Abstract
In vertebrates, gonadotropin-releasing hormone (GnRH) is a crucial decapeptide that activates the hypothalamic–pituitary–gonadal axis to ensure successful reproduction. Recently, a GnRH-like molecule has been isolated from a gastropod mollusk, Aplysia californica. This GnRH (ap-GnRH) is deduced to be an undecapeptide, and its function remains to be explored. Our previous study demonstrated that ap-GnRH did not stimulate a range of reproductive parameters. Instead, it affected acute behavioral and locomotive changes unrelated to reproduction. In this study, we used electrophysiology and retrograde tracing to further explore the central role of ap-GnRH. Sharp-electrode intracellular recordings revealed that ap-GnRH had diverse effects on central neurons that ranged from excitatory, inhibitory, to the alteration of membrane potential. Unexpectedly, extracellular recordings revealed that ap-GnRH suppressed the onset of electrical afterdischarge in bag cell neurons, suggesting an inhibitory effect on female reproduction. Lastly, using immunocytochemistry coupled with nickel backfill, we demonstrated that some ap-GnRH neurons projected to efferent nerves known to innervate the foot and parapodia, suggesting ap-GnRH may directly modulate the motor output of these peripheral tissues. Overall, our results suggested that in A. californica, ap-GnRH more likely functioned as a central modulator of complex behavior and motor regulation rather than as a conventional reproductive stimulator.
Keywords: GnRH, Aplysia californica, sea hare, reproduction, electrophysiology, gastropod mollusk, central nervous system
Introduction
In vertebrates, gonadotropin-releasing hormone (GnRH) is critical for the activation of the hypothalamo-pituitary–gonadal (HPG) axis and thus reproductive success (reviewed in Somoza et al., 2002). There is now evidence demonstrating that GnRH-like peptides are also present in non-chordate invertebrates (reviewed by Kah et al., 2007; Tsai and Zhang, 2008). A total of 26 variants of GnRH (Roch et al., 2011), including 2 from the mollusks (Iwakoshi et al., 2002; Zhang et al., 2008), have been identified. An additional four forms of GnRH-like molecules have been found in the owl limpet (Lottia gigantean), a polychaete worm (Capitella sp.), a sea urchin (Strongylocentrotus purpuratus), and a leech (Helobdella robusta) by searching within the expressed sequence tag database (Tsai and Zhang, 2008; Roch et al., 2011). GnRH from two species of cephalopod mollusk, common cuttlefish (Sepia officinalis; Di Cristo et al., 2009) and sword tip squid (Loligo edulis; Onitsuka et al., 2009) share the same peptide sequence as the common octopus (Octopus vulgaris; Iwakoshi et al., 2002). The isolated or deduced sequences of these GnRH peptides have either 11 or 12 amino acids with the insertion of 2 amino acids downstream of the N-terminal pyroglutamyl residue (Iwakoshi et al., 2002; Zhang et al., 2008; Di Cristo et al., 2009; Onitsuka et al., 2009; Tsai et al., 2010; Roch et al., 2011). The primary structure of these six novel forms of GnRHs differs markedly from that of chordate GnRHs, challenging the conventional belief that the decapeptide structure of chordate GnRHs is universally conserved. The diversification of GnRH structure further raises the possibility that non-chordate GnRH may have assumed diverse functions unrelated to reproduction during evolution (reviewed in Tsai and Zhang, 2008).
The physiological roles of two molluscan forms of GnRH have been examined previously in the O. vulgaris (reviewed in Minakata et al., 2009) and the sea hare (Aplysia californica; Tsai et al., 2010). In the octopus, early studies using heterologous vertebrate GnRH antisera suggested that GnRH played a role in the regulation of central nervous system (CNS) and reproductive tissues (Di Cosmo and Di Cristo, 1998; Di Cristo et al., 2002; Di Cristo and Di Cosmo, 2007). These findings are consistent with the results obtained from the native GnRH (oct-GnRH) which appeared to modulate reproductive function via the stimulation of oviductal contraction and gonadal steroid secretion (Kanda et al., 2006; Minakata et al., 2009). However, the wide distribution of oct-GnRH in the CNS of O. vulgaris also suggested a wide range of non-reproductive functions associated with motor and memory regulation (reviewed in Minakata et al., 2009). In A. californica, the native form of GnRH (ap-GnRH) acutely altered behaviors such as parapodial opening, substrate attachment, and feeding, but did not stimulate any reproductive parameters tested, including gametogenesis, gamete release, and the accumulation of a reproductive hormone, egg-laying hormone (ELH; Tsai et al., 2010). In this regard, the literature suggested that molluscan GnRH could modulate a wide range of motor behaviors unrelated to reproduction.
In the present study, we utilized extracellular and sharp-electrode intracellular recordings combined with retrograde labeling to explore the central effects of ap-GnRH that may underlie some of the behavioral changes observed previously (Tsai et al., 2010). In addition, we determined if ap-GnRH altered the ability of the neuroendocrine bag cell neurons (BCN) to undergo electrical afterdischarge (AD). As AD is a key event driving ELH secretion from BCN, and thus egg-laying (Kupfermann and Kandel, 1970; Wayne and Wong, 1994), it is a convenient target for reproductive modulation. Overall, our results strongly support a role of ap-GnRH as a modulator of activities in diverse central neurons. Further, ap-GnRH neurons project axons directly to nerves responsible for the motor innervation of the foot and the parapodia, suggesting a direct role in motor control. Lastly, to our surprise, ap-GnRH suppresses the ability of BCN to undergo AD, reflecting an inhibitory role in female reproduction.
Materials and Methods
Animals
Wild-caught A. californica were purchased from Alacrity Marine Biological Services (Redondo Beach, CA, USA) from January to August. All animals were kept in a 400-gal tank of Instant Ocean recirculated through biological and chemical filters. The water temperature was 15–18°C. Animals were fed Romaine lettuce daily. All sexually mature animals received an injection of atrial gland extract, which induces egg-laying (Nagle et al., 1985), to ensure they are competent to lay eggs. All experimental animals were anesthetized humanely by an injection of 1/3 body volume of ice-cold isotonic MgCl2 before sacrifice.
GnRH and other reagents
Synthetic ap-GnRH (pQNYHFSNGWYA-NH2) was manufactured by Genscript (Piscataway, NJ, USA) with >96% purity. All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
ap-GnRH extraction
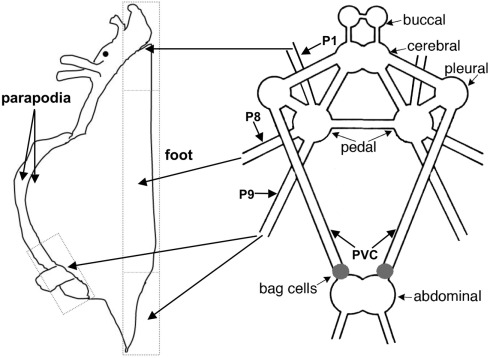
Pedal nerves P1, P8, and P9, and pleurovisceral connective nerve (PVC; Figure 1) were collected from different animals and snap-frozen on dry ice. These three pedal nerves innervate primarily the pedal and parapodial areas (Jahan-Parwar and Fredman, 1978; Kandel, 1979), regions previously shown to be modulated by ap-GnRH (Tsai et al., 2010). The PVC connects the pleural to abdominal ganglia and transmits stimulatory signals to the BCN to elicit AD, but is not known to be motor (Kandel, 1979; Figure 1). Three pairs of the same nerves were pooled and extracted for ap-GnRH as described in Tsai et al. (2010). The extracts were stored at −20°C until the measurement of ap-GnRH by a specific radioimmunoassay (RIA).
Figure 1.
The anatomy of the A. californica CNS and the projection of relevant nerves (P1, P8, and P9) to the foot and parapodia. P1 and P8 project to anterior and middle portions of the foot, respectively, and P9 projects to the posterior portion of the foot and the parapodia.
ap-GnRH iodination and RIA
The iodination of ap-GnRH was performed as previously described (Tsai et al., 2010). The homologous ap-GnRH RIA has been validated previously and found to be highly specific for ap-GnRH (Tsai et al., 2010). The limit of detection and the intra- and inter-assay coefficients of variation were 1.5 pg, 7.1%, and 8.5%, respectively. All samples were assayed in five serially diluted doses.
Retrograde labeling by nickel backfill
For nickel backfill, we employed a procedure modified from Fredman (1987). Briefly, pedal ganglia with right side nerves P1, P8, and P9 were pinned onto a Sylgard-lined dish containing sterile artificial seawater (ASW; 395 mM NaCl, 10 mM KCl, 10 mM CaCl2, 50 mM MgCl2, 28 mM Na2SO4, and 30 mM HEPES) supplemented with 10% hemolymph. Individual nerves were trimmed to a length of about 1–1.5 cm and the cut ends shocked osmotically in distilled water for 20 s. The cut ends were then placed in a chamber containing a nickel–lysine solution (0.38 M NiCl2 and 1.2 M l-lysine). The chamber was sealed with petroleum jelly and the whole ganglia incubated at 15°C for 72 h. The detection of NiCl2 uptake was carried out by incubating the ganglia in ASW containing 0.2% of a saturated dithiooxamide/dimethyl sulfoxide (DMSO) solution at room temperature for 1.5 h. Afterward, the ganglia were pinned and immersion-fixed in Bouin’s fixative overnight and stored in 70% ethanol. Fixed ganglia were dehydrated through ascending ethanol series, cleared in Histoclear II (National Diagnostics, Atlanta, GA, USA), embedded in paraffin, sectioned at 12-μm thickness and mounted on gelatin-subbed slides. Sections were either immediately cleared and coverslipped or processed for ap-GnRH immunocytochemistry (ICC) described below.
ap-GnRH ICC
Immunocytochemistry was performed using a specific ap-GnRH antiserum (AS203-2) on sections of pedal ganglia after nickel backfill. The ICC procedure followed the protocol established previously (Tsai et al., 2003, 2010).
Extracellular recording of BCN
The AD of BCN clusters from sexually mature animals competent to lay eggs was monitored in vitro to determine whether ap-GnRH modulated BCN activity (Zhang et al., 2000). Briefly, left and right BCN clusters with PVC attached were excised separately. AD was stimulated and recorded with suction electrodes placed on the PVC and distal BCN, respectively. In each study, one of the paired BCN clusters received vehicle (Veh) treatment (0.0002% DMSO in ASW), and one received ap-GnRH treatment (73 nM) for 30 min. The application of ap-GnRH at 73 nM was based on an earlier report in which 100 nM GnRH2 elicited an inhibitory effect on the duration of bag cell AD (Zhang et al., 2000). Treatments were randomized between experiments so Veh and ap-GnRH treatments were applied to right and left BCN clusters the same number of times. After treatment, AD was initiated by a Grass S44 stimulator using the following stimulus parameters: 10 V at 6 Hz and 2.5 ms duration for 30 s. BCN clusters were stimulated at an interval of 30 s until an AD was triggered. If no AD was triggered after five stimuli, BCN clusters were left to recover for 30 min before the second round of stimulus using the same protocol. BCN clusters failing to respond after the second round were judged as non-responsive. Compound action potentials (AP) in an AD were monitored by a Grass amplifier interfaced with LabScribe2 software (iWorx Systems, Dover, NH, USA).
Intracellular recordings
The ganglia were glued to a glass coverslip and anchored to the floor of a Sylgard-coated recording chamber. The connective tissue surrounding the ganglia was manually desheathed to expose the neurons. Intracellular recordings were obtained with glass microelectrodes filled with 1.5 M KCl (resistance = 10–20 MΩ). Signals were amplified and acquired with an AxoClamp 2B intracellular amplifier interfaced with LabScribe2 software. Only neurons with a resting membrane potential of −40 mV or lower, indicative of successful penetration and healthy cells, were included in the data analysis. All neurons recorded were noted for their relative sizes and for positions in the ganglia as superior, inferior, medial, lateral, dorsal, or ventral. These positions were used in our analysis to avoid the repeated inclusion of the same neurons from multiple recordings. Injections of depolarizing current were delivered with a S48 Grass stimulator to test evoked AP. A bridge-balanced circuit was used to adjust for the stimulus-induced voltage artifact.
After a successful penetration, the neuron was monitored for a few min until a stable voltage reading was obtained. Membrane potential was monitored during the following treatment sequence: Bath application of 0.002% DMSO (ap-GnRH vehicle) for 10 min, followed by perfusion with ASW at 7 μl/s for 15 min to wash out the Veh, followed by the bath application of 730 nM ap-GnRH for 10 min. The choice of 730 nM ap-GnRH was based on an earlier study (Goldberg et al., 1993) in which low micromolar range of mammalian GnRH (mGnRH) was shown to elicit diverse electrophysiological effects on central neurons of the pond snail, Helisoma trivolvis. At the end of 10-min Veh or ap-GnRH treatment period, a 2-s depolarizing current of 0.01, 0.1, and 1 nA was injected at 10-s intervals to test neuronal excitability. In some recordings, a second perfusion was performed to examine whether the effects of ap-GnRH could be washed out.
Analysis of intracellular electrophysiological recordings
A neuron was considered responsive to ap-GnRH if it exhibited one of the following three effects. First, an increase or a decrease of over 30% in maximum firing rate (MFR) was considered as excitatory or inhibitory, respectively. To assess MFR, the whole recording period (10 min) with either Veh or ap-GnRH treatment was divided into five blocks (2 min/block), and the spike number from a 10-s recording segment with the maximum firing frequency in each block counted. The sum of these 5 numbers represents the MFR of each neuron. Second, under ap-GnRH treatment, if a depolarizing current evoked a >15% increase (excitatory) or decrease (inhibitory) in the total number of AP fired within the first 400 ms compared to Veh, the neuron was considered responsive to ap-GnRH. Lastly, after 1 min of treatment, if ap-GnRH resulted in a change of membrane potential over the pre-treatment baseline that was 200% greater than seen with Veh treatment, the neuron was considered responsive.
Statistical analysis
Difference between treatment groups in the duration of AD, the number of compound AP fired during an AD, and the number of stimulations needed to trigger an AD was assessed by the Student’s t-test. Fisher’s exact test was used to determine the difference in percent BCN clusters capable of firing an AD between Veh and ap-GnRH treatments. One way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used to compare the levels of ap-GnRH in nerves. Differences were considered significant when P < 0.05.
Results
ap-GnRH neurons in pedal ganglia projected to nerves innervating the foot and parapodia
Figure 1 shows the anatomy of the A. californica CNS and the projection of relevant nerves (P1, P8, and P9) to the foot and parapodia. To determine if ap-GnRH neurons projected to the primary nerves responsible for the motor innervation of the foot and parapodia, pedal ganglia were labeled simultaneously via P1, P8, and P9 with a retrograde tracer, nickel chloride, followed by ap-GnRH ICC (Figure 2). In Figure 2A, four double-labeled neurons were observed. The nickel chloride labeling could be more clearly visualized in the same four neurons from the adjacent section (Figure 2B), which had not been processed for ap-GnRH ICC. Some ap-GnRH-immunoreactive (ir) neurons not labeled with nickel chloride were also observed (Figure 2A, asterisks), suggesting that not all ap-GnRH neurons projected to these nerves. The positions of four double-labeled neurons were in the superior (s) and medial (m) quadrant of pedal ganglia (Figure 2), consistent with the positions of ap-GnRH-ir neurons previously reported for these ganglia (Tsai et al., 2010). This experiment was repeated in three different animals. A total of 6, 8, and 10 double-labeled neurons were found in different individuals, respectively.
Figure 2.
Double-labeling of ap-GnRH neurons in the pedal ganglia of A. californica by ICC and nickel backfill. (A,B) are adjacent sections. After nickel backfill, sections were processed for nickel color detection with (A) or without (B) ap-GnRH ICC. Superior (s) and medial (m) orientations are denoted in (A). Blue color, nickel backfill; brown color, ap-GnRH immunostaining. The four double-labeled neurons in (A,B) are labeled by arrows, and the same neurons are indicated by arrows of the same shape. In (A,B), *represents ap-GnRH neurons not labeled by nickel. Scale bars = 100 μm.
ap-GnRH peptide was present in efferent motor nerve extracts
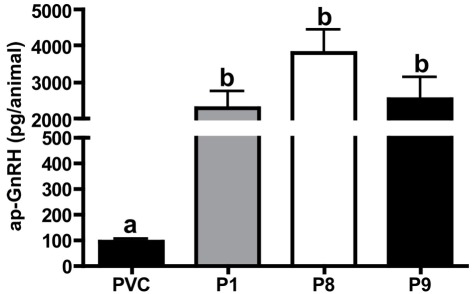
Extracts of nerves P1, P8, or P9, which were known to be motor, contained high levels of ap-GnRH, whereas the extract of PVC, which had no known motor function, had a very low level of ap-GnRH (Figure 3). ap-GnRH level in PVC was significantly lower than that in P1 (P < 0.05), P8 (P < 0.001), and P9 (P < 0.05), but there were no significant differences among extracts from different pedal nerves (Figure 3).
Figure 3.
Quantification of ap-GnRH peptide in nerve extracts by an ap-GnRH RIA. Each bar represents mean ± SEM. N = 5 for PVC, P8, and P9; N = 4 for P1.
ap-GnRH decreased the likelihood of AD in BCN
Using extracellular recording of BCN, we examined if ap-GnRH modulated the characteristics of AD and the ability of BCN to undergo an AD. The application of 73 nM ap-GnRH to BCN clusters did not significantly alter the number of compound AP fired during an AD (Figure 4A), the duration of AD (Figure 4B) and the number of stimulations needed to trigger an AD (Figure 4C) in sexually mature A. californica. However, ap-GnRH significantly decreased the number of responsive BCN clusters capable of initiating an AD under our stimulus protocol (P = 0.0188, Figure 4D).
Figure 4.
Treatment of BCN clusters with 73 nM ap-GnRH did not alter (A) the number of compound AP fired during an AD, (B) the duration of AD or (C) the number of stimulations needed to trigger an AD. (D) 73 nM ap-GnRH decreased the number of bag cell clusters capable of undergoing an AD (P = 0.0188). N = 26 for all groups.
ap-GnRH altered electrophysiological properties of central neurons
A total of 65 neurons have been recorded from buccal, cerebral, pedal, pleural, and abdominal ganglia. 29.2% of the recorded neurons were responsive to ap-GnRH treatment. The responses were diverse and included excitatory, inhibitory, and alteration of resting membrane potential. A summary of the effects was shown in Table 1.
Table 1.
Percentage of effects in the central ganglia in response to the addition of 730 nM ap-GnRH (N = 65).
| Ganglia | Number of recordings | Effects | ||||
|---|---|---|---|---|---|---|
| Excitatory (spikes only, spontaneous, and evoked) (%) | Inhibitory (spikes only, spontaneous, and evoked) (%) | Membrane potential change | Non-responding (%) | |||
| Depolarizing (%) | Hyperpolarizing (%) | |||||
| Pedal | 32 | 6.3 | 15.6 | 3.1 | 3.1 | 71.9 |
| Pleural | 4 | 0 | 0 | 0 | 25 | 75 |
| Cerebral | 6 | 0 | 0 | 0 | 16.7 | 83.3 |
| Abdominal | 15 | 6.7 | 20 | 0 | 20 | 53.3 |
| Buccal | 8 | 0 | 12.5 | 0 | 0 | 87.5 |
| Total | 65 | |||||
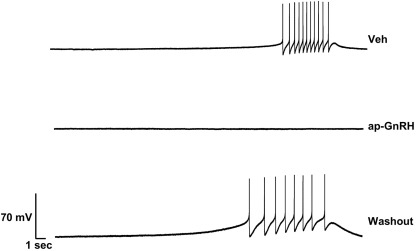
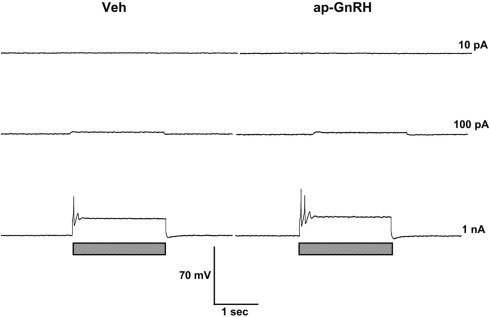
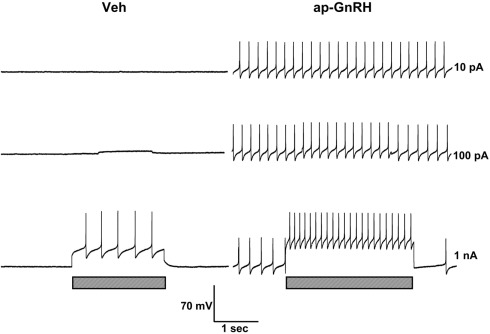
Representative recording traces of different effects of ap-GnRH are shown in Figures 5–8. ap-GnRH (730 nM) inhibited the spontaneous bursting of a neuron located on the inferior quadrant of the right abdominal ganglion (ventral surface; Figure 5). The regular bursting pattern exhibited by this neuron under Veh treatment (Figure 5, top) was suppressed with ap-GnRH (Figure 5, middle) but returned after a washout period (Figure 5, bottom). Not all neurons responded to ap-GnRH. For example, when treated with ap-GnRH, a neuron located on the superior quadrant of the right cerebral ganglion (ventral surface) remained quiescent with no apparent changes in firing pattern after current injections (Figure 6). Next, a neuron located on the superior quadrant of the left pedal ganglion (dorsal surface) was originally quiescent but responded to ap-GnRH with tonic firing and an increased firing rate after depolarization (Figure 7). Lastly, a neuron located on the lateral quadrant of the left pedal ganglion (ventral surface) was undergoing spontaneous tonic firing before treatment (Figure 8, top). The addition of ap-GnRH inhibited AP firing to reveal only the excitatory postsynaptic potential (EPSP; Figure 8, top and middle). The AP firing remained inhibited even after depolarization to a plateau potential by current injection of 1 nA.
Figure 5.
Effect of ap-GnRH on a spontaneously bursting neuron located on the inferior quadrant of the right abdominal ganglion (ventral surface). The addition of 730 nM ap-GnRH inhibited the spontaneous bursting of the neuron, but the activity was recovered after a washout period.
Figure 8.
The inhibitory effect of ap-GnRH on a neuron located on the lateral quadrant of the left pedal ganglion (ventral surface). The addition of 730 nM ap-GnRH inhibited the spontaneous firing (top, middle, and bottom before current injection) and decreased evoked AP when depolarized to a plateau potential (bottom). Left panels represent recording traces of the neuron treated with Veh. Right panels represent the same neuron treated with 730 nM ap-GnRH. Current injections (indicated by hatched bars on bottom) were given at 10 pA (top), 100 pA (middle), and 1 nA (bottom).
Figure 6.
ap-GnRH did not alter the firing pattern and the evoked AP of a neuron located on the superior quadrant of the right cerebral ganglion (ventral surface). Left panels represent recording traces of the neuron treated with Veh. Right panels represent the same neuron treated with 730 nM ap-GnRH. Current injections (indicated by hatched bars on bottom) were given at 10 pA (top), 100 pA (middle), and 1 nA (bottom).
Figure 7.
ap-GnRH was excitatory to a neuron located on the superior quadrant of the left pedal ganglion (dorsal surface). The addition of 730 nM ap-GnRH triggered the spontaneous firing (top, middle, and bottom before current injection) and increased the firing frequency of evoked AP when depolarized to a plateau potential (bottom). Left panels represent recording traces of the neuron treated with Veh. Right panels represent the same neuron treated with 730 nM ap-GnRH. Current injections (indicated by hatched bars on bottom) were given at 10 pA (top), 100 pA (middle), and 1 nA (bottom).
Discussion
Although the structure of multiple non-chordate GnRHs has recently been elucidated, many are still considered peptides in search of a function. At present, the primary physiological roles of these peptides have not yet emerged clearly from the limited functional studies (reviewed in Tsai and Zhang, 2008). The goal of the current study is therefore to fill some of these knowledge gaps by exploring the central functions of ap-GnRH. Our results show that ap-GnRH exerts diverse effects on all central ganglia and is directly positioned for the modulation of foot and parapodial movement. Unexpectedly, ap-GnRH reduces the likelihood of BCN clusters to undergo AD, suggesting an inhibitory effect on female reproduction.
We previously showed that ap-GnRH injection enhanced parapodial opening and substrate attachment in A. californica, all of which could be accomplished by efferent input from the pedal ganglia to the foot and parapodia. In A. californica, the pedal nerve P1 innervates the anterior part of foot, and P8 innervates the middle part of foot. P9 innervates both the posterior parts of the foot and parapodia (Jahan-Parwar and Fredman, 1978). The uptake of nickel chloride by some ap-GnRH neurons from these nerves supported the direct projection of ap-GnRH neurons to these efferent nerves. Interestingly, in A. californica, the majority of motor neurons in the pedal ganglia were located medially and rostrally (Hening et al., 1979), a location consistent with double-labeled neurons found in the present study. Further, motor neurons in A. brasiliana that innervate parapodial muscles to control swimming were also located in the same position of the pedal ganglia (McPherson and Blankenship, 1991). The neuroanatomical overlap between identified motor neurons and ap-GnRH neurons, coupled with the observation that ap-GnRH neurons project to efferent motor nerves (P1, P8, and P9), provided the first evidence that some ap-GnRH neurons may also function as motor neurons.
In addition to directly modulating the effector muscles, ap-GnRH may also affect motor functions via direct or trans-synaptic modulation of motor neurons. Indeed, we have observed a wide range of electrophysiological effects elicited by ap-GnRH within the CNS. A cognate receptor for ap-GnRH has not yet been identified, but the conserved nature of GnRH receptor (GnRHR) family across animal phyla suggests the receptor for ap-GnRH is likely a G-protein coupled receptor (GPCR) that couples to either Gq/11 or Gs and utilizes inositol trisphosphate and diacylglycerol or cAMP as the primary second messengers (Millar et al., 2004). The cascades triggered by these pathways could be rather diverse and could explain the highly divergent effects seen in our intracellular recordings. In support of this notion, a wide range of electrophysiological effects, including excitatory, inhibitory, and alteration of input resistance, had also been observed when central neurons of the pond snails were treated with mGnRH (Goldberg et al., 1993).
The total levels of ap-GnRH in extracts of P1, P8, or P9 exceeded those previously reported for the whole pedal ganglia (3500 pg/animal; Tsai et al., 2010). This raised the question of whether pedal ganglia could supply enough ap-GnRH to the nerves, and whether these nerves synthesized ap-GnRH locally. A similar situation was observed for the pedal peptide (Pep) in A. californica, a peptide present at higher levels in the pedal nerves than the pedal ganglia proper (Hall and Lloyd, 1990; Pearson and Llody, 1990). However, Pep was conventionally synthesized in the pedal ganglia proper and subsequently transported to and accumulated in the nerves. Likewise, ap-GnRH could be accumulated in the nerves in a similar fashion. An interesting dichotomy was that Pep decreased foot tonus to cause foot to lose its grip of the substrate (Hall and Lloyd, 1990), whereas ap-GnRH increased foot tonus to improve substrate attachment (Tsai et al., 2010). It is tantalizing to hypothesize that both ap-GnRH and Pep are present in the same ganglia and nerves to coordinate different actions by pedal motor neurons on the effector muscles.
One way that ap-GnRH could exert the observed electrophysiological effects on the recorded neurons was by the direct modulation of ion channels and current types. In bullfrog sympathetic neurons, a well characterized model for GnRH peptidergic neurotransmission, GnRH2 promoted a late, slow EPSP by inhibiting a voltage-dependent K+ current (Jones, 1987a,b). This enhanced the response to other synaptic inputs, decreased spike frequency adaption and promoted sustained repetitive firing (Jones, 1987b). GnRH2 also partially reduced the N-type Ca2+ current and slowed the activity of the residual current (Elmslie et al., 1990). In addition to direct modulation, ap-GnRH could target the afferents of the recorded neurons to elicit trans-synaptic changes. As the neurocircuits governing movement are complex, it is at present difficult to identify these afferent targets, but the presence of ample sensory–motor connections and interneurons within the central ganglia certainly provides neural substrates for such regulation (Matsumoto et al., 1988; Bley and Tsien, 1990; Kawaski et al., 2007). Overall, ap-GnRH, through both direct and trans-synaptic mechanisms, can generate the diverse responses observed in our intracellular recordings.
In response to a brief stimulation from the cerebral ganglia or PVC, BCN can be depolarized to undergo an AD lasting for up to 30 min (Kupfermann and Kandel, 1970; Wayne and Wong, 1994). AD is a key driver for the release of ELH (Wayne and Wong, 1994), which in turn triggers ovulation and egg-laying behavior. Several peptides have been shown to modulate AD onset by altering ion channels and second messengers (reviewed in Conn and Kaczmarek, 1989). For example, peptide FMRFamide suppressed the initiation of the AD in response to electrical stimulation by decreasing voltage-activated Ca2+ current and by activating an outward current carried by K+ and Cl− (Fisher et al., 1993). Also, bag cell peptides (BCPs) were capable of depolarizing BCN by inducing a voltage-dependent inward Na+ current (Loechner and Kaczmarek, 1994). Further, the second messenger cAMP could facilitate the initiation of an AD (Kaczmarek et al., 1978) in part by decreasing the delayed K+ current and the A current (Kaczmarek and Strumwasser, 1981, 1984). We surmised that ap-GnRH could enhance or interfere with one of these mechanisms to inhibit the initiation of the AD. That said, since our BCN studies were performed in vitro, the overall reproductive impact of ap-GnRH was less clear. Our data suggested that 30.7% of BCN preparations became less likely to undergo an AD when treated with ap-GnRH. If we extrapolated this number to the whole animal, the negative reproductive consequence could be rather significant.
Although the primary role of GnRH in vertebrates is reproductive stimulation, there are ample reports on the non-reproductive roles of GnRH in both chordates and protostomes (Skinner et al., 2009). For example, GnRH modulates odorant response in the olfactory epithelium of the axolotl (Park and Eisthen, 2003) and regulates the development of the eye and brain in the zebrafish (Wu et al., 2006). In the O. vulgaris, GnRH promotes gonadal steroid synthesis and oviducal contraction (Iwakoshi-Ukena et al., 2004; Kanda et al., 2006), but also appears to be involved in movement and postural regulation unrelated to reproduction (Minakata et al., 2009). The diverse effects elicited by GnRH suggest the evolutionary trajectory of GnRH as a multifunctional neuropeptide.
In sum, the present study provides compelling evidence for a central role of ap-GnRH with links to motor modulation. Further, the negative effect of ap-GnRH on AD onset continues to support our previous views that ap-GnRH is not stimulatory to either BCN activity (Zhang et al., 2000) or egg-laying (Tsai et al., 2010). As such, we believe that the function of ap-GnRH, at least in A. californica, is not directed primarily toward reproductive stimulation. The direct projection of some ap-GnRH neurons to efferent motor nerves, the exceptional accumulation of ap-GnRH peptide in these nerves, and the ability of ap-GnRH to alter electrophysiological properties of diverse central neurons all point to what appear to be unconventional alternatives for the primary functions of this peptide.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We appreciate Dr. Janet Casagrand’s technical assistance on electrophysiological recordings, and Dr. Sue Moenter’s helpful comments on data interpretation. This work was supported by NSF grant IOS 0743818 to Pei-San Tsai.
References
- Bley K. R., Tsien R. W. (1990). Inhibition of Ca2+ and K+ channels in sympathetic neurons by neuropeptides and other ganglionic transmitters. Neuron 4, 379–391 10.1016/0896-6273(90)90050-P [DOI] [PubMed] [Google Scholar]
- Conn P. J., Kaczmarek L. K. (1989). The bag cell neurons of Aplysia. Mol. Neurobiol. 3, 237–273 10.1007/BF02740607 [DOI] [PubMed] [Google Scholar]
- Di Cosmo A., Di Cristo C. (1998). Neuropeptidergic control of the optic gland of Octopus vulgaris: FMRF-amide and GnRH immunoreactivity. J. Comp. Neurol. 398, 1–12 [DOI] [PubMed] [Google Scholar]
- Di Cristo C., De Lisa E., Di Cosmo A. (2009). GnRH in the brain and ovary of Sepia officinalis. Peptides 30, 531–537 [DOI] [PubMed] [Google Scholar]
- Di Cristo C., Di Cosmo A. (2007). Neuropeptidergic control of Octopus oviducal gland. Peptides 28, 163–168 10.1016/j.peptides.2006.09.016 [DOI] [PubMed] [Google Scholar]
- Di Cristo C., Paolucci M., Iglesias J., Sanchez J., Di Cosmo A. (2002). Presence of two neuropeptides in the fusiform ganglion and reproductive ducts of Octopus vulgaris: FMRFamide and gonadotropin-releasing hormone (GnRH). J. Exp. Zool. 292, 267–276 [DOI] [PubMed] [Google Scholar]
- Elmslie K. S., Zhou W., Jones S. W. (1990). LHRH and GTP-Γ-S modify calcium current activation in bullfrog sympathetic neurons. Neuron 5, 75–80 10.1016/0896-6273(90)90035-E [DOI] [PubMed] [Google Scholar]
- Fisher T., Lin C. H., Kaczmarek L. K. (1993). The peptide FMRFa terminates a discharge in Aplysia bag cell neurons by modulating calcium, potassium, and chloride conductances. J. Neurophysiol. 69, 2164–2173 [DOI] [PubMed] [Google Scholar]
- Fredman S. M. (1987). Intracellular staining of neurons with nickel-lysine. J. Neurosci. Methods 20, 181–194 10.1016/0165-0270(87)90050-1 [DOI] [PubMed] [Google Scholar]
- Goldberg J. I., Garofalo R., Price C. J., Chang J. P. (1993). Presence and biological activity of a GnRH-like factor in the nervous system of Helisoma trivolvis. J. Comp. Neurol. 336, 571–582 10.1002/cne.903360409 [DOI] [PubMed] [Google Scholar]
- Hall J. D., Lloyd P. E. (1990). Involvement of pedal peptide in locomotion in Aplysia: modulation of foot muscle contraction. J. Neurobiol. 21, 858–868 10.1002/neu.480210604 [DOI] [PubMed] [Google Scholar]
- Hening W. A., Walters E. T., Carew T. J., Kandel E. R. (1979). Motorneuronal control of locomotion in Aplysia. Brain Res. 179, 231–253 10.1016/0006-8993(79)90441-4 [DOI] [PubMed] [Google Scholar]
- Iwakoshi E., Takuwa-Kuroda K., Fujisawa Y., Hisada M., Ukena K., Tsutsui K., Minakata H. (2002). Isolation and characterization of a GnRH-like peptide from Octopus vulgaris. Biochem. Biophys. Res. Commun. 291, 1187–1193 10.1006/bbrc.2002.6594 [DOI] [PubMed] [Google Scholar]
- Iwakoshi-Ukena E., Ukena K., Takuwa-Kuroda K., Kanda A., Tsutsui K., Minakata H. (2004). Expression and distribution of octopus gonadotropin-releasing hormone in the central nervous system and peripheral organs of the octopus (Octopus vulgaris) by in situ hybridization and immunohistochemistry. J. Comp. Neurol. 477, 310–323 10.1002/cne.20260 [DOI] [PubMed] [Google Scholar]
- Jahan-Parwar B., Fredman S. M. (1978). Control of pedal and parapodial movements in Aplysia. I. Proprioceptive and tactile reflexes. J. Neurophysiol. 41, 600–608 [DOI] [PubMed] [Google Scholar]
- Jones S. W. (1987a). Chicken II luteinizing hormone-releasing hormone inhibits the M-current of bullfrog sympathetic neurons. Neurosci. Lett. 80, 180–184 10.1016/0304-3940(87)90650-1 [DOI] [PubMed] [Google Scholar]
- Jones S. W. (1987b). Luteinizing hormone-releasing hormone as a neurotransmitter in bullfrog sympathetic ganglia. Ann. N. Y. Acad. Sci. 519, 310–322 10.1111/j.1749-6632.1987.tb36306.x [DOI] [PubMed] [Google Scholar]
- Kaczmarek L. K., Jennings K., Strumwasser F. (1978). Neurotransmitter modulation, phosphodiesterase inhibitor effects, and cyclic AMP correlates of afterdischarge in peptidergic neuritis. Proc. Natl. Acad. Sci. U.S.A. 75, 5200–5204 10.1073/pnas.75.10.5200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek L. K., Strumwasser F. (1981). The expression of long lasting afterdischarge by isolated Aplysia bag cell neurons. J. Neurosci. 1, 626–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek L. K., Strumwasser F. (1984). A voltage-clamp analysis of currents underlying cyclic AMP-induced membrane modulation in isolated peptidergic neurons of Aplysia. J. Neurophysiol. 52, 340–349 [DOI] [PubMed] [Google Scholar]
- Kah O., Lethimonier C., Somoza G., Guilgur L. G., Vaillant C., Lareyre J. J. (2007). GnRH and GnRH receptors in metazoa: a historical, comparative, and evolutive perspective. Gen. Comp. Endocrinol. 153, 346–364 10.1016/j.ygcen.2007.01.030 [DOI] [PubMed] [Google Scholar]
- Kanda A., Takahashi T., Satake H., Minakata H. (2006). Molecular and functional characterization of a novel gonadotropin-releasing-hormone receptor isolated from the common octopus (Octopus vulgaris). Biochem. J. 395, 125–135 10.1042/BJ20051615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. (1979). Behavioral Biology of Aplysia. San Francisco: W. H. Freeman and Company [Google Scholar]
- Kawaski S., Kimura S., Watanabe S., Fujita R., Matsumoto M., Sasaki K. (2007). Augmenting effect of serotonin on the voltage-dependent Ca2+ current and subsequently activated K+ current in Aplysia neurons. Tohoku J. Exp. Med. 211, 31–41 10.1620/tjem.211.31 [DOI] [PubMed] [Google Scholar]
- Kupfermann I., Kandel E. R. (1970). Electrophysiological properties and functional interconnections of two symmetrical neurosecretory clusters (bag cells) in abdominal ganglion of Aplysia. J. Neurophysiol. 33, 865–876 [DOI] [PubMed] [Google Scholar]
- Loechner K. J., Kaczmarek L. K. (1994). Autoactive peptides act at three distinct receptors to depolarize the bag cell neurons of Aplysia. J. Neurophysiol. 71, 195–203 [DOI] [PubMed] [Google Scholar]
- Matsumoto M., Sasaki K., Sato M., Shozushima M., Takashima K. (1988). Dopamine-induced depolarizing responses associated with negative slope conductance in LB-cluster neurons of Aplysia. J. Physiol. 407, 199–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson D. R., Blankenship J. E. (1991). Neural control of swimming in Aplysia brasiliana. I. Innervation of parapodial muscle by pedal ganglion motoneurons. J. Neurophysiol. 66, 1338–1351 [DOI] [PubMed] [Google Scholar]
- Millar R. P., Lu Z. L., Pawson A. J., Flanagan C. A., Morgan K., Maudsley S. R. (2004). Gonadotropin-releasing hormone receptors. Endocr. Rev. 25, 235–275 10.1385/ENDO:25:3:235 [DOI] [PubMed] [Google Scholar]
- Minakata H., Shigeno S., Kano N., Haraguchi S., Osugi T., Tsutsui K. (2009). Octopus gonadotropin-releasing hormone: a multifunctional peptide in the endocrine and nervous systems of the cephalopod. J. Neuroendocrinol. 21, 322–326 10.1111/j.1365-2826.2009.01852.x [DOI] [PubMed] [Google Scholar]
- Nagle G. T., Painter S. D., Kelner K. L., Blankenship J. E. (1985). Atrial gland cells synthesize a family of peptides that can induce egg laying in Aplysia. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 156, 43–55 10.1007/BF00692925 [DOI] [PubMed] [Google Scholar]
- Onitsuka C., Yamaguchi A., Kanamaru H., Oikawa S., Takeda T., Matsuyama M. (2009). Molecular cloning and expression analysis of a GnRH-like dodecapeptide in the swordtip squid, Loligo edulis. Zool. Sci. 26, 203–208 10.2108/zsj.26.203 [DOI] [PubMed] [Google Scholar]
- Park D., Eisthen H. L. (2003). Gonadotropin releasing hormone (GnRH) modulates odorant responses in the peripheral olfactory system of axolotls. J. Neurophysiol. 90, 731–738 10.1152/jn.01162.2002 [DOI] [PubMed] [Google Scholar]
- Pearson W. L., Llody P. E. (1990). Distribution and characterization of pedal peptide immunoreactivity in Aplysia. J. Neurobiol. 21, 883–892 10.1002/neu.480210606 [DOI] [PubMed] [Google Scholar]
- Roch G. J., Busby E. R., Sherwood N. M. (2011). Evolution of GnRH: diving deeper. Gen. Comp. Endocrinol. 171, 1–16 10.1016/j.ygcen.2010.12.014 [DOI] [PubMed] [Google Scholar]
- Skinner D. C., Albertson A. J., Navratil A., Smith A., Mignot M., Talbott H., Scanlan-Blake N. (2009). Effects of gonadotropin-releasing hormone outside the hypothalamic-pituitary-reproductive axis. J. Neuroendocrinol. 21, 282–292 10.1111/j.1365-2826.2009.01842.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somoza G. M., Miranda L. A., Strobl-Mazzulla P., Guilgur L. G. (2002). Gonadotropin-releasing hormone (GnRH): from fish to mammalian brains. Cell. Mol. Neurobiol. 22, 589–609 10.1023/A:1021888420271 [DOI] [PubMed] [Google Scholar]
- Tsai P. S., Maldonado T. A., Lunden J. B. (2003). Localization of gonadotropin-releasing hormone in the central nervous system and a peripheral chemosensory organ of Aplysia californica. Gen. Comp. Endocrinol. 130, 20–28 10.1016/S0016-6480(02)00519-1 [DOI] [PubMed] [Google Scholar]
- Tsai P. S., Sun B., Rochester J. R., Wayne N. L. (2010). Gonadotropin-releasing hormone-like molecule is not an acute reproductive activator in the gastropod, Aplysia californica. Gen. Comp. Endocrinol. 166, 280–288 10.1016/j.ygcen.2009.09.009 [DOI] [PubMed] [Google Scholar]
- Tsai P. S., Zhang L. (2008). The emergence and loss of gonadotropin-releasing hormone in protostomes: orthology, phylogeny, structure, and function. Biol. Reprod. 79, 798–805 10.1095/biolreprod.108.070185 [DOI] [PubMed] [Google Scholar]
- Wayne N. L., Wong H. (1994). Persistence of hormone secretion from neuroendocrine cells of Aplysia after termination of electrical after discharge. Endocrinology 134, 1046–1054 10.1210/en.134.3.1046 [DOI] [PubMed] [Google Scholar]
- Wu S., Page L., Sherwood N. M. (2006). A role for GnRH in early brain regionalization and eye development in zebra fish. Mol. Cell. Endocrinol. 257–258, 47–64. [DOI] [PubMed] [Google Scholar]
- Zhang L., Tello J. A., Zhang W., Tsai P. S. (2008). Molecular cloning, expression pattern, and immunocytochemical localization of a gonadotropin-releasing hormone-like molecule in the gastropod mollusk, Aplysia californica. Gen. Comp. Endocrinol. 156, 201–209 10.1016/j.ygcen.2007.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wayne N. L., Sherwood N. M., Postigo H. R., Tsai P. S. (2000). Biological and immunological characterization of multiple GnRH in an opisthobranch mollusk, Aplysia californica. Gen. Comp. Endocrinol. 118, 77–89 10.1006/gcen.2000.7457 [DOI] [PubMed] [Google Scholar]