Abstract
This study examines the efficacy and toxicity of two stereotactic body radiation therapy (SBRT) dose regimens for treatment of early prostate cancer. Forty-one patients treated with 35 Gy were matched with 41 patients treated with 36.25 Gy. Both patient groups received SBRT in five fractions over five consecutive days using the CyberKnife. Each group had 37 low-risk patients and 4 intermediate-risk patients. No statistically significant differences were present for age, prostate volume, PSA, Gleason score, stage, or risk between the groups. The dose was prescribed to the 83–87% isodose line to cover the prostate and a 5-mm margin all around, except 3 mm posteriorly. The overall median follow-up is 51 months (range, 45–58 months) with a median 54 and 48 months follow-up for the 35 and 36.25-Gy dose groups, respectively. One biochemical failure occurred in each group yielding a 97.5% freedom from biochemical failure. The PSA response has been favorable for all patients with a mean PSA of 0.1 ng/ml at 4-years. Overall toxicity has been mild with 5% late grade 2 rectal toxicity in both dose groups. Late grade 1 urinary toxicity was equivalent between groups; grade 2 urinary toxicity was 5% (2/41 patients) and 10% (4/41 patients) in the 35-Gy and 36.25-Gy dose groups (p = 0.6969), respectively. Overall, the highly favorable PSA response, limited biochemical failures, limited toxicity, and limited impact on quality of life in these low- to low-intermediate-risk patients are supportive of excellent long-term results for CyberKnife delivered SBRT.
Keywords: stereotactic body radiation therapy, prostate, dose, α/β ratio, CyberKnife
Introduction
As has been seen for other malignancies such as lung, liver, spine, and kidney (Svedman et al., 2008; Gagnon et al., 2009; Rusthoven et al., 2009; Martin and Gaya, 2010; Timmerman et al., 2010), evidence is rapidly accumulating that supports acceptable disease control and acute and late toxicity of stereotactic body radiotherapy (SBRT) for low-risk prostate cancer (Friedland et al., 2009; King et al., 2009; Bolzicco et al., 2010; Fuller et al., 2010; Jabbari et al., 2010; Katz et al., 2010; Meier et al., 2010; Boike et al., 2011; Freeman and King, 2011; Townsend et al., 2011). Indeed, initial studies on low-risk patients support SBRT’s potential for clinical efficacy while limiting treatment-related morbidity and maintaining quality of life (QOL; Friedland et al., 2009; King et al., 2009; Katz et al., 2010). Longer-term results report 93% biochemical control at a median 5 years follow-up (Freeman and King, 2011). Additional publications with varying follow-up lengths, numbers of patients, and risk categories continue to support these findings (Bolzicco et al., 2010; Jabbari et al., 2010; Boike et al., 2011; Townsend et al., 2011). Furthermore, industry sponsored multi-institution clinical studies have reported promising preliminary results (Fuller et al., 2010; Meier et al., 2010).
Stereotactic body radiotherapy delivers a large radiation dose in few fractions, typically four to five fractions of 7–10 Gy. This approach takes advantage of the prostate’s low α/β ratio. While debate continues on the exact value of the α/β ratio evidence from a variety of sources suggest that the α/β ratio resides in the 1.4- to 1.5-Gy range (Brenner and Hall, 1999; Fowler et al., 2001, 2003; King and Fowler, 2001; Brenner et al., 2002). Furthermore, supporting evidence for this low α/β ratio value continues to accumulate; a recent report by Miralbell et al. (2011) concluded, based on seven datasets with over 5000 patients, that the α/β ratio for prostate cancer is 1.4 Gy.
Given that increased dose, particularly for intermediate- and high-risk localized prostate cancer patients, has shown improved biochemical control and cause-specific survival for EBRT as well as intensity-modulated radiation therapy (IMRT) and brachytherapy treatments (Pollack et al., 2002; Kupelian et al., 2004; Zelefsky et al., 2006; Stone et al., 2010), the low α/β ratio of prostate cancer offers the opportunity, via hypofractionation, for even further dose escalation. Indeed, in the case of SBRT, assuming the prostate α/β ratio is 1.4–1.5 Gy then the equivalent dose (EQD) for SBRT’s hypofractionated dose schemes typically range from 90.15–140 Gy. Thus, SBRT offers a higher EQD1.8 than conventional fractionation schemes that reside around 70 Gy (Kuban et al., 2008), dose-escalated fractionation schemes ranging up to roughly 80 Gy (Zelefsky et al., 1998, 2006; Hanks et al., 2000), moderate hypofractionation (Kupelian et al., 2005) at 84.8 Gy, and even “ultra-high” IMRT doses at 86.4 Gy (Cahlon et al., 2008). While long-term results are not yet available, the on-going favorable PSA and biochemical control results of SBRT delivered in the 90- to 96-Gy EQD1.8 range for low-risk patients are promising. As such, the purpose of this study is to examine the toxicity, PSA nadirs and 4-year efficacy of two dose regimens for CyberKnife delivered SBRT treatment of prostate cancer. Specifically, we examine SBRT delivery of a total dose of 35 Gy (EQD1.8 91 Gy) and 36.25 Gy (EQD1.8 96 Gy) both delivered in five daily fractions using the CyberKnife.
Materials and Methods
Patients and treatment
Between April 2006 and July 2008, 304 patients with organ-confined prostate cancer were treated with SBRT at Winthrop University Hospital in Mineola, NY, USA. All patients signed consent statements and were informed of the potential risks involved with this treatment. Institutional IRB-approval was obtained on the treatment protocol. Details of the treatment have been previously published (Katz et al., 2010). Briefly, a total dose of either 35 or 36.25 Gy was delivered in 5 Gy fractions over consecutive days using CyberKnife (Accuray Inc., Sunnyvale, CA, USA) SBRT. The planning target volume (PTV) equaled the prostate plus a 5-mm margin (3 mm posteriorly). The dose was prescribed to the 83–87% isodose line covering 95% of the PTV. The D50 to bladder and rectum were to be less than 50% of Dmax, the D50 to the penile bulb was to be less than 45%, there were no constraints on the urethra. All patients received a 1500-mg amifostine enema 15 min prior to each fraction after a bowel prep that included Dulcolax ® (Boehringer Ingelheim, Germany) and a Fleet Enema. Four gold fiducials were tracked during each fraction, including translations and rotations, and beam aim automatically corrected when motion was detected. Each fraction was delivered in 45 min with two collimators using 140–170 beams.
Matched pair
For the purposes of this report, the low dose (35 Gy) patients were matched with the higher dose (36.25 Gy) patients by risk and in the order of treatment. Only patients who had not received neoadjuvant hormonal therapy were considered eligible for the pair matching. In addition, three patients in the 35-Gy group and three in the 36.25-Gy group who had died from causes other than prostate cancer were excluded from the pair matching. Specifically, each low dose patient that had not received neoadjuvant hormonal therapy was matched with a higher dose patient who had the same risk and who had not received neoadjuvant hormonal therapy. The higher dose patients were selected for pair matching in order from the time of treatment. This resulted in a match of 41 patients in each dose group.
Follow-up and statistical analysis
All patients were scheduled for follow-up 3 weeks after final treatment, 4 months later and then every 6 months thereafter. PSA tests were performed 3 and 6 months after treatment, and every 6 months thereafter. QOL was assessed using the expanded prostate cancer index composite (EPIC) questionnaire (Wei et al., 2000) at every follow-up visit during the first year and every 12 months thereafter. Toxicity was assessed using the Radiation Therapy Oncology Group (RTOG) urinary and rectal toxicity scale (Cox et al., 1995) at every follow-up visit. Biochemical failure was assessed using the Phoenix definition (Roach et al., 2006). Statistical independence for patient characteristics was assessed for continuous variables using the Student’s t-test whereas discrete variables were assessed using the Fisher’s exact test. All statistical analysis was performed using Prism (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Matched pair
A total of 41 patients from each dose group were included in this analysis. No statistically significant differences were present for age, baseline PSA, Gleason score, prostate volume, stage, or risk between the two dose groups (Table 1). Specifically, each group had 37 low-risk patients (Gleason Score 6 and PSA < 10 ng/ml) and 4 intermediate-risk patients (Gleason Score 7 or PSA > 10 and <20 ng/ml). Median baseline PSAs were 5.46 and 5.52 ng/ml for the low- and intermediate-risk groups, respectively. Median ages were 70.2 and 69.8 years, respectively. The median number of positive cores in each group was 2. The mean prostate volume was 48.23 cc (range, 28–108 cc). Dose constraints were met with the mean D50’s 42% of Dmax for the rectum/bladder and less than 40% of Dmax for the penile bulb. The mean dose to the testes was 5.1 Gy.
Table 1.
Patient characteristics detailed by overall cohort and dose.
| Characteristic | All patients (n = 82) | 35 Gy group (n = 41) | 36.25 Gy group (n = 41) | p-Value |
|---|---|---|---|---|
| Low-risk | 74 | 37 | 37 | 1 |
| Intermediate risk | 4 | 4 | 4 | 1 |
| T-STAGE | ||||
| T1c | 64 | 38 | 38 | 1 |
| T2a | 6 | 3 | 3 | 1 |
| Median PSA, range (ng/ml) | 5.35, 0.9–13.2 | 5.3, 0.9–12.05 | 5.4, 1.27–13.2 | 0.5064 |
| Median age, range (years) | 70, 45–84 | 71, 56–84 | 69, 4–84 | 0.7569 |
| Median prostate volume (cc) | 48.23, 28–108 | 49.05, 28–108 | 47.41, 29–104 | 0.821 |
Clinical outcomes
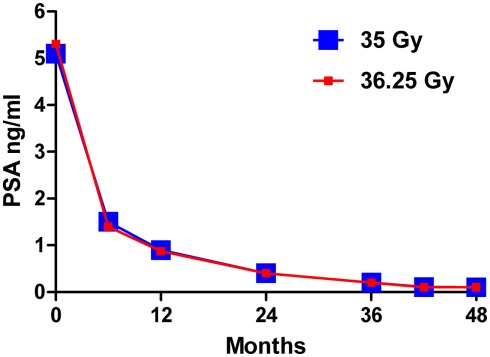
The overall median follow-up is 51 months (range, 45–58 months) with a median 54 months (range, 51–58 months) and 48 months (range, 45–52 months) follow-up for the 35-Gy and 36.25-Gy dose groups, respectively. The PSA response (Figure 1) has been favorable for all patients with a median PSA of 0.2, 0.1, and 0.1 ng/ml at 36, 42, and 48 months, respectively, with no statistically significant differences observed between the dose groups at latest follow-up (p = 0.8130) or as a function of time (p = 0.7704). To date, one biochemical failure has occurred in each dose group. For the 35-Gy dose group a low-risk patient failed at 50 months. For the 36.25-Gy dose group a low-risk patient failed at 36 months. Both patients had distant metastases. The resulting overall 4-year freedom from biochemical relapse is 97.5%.
Figure 1.
Median PSA response over time for the 35-Gy (blue) and 36.25 Gy (red) dose groups.
Toxicity and quality of life
Late grade 2 rectal toxicity was 5% for both groups. Late grade 2 urinary toxicity, consisting of dysuria, urgency, and incontinence, occurred in 2/41 (5%) patients for the 35-Gy group and in 4/41 (10%) patients for the 36.25-Gy group. Of those patients that were cystoscoped prostatic urethral inflammation was the cause of the symptoms. The differences in late grade rectal and urinary toxicity were not statistically significant (p = 0.8987 and p = 0.6969, respectively). No grade 3 or 4 toxicities were observed. Table 2 summarizes all observed late toxicity.
Table 2.
Summary of RTOG late toxicity.
| All patients (n = 82) | 35 Gy group (n = 41) | 36.25 Gy group (n = 41) | p-Value | |
|---|---|---|---|---|
| URINARY | ||||
| Grade 0 | 72 | 37 | 35 | 0.6969 |
| Grade 1 | 4 | 2 | 2 | |
| Grade 2 | 6 | 2 | 4 | |
| RECTAL | ||||
| Grade 0 | 73 | 37 | 36 | 0.8987 |
| Grade 1 | 5 | 2 | 3 | |
| Grade 2 | 4 | 2 | 2 | |
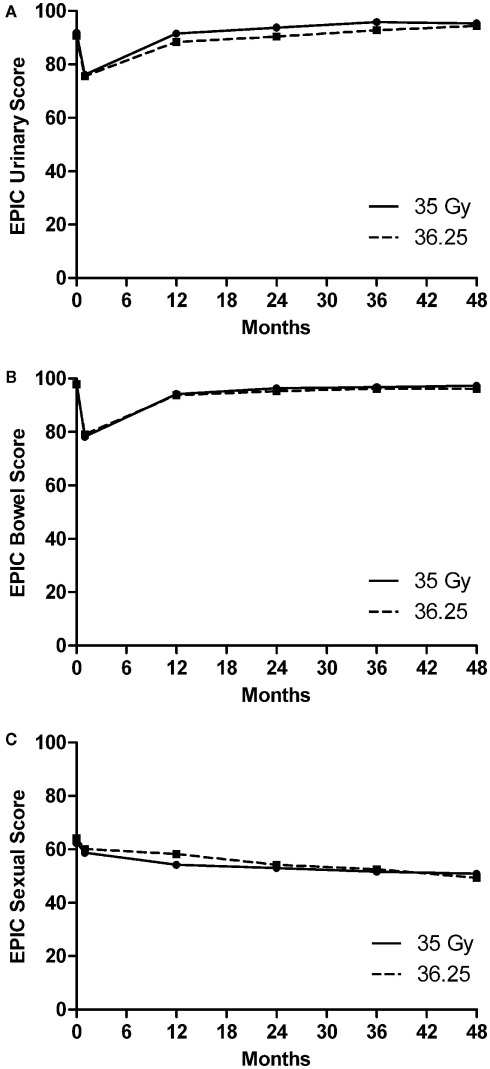
Figure 2 plots the mean EPIC scores for bowel, urinary, and sexual QOL along with patient response rates for both dose groups. All mean EPIC QOL scores initially decreased. The mean bowel and urinary QOL scores subsequently returned to baseline values. No statistically significant differences over time were observed between dose groups for the mean bowel and sexual QOL, however, for the mean urinary QOL a small, but significant difference (p = 0.0001) was observed over time with the lower dose group having a better QOL. This difference dissipated over time; comparison of the mean urinary QOL scores for the two dose groups as a function of time at 36 and 48 months showed no significant difference (p = 0.4999).
Figure 2.
Plots of mean EPIC quality of life over time from SBRT treatment for the 35-Gy (solid line) and 36.25 Gy (dashed line) group for (A) urinary, (B) bowel, and (C) sexual function.
Discussion
This matched pair analysis shows that at 4 years follow-up, CyberKnife delivered SBRT produces highly promising clinical outcomes, overall limited toxicity and minimal impact on patient QOL, regardless of whether a total dose of 35 or 36.25 Gy was delivered for low- to low-intermediate-risk patients. While longer follow-up is needed to confirm the durability of the current clinical outcomes, these results are supportive of a low α/β ratio. Results from Cahlon et al. (2008) using ultra-high dose IMRT to 86.4 Gy yields even higher rates of control than seen with 81 Gy (Zelefsky et al., 2006). At 4 years median follow-up with 35 Gy, the results in the current study show a similarly high freedom from relapse to the 98% 4-year actuarial rates of Cahlon et al. (2008) and an even lower median PSA at 0.10 ng/ml. If the α/β ratio is not 1.5 Gy but higher, say 3 Gy, then the EQD1.8 at the 35 Gy dose would only be 72 Gy. Yet, in order to achieve the results observed in this study, in comparison to those obtained with delivery of 86.4 Gy, it is reasonable to assume a comparable or higher EQD1.8 was delivered. Indeed, with an α/β ratio of 1.5 Gy, the total delivered dose of 35 Gy is equivalent to a EQD1.8 of about 91 Gy, which is consistent with the observed rates of biochemical control.
The PSA nadirs reached in both groups of the current study are suggestive of excellent long-term outcomes (Fowler, 2005). An increasingly large body of data in the literature supports the predictive value of the PSA nadir (Grimm et al., 2001; Ray et al., 2006; Alcantara et al., 2007; Stock et al., 2009; Zelefsky et al., 2009; Lamb et al., 2011). Specifically, following an analysis of 742 patients treated with brachytherapy or external beam radiotherapy, Stock et al. (2009) found that the 5-year PSA value is prognostic. They further found that patients with a PSA value of less than 0.2 ng/ml were unlikely to undergo biochemical failure. Zelefsky et al. (2009) concluded that the 2-year PSA nadir is a predictor of long-term prostate cancer mortality. While the median follow-up in the present study is only 4 years, the median PSA is 0.1 ng/ml with 71% of patients having a PSA value of less than 0.2 ng/ml. In fact, it appears that not all patients have reached their ultimate nadir, as their PSAs are still slowly dropping. These low PSA readings are comparable to those achieved with high-dose-rate (HDR) brachytherapy (Martinez et al., 2001, 2009), supporting the equivalence of these hypofractionated dose schemes with HDR. It also suggests that the results with the two dose schemes used in this study will not diverge over time.
In terms of toxicity, observed differences in urinary toxicity between the dose groups were not statistically significant; late grade 3–5 toxicity was not observed in either dose group. While 4/41 patients in the higher dose group exhibited late grade 2 urinary toxicity compared with 2/41 patients in the lower dose group (grade 1 urinary toxicity was equal between groups), the small number of patients and relatively short follow-up does not allow firm conclusions regarding the effect of dose on toxicity. Still, an α/β ratio of 3 Gy for the urethra suggests that the higher dose could increase the rate of complications as the EQD1.8 rises from 72 to 78 Gy. The potential for increased urinary toxicity at higher doses should encourage careful attention to dose constraints, and perhaps inclusion of urethral dose constraints. If biochemical control between dose groups remains comparable with longer follow-up, it may be possible to treat with the lower dose which may decrease the likelihood of urinary toxicity.
It should be emphasized that this study compares two doses that were prescribed identically, with daily fractions and coverage of the PTV at 83–87% of the Dmax. When comparing the relative benefits and toxicities of other doses used in other studies, one must be careful to discern how the dose prescription is defined. For instance, one must take into account that IMRT based plans will be more homogeneous, may impose less urethral dose and may deliver less dose to the gross tumor volume (GTV) than a CyberKnife SBRT plan that delivers the same dose to the PTV. Even when comparing different CyberKnife doses there is variability in how the dose prescription is defined. Specifically, in this study the prostate GTV received at least 7% more dose than the PTV, yielding respective doses of about 37.50 and 38.75 Gy. In contrast, in a multi-institutional CyberKnife SBRT clinical study (Meier et al., 2010), the PTV is covered at 36.25 Gy, but the prostate GTV receives at least 40 Gy. Even more confounding to direct comparisons is the HDR-like dosimetry used in some centers (Fuller, 2008; Jabbari et al., 2010) whereby the prescription dose is 38 Gy delivered in four fractions, but the delivered dose to the peripheral zone is much higher and the urethra is contoured and urethral dose constrained (Fuller, 2008; Jabbari et al., 2010). Also, in two recently published studies patients were treated every other day (King et al., 2009; Boike et al., 2011), which may impact the efficacy and toxicity due to repair that may take place in the 48-h period between fractions. Thus, while comparing the reported toxicity and outcomes of various studies is important, it is equally important to note the differences in both prescription dose as well as the overall treatment planning and dose delivery since these factors can also affect the expected outcomes.
Conclusion
The highly favorable PSA response, limited biochemical failures, and overall limited toxicity and impact on QOL in these low- to low-intermediate-risk patients, obtained regardless of the dose delivered, are supportive of excellent long-term results for CyberKnife delivered SBRT. A small, non-significant increase in the rate of late grade 2 urethral toxicity, with no increase in grade 1 toxicity, was observed in the higher dose group, but no higher rate of biochemical disease free survival was seen. Further follow-up will be necessary to validate these observations with the current data and larger populations of patients with longer follow-up should be compared to confirm these findings. Nevertheless, it should be noted that such a trend would also be true for even higher delivered SBRT doses. That is, treatment regimes delivering total doses of 38 Gy in four fractions (Fuller et al., 2010), 40 Gy in five fractions (Meier et al., 2010), and 50 Gy in five fractions (Boike et al., 2011) may well observe higher toxicity. The question is whether delivery of higher doses will result in a corresponding improvement in disease control or if further dose escalation is unwarranted in the population of low- to low-intermediate-risk organ confined prostate cancer patients.
Conflict of Interest Statement
Dr. Katz has received speaker's honoraria from Accuray, Inc., Sunnyvale, CA, USA.
Acknowledgments
We gratefully acknowledge the editorial assistance of Pam Commike, Ph.D., Accuray Incorporated. The views expressed here are entirely the authors’; Accuray did not provide assistance with data collection, compilation, or interpretation.
References
- Alcantara P., Hanlon A., Buyyounouski M. K., Horwitz E. M., Pollack A. (2007). Prostate-specific antigen nadir within 12 months of prostate cancer radiotherapy predicts metastasis and death. Cancer 109, 41–47 10.1002/cncr.22341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boike T. P., Lotan Y., Cho L. C., Brindle J., Derose P., Xie X. J., Yan J., Foster R., Pistenmaa D., Perkins A., Cooley S., Timmerman R. (2011). Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. J. Clin. Oncol. 29, 2020–2026 10.1200/JCO.2010.31.4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolzicco G., Favretto M. S., Scremin E., Tambone C., Tasca A., Guglielmi R. (2010). Image-guided stereotactic body radiation therapy for clinically localized prostate cancer: preliminary clinical results. Technol. Cancer Res. Treat. 9, 473–477 [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Hall E. J. (1999). Fractionation and protraction for radiotherapy of prostate carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 43, 1095–1101 10.1016/S0360-3016(98)00438-6 [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Martinez A. A., Edmundson G. K., Mitchell C., Thames H. D., Armour E. P. (2002). Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int. J. Radiat. Oncol. Biol. Phys. 52, 6–13 10.1016/S0360-3016(01)02664-5 [DOI] [PubMed] [Google Scholar]
- Cahlon O., Zelefsky M. J., Shippy A., Chan H., Fuks Z., Yamada Y., Hunt M., Greenstein S., Amols H. (2008). Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: toxicity and biochemical outcomes. Int. J. Radiat. Oncol. Biol. Phys. 71, 330–337 10.1016/j.ijrobp.2007.10.004 [DOI] [PubMed] [Google Scholar]
- Cox J. D., Stetz J., Pajak T. F. (1995). Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 31, 1341–1346 10.1016/0360-3016(95)00060-C [DOI] [PubMed] [Google Scholar]
- Fowler J., Chappell R., Ritter M. (2001). Is alpha/beta for prostate tumors really low? Int. J. Radiat. Oncol. Biol. Phys. 50, 1021–1031 10.1016/S0360-3016(01)01607-8 [DOI] [PubMed] [Google Scholar]
- Fowler J. F. (2005). The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncol. 44, 265–276 10.1080/02841860410002824 [DOI] [PubMed] [Google Scholar]
- Fowler J. F., Ritter M. A., Chappell R. J., Brenner D. J. (2003). What hypofractionated protocols should be tested for prostate cancer? Int. J. Radiat. Oncol. Biol. Phys. 56, 1093–1104 10.1016/S0360-3016(03)00132-9 [DOI] [PubMed] [Google Scholar]
- Freeman D. E., King C. R. (2011). Stereotactic body radiotherapy for low-risk prostate cancer: five-year outcomes. Radiat. Oncol. 6, 3. 10.1186/1748-717X-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland J. L., Freeman D. E., Masterson-Mcgary M. E., Spellberg D. M. (2009). Stereotactic body radiotherapy: an emerging treatment approach for localized prostate cancer. Technol. Cancer Res. Treat. 8, 387–392 [DOI] [PubMed] [Google Scholar]
- Fuller D. B. (2008). CyberKnife Radiosurgery For Low and Intermediate Risk Prostate Cancer: Emulating HDR Brachytherapy Dosimetry. Available at: http://clinicaltrials.gov/ct2/show/NCT00643617
- Fuller D. B., Mardirossian G., Wong D., Mckellar H. (2010). Prospective evaluation of stereotactic radiotherapy for low and intermediate risk prostate cancer: emulating HDR brachytherapy dose distribution. Int. J. Radiat. Oncol. Biol. Phys. 78, S358–S359 10.1016/j.ijrobp.2010.07.845 [DOI] [Google Scholar]
- Gagnon G. J., Nasr N. M., Liao J. J., Molzahn I., Marsh D., Mcrae D., Henderson F. C. Sr. (2009). Treatment of spinal tumors using cyberknife fractionated stereotactic radiosurgery: pain and quality-of-life assessment after treatment in 200 patients. Neurosurgery 64, 297–306; discussion 306–297. 10.1227/01.NEU.0000338072.30246.BD [DOI] [PubMed] [Google Scholar]
- Grimm P. D., Blasko J. C., Sylvester J. E., Meier R. M., Cavanagh W. (2001). 10-Year biochemical (prostate-specific antigen) control of prostate cancer with (125)I brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 51, 31–40 10.1016/S0360-3016(01)01601-7 [DOI] [PubMed] [Google Scholar]
- Hanks G. E., Hanlon A. L., Pinover W. H., Horwitz E. M., Price R. A., Schultheiss T. (2000). Dose selection for prostate cancer patients based on dose comparison and dose response studies. Int. J. Radiat. Oncol. Biol. Phys. 46, 823–832 10.1016/S0360-3016(99)00498-8 [DOI] [PubMed] [Google Scholar]
- Jabbari S., Weinberg V. K., Kaprealian T., Hsu I. C., Ma L., Chuang C., Descovich M., Shiao S., Shinohara K., Roach M., III, Gottschalk A. R. (2010). Stereotactic body radiotherapy as monotherapy or post-external beam radiotherapy boost for prostate cancer: technique, early toxicity, and PSA response. Int. J. Radiat. Oncol. Biol. Phys. (in press). 10.1016/j.ijrobp.2009.01.029 [DOI] [PubMed] [Google Scholar]
- Katz A. J., Santoro M., Ashley R., Diblasio F., Witten M. (2010). Stereotactic body radiotherapy for organ-confined prostate cancer. BMC Urol. 10, 1. 10.1186/1471-2490-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. R., Brooks J. D., Gill H., Pawlicki T., Cotrutz C., Presti J. C., Jr. (2009). Stereotactic body radiotherapy for localized prostate cancer: interim results of a prospective phase II clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 73, 1043–1048 10.1016/j.ijrobp.2008.05.041 [DOI] [PubMed] [Google Scholar]
- King C. R., Fowler J. F. (2001). A simple analytic derivation suggests that prostate cancer alpha/beta ratio is low. Int. J. Radiat. Oncol. Biol. Phys. 51, 213–214 10.1016/S0360-3016(01)01651-0 [DOI] [PubMed] [Google Scholar]
- Kuban D. A., Tucker S. L., Dong L., Starkschall G., Huang E. H., Cheung M. R., Lee A. K., Pollack A. (2008). Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 70, 67–74 10.1016/j.ijrobp.2007.06.054 [DOI] [PubMed] [Google Scholar]
- Kupelian P., Kuban D., Thames H., Levy L., Horwitz E., Martinez A., Michalski J., Pisansky T., Sandler H., Shipley W., Zelefsky M., Zietman A. (2005). Improved biochemical relapse-free survival with increased external radiation doses in patients with localized prostate cancer: the combined experience of nine institutions in patients treated in 1994 and 1995. Int. J. Radiat. Oncol. Biol. Phys. 61, 415–419 10.1016/j.ijrobp.2004.05.018 [DOI] [PubMed] [Google Scholar]
- Kupelian P. A., Potters L., Khuntia D., Ciezki J. P., Reddy C. A., Reuther A. M., Carlson T. P., Klein E. A. (2004). Radical prostatectomy, external beam radiotherapy <72 Gy, external beam radiotherapy > or =72 Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1-T2 prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 58, 25–33 10.1016/S0360-3016(03)00784-3 [DOI] [PubMed] [Google Scholar]
- Lamb D. S., Denham J. W., Joseph D., Matthews J., Atkinson C., Spry N. A., Duchesne G., Ebert M., Steigler A., Delahunt B., D’este C. (2011). A comparison of the prognostic value of early PSA test-based variables following external beam radiotherapy, with or without preceding androgen deprivation: analysis of data from the TROG 96.01 randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 79, 385–391 10.1016/j.ijrobp.2009.10.071 [DOI] [PubMed] [Google Scholar]
- Martin A., Gaya A. (2010). Stereotactic body radiotherapy: a review. Clin. Oncol. (R. Coll. Radiol.) 22, 157–172 10.1016/j.clon.2009.12.003 [DOI] [PubMed] [Google Scholar]
- Martinez A. A., Demanes J., Vargas C., Schour L., Ghilezan M., Gustafson G. S. (2009). High-dose-rate prostate brachytherapy: an excellent accelerated-hypofractionated treatment for favorable prostate cancer. Am. J. Clin. Oncol. 33, 481–488 10.1097/COC.0b013e3181b9cd2f [DOI] [PubMed] [Google Scholar]
- Martinez A. A., Pataki I., Edmundson G., Sebastian E., Brabbins D., Gustafson G. (2001). Phase II prospective study of the use of conformal high-dose-rate brachytherapy as monotherapy for the treatment of favorable stage prostate cancer: a feasibility report. Int. J. Radiat. Oncol. Biol. Phys. 49, 61–69 10.1016/S0360-3016(00)01463-2 [DOI] [PubMed] [Google Scholar]
- Meier R., Beckman A., Kaplan I., Mohideen N., Shieh E., Henning G., Walz B., Cotrutz C., Sanda M. (2010). Stereotactic radiotherapy for organ-confined prostate cancer: early toxicity and quality of life outcomes from a multi-institutional trial. Int. J. Radiat. Oncol. Biol. Phys. 78, S57. 10.1016/j.ijrobp.2010.07.167 [DOI] [Google Scholar]
- Miralbell R., Roberts S. A., Zubizarreta E., Hendry J. H. (2011). Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: alpha/beta=1.4 (0.9-2.2) Gy. Int. J. Radiat. Oncol. Biol. Phys. (in press). 10.1016/j.ijrobp.2010.10.075 [DOI] [PubMed] [Google Scholar]
- Pollack A., Zagars G. K., Starkschall G., Antolak J. A., Lee J. J., Huang E., Von Eschenbach A. C., Kuban D. A., Rosen I. (2002). Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 53, 1097–1105 10.1016/S0360-3016(02)02829-8 [DOI] [PubMed] [Google Scholar]
- Ray M. E., Thames H. D., Levy L. B., Horwitz E. M., Kupelian P. A., Martinez A. A., Michalski J. M., Pisansky T. M., Shipley W. U., Zelefsky M. J., Zietman A. L., Kuban D. A. (2006). PSA nadir predicts biochemical and distant failures after external beam radiotherapy for prostate cancer: a multi-institutional analysis. Int. J. Radiat. Oncol. Biol. Phys. 64, 1140–1150 10.1016/j.ijrobp.2005.07.006 [DOI] [PubMed] [Google Scholar]
- Roach M., III, Hanks G., Thames H., Jr., Schellhammer P., Shipley W. U., Sokol G. H., Sandler H. (2006). Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 65, 965–974 10.1016/j.ijrobp.2006.04.029 [DOI] [PubMed] [Google Scholar]
- Rusthoven K. E., Kavanagh B. D., Cardenes H., Stieber V. W., Burri S. H., Feigenberg S. J., Chidel M. A., Pugh T. J., Franklin W., Kane M., Gaspar L. E., Schefter T. E. (2009). Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J. Clin. Oncol. 27, 1572–1578 10.1200/JCO.2008.19.6329 [DOI] [PubMed] [Google Scholar]
- Stock R. G., Klein T. J., Cesaretti J. A., Stone N. N. (2009). Prognostic significance of 5-year PSA value for predicting prostate cancer recurrence after brachytherapy alone and combined with hormonal therapy and/or external beam radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 74, 753–758 10.1016/j.ijrobp.2008.08.049 [DOI] [PubMed] [Google Scholar]
- Stone N. N., Stock R. G., Cesaretti J. A., Unger P. (2010). Local control following permanent prostate brachytherapy: effect of high biologically effective dose on biopsy results and oncologic outcomes. Int. J. Radiat. Oncol. Biol. Phys. 76, 355–360 10.1016/j.ijrobp.2009.01.078 [DOI] [PubMed] [Google Scholar]
- Svedman C., Karlsson K., Rutkowska E., Sandstrom P., Blomgren H., Lax I., Wersall P. (2008). Stereotactic body radiotherapy of primary and metastatic renal lesions for patients with only one functioning kidney. Acta Oncol. 47, 1578–1583 10.1080/02841860802123196 [DOI] [PubMed] [Google Scholar]
- Timmerman R., Paulus R., Galvin J., Michalski J., Straube W., Bradley J., Fakiris A., Bezjak A., Videtic G., Johnstone D., Fowler J., Gore E., Choy H. (2010). Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 303, 1070–1076 10.1001/jama.2010.778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend N. C., Huth B. J., Ding W., Garber B., Mooreville M., Arrigo S., Lamond J., Brady L. W. (2011). Acute toxicity after cyberknife-delivered hypofractionated radiotherapy for treatment of prostate cancer. Am. J. Clin. Oncol. 34, 6–10 10.1097/COC.0b013e3181c4c7c4 [DOI] [PubMed] [Google Scholar]
- Wei J. T., Dunn R. L., Litwin M. S., Sandler H. M., Sanda M. G. (2000). Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 56, 899–905 10.1016/S0090-4295(00)00858-X [DOI] [PubMed] [Google Scholar]
- Zelefsky M. J., Chan H., Hunt M., Yamada Y., Shippy A. M., Amols H. (2006). Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J. Urol. 176, 1415–1419 10.1016/j.juro.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Zelefsky M. J., Leibel S. A., Gaudin P. B., Kutcher G. J., Fleshner N. E., Venkatramen E. S., Reuter V. E., Fair W. R., Ling C. C., Fuks Z. (1998). Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 41, 491–500 10.1016/S0360-3016(98)00091-1 [DOI] [PubMed] [Google Scholar]
- Zelefsky M. J., Shi W., Yamada Y., Kollmeier M. A., Cox B., Park J., Seshan V. E. (2009). Postradiotherapy 2-year prostate-specific antigen nadir as a predictor of long-term prostate cancer mortality. Int. J. Radiat. Oncol. Biol. Phys. 75, 1350–1356 10.1016/j.ijrobp.2008.12.067 [DOI] [PubMed] [Google Scholar]