Abstract
Recent findings showed the presence of a reciprocal relationship between thyroid hormones and ghrelin, although the exact mechanism is not known. Design: Our study is addressed to evaluate the effect of acute exogenous rhTSH administration on serum ghrelin levels in athyreotic patients on replacement l-thyroxine therapy. The study group included 50 patients (16 males and 34 females) submitted to total thyroidectomy and 131-iodine remnant ablation for differentiated thyroid cancer on l-thyroxine therapy. Mean age was 47.5 ± 16.5 years and mean BMI was 25.6 ± 5.01 kg/m2. rhTSH was administrated at the dosage of 0.9 mg i.m. once daily for two consecutive days. Blood samples were taken between 08.00 and 09.00 after a overnight fasting for measurement of TSH, FT3, FT4, and ghrelin before the first administration of rhTSH and for measurement of TSH and ghrelin 24, 48, 72, and 96 h after the first administration of rhTSH. Results: Mean ± SD values of basal TSH were 0.54 ± 0.77 μU/ml without significant difference between females and males. As expected, after rhTSH administration TSH concentrations increased at 24 and 48 h with peak TSH values ranging from 20.20 to 313 μU/ml (mean ± SD 98.4 ± 66.7 μU/ml). Mean ± SD values of basal ghrelin were 1085 ± 373 pg/ml without significant difference between males and females. After rhTSH administration ghrelin concentrations decreased significantly (p < 0.01) at 24 h (mean ± SD 934 ± 314 pg/ml p < 0.01) and returned to pre-treatment levels at 96 h. Conclusion: Our study demonstrates that acute exogenous TSH administration has a suppressive effect on ghrelin secretion independent from changes in thyroid status.
Keywords: TSH, ghrelin
Introduction
Ghrelin, an acylated 28-amino acid ligand for the growth hormone secretagogue receptor (GHS-R), is produced principally by the neuroendocrine X/A cells of the gastric mucosa (Kojima and Kangawa, 2005). It was also identified in the pituitary gland, hypothalamus, pancreas, and others tissues (Date and Kojima, 2000). At the hypothalamic level, ghrelin stimulates GH release and regulates appetite and energy balance (Waren and Small, 2000; Ghigo and Arvat, 2001). Increased levels are seen in catabolic conditions (Misra and Miller, 2005), and decreased levels are found in obese patients (Marchesini and Bianchi, 2004). Moreover, an inverse correlation between serum ghrelin levels and resting energy expenditure (REE) has been recorded in healthy women (Kamiji and Troncon, 2010). Thyroid disease is associated with changes in appetite, food intake, and REE. Previous studies have reported decreased levels of ghrelin in hyperthyroidism (Altinova and Toruner, 2006a) and increased levels in hypothyroid patients (Gjedde and Vestergaard, 2008; Kosowicz and Baumann-Antczak, 2011). On the other hand it has been recently demonstrated the presence of mRNA of GHS-R in thyroid tissues (Ueberberg and Unger, 2009) and a stimulating effect of ghrelin on FT4 secretion in vivo (Kluge and Riedl, 2010), suggesting a reciprocal effect between ghrelin and thyroid hormones. Since previous studies were conducted in patients with abnormal thyroid hormone levels (hyperthyroidism and hypothyroidism) they could not assess the direct role of TSH on ghrelin secretion. The present study was designed in athyreotic patients in stable replacement l-thyroxine therapy and normal TSH to study the effect of exogenous TSH administration independent of thyroid status.
Patients and Methods
The study group included 50 patients (16 males and 34 females) submitted to total thyroidectomy and 131-iodine remnant ablation for differentiated thyroid cancer. Mean age was 47.5 ± 16.5 years and mean BMI was 25.6 ± 5.01 kg/m2. Twenty-eight patients with persistent disease or at high risk, received l-thyroxine in suppressive or semisuppressive doses (target TSH <0.5 μIU/mL), 22 patients, who had previous evidence of complete remission, were treated with replacement doses of l-thyroxine aimed to maintain normal TSH concentration.
Patients were scheduled to receive rhTSH (Thyrogen; Genzyme Therapeutics, Cambridge, MA, USA) for periodic staging of their disease. rhTSH was administrated at the dosage of 0.9 mg i.m. once daily for two consecutive days according to conventional protocol (Pacini and Ladenson, 2006). Blood samples were taken between 08.00 and 09.00 after a overnight fasting for measurement of TSH, FT3, FT4, and ghrelin before the first administration of rhTSH and for measurement of only TSH and ghrelin 24, 48, 72, and 96 h after the first administration of rhTSH.
Serum total ghrelin was determined by a validated radioimmunoassay (RIA; Mediagnost, Reutlingen, Germany) with a functional sensitivity described by the manufacturer of 0.04 ng/ml; the normal range is 600–1400 pg/ml. Serum TSH was determined by a commercially chemiluminescent assay (Immulite 2000, third generation TSH, Los Angeles, CA, USA; EURO/DPC limited Lamberies, Gwynedd, UK).
Statistical analysis
Data are presented as mean ± SD. Statistical analysis was performed using the Mann–Whitney test when comparing non-parametric data. Areas under the serum concentration curves (AUC) of ghrelin and TSH were calculated for the intervals 0–96 h by applying the trapezoid rule. One-way ANOVA was conducted to compare serum ghrelin concentration at various time points. Post hoc comparisons were conducted using Bonferroni’s correction. Correlations between the variables were analyzed by Pearson analysis using the Graph Pad Prism version 3.0. Statistical significance was defined as p value < 0.05.
Results
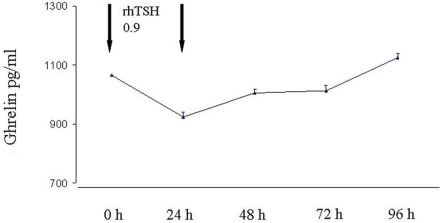
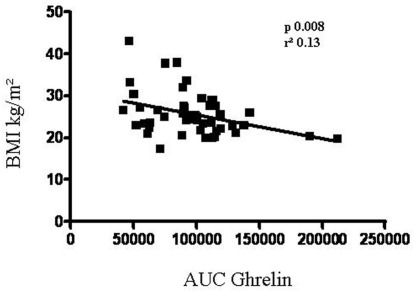
Mean ± SD values of basal TSH were 0.54 ± 0.77 μU/ml without significant difference between females and males. As expected, after rhTSH administration TSH concentrations increased at 24 and 48 h with peak TSH values ranging 20.2–313 μU/ml (mean ± SD 98.4 ± 66.7 μU/ml). In most patients (42 patients) the peak serum TSH was observed 48 h after the second rhTSH injection. The TSH–AUC was positively associated with age (r = 0.55, p < 0.0001), but not with BMI. Mean ± SD values of basal ghrelin were 1085 ± 373 pg/ml without significant difference between males and females. After rhTSH administration ghrelin concentrations decreased significantly (p < 0.01) at 24 h (mean ± SD 934 ± 314 pg/ml p < 0.01) and returned to pre-treatment levels at 96 h (Figure 1). As shown in Figure 2 a negative correlation between ghrelin AUC and BMI was observed (r = −0.37, p = 0.008).
Figure 1.
Changes of serum ghrelin concentrations after rhTSH stimulation.
Figure 2.
Correlation between ghrelin AUC after rhTSH administration and BMI.
Discussion
In vitro studies demonstrated that both ghrelin and GHR-S are expressed in normal and neoplastic thyroid glands (Gnanapavan and Kola, 2002; Zhang and Wang, 2006; Karaoglu and Aydin, 2009; Ueberberg and Unger, 2009) and a reciprocal relationship between thyroid hormones and ghrelin levels has been demonstrated (Chrysanthia and Franchi, 2007). In vivo some studies have shown that ghrelin levels are reduced in overt hyperthyroidism, but not in subclinical hyperthyroidism (Tanda and Lombardi, 2009), and are normalized after correction of hyperthyroidism (Riis and Hansen, 2003). However it is not clear whether ghrelin reduction is a consequence of excess thyroid hormones or secondary to the hyperinsulinemic status associated with hyperthyroidism (Gimenez-Palop and Gimenez-Perez, 2005). More conflicting data are reported in hypothyroid patients with studies showing higher, normal, and even lower serum ghrelin levels (Gimenez-Palop and Gimenez-Perez, 2005; Altinova and Toruner, 2006b; Gjedde and Vestergaard, 2008; Tanda and Lombardi, 2009).
Our model of athyreotic patients replaced with l-thyroxine consented to study the effect of TSH on ghrelin concentrations independently of thyroid status. We observed a decrease of ghrelin levels in the first 24 h from rhTSH administration returning to baseline thereafter. We did not observe gender difference in TSH-stimulated ghrelin concentrations, but, as expected, we found a negative correlation between basal and TSH-stimulated ghrelin levels and BMI. The observation that the correlation persists also after TSH stimulation is an indication that the effect of TSH is a true biological phenomenon rather than a fortuitous finding. Although we injected pharmacological doses of rhTSH, we want to notice that the TSH levels achieved in the blood are similar to those obtained after thyroid hormone withdrawal in thyroid cancer patients undergoing routine follow-up examination.
In conclusion, our study shows that a modulator action of TSH is operating at gastric mucosa on ghrelin secretion, at least at acute pharmacological doses.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
BMI, body mass index; GHS-R, growth hormone secretagogue receptor; TSH, thyroid stimulating hormone.
References
- Altinova A. E., Toruner F. B. (2006a). Reduced serum acylated ghrelin levels in patients with hyperthyroidism. Horm. Res. 65, 295–299 10.1159/000092603 [DOI] [PubMed] [Google Scholar]
- Altinova A. E., Toruner F. (2006b). Serum ghrelin levels in patients with Hashimoto’s thyroiditis. Thyroid 16, 1259–1264 10.1089/thy.2006.16.1259 [DOI] [PubMed] [Google Scholar]
- Chrysanthia A. L., Franchi G. (2007). Ghrelin in neuroendocrine organs and tumors. Pituitary 10, 213–225 10.1007/s11102-007-0023-0 [DOI] [PubMed] [Google Scholar]
- Date Y., Kojima M. (2000). Ghrelin, a novel growth hormone-realising acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141, 4255–4261 10.1210/en.141.11.4255 [DOI] [PubMed] [Google Scholar]
- Ghigo E., Arvat E. (2001). Biologic activities of growth hormone secretagogues in humans. Endocrine 14, 87–93 10.1385/ENDO:14:1:087 [DOI] [PubMed] [Google Scholar]
- Gimenez-Palop O., Gimenez-Perez G. (2005). Circulating ghrelin in thyroid dysfunction is related to insulin-resistance and hunger, food-intake or anthropometric changes. Eur. J. Endocrinol. 153, 73–79 10.1530/eje.1.01934 [DOI] [PubMed] [Google Scholar]
- Gjedde S., Vestergaard E. T. (2008). Serum ghrelin levels are increased in hypothyroid patients and become normalized by L-thyroxine treatment. J. Clin. Endocrinol. Metab. 93, 2277–2280 10.1210/jc.2007-2619 [DOI] [PubMed] [Google Scholar]
- Gnanapavan S., Kola B. (2002). The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 87, 2988–2991 10.1210/jc.87.6.2988 [DOI] [PubMed] [Google Scholar]
- Kamiji M. M., Troncon L. E. (2010). Ghrelin and PYY (3–36) in gastrectomized and vagotomized patients: relations with appetite, energy intake, and resting energy expenditure. Eur. J. Clin. Nutr. 64, 845–852 10.1038/ejcn.2010.88 [DOI] [PubMed] [Google Scholar]
- Karaoglu A., Aydin S. (2009). Expression of obestatin and ghrelin in papillary thyroid carcinoma. Mol. Cell. Biochem. 323, 113–118 10.1007/s11010-008-9969-0 [DOI] [PubMed] [Google Scholar]
- Kluge M., Riedl S. (2010). Ghrelin affects the hypothalamus-pituitary-thyroid axis in humans by increasing free thyroxine and decreasing TSH in plasma. Eur. J. Endocrinol. 162, 1059–1065 10.1530/EJE-10-0094 [DOI] [PubMed] [Google Scholar]
- Kojima M., Kangawa K. (2005). Ghrelin: structure and function. Physiol. Rev. 85, 495–522 10.1152/physrev.00012.2004 [DOI] [PubMed] [Google Scholar]
- Kosowicz J., Baumann-Antczak A. (2011). Thyroid hormones affect plasma ghrelin and obestatin levels. Horm. Metab. Res. 43, 121–125 10.1055/s-0030-1269853 [DOI] [PubMed] [Google Scholar]
- Marchesini G., Bianchi G. (2004). Plasma ghrelin concentrations, food intake, and anorexia in liver failure. J. Clin. Endocrinol. Metab. 89, 2136–2141 10.1210/jc.2003-031771 [DOI] [PubMed] [Google Scholar]
- Misra M., Miller K. K. (2005). Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am. J. Physiol. Endocrinol. Metab. 289, 347–356 10.1152/ajpendo.00615.2004 [DOI] [PubMed] [Google Scholar]
- Pacini F., Ladenson P. W. (2006). Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J. Clin. Endocrinol. Metab. 91, 926–932 10.1210/jc.2005-1651 [DOI] [PubMed] [Google Scholar]
- Riis A. L., Hansen T. K. (2003). Hyperthyroidism is associated with suppressed circulating ghrelin levels. J. Clin. Endocrinol. Metab. 88, 853–857 10.1210/jc.2002-021302 [DOI] [PubMed] [Google Scholar]
- Tanda M. L., Lombardi V. (2009). Plasma total and acylated ghrelin concentrations in patients with clinical and subclinical thryoid dysfunction. J. Endocrinol. Invest. 32, 74–78 [DOI] [PubMed] [Google Scholar]
- Ueberberg B., Unger N. (2009). Expression of ghrelin and its receptor in human tissues. Horm. Metab. Res. 41, 814–821 10.1055/s-0029-1233462 [DOI] [PubMed] [Google Scholar]
- Waren A. M., Small C. J. (2000). The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141, 4325–4328 10.1210/en.141.11.4325 [DOI] [PubMed] [Google Scholar]
- Zhang Y. F., Wang H. N. (2006). Ghrelin expression in the tissues of different thyroid diseases. Beijing Da Xue Xue Bao 38, 193–196 [PubMed] [Google Scholar]