Abstract
Fine-needle aspiration biopsy is regarded as an important tool for diagnosing thyroid lesions because of its simplicity, safety, and cost–effectiveness. Its role in correctly characterizing the group of indeterminate lesions or follicular-patterned neoplasms (FN) might be more decisive. Liquid-based cytology (LBC) is a technique based on the use of a semi-automated device that has gained popularity as a method of collecting and processing both gynecologic and non-gynecologic cytologic specimens. It achieves a diagnostic sensitivity as accurate as conventional preparations especially for its excellent cell preservation and for the lack of background which decrease the amount of inadequate diagnoses. Moreover, the cellular material which has been stored in the preservative solution could be effectively used for the application of immunocytochemical and molecular techniques especially for the Follicular proliferations. In many cases the cytologic features are similar in both methods but the colloid film and the lymphocytic component are more easily evaluated on direct smears whereas nuclear details and colloid globules are better evaluated in LBC slides. The LBC-processed biopsies represent a valid alternative to conventional cytology. The possibility of applying special techniques enhance the efficacy of the cytological diagnosis of thyroid lesions.
Keywords: thyroid nodules, fine-needle aspiration cytology, liquid-based cytology
Introduction
Fine-needle aspiration biopsy (FNAB) represents an invaluable diagnostic tool for characterizing thyroid nodules with a worldwide consensus for its simplicity, safety (Galera-Davidson and Gonzalez-Campora, 2008), and regarded as the most accurate and cost–effective method for the selection of surgical patients.
The liquid-based cytology (LBC) technique, originally developed for application to gynecologic cervical smears, has progressively gained consensus for both non-gynecologic and fine-needle aspiration cytological material (Biscotti et al., 1995; Rossi and Fadda, 2008; Rossi et al., 2009).
This method is based on a two-step procedure: (1) the fixation of the material in an methanol-based solution and (2) the automated processing of the material to obtain a thin layer of cells with a computer-assisted device. The two most common methods for processing the cytologic samples are: ThinPrep2000™ (Hologic Co., Marlborough, MA, USA), in which the cells are aspirated from a methanol-based solution (Cytolyt™) then filtered and transferred onto a positively charged slide with a gentle positive pressure; in the method SurePath™ (TriPath Imaging, Burlington, NC, USA) the cells are collected in an ethanol-based solution (CytoRich™), centrifuged twice then slowly sedimentated onto a poly-l-lysinated slide and eventually stained with a specific hematoxylin–eosin stain. The final result for both methods is one slide for each lesion with all cells concentrated in the central area of the slide with a sensitivity of 77% and a specificity of 81% (Geers and Bourgain, 2011).
Despite the initial controversy regarding the efficacy of the use of Thin Prep alone (Cochand-Priollet et al., 2003; Fadda et al., 2006; Rossi et al., 2010) good results have been achieved by many groups in different countries, especially in the recent years. Since November 2003 until 2011 the majority of about 22,000 FNABs carried out in the “Agostino Gemelli” School of Medicine and Hospital of Rome have been processed by ThinPrep2000™ alone. This experience has been reported in many studies published since 2005 where the efficacy of the ThinPrep2000™ technique for a correct pre-operative diagnosis of more than 500 malignant neoplasms is highlighted. In the study by Rossi et al. (2009), three parameters of efficacy (inadequacy, indeterminacy, and malignancy rates) were chosen for evaluating the efficacy of ThinPrep2000™ in comparison with CS alone and combining ThinPrep2000™ and CS in more than 10,000 thyroid FNAB showing that ThinPrep2000™ alone was as effective as CS in decreasing both inadequate and indeterminate diagnoses (Fadda et al., 2011b). The study of Geers and Bourgain (2011) using the SurePath method achieves controversial results in terms of inadequacy rate between LBC and SurePath.
The ThinPrep2000™ material stored in the vial can be used for additional techniques such as immunocytochemistry, flow cytometry, and molecular biology (Cochand-Priollet et al., 2003; Rossi et al., 2005).
Cytology of LBC-Processed Thyroid Lesions
Benign lesions
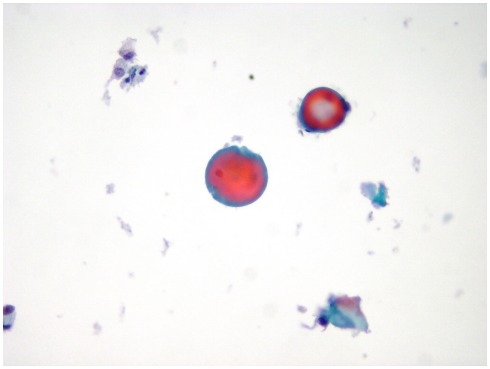
The morphologic picture of LBC differs mainly from CS in two aspects: (a) the cells in each slide are a monolayered representative sample of the entire material collected in the vial with a variable amount of cells which remains in the preservative solution; (b) the automated process causes some changes in both cellular and background morphology. One of the most important change, occurring in LBC slides, is the appearance of the fragmented colloid which is present as small droplets in the background of a benign nodule with a quantitative detection whereas in CS the colloid usually does not require a quantization (Figure 1; Biscotti et al., 1995). The LBC picture of a thyroiditis is similar to CS with the exception of the amount of lymphocytes in the background which can be higher than normal because of the spinning of the material before the automated process. When a thyroiditis is suspected, the detection of lympho-epithelial clusters in an inflammatory background is the pivotal clue for the diagnosis and warrants a simple follow-up for the patient (Das, 2006).
Figure 1.
A case of colloid goiter exhibiting small clusters of monomorphic follicular cells mixed with colloid droplets (LBC, Papanicolaou, 400×).
Follicular-patterned lesions
There are very few differences in the cytologic pictures of follicular neoplasms (FN) in LBC versus CS based upon the identification of microfollicles made up of medium-sized thyrocytes in scant colloid. The amount and morphology of the follicular cells allow the inclusion of an individual lesion in one of the categories that have been recently devised in Europe and in the USA with three possible scenario (British Thyroid Association, 2007; Baloch et al., 2008; Fadda et al., 2010):
-
(1)
The first scenario is represented by a lesion which high cellularity but the cells are monomorphous with occasional enlarged nuclei. This lesion may correspond to the cellular adenomatous nodule and is usually included in the non-neoplastic category of the European classifications (Thy 2 by BTA classification, TIR 2 by Italian classification,). On the other hand, the Bethesda classification has established a different category for this picture which is defined as “follicular lesion with undetermined significance (FLUS)” or “atypical cells of undetermined significance (ACUS or AUS)” with a different risk of malignant occurrence which in the American classification is stated between 5 and 15% (mostly follicular carcinoma) whereas in the European systems is closer to non-neoplastic lesions (British Thyroid Association, 2007; Baloch et al., 2008; Fadda et al., 2010).
-
(2)
The second scenario is represented by a lesion mostly follicular-structured and made up of medium-sized thyrocytes with rounded nuclei and central nucleolus (Follicular Neoplasm) with a malignancy risk between 20 and 30%. The action, although debated in the literature, results in the surgical removal of the lesion which could histologically correspond to both a follicular adenoma or an adenomatous nodule in a goiter (70–80% of cases) but also follicular carcinoma or a follicular variant of a papillary carcinoma (PC) cannot be ruled out only on morphology. The same diagnostic criteria and therapeutic action is applied to the FN composed mostly by oxyphilic (or Hurthle) cells which is defined when follicles are made up of more than 80% of oxyphilic cells and it should be included in the FN category (Thy 3 of the BTA, TIR 3 of the Italian classifications). The colloid amount may be scant (but sometimes is abundant) and features of old hemorrhage (hemosiderin-laden histiocytes) may coexist (Giorgadze et al., 2004; British Thyroid Association, 2007; Baloch et al., 2008).
-
(3)
The third scenario is represented by follicular-structured lesions, composed by thyrocytes with elongated and clear nuclei, sometimes with grooves and peripheral nucleoli without papillae, psammomatous bodies, or nuclear pseudoinclusions with a risk of malignancy ranging between 50 and 70%. This category warrants the surgical removal of the nodule as a follicular variant of a PC is very likely to be found at the histological examination (more than 90% of cases).
Malignant tumors
The cytological diagnosis of thyroid malignancy does not differ substantially in LBC preparations as the clear background facilitates the identification and characterization of the cellular details.
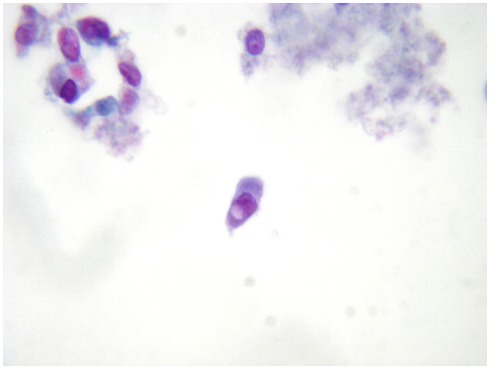
The most important malignant tumor which can be appropriately identified on LBC preparations is PC which is easily when nuclear pseudoinclusions are detected (Figure 2) papillary structures and psammoma bodies are seldom identified. In the earlier reports, the difficulty in detecting the distinctive nuclear features of PC was one of the most important objections against the adoption of the thyroid LBC cytology.
Figure 2.
A distinctive nuclear pseudoinclusion in a fine-needle aspiration biopsy from a thyroid papillary carcinoma (LBC, Papanicolau, 400×).
Medullary thyroid carcinoma (MTC) is a difficult cytological diagnosis. The LBC technique offers the opportunity to detect the calcitonin expression in the neoplastic parafollicular cells and their concomitant negativity for thyroglobulin (Rossi et al., 2008).
Anaplastic thyroid carcinoma (ATC) is seldom seen in routine thyroid cytology. The LBC picture of ATC usually shows a background of necrotic debris with small clusters of large round or spindle cells with pleomorphic nuclei and prominent nucleoli which stains positive for cytokeratins (useful to confirm the epithelial origin) and negative for thyroglobulin and TTF-1. The LBC diagnosis of the large cell variant of malignant non-Hodgkin lymphoma usually does not constitute a problem and relies on the immunocytochemical expression of LCA, CD20, bcl-6, and other lymphoid antigens.
Lung, breast, kidney, large bowel, and laryngeal metastatic carcinomas to the thyroid gland may occasionally present as a single nodule mimicking a primary tumor in which necrotic debris or hemorrhagic material and clusters of neoplastic cells with features of adenocarcinoma or squamous cell carcinoma are detected on Cs and ThinPrep2000™
Special Techniques
Immunocytochemistry
Immunocytochemistry was introduced in the early 1970s for the definition of the nature of the lesions, especially in the differential diagnosis between follicular and C-cell derived neoplasms and in the identification of primary or metastatic thyroid neoplasms (e.g., malignant lymphoma) even if the main role is the identification of the markers of malignancy which may distinguish malignant from benign lesions regardless of the presence of capsular and vascular invasion (Rossi et al., 2006).
There have been only few reported experiences in literature dealing with ICC applied on ThinPrep2000™. The use of ICC on the cells stored in the preservative ThinPrep2000™ solution yields excellent results with most immunoreagents in terms of staining pattern, intensity of the reaction and less amount of reagent due to the clear background and smaller size of the ThinPrep2000™ slide (Dabbs et al., 1997; Leung and Bedard, 1999; Rossi et al., 2006).
In our practice and based on the data in the literature, no “magic single marker” may be useful but only the concordance of a panel should be considered especially in cases of follicular lesions. The use of more than one immunomarker is a further guarantee for a correct diagnostic approach, especially when a concordant panel is expressed (Rossi et al., 2006) and with excellent results also in the differential diagnosis between benign and malignant follicular neoplasms (Dabbs et al., 1997; Leung and Bedard, 1999; Schmitt et al., 2008; Fadda et al., 2011a).
HBME-1, Galectin-3, and RET proto-oncogene have shown the best specificity and sensitivity in discriminating benign from malignant differentiated tumors. These data emerged from one of the paper of our group, in which the combination of nuclear pleomorphism and positivity of the panel resulted in 75% specificity and 89% diagnostic accuracy of FNAB (Rossi et al., 2005). In another study recently published by our group, the complete immunocytochemical panel (made up of HBME-1 and Galectin-3) was positive in 83.3% of malignancy and negative in 87.5% benign histological cases. In the group of FN/AUS (according to the Bethesda system), the expression of HBME-1 and Galectin-3 on LBC can effectively distinguish lesions which need immediate surgery (high risk FN) from those which can be followed-up (low risk FN).
Molecular techniques
Recent advances in molecular genetics of thyroid cancer are being applied for developing new diagnostic markers for FNA samples in an attempt to differentiate benign from malignant thyroid nodules (Nikiforova and Nikiforov, 2009). PC, the most common thyroid malignancy, may carry BRAF, Ret/PTC, or RAS mutations (Nikiforova and Nikiforov, 2009). These mutually exclusive somatic mutations are found in more than 70% of PCs with a more aggressive tumor behavior such as extra-thyroidal extension, advanced tumor stage at presentation and lymph node or distant metastases (Xing, 2007).
Several studies have demonstrated the feasibility of detecting BRAF, RET/PTC, or RAS mutation in thyroid FNA samples and have shown that this may improve the cytological FNA diagnosis (Nikiforova and Nikiforov, 2009; Ohori et al., 2010; Fadda et al., 2011b).
A recent paper by Nikiforova and Nikiforov (2009) explored the diagnostic utility of molecular testing for a panel of molecular mutations consisting on BRAF, RAS, RET/PTC, and PAX8–PPARγ in 480 FNA samples from thyroid nodules which were prospectively tested and yielded 32 mutations with 31 malignant surgical diagnoses and only one case of follicular adenoma.
The possible diagnostic use of molecular markers is reflected in the last guidelines published by the American Thyroid Association. These guidelines indicate that the use of molecular markers such as BRAF, RAS, RET/PTC, and PAX8–PPARγ may be considered (with low recommendation rate) for patients with indeterminate FNA cytology to help guide their clinical management.
As resulted in our preliminary study the evaluation of BRAF positivity could be a prognostic value for the presence of nodal metastases and the presence of BRAF positivity performances to the surgical neck central dissection and also to the detection of any other nodal possible implication (Fadda et al., 2011b).
Liquid-based cytology can offer the possibility of immunocytochemical evaluation and molecular testings for common somatic mutations in thyroid FNAB as the nucleic acids are stable in the preservative solution up to 6 months after the sampling. In this setting, the future possibility of a guideline encompassing the combined use of immunocytochemistry and molecular tests for supplementing the morphologic diagnosis could be the starting point for a complete pre-operative assessment of a thyroid lesion.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Baloch Z. W., LiVolsi V. A., Asa S. L., Rosai J., Merino M. J., Randolph G., Vielh P., DeMay R. M., Sidawy M. K., Frable W. J. (2008). Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Fine-Needle Aspiration State-of-Science Conference. Diagn. Cytopathol. 36, 425–437 10.1002/dc.20830 [DOI] [PubMed] [Google Scholar]
- Biscotti C. V., Hollow J. A., Toddy S. M., Easly K. A. (1995). Thin prep versus conventional smear cytological preparations in the analysis of thyroid fine-needle aspiration specimens. Am. J. Clin. Pathol. 104, 150–153 [DOI] [PubMed] [Google Scholar]
- British Thyroid Association. (2007). Guidelines for the Management of Thyroid Cancer, 2nd Edn Suffolk: The Lavenham Press [Google Scholar]
- Cochand-Priollet B., Prat J. J., Polivka M., Thienpont L., Dahan H., Wassef M., Guillauseau P. J. (2003). Thyroid fine needle aspiration: the morphological features on ThinPrep slide preparations. Eighty cases with histological control. Cytopathology 14, 343–349 [DOI] [PubMed] [Google Scholar]
- Dabbs D. J., Abendroth C. S., Grenko R. T., Wang X., Radcliffe G. E. (1997). Immunocytochemistry on the ThinPrep processor. Diagn. Cytopathol. 17, 388–392 [DOI] [PubMed] [Google Scholar]
- Das D. K. (2006). Marginal vacuoles (fire-flare appearance) in fine needle aspiration smears of thyroid lesions: does it represent diffusing out of thyroid hormones at the base of follicular cells? Diagn. Cytopathol. 34, 277–283 10.1002/dc.20416 [DOI] [PubMed] [Google Scholar]
- Fadda G., Basolo F., Bondi A., Bussolati G., Crescenzi A., Nappi O., Nardi F., Papotti M., Taddei G., Palombini L., SIAPEC-IAP Italian Consensus Working Group (2010). Cytological classification of thyroid nodules. Proposal of the SIAPEC-IAP Italian Consensus Working Group. Pathologica 102, 405–408 [PubMed] [Google Scholar]
- Fadda G., Rossi E. D., Raffaelli M., Mule A., Pontecorvi A., Miraglia A., Lombardi C. P., and Vecchio F. M. (2006). Fine-Needle aspiration biopsy of thyroid lesions processed by thin-layer cytology: one-year institutional experience with histologic correlation. Thyroid 16, 975–981 10.1089/thy.2006.16.975 [DOI] [PubMed] [Google Scholar]
- Fadda G., Rossi E. D., Raffaelli M., Pontecorvi A., Sioletic S., Morassi F., Lombardi C. P., Zannoni G. F., Rindi G. (2011a). Follicular thyroid neoplasms can be classified as low-and high risk according to HBME-1 and Galectin 3 expression on liquid based fine needle cytology. Eur. J. Endocrinol. 165, 447–453 10.1530/EJE-11-0181 [DOI] [PubMed] [Google Scholar]
- Fadda G., Rossi E. D., Martini M., Zannoni G. F., Vellone V. G., Pontecorvi A., Lombardi C. P., Larocca L. M., Rindi G. (2011b). Braf mutation (V600E) in papillary carcinoma identified on LBC processed thyroid aspiration biopsies. Mod. Pathol. 24, 89A [Google Scholar]
- Galera-Davidson H., Gonzalez-Campora R. (2008). Thyroid in: Bibbo M and Wilbur D: Comprehensive Cytopathology, 3rd Edn, Chap. 23. Philadelphia: Saunders Elsevier, 633–670 [Google Scholar]
- Geers A. J., Bourgain C. (2011). Liquid based FNAC of thyroid: a 4-year survey with Sure Path. Cancer Cytopathol. 119, 58–67 10.1002/cncy.20125 [DOI] [PubMed] [Google Scholar]
- Giorgadze T. A., Rossi E. D., Fadda G., Gupta P. K., LiVolsi V. A., Baloch Z. W. (2004). Does the fine-needle aspiration diagnosis of “Hurthle cell neoplasm/Follicular neoplasm with oncocytic features” denote increased risk of malignancy? Diagn. Cytopathol. 31, 307–312 10.1002/dc.20132 [DOI] [PubMed] [Google Scholar]
- Leung S. W., Bedard Y. C. (1999). Immunocytochemical staining on thin prep processed smears. Mod. Pathol. 9, 304–306 [PubMed] [Google Scholar]
- Nikiforova M. N., Nikiforov Y. (2009). Molecular diagnostics and predictors in thyroid cancer. Thyroid 19, 1351–1361 10.1089/thy.2009.0240 [DOI] [PubMed] [Google Scholar]
- Ohori N. P., Nikiforova M. N., Schoedel K. E., LeBeau S. O., Hodak S. P., Seethala R. R., Carty S. E., Ogilvie J. B., Yip L., Nikiforov Y. E. (2010). Contribution of molecular testing to thyroid fine needle aspiration cytology of “follicular lesion of undetermined significance/Atypia of undetermined significance.” Cancer Cytopathol. 118, 17–23 10.1002/cncy.20063 [DOI] [PubMed] [Google Scholar]
- Rossi E. D., Fadda G. (2008). Thin-layer liquid-based preparation of exfoliative non-gynaecologic and fine-needle aspiration biopsy cytology. Diagn. Histopathol. 14, 563–570 10.1016/j.mpdhp.2008.08.004 [DOI] [Google Scholar]
- Rossi E. D., Morassi F., Santeusanio G., Zannoni G. F., Fadda G. (2010). Thyroid fine-needle aspiration cytology processed by Thin Prep: an additional slide decreased the number of inadequate results. Cytopathology 21, 97–102 10.1111/j.1365-2303.2009.00659.x [DOI] [PubMed] [Google Scholar]
- Rossi E. D., Raffaelli M., Minimo C., Mule A., Lombardi C. P., Vecchio F. M., Fadda G. (2005). Immunocytochemical evaluation of thyroid neoplasms on thin-layer smears from fine-needle aspiration biopsies. Cancer Cytopathol. 105, 87–95 [DOI] [PubMed] [Google Scholar]
- Rossi E. D., Raffaelli M., Mule A., Miraglia A., Lombardi C. P., Vecchio F. M., Fadda G. (2006). Simultaneous immunohistochemical expression of HBME-1 and Galectine 3 differentiates papillary carcinomas from hyperfunctioning lesions of the thyroid. Histopathology 48, 795–800 10.1111/j.1365-2559.2006.02363.x [DOI] [PubMed] [Google Scholar]
- Rossi E. D., Raffaelli M., Mule A., Raffaelli M., Mulè A., Zannoni G. F., Pontecorvi A., Santeusanio G., Minimo C., Fadda G. (2008). Relevance of immunocytochemistry on thin-layer cytology in thyroid lesions suspicious for medullary carcinoma. A case-control study. Appl. Immunohistochem. Mol. Morphol. 16, 548–555 10.1097/PAI.0b013e3181690ca3 [DOI] [PubMed] [Google Scholar]
- Rossi E. D., Raffaelli M., Zannoni G. F., Pontecorvi A., Mule A., Calla C., Lombardi C. P., Fadda G. (2009). Diagnostic efficacy of conventional as compared to liquid-based cytology in thyroid lesions. Evaluation of 10,360 fine needle aspiration cytology cases. Acta Cytol. 53, 659–666 10.1159/000325407 [DOI] [PubMed] [Google Scholar]
- Schmitt F. C., Longatto-Filho A., Valent A., Vielh P. (2008). Molecular techniques in cytopathology practise. J. Clin. Pathol. 61, 257–268 [DOI] [PubMed] [Google Scholar]
- Xing M. (2007). Braf mutationin papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr. Rev. 28, 742–762 10.1210/er.2007-0007 [DOI] [PubMed] [Google Scholar]