83)90249-6 [DOI] [PubMed] [Google Scholar]
- Levitsky D. A., DeRosimo L. (2010). One day of food restriction does not result in an increase in subsequent daily food intake in humans. Physiol. Behav. 99, 495–499 10.1016/j.physbeh.2009.12.020 [DOI] [PubMed] [Google Scholar]
- Li A. J., Wang Q., Ritter S. (2011). Participation of hindbrain AMP-activated protein kinase in glucoprivic feeding. Diabetes 60, 436–442 10.2337/db10-0331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. F., Bowe J. E., Lightman S. L., O’Byrne K. T. (2005). Role of corticotropin-releasing factor receptor-2 in stress-induced suppression of pulsatile luteinizing hormone secretion in the rat. Endocrinology 146, 318–322 10.1210/en.2004-0950 [DOI] [PubMed] [Google Scholar]
- Lisk R. D., Ciaccio L. A., Catanzaro C. (1983). Mating behavior of the golden hamster under seminatural conditions. Anim. Behav. 31, 659–666 10.1016/S0003-3472(83)80221-8 [DOI] [Google Scholar]
- Lisk R. D., Zeiss J., Ciaccio L. A. (1972). The influence of olfaction on sexual behavior in the male golden hamster (Mesocricetus auratus). J. Exp. Zool. 181, 69–78 10.1002/jez.1401810108 [DOI] [PubMed] [Google Scholar]
- Lively K. M., Piacsek B. E. (1988). Gonadotropin-releasing hormone sensitivity in underfed prepubertal female rats. Am. J. Physiol. 255, E482–E487 [DOI] [PubMed] [Google Scholar]
- Loftus T. M., Jaworsky D. E., Frehywot G. L., Townsend C. A., Ronnett G. V., Lane M. D., Kuhajda F. P. (2000). Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science 288, 2379–2381 10.1126/science.288.5475.2379 [DOI] [PubMed] [Google Scholar]
- Lorenz K. (1950). The comparative method in studying innate behavior patterns. Symp. Soc. Exp. Biol. 1950, 221–268 [Google Scholar]
- Loucks A. B. (2003a). Energy availability, not body fatness, regulates reproductive function in women. Exerc. Sport Sci. Rev. 31, 144–148 10.1097/00003677-200307000-00008 [DOI] [PubMed] [Google Scholar]
- Loucks A. B. (2003b). Introduction to menstrual disturbances in athletes. Med. Sci. Sports Exerc. 35, 1551–1552 10.1249/01.MSS.0000084531.33556.7B [DOI] [PubMed] [Google Scholar]
- Loucks A. B., Thuma J. R. (2003). Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J. Clin. Endocrinol. Metab. 88, 297–311 10.1210/jc.2002-020369 [DOI] [PubMed] [Google Scholar]
- Louis-Sylvestre J. (1987). Adaptation of food ingestion to energy expenditure. Reprod. Nutr. Dev. 27, 171–188 10.1051/rnd:19870204 [DOI] [PubMed] [Google Scholar]
- MacArthur R. H. P. E. R. (1966). On optimal use of a patchy environment. Am. Nat. 100, 603–609 10.1086/282425 [DOI] [Google Scholar]
- Manning J. M., Bronson F. H. (1990). The effects of low temperature and food intake on ovulation in domestic mice. Physiol. Zool. 63, 938–948 [Google Scholar]
- Manning J. M., Bronson F. H. (1991). Suppression of puberty in rats by exercise: effects on hormone levels and reversal with GnRH infusion. Am. J. Physiol. 260, R717–R723 [DOI] [PubMed] [Google Scholar]
- McClure T. J. (1962). Infertility in female rodents caused by temporary inanition at or about the time of implantation. J. Reprod. Fertil. 4, 241 [Google Scholar]
- McKay L. D., Kenney N. J., Edens N. K., Williams R. H., Woods S. C. (1981). Intracerebroventricular beta-endorphin increases food intake of rats. Life Sci. 29, 1429–1434 10.1016/0024-3205(81)90006-0 [DOI] [PubMed] [Google Scholar]
- McLaughlin C. L., Baile C. A., Peikin S. R. (1983). Hyperphagia during lactation: satiety response to CCK and growth of the pancreas. Am. J. Physiol. 244, E61–E65 [DOI] [PubMed] [Google Scholar]
- Medina C. L., Nagatani S., Darling T. A., Bucholtz D. C., Tsukamura H., Maeda K., Foster D. L. (1998). Glucose availability modulates the timing of the luteinizing hormone surge in the ewe. J. Neuroendocrinol. 10, 785–792 10.1046/j.1365-2826.1998.00264.x [DOI] [PubMed] [Google Scholar]
- Meisel R. L., Mullins A. J. (2006). Sexual experience in female rodents: cellular mechanisms and functional consequences. Brain Res. 1126, 56–65 10.1016/j.brainres.2006.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mela D. J., Aaron J. I., Gatenby S. J. (1996). Relationships of consumer characteristics and food deprivation to food purchasing behavior. Physiol. Behav. 60, 1331–1335 10.1016/S0031-9384(96)00241-7 [DOI] [PubMed] [Google Scholar]
- Meuwissen I., Over R. (1992). Sexual arousal across phases of the human menstrual cycle. Arch. Sex. Behav. 21, 101–119 10.1007/BF01542588 [DOI] [PubMed] [Google Scholar]
- Meyer A. H., Langhans W., Scharrer E. (1989). Vasopressin reduces food intake in goats. Q. J. Exp. Physiol. 74, 465–473 [DOI] [PubMed] [Google Scholar]
- Michopoulos V., Wilson M. E. (2011). Body weight decreases induced by estradiol in female rhesus monkeys are dependent upon social status. Physiol. Behav. 102, 382–388 10.1016/j.physbeh.2010.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millington G. W. (2007). The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutr. Metab. (Lond) 4, 18. 10.1186/1743-7075-4-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y., Kim Y. B., Peroni O. D., Fryer L. G., Muller C., Carling D., Kahn B. B. (2002). Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415, 339–343 10.1038/415339a [DOI] [PubMed] [Google Scholar]
- Minokoshi Y., Shiuchi T., Lee S., Suzuki A., Okamoto S. (2008). Role of hypothalamic AMP-kinase in food intake regulation. Nutrition 24, 786–790 10.1016/j.nut.2008.06.002 [DOI] [PubMed] [Google Scholar]
- Morin L. P. (1975). Effects of various feeding regimens and photoperiod or pinealectomy on ovulation in the hamster. Biol. Reprod. 13, 99–103 10.1095/biolreprod13.1.99 [DOI] [PubMed] [Google Scholar]
- Morin L. P. (1986). Environment and hamster reproduction: responses to phase-specific starvation during estrous cycle. Am. Physiol. Soc. 251, R663–R669 [DOI] [PubMed] [Google Scholar]
- Moriyama R., Reyes B. A., Tsukamura H., Maeda K. (2003). Glucoprivation-induced Fos expression in the hypothalamus and medulla oblongata in female rats. J. Reprod. Dev. 49, 151–157 10.1262/jrd.49.151 [DOI] [PubMed] [Google Scholar]
- Moss R. L., McCann S. M. (1975). Action of luteinizing hormone-releasing factor (lrf) in the initiation of lordosis behavior in the estrone-primed ovariectomized female rat. Neuroendocrinology 17, 309–318 10.1159/000122369 [DOI] [PubMed] [Google Scholar]
- Murahashi K., Bucholtz D. C., Nagatani S., Tsukahara S., Tsukamura H., Foster D. L., Maeda K. I. (1996). Suppression of luteinizing hormone pulses by restriction of glucose availability is mediated by sensors in the brain stem. Endocrinology 137, 1171–1176 10.1210/en.137.4.1171 [DOI] [PubMed] [Google Scholar]
- Nagatani S., Bucholtz D. C., Murahashi K., Estacio M. A., Tsukamura H., Foster D. L., Maeda K. I. (1996). Reduction of glucose availability suppresses pulsatile luteinizing hormone release in female and male rats. Endocrinology 137, 1166–1170 10.1210/en.137.8.3183 [DOI] [PubMed] [Google Scholar]
- Nagatani S., Murahashi K., Bucholtz D. C., Tsukamura H., Estacio M. A. C., Foster D., Maeda K.-I. (1995). Suppression of LH pulses by reducing glucose availability is mediated by sensors in the lower brain stem. Soc. Neurosci. Abstr. 21, 1895 [Google Scholar]
- Nance D. M., Gorski R. A. (1977). Sex and hormone dependent alterations in responsiveness to caloric dilution. Physiol. Behav. 19, 679–683 10.1016/0031-9384(77)90173-1 [DOI] [PubMed] [Google Scholar]
- Neel J. V. (1962). Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am. J. Hum. Genet. 14, 353–362 [PMC free article] [PubMed] [Google Scholar]
- Neel J. V. (1999). The “thrifty genotype” in 1998. Nutr. Rev. 57, S2–S9 10.1111/j.1753-4887.1999.tb01782.x [DOI] [PubMed] [Google Scholar]
- Nemoto T., Iwasaki-Sekino A., Yamauchi N., Shibasaki T. (2010). Role of urocortin 2 secreted by the pituitary in the stress-induced suppression of luteinizing hormone secretion in rats. Am. J. Physiol. Endocrinol. Metab. 299, E567–E575 10.1152/ajpendo.00163.2010 [DOI] [PubMed] [Google Scholar]
- Nillius S. J., Fries H., Wide L. (1975). Successful induction of follicular maturation and ovulation by prolonged treatment with LH-releasing hormone in women with anorexia nervosa. Am. J. Obstet. Gynecol. 122, 921–928 [DOI] [PubMed] [Google Scholar]
- Olson B. R., Drutarosky M. D., Stricker E. M., Verbalis J. G. (1991). Brain oxytocin receptors mediate corticotropin-releasing hormone-induced anorexia. Am. J. Physiol. 260, R448–R452 [DOI] [PubMed] [Google Scholar]
- Olster D. H., Ferin M. (1987). Corticotropin-releasing hormone inhibits gonadotropin secretion in the ovariectomized rhesus monkey. J. Clin. Endocrinol. Metab. 65, 262–267 10.1210/jcem-65-2-262 [DOI] [PubMed] [Google Scholar]
- O’Malley D., Reimann F., Simpson A. K., Gribble F. M. (2006). Sodium-coupled glucose cotransporters contribute to hypothalamic glucose sensing. Diabetes 55, 3381–3386 10.2337/db06-0531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicker A. K., Mangels R. A., Powers J. B., Wade G. N., Schneider J. E. (1998). AP lesions block suppression of estrous behavior, but not estrous cyclicity, in food-deprived Syrian hamsters. Am. J. Physiol. 275, R158–R164 [DOI] [PubMed] [Google Scholar]
- Parent A. S., Lebrethon M. C., Gerard A., Vandersmissen E., Bourguignon J. P. (2000). Leptin effects on pulsatile gonadotropin releasing hormone secretion from the adult rat hypothalamus and interaction with cocaine and amphetamine regulated transcript peptide and neuropeptide Y. Regul. Pept. 92, 17–24 10.1016/S0167-0115(00)00144-0 [DOI] [PubMed] [Google Scholar]
- Pelleymounter M. A., Cullen M. J., Baker M. B., Hecht R., Winters D., Boone T., Collins F. (1995). Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269, 540–549 10.1126/science.7624776 [DOI] [PubMed] [Google Scholar]
- Perera A. D., Verbalis J. G., Mikuma N., Majumdar S. S., Plant T. M. (1993). Cholecystokinin stimulates gonadotropin-releasing hormone release in the monkey (Macaca mulatta). Endocrinology 132, 1723–1728 10.1210/en.132.4.1723 [DOI] [PubMed] [Google Scholar]
- Perrigo G. (1987). Breeding and feeding strategies in deer mice and house mice when females are challenged to work for their food. Anim. Behav. 35, 1298–1316 10.1016/S0003-3472(87)80002-7 [DOI] [Google Scholar]
- Peterson A. D., Baumgardt B. R. (1971). Influence of level of energy demand on the ability of rats to compensate for diet dilution. J. Nutr. 101, 1069–1074 [DOI] [PubMed] [Google Scholar]
- Pocai A., Lam T. K., Obici S., Gutierrez-Juarez R., Muse E. D., Arduini A., Rossetti L. (2006). Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J. Clin. Invest. 116, 1081–1091 10.1172/JCI26640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper K. R. (1963). “Science as falsification,” in Conjectures and Refutations, ed. Popper K. R. (London: Routledge and Keagan Paul; ), 33–39 [Google Scholar]
- Presse F., Sorokovsky I., Max J. P., Nicolaidis S., Nahon J. L. (1996). Melanin-concentrating hormone is a potent anorectic peptide regulated by food-deprivation and glucopenia in the rat. Neuroscience 71, 735–745 10.1016/0306-4522(95)00481-5 [DOI] [PubMed] [Google Scholar]
- Proulx K., Cota D., Woods S. C., Seeley R. J. (2008). Fatty acid synthase inhibitors modulate energy balance via mammalian target of rapamycin complex 1 signaling in the central nervous system. Diabetes 57, 3231–3238 10.2337/db07-1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu S., Jain M. R., Kalra P. S., Kalra S. P. (1998). Orexins, a novel family of hypothalamic neuropeptides, modulate pituitary luteinizing hormone secretion in an ovarian steroid-dependent manner. Regul. Pept. 78, 133–136 10.1016/S0167-0115(98)00128-1 [DOI] [PubMed] [Google Scholar]
- Rawson N. E., Blum H., Osbakken M. D., Friedman M. I. (1994). Hepatic phosphate trapping, decreased ATP, and increased feeding after 2,5-anhydro-D-mannitol. Am. J. Physiol. 266, R112–R117 [DOI] [PubMed] [Google Scholar]
- Rawson N. E., Friedman M. I. (1994). Phosphate loading prevents the decrease in ATP and increase in food intake produced by 2,5-anhydro-D-mannitol. Am. J. Physiol. 266, R1792–R1796 [DOI] [PubMed] [Google Scholar]
- Rawson N. E., Ji H., Friedman M. I. (2003). 2,5-Anhydro-D-mannitol increases hepatocyte calcium: implications for a hepatic hunger stimulus. Biochim. Biophys. Acta 1642, 59–66 10.1016/S0167-4889(03)00099-5 [DOI] [PubMed] [Google Scholar]
- Ritter R. C., Slusser P. G., Stone S. (1981). Glucoreceptors controlling feeding and blood glucose are in the hindbrain. Science 213, 451–453 10.1126/science.6264602 [DOI] [PubMed] [Google Scholar]
- Ritter S. (1986). “Glucoprivation and the glucoprivic control of food intake,” in Feeding Behavior Neural and Humoral Controls (New York, NY: Academic Press, Inc.), 271–303 [Google Scholar]
- Ritter S., Bugarith K., Dinh T. T. (2001). Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J. Comp. Neurol. 432, 197–216 10.1002/cne.1097 [DOI] [PubMed] [Google Scholar]
- Ritter S., Calingasan N. Y. (1994). “Neural substrates for metabolic controls of feeding,” in Appetite and Body Weight Regulation: Sugar, Fat, and Macronutrient Substitutes, 1st Edn, eds Fernstrom J. D., Miller G. D. (Boca Raton: CRC Press, Inc.), 77–94 [Google Scholar]
- Ritter S., Dinh T. T., Li A. J. (2006). Hindbrain catecholamine neurons control multiple glucoregulatory responses. Physiol. Behav. 89, 490–500 10.1016/j.physbeh.2006.05.036 [DOI] [PubMed] [Google Scholar]
- Ritter S., Dinh T. T., Zhang Y. (2000). Localization of hindbrain glucoreceptive sites controlling food intake and blood glucose. Brain Res. 856, 37–47 10.1016/S0006-8993(99)02327-6 [DOI] [PubMed] [Google Scholar]
- Ritter S., Li A. J., Wang Q., Dinh T. T. (2011). Minireview: the value of looking backward: the essential role of the hindbrain in counterregulatory responses to glucose deficit. Endocrinology 152, 4019–4032 10.1210/en.2010-1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter S., Taylor J. S. (1990). Vagal sensory neurons are required for lipoprivic but not glucoprivic feeding in rats. Am. J. Physiol. 258, R1395–R1401 [DOI] [PubMed] [Google Scholar]
- Rivier C. L. (2008). Urocortin 1 inhibits rat leydig cell function. Endocrinology 149, 6425–6432 10.1210/en.2008-0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa J., Garcia-Galiano D., Varela L., Sanchez-Garrido M. A., Pineda R., Castellano J. M., Ruiz-Pino F., Romero M., Aguilar E., Lopez M., Gaytan F., Dieguez C., Pinilla L., Tena-Sempere M. (2009). The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology 150, 5016–5026 10.1210/en.2009-0096 [DOI] [PubMed] [Google Scholar]
- Roa J., Vigo E., Castellano J. M., Navarro V. M., Fernandez-Fernandez R., Casanueva F. F., Dieguez C., Aguilar E., Pinilla L., Tena-Sempere M. (2006). Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female Rat. Endocrinology 147, 2864–2878 10.1210/en.2005-1463 [DOI] [PubMed] [Google Scholar]
- Roland A. V., Moenter S. M. (2011a). Glucosensing by GnRH neurons: inhibition by androgens and involvement of AMP-activated protein kinase. Mol. Endocrinol. 25, 847–858 10.1210/me.2010-0508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland A. V., Moenter S. M. (2011b). Prenatal androgenization of female mice programs an increase in firing activity of gonadotropin-releasing hormone (GnRH) neurons that is reversed by metformin treatment in adulthood. Endocrinology 152, 618–628 10.1210/en.2010-0823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland A. V., Moenter S. M. (2011c). Regulation of gonadotropin-releasing hormone neurons by glucose. Trends Endocrinol. Metab. 22, 443–449 10.1016/j.tem.2011.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnekleiv O. K., Ojeda S. R., Mccann S. M. (1978). Undernutrition, puberty and the development of estrogen positive feedback in the female rat. Biol. Reprod. 19, 414–424 10.1095/biolreprod19.2.414 [DOI] [PubMed] [Google Scholar]
- Rosetta L., Mascie-Taylor C. G. (2009). Factors in the regulation of fertility in deprived populations. Ann. Hum. Biol. 36, 642–652 10.1080/03014460903103921 [DOI] [PubMed] [Google Scholar]
- Rossetti L., Massillon D., Barzilai N., Vuguin P., Chen W., Hawkins M., Wu J., Wang J. (1997). Short term effects of leptin on hepatic gluconeogenesis and in vivo insulin action. J. Biol. Chem. 272, 27758–27763 10.1074/jbc.272.44.27758 [DOI] [PubMed] [Google Scholar]
- Rossi M., Kim M. S., Morgan D. G., Small C. J., Edwards C. M., Sunter D., Abusnana S., Goldstone A. P., Russell S. H., Stanley S. A., Smith D. M., Yagaloff K., Ghatei M. A., Bloom S. R. (1998). A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology 139, 4428–4431 10.1210/en.139.10.4428 [DOI] [PubMed] [Google Scholar]
- Rowland N. (1982). Failure by deprived hamsters to increase food intake: some behavioral and physiological determinants. J. Comp. Physiol. Psychol. 96, 591–603 10.1037/h0077905 [DOI] [PubMed] [Google Scholar]
- Rowland N. (1984). Effects of chronic cold exposure on wheel running, food intake and fatty acid synthesis in Syrian hamsters. Physiol. Behav. 33, 253–256 10.1016/0031-9384(84)90107-0 [DOI] [PubMed] [Google Scholar]
- Sahu A., Crowley W. R., Tatemoto K., Balasubramaniam A., Kalra S. P. (1987). Effects of neuropeptide Y, NPY analog (norleucine4-NPY), galanin and neuropeptide K on LH release in ovariectomized (ovx) and ovx estrogen, progesterone-treated rats. Peptides 8, 921–926 10.1016/0196-9781(87)90081-7 [DOI] [PubMed] [Google Scholar]
- Sajapitak S., Iwata K., Shahab M., Uenoyama Y., Yamada S., Kinoshita M., Bari F. Y., I’Anson H., Tsukamura H., Maeda K. (2008). Central lipoprivation-induced suppression of luteinizing hormone pulses is mediated by paraventricular catecholaminergic inputs in female rats. Endocrinology 149, 3016–3024 10.1210/en.2008-0016 [DOI] [PubMed] [Google Scholar]
- Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R. M., Tanaka H., William S. C., Richardson J. A., Kozlowski G. P., Wilson S., Arch J. R. S., Buckingham R. E., Haynes A. C., Carr S. A., Annan R. S., Mcnulty D. E., Liu W. S., Terrett J. A., Elshourbagy N. A., Bergsma D. J., Yanagisawa M. (1998). Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585 10.1016/S0092-8674(00)80949-6 [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W., Grzanna R., Howe P. R., Bloom S. R., Polak J. M. (1985). Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J. Comp. Neurol. 241, 138–153 10.1002/cne.902410203 [DOI] [PubMed] [Google Scholar]
- Schioth H. B., Kakizaki Y., Kohsaka A., Suda T., Watanobe H. (2001). Agouti-related peptide prevents steroid-induced luteinizing hormone and prolactin surges in female rats. Neuroreport 12, 687–690 10.1097/00001756-200103260-00014 [DOI] [PubMed] [Google Scholar]
- Schneider J. E. (2000). Leptin and related peptides. Horm. Behav. 37, 258–260 10.1006/hbeh.2000.1594 [DOI] [PubMed] [Google Scholar]
- Schneider J. E. (2004). Energy balance and reproduction. Physiol. Behav. 81, 289–317 10.1016/j.physbeh.2004.02.007 [DOI] [PubMed] [Google Scholar]
- Schneider J. E. (2006). Metabolic and hormonal control of the desire for food and sex: implications for obesity and eating disorders. Horm. Behav. 50, 562–571 10.1016/j.yhbeh.2006.06.023 [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Blum R. M., Wade G. N. (2000a). Metabolic control of food intake and estrous cycles in Syrian hamsters. I. Plasma insulin and leptin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R476–R485 [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Zhou D., Blum R. M. (2000b). Leptin and metabolic control of reproduction. Horm. Behav. 37, 306–326 10.1006/hbeh.2000.1594 [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Casper J. F., Barisich A., Schoengold C., Cherry S., Surico J., Debarba A., Rabold E. (2007). Food deprivation and leptin prioritize ingestive and sex behavior without affecting estrous cycles in Syrian hamsters. Horm. Behav. 51, 413–427 10.1016/j.yhbeh.2006.09.006 [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Finnerty B. C., Swann J. M., Gabriel J. M. (1995). Glucoprivic treatments that induce anestrus, but do not affect food intake, increase FOS-like immunoreactivity in the area postrema and nucleus of the solitary tract in Syrian hamsters. Brain Res. 698, 107–113 10.1016/0006-8993(95)00860-S [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Goldman M. D., Tang S., Bean B., Ji H., Friedman M. I. (1998). Leptin indirectly affects estrous cycles by increasing metabolic fuel oxidation. Horm. Behav. 33, 217–228 10.1006/hbeh.1998.1453 [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Wade G. N. (1989). Availability of metabolic fuels controls estrous cyclicity of Syrian hamsters. Science 244, 1326–1328 10.1126/science.244.4907.904-c [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Wade G. N. (1990a). Decreased availability of metabolic fuels induces anestrus in golden hamsters. Am. J. Physiol. 258, R750–R755 [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Wade G. N. (1990b). Effects of diet and body fat content on cold-induced anestrus in Syrian hamsters. Am. J. Physiol. 259, R1198–R1204 [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Zhou D. (1999). Interactive effects of central leptin and peripheral fuel oxidation on estrous cyclicity. Am. J. Physiol. 277, R1020–R1024 [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Zhu Y. (1994). Caudal brain stem plays a role in metabolic control of estrous cycles in Syrian hamsters. Brain Res. 661 10.1016/0006-8993(94)90012-4 [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Zhu Y., Swann J. M., Gabriel J. M. (1993). Glucose detectors in the caudal brain stem control estrous cycles in Syrian hamsters. Soc. Neurosci. Abstr. 19, 19 [Google Scholar]
- Shah S. N., Nyby J. G. (2010). Ghrelin’s quick inhibition of androgen-dependent behaviors of male house mice (Mus musculus). Horm. Behav. 57, 291–296 10.1016/j.yhbeh.2009.12.010 [DOI] [PubMed] [Google Scholar]
- Shahab M., Sajapitak S., Tsukamura H., Kinoshita M., Matsuyama S., Ohkura S., Yamada S., Uenoyama Y., I’Anson H., Maeda K. (2006). Acute lipoprivation suppresses pulsatile luteinizing hormone secretion without affecting food intake in female rats. J. Reprod. Dev. 52, 763–772 10.1262/jrd.18066 [DOI] [PubMed] [Google Scholar]
- Shahab M., Zaman W., Bashir K., Arslan M. (1997). Fasting-induced suppression of hypothalamic-pituitary-gonadal axis in the adult rhesus monkey: evidence for involvement of excitatory amino acid neurotransmitters. Life Sci. 61, 1293–1300 10.1016/S0024-3205(97)00674-7 [DOI] [PubMed] [Google Scholar]
- Sherrington C. S. (1906). The Integrative Action of the Nervous System. New York: Scribner [Google Scholar]
- Shimabukuro M., Koyama K., Chen G., Wang M. Y., Trieu F., Lee Y., Newgard C. B., Unger R. H. (1997). Direct antidiabetic effect of leptin through triglyceride depletion of tissues. Proc. Natl. Acad. Sci. U.S.A. 94, 4637–4641 10.1073/pnas.94.9.4637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H., Shargill N. S., Bray G. A., Yen T. T., Gesellchen P. D. (1989). Effects of MSH on food intake, body weight and coat color of the yellow obese mouse. Life Sci. 45, 543–552 10.1016/0024-3205(89)90105-7 [DOI] [PubMed] [Google Scholar]
- Silverman H. J., Zucker I. (1976). Absence of post-fast food compensation in the golden hamster (Mesocricetus auratus). Physiol. Behav. 17, 271–285 10.1016/0031-9384(76)90076-7 [DOI] [PubMed] [Google Scholar]
- Sirinathsinghji D. J., Whittington P. E., Audsley A., Fraser H. M. (1983). beta-Endorphin regulates lordosis in female rats by modulating LH-RH release. Nature 301, 62–64 10.1038/301062a0 [DOI] [PubMed] [Google Scholar]
- Skibicka K. P., Grill H. J. (2009). Hindbrain leptin stimulation induces anorexia and hyperthermia mediated by hindbrain melanocortin receptors. Endocrinology 150, 1705–1711 10.1210/en.2009-0804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. P., Epstein A. N. (1969). Increased feeding in response to decreased glucose utilization in the rat and monkey. Am. J. Physiol. 217, 1083–1087 [DOI] [PubMed] [Google Scholar]
- Smith J. T., Coolen L. M., Kriegsfeld L. J., Sari I. P., Jaafarzadehshirazi M. R., Maltby M., Bateman K., Goodman R. L., Tilbrook A. J., Ubuka T., Bentley G. E., Clarke I. J., Lehman M. N. (2008). Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology 149, 5770–5782 10.1210/en.2007-1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina M., Merlo-Pich E., Chan R. K., Basso A. M., Rivier J., Vale W., Koob G. F. (1996). Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science 273, 1561–1564 10.1126/science.273.5281.1561 [DOI] [PubMed] [Google Scholar]
- Sprangers S. A., Piacsek B. E. (1988). Increased suppression of luteinizing hormone secretion by chronic and acute estradiol administration in underfed adult female rats. Biol. Reprod. 39, 81–87 10.1095/biolreprod39.1.81 [DOI] [PubMed] [Google Scholar]
- Stanislaw H., Rice F. J. (1988). Correlation between sexual desire and menstrual cycle characteristics. Arch. Sex. Behav. 17, 499–508 10.1007/BF01542338 [DOI] [PubMed] [Google Scholar]
- Stanley B. G., Leibowitz S. F. (1984). Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 35, 2635–2642 10.1016/0024-3205(84)90032-8 [DOI] [PubMed] [Google Scholar]
- Stanley S. A., Small C. J., Kim M. S., Heath M. M., Seal L. J., Russell S. H., Ghatei M. A., Bloom S. R. (1999). Agouti related peptide (Agrp) stimulates the hypothalamo pituitary gonadal axis in vivo and in vitro in male rats. Endocrinology 140, 5459–5462 10.1210/en.140.11.5459 [DOI] [PubMed] [Google Scholar]
- Steel E. (1981). Control of proceptive and receptive behavior by ovarian hormones in the Syrian hamster (Mesocricetus auratus). Horm. Behav. 15, 141–156 10.1016/0018-506X(81)90024-6 [DOI] [PubMed] [Google Scholar]
- Stengel A., Wang L., Goebel-Stengel M., Tache Y. (2011). Centrally injected kisspeptin reduces food intake by increasing meal intervals in mice. Neuroreport 22, 253–257 10.1097/WNR.0b013e32834558df [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski L. A., Schneider J. E., Friedman M. I., Ji H., Kurose Y., Blache D., Rao A., Dunshea F. R., Clarke I. J. (2007). Changes in insulin, glucose and ketone bodies, but not leptin or body fat content precede restoration of luteinising hormone secretion in ewes. J. Neuroendocrinol. 19, 449–460 10.1111/j.1365-2826.2007.01551.x [DOI] [PubMed] [Google Scholar]
- Szymanski L. A., Tabaac B. J., Schneider J. E. (2009). Signals that link energy to reproduction: gastric fill, bulk intake, or caloric intake? Physiol. Behav. 96, 540–547 10.1016/j.physbeh.2008.12.005 [DOI] [PubMed] [Google Scholar]
- Tachibana T., Sato M., Takahashi H., Ukena K., Tsutsui K., Furuse M. (2005). Gonadotropin-inhibiting hormone stimulates feeding behavior in chicks. Brain Res. 1050, 94–100 10.1016/j.brainres.2005.05.035 [DOI] [PubMed] [Google Scholar]
- Temple J. L., Millar R. P., Rissman E. F. (2003). An evolutionarily conserved form of gonadotropin-releasing hormone coordinates energy and reproductive behavior. Endocrinology 144, 13–19 10.1210/en.2002-220883 [DOI] [PubMed] [Google Scholar]
- Temple J. L., Schneider J. E., Scott D. K., Korutz A., Rissman E. F. (2002). Mating behavior is controlled by acute changes in metabolic fuels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R782–R790 [DOI] [PubMed] [Google Scholar]
- Terry K. K., Chatman L. A., Foley G. L., Kadyszewski E., Fleeman T. L., Hurtt M. E., Chapin R. E. (2005). Effects of feed restriction on fertility in female rats. Birth Defects Res. B Dev. Reprod. Toxicol. 74, 431–441 10.1002/bdrb.20060 [DOI] [PubMed] [Google Scholar]
- Teubner B. J., Bartness T. J. (2010). Cholecystokinin-33 acutely attenuates food foraging, hoarding and intake in Siberian hamsters. Peptides 31, 618–624 10.1016/j.peptides.2009.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teubner B. J., Keen-Rhinehart E., Bartness T. J. (2011). Third Ventricular co-injection of sub-threshold doses of NPY and AgRP stimulate food hoarding, foraging, intake and neural activation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R37–R48 10.1152/ajpregu.00475.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. B., Mercer J. E., Karalis T., Rao A., Cummins J. T., Clarke I. J. (1990). Effect of restricted feeding on the concentrations of growth hormone (GH), gonadotropins, and prolactin (PRL) in plasma, and on the amounts of messenger ribonucleic acid for GH, gonadotropin subunits, and PRL in the pituitary glands of adult ovariectomized ewes. Endocrinology 126, 1361–1367 10.1210/endo-126-3-1361 [DOI] [PubMed] [Google Scholar]
- Tom G. (1983). Effect of deprivation on the grocery shopping behavior of obese and nonobese consumers. Int. J. Obes. 7, 307–311 [PubMed] [Google Scholar]
- Tordoff M. G., Rawson N., Friedman M. I. (1991). 2,5-anhydro-D-mannitol acts in liver to initiate feeding. Am. J. Physiol. 261, R283–R288 [DOI] [PubMed] [Google Scholar]
- Trujillo M. L., Spuch C., Carro E., Senaris R. (2011). Hyperphagia and central mechanisms for leptin resistance during pregnancy. Endocrinology 152, 1355–1365 10.1210/en.2010-0975 [DOI] [PubMed] [Google Scholar]
- Tsai A. C., Rosenberg R., Borer K. T. (1982). Metabolic alterations induced by voluntary exercise and discontinuation of exercise in hamsters. Am. J. Clin. Nutr. 35, 943–949 [DOI] [PubMed] [Google Scholar]
- Tsukamura H., Thompson R. C., Tsukahara S., Ohkura S., Maekawa F., Moriyama R., Niwa Y., Foster D. L., Maeda K. (2000a). Intracerebroventricular administration of melanin-concentrating hormone suppresses pulsatile luteinizing hormone release in the female rat. J. Neuroendocrinol. 12, 529–534 10.1046/j.1365-2826.2000.00482.x [DOI] [PubMed] [Google Scholar]
- Tsukamura H., Tsukahara S., Maekawa F., Moriyama R., Reyes B. A., Sakai T., Niwa Y., Foster D. L. (2000b). Peripheral or central administration of motilin suppresses LH release in female rats: a novel role for motilin. J. Neuroendocrinol. 12, 403–408 10.1046/j.1365-2826.2000.00467.x [DOI] [PubMed] [Google Scholar]
- Turton M. D., O’Shea D., Gunn I., Beak S. A., Edwards C. M., Meeran K., Choi S. J., Taylor G. M., Heath M. M., Lambert P. D., Wilding J. P., Smith D. M., Ghatei M. A., Herbert J., Bloom S. R. (1996). A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379, 69–72 10.1038/379069a0 [DOI] [PubMed] [Google Scholar]
- Van Der Kolk N., Madison F. N., Mohr M., Eberhard N., Kofler B., Fraley G. S. (2010). Alarin stimulates food intake in male rats and LH secretion in castrated male rats. Neuropeptides 44, 333–340 10.1016/j.npep.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Walt E. N., Wilmsen E. N., Jenkins T. (1978). Unusual sex hormone patterns among desert dwelling hunter-gatherers. J. Clin. Endocrinol. Metab. 46, 658–663 10.1210/jcem-46-4-658 [DOI] [PubMed] [Google Scholar]
- Van Itallie T. B., Kissileff H. R. (1990). “Human obesity: a problem in body energy economics,” in Handbook of Neurobiology: Neurobiology of Food and Fluid Intake, ed. Stricker E. M. (New York, NY: Plenum Press; ), 207–342 [Google Scholar]
- Vijayan E., McCann S. M. (1977). Suppression of feeding and drinking activity in rats following intraventricular injection of thyrotropin releasing hormone (TRH). Endocrinology 100, 1727–1730 10.1210/endo-100-6-1727 [DOI] [PubMed] [Google Scholar]
- Wade G. N. (1975). Some effects of ovarian hormones on food intake and body weight in female rats. J. Comp. Physiol. Psychol. 88, 183–193 10.1037/h0076186 [DOI] [PubMed] [Google Scholar]
- Wade G. N., Gray J. M. (1979). Gonadal effects on food intake and adiposity: a metabolic hypothesis. Physiol. Behav. 22, 583–593 10.1016/0031-9384(79)90028-3 [DOI] [PubMed] [Google Scholar]
- Wade G. N., Lempicki R. L., Panicker A. K., Frisbee R. M., Blaustein J. D. (1997). Leptin facilitates and inhibits sexual behavior in female hamsters. Am. J. Physiol. 272, R1354–R1358 [DOI] [PubMed] [Google Scholar]
- Wade G. N., Schneider J. E. (1992). Metabolic fuels and reproduction in female mammals. Neurosci. Biobehav. Rev. 16, 235–272 10.1016/S0149-7634(05)80183-6 [DOI] [PubMed] [Google Scholar]
- Wade G. N., Zucker I. (1970). Modulation of food intake and locomotor activity in female rats by diencephalic hormone implants. J. Comp. Physiol. Psychol. 72, 328–336 10.1037/h0029461 [DOI] [PubMed] [Google Scholar]
- Wallen K. (2000). “Risky business,” in Reproduction in Context: Social and Environmental Influences on Reproduction, ed. Schneider K. W. A. J. E. (Cambridge, MA: MIT Press; ). [Google Scholar]
- Wallen K. (2001). Sex and context: hormones and primate sexual motivation. Horm. Behav. 40, 339–357 10.1006/hbeh.2001.1696 [DOI] [PubMed] [Google Scholar]
- Wang J., Liu R., Hawkins M., Barzilai N., Rossetti L. (1998). A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 393, 684–688 10.1038/31474 [DOI] [PubMed] [Google Scholar]
- Whitman D. C., Albers H. E. (1995). Role of oxytocin in the hypothalamic regulation of sexual receptivity in hamsters. Brain Res. 680, 73–79 10.1016/0006-8993(95)00233-G [DOI] [PubMed] [Google Scholar]
- Williams D. L., Bowers R. R., Bartness T. J., Kaplan J. M., Grill H. J. (2003). Brainstem melanocortin 3/4 receptor stimulation increases uncoupling protein gene expression in brown fat. Endocrinology 144, 4692–4697 10.1210/en.2003-0381 [DOI] [PubMed] [Google Scholar]
- Williams D. L., Kaplan J. M., Grill H. J. (2000). The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology 141, 1332–1337 10.1210/en.141.4.1332 [DOI] [PubMed] [Google Scholar]
- Wirtshafter D., Davis J. D. (1977). Set points, settling points, and the control of body weight. Physiol. Behav. 19, 75–78 10.1016/0031-9384(77)90162-7 [DOI] [PubMed] [Google Scholar]
- Wise R. A. (2004). Dopamine and food reward: back to the elements. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R13. 10.1152/ajpregu.00590.2003 [DOI] [PubMed] [Google Scholar]
- Wolfgang M. J., Cha S. H., Millington D. S., Cline G., Shulman G. I., Suwa A., Asaumi M., Kurama T., Shimokawa T., Lane M. D. (2008). Brain-specific carnitine palmitoyl-transferase-1c: role in CNS fatty acid metabolism, food intake, and body weight. J. Neurochem. 105, 1550–1559 10.1111/j.1471-4159.2008.05255.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A. D., Bartness T. J. (1996). Caloric density affects food hoarding and intake by Siberian hamsters. Physiol. Behav. 59, 897–903 10.1016/0031-9384(95)02167-1 [DOI] [PubMed] [Google Scholar]
- Woodside B., Abizaid A., Walker C. (2000). Changes in leptin levels during lactation: implications for lactational hyperphagia and anovulation. Horm. Behav. 37, 353–365 10.1006/hbeh.2000.1598 [DOI] [PubMed] [Google Scholar]
- Wren A. M., Small C. J., Ward H. L., Murphy K. G., Dakin C. L., Taheri S., Kennedy A. R., Roberts G. H., Morgan D. G., Ghatei M. A., Bloom S. R. (2000). The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141, 4325–4328 10.1210/en.141.11.4325 [DOI] [PubMed] [Google Scholar]
- Wright E. M. (2001). Renal Na(+)-glucose cotransporters. Am. J. Physiol. Renal. Physiol. 280, F10–F18 [DOI] [PubMed] [Google Scholar]
- Yeo S. H., Herbison A. E. (2011). Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology 152, 2387–2399 10.1210/en.2011-0164 [DOI] [PubMed] [Google Scholar]
- Zucker I. (1969). Hormonal determinants of sex differences in saccharin preference, food intake and body weight. Physiol. Behav. 4, 595–602 10.1016/0031-9384(69)90160-7 [DOI] [Google Scholar]
- Zucker I. (1972). Body weight and age as factors determining estrogen responsiveness in the rat feeding system. Behav. Biol. 7, 527–542 10.1016/S0091-6773(72)80215-3 [DOI] [PubMed] [Google Scholar]
- Zucker I., Wade G. N., Ziegler R. (1972). Sexual and hormonal influences on eating, taste preferences, and body weight of hamsters. Physiol. Behav. 8, 101–111 10.1016/0031-9384(72)90135-7 [DOI] [PubMed] [Google Scholar]