Abstract
The phage shock protein (Psp) system is a conserved extracytoplasmic stress response in bacteria that is essential for virulence of the human pathogen Yersinia enterocolitica. This article summarizes some recent findings about Y. enterocolitica Psp system function. Increased psp gene expression requires the transcription factor PspF, but under non-inducing conditions PspF is inhibited by an interaction with another protein, PspA, in the cytoplasm. A Psp-inducing stimulus causes PspA to relocate to the cytoplasmic membrane, freeing PspF to induce psp gene expression. This PspA relocation requires the integral cytoplasmic membrane proteins, PspB and PspC, which might sense an inducing trigger and sequester PspA by direct interaction. The subsequent induction of psp gene expression increases the PspA concentration, which also allows it to contact the membrane directly, perhaps for its physiological function. Mutational analysis of the PspB and PspC proteins has revealed that they both positively and negatively regulate psp gene expression and has also identified PspC domains associated with each function. We also compare the contrasting physiological roles of the Psp system in the virulence of Y. enterocolitica and Salmonella enterica sv. Typhimurium (S. Typhimurium). In S. Typhimurium, PspA maintains the proton motive force, which provides the energy needed to drive ion importers required for survival within macrophages. In contrast, in the extracellular pathogen Y. enterocolitica, PspB and PspC, but not PspA, are the Psp components needed for virulence. PspBC protect Y. enterocolitica from damage caused by the secretin component of its type 3 secretion system, an essential virulence factor.
Keywords: Yersinia, stress-response, secretin, membrane, virulence
Introduction
Yersinia is a Gram-negative bacterial genus within the family Enterobacteriaceae and is comprised of several species. Three of these species are considered primary human pathogens: Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica. Y. pestis is the etiologic agent of plague (Perry and Fetherston, 1997), whereas Y. enterocolitica and Y. pseudotuberculosis cause a self-limiting enteritis that only rarely progresses to a systemic infection (Cornelis et al., 1987). Despite these different diseases, the three pathogenic species share a number of characteristics, including an approximately 70 kb virulence plasmid (e.g. Portnoy and Martinez, 1985) encoding the Ysc-Yop type three secretion system (T3SS; e.g. Cornelis, 2002). This system is essential for the virulence of all three pathogenic species in mouse models of infection.
T3SSs are widely conserved in pathogenic Gram-negative bacteria where they function to traffic cytotoxic substrates from bacterial to host cell cytoplasm (Cornelis, 2006). A critical component of all T3SSs is an oligomeric outer membrane pore-forming protein known as a secretin (Genin and Boucher, 1994; Korotkov et al., 2011). However, production of the Y. enterocolitica Ysc-Yop T3SS, and more specifically production of its secretin component YscC, triggers an envelope stress response called the phage shock protein (Psp) system (Darwin and Miller, 2001). Mutational inactivation of the Psp system renders Y. enterocolitica sensitive to production of the YscC secretin alone, sensitive to production of the native Ysc-Yop T3SS, and avirulent in mice (Darwin and Miller, 1999, 2001). In this review we will describe some of our work to understand the regulation and function of the Y. enterocolitica Psp system, with a particular focus on more recent investigations into how the system is regulated. We also refer the reader to other general reviews of the Psp system (Darwin, 2005; Joly et al., 2010).
Original discovery of the Psp system
The Psp system was first described in Escherichia coli K-12 in a paper from Peter Model’s laboratory over twenty years ago (Brissette et al., 1990). Their report identified an approximately 25 kDa E. coli protein that was massively produced during infection by filamentous phage f1. This protein was named phage shock protein A (PspA) and subsequently found to be encoded by the first gene of the pspABCDE operon (Brissette et al., 1991). pspA operon expression was also shown to be induced by other triggers, including heat, ethanol, and osmotic shock, all of which have the capacity to compromise the cytoplasmic membrane (e.g. Brissette et al., 1990; Kleerebezem and Tommassen, 1993; Weiner and Model, 1994). The induction of PspA synthesis during phage f1 infection was due specifically to production of the phage-encoded protein IV (Brissette et al., 1990), which is a member of the above-described secretin family. Other work has indicated that it is the mislocalization of a secretin into the cytoplasmic membrane that is a potent Psp-inducing signal (Guilvout et al., 2006). Taken together, these and other observations suggest a link between cytoplasmic membrane disruption and Psp system function.
Discovery of the Psp system in Y. enterocolitica
Random identification of transposon insertion mutants attenuated in a mouse model of systemic infection led to the discovery of the Y. enterocolitica Psp system. Specifically, a pspC transposon insertion mutant was completely avirulent in a mouse model of infection (Darwin and Miller, 1999). Subsequent work showed that production of the Ysc-Yop T3SS, or just its YscC secretin component, caused a severe growth defect in the pspC null mutant (Darwin and Miller, 2001). This was a striking discovery because all of the phenotypes described previously for E. coli psp null mutants were subtle (e.g. survival defect during prolonged stationary phase at alkaline pH; Weiner and Model, 1994).
Eight genes encode the Y. enterocolitica Psp system. Seven are at a single locus, which is made up of the pspABCDycjXF operon and the divergently transcribed pspF gene. The eighth is the unlinked pspG gene (Green and Darwin, 2004). Of these, individual disruption of the pspD, ycjX, ycjF, and pspG genes has not been reported to cause any detectable phenotype. This is in sharp contrast to the pspFABC genes for which removal of any one of them results in significant effects on the regulation of psp gene expression and/or stress- tolerance (reviewed by Darwin, 2005). For this reason we consider the PspF, -A, -B, and -C proteins to be the core Y. enterocolitica Psp system and our work has focused primarily on understanding their functions.
The PspF, -A, -B, and -C proteins control psp gene expression
The PspF, -A, -B, and -C proteins are needed for normal regulation of psp gene expression. PspF is a DNA-binding protein required for expression of the σ54-dependent promoters located upstream of pspA and pspG (Jovanovic et al., 1996; Green and Darwin, 2004). In contrast, PspA negatively regulates these promoters. Work in E. coli has demonstrated that PspA mediates this negative effect by forming a complex with PspF and inhibiting its transcription activation ability (Dworkin et al., 2000; Elderkin et al., 2002). Finally, the integral cytoplasmic membrane proteins PspB and -C are required for stress-dependent induction of the pspA and pspG promoters, which is probably achieved by modulating the PspA-PspF inhibitory complex, as described below.
Activation of the Y. enterocolitica Psp response is essential for its virulence. Therefore, a detailed understanding of the molecular events underlying the regulation of psp gene expression is one of the areas into which we have devoted considerable attention. Some of our more recent work is summarized below.
Activation of the Y. enterocolitica Psp system involves a change in PspA location
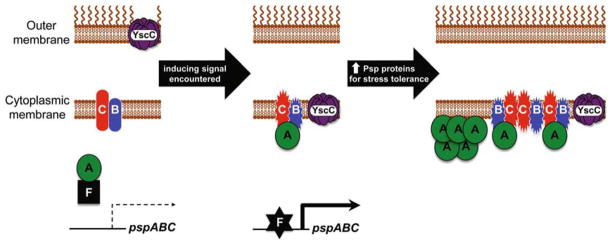
We recently published a study that provided an improved spatial understanding of how integral (PspBC) and peripheral (PspA) cytoplasmic membrane proteins communicate with each other and with the soluble transcriptional activator PspF (Yamaguchi et al., 2010). The work was founded on the hypothesis that the communication probably involved a change in the location of PspF and/or PspA, with two proposals for how this might work. First, that PspA is always membrane-associated, perhaps in complex with PspBC. In this scenario, under non-Psp inducing conditions PspA would sequester PspF into this membrane bound complex. However, in Psp-inducing conditions a signal transmitted via PspBC would cause PspA to release PspF to the cytoplasm where it could activate transcription. In the second hypothesis, the PspA-PspF inhibitory complex would form in the cytoplasm. When a Psp-inducing stimulus is encountered the PspBC proteins would then interact with PspA, moving PspA into a membrane-associated complex and freeing PspF to activate psp gene expression.
We distinguished between these hypotheses by separating Y. enterocolitica cells into soluble and insoluble (membrane) fractions and determining the location of PspF, -A, -B, and -C by immnoblot analysis (Yamaguchi et al., 2010). Those experiments revealed that the locations of PspF, -B, and -C do not change. PspF was always soluble and PspB and -C were always in the membrane. However, under non-Psp- inducing conditions PspA was soluble, whereas upon Psp system induction the majority was now associated with the membrane. These data supported our second hypothesis, with an inhibitory PspA-PspF complex forming in the cytoplasm under non-inducing conditions and PspA moving to the membrane upon induction (Fig. 1). Subsequent experiments revealed that the relocation of PspA to the membrane was dependent on PspBC and probably involves a direct protein-protein interaction with them (Yamaguchi et al., 2010).
Fig. 1. Model for activation of the Y. enterocolitica Psp system.
In non-inducing conditions PspB and PspC are in an “off” state and PspA forms an inhibitory complex with PspF in the cytoplasm. Secretin mislocalization to the cytoplasmic membrane generates an inducing signal that switches PspB and PspC to their “on” state, allowing them to sequester PspA by protein-protein interaction. PspF becomes active, induces the pspA promoter, and the concentration of PspA, -B, and -C increases to the levels required for stress-relief and/or prevention. The increase in PspA concentration also compensates for a low phospholipid binding affinity, allowing it to bind to the membrane directly only after Psp system induction.
We also considered the potential impact of the increased PspA protein concentration that happens after a Psp-inducing stimulus is encountered. However, our experiments were able to determine that the PspBC-dependent relocation of PspA to the membrane happened independently of any increase in PspA concentration (Yamaguchi et al., 2010). Thus, the basal level Psp proteins are sufficient to act as a stress-responsive transcriptional switch. Nevertheless, we also discovered that artificially elevating PspA concentration in the absence of a Psp-inducing stimulus caused it to associate with the membrane in a PspBC-independent manner. Therefore, it appears that PspA has two different mechanisms to locate to the membrane. First, by a direct interaction with one or both of the integral membrane proteins PspB and -C. Second, by direct interaction with the membrane phospholipids or with some other unidentified membrane component.
These data contributed to our current model that describes the situation before, during and after activation of the Y. enterocolitica Psp response (Fig. 1 and Yamaguchi et al., 2010). According to this model, in an uniduced cell, PspA forms an inhibitory complex with PspF in the cytoplasm, perhaps with PspF also bound to DNA. The integral cytoplasmic membrane proteins PspB and PspC might switch between two hypothetical states (e.g. conformations) depending on the presence or absence of a Psp-inducing signal. In the uninduced cell they are in their “off” state. However, when an inducing stimulus is encountered, such as a mislocalized secretin protein, PspB and/or PspC respond by changing into their “on” state. This allows them to interact with PspA, sequestering it to the membrane and freeing PspF to activate transcription. All of these events occur with only the basal level of Psp proteins. However, once this regulatory switch mechanism has activated, the subsequent increase in PspA concentration compensates for its inherent low binding affinity for the phospholipid membrane. As a result, at high concentration PspA is able to interact directly with the membrane to facilitate its poorly-understood physiological function.
PspB and PspC regulate psp gene expression in multiple ways
Work on both the E. coli and Y. enterocolitica Psp systems has shown that PspB and -C are required for stress-dependent induction of psp gene expression in most conditions (e.g. Weiner et al., 1991; Kleerebezem et al., 1996; Maxson and Darwin, 2006; Gueguen et al., 2009). PspB and -C are small integral cytoplasmic membrane proteins that interact with each another (Maxson and Darwin, 2006). Their locations are consistent with the proposal that one or both of them acts to detect a Psp-inducing signal, such as altered membrane properties, and to transmit this information to PspA by direct protein-protein interaction (see above). In the Y. enterocolitica Psp system we have studied various aspects of PspB and -C function. We view them as multi-functional components of the Psp response because, in addition to regulating psp gene expression, they also help to alleviate secretin-induced stress (Maxson and Darwin, 2006 and see below).
To better understand PspB and -C functions in Y. enterocolitica we isolated and characterized altered function mutants (Gueguen et al., 2009). Alanine substitution of some highly conserved amino acids in PspC resulted in activation of pspA promoter expression in the absence of a Psp-inducing signal. Subsequent random mutagenesis and truncation analysis provided many PspC and PspB mutants with similar constitutive regulatory activity (Gueguen et al., 2009). This suggests that an interaction between PspB and PspC might be important for negative regulation of psp gene expression, providing a third function for these proteins (secretin-stress tolerance, positive regulation of psp gene expression, and negative regulation of psp gene expression). Specifically, the N-terminus of PspC appears to be important for this negative regulatory function because removing it also causes constitutive psp gene expression (Gueguen et al., 2009).
This mutational investigation also allowed us to isolate PspC (but not PspB) mutants that lost only their ability to activate psp gene expression in response to stress (Gueguen et al., 2009). These mutations mapped to a region within the C-terminus of PspC that is predicted to contain a leucine- zipper like amphipathic helix. Truncation analysis confirmed the importance of this region for positive regulatory function. This domain of PspC might be needed to sense a Psp-inducing signal. Alternatively, it might facilitate psp gene activation by using the hydrophobic face of the amphipathic helix to dimerize (Gueguen et al., 2011) and/or to interact with other Psp proteins as part of the signal transduction pathway.
Finally, in addition to their places in the signal transduction pathway regulating psp gene transcription, we also discovered that PspB controls the level of PspC post-transcriptionally in Y. enterocolitica (Gueguen et al., 2009). Subsequent experiments revealed that PspB protects PspC form proteolysis by the cytoplasmic membrane protease FtsH, in both Y. enterocolitica and E. coli (Singh and Darwin, 2011). In the absence of PspB, PspC production is toxic and we hypothesize that this mechanism has evolved to rapidly remove it from the cell. This is analogous to FtsH-dependent degradation of the E. coli SecY and AtpB proteins produced in excess of their binding partners (Kihara et al., 1995; Akiyama et al., 1996). SecY and AtpB function in complexes that move proteins or protons, respectively, across the cytoplasmic membrane. If they fail to assemble into their native complexes their unregulated sub-reactions might compromise cytoplasmic membrane integrity, making rapid FtsH-dependent degradation critical. Therefore, perhaps PspC might also compromise membrane integrity if it fails to interact with PspB. In fact, experiments in E. coli suggest that PspC does compromise membrane potential unless it is co-produced with PspB (Jovanovic et al., 2010). These observations might offer a clue into one function of the PspBC complex, such as a carefully controlled role in altering cytoplasmic membrane properties, with PspB controlling this activity of PspC. However, this is speculative and would require considerable further experimentation to investigate fully.
The specific link between secretins and induction of the Psp system
The Psp system has been linked to secretin proteins since its original discovery. The massive induction of PspA synthesis observed by Model and his colleagues when E. coli was infected with filamentous phage f1 was caused by the phage-encoded secretin, protein IV (Brissette et al., 1990). Furthermore, the relationship between secretins and the Psp system is remarkably specific. Microarray analysis showed that when the protein IV secretin was produced in E. coli or Salmonella enterica, the only significantly upregulated genes were the psp genes, and no genes were downregulated (Lloyd et al., 2004). Our laboratory reported a similarly specific transcriptional response to the production of a secretin in Y. enterocolitica (Seo et al., 2007).
The normal function of all secretins requires them to be inserted into the outer membrane. However, there is plenty of data suggesting that they can also mislocalize to the cytoplasmic membrane in some conditions (e.g. Koster et al., 1997; Burghout et al., 2004; Guilvout et al., 2006). Most notably, the PulD secretin of the Klebsiella oxytoca type 2 secretion system was observed as an apparent oligomeric pore mislocalized in the E. coli cytoplasmic membrane, and this event was associated with increased PspA synthesis (Guilvout et al., 2006). Therefore, it seems likely that it is the mislocalization of a secretin into the inner membrane that induces the Psp system. This is consistent with the idea that the Psp system is induced by altered cytoplasmic membrane properties. For example, in E. coli the Psp response is activated by heat-, osmotic-, or ethanol-shock, all of which might compromise the cytoplasmic membrane permeability barrier (Brissette et al., 1990; Kleerebezem and Tommassen, 1993; Weiner and Model, 1994). However, unlike secretin mislocalization, which only induces psp gene expression, the environmental inducers have pleotropic effects on gene expression. It is not yet known why secretin mislocalization is such a specific inducer of the Psp response.
The PspB and PspC proteins alleviate secretin-induced stress
Prior to our discovery of the Y. enterocolitica Psp system, the absence of a functional Psp response in E. coli had been associated with only subtle and difficult to detect phenotypes. For example, a survival defect in prolonged stationary phase at highly alkaline pH (Weiner and Model, 1994), or reduced proton motive force and Sec-dependent protein translocation (e.g. Kleerebezem and Tommassen, 1993; Kleerebezem et al., 1996). It is the PspA protein alone that has been associated with preventing these physiological defects from occurring. This is consistent with the fact that PspA is a highly abundant protein under Psp-inducing conditions (e.g. Brissette et al., 1990). It has been proposed that PspA might somehow help to maintain cytoplasmic membrane integrity and the proton motive force. This proposal is supported by both in vivo and in vitro experiments (Kleerebezem et al., 1996; Kobayashi et al., 2007; Standar et al., 2008). However, exactly how PspA achieves this physiological function is unknown.
In contrast to the subtle phenotypes described for E. coli, the originally described Y. enterocolitica pspC null mutant had much more severe and easily detected phenotypes. It was completely avirulent in a mouse model of infection, growth-sensitive to production of the native Ysc T3SS in the laboratory and killed by production and mislocalization of the YscC secretin (Darwin and Miller, 1999, 2001). The severe sensitivity of Y. enterocolitica psp null mutants extends to any secretin that we have tested (e.g. Maxson and Darwin, 2006; Seo et al., 2007, 2009). Furthermore, we now know that this severe secretin-sensitivity phenotype also occurs in E. coli and Salmonella enterica serovar Typhimurium (Seo et al., 2007, 2009). Therefore, not only is psp gene expression induced by secretin mislocalization, but a functional Psp system is also essential for survival when this event occurs.
Despite all of the evidence supporting the role of PspA as an important physiological effector in E. coli, a non-polar Y. enterocolitica pspA null mutant is insensitive to secretin production (Darwin and Miller, 2001). Therefore, whilst PspA almost certainly does play an important physiological role during some Psp-inducing conditions, it is not required to tolerate secretin-stress. However, we have shown that the small inner membrane proteins PspB and PspC are required (Maxson and Darwin, 2006; Gueguen et al., 2009). Therefore, PspB and -C are dual function proteins involved in both regulating psp gene expression and in mitigating secretin-induced stress. Genetic analysis suggests that these functions are independent of one another (Maxson and Darwin, 2006; Gueguen et al., 2009).
One size does not fit all: contrasting roles of the Psp system in Y. enterocolitica and Salmonella enterica serovar Typhimurium virulence
The Y. enterocolitica Ysc T3SS is essential for virulence in an animal model of infection. In the laboratory, strains with various psp gene deletions are sensitive to YscC secretin production either alone or as part of the native Ysc T3SS (Darwin and Miller, 2001). Importantly, the level of attenuation of different psp null mutants in mice correlates with their sensitivity to YscC in the laboratory (e.g. Green and Darwin, 2004). Together, this strongly suggests that the requirement of the Y. enterocolitica Psp system for virulence is due to its role in mitigating secretin-induced stress caused by production of the Ysc T3SS during infection.
The Psp system is also required for virulence in Salmonella enterica serovar Typhimurium (S. Typhimurium; Karlinsey et al., 2010). However, the reason is different to the situation in Y. enterocolitica, and so is the Psp protein involved (Fig. 2). A S. Typhimurium pspA null strain is attenuated in mice that posses the natural resistance-associated macrophage protein 1 (Nramp1) but is not attenuated in Nramp1-defective mice (Karlinsey et al., 2010). Nramp1 limits the availability of divalent metal ions within the macrophage phagosome, which restricts the growth of pathogens that reside there, such as S. Typhimurium. To counter this, S. Typhimurium utilizes a number of energy-dependent transporters to accumulate these ions under such limiting conditions. Ferric Fang’s group have demonstrated that the function of these transporters is compromised in a pspA null strain, presumably due to the role of PspA in helping maintain the protein motive force (Karlinsey et al., 2010). Thus, in S. Typhimurium, PspA is needed to help maintain the proton motive force during infection, which in turn allows S. Typhimurium to power essential metal ion importers needed to survive in Nramp1-positive macrophages.
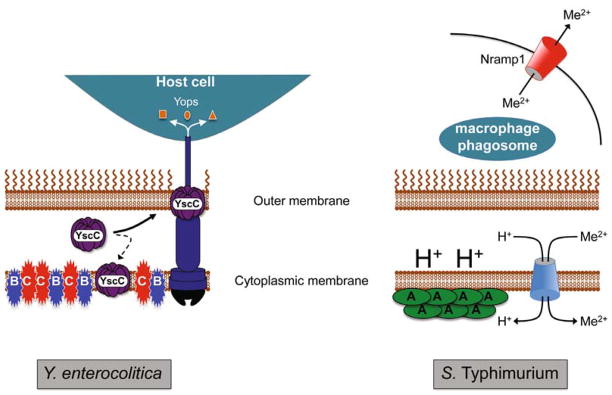
Fig. 2. Summary of the contrasting roles of the Psp system in supporting the virulence of Y. enterocolitica and S. enterica sv. Typhimurium (S. Typhimurium).
In the extracellular pathogen Y. enterocolitica (left) the Ysc T3SS must be produced during host infection to disarm the functions of host immune cells via the function of the exported Yop effector proteins. However, some of the YscC secretin component mislocalizes to the cytoplasmic membrane with the potential to compromise membrane integrity. Secretin mislocalization induces the Psp system and under these conditions the integral cytoplasmic membrane proteins PspB and PspC (but not PspA) are essential for Y. enterocolitica viability. It is not yet known how PspBC prevent or counteract secretin-toxicity. In the intracellular pathogen S. Typhimurium (right) the available divalent cation concentration within the macrophage phagosome is maintained at a low level by the action of the host Nramp1 protein. As a result, S. Typhimurium relies on proton (H+)-dependent and ATP-dependent (not shown) transporters to accumulate divalent cations (Me2+). In this situation, the role of PspA in maintaining the proton motive force is essential because it ensures an energy supply for metal ion import. How PspA achieves this function and the source of the Psp-inducing signal during host infection by S. Typhimurium is unknown.
It is interesting that the intracellular pathogen S. Typhimurium and the extracellular pathogen Y. enterocolitica both need a functional Psp system for virulence, but for quite distinct reasons (Fig. 2). In S. Typhimurium, PspA is the critical component, which facilitates intracellular survival due to its role in maintaining the protein motive force. In contrast, in Y. enterocolitica PspB and PspC appear to be the critical components, which must protect the bacteria from secretin-induced cell injury.
Closing comments and some outstanding questions
This review has focused primarily on our more recent work to understand the regulation and function of the Psp system in Y. enterocolitica. As such, we apologize to the authors of much of the work done on the Psp system in other bacteria, most notably E. coli, which we have been unable to discuss here. For further information, we refer readers to a recent comprehensive review that covers many of the E. coli studies in particular (Joly et al., 2010).
We still have many questions to answer about the Psp system in Y. enterocolitica. Chief amongst these are the exact nature of the inducing signal(s) and the mechanism by which PspB and PspC protect the bacterial cell from secretin-induced death. It is also intriguing that the most abundant Psp protein, PspA, is not required for the latter role. One possibility is that the Psp system might counteract different defects of the cytoplasmic membrane, with PspA being required for some and PspBC for others. For example, PspA might specifically help to maintain the protein motive force, whereas PspBC might correct or prevent a more severe defect caused by the insertion of a secretin into the cytoplasmic membrane. Investigating these aspects of Psp system function remains the motivation for our continued work with this intriguing and enigmatic stress response.
Acknowledgments
Work in our laboratory is supported by Award Number R01AI052148 from the National Institute of Allergy and Infectious Diseases (NIAID). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the National Institutes of Health. AJD holds an Investigators in Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund. SY was supported in part by a Vilcek Endowment Fellowship Award from the NYU School of Medicine Department of Microbiology.
References
- Akiyama Y, Kihara A, Ito K. Subunit a of proton ATPase F0 sector is a substrate of the FtsH protease in Escherichia coli. FEBS Lett. 1996;399:26–28. doi: 10.1016/s0014-5793(96)01283-5. [DOI] [PubMed] [Google Scholar]
- Brissette JL, Russel M, Weiner L, Model P. Phage shock protein, a stress protein of Escherichia coli. Proc Natl Acad Sci USA. 1990;87:862–866. doi: 10.1073/pnas.87.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissette JL, Weiner L, Ripmaster TL, Model P. Characterization and sequence of the Escherichia coli stress-induced psp operon. J Mol Biol. 1991;220:35–48. doi: 10.1016/0022-2836(91)90379-k. [DOI] [PubMed] [Google Scholar]
- Burghout P, Beckers F, de Wit E, van Boxtel R, Cornelis GR, Tommassen J, Koster M. Role of the pilot protein YscW in the biogenesis of the YscC secretin in Yersinia enterocolitica. J Bacteriol. 2004;186:5366–5375. doi: 10.1128/JB.186.16.5366-5375.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis GR. The Yersinia Ysc-Yop “Type III” weaponry. Nat Rev Mol Cell Biol. 2002;3:742–754. doi: 10.1038/nrm932. [DOI] [PubMed] [Google Scholar]
- Cornelis GR. The type III secretion injectisome. Nat Rev Microbiol. 2006;4:811–825. doi: 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]
- Cornelis G, Laroche Y, Balligand G, Sory MP, Wauters G. Yersinia enterocolitica, a primary model for bacterial invasiveness. Rev Infect Dis. 1987;9:64–87. doi: 10.1093/clinids/9.1.64. [DOI] [PubMed] [Google Scholar]
- Darwin AJ. The phage-shock-protein response. Mol Microbiol. 2005;57:621–628. doi: 10.1111/j.1365-2958.2005.04694.x. [DOI] [PubMed] [Google Scholar]
- Darwin AJ, Miller VL. Identification of Yersinia enterocolitica genes affecting survival in an animal host using signature-tagged transposon mutagenesis. Mol Microbiol. 1999;32:51–62. doi: 10.1046/j.1365-2958.1999.01324.x. [DOI] [PubMed] [Google Scholar]
- Darwin AJ, Miller VL. The psp locus of Yersinia enterocolitica is required for virulence and for growth in vitro when the Ysc type III secretion system is produced. Mol Microbiol. 2001;39:429–444. doi: 10.1046/j.1365-2958.2001.02235.x. [DOI] [PubMed] [Google Scholar]
- Dworkin J, Jovanovic G, Model P. The PspA protein of Escherichia coli is a negative regulator of σ54-dependent transcription. J Bacteriol. 2000;182:311–319. doi: 10.1128/jb.182.2.311-319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elderkin S, Jones S, Schumacher J, Studholme D, Buck M. Mechanism of action of the Escherichia coli phage shock protein PspA in repression of the AAA family transcription factor PspF. J Mol Biol. 2002;320:23–37. doi: 10.1016/S0022-2836(02)00404-7. [DOI] [PubMed] [Google Scholar]
- Genin S, Boucher CA. A superfamily of proteins involved in different secretion pathways in Gram-negative bacteria: modular structure and specificity of the N-terminal domain. Mol Gen Genet. 1994;243:112–118. doi: 10.1007/BF00283883. [DOI] [PubMed] [Google Scholar]
- Green RC, Darwin AJ. PspG, a new member of the Yersinia enterocolitica phage shock protein regulon. J Bacteriol. 2004;186:4910–4920. doi: 10.1128/JB.186.15.4910-4920.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueguen E, Flores-Kim J, Darwin AJ. The Yersinia enterocolitica phage shock proteins B and C can form homodimers and heterodimers in vivo with the possibility of close association between multiple domains. J Bacteriol. 2011;193:5747–5758. doi: 10.1128/JB.05080-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueguen E, Savitzky DC, Darwin AJ. Analysis of the Yersinia enterocolitica PspBC proteins defines functional domains, essential amino acids and new roles within the phage- shock-protein response. Mol Microbiol. 2009;74:619–633. doi: 10.1111/j.1365-2958.2009.06885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilvout I, Chami M, Engel A, Pugsley AP, Bayan N. Bacterial outer membrane secretin PulD assembles and inserts into the inner membrane in the absence of its pilotin. EMBO J. 2006;25:5241–5249. doi: 10.1038/sj.emboj.7601402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly N, Engl C, Jovanovic G, Huvet M, Toni T, Sheng X, Stumpf MPH, Buck M. Managing membrane stress: the phage shock protein (Psp) response, from molecular mechanisms to physiology. FEMS Microbiol Rev. 2010;34:797–827. doi: 10.1111/j.1574-6976.2010.00240.x. [DOI] [PubMed] [Google Scholar]
- Jovanovic G, Engl C, Mayhew AJ, Burrows PC, Buck M. Properties of the phage shock protein (Psp) regulatory complex that govern signal transduction and induction of the Psp response in Escherichia coli. Microbiology. 2010;156:2920–2932. doi: 10.1099/mic.0.040055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic G, Weiner L, Model P. Identification, nucleotide sequence, and characterization of PspF, the transcriptional activator of the Escherichia coli stress-induced psp operon. J Bacteriol. 1996;178:1936–1945. doi: 10.1128/jb.178.7.1936-1945.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlinsey JE, Maguire ME, Becker LA, Crouch ML, Fang FC. The phage shock protein PspA facilitates divalent metal transport and is required for virulence of Salmonella enterica sv. Typhimurium. Mol Microbiol. 2010;78:669–685. doi: 10.1111/j.1365-2958.2010.07357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara A, Akiyama Y, Ito K. FtsH is required for proteolytic elimination of uncomplexed forms of SecY, an essential protein translocase subunit. Proc Natl Acad Sci USA. 1995;92:4532–4536. doi: 10.1073/pnas.92.10.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleerebezem M, Crielaard W, Tommassen J. Involvement of stress protein PspA (phage shock protein A) of Escherichia coli in maintenance of the protonmotive force under stress conditions. EMBO J. 1996;15:162–171. [PMC free article] [PubMed] [Google Scholar]
- Kleerebezem M, Tommassen J. Expression of the pspA gene stimulates efficient protein export in Escherichia coli. Mol Microbiol. 1993;7:947–956. doi: 10.1111/j.1365-2958.1993.tb01186.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi R, Suzuki T, Yoshida M. Escherichia coli phage-shock protein A (PspA) binds to membrane phospholipids and repairs proton leakage of the damaged membranes. Mol Microbiol. 2007;66:100–109. doi: 10.1111/j.1365-2958.2007.05893.x. [DOI] [PubMed] [Google Scholar]
- Korotkov KV, Gonen T, Hol WG. Secretins: dynamic channels for protein transport across membranes. Trends Biochem Sci. 2011;36:433–443. doi: 10.1016/j.tibs.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster M, Bitter W, de Cock H, Allaoui A, Cornelis GR, Tommassen J. The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocolitica forms a ring-shaped multimeric complex. Mol Microbiol. 1997;26:789–797. doi: 10.1046/j.1365-2958.1997.6141981.x. [DOI] [PubMed] [Google Scholar]
- Lloyd LJ, Jones SE, Jovanovic G, Gyaneshwar P, Rolfe MD, Thompson A, Hinton JC, Buck M. Identification of a new member of the phage shock protein response in Escherichia coli, the phage shock protein G (PspG) J Biol Chem. 2004;279:55707–55714. doi: 10.1074/jbc.M408994200. [DOI] [PubMed] [Google Scholar]
- Maxson ME, Darwin AJ. PspB and PspC of Yersinia enterocolitica are dual function proteins: regulators and effectors of the phage-shock-protein response. Mol Microbiol. 2006;59:1610–1623. doi: 10.1111/j.1365-2958.2006.05047.x. [DOI] [PubMed] [Google Scholar]
- Perry RD, Fetherston JD. Yersinia pestis--etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy DA, Martinez RJ. Role of a plasmid in the pathogenicity of Yersinia species. Curr Top Microbiol Immunol. 1985;118:29–51. doi: 10.1007/978-3-642-70586-1_3. [DOI] [PubMed] [Google Scholar]
- Seo J, Brencic A, Darwin AJ. Analysis of secretin-induced stress in Pseudomonas aeruginosa suggests prevention rather than response and identifies a novel protein involved in secretin function. J Bacteriol. 2009;191:898–908. doi: 10.1128/JB.01443-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J, Savitzky DC, Ford E, Darwin AJ. Global analysis of tolerance to secretin-induced stress in Yersinia enterocolitica suggests that the phage-shock-protein system may be a remarkably self-contained stress response. Mol Microbiol. 2007;65:714–727. doi: 10.1111/j.1365-2958.2007.05821.x. [DOI] [PubMed] [Google Scholar]
- Singh S, Darwin AJ. FtsH-dependent degradation of phage shock protein C in Yersinia enterocolitica and Escherichia coli. J Bacteriol. 2011;193:6436–6442. doi: 10.1128/JB.05942-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standar K, Mehner D, Osadnik H, Berthelmann F, Hause G, Lunsdorf H, Bruser T. PspA can form large scaffolds in Escherichia coli. FEBS Lett. 2008;582:3585–3589. doi: 10.1016/j.febslet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Weiner L, Brissette JL, Model P. Stress-induced expression of the Escherichia coli phage shock protein operon is dependent on σ54 and modulated by positive and negative feedback mechanisms. Genes Dev. 1991;5:1912–1923. doi: 10.1101/gad.5.10.1912. [DOI] [PubMed] [Google Scholar]
- Weiner W, Model P. Role of an Escherichia coli stress- response operon in stationary-phase survival. Proc Natl Acad Sci USA. 1994;91:2191–2195. doi: 10.1073/pnas.91.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Gueguen E, Horstman NK, Darwin AJ. Membrane association of PspA depends on activation of the phage-shock-protein response in Yersinia enterocolitica. Mol Microbiol. 2010;78:429–443. doi: 10.1111/j.1365-2958.2010.07344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]