Abstract
The ubiquitin proteasome system (UPS) is required for normal cell proliferation, vertebrate development, and cancer cell transformation. The UPS consists of multiple proteins that work in concert to target a protein for degradation via the 26S proteasome. Chains of an 8.5-kDa protein called ubiquitin are attached to substrates, thus allowing recognition by the 26S proteasome. Enzymes called ubiquitin ligases or E3s mediate specific attachment to substrates. Although there are over 600 different ubiquitin ligases, the Skp1–Cullin–F-box (SCF) complexes and the anaphase promoting complex/cyclosome (APC/C) are the most studied. SCF involvement in cancer has been known for some time while APC/C’s cancer role has recently emerged. In this review we will discuss the importance of APC/C to normal cell proliferation and development, underscoring its possible contribution to transformation. We will also examine the hypothesis that modulating a specific interaction of the APC/C may be therapeutically attractive in specific cancer subtypes. Finally, given that the APC/C pathway is relatively new as a cancer target, therapeutic interventions affecting APC/C activity may be beneficial in cancers that are resistant to classical chemotherapy.
Keywords: ubiquitin, cell cycle, differentiation, cancer, ubiquitin ligase, cancer therapy
Introduction
Any discussion of the anaphase promoting complex/cyclosome (APC/C) pathway as a possible therapeutic target has to start with the question of what makes APC/C unique among the many ubiquitin ligases present in human cells. On one level APC/C is mechanistically similar to the Skp1–Cullin–F-box (SCF) ubiquitin ligases where there are core subunits and an adaptor protein that directly binds to a substrate. In SCFs, the four subunits are Skp1 (scaffold protein), Cul1 (scaffold protein), RING-finger component (Rbx1), and the variable adaptor protein or F-box protein that recognizes substrates (Zheng et al., 2002). In APC/C there are 13 subunits as well as variable adaptor proteins termed Cdc20 (or Fizzy; Fzy), Cdh1 (or Fizzy-related; Fzr), Cortex, Ama1, or Mfr1 (Acquaviva and Pines, 2006; Hutchins et al., 2010; Kops et al., 2010). However, beyond these similarities regarding adaptors and subunits present in APC/C and SCF ligases, large differences in size and structure exist between these two types of ligases. By contrast to SCF ubiquitin ligases, there is only limited knowledge regarding APC/C structure mainly because the enormous size and complexity of the holoenzyme present significant challenges for structure determination at the atomic level. The first structural insights into the APC/C was obtained by cryo-EM of complexes purified from human cells, Xenopus laevis egg extracts, and budding yeast (Gieffers et al., 2001; Dube et al., 2005; Passmore et al., 2005). 3D modeling showed that in all cases the APC/C is an asymmetric triangular complex (200 by 230 Å in size), composed of an outer wall and an internal cavity. Cdh1 and the Cullin domain of the Apc2 subunit are located on the outside of the complex, making it plausible that ubiquitination reactions occur on the outside and not inside the cavity. An emerging view of the APC/C is that of a four-part enzyme composed of a structural arm or scaffolding unit made of Apc1, Apc4, and Apc5, a catalytic arm consisting of Apc2, Apc11, and Doc1 (or Apc10), a tetratricopeptide repeat (TPR) arm made of Cdc23, Cdc16, and Cdc27, which mediates binding to activators and coactivators (Cdc20, Cdh1, Cortex). Other subunits such as Cdc26, Apc9, and Swm1 stabilize the TPR arm (Schwickart et al., 2004; Thornton and Toczyski, 2006). The TPR subunits have 12–15 copies of the 34-amino acid long TPR. They facilitate interactions between subunits and the assembly of multisubunit complexes (Zachariae and Nasmyth, 1999). A pseudo-atomic model of the yeast APC/C obtained by reconstitution studies of the holoenzyme and its subcomplexes has revealed that the TPR arm along with the structural arm coordinate the juxtaposition of the catalytic arm and the TPR phosphorylation sites relative to the coactivators, substrates, and regulators (Schreiber et al., 2011).
A previously unidentified APC/C subunit, Apc16, was reported recently (Hutchins et al., 2010; Kops et al., 2010). It is a small protein of 11.7 kDa in size encoded by Chromosome 10 open reading frame 104 (C10orf104) in humans. Apc16 may facilitate Cdc27 hyperphosphorylation, although it is not essential for assembly of the holocomplex (Kops et al., 2010). Therefore, the APC/C is composed of multiple subunits, some of which are newly discovered. However, the minimum ubiquitin ligase module of the APC/C that can catalyze ubiquitination is comprised of just two subunits – the Apc2 Cullin subunit and the Apc11 RING subunit (Gmachl et al., 2000; Leverson et al., 2000; Tang et al., 2001), which are analogous to the Cullin and Rbx1 subunits of the SCF complex (Barford, 2011). Thus, while we still do not have a complete understanding of APC/C structure, we are beginning to understand the general architecture of the complex, and possibly achieve an atomic level resolution of APC/C subcomplexes. These subcomplexes may provide multiple binding sites for small molecules that would perhaps make APC/C unique among ubiquitin ligases as a therapeutic target.
APC/C Activity
Another attractive aspect of the APC/C as a drug target is that it binds a unique set of enzymes required for transferring ubiquitin to substrates. The process of ubiquitination begins with the ubiquitin-activating enzyme E1 binding to and activating ubiquitin in an ATP-dependent manner. This activated ubiquitin is then transferred to a ubiquitin-conjugating enzyme or E2. The ubiquitin ligases or E3 enzymes then associate with E2s to catalyze the ubiquitin transfer to the ε-amino group of lysine residues on substrate proteins (Ye and Rape, 2009). Multiple ubiquitin molecules can be linked together in different ways to form polyubiquitin chains that satisfy different objectives. In yeast, chains linked via Lysine 48 of ubiquitin (K48 chains) are a “proteolytic signal” whereas those linked via Lysine 63 (K63 chains) function as molecular scaffolds. In higher eukaryotes, the APC/C is known to build atypical K11-linked polyubiquitin chains on its substrates in association with its unique E2 partner, Ube2C (or UbcH10; Wickliffe et al., 2011b). Ube2C only initiates chain formation, however. Chain elongation is carried out by a K11-specific E2 called Ube2S (or E2–EPF) that works with both APC/CCdc20 and APC/CCdh1 (Garnett et al., 2009; Wu et al., 2010; Wickliffe et al., 2011a). The importance of this dual regulation of APC/C activity via Ubch10 and Ube2S is underscored by the finding that removing K11-specific E2s causes defects in spindle assembly and mitotic progression (Williamson et al., 2009).
One of the most attractive means of attenuating APC/C activity pharmacologically is by modulating the adaptor protein–substrate binding reaction. Early studies in yeast identified the Cdc20 and Cdh1 substrate binding adaptor proteins as required for APC/C activity (Visintin et al., 1997). Subsequent studies in multiple experimental systems demonstrated the biochemical requirements for these adaptors to bind their substrates (Fang et al., 1998). Although exceptions exist, Cdc20 and Cdh1 bind substrates containing the sequence elements RXXLXXXXN/D/E or destruction boxes (D-boxes), while Cdh1 can also bind substrates containing KEN sequences (Glotzer et al., 1991; Pfleger and Kirschner, 2000). Substrate binding initiates ubiquitination mediated by APC/CCdc20 or APC/CCdh1. However, APC/CCdc20 or APC/CCdh1 mediated substrate binding is controlled during the cell cycle using an overlapping series of regulatory mechanisms. Inhibitory complexes control APC/C activity during the cell cycle, thus limiting its activity to defined temporal windows (for reviews see Manchado et al., 2010; Qiao et al., 2010; Pines, 2011). One of the most important examples of this is the exquisite control of APC/CCdc20 activity during mitosis. In early mitosis, APC/CCdc20 activity is curtailed by the spindle assembly checkpoint (SAC), which monitors the attachment of kinetochores to the mitotic spindle. An inhibitory complex containing Mad2, Bub3, and BubR1 proteins sequesters Cdc20 and renders it unable to bind substrates (Kim and Yu, 2011). After the checkpoint is switched off, this complex is released from APC/CCdc20, which initiates the metaphase to anaphase transition by mediating degradation of key proteins such as securin, shugoshin, and cyclin B1 (Kim and Yu, 2011). Securin and cyclin B1 degradation activates separase, which cleaves the cohesin complex that holds sister chromatids together while shugoshin destruction relieves sister chromatid cohesion at the centromere (Wang and Dai, 2005). These reactions are essential for the metaphase to anaphase transition, which is inhibited when APC/C activity is abrogated pharmacologically or by siRNA depletion or genetic disruption of Cdc20 (Huang et al., 2009; Manchado et al., 2010; Zeng et al., 2010). Cells lacking APC/CCdc20 eventually undergo mitotic catastrophe and die in a manner reminiscent of microtubule inhibition (Zeng et al., 2010). However, APC/C inhibitory molecules may have the added advantage that they will not have the usual off-target effects of microtubule inhibition well-known during chemotherapy treatment (Huang et al., 2009; Manchado et al., 2010).
APC/CCdh1 Controls Several Cell Cycle Transitions
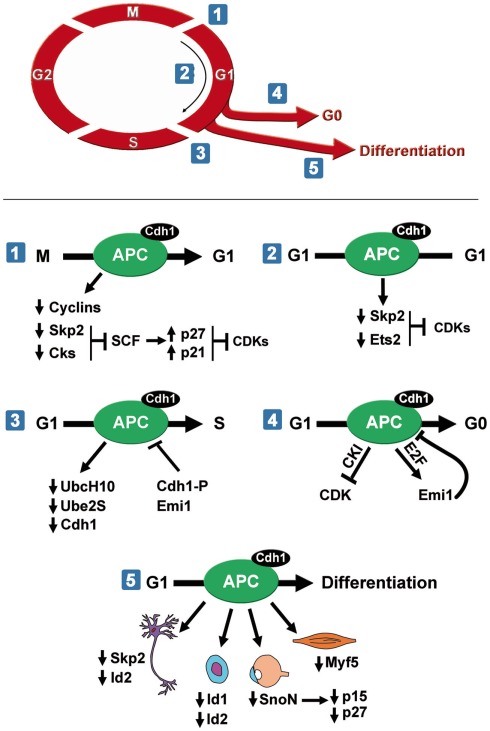
Small molecules that inhibit substrate ubiquitination via APC/CCdc20 also reduce APC/CCdh1 activity (Zeng et al., 2010). Since the APC/CCdh1 window of activity during the cell cycle is larger than that of APC/CCdc20, it may be easier to modulate APC/CCdh1 specific pathways necessary for growth of a particular cancer cell. APC/CCdh1 controls mitotic exit and maintains the G1-phase in cycling cells (Figure 1). Outside the cell cycle, APC/CCdh1 is required for quiescence, cell cycle exit, and differentiation (Figure 1). These multiple roles suggest the presence of distinct upstream regulatory and signaling pathways controlling APC/CCdh1 within temporal or developmental windows. Importantly, these pathways will likely provide unique interactions with APC/C that can be targeted pharmacologically.
Figure 1.
APC/CCdh1 regulates several cell cycle phases. (1, 2) APC/CCdh1promotes mitotic exit and maintains G1-phase by inhibiting CDK activity. (3) APC/CCdh1 needs to be inactivated for cells to enter S phase. APC/CCdh1 promotes cell cycle exit to a quiescent state (4) or differentiation (5). APC/CCdh1 induces differentiation of neurons, hematopoietic cells, lens cells, and myocytes.
APC/CCdh1 is required for mitotic exit and G1 maintenance
APC/CCdh1 targets multiple substrates for degradation during mitosis (Figure 1). Foremost among these are the mitotic cyclins, whose degradation ablates cyclin-dependent kinase 1 (CDK1) activity (Brandeis and Hunt, 1996; Irniger and Nasmyth, 1997). APC/C also reduces activity of multiple CDKs by initiating degradation of two components of the SCF ubiquitin ligase, the F-box protein Skp2 and an accessory protein, Cks1 (Bashir et al., 2004; Wei et al., 2004). Degradation of these two substrates raises the levels of the cyclin-dependent kinase inhibitors p27Kip1 and p21Cip1, which are normally targeted for degradation in an SCFSkp2 and Cks1 dependent manner (Ganoth et al., 2001; Bornstein et al., 2003). CDK inhibitors associate with specific cyclins and CDKs, preventing them from binding to ATP, and hence blocking their catalytic activity. Thus, high p27Kip1 and p21Cip1 levels maintain an early G1 state by reducing CDK activity. Another mechanism to promote G1 by reducing CDK activity involves APC/CCdh1 mediated inhibition of an activator of S phase entry, cyclin D1. APC/CCdh1 initiates degradation of the transcription factor Ets2 (Li et al., 2008), which normally controls cyclin D1 levels (Albanese et al., 1995). Consistent with these findings, depletion of Cdh1 by siRNA stabilizes Skp2 and Ets2, resulting in p21Cip1 and p27Kip1 degradation and cyclin D1 elevation in G1, followed by premature S phase entry (Wei et al., 2004; Li et al., 2008) and increased proliferation (Bashir et al., 2004). Thus, APC/CCdh1 function during mitotic exit is coupled to G1 maintenance. It initiates a sharp decrease in CDK1 activity during mitotic exit by targeting the mitotic cyclins for destruction and subsequently remains active in early G1 to ensure that they remain inactive. In addition, it ensures that other cyclin-dependent kinases, namely CDK2/cyclin E, CDK2/cyclin A, CDK4/cyclin D, and CDK6/cyclin D are inactive since it maintains high p27Kip1 levels and low cyclin D1 levels through Skp2 and Ets2 degradation.
One question that immediately arises from these studies is that if APC/C keeps CDKs inactive in early G1, how do cells eventually reach S phase. The answer lies in the multiple mechanisms that decrease APC activity (Figure 1). For instance, the ubiquitination of the APC/C-specific ubiquitin-conjugating enzyme (E2) UbcH10 by APC/CCdh1 provides a negative feedback mechanism that eventually dampens APC/CCdh1 activity (Rape and Kirschner, 2004; Rape et al., 2006). Further, Cdh1 is inactivated by both phosphorylation and degradation (Lukas et al., 1999; Listovsky et al., 2004; Benmaamar and Pagano, 2005). Finally, E2F activates the transcription of the APC/C pseudo-substrate early mitotic inhibitor-1 (Emi1)/Rca1 in late G1, which inhibits APC/CCdh1 activity (Hsu et al., 2002). These distinct mechanisms ensure that APC/CCdh1 activity remains low from late G1 until the subsequent metaphase when it is activated via reduction of CDK and Emi1 activity (Kotani et al., 1998; Hsu et al., 2002; Figure 1).
APC/CCdh1 controls pre-replication complex formation
One of the main reasons APC/CCdh1 activity must remain low during S phase is that it is an inhibitor of pre-replication complex formation required for S phase entry (Diffley, 2004). APC/CCdh1 controls the formation of the pre-replication complexes by modulating levels of three major regulators of the formation of the complex: Orc1, Cdc6, and geminin. For DNA replication to proceed, cells need to alternate between periods of low CDK activity and low geminin levels, in which the pre-replicative complexes (preRCs) are assembled; and periods of high CDK activity and high geminin levels in which origin firing and DNA replication occurs (McGarry and Kirschner, 1998; Petersen et al., 2000; Araki et al., 2003). APC/CCdh1 is crucial to properly regulate the switch between these two states. For instance, Orc1 and Cdc6 are degraded via APC/CCdh1 during early G1 (Petersen et al., 2000; Araki et al., 2003). Further, geminin levels are tightly controlled by APC/CCdh1. Geminin prohibits initiation of DNA replication at inappropriate times of the cell cycle by preventing MCM recruitment at the replication origins. During G1 the APC/C is active and as a consequence, geminin concentration is low. At the G1–S transition, APC/C is inactivated and geminin begins to accumulate. However, geminin concentration is not sufficient to inhibit a first wave of preRC formation and DNA replication begins. As S phase progresses, geminin accumulates and inhibits subsequent recruitment of MCMs to the replication origins (McGarry and Kirschner, 1998), and therefore re-duplication is avoided (McGarry and Kirschner, 1998; Wohlschlegel et al., 2000).
APC/CCdh1 promotes cell cycle exit and quiescence
Multiple studies suggest that a major APC/CCdh1 function outside of the cell cycle is limiting CDK activity required for cell cycle progression (Qiao et al., 2010). APC/CCdh1 regulates CDK inhibitors that reduce CDK activity and initiate cell cycle exit. Adding a second layer of regulation to APC/CCdh1 activity, Binné et al. (2007) connected retinoblastoma protein (pRb) to APC/CCdh1, Skp2, and CDK inhibitor dependent cell cycle exit. These studies demonstrated that hypophosphorylated pRb associates with the APC/C specifically when activated by Cdh1, thus promoting Skp2 degradation and accumulation of p27Kip1 and p21Cip1. This important finding linked extracellular signaling mechanisms, which normally control pRb activity to APC/C dependent degradation of Skp2 and initiation of cell cycle exit.
Once cells have exited the cell cycle, APC/C activity is required to maintain quiescence or differentiation. For instance, APCCdh1 inactivation by deleting its Apc2 subunit in adult hepatocytes induced these otherwise quiescent cells to re-enter the cell cycle (Wirth et al., 2004). Similarly, APCCdh1 has also been proposed to block postmitotic differentiated neurons from inappropriate cycling and apoptosis (Almeida et al., 2005; Jackson, 2006).
APC/CCdh1 regulates cell cycle exit and cell differentiation
APC/CCdh1 activity promotes cell cycle exit and differentiation since Cdh1 depletion reduces differentiation of muscle, lens, hematopoietic, and neuronal cells (Lasorella and Iavarone, 2006; Li et al., 2007; Wu et al., 2007; Garcia-Higuera et al., 2008). Although our knowledge of the involvement of APC/CCdh1 in the differentiation of other tissues awaits further investigation, its critical function in the degradation of cell cycle proteins suggests it likely plays relevant roles in linking quiescence and differentiation in most cell types. Further, since cancer progression is often thought to involve a dedifferentiation process (Daley, 2008; Trosko, 2009), understanding APC/C’s involvement in normal cell cycle exit and differentiation will give us clues as to how APC/C regulation or substrate targeting may be misregulated during tumorigenesis.
Neuronal differentiation
Following a period of proliferation, neural progenitors differentiate into postmitotic neurons. Since Cdh1 levels are (Gieffers et al., 1999; Stegmuller and Bonni, 2005) higher in postmitotic neurons relative to their neural progenitors (Yao et al., 2010), APC/CCdh1 may play a role in neuronal cell cycle exit. Consistent with this notion, an increase in APC/CCdh1 activity and a decrease of APC/CCdh1 substrates has been observed during terminal differentiation (Almeida et al., 2005; Yao et al., 2010). By contrast, upregulation of APC/CCdh1 substrates has been described in human neural tumors, suggesting that APC/CCdh1 activity is attenuated under these conditions (Lasorella and Iavarone, 2006; Eckerle et al., 2009).
Inhibitor of DNA binding 2
One of the targets that couples APC/CCdh1 activity with neuronal differentiation is inhibitor of DNA binding 2 (Id2, also known as inhibitor of differentiation 2). In the developing nervous system, Id2 has been shown to inhibit the activity of neurogenic basic helix–loop–helix (bHLH) transcription factors required for neuronal differentiation (Yokota, 2001; Perk et al., 2005; Rothschild et al., 2006; Jung et al., 2010). APC/CCdh1 targets Id2 for destruction, which couples cell cycle exit, differentiation, and axonal growth during the differentiation of diverse neuronal types (Lasorella and Iavarone, 2006; Yao et al., 2010). Thus, Id2 protein downregulation via proteolysis is essential for neuronal differentiation, suggesting that deregulation of Id2 destruction may underlie neural tumors.
Deregulated Id2 expression prevents cell cycle arrest via a wide range of signals (Lasorella et al., 2002; Kowanetz et al., 2004; Baghdoyan et al., 2005). Id2 downregulation via degradation allows the bHLH transcription factor E47 levels to increase and subsequently promote neural cell differentiation through induction of the CDK inhibitor p57Kip2 in the developing mouse brain (Rothschild et al., 2006; Tury et al., 2011). Similarly, decreasing Id2 levels increases expression of known mediators of neuronal differentiation such as Hes1 and Ascl1 (Mash1) transcription factors (Havrda et al., 2008) and NeuroD/E47 (Jung et al., 2010). Under certain experimental conditions, ectopic Id2 is able to drive terminally differentiated cells back into the cell cycle (Chaudhary et al., 2005). Importantly, the observation that the most aggressive tumors frequently contain the highest levels of Id proteins raises the possibility that deregulating Id protein stability by APC/CCdh1 might also contribute to Id accumulation in cancer.
Skp2
The APC/C substrate Skp2 controls the G1 to S transition by eliminating numerous regulatory proteins that inhibit S phase entry (Reed, 2008). The SCFSkp2 target p27Kip1 plays a large role in cell cycle exit and differentiation, particularly neuronal differentiation (Durand et al., 1998; Vernon et al., 2003; Tarui et al., 2005; Nguyen et al., 2006, 2007). Genetic disruption of p27Kip1 causes a general increase in cell proliferation (Fero et al., 1996; Carruthers et al., 2003), including neurogenesis in the cortex and spinal cord (Nguyen et al., 2007; Li et al., 2009). Control of p27Kip1 is dual, subject to the accumulation of both cyclin-CDK complexes, which promote its phosphorylation, and Skp2, which promote the ubiquitination of phosphorylated p27Kip1 (Carrano et al., 1999; Montagnoli et al., 1999). Harmey et al. (2009) described an essential role for APC/CCdh1 in cerebellar granule progenitors by mediating Skp2 destruction, thus coordinating cell cycle exit and terminal differentiation. Similar studies uncovered APC/CCdh1–Skp2 dependent differentiation of human embryonic stem cells (Bar-On et al., 2010). Collectively, these data suggested that two well-known inducers of neuronal differentiation, nerve growth factor (NGF), and retinoic acid, hyperactivate APC/CCdh1. NGF rapidly induced APC/CCdh1 activity and promoted degradation of APC substrates, including cyclin B1 and the F-box protein Skp2 (Harmey et al., 2009). Similarly, retinoic acid promoted neuronal differentiation through increasing APC/CCdh1 activity (Cuende et al., 2008; Yao et al., 2010). Further, retinoic acid induced nuclear accumulation of Cdh1, enhancing APC/CCdh1 activity, Skp2 destabilization, and p27Kip1 accumulation (Cuende et al., 2008) by reducing expression of Rae1, a nuclear export factor that limits APC/CCdh1 activity in mitosis (Yao et al., 2010). Similar to NGF and retinoic acid, bone morphogenetic protein 2 (BMP2) promotes cell cycle arrest via downregulation of Skp2 and accumulation of p27Kip1 in a neuroblastoma cell line (Nakamura et al., 2003). Thus, Skp2 degradation via APC/CCdh1 is linked to neuronal differentiation and various signaling pathways. Disruption of signaling pathways controlling the APC/C–Skp2 axis during neuronal differentiation may lead to disruption of homeostasis. Consistent with this possibility, reduced Skp2 and cyclin B1 expression has been seen in a transgenic model of Down syndrome where alteration of cell cycle rate and reduction of neurogenesis in the cerebellum was described (Contestabile et al., 2009). Moreover, Skp2 transcript levels gradually increase with the aggressiveness of neuroblastoma subtype (Westermann et al., 2007), making the regulation of Skp2 by APC/CCdh1 an attractive target in tumorigenesis.
Muscle cell differentiation
Myogenesis is a multistep process that sequentially requires the proliferation of committed myoblasts, the differentiation of myoblasts into postmitotic myocytes, and finally fusion of myocytes to form a multinucleated myotube with contractile capability. In muscle, APC/CCdh1 drives cell differentiation through the destruction of two proteins, Skp2 and Myf5 (Li et al., 2007; Figure 1). Elimination of Skp2 leads to the accumulation of the CDK inhibitors p21Cip1 and p27Kip1 in myoblasts, allowing cell cycle withdrawal. Consistent with a role of p21Cip1 in myotube formation, mice lacking p21Cip1 fail to form myotubes (Zhang et al., 1999). Thus, the APC/C–Skp2–p21Cip1/p27Kip1 axis is likely to be essential for muscle development.
Coupled to APC/C mediated destruction of Skp2 in muscle is APC/C targeting of Myf5. Myf5 is a bHLH transcription factor that regulates myoblast proliferation and homeostasis (Gayraud-Morel et al., 2007). Its expression is restricted to dividing and undifferentiated cells. Myf5 is not directly involved in the decision to differentiate per se but in Myf5 null animals, differentiation is delayed during early regeneration and Myf5 null mutants are characterized by a subtle progressive myopathy and muscle regeneration deficits (Gayraud-Morel et al., 2007). Degradation of Myf5 by APC/CCdh1 facilitates myogenic fusion, a process required for myoblast differentiation (Gayraud-Morel et al., 2007). Importantly, impaired degradation of both Skp2 and Myf5 seems to have a role in muscle cancer since overexpression of Skp2 and Myf5 are found in cervical carcinoma and rhabdomyosarcomas, respectively (Tamamori-Adachi et al., 2004; Zibat et al., 2010).
Lens cell differentiation
Proper lens differentiation requires precise temporal control of the cell cycle and the coordination of cell cycle exit with differentiation cues and signaling pathways (Zhu and Skoultchi, 2001). APC/CCdh1 has been identified as a crucial regulator of lens differentiation that induces SnoN degradation (Wu et al., 2007). SnoN, a critical transcriptional corepressor of the TGF-β pathway, has been proposed to be a functional switch controlling the expression of p15 and p21Cip1 (Zhu et al., 2005). p21Cip1 and p15 are two essential cell cycle inhibitors that contribute to cell cycle arrest via downregulation of cyclin D/CDK4/6 activity (Reynisdottir et al., 1995). The upregulation of the p21Cip1 and p15 inhibitors is necessary for coupling cell cycle withdrawal in response to TGF-β signaling and the initiation of lens differentiation. Moreover, Cdh1 depletion was shown to attenuate induction of p15 and p21Cip1 and significantly block lens differentiation (Wu et al., 2007). Impaired regulation of SnoN has not been related to corneal tumors but given the importance of APC/CCdh1 in lens differentiation, further studies could open new potential targets in these carcinomas.
Hematopoietic cell differentiation
Hematopoiesis gives rise to all blood cells through a complex series of proliferation and differentiation events that occur throughout lifespan (Kawamoto et al., 2010). The hematopoietic system consists of a large array of differentiated blood cells including erythrocytes and cells of the myeloid and lymphoid lineages. Evidence supporting a role for APC/CCdh1 in hematopoiesis comes from Cdh1 knockout mice. Cdh1 heterozygous mice develop B-cell lymphoma and myelodysplastic disorder (Garcia-Higuera et al., 2008). Although how APC/CCdh1 regulates hematopoiesis remains to be investigated, targeted degradation of Id protein could be one mechanism (Figure 1). While Id proteins are downregulated during cell cycle exit, overexpression of Id proteins in terminally differentiated cells triggers cell cycle re-entry. Id proteins modulate cellular proliferation and differentiation in hematopoietic cells (Perk et al., 2005). Id1 is essential for hematopoietic stem cell maintenance and hematopoietic development (Perry et al., 2007) and the balance between Id1 and E-protein regulates myeloid-versus-lymphoid lineage commitment (Cochrane et al., 2009). Dysfunction of Id2 in mice or cultured cells induces lymphoid differentiation, whereas Id2 overexpression inhibits lymphoid and myeloid differentiation (Perk et al., 2005; Ji et al., 2008). Furthermore, in addition to an Id1 and Id2 requirement for the proliferation and differentiation of hematopoietic precursors, these factors may contribute to the development of myeloid malignancies through enhanced proliferation or inhibited differentiation (Perk et al., 2005).
APC/CCdh1 Preserves Chromosome Integrity
Loss of Cdh1 function produces precocious initiation of DNA synthesis, leading to lower S phase progression, which results in stalled replication forks and under-replicated DNA (Garcia-Higuera et al., 2008). These replicative defects, as well as the upregulation of mitotic kinases, can ultimately lead to genetic damage (Garcia-Higuera et al., 2008; Cotto-Rios et al., 2011). Cdh1-deficient cells exhibit defects in mitotic exit and cytokinesis and accumulate a variety of genomic aberrations (Engelbert et al., 2008; Garcia-Higuera et al., 2008). Such genomic aberrations may be a consequence of the inability of APC/CCdh1 to properly mediate a response to DNA damage in Cdh1 null cells. In G1, APC/CCdh1 targets the ubiquitin specific protease USP1 for proteasome dependent degradation (Cotto-Rios et al., 2011). Since USP1 counteracts monoubiquitination of the DNA repair protein proliferating cell nuclear antigen (PCNA), APC/CCdh1 dependent USP1 destruction allows a permissive environment during G1 for PCNA monoubiquitination, which is required for UV-mediated DNA gap repair (Cotto-Rios et al., 2011). In addition to USP1, APC/CCdh1 controls the levels of multiple proteins involved in the DNA damage checkpoint response and DNA repair including Claspin, Rad17, thymidine kinase 1, and the ribonucleotide reductase subunit (Chabes et al., 2003; Ke et al., 2005; Bassermann et al., 2008; Gao et al., 2009a; Zhang et al., 2010). During G2, the APC/CCdh1 substrate polo-kinase 1 (Plk1) controls CDK1 activation and recovery from DNA replication stresses (Watanabe et al., 2004; Mamely et al., 2006). Cells exposed to genotoxic stress in G2 need to arrest the cell cycle and repair damaged DNA. One mechanism to achieve this is to reduce levels of proteins such as Plk1 and mitotic cyclins, which are required for mitotic entry. Although normally inactive during G2, APC/CCdh1 is activated via Cdh1 dephosphorylation during genotoxic stress, which is mediated by the Cdc14B phosphatase (Bassermann et al., 2008). Therefore, participation of APC/CCdh1 in the G2 DNA damage response suggests another mechanism for genomic instability observed in Cdh1 null cells.
APC/CCdh1 Modulators as Tumor Suppressor Proteins or Oncogenes
Considering the prominent role of APC/C in cell cycle regulation and genomic stability, it is tempting to speculate that its dysregulation triggers a major perturbation of cell cycle progression and contributes to cell transformation. In fact, hemizygous frameshift and point mutations in several subunits of APC/C have been found in colon cancer cell lines and tumors (Wang et al., 2003). Mutations in Apc3, Apc6, and Apc8 were found in colon cancer, breast cancer, neuroblastoma, hepatocarcinoma, melanoma, glioma, choriocarcinoma, endometrial cancer, ovarian cancer, and prostate carcinoma (Wang et al., 2003). Overexpression studies of Apc8 suggested that APC/C mutations can act in a dominant-negative manner to inhibit its function and cause inappropriate cell cycle progression (Wang et al., 2003). Moreover, the APC/C cofactor Cdh1 has been described as a tumor suppressor (Garcia-Higuera et al., 2008). Knockdown or inhibition of Cdh1 is associated with centrosome amplification and chromosome mis-segregation, and is implicated in genomic instability and tumorigenesis (Ross and Cohen-Fix, 2003; Wasch and Engelbert, 2005; Engelbert et al., 2008; Garcia-Higuera et al., 2008). Cdh1-deficient mice display genomic instability and heterozygous Cdh1 mice develop epithelial tumors, such as mammary gland adenocarcinomas and fibroadenomas, which are not observed in wild-type mice. Furthermore, these tumors contain one Cdh1 wild-type allele, suggesting that Cdh1 is haploinsufficient for tumor suppression. In addition to mutations in APC/C or Cdh1 subunits, it is tempting to speculate that alterations of APC/C upstream regulators may lead to transformation (Table 1).
Table 1.
Modulators of APC/CCdh1 dysregulated in cancer.
| APC/C regulator | Effects on APC/C | Major cell cycle function (s) | Role in tumorigenesis | Relevant cancers | Relevant reference |
|---|---|---|---|---|---|
| PTEN | Activator. Promotes the interaction between APC/C and Cdh1 | Promotes cell differentiation. Downregulation promotes cell proliferation | Acts as a tumor suppressor. Null mice die and heterozygous develop tumors | Lymphoid system, endometrium, thyroids, central nervous system, skin, prostate, breast | Chalhoub et al. (2009), Hollander et al. (2011), Song et al. (2011), Uddin et al. (2004), Kwon et al. (2008) |
| pRB | Activator. Promotes degradation of Skp2 by interaction with APC/C and Skp2 | Blocks progression of G1/S. Downregulation promotes cell proliferation | Acts as a tumor suppressor. Prevents integration of DNA damage | Lung, breast, eye | Chen et al. (2009), Classon and Harlow (2002), Ji et al. (2004), Binné et al. (2007), Sabado Alvarez (2008) |
| Sirt2 | Activator. Acetylates Cdh1 and Cdc20 promoting their interaction with APC/C | Regulates chromosomal condensation during mitosis. Maintains genome integrity | Acts as a tumor suppressor. Decrease of function promotes genome instability | Breast, liver, brain, kidney, and prostate cancers | North and Verdin (2007), Kim et al. (2011) |
| PSMA | Activator. Associates with Cdc27 | Unknown | Acts as an oncogene. Overexpression promotes premature activation of APC/C and aneuploidy | Prostate cancer | Rajasekaran et al. (2005, 2008), Burger et al. (2002), Chang et al. (1999, 2001) |
| Emi1 | Inhibitor. Binds to APC/C | Permits accumulation of cyclins in G1/S | Overexpression with p53 knock down promotes proliferation and chromosomal instability | Kidney, liver, lung, endometrium, lymphoid system, ovarium, lung | Hsu et al. (2002), Miller et al. (2006), Lehman et al. (2006), Gütgemann et al. (2008) |
| MAK | Inhibitor. Phosphorylates Cdh1 | Promotes stabilization of APC/CCdh1 substrates | Acts as an oncogene. Overexpression promotes extra-centrosomes | Prostate cancer | Wang and Kung (2011) |
Retinoblastoma protein
A well-established tumor suppressor protein that positively regulates APC/C activity is the pRB. pRB plays multiple roles during the cell cycle; it blocks cell cycle progression from G1 to S phase, and guards the cell from replicating damaged DNA, thus preventing the integration of mutations into the genome. This tumor suppressor function requires binding and repressing the E2F family transcription factors (Classon and Harlow, 2002; Chen et al., 2009). Reversion of the pRb–E2F interaction occurs after mitogenic stimuli sufficient for the activation of CDKs that phosphorylate pRB in the nucleus. Subsequently, phospho-pRb is released from E2Fs, which can then induce S phase entry (Thoma et al., 2011). Consistent with a direct inhibitory role in S phase entry, loss of various pRBs leads to unscheduled cell proliferation in many tissues (Vidal and Koff, 2000; Cobrinik, 2005; Marx et al., 2010). Accordingly, mutations in Rb are found in a wide variety of cancers mainly in the lung, breast, and eye (Meraldi et al., 1999; Sabado Alvarez, 2008).
Retinoblastoma protein also possesses E2F-independent functions that contribute to cell cycle control. pRB interacts with both Skp2 (Ji et al., 2004) and with APC/CCdh1 through distinct surfaces (Binné et al., 2007). pRB needs to interact with both proteins to target Skp2 for ubiquitin-mediated degradation and promote p27Kip1 accumulation and cell cycle exit. Interestingly, similar to E2F1, Cdh1 only interacts with the hypophosphorylated (active) form of pRb, and phosphorylation of pRb by cyclin A/CDK2 kinase abolished the ability of Rb to interact with both Cdh1 and E2F1. Therefore Cdh1, by competing with pRb interaction, can also regulate the activity of the E2F1 transcription factor (Sorensen et al., 2000; Gao et al., 2009a). Further, given that both pRB and Cdh1 have tumor suppressive functions, whether the pRB–APC/CCdh1 interaction is misregulated during tumorigenesis should be investigated (Figure 2).
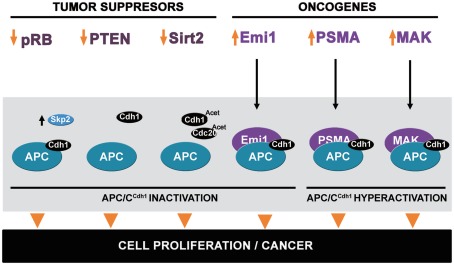
Figure 2.
Dysregulation of APC/CCdh1 modulators can promote cancer. Decreasing expression of the tumor suppressors pRB, PTEN, and Sirt2 may promote APC/CCdh1 inactivation, leading to cell proliferation and tumorigenesis. pRB downregulation may decrease Skp2 destruction. Decreasing PTEN levels might reduce APC/C activity. Decrease of Sirt2 could enhance the acetylation of the coactivators Cdh1 and Cdc20, lowering the capacity to interact with APC/C. In addition to the tumor suppressors, the oncogenes Emi1, PSMA, and MAK also regulate APC/CCdh1 activity. Emi1 binds as a pseudo-substrate and leads to APC/C inactivation. PSMA and MAK increase APC/CCdh1 activity and produce genomic instability.
PTEN
Recent studies have demonstrated that the well-characterized tumor suppressor protein PTEN is a positive regulator of APC/C activity. PTEN (Cairns et al., 1997; Steck et al., 1997; Feilotter et al., 1998; Gray et al., 1998; Li and Sun, 1998) encodes a lipid phosphatase, which plays a crucial role in adhesion, migration, growth, and apoptosis. Pten-null mice die embryonically, but heterozygous mice survive and develop tumors in the lymphoid system, endometrium, prostate, and the thyroid (Di Cristofano et al., 1998; Podsypanina et al., 1999). Pten somatic mutations occur in a large percentage of human cancers, with the highest numbers found in endometrial, central nervous system, skin, and prostate cancers (Chalhoub and Baker, 2009).
The effects observed after PTEN loss have been attributed to activation of the PI3K/AKT pathway as PTEN can dephosphorylate phosphoinositide-3,4,5-triphosphate (PIP3), a potent activator of Akt1 (Maehama and Dixon, 1998). PTEN inactivation by phosphorylation or oxidation in human cancer results in elevated Akt1 activity and abnormal growth regulation (Silva et al., 2008; Chalhoub and Baker, 2009; McCubrey et al., 2011). However, functions independent of its phosphatase activity have been described (Maier et al., 1999; Georgescu et al., 2000; Koul et al., 2002a,b; Gildea et al., 2004; Blanco-Aparicio et al., 2007). Recently, a novel interaction between PTEN and APC/CCdh1 has been reported (Song et al., 2011). Song et al. (2011) demonstrated that nuclear PTEN directly enhances the activity of APC/C by promoting its association with Cdh1 in a phosphatase-independent manner. Conversely, PTEN loss impairs the activity of the APC/CCdh1 complex. Therefore, loss of PTEN function decreases APC/CCdh1 activity, suggesting novel molecular pathways that may be involved in the progression and initiation of tumorigenesis. This in turn highlights the complexity of the dose-dependent tumor promoting and fail-safe cellular responses evoked by PTEN loss. Accordingly, tumor-derived mutations that do not affect the phosphatase enzymatic activity of PTEN in vitro have also been identified. These were found to affect the compartmentalization of PTEN in the cell, either by sequestering PTEN in the nucleus (Denning et al., 2007) or by interfering with PTEN’s recruitment to the plasma membrane (Lee et al., 1999; Georgescu et al., 2000; Walker et al., 2004), thus possibly negatively impacting the PTEN–APC/CCdh1 interaction. Consistent with this possibility, APC/CCdh1 substrates are overexpressed in tumors containing PTEN deficiency (Marino et al., 2002; Gao et al., 2009a; Liu et al., 2011b). Further, PTEN overexpression has been found to suppress tumorigenesis by inducing G1 cell cycle arrest in human glioblastoma cells (Li and Sun, 1998). Given the importance of PTEN and APC/CCdh1 for cancer progression, it will be crucial to determine whether tumors containing PTEN misregulation have both hyeractivation of the Akt1 pathway and dysregulation of APC/CCdh1 function (Figure 2).
Early mitotic inhibitor-1
The Emi1 is an APC/C inhibitor, which is overexpressed in multiple tumors. Emi1 is a key cell cycle regulator that is required for accumulation of mitotic cyclins and other critical cell cycle regulators during S phase and G2 (Hsu et al., 2002). At the G1/S transition, Emi1 functions as a pseudo-substrate inhibitor of the APC/C (Miller et al., 2006), allowing substrates to accumulate (Guardavaccaro et al., 2003; Miller et al., 2006). In early mitosis, Emi1 is phosphorylated by Plk1 (Hansen et al., 2004), which triggers SCFβTrCP-dependent ubiquitination and destruction, thus inducing APC/C activation and mitotic progression (Margottin-Goguet et al., 2003). Emi1 overexpression leads to unscheduled cell proliferation, tetraploidy, and chromosomal instability in p53-deficient cells (Lehman et al., 2006; Figure 2). In p53 wild-type cells, the induction of tetraploidy and aneuploidy by overexpressing APC/C inhibitors like Emi1 typically leads to G1 arrest or apoptosis. Indeed, Emi1 overexpression causes mitotic catastrophe and genomic instability through APC/C misregulation, and thus potentially contributes to tumorigenesis (Hsu et al., 2002). Upregulation of Emi1 mRNA has been found in a variety of malignant tumors compared to matched normal and benign tumor tissue. Notably, Emi1 protein is highly expressed in renal cell carcinomas, cervical adenocarcinomas, hepatocellular carcinomas, oligodendrogliomas, lung adenocarcinomas, endometrial cancers, of melanomas, many lymphomas and ovarian clear cell carcinoma (Lehman et al., 2006; Gütgemann et al., 2008). Emi1 expression is also related to the pRB/E2F pathway since Emi1 is a target of the E2F transcription factor. At the G1-S transition Emi1 is transcriptionally induced by E2F, thus accelerating S phase entry (Hsu et al., 2002). The importance of this regulation is underscored by the finding that lack of pRb repression of E2F-mediated transcription causes misregulation of Emi1 and APC/C substrates in malignant tumors (Lehman et al., 2006).
Sirt2
Recently, the histone deacetylase (HDAC) SIRT2 has been shown to positively regulate APC/C activity (Kim et al., 2011). Histone acetyltransferases (HATs) and HDACs are enzymes controlling protein acetylation. Both target histones, whereas HATs catalyze the acetylation of histones and relax chromatin to increase accessibility of transcription factors to promoters of target genes, HDACs remove acetyl groups from histones and repress transcription (Strahl and Allis, 2000). Most of them have been found in transcription factor complexes and, therefore, have been considered to regulate transcription by modulating acetylation levels of chromatin (Kuo and Allis, 1998; Kouzarides, 1999). HDACs are important for cell cycle progression and their inhibition promote cell arrest in G1 and G2 (Marks et al., 2001; Johnstone and Licht, 2003; Noh and Lee, 2003). HDACs also have an important role in heterochromatin formation and maintenance (Olsson et al., 1999; Taddei et al., 2001) and are implicated in chromosome segregation (Nakayama et al., 2003; Silverstein et al., 2003; Kimata et al., 2008).
Several HDACs have a role in tumorigenesis, acting as a suppressors or promoters (Lagger et al., 2002; Glaser et al., 2003; Saunders and Verdin, 2007; Wang et al., 2008a,b; Deng, 2009; Bell et al., 2011; Kim et al., 2011) and HDAC inhibitors are a promising class of anti-cancer agents (Johnstone and Licht, 2003; Yoshida et al., 2003; Bolden et al., 2006). SIRT2 is predominantly localized in the cytoplasm where it deacetylates microtubules (North et al., 2003). During mitosis, SIRT2 is localized to chromosomes regulating chromosomal condensation (Vaquero et al., 2006; Inoue et al., 2007), and is also associated with mitotic structures, including the centrosome, mitotic spindle, and midbody, presumably to ensure normal cell division (North and Verdin, 2007). Kim et al. (2011) demonstrated that SIRT2 regulates APC/C activity through deacetylation of its coactivators Cdh1 and Cdc20. Deacetylation of Cdh1 and Cdc20 by SIRT2 enhances the interaction of these coactivators with Cdc27, leading to activation of APC/C. In Sirt2 mutant mice, reduced APC/C activity results in tumor formation by producing genomic instability associated with centrosome amplification, aneuploidy mitotic cell death, and spontaneous tumor formation. These deficiencies seem to be caused by a combined effect of altered expression of mitotic regulators that are controlled by APC/C (Figure 2). Accordingly, SIRT2 expression is reduced in several human malignancies including breast, liver, brain, kidney, and prostate cancers (Kim et al., 2011).
PSMA and MAK
Mitotic defects associated with chromosomal aberrations are often observed in prostate cancer cells and tissue, suggesting possible misregulation by APC/C (Tribukait, 1991; Beheshti et al., 2001; Pihan et al., 2001). Two regulators of the APC/C have been found to be clearly involved in prostate cancer, prostate specific membrane antigen (PMSA) and male germ cell-associated kinase (MAK). PSMA is a type II transmembrane protein that exhibits both N-acetylated alpha-linked acidic peptidase (NAALADase) and folate hydrolase activities (Ghosh and Heston, 2004; Rajasekaran et al., 2005). PSMA is localized to secretory cells within the prostatic epithelium although its physiological and pathological functions remain unclear. PSMA is upregulated in advanced prostate carcinoma and metastatic disease (Rajasekaran et al., 2005), and is a feature of practically every prostatic tissue examined (Rajasekaran et al., 2005, 2008). PSMA is absent or moderately expressed in hyperplastic and benign tissues, while malignant tissues have high levels, demonstrating that PSMA expression increases proportionally to tumor aggressiveness (Troyer et al., 1995; Kawakami and Nakayama, 1997; Liu et al., 1997; Silver et al., 1997; Sweat et al., 1998; Chang et al., 1999, 2001; Burger et al., 2002; Ross et al., 2003). PSMA is localized to a membrane compartment in the vicinity of centrosomes at the spindle poles and associates with the APC/C subunit Cdc27 leading to premature activation of APC/C, and induction of aneuploidy (Rajasekaran et al., 2008). Increased APC/C activity observed in PSMA expressing cells is sufficient to impair the mitotic spindle checkpoint, which agrees with the finding that PSMA expressing cells exit mitosis prematurely (Figure 2). Therefore, PSMA may have a causal role in the progression of prostate cancer.
Male germ cell-associated kinase belongs to a protein kinase family characterized by a catalytic domain resembling a hybrid of the TXY motif found in mitogen-activated protein kinases (MAPK) and the TY motif in CDKs (Payne et al., 1991; Brown et al., 1999; Xia et al., 2002; Fu et al., 2005). MAK expression is elevated in castration-resistant prostate cancer cell lines and is generally overexpressed in prostate tumors, thus possibly contributing to malignancy via aberrant regulation of mitosis (Wang and Kung, 2011). MAK protein has a dynamic subcellular localization during the cell cycle: it is localized to the mitotic spindle and centrosomes during metaphase and anaphase, and to the mitotic midbody from anaphase to telophase. MAK negatively regulates APC/CCdh1 through interaction and phosphorylation of Cdh1 in CDK phosphorylated sites, in a manner reminiscent of CDK-dependent inactivation of Cdh1 (Wang and Kung, 2011; Figure 2). The phosphorylation of MAK increases between S and G2, peaks at early mitosis, and drastically decreases at the end of mitosis. This promotes the dissociation of Cdh1 and APC/C thus decreasing APC/CCdh1 activity and promoting the stabilization of the substrates Aurora kinase A and Plk1. As overexpression of Aurora-A is known to induce centrosome amplification (Zhou et al., 1998), the extra-centrosomes observed in MAK overexpressed cells is likely due to cellular accumulation of Aurora-A.
APC/C Substrates in Cancer
Many APC/C substrates have been implicated in a variety of human cancers (Table 2). For those substrates that are overexpressed in cancer it is important to note that overexpression of an APC/C substrate influences degradation of other APC/C substrates (Rape and Kirschner, 2004), suggesting that global estimates of APC/C substrate levels are required in normal and disease states. Further, while there are arguably many APC/C substrates that may be important for cancer progression, classifying their relative importance and druggability is essential.
Table 2.
Substrates of APC/C overexpressed in cancer.
| APC/C substrate | Major cell cycle function(s) | Role in tumorigenesis | Relevant cancers | Relevant reference |
|---|---|---|---|---|
| Nek2 | Regulation of centrosome separation and spindle formation | Formation of multinucleated cells with supernumerary centrosomes | Cervical cancer, B-cell lymphoma, pediatric osteosarcoma, breast cancer, leukemia, ovarian cancer | Wai et al. (2002), de Vos et al. (2003), Hayward et al. (2004) |
| Cdc20 | Essential APC/C coactivator in early mitosis | Spindle checkpoint defects and premature Securin destruction leading to mitotic slippage | Oral squamous cell carcinomas, breast cancer | Yuan et al. (2006), Mondal et al. (2007) |
| Plk1 | Activation of MPF by phosphorylation of Cdc25C and Cyclin B Assembling the mitotic spindle | Overexpression in cancer drives cells into mitosis | Lung, ovarian, breast, colon, prostate and pancreatic cancers, head/neck squamous cell carcinomas, melanomas | Wolf et al. (1997), Knecht et al. (1999), Strebhardt et al. (2000), Wolf et al. (2000), Macmillan et al. (2001), Gray et al. (2004), Weichert et al. (2004) |
| Aurora-A | Controls centrosome maturation and mitotic spindle formation | Induces centrosome amplification and aneuploidy by hindering APC/CCdh1 mediated destruction of Centrin | Bladder, lung, and colon cancers | Bischoff et al. (1998), Zhou et al. (1998), Sen et al. (2002), Gu et al. (2007) |
| HEC1 | Controls kinetochore microtubule dynamics as part of the NDC80 complex | Chromosome mis-segregation | Breast cancer | Bieche et al. (2011) |
| JNK | Central role in stress response pathways Ensures correct timing of mitotic entry by phosphorylating Cdc25C | Supports survival of tumor cells by controlling cell cycle arrest and apoptosis | Brain tumors such as glioblastoma, prostate cancer, gastrointestinal cancers, melanomas | Potapova et al. (2000), Antonyak et al. (2002), Tsuiki et al. (2003), Yang et al. (2003), Xia et al. (2006), Lopez-Bergami et al. (2007), Alexaki et al. (2008) |
| Ect2 | Positive regulator of the Rho GTPase pathway that controls actin cytoskeleton functions like cytokinesis | Ensures proper cytokinesis in tumor cells | Glioblastoma, non-small cell lung cancer | Salhia et al. (2008), Justilien et al. (2011) |
| Skp2 | F-box protein that functions as part of SCFSkp2 to degrade Cdk inhibitors p27Kip1 and p21Cip1 | Acts as an oncogene that destroys tumor suppressor proteins | Breast, prostate, liver and pancreatic cancers, melanomas, lymphomas | Lim et al. (2002), Radke et al. (2005), Lu et al. (2009), Rose et al. (2011), Schuler et al. (2011) |
| Ube2C (UbcH10) | Principal E2 enzyme the APC/C collaborates with | Chromosome abnormalities and mitotic slippage | Gastric carcinomas, cancers of the lung, prostate, breast, ovary, bladder, thyroid, uterus, and esophagus | Okamoto et al. (2003), Pallante et al. (2005), Berlingieri et al. (2007), Jiang et al. (2008), van Ree et al. (2010) |
NIMA-related kinase 2
The proteolysis of APC/C substrates starts as cells make the transition from G2 to M phase. One of the earliest targets is the NIMA-related kinase 2 (Nek2) family of serine/threonine protein kinases. They are implicated in the regulation of centrosome separation and spindle formation. Nek2A, along with cyclin A are unique APC/CCdc20 substrates that get degraded during prometaphase even when the SAC is active. A C-terminal dipeptide methionine–arginine (MR) tail enables Nek2A to directly bind the APC/C independently of Cdc20 (Hayes et al., 2006). Nek2 levels are upregulated in human breast cancer, pediatric osteosarcoma, and B-cell lymphomas (Wai et al., 2002; de Vos et al., 2003; Hayward et al., 2004).
Cell division cycle protein 20
Cell division cycle protein 20 is an essential coactivator of the APC/C during mitosis. Multiple studies have indicated that maintaining appropriate Cdc20 levels is important for cellular homeostasis. When this is disturbed and Cdc20 is overexpressed, tumorigenesis can occur. For instance, oral squamous cell carcinomas (OSCC) and breast cancer cells overexpress Cdc20 causing premature anaphase and aneuploidy linked to tumor formation (Yuan et al., 2006; Mondal et al., 2007). Importantly, even a twofold overexpression of Cdc20 leads to spindle checkpoint defects and early Pds1 (securin) destruction producing aneuploidy (Pan and Chen, 2004).
One of the major ways Cdc20 protein levels are controlled is via the SAC. When the SAC is active during metaphase, the checkpoint proteins Mad2, BubR1, and Bub3 bind to Cdc20 and convert it into a substrate of APC/CCdc20. This allows for the ubiquitination and degradation of Cdc20 by APC/CCdc20 (Nilsson et al., 2008; Ge et al., 2009). Thus, SAC activation inhibits Cdc20 and arrests cells in metaphase. Inactivation of SAC at the end of metaphase relieves this inhibition and Cdc20 promotes mitotic exit by degrading key substrates such as the mitotic cyclins. The importance of SAC dependent control of Cdc20 levels is underscored by the finding that anti-mitotic cancer drugs that activate the SAC cause cell cycle arrest. These drugs cause a prolonged metaphase arrest that may eventually lead to cell death. However, during this prolonged metaphase arrest, some Cdc20 escapes inhibition by the SAC and as a result, cyclin B1 levels start to fall and cells exit mitosis, thereby initiating mitotic slippage (Nilsson, 2011). This may be especially true in some cancer subtypes since the frequency of mitotic slippage is hastened in tumor cells lacking p53 and pRb (Depamphilis, 2011). Further, mitotic slippage reduces the sensitivity of tumor cells to chemotherapeutics. Thus, alternative means of inhibiting Cdc20 are needed.
Recent evidence suggests that targeting Cdc20 directly could be a better alternative than targeting the SAC in cancer therapy (Huang et al., 2009; Manchado et al., 2010). Knockdown of Cdc20 by siRNA induces mitotic arrest and apoptosis in various cancer cell lines that are otherwise resistant to apoptosis and prone to mitotic slippage (Huang et al., 2009). Further, genetic ablation of the Cdc20 gene in murine skin tumor and aggressive fibrosarcoma models results in complete tumor regression (Manchado et al., 2010). Cdc20 deletion induces metaphase arrest and apoptosis in tumor cells both in vitro and in vivo. Importantly, the activities of Cdk1 and Mast1 kinases are required for this arrest. When Cdk1 and Mast1 kinases are inhibited, Cdc20 null cells exit mitosis via the activities of the phosphatases PP2A/B55α and PP2A/B55δ (Manchado et al., 2010). Thus, small molecules attenuating Cdc20 coupled with those inhibiting these phosphatases may be effective therapeutically.
Geminin
Another APC/C substrate degraded at the metaphase–anaphase transition is geminin. It is a small 25 kDa protein that inhibits DNA re-replication during S phase. Geminin levels accumulate during S, G2, and early mitosis. It suppresses the DNA replication factor CDT1 that is required for the formation of preRCs. Depletion of geminin by siRNA in tumor cells leads to DNA re-replication, cell cycle arrest, and apoptosis. However, regulation of CDT1 by geminin is found to be rate limiting for initiation of DNA replication only in cancer cells and not in normal or immortalized cells. Normal cells show the same effect only when cyclin A is co-depleted with geminin (Zhu and Depamphilis, 2009). Thus it has been proposed that inhibition of geminin activity can be used to selectively kill cancer cells (Zhu and Depamphilis, 2009).
Polo-like kinase 1
Toward the end of anaphase, the pro-mitotic regulatory kinase Plk1, is degraded by APC/CCdh1 in a D-box dependent manner (Lindon and Pines, 2004). Plk1 is a major kinase in eukaryotic cells involved in a variety of processes such as activation of the maturation promoting factor (MPF) by phosphorylation of Cdc25C and cyclin B1, bipolar spindle formation, and maturation of the centrosome (Ohkura et al., 1995; Lane and Nigg, 1996; Abrieu et al., 1998; Qian et al., 1998; Eckerdt et al., 2005). Elevated Plk1 levels are present in a broad spectrum of cancers including breast cancer, colorectal cancer, ovarian cancer, melanomas, pancreatic cancer, prostate cancer, lung cancer, and squamous cell carcinomas of the head and neck (Wolf et al., 1997, 2000; Knecht et al., 1999; Strebhardt et al., 2000; Macmillan et al., 2001; Gray et al., 2004; Weichert et al., 2004). This broad range of expression makes Plk1 a prime target in cancer (Strebhardt and Ullrich, 2006).
Aurora-A
Another APC/CCdh1 substrate during late mitosis is Aurora-A (STK15/BTAK), a serine/threonine kinase localized to the centrosome controlling mitotic spindle assembly and centrosome maturation. It is involved in a variety of human cancers (Bischoff et al., 1998; Zhou et al., 1998; Sen et al., 2002; Mondal et al., 2007). Ectopic expression of Aurora-A leads to chromosome instability and centrosome amplification (Miyoshi et al., 2001). One way it achieves this is by phosphorylating and regulating Centrin, the calcium-binding phosphoprotein located in the centrosome. Phosphorylated Centrin is more stable against APC/CCdh1 mediated destruction and an excess of it is thought to promote centrosome amplification in cancer (Lukasiewicz et al., 2011). Aurora-A itself becomes resistant to APC/CCdh1 mediated degradation when it is constitutively phosphorylated on Ser 51, and consequently gets overexpressed in head and neck cancers (Kitajima et al., 2007).
High expression in cancer 1
High expression in cancer 1 (HEC1), a recently identified APC/CCdh1 substrate, is part of the NDC80 complex controlling kinetochore microtubule dynamics. HEC1 levels peak during early mitosis and fall by telophase (Lipkowitz and Weissman, 2011). The mitotic regulatory kinase Nek2 phosphorylates HEC1 on Ser 165 thus activating it during G2/M. Inactivation of HEC1 results in severe chromosome segregation defects (Chen et al., 1997, 2002; Zheng et al., 1999). HEC1 is considered as a candidate marker for breast lesions likely to undergo malignant transformation because it is significantly overexpressed in benign breast tumors (Bieche et al., 2011). A small molecule called INH1, which binds to HEC1 and specifically disrupts the HEC1–Nek2 interaction, is found to suppress human breast cancer cell proliferation in culture as well as tumor growth in nude mice bearing xenografts (Wu et al., 2008).
c-Jun NH2-terminal kinase
c-Jun NH2-terminal kinase (JNKs), a class of MAPK, lead the cell’s response to stress stimuli such as heat shock, radiation, and cytotoxic and genotoxic stress. Recently, nuclear JNK was found to be a substrate of APC/CCdh1 during late mitosis and G1 (Gutierrez et al., 2010a). JNK, in turn, controls APC/CCdh1 by phosphorylating Cdh1 at three residues (Yu et al., 1998; Summers et al., 2008) to prevent premature Cdh1 association with the APC/C during G2. JNK-induced phosphorylation of key regulators not only controls cell survival and differentiation, but also cell cycle progression. JNK phosphorylates Cdc25C at Ser 168 in G2, downregulating its phosphatase activity required for CDK1 activation (Gutierrez et al., 2010b). This is required for the correct timing of mitotic entry and the proper establishment of G2/M checkpoint upon UV irradiation. However, constitutively active JNK causes defects in cell cycle progression. Indeed, many human tumors have been reported to require JNK activity for their growth and survival (Potapova et al., 2000; Antonyak et al., 2002; Tsuiki et al., 2003; Yang et al., 2003; Lopez-Bergami et al., 2007; Alexaki et al., 2008). In gastrointestinal cancers, a small molecule inhibitor of JNK shows promise as a therapeutic agent, because it induces cell cycle arrest and apoptosis (Xia et al., 2006).
Ect2
The oncogenic protein Ect2 is ubiquitinated by APC/CCdh1 shortly after completion of mitosis (Liot et al., 2011). Ect2 is a positive regulator of the Rho GTPase pathway that controls cell cycle progression and cytokinesis (Bustelo et al., 2007). Many human tumors overexpress Ect2 (Sano et al., 2006; Salhia et al., 2008; Justilien and Fields, 2009; Justilien et al., 2011). Until now, this overexpression was believed to be a result of gene amplification and transcriptional upregulation (Seguin et al., 2009; Liot et al., 2011). But the newly discovered APC/CCdh1 dependent proteolysis of Ect2 shows that impaired destruction can also be a reason for increased Ect2 levels in tumor cells, which possibly asserts the role of Cdh1 as a tumor suppressor protein.
Skp2
Skp2 is one of the most important substrates targeted by APC/CCdh1 during mitotic exit. An SCF containing Skp2 targets the essential cell cycle substrates p27Kip1, p21Cip1, and FOXO1 for degradation (Yu et al., 1998; Tsvetkov et al., 1999; Huang et al., 2005). Destruction of the CDK inhibitor p27Kip1 is a prerequisite for entry into mitosis. Once cells exit mitosis, APC/CCdh1 targets Skp2 for proteolysis in G1 (Bashir et al., 2004; Wei et al., 2004). Skp2 functions as an oncogene because most of its ubiquitination targets are tumor suppressor proteins. Thus overexpression of Skp2 is common in many cancers including breast cancer, prostate cancer, pancreatic cancer, hepatocellular carcinomas, melanomas, and malignant lymphomas (Lim et al., 2002; Yang et al., 2002; Radke et al., 2005; Lu et al., 2009; Rose et al., 2011; Schuler et al., 2011). Importantly, the Akt1 serine/threonine kinase phosphorylates Skp2 at Ser 72 promoting its cytoplasmic translocation and impairing degradation by APC/CCdh1 (Gao et al., 2009b,c; Lin et al., 2009). Since the Akt1 pathway is also found to be hyperactive in cancer cells, it may contribute significantly to overexpression of Skp2 protein found in some cancers (Gao et al., 2009b,c). Opposing the Akt1 pathway is PTEN, which is frequently misregulated in human brain, breast, prostate cancers, and leukemias (Li et al., 1997; Dahia et al., 1999). Thus, PTEN misregulation may contribute to Akt1 hyperactivation and increased levels of Skp2 in tumors.
Ube2C (UbcH10)
Ube2C is an E2 enzyme that works exclusively with the APC/C throughout the cell cycle. However, Ube2C levels peak during mitosis and fall as cells enter G1, finally becoming a substrate for APC/CCdh1 at the end of G1 (Rape and Kirschner, 2004; Summers et al., 2008). When Ube2C is overexpressed in mouse embryonic fibroblasts (MEFs), precocious cyclin B1 degradation, mitotic slippage, and chromosome abnormalities have been observed (van Ree et al., 2010). Importantly, elevated Ube2C levels is a common feature in a wide variety of human cancers including lung, prostate, breast, bladder, ovarian, uterine, thyroid, esophageal, and gastric carcinomas (Okamoto et al., 2003; Pallante et al., 2005; Berlingieri et al., 2007; Jiang et al., 2008; van Ree et al., 2010). Further, induced expression of high levels of Ube2C hastens tumor formation in transgenic mice (van Ree et al., 2010).
Modulating APC/CCdh1 Activity in Cancer
Targeting APC/CCdh1substrates
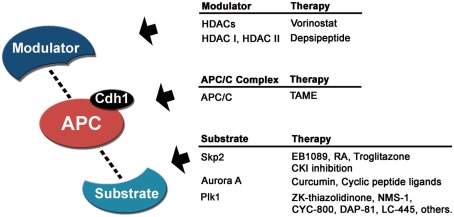
Several APC/C substrates are overexpressed in various cancers, making them prime targets for small molecule inhibition (Table 2). One potential APC/C substrate that could be targeted via such a strategy is the proto-oncogene Skp2 (Fujita et al., 2008, 2009). Downregulation of Skp2 by antisense RNA treatment induces apoptosis in lung cancer cells (Yokoi et al., 2003). Therefore, one possibility is utilizing compounds affecting Skp2 expression. For instance, treatment with EB1089 (vitamin D analog) reduces Skp2 expression in treated cancer cells, thus promoting increased stability of p27Kip1 protein and subsequent growth arrest (Lin et al., 2003; Figure 3). Skp2 expression is also affected by treatment with retinoic acid, thus altering the ability of p27Kip1 to be ubiquitinated (Nakamura et al., 2003). Furthermore, the PPARg agonist Troglitazone reduces Skp2 mRNA levels, leading to p27Kip1 accumulation (Koga et al., 2003).
Figure 3.
Cancer therapeutics related to APC/CCdh1. Targeting the modulators, the APC/CCdh1 complex or its substrates could be a good anti-cancer strategy. Some pharmacological treatments that regulate APC/C activity or substrate levels are currently in clinical trials or under development.
Since the APC/C mediates Skp2 ubiquitination, enhancing APC/C dependent degradation of Skp2 in cancer would also be therapeutically attractive. One possible means of achieving this is to inhibit casein kinase 1 (CK1), which controls APC/C dependent degradation of Skp2 (Gao et al., 2009b,c). CK1 inhibition induces Skp2 degradation since CK1 dependent phosphorylation normally inhibits Skp2 nuclear translocation and interaction with APC/CCdh1 (Gao et al., 2009b,c). An alternative strategy, however, could be increasing the affinity of Skp2 or other oncogenic substrates for APC/C, thus reducing their protein levels after ubiquitination and degradation. The advent of high-throughput screens to study APC/C-dependent degradation and protein–protein interactions will facilitate identification of small molecule agonists of the APC/C–Skp2 interaction (Madoux et al., 2010; Zeng et al., 2010).
Another potential APC/C cancer target is the substrate Aurora-A, since its overexpression is known to induce centrosome amplification and is overexpressed in human cancer cells (Zhou et al., 1998). Recently, several compounds have been found to target Aurora-A (Figure 3). Curcumin, an active compound in turmeric and curry that has been proven to induce tumor apoptosis and inhibit tumor proliferation, invasion, angiogenesis, and metastasis, has been shown to reduce Aurora-A mRNA expression in human bladder cancer cells (Liu et al., 2011a). These curcumin-induced phenomena were similar to those using Aurora-A small interfering RNA and were attenuated by ectopic expression of Aurora-A. Also, several cyclic peptide ligands inhibiting Aurora-A kinase activity have been developed (Shomin et al., 2011), although future studies regarding proliferation of cancer cells still have to be performed.
In addition to Aurora-A inhibition, there is pharmaceutical interest in targeting Plk1 as Plk1 depletion in cancer cells dramatically inhibits cell proliferation and decreases viability (Liu and Erikson, 2003; Xu et al., 2011; Yoon et al., 2011). Depletion of Plk1 perturbs spindle assembly, which leads to activation of the mitotic checkpoint, prolonged mitotic arrest, and eventually apoptosis (Liu and Erikson, 2003). Thus, given the known functions and effects of Plk1 inhibition, coupled with its wide overexpression pattern in cancer, a naturally occurring Plk1 inhibitor with low or no toxicity will be immensely useful in prevention as well as treatment of cancer. Numerous Plk1 inhibitors are in development to evaluate their potential as treatments in oncology, some of them in preclinical and clinical phase I/II development (Schöffski, 2009; Figure 3). It will be interesting to determine whether enhancing APC/C mediated degradation of Plk1 yield similar therapeutic benefits as Plk1 inhibitors currently in clinical trials.
Targeting APC/CCdh1 activity
Several lines of evidence suggest that modulation of the APC/C complex is a good anti-cancer therapeutic strategy. Downregulation of the catalytic subunits Apc2 and Apc11 leads to growth arrest and cell death in HeLa cells (Pray et al., 2002). Also, a small molecule, tosyl-l-arginine methyl ester (TAME), which binds to APC/C and prevents its activation by Cdc20 and Cdh1, produces mitotic arrest (Zeng et al., 2010; Figure 3). Furthermore, expression of several APC/C subunits is higher in tumor relative to normal tissue (Wang et al., 2003). Collectively, these studies suggest that pharmacological interference with APC/C activity through disruption of protein–protein interaction is likely to have potent anti-tumor activity (Pray et al., 2002; Huang et al., 2009).
Targeting upstream regulators of the APC/C complex controlling mitosis is also a promising strategy. Overexpression of APC/C inhibitors such as Emi1 in mammalian cells impedes cell cycle progression and results in cell death (Reimann et al., 2001). HDACs might directly target APC/C to ensure proper chromosome segregation and anti-tumor effects of HDAC inhibitors could be attributed to this deregulation (Kimata et al., 2008). There are emerging HDAC inhibitors that have been clinically validated as therapeutic agents in cancer patients with hematologic malignancies. Two HDAC inhibitors, vorinostat (pan-HDAC inhibitor) and depsipeptide (HDAC 1 and II inhibitor) have been approved by the FDA and are under further clinical investigation (Figure 3). HDAC inhibitors are well tolerated and clinically effective against hematologic cancers (Duvic and Vu, 2007; Luu et al., 2008; Modesitt et al., 2008).
Concluding Remarks
The APC/C is a unique ubiquitin ligase since it possesses a distinct structure and interacts with specific E2 enzymes required for mediating substrate ubiquitination. Further, APC/CCdc20 activity is essential in vitro and in vivo, suggesting that no other ubiquitin ligase can substitute for APC/C’s role during mitosis (Li et al., 2007; Manchado et al., 2010). Similarly, APC/CCdh1 activity is required for cell cycle traverse, differentiation, placental formation, and for inhibiting tumorigenesis (Garcia-Higuera et al., 2008; Li et al., 2008). These exciting studies suggest that APC/C plays a large role in normal cell proliferation, which may be misregulated during cancer. Consistent with this notion are new studies linking APC/C substrates and regulators to tumor formation. Thus, it may be attractive to modulate APC/C substrate or regulator interaction pharmacologically to ablate tumor growth, which would have the advantage that specific interactions relevant to a cancer type can be uniquely targeted.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Ms. Satu Hyvarinen for helpful discussions and critical reading of the manuscript. We thank all members of the Wahlestedt laboratory and the Center for Therapeutic Innovation for helpful discussions. We apologize if we have omitted studies linking APC/C modulators and substrates to differentiation or tumorigenesis due to space constraints. This work was supported by R01NS067289-03.
Abbreviations
APC/C, anaphase promoting complex/cyclosome; bHLH, basic helix–loop–helix; BMP, bone morphogenetic protein; Cdc20, cell division cycle protein 20 or Fizzy, Fzy; Cdh1, Cdc20 homolog 1, or Fizzy-related, Fzr; Cdk, cyclin-dependent kinase; CK1, casein kinase 1; D-boxes, destruction boxes; E1, ubiquitin-activating enzyme; E2, ubiquitin-conjugating enzyme; E3, ubiquitin ligases; Emi1, early mitotic inhibitor-1/Rca1; HAT, histone acetyltransferase; HDAC, histone deacetylase; HEC1, high expression in cancer 1; Id1, inhibitor of DNA binding 1; Id2, inhibitor of DNA binding 2, also known as inhibitor of differentiation 2; JNK, c-Jun NH2-terminal kinase; MAK, male germ cell-associated kinase; MCM, minichromosome maintenance; MEF, mouse embryonic fibroblast; MR, methionine–arginine; NAALADase, N-acetylated alpha-linked acidic peptidase; Nek2, NIMA-related kinase 2; NGF, nerve growth factor; OSCC, oral squamous cell carcinomas; PC, proteasome/cyclosome; PCNA, proliferating cell nuclear antigen; PIP3, phosphoinositide-3,4,5-triphosphate; Plk1, polo-like kinase 1; PMSA, prostate specific membrane antigen; pRb, retinoblastoma protein; preRC, pre-replicative complex; Rbx1, RING-finger component; SCF, Skp1–Cullin–F-box; TAME, tosyl-l-arginine methyl ester; TGF-β, transforming growth factor beta; TPR, tetratricopeptide repeat; UPS, ubiquitin proteasome system.
References
- Abrieu A., Brassac T., Galas S., Fisher D., Labbe J. C., Doree M. (1998). The polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J. Cell Sci. 111, 1751–1757 [DOI] [PubMed] [Google Scholar]
- Acquaviva C., Pines J. (2006). The anaphase-promoting complex/cyclosome: APC/C. J. Cell Sci. 119, 2401–2404 10.1242/jcs.02937 [DOI] [PubMed] [Google Scholar]
- Albanese C., Johnson J., Watanabe G., Eklund N., Vu D., Arnold A., Pestell R. G. (1995). Transforming p21ras mutants and c-ets-2 activate the cyclin D1 promoter through distinguishable regions. J. Biol. Chem. 270, 23589–23597 10.1074/jbc.270.40.23589 [DOI] [PubMed] [Google Scholar]
- Alexaki V. I., Javelaud D., Mauviel A. (2008). JNK supports survival in melanoma cells by controlling cell cycle arrest and apoptosis. Pigment Cell Melanoma Res. 21, 429–438 10.1111/j.1755-148X.2008.00466.x [DOI] [PubMed] [Google Scholar]
- Almeida A., Bolanos J. P., Moreno S. (2005). Cdh1/Hct1-APC is essential for the survival of postmitotic neurons. J. Neurosci. 25, 8115–8121 10.1523/JNEUROSCI.1143-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonyak M. A., Kenyon L. C., Godwin A. K., James D. C., Emlet D. R., Okamoto I., Tnani M., Holgado-Madruga M., Moscatello D. K., Wong A. J. (2002). Elevated JNK activation contributes to the pathogenesis of human brain tumors. Oncogene 21, 5038–5046 10.1038/sj.onc.1205593 [DOI] [PubMed] [Google Scholar]
- Araki M., Wharton R. P., Tang Z., Yu H., Asano M. (2003). Degradation of origin recognition complex large subunit by the anaphase-promoting complex in drosophila. EMBO J. 22, 6115–6126 10.1093/emboj/cdg573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghdoyan S., Lamartine J., Castel D., Pitaval A., Roupioz Y., Franco N., Duarte M., Martin M. T., Gidrol X. (2005). Id2 reverses cell cycle arrest induced by {gamma}-irradiation in human HaCaT keratinocytes. J. Biol. Chem. 280, 15836–15841 10.1074/jbc.M414216200 [DOI] [PubMed] [Google Scholar]
- Barford D. (2011). Structure, function and mechanism of the anaphase promoting complex (APC/C). Q. Rev. Biophys. 44, 153–190 10.1017/S0033583510000259 [DOI] [PubMed] [Google Scholar]
- Bar-On O., Shapira M., Skorecki K., Hershko A., Hershko D. D. (2010). Regulation of APC/C (Cdh1) ubiquitin ligase in differentiation of human embryonic stem cells. Cell Cycle 9, 1986–1989 10.4161/cc.9.10.11727 [DOI] [PubMed] [Google Scholar]
- Bashir T., Dorrello N. V., Amador V., Guardavaccaro D., Pagano M. (2004). Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature 428, 190–193 10.1038/nature02330 [DOI] [PubMed] [Google Scholar]
- Bassermann F., Frescas D., Guardavaccaro D., Busino L., Peschiaroli A., Pagano M. (2008). The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell 134, 256–267 10.1016/j.cell.2008.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beheshti B., Park P. C., Sweet J. M., Trachtenberg J., Jewett M. A., Squire J. A. (2001). Evidence of chromosomal instability in prostate cancer determined by spectral karyotyping (SKY) and interphase fish analysis. Neoplasia 3, 62–69 10.1038/sj.neo.7900125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E. L., Emerling B. M., Ricoult S. J., Guarente L. (2011). SirT3 suppresses hypoxia inducible factor 1alpha and tumor growth by inhibiting mitochondrial ROS production. Oncogene 30, 2986–2996 10.1038/onc.2011.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmaamar R., Pagano M. (2005). Involvement of the SCF complex in the control of Cdh1 degradation in S-phase. Cell Cycle 4, 1230–1232 10.4161/cc.4.9.2048 [DOI] [PubMed] [Google Scholar]
- Berlingieri M. T., Pallante P., Guida M., Nappi C., Masciullo V., Scambia G., Ferraro A., Leone V., Sboner A., Barbareschi M., Ferro A., Troncone G., Fusco A. (2007). UbcH10 expression may be a useful tool in the prognosis of ovarian carcinomas. Oncogene 26, 2136–2140 10.1038/sj.onc.1210010 [DOI] [PubMed] [Google Scholar]
- Bieche I., Vacher S., Lallemand F., Tozlu-Kara S., Bennani H., Beuzelin M., Driouch K., Rouleau E., Lerebours F., Ripoche H., Cizeron-Clairac G., Spyratos F., Lidereau R. (2011). Expression analysis of mitotic spindle checkpoint genes in breast carcinoma: role of NDC80/HEC1 in early breast tumorigenicity, and a two-gene signature for aneuploidy. Mol. Cancer 10, 23. 10.1186/1476-4598-10-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binné U. K., Classon M. K., Dick F. A., Wei W., Rape M., Kaelin W. G., Jr, Näär A. M., Dyson N. J. (2007). Retinoblastoma protein and anaphase-promoting complex physically interact and functionally cooperate during cell-cycle exit. Nat. Cell Biol. 9, 225–232 10.1038/ncb1532 [DOI] [PubMed] [Google Scholar]
- Bischoff J. R., Anderson L., Zhu Y., Mossie K., Ng L., Souza B., Schryver B., Flanagan P., Clairvoyant F., Ginther C., Chan C. S., Novotny M., Slamon D. J., Plowman G. D. (1998). A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 17, 3052–3065 10.1093/emboj/17.11.3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Aparicio C., Renner O., Leal J. F., Carnero A. (2007). PTEN, more than the AKT pathway. Carcinogenesis 28, 1379–1386 10.1093/carcin/bgm052 [DOI] [PubMed] [Google Scholar]
- Bolden J. E., Peart M. J., Johnstone R. W. (2006). Anticancer activities of histone deacetylase inhibitors. Nat. Rev. Drug Discov. 5, 769–784 10.1038/nrd2133 [DOI] [PubMed] [Google Scholar]
- Bornstein G., Bloom J., Sitry-Shevah D., Nakayama K., Pagano M., Hershko A. (2003). Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J. Biol. Chem. 278, 25752–25757 10.1074/jbc.M301774200 [DOI] [PubMed] [Google Scholar]
- Brandeis M., Hunt T. (1996). The proteolysis of mitotic cyclins in mammalian cells persists from the end of mitosis until the onset of S phase. EMBO J. 15, 5280–5289 [PMC free article] [PubMed] [Google Scholar]
- Brown N. R., Noble M. E., Lawrie A. M., Morris M. C., Tunnah P., Divita G., Johnson L. N., Endicott J. A. (1999). Effects of phosphorylation of threonine 160 on cyclin-dependent kinase 2 structure and activity. J. Biol. Chem. 274, 8746–8756 10.1074/jbc.274.13.8797 [DOI] [PubMed] [Google Scholar]
- Burger M. J., Tebay M. A., Keith P. A., Samaratunga H. M., Clements J., Lavin M. F., Gardiner R. A. (2002). Expression analysis of delta-catenin and prostate-specific membrane antigen: their potential as diagnostic markers for prostate cancer. Int. J. Cancer 100, 228–237 10.1002/ijc.10468 [DOI] [PubMed] [Google Scholar]
- Bustelo X. R., Sauzeau V., Berenjeno I. M. (2007). GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays 29, 356–370 10.1002/bies.20558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns P., Okami K., Halachmi S., Halachmi N., Esteller M., Herman J. G., Jen J., Isaacs W. B., Bova G. S., Sidransky D. (1997). Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res. 57, 4997–5000 [PubMed] [Google Scholar]
- Carrano A. C., Eytan E., Hershko A., Pagano M. (1999). SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1, 193–199 10.1038/12013 [DOI] [PubMed] [Google Scholar]
- Carruthers S., Mason J., Papalopulu N. (2003). Depletion of the cell-cycle inhibitor p27(Xic1) impairs neuronal differentiation and increases the number of ElrC(+) progenitor cells in Xenopus tropicalis. Mech. Dev. 120, 607–616 10.1016/S0925-4773(03)00010-8 [DOI] [PubMed] [Google Scholar]
- Chabes A. L., Pfleger C. M., Kirschner M. W., Thelander L. (2003). Mouse ribonucleotide reductase R2 protein: a new target for anaphase-promoting complex-Cdh1-mediated proteolysis. Proc. Natl. Acad. Sci. U.S.A. 100, 3925–3929 10.1073/pnas.0330774100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalhoub N., Baker S. J. (2009). PTEN and the PI3-kinase pathway in cancer. Annu. Rev. Pathol. 4, 127–150 10.1146/annurev.pathol.4.110807.092311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. S., Reuter V. E., Heston W. D., Bander N. H., Grauer L. S., Gaudin P. B. (1999). Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 59, 3192–3198 [PubMed] [Google Scholar]
- Chang S. S., Reuter V. E., Heston W. D., Gaudin P. B. (2001). Comparison of anti-prostate-specific membrane antigen antibodies and other immunomarkers in metastatic prostate carcinoma. Urology 57, 1179–1183 10.1016/S0090-4295(01)00998-0 [DOI] [PubMed] [Google Scholar]
- Chaudhary J., Sadler-Riggleman I., Ague J. M., Skinner M. K. (2005). The helix-loop-helix inhibitor of differentiation (ID) proteins induce post-mitotic terminally differentiated sertoli cells to re-enter the cell cycle and proliferate. Biol. Reprod. 72, 1205–1217 10.1095/biolreprod.104.035717 [DOI] [PubMed] [Google Scholar]
- Chen H. Z., Tsai S. Y., Leone G. (2009). Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat. Rev. Cancer 9, 785–797 10.1038/nrc2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Riley D. J., Chen P. L., Lee W. H. (1997). HEC, a novel nuclear protein rich in leucine heptad repeats specifically involved in mitosis. Mol. Cell. Biol. 17, 6049–6056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Riley D. J., Zheng L., Chen P. L., Lee W. H. (2002). Phosphorylation of the mitotic regulator protein Hec1 by Nek2 kinase is essential for faithful chromosome segregation. J. Biol. Chem. 277, 49408–49416 10.1074/jbc.M202076200 [DOI] [PubMed] [Google Scholar]
- Classon M., Harlow E. (2002). The retinoblastoma tumour suppressor in development and cancer. Nat. Rev. Cancer 2, 910–917 10.1038/nrc950 [DOI] [PubMed] [Google Scholar]
- Cobrinik D. (2005). Pocket proteins and cell cycle control. Oncogene 24, 2796–2809 10.1038/sj.onc.1208619 [DOI] [PubMed] [Google Scholar]
- Cochrane S. W., Zhao Y., Welner R. S., Sun X. H. (2009). Balance between id and E proteins regulates myeloid-versus-lymphoid lineage decisions. Blood 113, 1016–1026 10.1182/blood-2008-06-164996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contestabile A., Fila T., Bartesaghi R., Ciani E. (2009). Cell cycle elongation impairs proliferation of cerebellar granule cell precursors in the Ts65Dn mouse, an animal model for down syndrome. Brain Pathol. 19, 224–237 10.1111/j.1750-3639.2008.00168.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotto-Rios X. M., Jones M. J., Busino L., Pagano M., Huang T. T. (2011). APC/CCdh1-dependent proteolysis of USP1 regulates the response to UV-mediated DNA damage. J. Cell Biol. 194, 177–186 10.1083/jcb.201101062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuende J., Moreno S., Bolanos J. P., Almeida A. (2008). Retinoic acid downregulates Rae1 leading to APC(Cdh1) activation and neuroblastoma SH-SY5Y differentiation. Oncogene 27, 3339–3344 10.1038/sj.onc.1210987 [DOI] [PubMed] [Google Scholar]
- Dahia P. L., Aguiar R. C., Alberta J., Kum J. B., Caron S., Sill H., Marsh D. J., Ritz J., Freedman A., Stiles C., Eng C. (1999). PTEN is inversely correlated with the cell survival factor Akt/PKB and is inactivated via multiple mechanismsin haematological malignancies. Hum. Mol. Genet. 8, 185–193 10.1093/hmg/8.2.185 [DOI] [PubMed] [Google Scholar]
- Daley G. Q. (2008). Common themes of dedifferentiation in somatic cell reprogramming and cancer. Cold Spring Harb. Symp. Quant. Biol. 73, 171–174 10.1101/sqb.2008.73.041 [DOI] [PubMed] [Google Scholar]
- de Vos S., Hofmann W. K., Grogan T. M., Krug U., Schrage M., Miller T. P., Braun J. G., Wachsman W., Koeffler H. P., Said J. W. (2003). Gene expression profile of serial samples of transformed B-cell lymphomas. Lab. Invest. 83, 271–285 [DOI] [PubMed] [Google Scholar]
- Deng C. X. (2009). SIRT1, is it a tumor promoter or tumor suppressor? Int. J. Biol. Sci. 5, 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning G., Jean-Joseph B., Prince C., Durden D. L., Vogt P. K. (2007). A short N-terminal sequence of PTEN controls cytoplasmic localization and is required for suppression of cell growth. Oncogene 26, 3930–3940 10.1038/sj.onc.1210175 [DOI] [PubMed] [Google Scholar]
- Depamphilis M. L. (2011). Spotlight on geminin. Breast Cancer Res. 13, 109. 10.1186/bcr3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A., Pesce B., Cordon-Cardo C., Pandolfi P. P. (1998). Pten is essential for embryonic development and tumour suppression. Nat. Genet. 19, 348–355 10.1038/1235 [DOI] [PubMed] [Google Scholar]
- Diffley J. F. (2004). Regulation of early events in chromosome replication. Curr. Biol. 14, R778–R786 10.1016/j.cub.2004.09.019 [DOI] [PubMed] [Google Scholar]
- Dube P., Herzog F., Gieffers C., Sander B., Riedel D., Muller S. A., Engel A., Peters J. M., Stark H. (2005). Localization of the coactivator Cdh1 and the cullin subunit Apc2 in a cryo-electron microscopy model of vertebrate APC/C. Mol. Cell 20, 867–879 10.1016/j.molcel.2005.11.008 [DOI] [PubMed] [Google Scholar]
- Durand B., Fero M. L., Roberts J. M., Raff M. C. (1998). p27Kip1 alters the response of cells to mitogen and is part of a cell-intrinsic timer that arrests the cell cycle and initiates differentiation. Curr. Biol. 8, 431–440 10.1016/S0960-9822(98)70177-0 [DOI] [PubMed] [Google Scholar]
- Duvic M., Vu J. (2007). Vorinostat: a new oral histone deacetylase inhibitor approved for cutaneous T-cell lymphoma. Expert Opin. Investig. Drugs 16, 1111–1120 10.1517/13543784.16.7.1111 [DOI] [PubMed] [Google Scholar]
- Eckerdt F., Yuan J., Strebhardt K. (2005). Polo-like kinases and oncogenesis. Oncogene 24, 267–276 10.1038/sj.onc.1208273 [DOI] [PubMed] [Google Scholar]
- Eckerle I., Muth D., Batzler J., Henrich K. O., Lutz W., Fischer M., Witt O., Schwab M., Westermann F. (2009). Regulation of BIRC5 and its isoform BIRC5-2B in neuroblastoma. Cancer Lett. 285, 99–107 10.1016/j.canlet.2009.05.007 [DOI] [PubMed] [Google Scholar]
- Engelbert D., Schnerch D., Baumgarten A., Wasch R. (2008). The ubiquitin ligase APC(Cdh1) is required to maintain genome integrity in primary human cells. Oncogene 27, 907–917 10.1038/sj.onc.1210703 [DOI] [PubMed] [Google Scholar]
- Fang G., Yu H., Kirschne M. W. (1998). Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol. Cell 2, 163–171 10.1016/S1097-2765(00)80126-4 [DOI] [PubMed] [Google Scholar]
- Feilotter H. E., Nagai M. A., Boag A. H., Eng C., Mulligan L. M. (1998). Analysis of PTEN and the 10q23 region in primary prostate carcinomas. Oncogene 16, 1743–1748 10.1038/sj.onc.1200205 [DOI] [PubMed] [Google Scholar]
- Fero M. L., Rivkin M., Tasch M., Porter P., Carow C. E., Firpo E., Polyak K., Tsai L. H., Broudy V., Perlmutter R. M., Kaushansky K., Roberts J. M. (1996). A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell 85, 733–744 10.1016/S0092-8674(00)81239-8 [DOI] [PubMed] [Google Scholar]
- Fu Z., Schroeder M. J., Shabanowitz J., Kaldis P., Togawa K., Rustgi A. K., Hunt D. F., Sturgill T. W. (2005). Activation of a nuclear Cdc2-related kinase within a mitogen-activated protein kinase-like TDY motif by autophosphorylation and cyclin-dependent protein kinase-activating kinase. Mol. Cell. Biol. 25, 6047–6064 10.1128/MCB.25.14.6047-6064.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Liu W., Doihara H., Wan Y. (2008). Regulation of Skp2-p27 axis by the Cdh1/anaphase-promoting complex pathway in colorectal tumorigenesis. Am. J. Pathol. 173, 217–228 10.2353/ajpath.2008.070957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Liu W., Doihara H., Wan Y. (2009). An in vivo study of Cdh1/APC in breast cancer formation. Int. J. Cancer 125, 826–836 10.1002/ijc.24399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganoth D., Bornstein G., Ko T. K., Larsen B., Tyers M., Pagano M., Hershko A. (2001). The cell-cycle regulatory protein Cks1 is required for SCF(Skp2)-mediated ubiquitinylation of p27. Nat. Cell Biol. 3, 321–324 10.1038/35060126 [DOI] [PubMed] [Google Scholar]
- Gao D., Inuzuka H., Korenjak M., Tseng A., Wu T., Wan L., Kirschner M., Dyson N., Wei W. (2009a). Cdh1 regulates cell cycle through modulating the claspin/Chk1 and the Rb/E2F1 pathways. Mol. Biol. Cell 20, 3305–3316 10.1091/mbc.E09-03-0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D., Inuzuka H., Tseng A., Chin R. Y., Toker A., Wei W. (2009b). Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nat. Cell Biol. 11, 397–408 10.1038/ncb1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D., Inuzuka H., Tseng A., Wei W. (2009c). Akt finds its new path to regulate cell cycle through modulating Skp2 activity and its destruction by APC/Cdh1. Cell Div. 4, 11. 10.1186/1747-1028-4-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Higuera I., Manchado E., Dubus P., Canamero M., Mendez J., Moreno S., Malumbres M. (2008). Genomic stability and tumour suppression by the APC/C cofactor Cdh1. Nat. Cell Biol. 10, 802–811 10.1038/ncb1742 [DOI] [PubMed] [Google Scholar]
- Garnett M. J., Mansfeld J., Godwin C., Matsusaka T., Wu J., Russell P., Pines J., Venkitaraman A. R. (2009). UBE2S elongates ubiquitin chains on APC/C substrates to promote mitotic exit. Nat. Cell Biol. 11, 1363–1369 10.1038/ncb1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayraud-Morel B., Chretien F., Flamant P., Gomes D., Zammit P. S., Tajbakhsh S. (2007). A role for the myogenic determination gene Myf5 in adult regenerative myogenesis. Dev. Biol. 312, 13–28 10.1016/j.ydbio.2007.08.059 [DOI] [PubMed] [Google Scholar]
- Ge S., Skaar J. R., Pagano M. (2009). APC/C- and Mad2-mediated degradation of Cdc20 during spindle checkpoint activation. Cell Cycle 8, 167–171 10.4161/cc.8.1.7606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu M. M., Kirsch K. H., Kaloudis P., Yang H., Pavletich N. P., Hanafusa H. (2000). Stabilization and productive positioning roles of the C2 domain of PTEN tumor suppressor. Cancer Res. 60, 7033–7038 [PubMed] [Google Scholar]
- Ghosh A., Heston W. D. (2004). Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J. Cell. Biochem. 91, 528–539 10.1002/jcb.10789 [DOI] [PubMed] [Google Scholar]
- Gieffers C., Dube P., Harris J. R., Stark H., Peters J. M. (2001). Three-dimensional structure of the anaphase-promoting complex. Mol. Cell 7, 907–913 10.1016/S1097-2765(01)00234-9 [DOI] [PubMed] [Google Scholar]
- Gieffers C., Peters B. H., Kramer E. R., Dotti C. G., Peters J. M. (1999). Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proc. Natl. Acad. Sci. U.S.A. 96, 11317–11322 10.1073/pnas.96.20.11317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildea J. J., Herlevsen M., Harding M. A., Gulding K. M., Moskaluk C. A., Frierson H. F., Theodorescu D. (2004). PTEN can inhibit in vitro organotypic and in vivo orthotopic invasion of human bladder cancer cells even in the absence of its lipid phosphatase activity. Oncogene 23, 6788–6797 10.1038/sj.onc.1207599 [DOI] [PubMed] [Google Scholar]
- Glaser K. B., Li J., Staver M. J., Wei R. Q., Albert D. H., Davidsen S. K. (2003). Role of class I and class II histone deacetylases in carcinoma cells using siRNA. Biochem. Biophys. Res. Commun. 310, 529–536 10.1016/j.bbrc.2003.09.043 [DOI] [PubMed] [Google Scholar]
- Glotzer M., Murray A. W., Kirschner M. W. (1991). Cyclin is degraded by the ubiquitin pathway. Nature 349, 132–138 10.1038/349132a0 [DOI] [PubMed] [Google Scholar]
- Gmachl M., Gieffers C., Podtelejnikov A. V., Mann M., Peters J. M. (2000). The RING-H2 finger protein APC11 and the E2 enzyme UBC4 are sufficient to ubiquitinate substrates of the anaphase-promoting complex. Proc. Natl. Acad. Sci. U.S.A. 97, 8973–8978 10.1073/pnas.97.16.8973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray I. C., Stewart L. M., Phillips S. M., Hamilton J. A., Gray N. E., Watson G. J., Spurr N. K., Snary D. (1998). Mutation and expression analysis of the putative prostate tumour-suppressor gene PTEN. Br. J. Cancer 78, 1296–1300 10.1038/bjc.1998.674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. J., Bearss D. J., Han H., Nagle R., Tsao M. S., Dean N., Von Hoff D. D. (2004). Identification of human polo-like kinase 1 as a potential therapeutic target in pancreatic cancer. Mol. Cancer Ther. 3, 641–646 10.4161/cbt.3.7.918 [DOI] [PubMed] [Google Scholar]
- Gu J., Gong Y., Huang M., Lu C., Spitz M. R., Wu X. (2007). Polymorphisms of STK15 (aurora-A) gene and lung cancer risk in Caucasians. Carcinogenesis 28, 350–355 10.1093/carcin/bgl149 [DOI] [PubMed] [Google Scholar]
- Guardavaccaro D., Kudo Y., Boulaire J., Barchi M., Busino L., Donzelli M., Margottin-Goguet F., Jackson P. K., Yamasaki L., Pagano M. (2003). Control of meiotic and mitotic progression by the F box protein beta-Trcp1 in vivo. Dev. Cell 4, 799–812 10.1016/S1534-5807(03)00154-0 [DOI] [PubMed] [Google Scholar]
- Gütgemann I., Lehman N. L., Jackson P. K., Longacre T. A. (2008). Emi1 protein accumulation implicates misregulation of the anaphase promoting complex/cyclosome pathway in ovarian clear cell carcinoma. Mod. Pathol. 21, 445–454 10.1038/modpathol.3801022 [DOI] [PubMed] [Google Scholar]
- Gutierrez G. J., Tsuji T., Chen M., Jiang W., Ronai Z. A. (2010a). Interplay between Cdh1 and JNK activity during the cell cycle. Nat. Cell Biol. 12, 686–695 10.1038/ncb2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez G. J., Tsuji T., Cross J. V., Davis R. J., Templeton D. J., Jiang W., Ronai Z. A. (2010b). JNK-mediated phosphorylation of Cdc25C regulates cell cycle entry and G(2)/M DNA damage checkpoint. J. Biol. Chem. 285, 14217–14228 10.1074/jbc.M110.121848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen D. V., Loktev A. V., Ban K. H., Jackson P. K. (2004). Plk1 regulates activation of the anaphase promoting complex by phosphorylating and triggering SCFbetaTrCP-dependent destruction of the APC inhibitor Emi1. Mol. Biol. Cell 15, 5623–5634 10.1091/mbc.E04-07-0598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmey D., Smith A., Simanski S., Moussa C. Z., Ayad N. G. (2009). The anaphase promoting complex induces substrate degradation during neuronal differentiation. J. Biol. Chem. 284, 4317–4323 10.1074/jbc.M804944200 [DOI] [PubMed] [Google Scholar]
- Havrda M. C., Harris B. T., Mantani A., Ward N. M., Paolella B. R., Cuzon V. C., Yeh H. H., Israel M. A. (2008). Id2 is required for specification of dopaminergic neurons during adult olfactory neurogenesis. J. Neurosci. 28, 14074–14086 10.1523/JNEUROSCI.3188-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes M. J., Kimata Y., Wattam S. L., Lindon C., Mao G., Yamano H., Fry A. M. (2006). Early mitotic degradation of Nek2A depends on Cdc20-independent interaction with the APC/C. Nat. Cell Biol. 8, 607–614 10.1038/ncb1006-1052 [DOI] [PubMed] [Google Scholar]
- Hayward D. G., Clarke R. B., Faragher A. J., Pillai M. R., Hagan I. M., Fry A. M. (2004). The centrosomal kinase Nek2 displays elevated levels of protein expression in human breast cancer. Cancer Res. 64, 7370–7376 10.1158/0008-5472.CAN-04-0960 [DOI] [PubMed] [Google Scholar]
- Hollander M. C., Blumenthal G. M., Dennis P. A. (2011). PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat. Rev. Cancer 11, 289–301 10.1038/nrc3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J. Y., Reimann J. D., Sorensen C. S., Lukas J., Jackson P. K. (2002). E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1). Nat. Cell Biol. 4, 358–366 10.1038/ncb785 [DOI] [PubMed] [Google Scholar]
- Huang H., Regan K. M., Wang F., Wang D., Smith D. I., van Deursen J. M., Tindall D. J. (2005). Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc. Natl. Acad. Sci. U.S.A. 102, 1649–1654 10.1073/pnas.0504184102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. C., Shi J., Orth J. D., Mitchison T. J. (2009). Evidence that mitotic exit is a better cancer therapeutic target than spindle assembly. Cancer Cell 16, 347–358 10.1016/j.ccr.2009.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins J. R., Toyoda Y., Hegemann B., Poser I., Heriche J. K., Sykora M. M., Augsburg M., Hudecz O., Buschhorn B. A., Bulkescher J., Conrad C., Comartin D., Schleiffer A., Sarov M., Pozniakovsky A., Slabicki M. M., Schloissnig S., Steinmacher I., Leuschner M., Ssykor A., Lawo S., Pelletier L., Stark H., Nasmyth K., Ellenberg J., Durbin R., Buchholz F., Mechtler K., Hyman A. A., Peters J. M. (2010). Systematic analysis of human protein complexes identifies chromosome segregation proteins. Science 328, 593–599 10.1126/science.1181348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Hiratsuka M., Osaki M., Yamada H., Kishimoto I., Yamaguchi S., Nakano S., Katoh M., Ito H., Oshimura M. (2007). SIRT2, a tubulin deacetylase, acts to block the entry to chromosome condensation in response to mitotic stress. Oncogene 26, 945–957 10.1038/sj.onc.1210226 [DOI] [PubMed] [Google Scholar]
- Irniger S., Nasmyth K. (1997). The anaphase-promoting complex is required in G1 arrested yeast cells to inhibit B-type cyclin accumulation and to prevent uncontrolled entry into S-phase. J. Cell Sci. 110, 1523–1531 [DOI] [PubMed] [Google Scholar]
- Jackson P. K. (2006). Developmental neurobiology: a destructive switch for neurons. Nature 442, 365–366 10.1038/442365a [DOI] [PubMed] [Google Scholar]
- Ji M., Li H., Suh H. C., Klarmann K. D., Yokota Y., Keller J. R. (2008). Id2 intrinsically regulates lymphoid and erythroid development via interaction with different target proteins. Blood 112, 1068–1077 10.1182/blood-2008-01-133504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji P., Jiang H., Rekhtman K., Bloom J., Ichetovkin M., Pagano M., Zhu L. (2004). An Rb-Skp2-p27 pathway mediates acute cell cycle inhibition by Rb and is retained in a partial-penetrance Rb mutant. Mol. Cell 16, 47–58 10.1016/j.molcel.2004.09.029 [DOI] [PubMed] [Google Scholar]
- Jiang L., Huang C. G., Lu Y. C., Luo C., Hu G. H., Liu H. M., Chen J. X., Han H. X. (2008). Expression of ubiquitin-conjugating enzyme E2C/UbcH10 in astrocytic tumors. Brain Res. 1201, 161–166 10.1016/j.brainres.2008.01.037 [DOI] [PubMed] [Google Scholar]
- Johnstone R. W., Licht J. D. (2003). Histone deacetylase inhibitors in cancer therapy: is transcription the primary target? Cancer Cell 4, 13–18 10.1016/S1535-6108(03)00165-X [DOI] [PubMed] [Google Scholar]
- Jung S., Park R. H., Kim S., Jeon Y. J., Ham D. S., Jung M. Y., Kim S. S., Lee Y. D., Park C. H., Suh-Kim H. (2010). Id proteins facilitate self-renewal and proliferation of neural stem cells. Stem Cells Dev. 19, 831–841 10.1089/scd.2009.0093 [DOI] [PubMed] [Google Scholar]
- Justilien V., Fields A. P. (2009). Ect2 links the PKCiota-Par6alpha complex to Rac1 activation and cellular transformation. Oncogene 28, 3597–3607 10.1038/onc.2009.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justilien V., Jameison L., Der C. J., Rossman K. L., Fields A. P. (2011). Oncogenic activity of Ect2 is regulated through protein kinase C iota-mediated phosphorylation. J. Biol. Chem. 286, 8149–8157 10.1074/jbc.M110.196113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami M., Nakayama J. (1997). Enhanced expression of prostate-specific membrane antigen gene in prostate cancer as revealed by in situ hybridization. Cancer Res. 57, 2321–2324 [PubMed] [Google Scholar]
- Kawamoto H., Wada H., Katsura Y. (2010). A revised scheme for developmental pathways of hematopoietic cells: the myeloid-based model. Int. Immunol. 22, 65–70 10.1093/intimm/dxp125 [DOI] [PubMed] [Google Scholar]
- Ke P. Y., Kuo Y. Y., Hu C. M., Chang Z. F. (2005). Control of dTTP pool size by anaphase promoting complex/cyclosome is essential for the maintenance of genetic stability. Genes Dev. 19, 1920–1933 10.1101/gad.1322905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. S., Vassilopoulos A., Wang R. H., Lahusen T., Xiao Z., Xu X., Li C., Veenstra T. D., Li B., Yu H., Ji J., Wang X. W., Park S. H., Cha Y. I., Gius D., Deng C. X. (2011). SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell 20, 487–499 10.1016/j.ccr.2011.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Yu H. (2011). Mutual regulation between the spindle checkpoint and APC/C. Semin. Cell Dev. Biol. 22, 551–558 10.1016/j.semcdb.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata Y., Matsuyama A., Nagao K., Furuya K., Obuse C., Yoshida M., Yanagida M. (2008). Diminishing HDACs by drugs or mutations promotes normal or abnormal sister chromatid separation by affecting APC/C and adherin. J. Cell Sci. 121, 1107–1118 10.1242/jcs.024224 [DOI] [PubMed] [Google Scholar]
- Kitajima S., Kudo Y., Ogawa I., Tatsuka M., Kawai H., Pagano M., Takata T. (2007). Constitutive phosphorylation of aurora-a on ser51 induces its stabilization and consequent overexpression in cancer. PLoS ONE 2, e944. 10.1371/journal.pone.0000944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht R., Elez R., Oechler M., Solbach C., von Ilberg C., Strebhardt K. (1999). Prognostic significance of polo-like kinase (PLK) expression in squamous cell carcinomas of the head and neck. Cancer Res. 59, 2794–2797 [PubMed] [Google Scholar]
- Koga H., Harada M., Ohtsubo M., Shishido S., Kumemura H., Hanada S., Taniguchi E., Yamashita K., Kumashiro R., Ueno T., Sata M. (2003). Troglitazone induces p27Kip1-associated cell-cycle arrest through down-regulating Skp2 in human hepatoma cells. Hepatology 37, 1086–1096 10.1053/jhep.2003.50186 [DOI] [PubMed] [Google Scholar]
- Kops G. J., Van Der Voet M., Manak M. S., Van Osch M. H., Naini S. M., Brear A., Mcleod I. X., Hentschel D. M., Yates J. R., III, Van Den Heuvel S., Shah J. V. (2010). APC16 is a conserved subunit of the anaphase-promoting complex/cyclosome. J. Cell Sci. 123, 1623–1633 10.1242/jcs.074104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Tugendreich S., Fujii M., Jorgensen P. M., Watanabe N., Hoog C., Hieter P., Todokoro K. (1998). PKA and MPF-activated polo-like kinase regulate anaphase-promoting complex activity and mitosis progression. Mol. Cell 1, 371–380 10.1016/S1097-2765(00)80037-4 [DOI] [PubMed] [Google Scholar]
- Koul D., Jasser S. A., Lu Y., Davies M. A., Shen R., Shi Y., Mills G. B., Yung W. K. A. (2002a). Motif analysis of the tumor suppressor gene MMAC/PTEN identifies tyrosines critical for tumor suppression and lipid phosphatase activity. Oncogene 21, 2357–2364 10.1038/sj.onc.1205296 [DOI] [PubMed] [Google Scholar]
- Koul D., Shen R., Garyali A., Ke L. D., Liu T. J., Yung W. K. (2002b). MMAC/PTEN tumor suppressor gene regulates vascular endothelial growth factor-mediated angiogenesis in prostate cancer. Int. J. Oncol. 21, 469–475 [PubMed] [Google Scholar]
- Kouzarides T. (1999). Histone acetylases and deacetylases in cell proliferation. Curr. Opin. Genet. Dev. 9, 40–48 10.1016/S0959-437X(99)80006-9 [DOI] [PubMed] [Google Scholar]
- Kowanetz M., Valcourt U., Bergstrom R., Heldin C. H., Moustakas A. (2004). Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor beta and bone morphogenetic protein. Mol. Cell. Biol. 24, 4241–4254 10.1128/MCB.24.10.4241-4254.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M. H., Allis C. D. (1998). Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays 20, 615–626 [DOI] [PubMed] [Google Scholar]
- Kwon C. H., Zhao D., Chen J., Alcantara S., Li Y., Burns D. K., Mason R. P., Lee E. Y., Wu H., Parada L. F. (2008). Pten haploinsufficiency accelerates formation of high-grade astrocytomas. Cancer Res. 68, 3286–3294 10.1158/0008-5472.CAN-08-1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagger G., O’Carroll D., Rembold M., Khier H., Tischler J., Weitzer G., Schuettengruber B., Hauser C., Brunmeir R., Jenuwein T., Seiser C. (2002). Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J. 21, 2672–2681 10.1093/emboj/21.11.2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H. A., Nigg E. A. (1996). Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J. Cell Biol. 135, 1701–1713 10.1083/jcb.135.6.1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasorella A., Boldrini R., Dominici C., Donfrancesco A., Yokota Y., Inserra A., Iavarone A. (2002). Id2 is critical for cellular proliferation and is the oncogenic effector of N-myc in human neuroblastoma. Cancer Res. 62, 301–306 [PubMed] [Google Scholar]
- Lasorella A., Iavarone A. (2006). The protein ENH is a cytoplasmic sequestration factor for Id2 in normal and tumor cells from the nervous system. Proc. Natl. Acad. Sci. U.S.A. 103, 4976–4981 10.1073/pnas.0600168103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. O., Yang H., Georgescu M. M., Di Cristofano A., Maehama T., Shi Y., Dixon J. E., Pandolfi P., Pavletich N. P. (1999). Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99, 323–334 10.1016/S0092-8674(00)81661-X [DOI] [PubMed] [Google Scholar]
- Lehman N. L., Verschuren E. W., Hsu J. Y., Cherry A. M., Jackson P. K. (2006). Overexpression of the anaphase promoting complex/cyclosome inhibitor Emi1 leads to tetraploidy and genomic instability of p53-deficient cells. Cell Cycle 5, 1569–1573 10.4161/cc.5.14.2925 [DOI] [PubMed] [Google Scholar]
- Leverson J. D., Joazeiro C. A., Page A. M., Huang H., Hieter P., Hunter T. (2000). The APC11 RING-H2 finger mediates E2-dependent ubiquitination. Mol. Biol. Cell 11, 2315–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. M., Sun H. (1998). PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells. Proc. Natl. Acad. Sci. U.S.A. 95, 15406–15411 10.1073/pnas.95.10.5718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Yen C., Liaw D., Podsypanina K., Bose S., Wang S. I., Puc J., Miliaresis C., Rodgers L., McCombie R., Bigner S. H., Giovanella B. C., Ittmann M., Tycko B., Hibshoosh H., Wigler M. H., Parsons R. (1997). PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275, 1943–1947 10.1126/science.275.5308.1943 [DOI] [PubMed] [Google Scholar]
- Li M., Shin Y. H., Hou L., Huang X., Wei Z., Klann E., Zhang P. (2008). The adaptor protein of the anaphase promoting complex Cdh1 is essential in maintaining replicative lifespan and in learning and memory. Nat. Cell Biol. 10, 1083–1089 10.1038/ncb1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Wu G., Wan Y. (2007). The dual effects of Cdh1/APC in myogenesis. FASEB J. 21, 3606–3617 10.1096/fj.06-7345com [DOI] [PubMed] [Google Scholar]
- Li X., Tang X., Jablonska B., Aguirre A., Gallo V., Luskin M. B. (2009). p27(KIP1) regulates neurogenesis in the rostral migratory stream and olfactory bulb of the postnatal mouse. J. Neurosci. 29, 2902–2914 10.1523/JNEUROSCI.3517-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M. S., Adamson A., Lin Z., Perez-Ordonez B., Jordan R. C., Tripp S., Perkins S. L., Elenitoba-Johnson K. S. (2002). Expression of Skp2, a p27(Kip1) ubiquitin ligase, in malignant lymphoma: correlation with p27(Kip1) and proliferation index. Blood 100, 2950–2956 10.1182/blood.V100.8.2950 [DOI] [PubMed] [Google Scholar]
- Lin H. K., Wang G., Chen Z., Teruya-Feldstein J., Liu Y., Chan C. H., Yang W. L., Erdjument-Bromage H., Nakayama K. I., Nimer S., Tempst P., Pandolfi P. P. (2009). Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/PKB. Nat. Cell Biol. 11, 420–432 10.1038/ncb1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., Wang T. T., Miller W. H., Jr., White J. H. (2003). Inhibition of F-box protein p45(SKP2) expression and stabilization of cyclin-dependent kinase inhibitor p27(KIP1) in vitamin D analog-treated cancer cells. Endocrinology 144, 749–753 10.1210/en.2002-220908 [DOI] [PubMed] [Google Scholar]
- Lindon C., Pines J. (2004). Ordered proteolysis in anaphase inactivates Plk1 to contribute to proper mitotic exit in human cells. J. Cell Biol. 164, 233–241 10.1083/jcb.200309035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liot C., Seguin L., Siret A., Crouin C., Schmidt S., Bertoglio J. (2011). APC(cdh1) mediates degradation of the oncogenic rho-GEF Ect2 after mitosis. PLoS ONE 6, e23676. 10.1371/journal.pone.0023676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkowitz S., Weissman A. M. (2011). RINGs of good and evil: RING finger ubiquitin ligases at the crossroads of tumour suppression and oncogenesis. Nat. Rev. Cancer 11, 629–643 10.1038/nrc3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listovsky T., Oren Y. S., Yudkovsky Y., Mahbubani H. M., Weiss A. M., Lebendiker M., Brandeis M. (2004). Mammalian Cdh1/Fzr mediates its own degradation. EMBO J. 23, 1619–1626 10.1038/sj.emboj.7600149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Moy P., Kim S., Xia Y., Rajasekaran A., Navarro V., Knudsen B., Bander N. H. (1997). Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 57, 3629–3634 [PubMed] [Google Scholar]
- Liu H. S., Ke C. S., Cheng H. C., Huang C. Y., Su C. L. (2011a). Curcumin-induced mitotic spindle defect and cell cycle arrest in human bladder cancer cells occurs partly through inhibition of aurora A. Mol. Pharmacol. 80, 638–646 10.1124/mol.111.072512 [DOI] [PubMed] [Google Scholar]
- Liu X. S., Song B., Elzey B. D., Ratliff T. L., Konieczny S. F., Cheng L., Ahmad N., Liu X. (2011b). Polo-like kinase 1 facilitates loss of pten tumor suppressor-induced prostate cancer formation. J. Biol. Chem. 286, 35795–35800 10.1074/jbc.M110.176982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Erikson R. L. (2003). Polo-like kinase (plk)1 depletion induces apoptosis in cancer cells. Proc. Natl. Acad. Sci. U.S.A. 100, 5789–5794 10.1073/pnas.0630387100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bergami P., Huang C., Goydos J. S., Yip D., Bar-Eli M., Herlyn M., Smalley K. S., Mahale A., Eroshkin A., Aaronson S., Ronai Z. (2007). Rewired ERK-JNK signaling pathways in melanoma. Cancer Cell 11, 447–460 10.1016/j.ccr.2007.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Ma J., Xue W., Cheng C., Wang Y., Zhao Y., Ke Q., Liu H., Liu Y., Li P., Cui X., He S., Shen A. (2009). The expression and prognosis of FOXO3a and Skp2 in human hepatocellular carcinoma. Pathol. Oncol. Res. 15, 679–687 10.1007/s12253-009-9171-z [DOI] [PubMed] [Google Scholar]
- Lukas C., Sorensen C. S., Kramer E., Santoni-Rugiu E., Lindeneg C., Peters J. M., Bartek J., Lukas J. (1999). Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature 401, 815–818 10.1038/44611 [DOI] [PubMed] [Google Scholar]
- Lukasiewicz K. B., Greenwood T. M., Negron V. C., Bruzek A. K., Salisbury J. L., Lingle W. L. (2011). Control of centrin stability by aurora A. PLoS ONE 6, e21291. 10.1371/journal.pone.0021291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu T. H., Morgan R. J., Leong L., Lim D., McNamara M., Portnow J., Frankel P., Smith D. D., Doroshow J. H., Wong C., Aparicio A., Gandara D. R., Somlo G. (2008). A phase II trial of vorinostat (suberoylanilide hydroxamic acid) in metastatic breast cancer: a California cancer consortium study. Clin. Cancer Res. 14, 7138–7142 10.1158/1078-0432.CCR-08-0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan J. C., Hudson J. W., Bull S., Dennis J. W., Swallow C. J. (2001). Comparative expression of the mitotic regulators SAK and PLK in colorectal cancer. Ann. Surg. Oncol. 8, 729–740 10.1007/s10434-001-0729-6 [DOI] [PubMed] [Google Scholar]
- Madoux F., Simanski S., Chase P., Mishra J. K., Roush W. R., Ayad N. G., Hodder P. (2010). An ultra-high throughput cell-based screen for wee1 degradation inhibitors. J. Biomol. Screen. 15, 907–917 10.1177/1087057110375848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T., Dixon J. E. (1998). The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273, 13375–13378 10.1074/jbc.273.22.13375 [DOI] [PubMed] [Google Scholar]
- Maier D., Jones G., Li X., Schonthal A. H., Gratzl O., Van Meir E. G., Merlo A. (1999). The PTEN lipid phosphatase domain is not required to inhibit invasion of glioma cells. Cancer Res. 59, 5479–5482 [PubMed] [Google Scholar]
- Mamely I., van Vugt M. A., Smits V. A., Semple J. I., Lemmens B., Perrakis A., Medema R. H., Freire R. (2006). Polo-like kinase-1 controls proteasome-dependent degradation of claspin during checkpoint recovery. Curr. Biol. 16, 1950–1955 10.1016/j.cub.2006.08.026 [DOI] [PubMed] [Google Scholar]
- Manchado E., Guillamot M., de Carcer G., Eguren M., Trickey M., Garcia-Higuera I., Moreno S., Yamano H., Canamero M., Malumbres M. (2010). Targeting mitotic exit leads to tumor regression in vivo: modulation by Cdk1, mastl, and the PP2A/B55alpha, delta phosphatase. Cancer Cell 18, 641–654 10.1016/j.ccr.2010.10.028 [DOI] [PubMed] [Google Scholar]
- Margottin-Goguet F., Hsu J. Y., Loktev A., Hsieh H. M., Reimann J. D., Jackson P. K. (2003). Prophase destruction of Emi1 by the SCF(betaTrCP/Slimb) ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Dev. Cell 4, 813–826 10.1016/S1534-5807(03)00153-9 [DOI] [PubMed] [Google Scholar]
- Marino S., Krimpenfort P., Leung C., van der Korput H. A., Trapman J., Camenisch I., Berns A., Brandner S. (2002). PTEN is essential for cell migration but not for fate determination and tumourigenesis in the cerebellum. Development 129, 3513–3522 [DOI] [PubMed] [Google Scholar]
- Marks P., Rifkind R. A., Richon V. M., Breslow R., Miller T., Kelly W. K. (2001). Histone deacetylases and cancer: causes and therapies. Nat. Rev. Cancer 1, 194–202 10.1038/35106079 [DOI] [PubMed] [Google Scholar]
- Marx M., Lebuhotel C., Laugier D., Chapelle A., Calothy G., Saule S. (2010). Down regulation of pRb in cultures of avian neuroretina cells promotes proliferation of reactive Muller-like cells and emergence of retinal stem/progenitors. Exp. Eye Res. 90, 791–801 10.1016/j.exer.2010.03.015 [DOI] [PubMed] [Google Scholar]
- McCubrey J. A., Steelman L. S., Kempf C. R., Chappell W. H., Abrams S. L., Stivala F., Malaponte G., Nicoletti F., Libra M., Bäsecke J., Maksimovic-Ivanic D., Mijatovic S., Montalto G., Cervello M., Cocco L., Martelli A. M. (2011). Therapeutic resistance resulting from mutations in Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR signaling pathways. J. Cell. Physiol. 226, 2762–2781 10.1002/jcp.22647 [DOI] [PubMed] [Google Scholar]
- McGarry T. J., Kirschner M. W. (1998). Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell 93, 1043–1053 10.1016/S0092-8674(00)81209-X [DOI] [PubMed] [Google Scholar]
- Meraldi P., Lukas J., Fry A. M., Bartek J., Nigg E. A. (1999). Centrosome duplication in mammalian somatic cells requires E2F and Cdk2-cyclin A. Nat. Cell Biol. 1, 88–93 10.1038/10054 [DOI] [PubMed] [Google Scholar]
- Miller J. J., Summers M. K., Hansen D. V., Nachury M. V., Lehman N. L., Loktev A., Jackson P. K. (2006). Emi1 stably binds and inhibits the anaphase-promoting complex/cyclosome as a pseudosubstrate inhibitor. Genes Dev. 20, 2410–2420 10.1101/gad.1454006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi Y., Iwao K., Egawa C., Noguchi S. (2001). Association of centrosomal kinase STK15/BTAK mRNA expression with chromosomal instability in human breast cancers. Int. J. Cancer 92, 370–373 10.1002/ijc.1200 [DOI] [PubMed] [Google Scholar]
- Modesitt S. C., Sill M., Hoffman J. S., Bender D. P., Gynecologic Oncology Group (2008). A phase II study of vorinostat in the treatment of persistent or recurrent epithelial ovarian or primary peritoneal carcinoma: a gynecologic oncology group study. Gynecol. Oncol. 109, 182–186 10.1016/j.ygyno.2008.01.009 [DOI] [PubMed] [Google Scholar]
- Mondal G., Sengupta S., Panda C. K., Gollin S. M., Saunders W. S., Roychoudhury S. (2007). Overexpression of Cdc20 leads to impairment of the spindle assembly checkpoint and aneuploidization in oral cancer. Carcinogenesis 28, 81–92 10.1093/carcin/bgl100 [DOI] [PubMed] [Google Scholar]
- Montagnoli A., Fiore F., Eytan E., Carrano A. C., Draetta G. F., Hershko A., Pagano M. (1999). Ubiquitination of p27 is regulated by cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 13, 1181–1189 10.1101/gad.13.9.1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Ozaki T., Koseki H., Nakagawara A., Sakiyama S. (2003). Accumulation of p27 KIP1 is associated with BMP2-induced growth arrest and neuronal differentiation of human neuroblastoma-derived cell lines. Biochem. Biophys. Res. Commun. 307, 206–213 10.1016/S0006-291X(03)01138-0 [DOI] [PubMed] [Google Scholar]
- Nakayama J., Xiao G., Noma K., Malikzay A., Bjerling P., Ekwall K., Kobayashi R., Grewal S. I. (2003). Alp13, an MRG family protein, is a component of fission yeast Clr6 histone deacetylase required for genomic integrity. EMBO J. 22, 2776–2787 10.1093/emboj/cdg248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L., Besson A., Heng J. I., Schuurmans C., Teboul L., Parras C., Philpott A., Roberts J. M., Guillemot F. (2007). p27Kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Bull. Mem. Acad. R. Med. Belg. 162, 310–314 [PubMed] [Google Scholar]
- Nguyen L., Besson A., Roberts J. M., Guillemot F. (2006). Coupling cell cycle exit, neuronal differentiation and migration in cortical neurogenesis. Cell Cycle 5, 2314–2318 10.4161/cc.5.20.3381 [DOI] [PubMed] [Google Scholar]
- Nilsson J. (2011). Cdc20 control of cell fate during prolonged mitotic arrest: do Cdc20 protein levels affect cell fate in response to antimitotic compounds? Bioessays 33, 903–909 10.1002/bies.201100094 [DOI] [PubMed] [Google Scholar]
- Nilsson J., Yekezare M., Minshull J., Pines J. (2008). The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction. Nat. Cell Biol. 10, 1411–1420 10.1038/ncb1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh E. J., Lee J. S. (2003). Functional interplay between modulation of histone deacetylase activity and its regulatory role in G2-M transition. Biochem. Biophys. Res. Commun. 310, 267–273 10.1016/j.bbrc.2003.09.013 [DOI] [PubMed] [Google Scholar]
- North B. J., Marshall B. L., Borra M. T., Denu J. M., Verdin E. (2003). The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol. Cell 11, 437–444 10.1016/S1097-2765(03)00038-8 [DOI] [PubMed] [Google Scholar]
- North B. J., Verdin E. (2007). Interphase nucleo-cytoplasmic shuttling and localization of SIRT2 during mitosis. PLoS ONE 2, e784. 10.1371/journal.pone.0000784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura H., Hagan I. M., Glover D. M. (1995). The conserved schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev. 9, 1059–1073 10.1101/gad.9.9.1059 [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Ozaki T., Miyazaki K., Aoyama M., Miyazaki M., Nakagawara A. (2003). UbcH10 is the cancer-related E2 ubiquitin-conjugating enzyme. Cancer Res. 63, 4167–4173 [PubMed] [Google Scholar]
- Olsson T. G., Silverstein R. A., Ekwall K., Sunnerhagen P. (1999). Transient inhibition of histone deacetylase activity overcomes silencing in the mating-type region in fission yeast. Curr. Genet. 35, 82–87 10.1007/s002940050436 [DOI] [PubMed] [Google Scholar]
- Pallante P., Berlingieri M. T., Troncone G., Kruhoffer M., Orntoft T. F., Viglietto G., Caleo A., Migliaccio I., Decaussin-Petrucci M., Santoro M., Palombini L., Fusco A. (2005). UbcH10 overexpression may represent a marker of anaplastic thyroid carcinomas. Br. J. Cancer 93, 464–471 10.1038/sj.bjc.6602721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J., Chen R. H. (2004). Spindle checkpoint regulates Cdc20p stability in Saccharomyces cerevisiae. Genes Dev. 18, 1439–1451 10.1101/gad.1184204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passmore L. A., Booth C. R., Venien-Bryan C., Ludtke S. J., Fioretto C., Johnson L. N., Chiu W., Barford D. (2005). Structural analysis of the anaphase-promoting complex reveals multiple active sites and insights into polyubiquitylation. Mol. Cell 20, 855–866 10.1016/j.molcel.2005.11.003 [DOI] [PubMed] [Google Scholar]
- Payne D. M., Rossomando A. J., Martino P., Erickson A. K., Her J. H., Shabanowitz J., Hunt D. F., Weber M. J., Sturgill T. W. (1991). Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 10, 885–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perk J., Iavarone A., Benezra R. (2005). Id family of helix-loop-helix proteins in cancer. Nat. Rev. Cancer 5, 603–614 10.1038/nrc1712 [DOI] [PubMed] [Google Scholar]
- Perry S. S., Zhao Y., Nie L., Cochrane S. W., Huang Z., Sun X. H. (2007). Id1, but not Id3, directs long-term repopulating hematopoietic stem-cell maintenance. Blood 110, 2351–2360 10.1182/blood-2007-01-069914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen B. O., Wagener C., Marinoni F., Kramer E. R., Melixetian M., Lazzerini Denchi E., Gieffers C., Matteucci C., Peters J. M., Helin K. (2000). Cell cycle- and cell growth-regulated proteolysis of mammalian CDC6 is dependent on APC-CDH1. Genes Dev. 14, 2330–2343 10.1101/gad.832500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfleger C. M., Kirschner M. W. (2000). The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 14, 655–665 [PMC free article] [PubMed] [Google Scholar]
- Pihan G. A., Purohit A., Wallace J., Malhotra R., Liotta L., Doxsey S. J. (2001). Centrosome defects can account for cellular and genetic changes that characterize prostate cancer progression. Cancer Res. 61, 2212–2219 [PubMed] [Google Scholar]
- Pines J. (2011). Cubism and the cell cycle: the many faces of the APC/C. Nat. Rev. Mol. Cell Biol. 12, 427–438 10.1038/nrm3132 [DOI] [PubMed] [Google Scholar]
- Podsypanina K., Ellenson L. H., Nemes A., Gu J., Tamura M., Yamada K. M., Cordon-Cardo C., Catoretti G., Fisher P. E., Parsons R. (1999). Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc. Natl. Acad. Sci. U.S.A. 96, 1563–1568 10.1073/pnas.96.4.1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapova O., Gorospe M., Bost F., Dean N. M., Gaarde W. A., Mercola D., Holbrook N. J. (2000). c-jun N-terminal kinase is essential for growth of human T98G glioblastoma cells. J. Biol. Chem. 275, 24767–24775 10.1074/jbc.M904591199 [DOI] [PubMed] [Google Scholar]
- Pray T. R., Parlati F., Huang J., Wong B. R., Payan D. G., Bennett M. K., Issakani S. D., Molineaux S., Demo S. D. (2002). Cell cycle regulatory E3 ubiquitin ligases as anticancer targets. Drug Resist. Updat. 5, 249–258 10.1016/S1368-7646(02)00121-8 [DOI] [PubMed] [Google Scholar]
- Qian Y. W., Erikson E., Li C., Maller J. L. (1998). Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis. Mol. Cell. Biol. 18, 4262–4271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao X., Zhang L., Gamper A. M., Fujita T., Wan Y. (2010). APC/C-Cdh1: from cell cycle to cellular differentiation and genomic integrity. Cell Cycle 9, 3904–3912 10.4161/cc.9.19.13585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke S., Pirkmaier A., Germain D. (2005). Differential expression of the F-box proteins Skp2 and Skp2B in breast cancer. Oncogene 24, 3448–3458 10.1038/sj.onc.1208328 [DOI] [PubMed] [Google Scholar]
- Rajasekaran A. K., Anilkumar G., Christiansen J. J. (2005). Is prostate-specific membrane antigen a multifunctional protein? Am. J. Physiol. Cell Physiol. 288, C975–C981 10.1152/ajpcell.00506.2004 [DOI] [PubMed] [Google Scholar]
- Rajasekaran S. A., Christiansen J. J., Schmid I., Oshima E., Ryazantsev S., Sakamoto K., Weinstein J., Rao N. P., Rajasekaran A. K. (2008). Prostate-specific membrane antigen associates with anaphase-promoting complex and induces chromosomal instability. Mol. Cancer Ther. 7, 2142–2151 10.1158/1535-7163.MCT-08-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rape M., Kirschner M. W. (2004). Autonomous regulation of the anaphase-promoting complex couples mitosis to S-phase entry. Nature 432, 588–595 10.1038/nature03023 [DOI] [PubMed] [Google Scholar]
- Rape M., Reddy S. K., Kirschner M. W. (2006). The processivity of multiubiquitination by the APC determines the order of substrate degradation. Cell 124, 89–103 10.1016/j.cell.2005.10.032 [DOI] [PubMed] [Google Scholar]
- Reed S. I. (2008). Deathproof: new insights on the role of skp2 in tumorigenesis. Cancer Cell 13, 88–89 10.1016/j.ccr.2008.01.023 [DOI] [PubMed] [Google Scholar]
- Reimann J. D., Freed E., Hsu J. Y., Kramer E. R., Peters J. M., Jackson P. K. (2001). Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell 105, 645–655 10.1016/S0092-8674(01)00361-0 [DOI] [PubMed] [Google Scholar]
- Reynisdottir I., Polyak K., Iavarone A., Massague J. (1995). Kip/Cip and Ink4 cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 9, 1831–1845 10.1101/gad.9.15.1831 [DOI] [PubMed] [Google Scholar]
- Rose A. E., Wang G., Hanniford D., Monni S., Tu T., Shapiro R. L., Berman R. S., Pavlick A. C., Pagano M., Darvishian F., Mazumdar M., Hernando E., Osman I. (2011). Clinical relevance of SKP2 alterations in metastatic melanoma. Pigment Cell Melanoma Res. 24, 197–206 10.1111/j.1755-148X.2010.00784.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. S., Sheehan C. E., Fisher H. A., Kaufman R. P., Jr, Kaur P., Gray K., Webb I., Gray G. S., Mosher R., Kallakury B. V. (2003). Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin. Cancer Res. 9, 6357–6362 [PubMed] [Google Scholar]
- Ross K. E., Cohen-Fix O. (2003). The role of Cdh1p in maintaining genomic stability in budding yeast. Genetics 165, 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild G., Zhao X., Iavarone A., Lasorella A. (2006). E proteins and Id2 converge on p57Kip2 to regulate cell cycle in neural cells. Mol. Cell. Biol. 26, 4351–4361 10.1128/MCB.01743-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabado Alvarez C. (2008). Molecular biology of retinoblastoma. Clin. Transl. Oncol. 10, 389–394 10.1007/s12094-008-0220-y [DOI] [PubMed] [Google Scholar]
- Salhia B., Tran N. L., Chan A., Wolf A., Nakada M., Rutka F., Ennis M., McDonough W. S., Berens M. E., Symons M., Rutka J. T. (2008). The guanine nucleotide exchange factors trio, Ect2, and Vav3 mediate the invasive behavior of glioblastoma. Am. J. Pathol. 173, 1828–1838 10.2353/ajpath.2008.080043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M., Genkai N., Yajima N., Tsuchiya N., Homma J., Tanaka R., Miki T., Yamanaka R. (2006). Expression level of ECT2 proto-oncogene correlates with prognosis in glioma patients. Oncol. Rep. 16, 1093–1098 [PubMed] [Google Scholar]
- Saunders L. R., Verdin E. (2007). Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene 26, 5489–5504 10.1038/sj.onc.1210616 [DOI] [PubMed] [Google Scholar]
- Schöffski P. (2009). Polo-like kinase (PLK) inhibitors in preclinical and early clinical development in oncology. Oncologist 14, 559–570 10.1634/theoncologist.2009-0010 [DOI] [PubMed] [Google Scholar]
- Schreiber A., Stengel F., Zhang Z., Enchev R. I., Kong E. H., Morris E. P., Robinson C. V., da Fonseca P. C., Barford D. (2011). Structural basis for the subunit assembly of the anaphase-promoting complex. Nature 470, 227–232 10.1038/nature09756 [DOI] [PubMed] [Google Scholar]
- Schuler S., Diersch S., Hamacher R., Schmid R. M., Saur D., Schneider G. (2011). SKP2 confers resistance of pancreatic cancer cells towards TRAIL-induced apoptosis. Int. J. Oncol. 38, 219–225 [PubMed] [Google Scholar]
- Schwickart M., Havlis J., Habermann B., Bogdanova A., Camasses A., Oelschlaegel T., Shevchenko A., Zachariae W. (2004). Swm1/Apc13 is an evolutionarily conserved subunit of the anaphase-promoting complex stabilizing the association of Cdc16 and Cdc27. Mol. Cell. Biol. 24, 3562–3576 10.1128/MCB.24.8.3562-3576.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin L., Liot C., Mzali R., Harada R., Siret A., Nepveu A., Bertoglio J. (2009). CUX1 and E2F1 regulate coordinated expression of the mitotic complex genes Ect2, MgcRacGAP, and MKLP1 in S phase. Mol. Cell. Biol. 29, 570–581 10.1128/MCB.01275-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S., Zhou H., Zhang R. D., Yoon D. S., Vakar-Lopez F., Ito S., Jiang F., Johnston D., Grossman H. B., Ruifrok A. C., Katz R. L., Brinkley W., Czerniak B. (2002). Amplification/overexpression of a mitotic kinase gene in human bladder cancer. J. Natl. Cancer Inst. 94, 1320–1329 10.1093/jnci/94.17.1320 [DOI] [PubMed] [Google Scholar]
- Shomin C. D., Restituyo E., Cox K. J., Ghosh I. (2011). Selection of cyclic-peptide inhibitors targeting aurora kinase A: problems and solutions. Bioorg. Med. Chem. 19, 6743–6749 10.1016/j.bmc.2011.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A., Yunes J. A., Cardoso B. A., Martins L. R., Jotta P. Y., Abecasis M., Nowill A. E., Leslie N. R., Cardoso A. A., Barata J. T. (2008). PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J. Clin. Invest. 118, 3762–3774 10.1172/JCI34616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver D. A., Pellicer I., Fair W. R., Heston W. D., Cordon-Cardo C. (1997). Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 3, 81–85 [PubMed] [Google Scholar]
- Silverstein R. A., Richardson W., Levin H., Allshire R., Ekwall K. (2003). A new role for the transcriptional corepressor SIN3; regulation of centromeres. Curr. Biol. 13, 68–72 10.1016/S0960-9822(02)01432-X [DOI] [PubMed] [Google Scholar]
- Song M. S., Carracedo A., Salmena L., Song S. J., Egia A., Malumbres M., Pandolfi P. P. (2011). Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell 144, 187–199 10.1016/j.cell.2010.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen C. S., Lukas C., Kramer E. R., Peters J. M., Bartek J., Lukas J. (2000). Nonperiodic activity of the human anaphase-promoting complex-Cdh1 ubiquitin ligase results in continuous DNA synthesis uncoupled from mitosis. Mol. Cell. Biol. 20, 7613–7623 10.1128/MCB.20.20.7613-7623.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck P. A., Pershouse M. A., Jasser S. A., Yung W. K., Lin H., Ligon A. H., Langford L. A., Baumgard M. L., Hattier T., Davis T., Frye C., Hu R., Swedlund B., Teng D. H., Tavtigian S. V. (1997). Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 15, 356–362 10.1038/ng0497-356 [DOI] [PubMed] [Google Scholar]
- Stegmuller J., Bonni A. (2005). Moving past proliferation: new roles for Cdh1-APC in postmitotic neurons. Trends Neurosci. 28, 596–601 10.1016/j.tins.2005.09.003 [DOI] [PubMed] [Google Scholar]
- Strahl B. D., Allis C. D. (2000). The language of covalent histone modifications. Nature 403, 41–45 10.1038/47412 [DOI] [PubMed] [Google Scholar]
- Strebhardt K., Kneisel L., Linhart C., Bernd A., Kaufmann R. (2000). Prognostic value of polo like kinase expression in melanomas. JAMA 283, 479–480 10.1001/jama.283.4.479 [DOI] [PubMed] [Google Scholar]
- Strebhardt K., Ullrich A. (2006). Targeting polo-like kinase 1 for cancer therapy. Nat. Rev. Cancer 6, 321–330 10.1038/nrc1841 [DOI] [PubMed] [Google Scholar]
- Summers M. K., Pan B., Mukhyala K., Jackson P. K. (2008). The unique N terminus of the UbcH10 E2 enzyme controls the threshold for APC activation and enhances checkpoint regulation of the APC. Mol. Cell 31, 544–556 10.1016/j.molcel.2008.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweat S. D., Pacelli A., Murphy G. P., Bostwick D. G. (1998). Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology 52, 637–640 10.1016/S0090-4295(98)00278-7 [DOI] [PubMed] [Google Scholar]
- Taddei A., Roche D., Sibarita J. B., Huart S., Maison C., Bailly D., Almouzni G. (2001). Localizing Replication Sites and Nuclear Proteins. Mapping Protein/DNA Interactions by Cross-linking. Paris: Institut national de la sante et de la recherche medicale (INSERM) [PubMed] [Google Scholar]
- Tamamori-Adachi M., Hayashida K., Nobori K., Omizu C., Yamada K., Sakamoto N., Kamura T., Fukuda K., Ogawa S., Nakayama K. I., Kitajima S. (2004). Down-regulation of p27Kip1 promotes cell proliferation of rat neonatal cardiomyocytes induced by nuclear expression of cyclin D1 and CDK4. evidence for impaired Skp2-dependent degradation of p27 in terminal differentiation. J. Biol. Chem. 279, 50429–50436 10.1074/jbc.M403084200 [DOI] [PubMed] [Google Scholar]
- Tang Z., Li B., Bharadwaj R., Zhu H., Ozkan E., Hakala K., Deisenhofer J., Yu H. (2001). APC2 cullin protein and APC11 RING protein comprise the minimal ubiquitin ligase module of the anaphase-promoting complex. Mol. Biol. Cell 12, 3839–3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarui T., Takahashi T., Nowakowski R. S., Hayes N. L., Bhide P. G., Caviness V. S. (2005). Overexpression of p27 kip 1, probability of cell cycle exit, and laminar destination of neocortical neurons. Cereb. Cortex 15, 1343–1355 10.1093/cercor/bhi017 [DOI] [PubMed] [Google Scholar]
- Thoma C. R., Toso A., Meraldi P., Krek W. (2011). Mechanisms of aneuploidy and its suppression by tumour suppressor proteins. Swiss. Med. Wkly. 141, w13170. [DOI] [PubMed] [Google Scholar]
- Thornton B. R., Toczyski D. P. (2006). Precise destruction: an emerging picture of the APC. Genes Dev. 20, 3069–3078 10.1101/gad.1478306 [DOI] [PubMed] [Google Scholar]
- Tribukait B. (1991). DNA flow cytometry in carcinoma of the prostate for diagnosis, prognosis and study of tumor biology. Acta Oncol. 30, 187–192 10.3109/02841869109092348 [DOI] [PubMed] [Google Scholar]
- Trosko J. E. (2009). Review paper: cancer stem cells and cancer nonstem cells: from adult stem cells or from reprogramming of differentiated somatic cells. Vet. Pathol. 46, 176–193 10.1354/vp.46-2-176 [DOI] [PubMed] [Google Scholar]
- Troyer J. K., Beckett M. L., Wright G. L., Jr. (1995). Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int. J. Cancer 62, 552–558 10.1002/ijc.2910620511 [DOI] [PubMed] [Google Scholar]
- Tsuiki H., Tnani M., Okamoto I., Kenyon L. C., Emlet D. R., Holgado-Madruga M., Lanham I. S., Joynes C. J., Vo K. T., Wong A. J. (2003). Constitutively active forms of c-jun NH2-terminal kinase are expressed in primary glial tumors. Cancer Res. 63, 250–255 [PubMed] [Google Scholar]
- Tsvetkov L. M., Yeh K. H., Lee S. J., Sun H., Zhang H. (1999). p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr. Biol. 9, 661–664 10.1016/S0960-9822(99)80290-5 [DOI] [PubMed] [Google Scholar]
- Tury A., Mairet-Coello G., DiCicco-Bloom E. (2011). The cyclin-dependent kinase inhibitor p57Kip2 regulates cell cycle exit, differentiation, and migration of embryonic cerebral cortical precursors. Cereb. Cortex 21, 1840–1856 10.1093/cercor/bhq254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin S., Hussain A., Al-Hussein K., Platanias L. C., Bhatia K. G. (2004). Inhibition of phosphatidylinositol 3’-kinase induces preferentially killing of PTEN-null T leukemias through AKT pathway. Biochem. Biophys. Res. Commun. 320, 932–38 10.1016/j.bbrc.2004.06.038 [DOI] [PubMed] [Google Scholar]
- van Ree J. H., Jeganathan K. B., Malureanu L., van Deursen J. M. (2010). Overexpression of the E2 ubiquitin-conjugating enzyme UbcH10 causes chromosome missegregation and tumor formation. J. Cell Biol. 188, 83–100 10.1083/jcb.200906147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero A., Scher M. B., Lee D. H., Sutton A., Cheng H. L., Alt F. W., Serrano L., Sternglanz R., Reinberg D. (2006). SirT2 is a histone deacetylase with preference for histone H4 lys 16 during mitosis. Genes Dev. 20, 1256–1261 10.1101/gad.1412706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon A. E., Devine C., Philpott A. (2003). The cdk inhibitor p27Xic1 is required for differentiation of primary neurones in Xenopus. Development 130, 85–92 10.1242/dev.00180 [DOI] [PubMed] [Google Scholar]
- Vidal A., Koff A. (2000). Cell-cycle inhibitors: three families united by a common cause. Gene 247, 1–15 10.1016/S0378-1119(00)00092-5 [DOI] [PubMed] [Google Scholar]
- Visintin R., Prinz S., Amon A. (1997). CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science 278, 460–463 10.1126/science.278.5337.460 [DOI] [PubMed] [Google Scholar]
- Wai D. H., Schaefer K. L., Schramm A., Korsching E., Van Valen F., Ozaki T., Boecker W., Schweigerer L., Dockhorn-Dworniczak B., Poremba C. (2002). Expression analysis of pediatric solid tumor cell lines using oligonucleotide microarrays. Int. J. Oncol. 20, 441–451 [PubMed] [Google Scholar]
- Walker S. M., Leslie N. R., Perera N. M., Batty I. H., Downes C. P. (2004). The tumour-suppressor function of PTEN requires an N-terminal lipid-binding motif. Biochem. J. 379, 301–307 10.1042/BJ20031839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Y., Kung H. J. (2011). Male germ cell-associated kinase is overexpressed in prostate cancer cells and causes mitotic defects via deregulation of APC/C(CDH1). Oncogene. [Epub ahead of print]. 10.1038/onc.2011.464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Moyret-Lalle C., Couzon F., Surbiguet-Clippe C., Saurin J. C., Lorca T., Navarro C., Puisieux A. (2003). Alterations of anaphase-promoting complex genes in human colon cancer cells. Oncogene 22, 1486–1490 10.1038/sj.onc.1206125 [DOI] [PubMed] [Google Scholar]
- Wang R. H., Sengupta K., Li C., Kim H. S., Cao L., Xiao C., Kim S., Xu X., Zheng Y., Chilton B., Jia R., Zheng Z. M., Appella E., Wang X. W., Ried T., Deng C. X. (2008a). Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell 14, 312–323 10.1016/j.ccr.2008.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. H., Zheng Y., Kim H. S., Xu X., Cao L., Luhasen T., Lee M. H., Xiao C., Vassilopoulos A., Chen W., Gardner K., Man Y. G., Hung M. C., Finkel T., Deng C. X. (2008b). Interplay among BRCA1, SIRT1, and survivin during BRCA1-associated tumorigenesis. Mol. Cell 32, 11–20 10.1016/j.molcel.2008.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Dai W. (2005). Shugoshin, a guardian for sister chromatid segregation. Exp. Cell Res. 310, 1–9 10.1016/j.yexcr.2005.07.018 [DOI] [PubMed] [Google Scholar]
- Wasch R., Engelbert D. (2005). Anaphase-promoting complex-dependent proteolysis of cell cycle regulators and genomic instability of cancer cells. Oncogene 24, 1–10 10.1038/sj.onc.1208017 [DOI] [PubMed] [Google Scholar]
- Watanabe N., Arai H., Nishihara Y., Taniguchi M., Watanabe N., Hunter T., Osada H. (2004). M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP. Proc. Natl. Acad. Sci. U.S.A. 101, 4419–4424 10.1073/pnas.0306992101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W., Ayad N. G., Wan Y., Zhang G. J., Kirschner M. W., Kaelin W. G., Jr. (2004). Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature 428, 194–198 10.1038/nature02381 [DOI] [PubMed] [Google Scholar]
- Weichert W., Schmidt M., Gekeler V., Denkert C., Stephan C., Jung K., Loening S., Dietel M., Kristiansen G. (2004). Polo-like kinase 1 is overexpressed in prostate cancer and linked to higher tumor grades. Prostate 60, 240–245 10.1002/pros.20050 [DOI] [PubMed] [Google Scholar]
- Westermann F., Henrich K. O., Wei J. S., Lutz W., Fischer M., Konig R., Wiedemeyer R., Ehemann V., Brors B., Ernestus K., Leuschner I., Benner A., Khan J., Schwab M. (2007). High Skp2 expression characterizes high-risk neuroblastomas independent of MYCN status. Clin. Cancer Res. 13, 4695–4703 10.1158/1078-0432.CCR-06-2818 [DOI] [PubMed] [Google Scholar]
- Wickliffe K. E., Lorenz S., Wemmer D. E., Kuriyan J., Rape M. (2011a). The mechanism of linkage-specific ubiquitin chain elongation by a single-subunit E2. Cell 144, 769–781 10.1016/j.cell.2011.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickliffe K. E., Williamson A., Meyer H. J., Kelly A., Rape M. (2011b). K11-linked ubiquitin chains as novel regulators of cell division. Trends Cell Biol. 21, 656–663 10.1016/j.tcb.2011.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A., Wickliffe K. E., Mellone B. G., Song L., Karpen G. H., Rape M. (2009). Identification of a physiological E2 module for the human anaphase-promoting complex. Proc. Natl. Acad. Sci. U.S.A. 106, 18213–18218 10.1073/pnas.0907887106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth K. G., Ricci R., Gimenez-Abian J. F., Taghybeeglu S., Kudo N. R., Jochum W., Vasseur-Cognet M., Nasmyth K. (2004). Loss of the anaphase-promoting complex in quiescent cells causes unscheduled hepatocyte proliferation. Genes Dev. 18, 88–98 10.1101/gad.285404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlschlegel J. A., Dwyer B. T., Dhar S. K., Cvetic C., Walter J. C., Dutta A. (2000). Inhibition of eukaryotic DNA replication by geminin binding to Cdt1. Science 290, 2309–2312 10.1126/science.290.5500.2309 [DOI] [PubMed] [Google Scholar]
- Wolf G., Elez R., Doermer A., Holtrich U., Ackermann H., Stutte H. J., Altmannsberger H. M., Rübsamen-Waigmann H., Strebhardt K. (1997). Prognostic significance of polo-like kinase (PLK) expression in non-small cell lung cancer. Oncogene 14, 543–549 10.1038/sj.onc.1200862 [DOI] [PubMed] [Google Scholar]
- Wolf G., Hildenbrand R., Schwar C., Grobholz R., Kaufmann M., Stutte H. J., Strebhardt K., Bleyl U. (2000). Polo-like kinase: a novel marker of proliferation: correlation with estrogen-receptor expression in human breast cancer. Pathol. Res. Pract. 196, 753–759 [DOI] [PubMed] [Google Scholar]
- Wu G., Glickstein S., Liu W., Fujita T., Li W., Yang Q., Duvoisin R., Wan Y. (2007). The anaphase-promoting complex coordinates initiation of lens differentiation. Mol. Biol. Cell 18, 1018–1029 10.1091/mbc.E06-09-0793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Qiu X. L., Zhou L., Zhu J., Chamberlin R., Lau J., Chen P. L., Lee W. H. (2008). Small molecule targeting the Hec1/Nek2 mitotic pathway suppresses tumor cell growth in culture and in animal. Cancer Res. 68, 8393–8399 10.1158/0008-5472.CAN-08-1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Merbl Y., Huo Y., Gallop J. L., Tzur A., Kirschner M. W. (2010). UBE2S drives elongation of K11-linked ubiquitin chains by the anaphase-promoting complex. Proc. Natl. Acad. Sci. U.S.A. 107, 1355–1360 10.1073/pnas.1001825107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H. H., He H., De Wang J., Gu Q., Lin M. C., Zou B., Yu L. F., Sun Y. W., Chan A. O., Kung H. F., Wong B. C. (2006). Induction of apoptosis and cell cycle arrest by a specific c-jun NH2-terminal kinase (JNK) inhibitor, SP-600125, in gastrointestinal cancers. Cancer Lett. 241, 268–274 10.1016/j.canlet.2005.10.031 [DOI] [PubMed] [Google Scholar]
- Xia L., Robinson D., Ma A. H., Chen H. C., Wu F., Qiu Y., Kung H. J. (2002). Identification of human male germ cell-associated kinase, a kinase transcriptionally activated by androgen in prostate cancer cells. J. Biol. Chem. 277, 35422–35433 10.1074/jbc.M201369200 [DOI] [PubMed] [Google Scholar]
- Xu W. J., Zhang S., Yang Y., Zhang N., Wang W., Liu S. Y., Tian H. W., Dai L., Xie Q., Zhao X., Wei Y. Q., Deng H. X. (2011). Efficient inhibition of human colorectal carcinoma growth by RNA interference targeting polo-like kinase 1 in vitro and in vivo. Cancer Biother. Radiopharm. 26, 427–436 10.1089/cbr.2010.0922 [DOI] [PubMed] [Google Scholar]
- Yang G., Ayala G., De Marzo A., Tian W., Frolov A., Wheeler T. M., Thompson T. C., Harper J. W. (2002). Elevated Skp2 protein expression in human prostate cancer: association with loss of the cyclin-dependent kinase inhibitor p27 and PTEN and with reduced recurrence-free survival. Clin. Cancer Res. 8, 3419–3426 [PubMed] [Google Scholar]
- Yang Y. M., Bost F., Charbono W., Dean N., McKay R., Rhim J. S., Depatie C., Mercola D. (2003). C-jun NH(2)-terminal kinase mediates proliferation and tumor growth of human prostate carcinoma. Clin. Cancer Res. 9, 391–401 [PubMed] [Google Scholar]
- Yao W., Qian W., Zhu C., Gui L., Qiu J., Zhang C. (2010). Cdh1-APC is involved in the differentiation of neural stem cells into neurons. Neuroreport 21, 39–44 10.1097/WNR.0b013e32833312fe [DOI] [PubMed] [Google Scholar]
- Ye Y., Rape M. (2009). Building ubiquitin chains: E2 enzymes at work. Nat. Rev. Mol. Cell Biol. 10, 755–764 10.1038/nrm2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi S., Yasui K., Iizasa T., Takahashi T., Fujisawa T., Inazawa J. (2003). Down-regulation of SKP2 induces apoptosis in lung-cancer cells. Cancer Sci. 94, 344–349 10.1111/j.1349-7006.2003.tb01444.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y. (2001). Id and development. Oncogene 20, 8290–8298 10.1038/sj.onc.1205090 [DOI] [PubMed] [Google Scholar]
- Yoon H. E., Kim S. A., Choi H. S., Ahn M. Y., Yoon J. H., Ahn S. G. (2011). Inhibition of Plk1 and Pin1 by 5′-nitro-indirubinoxime suppresses human lung cancer cells. Cancer Lett. [Epub ahead of print]. 10.1016/j.canlet.2011.10.029 [DOI] [PubMed] [Google Scholar]
- Yoshida M., Shimazu T., Matsuyama A. (2003). Protein deacetylases: enzymes with functional diversity as novel therapeutic targets. Prog. Cell Cycle Res. 5, 269–278 [PubMed] [Google Scholar]
- Yu Z. K., Gervais J. L., Zhang H. (1998). Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1) and cyclin D proteins. Proc. Natl. Acad. Sci. U.S.A. 95, 11324–11329 10.1073/pnas.95.14.7993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B., Xu Y., Woo J. H., Wang Y., Bae Y. K., Yoon D. S., Wersto R. P., Tully E., Wilsbach K., Gabrielson E. (2006). Increased expression of mitotic checkpoint genes in breast cancer cells with chromosomal instability. Clin. Cancer Res. 12, 405–410 10.1158/1078-0432.CCR-05-0903 [DOI] [PubMed] [Google Scholar]
- Zachariae W., Nasmyth K. (1999). Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev. 13, 2039–2058 10.1101/gad.13.16.2039 [DOI] [PubMed] [Google Scholar]
- Zeng X., Sigoillot F., Gaur S., Choi S., Pfaff K. L., Oh D. C., Hathaway N., Dimova N., Cuny G. D., King R. W. (2010). Pharmacologic inhibition of the anaphase-promoting complex induces a spindle checkpoint-dependent mitotic arrest in the absence of spindle damage. Cancer Cell 18, 382–395 10.1016/j.ccr.2010.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Park C. H., Wu J., Kim H., Liu W., Fujita T., Balasubramani M., Schreiber E. M., Wang X. F., Wan Y. (2010). Proteolysis of Rad17 by Cdh1/APC regulates checkpoint termination and recovery from genotoxic stress. EMBO J. 29, 1726–1737 10.1038/emboj.2010.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Wong C., Liu D., Finegold M., Harper J. W., Elledge S. J. (1999). p21(CIP1) and p57(KIP2) control muscle differentiation at the myogenin step. Genes Dev. 13, 213–224 10.1101/gad.13.18.2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Chen Y., Lee W. H. (1999). Hec1p, an evolutionarily conserved coiled-coil protein, modulates chromosome segregation through interaction with SMC proteins. Mol. Cell. Biol. 19, 5417–5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N., Schulman B. A., Song L., Miller J. J., Jeffrey P. D., Wang P., Chu C., Koepp D. M., Elledge S. J., Pagano M., Conaway R. C., Conaway J. W., Harper J. W., Pavletich N. P. (2002). Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature 416, 703–709 10.1038/416703a [DOI] [PubMed] [Google Scholar]
- Zhou H., Kuang J., Zhong L., Kuo W. L., Gray J. W., Sahin A., Brinkley B. R., Sen S. (1998). Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat. Genet. 20, 189–193 10.1038/2496 [DOI] [PubMed] [Google Scholar]
- Zhu L., Skoultchi A. I. (2001). Coordinating cell proliferation and differentiation. Curr. Opin. Genet. Dev. 11, 91–97 10.1016/S0959-437X(00)00162-3 [DOI] [PubMed] [Google Scholar]
- Zhu Q., Pearson-White S., Luo K. (2005). Requirement for the SnoN oncoprotein in transforming growth factor beta-induced oncogenic transformation of fibroblast cells. Mol. Cell. Biol. 25, 10731–10744 10.1128/MCB.25.24.10731-10744.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Depamphilis M. L. (2009). Selective killing of cancer cells by suppression of geminin activity. Cancer Res. 69, 4870–4877 10.1158/0008-5472.SABCS-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibat A., Missiaglia E., Rosenberger A., Pritchard-Jones K., Shipley J., Hahn H., Fulda S. (2010). Activation of the hedgehog pathway confers a poor prognosis in embryonal and fusion gene-negative alveolar rhabdomyosarcoma. Oncogene 29, 6323–6330 10.1038/onc.2010.368 [DOI] [PubMed] [Google Scholar]