Abstract
The transmembrane receptor Notch, a master developmental regulator, controls gliogenesis, neurogenesis, and neurite development in the nervous system. Estradiol, acting as a hormonal signal or as a neurosteroid, also regulates these developmental processes. Here we review recent evidence indicating that estradiol and Notch signaling interact in developing hippocampal neurons by a mechanism involving the putative membrane receptor G protein-coupled receptor 30. This interaction is relevant for the control of neuronal differentiation, since the downregulation of Notch signaling by estradiol results in the upregulation of neurogenin 3, which in turn promotes dendritogenesis.
Keywords: dendritogenesis, estrogen receptors, G protein-coupled estrogen receptor, G protein-coupled receptor 30, hairy and enhancer of split, neurogenin 3
Introduction: Developmental Actions of Estradiol
Estradiol is a neuroactive steroid that exerts hormonal as well as local paracrine or autocrine actions in the central nervous system, during development and in adulthood. Estradiol is produced in the ovary and in other tissues by the enzyme aromatase, which, in the brain, is expressed by specific neuronal populations (Roselli et al., 2009; Azcoitia et al., 2011). Brain aromatase and local estradiol synthesis participate in the regulation of brain development. For instance, aromatase is expressed by neural progenitor cells in vertebrates (Mouriec et al., 2008) and it is involved in the regulation of neural progenitor cell proliferation in the embryonic cerebral cortex of mice (Martinez-Cerdeno et al., 2006). In addition, many of the developmental effects of hormonal testosterone on male brain sexual differentiation are mediated by its local conversion to estradiol by neurons expressing the enzyme aromatase (MacLusky and Naftolin, 1981; Roselli et al., 2009). Furthermore, recent evidence indicate that estradiol also participates in the regulation of brain sexual differentiation in females (Bakker and Brock, 2010; Brock et al., 2011).
In addition to regulate the proliferation of neural progenitor cells (Martinez-Cerdeno et al., 2006), estradiol modulates the development of postmitotic neurons, affecting the growth of axons, dendrites and dendritic spines, and the formation of synapses (Ferreira and Caceres, 1991; Díaz et al., 1992; Duenas et al., 1996; Beyer and Karolczak, 2000; Woolley, 2000; Blacklock et al., 2005; Tsutsui, 2006; Miñano et al., 2008; Bender et al., 2010). These developmental actions depend in many cases of local estradiol synthesis (Kretz et al., 2004; Tsutsui, 2006; von Schassen et al., 2006; Hu et al., 2007; Sasahara et al., 2007).
Notch Signaling in Neural Development
Notch is a transmembrane receptor involved in cell to cell communication. The activation of Notch by transmembrane ligands, Delta-like, and Jagged, results in the cleavage of the molecule and the release of a Notch intracellular domain that is translocated to the cell nucleus and regulates transcriptional activity (Lai, 2004). Notch signaling controls cell-fate specification, differentiation, proliferation, and apoptosis in developing tissues (Artavanis-Tsakonas et al., 1999). In the nervous system, Notch signaling regulates neurogenesis and gliogenesis, not only during development but also in the adult brain (Louvi and Artavanis-Tsakonas, 2006; Ables et al., 2011; Pierfelice et al., 2011). In developing neurons, Notch signaling inhibits neurite outgrowth. In contrast, the inhibition of Notch signaling promotes neurite extension (Berezovska et al., 1999; Sestan et al., 1999; Salama-Cohen et al., 2005).
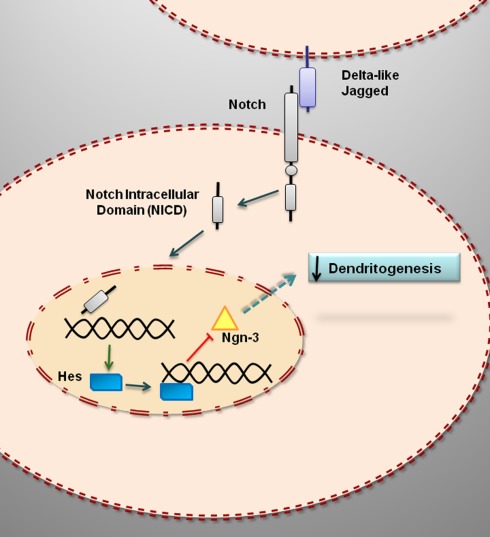
The activation of Notch signaling in developing hippocampal neurons in vitro (Figure 1) is associated with an increase in the expression of the transcription factors hairy and enhancer of split (Hes) 1 and 5 (Salama-Cohen et al., 2005). Hes transcription factors regulate the expression of several genes involved in cell differentiation. One of such genes is the basic helix-loop-helix transcription factor neurogenin 3 (Ngn3), which in developing pancreas is a proendocrine gene that causes the differentiation of the four endocrine cell lineages (Lee et al., 2001; Rukstalis and Habener, 2009) and that it is downregulated by Notch signaling via Hes1 (Lee et al., 2001). Activation of Notch signaling in neurons also induces a decrease in the expression of Ngn3 (Salama-Cohen et al., 2006). Ngn3 participates in different developmental events in the central nervous system. In the developing spinal cord, Ngn3 is expressed in glial precursor cells and it is necessary for the normal differentiation of mature oligodendrocytes and astrocytes (Lee et al., 2003). In chick embryos, Ngn3 promotes early retinal neurogenesis (Ma et al., 2009). In the hypothalamus, Ngn3 regulates the differentiation of several neuronal populations, including proopiomelanocortin (POMC) and neuropetide Y (NPY) neurons, which play a central role in energy balance and the control of food intake (Pelling et al., 2011). In hippocampal neurons, Ngn3 promotes the growth of dendrites and regulates the number of afferent GABAergic synaptic inputs (Salama-Cohen et al., 2006). Therefore, the activation of Notch in developing hippocampal neurons represses the process of dendritogenesis by the downregulation of Ngn3 (Figure 1).
Figure 1.
Notch signaling represses dendritogenesis in developing hippocampal neurons by downregulating the expression of neurogenin 3. The binding of Notch ligands (Delta-like, Jagged) results in the cleavage of Notch and the release of an active intracellular domain that is translocated to the cell nucleus where it enhances the transcription of target genes, such as Hes1, that repress the transcription of Ngn3. Ngn3 encodes for a protein, neurogenin 3, which promotes dendritogenesis.
Cross-Talk between Estradiol and Notch Signaling
Cross-talk between estradiol and Notch signaling has been detected in breast cancer cells and endothelial cells (Soares et al., 2004; Sobrino et al., 2009). Furthermore, the estrogenic compound genistein downregulates Notch-1 in prostate cancer cells (Wang et al., 2006, 2011). In breast cancer cells, estradiol decreases Notch transcriptional activity via an estrogen receptor (ER) α-mediated inhibition of Notch cleavage by γ-secretase (Rizzo et al., 2008). In turn, Notch-1 activates ERα-dependent transcription in these cells in the presence or absence of estradiol (Hao et al., 2010). Therefore, estradiol regulates Notch signaling and Notch signaling regulates estrogen signaling in breast cancer cells. It remains to be determined whether the cross-regulation of estrogen and Notch signaling also occurs in other cell types. Given the importance of Notch signaling for brain development, it is important to explore whether such interaction takes place in neural cells.
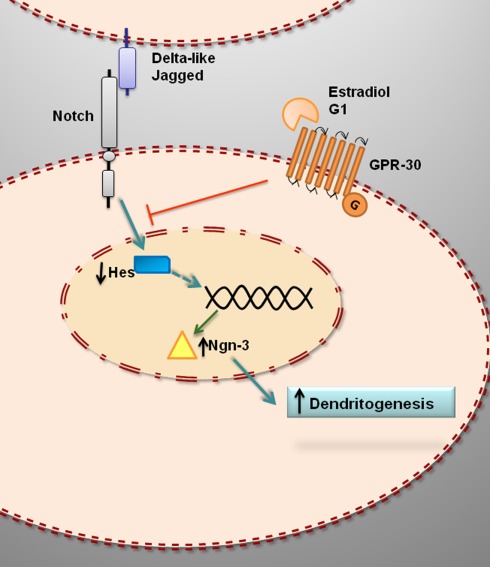
Recent studies have shown that estradiol reduces the levels of the intracellular transcriptionally active domain of Notch-1 in hippocampal slice cultures (Bender et al., 2010). This suggests that estradiol may decrease Notch-1 mediated transcription in hippocampal cells by reducing Notch-1 cleavage (Figure 2). In primary cultures of mice hippocampal neurons, estradiol decreases the expression of Hes1 and increases the expression of Ngn3 (Ruiz-Palmero et al., 2011). These findings further indicate that estradiol downregulates Notch signaling in hippocampal neurons (Figure 2).
Figure 2.
Hypothetical model for the estrogenic regulation of Ngn3 and dendritogenesis in primary hippocampal neurons. Estradiol downregulates Hes genes and upregulates Ngn3, which in turn promotes dendritogenesis. The effect of estradiol is not imitated by ERα or ERβ agonists and it is not blocked by ERα or ERβ antagonists. In contrast, G1, agonist of the putative membrane estrogen receptor GPR30, imitate the effect of estradiol on Ngn3 expression and dendritogenesis, suggesting that the action of estradiol is mediated by GPR30.
G protein-coupled receptor 30 (GPR30), also known as G protein-coupled estrogen receptor (GPER), is a putative membrane associated ER (Prossnitz et al., 2008; Olde and Leeb-Lundberg, 2009; Prossnitz and Maggiolini, 2009; Langer et al., 2010). GPR30 seems to be involved in the regulation of Notch signaling in hippocampal neurons, since G1, a ligand of GPR30 that imitates the effects of estradiol in different cell types and tissues (Terasawa et al., 2009; Zhang et al., 2010) also imitates the effect of estradiol on Ngn3 expression in hippocampal neurons (Ruiz-Palmero et al., 2011). In contrast, neither the ERα agonist 4,4′,4′′-(4-Propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol (PPT) nor the ERβ agonist 2,3-bis (4-Hydroxyphenyl)-propionitrile (DPN) affect the expression of Ngn3 in hippocampal neurons (Ruiz-Palmero et al., 2011). In addition, 1,3-Bis (4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy) phenol]-1H-pyrazole (MPP) and 4-[2-Phenyl-5,7-bis (trifluoromethyl) pyrazolo [1,5-a] pyrimidin-3-yl] phenol (PHTPP), selective antagonists of ERα and ERβ mediated transcription, respectively, do not antagonize the effect of estradiol on Ngn3 expression (Ruiz-Palmero et al., 2011). Furthermore, ICI 182,780 (ICI), antagonist of both ERα and ERβ mediated transcription and agonist of GPR30 (Thomas et al., 2005), not only does not block, but even imitates, the effect of estradiol on Ngn3 expression (Ruiz-Palmero et al., 2011). Therefore, estradiol may regulate Ngn3 levels in hippocampal neurons by a non-canonical mechanism, which probably is independent of classical nuclear ER mediated transcription.
Estradiol Promotes Dendritogenesis in Hippocampal Neurons by a Mechanism Involving Ngn3
The neuritogenic action of estradiol is mediated by the activation of the mitogen activated protein kinase (MAPK) cascade among other signaling mechanisms (Carrer et al., 2003, 2005; Dominguez et al., 2004; Gorosito and Cambiasso, 2008; Miñano et al., 2008). Recent studies have assessed whether Notch signaling is also involved in the neuritogenic actions of estradiol. Estradiol promotes dendritogenesis in primary hippocampal neurons in culture; this effect is imitated by G1 and it is not blocked by ICI (Ruiz-Palmero et al., 2011). Furthermore, neither G1 nor estradiol promote dendritogenesis in hippocampal neurons when Ngn3 is downregulated using Ngn3-specific siRNA oligonucleotides (Ruiz-Palmero et al., 2011). Therefore estradiol and G1 may act through common mechanisms to regulate Ngn3 expression and dendritogenesis by the inhibition of Notch signaling (Figure 2).
Conclusion
The studies reviewed here indicate that estradiol interacts with Notch signaling in the nervous system. Estradiol regulates dendritogenesis in developing hippocampal neurons through the modulation of Notch signaling and the upregulation of Ngn3 by a mechanism involving the putative membrane ER GPR30. Further studies are necessary to determine whether this mechanism also operates in other neuronal types. In addition, new experiments are needed to clarify the molecular mechanisms linking estrogen/GPR30 and Notch signaling in neurons.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge financial support from the Ministerio de Ciencia e Innovación, Spain (BFU2008-02950-C03-01/02), and from Comunidad de Madrid (CCG08-CSIC/SAL-3617).
References
- Ables J. L., Breunig J. J., Eisch A. J., Rakic P. (2011). Not(ch) just development: notch signalling in the adult brain. Nat. Rev. Neurosci. 12, 269–283 10.1038/nrn3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S., Rand M. D., Lake R. (1999). Notch signaling: cell fate control and signal integration in development. Science 284, 770–776 10.1126/science.284.5415.770 [DOI] [PubMed] [Google Scholar]
- Azcoitia I., Yague J. G., Garcia-Segura L. M. (2011). Estradiol synthesis within the human brain. Neuroscience 191, 139–147 [DOI] [PubMed] [Google Scholar]
- Bakker J., Brock O. (2010). Early oestrogens in shaping reproductive networks: evidence for a potential organisational role of oestradiol in female brain development. J. Neuroendocrinol. 22, 728–735 [DOI] [PubMed] [Google Scholar]
- Bender R. A., Zhou L., Wilkars W., Fester L., Lanowski J. S., Paysen D., König A., Rune G. M. (2010). Roles of 17ß-estradiol involve regulation of reelin expression and synaptogenesis in the dentate gyrus. Cereb. Cortex 20, 2985–2995 10.1093/cercor/bhq047 [DOI] [PubMed] [Google Scholar]
- Berezovska O., McLean P., Knowles R., Frosh M., Lu F. M., Lux S. E., Hyman B. T. (1999). Notch1 inhibits neurite outgrowth in postmitotic primary neurons. Neuroscience 93, 433–439 10.1016/S0306-4522(99)00157-8 [DOI] [PubMed] [Google Scholar]
- Beyer C., Karolczak M. (2000). Estrogenic stimulation of neurite growth in midbrain dopaminergic neurons depends on cAMP/protein kinase A signaling. J. Neurosci. Res. 59, 107–116 [DOI] [PubMed] [Google Scholar]
- Blacklock A. D., Johnson M. S., Krizsan-Agbas D., Smith P. G. (2005). Estrogen increases sensory nociceptor neuritogenesis in vitro by a direct, nerve growth factor-independent mechanism. Eur. J. Neurosci. 21, 2320–2328 10.1111/j.1460-9568.2005.04075.x [DOI] [PubMed] [Google Scholar]
- Brock O., Baum M. J., Bakker J. (2011). The development of female sexual behavior requires prepubertal estradiol. J. Neurosci. 31, 5574–5578 10.1523/JNEUROSCI.0209-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrer H. F., Cambiasso M. J., Brito V., Gorosito S. (2003). Neurotrophic factors and estradiol interact to control axogenic growth in hypothalamic neurons. Ann. N. Y. Acad. Sci. 1007, 306–316 10.1196/annals.1286.029 [DOI] [PubMed] [Google Scholar]
- Carrer H. F., Cambiasso M. J., Gorosito S. (2005). Effects of estrogen on neuronal growth and differentiation. J. Steroid Biochem. Mol. Biol. 93, 319–323 10.1016/j.jsbmb.2004.12.007 [DOI] [PubMed] [Google Scholar]
- Díaz H., Lorenzo A., Carrer H. F., Cáceres A. (1992). Time lapse study of neurite growth in hypothalamic dissociated neurons in culture: sex differences and estrogen effects. J. Neurosci. Res. 33, 266–281 10.1002/jnr.490330210 [DOI] [PubMed] [Google Scholar]
- Dominguez R., Jalali C., de Lacalle S. (2004). Morphological effects of estrogen on cholinergic neurons in vitro involves activation of extracellular signal-regulated kinases. J. Neurosci. 24, 982–990 10.1523/JNEUROSCI.2586-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duenas M., Torres-Aleman I., Naftolin F., Garcia-Segura L. M. (1996). Interaction of insulin-like growth factor-I and estradiol signaling pathways on hypothalamic neuronal differentiation. Neuroscience 74, 531–539 10.1016/0306-4522(96)00142-X [DOI] [PubMed] [Google Scholar]
- Ferreira A., Caceres A. (1991). Estrogen-enhanced neurite growth: evidence for a selective induction of Tau and stable microtubules. J. Neurosci. 11, 392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorosito S. V., Cambiasso M. J. (2008). Axogenic effect of estrogen in male rat hypothalamic neurons involves Ca(2+), protein kinase C, and extracellular signal-regulated kinase signaling. J. Neurosci. Res. 86, 145–157 10.1002/jnr.21466 [DOI] [PubMed] [Google Scholar]
- Hao L., Rizzo P., Osipo C., Pannuti A., Wyatt D., Cheung L. W., Sonenshein G., Osborne B. A., Miele L. (2010). Notch-1 activates estrogen receptor-alpha-dependent transcription via IKKalpha in breast cancer cells. Oncogene 29, 201–213 10.1038/onc.2009.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R., Cai W. Q., Wu X. G., Yang Z. (2007). Astrocyte-derived estrogen enhances synapse formation and synaptic transmission between cultured neonatal rat cortical neurons. Neuroscience 144, 1229–1240 10.1016/j.neuroscience.2006.09.056 [DOI] [PubMed] [Google Scholar]
- Kretz O., Fester L., Wehrenberg U., Zhou L., Brauckmann S., Zhao S., Prange-Kiel J., Naumann T., Jarry H., Frotscher M., Rune G. M. (2004). Hippocampal synapses depend on hippocampal estrogen synthesis. J. Neurosci. 24, 5913–5921 10.1523/JNEUROSCI.5186-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E. C. (2004). Notch signaling: control of cell communication and cell fate. Development 131, 965–973 10.1242/dev.01074 [DOI] [PubMed] [Google Scholar]
- Langer G., Bader B., Meoli L., Isensee J., Delbeck M., Noppinger P. R., Otto C. (2010). A critical review of fundamental controversies in the field of GPR30 research. Steroids 75, 603–610 10.1016/j.steroids.2009.12.006 [DOI] [PubMed] [Google Scholar]
- Lee J., Wu Y., Qi Y., Xue H., Liu Y., Scheel D., German M., Qiu M., Guillemot F., Rao M., Gradwohl G. (2003). Neurogenin3 participates in gliogenesis in the developing vertebrate spinal cord. Dev. Biol. 253, 84–98 10.1006/dbio.2002.0868 [DOI] [PubMed] [Google Scholar]
- Lee J. C., Smith S. B., Watada H., Lin J., Scheel D., Wang J., Mirmira R. G., German M. S. (2001). Regulation of the pancreatic pro-endocrine gene neurogenin3. Diabetes 50, 928–936 10.2337/diabetes.50.5.928 [DOI] [PubMed] [Google Scholar]
- Louvi A., Artavanis-Tsakonas S. (2006). Notch signalling in vertebrate neural development. Nat. Rev. Neurosci. 7, 93–102 10.1038/nrn1847 [DOI] [PubMed] [Google Scholar]
- Ma W., Yan R. T., Mao W., Wang S. Z. (2009). Neurogenin3 promotes early retinal neurogenesis. Mol. Cell. Neurosci. 40, 187–198 10.1016/j.mcn.2008.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky N. J., Naftolin F. (1981). Sexual differentiation of the central nervous system. Science 211, 1294–1302 10.1126/science.6163211 [DOI] [PubMed] [Google Scholar]
- Martinez-Cerdeno V., Noctor S. C., Kriegstein A. R. (2006). Estradiol stimulates progenitor cell division in the ventricular and subventricular zones of the embryonic neocortex. Eur. J. Neurosci. 24, 3475–3488 10.1111/j.1460-9568.2006.05239.x [DOI] [PubMed] [Google Scholar]
- Miñano A., Xifró X., Pérez V., Barneda-Zahonero B., Saura C. A., Rodríguez-Alvarez J. (2008). Estradiol facilitates neurite maintenance by a Src/Ras/ERK signalling pathway. Mol. Cell. Neurosci. 39, 143–151 10.1016/j.mcn.2008.06.001 [DOI] [PubMed] [Google Scholar]
- Mouriec K., Pellegrini E., Anglade I., Menuet A., Adrio F., Thieulant M. L., Pakdel F., Kah O. (2008). Synthesis of estrogens in progenitor cells of adult fish brain: evolutive novelty or exaggeration of a more general mechanism implicating estrogens in neurogenesis? Brain Res. Bull. 75, 274–280 10.1016/j.brainresbull.2007.10.030 [DOI] [PubMed] [Google Scholar]
- Olde B., Leeb-Lundberg L. M. (2009). GPR30/GPER1: searching for a role in estrogen physiology. Trends Endocrinol. Metab. 20, 409–416 10.1016/j.tem.2009.04.006 [DOI] [PubMed] [Google Scholar]
- Pelling M., Anthwal N., McNay D., Gradwohl G., Leiter A. B., Guillemot F., Ang S. L. (2011). Differential requirements for neurogenin 3 in the development of POMC and NPY neurons in the hypothalamus. Dev. Biol. 349, 406–416 10.1016/j.ydbio.2010.11.007 [DOI] [PubMed] [Google Scholar]
- Pierfelice T., Alberi L., Gaiano N. (2011). Notch in the vertebrate nervous system: an old dog with new tricks. Neuron 69, 840–855 10.1016/j.neuron.2011.02.031 [DOI] [PubMed] [Google Scholar]
- Prossnitz E. R., Maggiolini M. (2009). Mechanisms of estrogen signaling and gene expression via GPR30. Mol. Cell. Endocrinol. 308, 32–38 10.1016/j.mce.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz E. R., Oprea T. I., Sklar L. A., Arterburn J. B. (2008). The ins and outs of GPR30: a transmembrane estrogen receptor. J. Steroid Biochem. Mol. Biol. 109, 350–353 10.1016/j.jsbmb.2008.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo P., Miao H., D’Souza G., Osipo C., Song L. L., Yun J., Zhao H., Mascarenhas J., Wyatt D., Antico G., Hao L., Yao K., Rajan P., Hicks C., Siziopikou K., Selvaggi S., Bashir A., Bhandari D., Marchese A., Lendahl U., Qin J. Z., Tonetti D. A., Albain K., Nickoloff B. J., Miele L. (2008). Cross-talk between notch and the estrogen receptor in breast cancer suggests novel therapeutic approaches. Cancer Res. 68, 5226–5235 10.1158/0008-5472.CAN-07-5744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli C. E., Liu M., Hurn P. D. (2009). Brain aromatization: classic roles and new perspectives. Semin. Reprod. Med. 27, 207–217 10.1055/s-0029-1216274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Palmero I., Simon-Areces J., Garcia-Segura L. M., Arevalo M. A. (2011). Notch/neurogenin 3 signalling is involved in the neuritogenic actions of oestradiol in developing hippocampal neurones. J. Neuroendocrinol. 23, 355–364 10.1111/j.1365-2826.2011.02110.x [DOI] [PubMed] [Google Scholar]
- Rukstalis J. M., Habener J. F. (2009). Neurogenin3: a master regulator of pancreatic islet differentiation and regeneration. Islets 1, 177–184 10.4161/isl.1.3.9877 [DOI] [PubMed] [Google Scholar]
- Salama-Cohen P., Arevalo M. A., Grantyn R., Rodriguez-Tebar A. (2006). Notch and NGF/p75(NTR) control dendrite morphology and the balance of excitatory/inhibitory synaptic input to hippocampal neurones through neurogenin 3. J. Neurochem. 97, 1269–1278 10.1111/j.1471-4159.2006.03783.x [DOI] [PubMed] [Google Scholar]
- Salama-Cohen P., Arevalo M. A., Meier J., Grantyn R., Rodriguez-Tebar A. (2005). NGF controls dendrite development in hippocampal neurons by binding to p75(NTR) and modulating the cellular targets of notch. Mol. Biol. Cell 16, 339–347 10.1091/mbc.E04-05-0438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasahara K., Shikimi H., Haraguchi S., Sakamoto H., Honda S., Harada N., Tsutsui K. (2007). Mode of action and functional significance of estrogen-inducing dendritic growth, spinogenesis, and synaptogenesis in the developing Purkinje cell. J. Neurosci. 27, 7408–7417 10.1523/JNEUROSCI.0710-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestan N., Artavanis-Tsakonas S., Rakic P. (1999). Contact-dependent inhibition of cortical neurite growth mediated by notch signaling. Science 286, 741–746 10.1126/science.286.5440.741 [DOI] [PubMed] [Google Scholar]
- Soares R., Balogh G., Guo S., Gartner F., Russo J., Schmitt F. (2004). Evidence for the notch signaling pathway on the role of estrogen in angiogenesis. Mol. Endocrinol. 18, 2333–2343 10.1210/me.2003-0362 [DOI] [PubMed] [Google Scholar]
- Sobrino A., Mata M., Laguna-Fernandez A., Novella S., Oviedo P. J., García-Pérez M. A., Tarín J. J., Cano A., Hermenegildo C. (2009). Estradiol stimulates vasodilatory and metabolic pathways in cultured human endothelial cells. PLoS ONE 4, e8242. 10.1371/journal.pone.0008242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E., Noel S. D., Keen K. L. (2009). Rapid action of oestrogen in luteinising hormone-releasing hormone neurones: the role of GPR30. J. Neuroendocrinol. 21, 316–321 10.1111/j.1365-2826.2009.01839.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Pang Y., Filardo E. J., Dong J. (2005). Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146, 624–632 10.1210/en.2004-1064 [DOI] [PubMed] [Google Scholar]
- Tsutsui K. (2006). Biosynthesis and organizing action of neurosteroids in the developing Purkinje cell. Cerebellum 5, 89–96 10.1080/14734220600697211 [DOI] [PubMed] [Google Scholar]
- von Schassen C., Fester L., Prange-Kiel J., Lohse C., Huber C., Böttner M., Rune G. M. (2006). Oestrogen synthesis in the hippocampus: role in axon outgrowth. J. Neuroendocrinol. 18, 847–856 10.1111/j.1365-2826.2006.01484.x [DOI] [PubMed] [Google Scholar]
- Wang Z., Li Y., Ahmad A., Banerjee S., Azmi A. S., Kong D., Wojewoda C., Miele L., Sarkar F. H. (2011). Down-regulation of Notch-1 is associated with Akt and FoxM1 in inducing cell growth inhibition and apoptosis in prostate cancer cells. J. Cell. Biochem. 112, 78–88 10.1002/jcb.23027 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang Z., Zhang Y., Banerjee S., Li Y., Sarkar F. H. (2006). Inhibition of nuclear factor kappab activity by genistein is mediated via Notch-1 signaling pathway in pancreatic cancer cells. Int. J. Cancer 118, 1930–1936 10.1002/ijc.21499 [DOI] [PubMed] [Google Scholar]
- Woolley C. S. (2000). Effects of oestradiol on hippocampal circuitry. Novartis Found. Symp. 230, 173–180 [DOI] [PubMed] [Google Scholar]
- Zhang B., Subramanian S., Dziennis S., Jia J., Uchida M., Akiyoshi K., Migliati E., Lewis A. D., Vandenbark A. A., Offner H., Hurn P. D. (2010). Estradiol and G1 reduce infarct size and improve immunosuppression after experimental stroke. J. Immunol. 184, 4087–4094 10.4049/jimmunol.0990118 [DOI] [PMC free article] [PubMed] [Google Scholar]