Abstract
Sex steroids play essential roles in the modulation of synaptic plasticity and neuroprotection in the hippocampus. Accumulating evidence shows that hippocampal neurons synthesize both estrogen and androgen. Recently, we also revealed the hippocampal synthesis of corticosteroids. The accurate concentrations of these hippocampus-synthesized steroids are determined by liquid chromatography–tandem mass-spectrometry in combination with novel derivatization. The hippocampal levels of 17β-estradiol (E2), testosterone (T), dihydrotestosterone (DHT), and corticosterone (CORT), are 5–15 nM, and these levels are sufficient to modulate synaptic plasticity. Hippocampal E2 modulates memory-related synaptic plasticity not only slowly/genomically but also rapidly/non-genomically. Slow actions of E2 occur via classical nuclear receptors (ERα or ERβ), while rapid E2 actions occur via synapse-localized or extranuclear ERα or ERβ. Nanomolar concentrations of E2 change rapidly the density and morphology of spines in hippocampal neurons. ERα, but not ERβ, drives this enhancement/suppression of spinogenesis in adult animals. Nanomolar concentrations of androgens (T and DHT) and CORT also increase the spine density. Kinase networks are involved downstream of ERα and androgen receptor. Newly developed Spiso-3D mathematical analysis is useful to distinguish these complex effects by sex steroids and kinases. Significant advance has been achieved in investigations of rapid modulation by E2 of the long-term depression or the long-term potentiation.
Keywords: hippocampus, sex steroid, corticosteroid, estrogen, androgen, synaptic plasticity, spinogenesis
Introduction
Local steroidogenesis systems in the brain, initiated with the conversion of cholesterol, have been extensively investigated since 1990s (Baulieu, 1997). In particular, hippocampal synthesis of sex steroids is very attractive issue, because the hippocampus is a center for learning and memory. The localization of steroidogenic enzymes including cytochrome P450scc, P450(17α), and P450arom, and production of testosterone (T), dihydrotestosterone (DHT), and 17β-estradiol (E2) were demonstrated in adult male hippocampus (Kimoto et al., 2001; Kawato et al., 2002; Hojo et al., 2004). The level of E2, T, and DHT was 5–15 nM which is sufficient to modulate synaptic plasticity (Hojo et al., 2009).
For decades, genomic/slow neuromodulatory actions have been extensively investigated for circulating gonadal sex hormones in the hippocampus (Woolley and McEwen, 1994; Woolley, 1998; Foy et al., 1999; Pozzo-Miller et al., 1999; Bi et al., 2000; Shibuya et al., 2003). Modulation of dendritic spines has been extensively studied as a typical model of synaptic plasticity, because synapse is a site of memory storage and spine is a postsynaptic structure. Slow modulation of synaptogenesis or electrophysiological properties is investigated by estrogen replacement for ovariectomized (OVX) female rats (Woolley and McEwen, 1994; Woolley, 1998; Foy et al., 1999; Pozzo-Miller et al., 1999; Bi et al., 2000; Shibuya et al., 2003). An increase of synapses or an enhancement of synaptic transmission is observed upon s.c. injection of estrogen. Slow modulation of spines (postsynaptic structures) is also observed in slice cultures (Woolley and McEwen, 1994; Woolley, 1998; Foy et al., 1999; Pozzo-Miller et al., 1999; Bi et al., 2000; Shibuya et al., 2003). These slow genomic effects are mediated via nuclear estrogen receptors ERα/ERβ to initiate transcription processes.
The rapid effect of estradiol (E2; within 1–2 h) also occurs by modulating spine density or electrophysiological properties of the hippocampal slices (Teyler et al., 1980; Foy et al., 1999; Bi et al., 2000; Mukai et al., 2006a). These rapid modulations, relating to memory formation processes, may favor locally synthesized steroids rather than low level circulating gonadal hormones which need to go across brain tissues in order to reach estrogen receptors in the target neurons. Rather than being a limiting factor, a weak activity of sex steroid production in the hippocampus is sufficient for the local usage within small neurons (i.e., an intracrine system). This intracrine system contrasts with the endocrine organs in which high expression levels of steroidogenic enzymes are necessary to supply steroids to many other organs via the blood circulation. For brain-derived sex hormones, the essential functions may be the rapid and continuous modulation of synaptic plasticity and cognitive functions.
Hippocampal synthesis and action of corticosteroids have not been well clarified, in comparison with sex steroids. Recently we demonstrated the existence of complete pathway of corticosteroid synthesis in the hippocampus which synthesizes a low level of corticosterone (CORT; ∼7 nM; Higo et al., 2011). Low dose CORT (10 nM) may have beneficial effects such as enhancement of spinogenesis (Higo et al., 2011) and increase in both the expression and phosphorylation of Erk MAP kinase (Revest et al., 2005). These beneficial effects are very different from the high dose CORT (in the order of micro molar) released from adrenal under stressful conditions, which elicits the deleterious effects (e.g., neuronal cell death or shrinkage of dendrites; Sapolsky et al., 1985; Woolley et al., 1990). Hippocampus-synthesized corticosteroids (∼7 nM) may play an essential role in important physiological functions such as modulation of synaptic plasticity.
Hippocampal Synthesis of Sex Steroids and Corticosteroids
Synthesis of sex steroids in adult male hippocampus
Neuronal localization of steroidogenic enzymes in the hippocampus
In earlier studies, glial cells were thought to be a major place for steroidogenesis, because the white matter including glial cells had been stained with anti-P450scc antisera, throughout the adult rat brain (Le Goascogne et al., 1987). However, this white matter staining of cytochrome P450scc antisera may be an artifact which is due to the non-specific adsorption of the non-purified bovine antisera in rat hippocampus.
The role of neurons in steroid synthesis in mammalian brains had long been difficult to determine. P450(17α) was thought to be absent of in both neurons and glial cells due to the fact that many attempts to demonstrate the immunohistochemical reactivity in the rat brain had been unsuccessful for almost two decades (Le Goascogne et al., 1991). We overcame difficulties of non-specific immunostaining by using affinity–column-purified antibodies (Shinzawa et al., 1988; Jakab et al., 1993; instead of using non-purified antisera) in order to avoid cross-reaction with IgG with unknown proteins having similar antigen sequences, as well as using fresh frozen slices of hippocampus (instead of using paraffin sections).
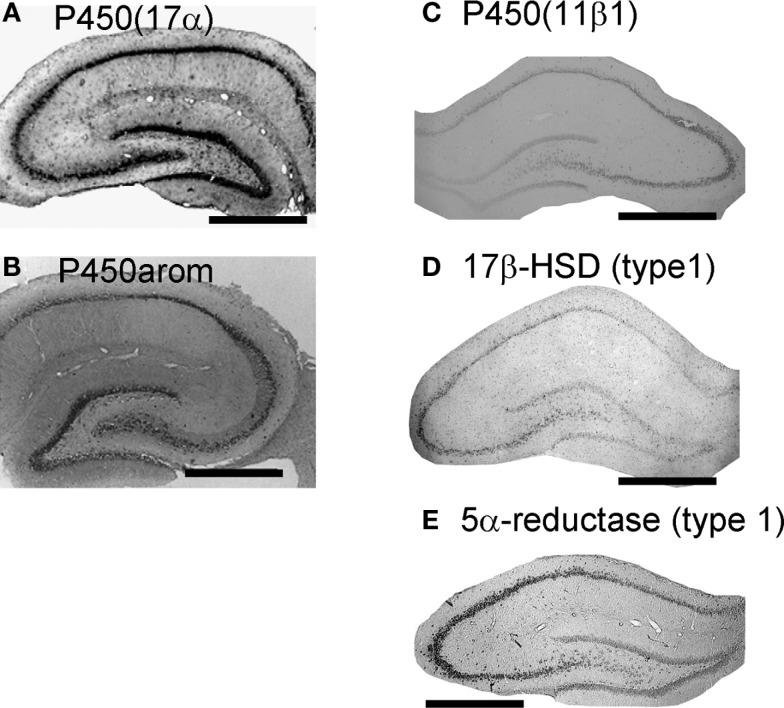
Localization of cytochromes P450scc, P450(17α) and P450arom was observed in pyramidal neurons in CA1–CA3, as well as in granule cells in the dentate gyrus (DG), by means of the immunohistochemical staining of hippocampal slices from adult (12 week; Figure 1) and developmental rats (Kimoto et al., 2001; Kawato et al., 2002; Shibuya et al., 2003; Hojo et al., 2004; Higo et al., 2009). The co-localization of P450s and NeuN (marker of neuron) confirmed the expression of P450s in these neurons (Kimoto et al., 2001; Kawato et al., 2002; Hojo et al., 2004). Astroglial cells had weak expression of P450(17α) or P450scc, however, oligodendroglial cells did not express these P450s (Figure 1; Kimoto et al., 2001; Kawato et al., 2002; Hojo et al., 2004).
Figure 1.
Localization of the enzymes required for synthesis of sex steroids and corticosteroids in the hippocampus. (A) and (B) Immunohistochemical staining of P450(17α) (A) and P450arom (B) in the coronal section of the adult male rat hippocampus. (C–E) In situ hybridization analysis of P450 (11β1) (C), 17β-HSD (type 1) (D) and 5α-reductase (type 1) (E) with antisense probes. All steroidogenic enzymes are mainly expressed in neurons, although a weak expression is observed in some glial cells. Scale bar, 800 μm. Modified from Hojo et al. (2004, 2009), Higo et al. (2011).
17β-HSD and 5α-reductase are necessary to synthesize androgens (testosterone; T and dihydrotestosterone; DHT). The neuronal localization of 17β-HSD (type 1) and 5α-reductase (types 1 and 2) was demonstrated with in situ hybridization in mouse and rat hippocampus (Agis-Balboa et al., 2006; Hojo et al., 2009; Figure 1). StAR was co-localized with P450s (Zwain and Yen, 1999; Kimoto et al., 2001; Wehrenberg et al., 2001). These results imply that pyramidal neurons and granule neurons are equipped with complete steroidogenic systems which catalyze the conversion of cholesterol to pregnenolone (PREG), dehydroepiandrosterone (DHEA), T, DHT, and estradiol (E2). Due to a weak immunostaining of P450s in glial cells, the activity of neurosteroidogenesis in glial cells is probably much lower than that of neurons.
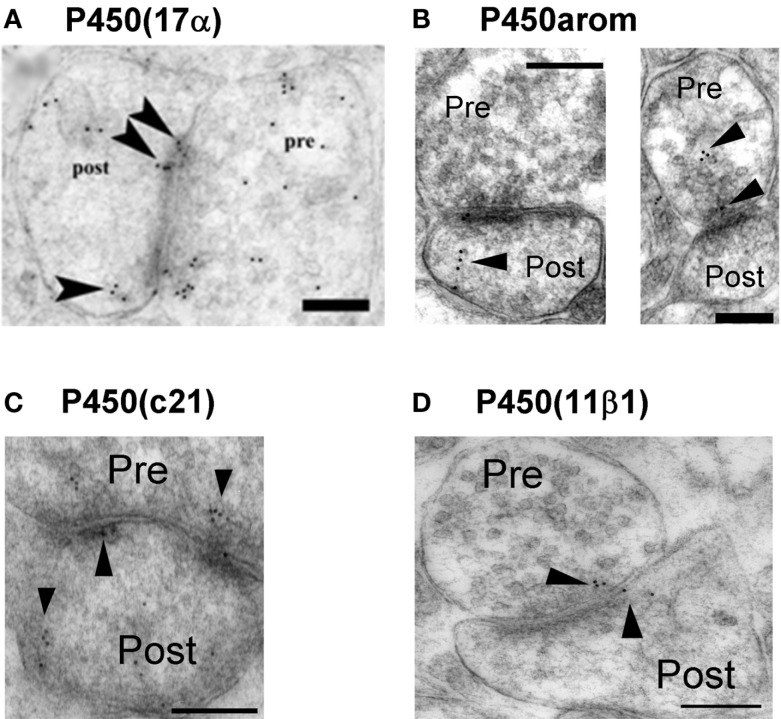
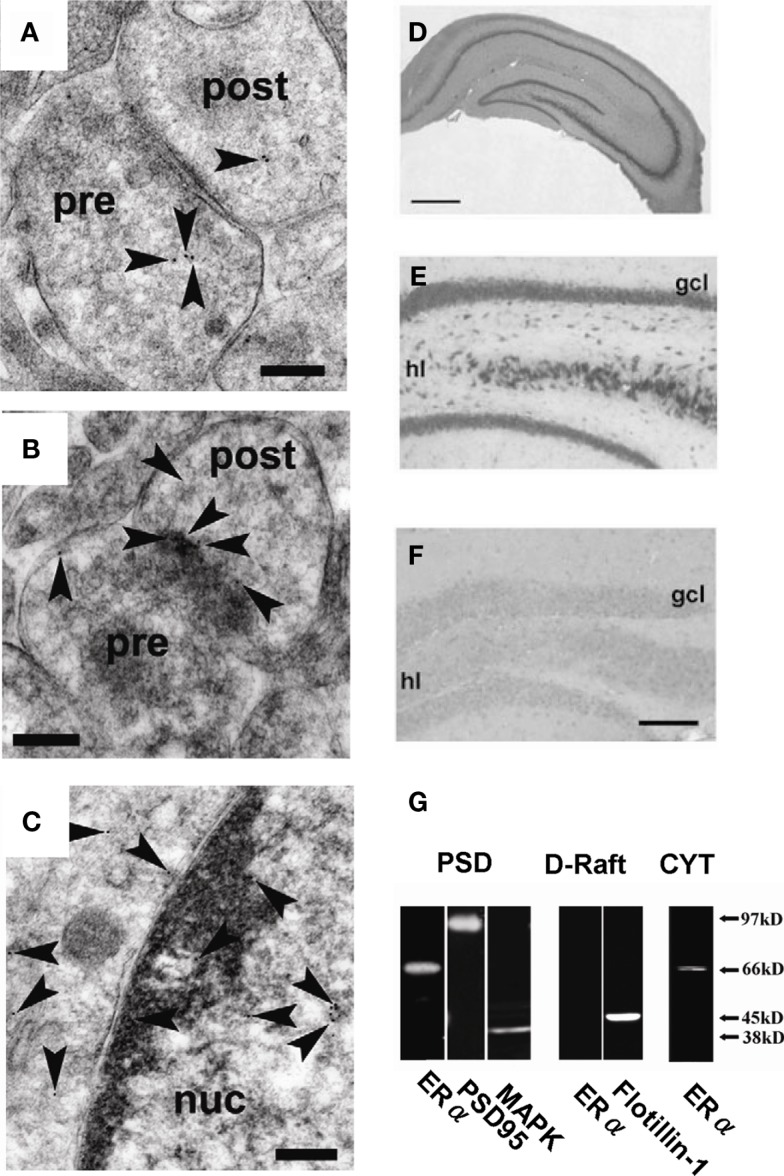
Are these steroidogenic enzymes localized at synapses? An immunoelectron microscopic analysis using post-embedding immunogold method is very useful to determine the intraneuronal localization of P450(17α) and P450arom in the hippocampal neurons of adult male rats. Surprisingly, we observed that both P450(17α) and P450arom were localized not only in the endoplasmic reticulum but also in the presynaptic region as well as the postsynaptic region of pyramidal neurons in the CA1 and CA3 regions and of granule neurons in DG (Figure 2). These results suggest “synaptic” synthesis of estrogens and androgens, in addition to classical microsomal synthesis of sex steroids. The existence of these steroidogenic proteins was confirmed by Western immunoblot analyses (Kimoto et al., 2001; Kawato et al., 2002; Hojo et al., 2004; Mukai et al., 2010). The molecular weights obtained for P450scc, P450(17α), and P450arom were identical to those obtained from peripheral steroidogenic organs. The relative levels of these P450s in the hippocampus were approximately 1/1000 (P450scc) and 1/300 [P450(17α) and P450arom] of that in the testis [P450scc and P450(17α)] and the ovary (P450arom), respectively.
Figure 2.
Synaptic localization of cytochromes P450 (17α), P450arom, P450 (c21), and P450 (11β1) in the hippocampus. Immunoelectron microscopic analysis of the distribution of P450 (17α) (A), P450arom (B), P450 (c21) (C), and P450 (11β1) (D) within synapses, in the hippocampal CA1 region. Gold particles (indicated by arrow heads) are observed in the presynaptic region (Pre), and the postsynaptic region (Post). Scale bar: 200 nm. Modified from Hojo et al. (2004), Higo et al. (2011).
In the brain regions other than the hippocampus (e.g., hypothalamus or amygdale, etc.,), the synaptic localization of P450arom is observed in earlier publications, with immunoelectron microscopy (EM) studies of the brains of a variety of species including quail, rats, monkeys, and humans (Jakab et al., 1993; Naftolin et al., 1996; Balthazart and Ball, 2006).
Pathway of synthesis of sex steroids
A direct demonstration of the neuronal synthesis of PREG, DHEA, T, and 17β-E2 in adult mammals is reported in early 2000s (Kimoto et al., 2001; Kawato et al., 2002; Prange-Kiel et al., 2003; Hojo et al., 2004). It had been assumed that T is supplied to the male brain such as hypothalamus, via the blood circulation, where T is converted to E2 by P450arom (Baulieu, 1997; Baulieu and Robel, 1998). The absence of P450(17α) activity in the brain of adult mammals had been reported in a number of studies (Le Goascogne et al., 1991; Baulieu and Robel, 1998; Mensah-Nyagan et al., 1999; Kibaly et al., 2005). Incubations of [3H]-PREG with brain slices, homogenates and microsomes, primary cultures of mixed glial cells, or astrocytes and neurons from rat and mouse embryos, had failed to produce a radioactive metabolite [3H]-DHEA (Baulieu and Robel, 1998).
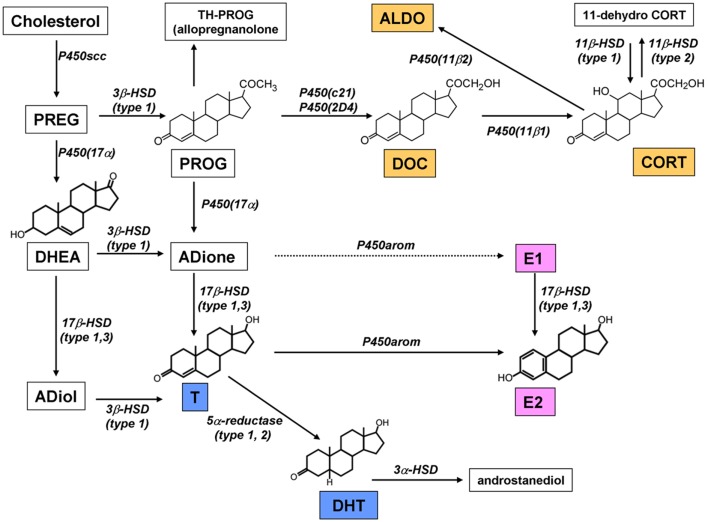
We succeeded in demonstration of the synthesis of DHEA, T, and E2 in the adult (12 week) hippocampal slices by means of careful HPLC analysis (Kawato et al., 2002; Hojo et al., 2004, 2009). The purification of neurosteroids from very fatty brain tissues requires the combination of several sophisticated methods, which included purification with organic solvent, solid column chromatography, and normal phase HPLC (Wang et al., 1997; Kimoto et al., 2001; Hojo et al., 2004). The significant conversion from [3H]-PREG to [3H]-DHEA, from [3H]-DHEA to [3H]-androstenediol (ADiol), [3H]-T, and [3H]-E2 was observed after incubation with the slices for 5 h (Figure 3; Hojo et al., 2004). The rate of production for [3H]-E2 from [3H]-T was very slow, and the production rate of [3H]-DHT from [3H]-T was much more rapid than that of E2. These activities were abolished by the application of specific inhibitors of cytochrome P450s. Surprisingly, [3H]-E2 was extremely stable and not significantly converted to other steroid metabolites such as estrone. On the other hand, DHT was rapidly converted to 3α, 5α-androstanediol (Figure 3). E2 synthesis is also demonstrated in cultured hippocampal slices from neonatal rats in the absence and presence of letrozole, an inhibitor of P450arom. After 4 days treatment with letrozole, the amount of E2 released into the medium was significantly decreased (Kretz et al., 2004).
Figure 3.
Pathway of steroid synthesis in the rat hippocampus. The hippocampus synthesizes estrogen (pink), androgen (blue), and corticosteroids (orange). All these steroids are probably synthesized within single neurons. Note that the chain arrow from ADione to E1 indicates an extremely weak conversion in male (Hojo et al., 2009). This mode of synthesis is different from that in peripheral steroidogenic organs.
To demonstrate the rapid net production of neurosteroids upon synaptic stimulation, the NMDA-induced production of PREG and E2 was investigated in hippocampal slices (Kimoto et al., 2001; Kawato et al., 2002; Hojo et al., 2004). Upon stimulation with NMDA for 30 min, the hippocampal level of PREG and E2 increased to approximately two-fold of the basal levels. This implies that the NMDA-induced Ca2+ influx drives net production of PREG and E2. As a slow/genetic modulator of steroidogenesis, cis-retinoic acid is shown to elevate E2 and T production via retinoic acid receptor in the cultured hippocampal slices (Munetsuna et al., 2009).
Hippocampus-synthesized and circulation-derived sex steroids
Is the level of hippocampus-synthesized sex steroids sufficiently high to allow action as local mediators? Is the level of circulation-derived sex steroids higher than that of hippocampus-synthesized sex steroids?
To answer these questions, an accurate determination of the concentration of E2 and other steroids is necessary for an understanding/explanation of its modulatory action on synaptic plasticity including spinogenesis, long-term potentiation (LTP), or long-term depression (LTD).
Because of technical problems including poor purification procedures of steroids from fatty brain tissues, the accurate determination of the E2 concentration in whole hippocampal tissues, slices, or cultured neurons had been difficult. By combination of steroid purification with solid phase C18 column and radioimmunoassay (RIA), the concentration of E2 was determined to be ∼0.6 nM (basal) and 1.3 nM after the NMDA stimulation, respectively, in the adult male rat hippocampus (Hojo et al., 2004). RIA is a very sensitive method for steroid detection, but has uncertainty regarding specificity and accuracy due to problems of antisera.
For a direct determination of steroids, mass-spectrometric assay is much better than RIA. However, even with mass-spectrometric assay, the presence of 17β-E2, DHT, and estrone (E1) had not yet been observed, although DHEA and T have been observed in the whole brain extracts (Liere et al., 2000; Liu et al., 2003; Ebner et al., 2006; Higashi et al., 2006; Caruso et al., 2010). Applied mass-spectrometric assay includes gas chromatography with mass-spectrometry (GC-MS/MS), liquid chromatography with mass-spectrometry (LC–MS), and liquid chromatography with tandem-mass-spectrometry (LC-MS/MS).
We therefore substantially improved the determination methodology using LC-MS/MS in combination with picolinoyl-derivatization (for induced ionization) of pre-purified E2/T/DHT/E1 fractions obtained from purification by normal phase HPLC (Yamashita et al., 2007a,b; Hojo et al., 2009). E2 was further derivatized with pentafluorobenzyl in order to elevate evaporation probability. We achieved the good limits of quantification which are 0.3 pg (17β-E2) and 1 pg (T, DHT, E1) per 0.1 g of hippocampal tissue, or 1 mL of plasma, respectively (Hojo et al., 2009).
Pre-purification of E2/T/DHT/E1 fractions via normal phase HPLC is necessary to remove contaminating fats, lipids, and mixed steroids, since reverse phase LC included in LC-MS/MS is not suitable for this kind of purification of non-charged steroids. By improved LC-MS/MS analysis, basal level of E2 is determined to be ∼8 nM in the hippocampus of male rats (Hojo et al., 2008, 2009). To our knowledge, possible reasons why other group could not detect E2 with LC–MS in the brain are as follows: (1) highly contaminating fats (may also be many steroids) from brain tissues, and (2) usage of unsuitable or no derivatization of steroids. Therefore, pre-purification step using normal phase HPLC before steroid-derivatization was very important to achieve high precision and good reproducibility of LC-MS/MS determination.
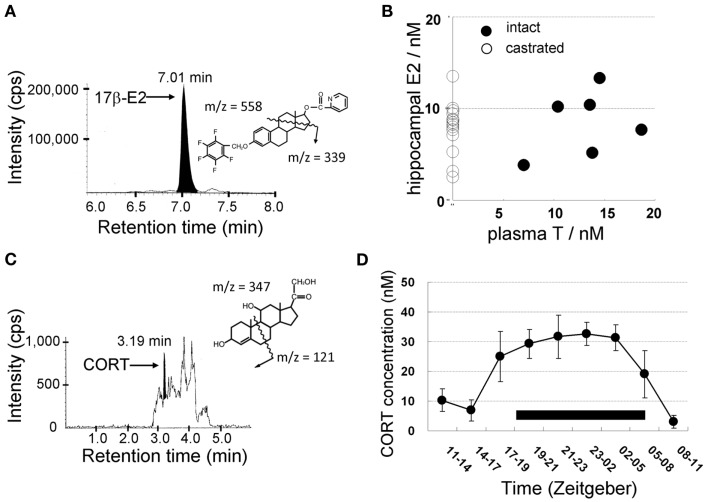
From LC-MS/MS analysis of male hippocampal tissues, the level of E2, T, and DHT is ∼8, 17, and 7 nM, respectively (Table 1). Surprisingly, the E2 level is not decreased by castration to deplete circulating T which is a precursor for E2 synthesis (Figure 4; Table 1; Hojo et al., 2009). On the other hand, castration decreases hippocampal T to 3 nM which is hippocampus-synthesized T. Hippocampal E2 may be preferentially synthesized from hippocampal T, rather than from circulating T which is preferentially converted to DHT, since castration decreases hippocampal DHT considerably to 0.2 nM.
Table 1.
Mass-spectrometric analysis of the concentration of steroids in the hippocampus and plasma of adult rats.
| Sex steroids | Hippocampus (freshly isolated)a |
Plasma |
Hippocampus (acute slice)b | ||
|---|---|---|---|---|---|
| Intactc | castrated | Intact | Castrated | ||
| 17β-E2 (ng/g wet weight or mL) | 2.3d (ne = 6) | 1.9 (n = 16) | 0.004 (n = 5) | 0.002 (n = 14) | |
| 17β-E2 (nM)f | 8.4 | 6.9 | 0.014 | 0.006 | <0.5 (n = 5) |
| T(ng/g wet weight or mL) | 4.9 (n = 8) | 0.9 (n = 16) | 4.2 (n = 8) | 0.06 (n = 16) | |
| T (nM) | 16.9 | 3.1 | 14.6 | 0.20 | <1.0 (n = 3) |
| DHT (ng/g wet weight or mL) | 1.9 (n = 8) | 0.06 (n = 16) | 0.18 (n = 8) | 0.012 (n = 16) | |
| DHT (nM) | 6.6 | 0.22 | 0.63 | 0.04 | |
| Corticosteroids |
Hippocampus (freshly isolated) |
Plasma |
Hippocampus (acute slice) | ||
| Intact | ADX | Intact | ADX | ||
| CORT (ng/g wet weight or mL) | 128.1 (n = 8) | 2.4 (n = 11) | 510.3 (n = 8) | 0.8 (n = 11) | 0.67 (n = 5) |
| CORT (nM) | 369.8 | 6.9 | 1472.8 | 2.3 | 1.9 |
| DOC (ng/g wet weight or mL) | 1.9 (n = 12) | 1.9 (n = 23) | 1.3 (n = 12) | 0.5 (n = 23) | |
| DOC (nM) | 5.9 | 5.8 | 3.8 | 1.4 | |
aHippocampus was homogenized immediately after dissection from a decapitated head. This condition reflects the basal concentration of steroids in hippocampus.
b“Acute slice” represents hippocampal slices incubated in ACSF for 2 h. Acute slices are usually used for electrophysiological or spinogenesis experiments. Hippocampal steroids are diffused into ACSF and their levels become much lower than that in freshly isolated hippocampus.
cIntact shows the averaged values from intact and sham-operated rats, because there were no significant differences between these two groups of rats.
dData are expressed as mean.
eNumber of animals (i.e., the number of hippocampi).
Figure 4.
Mass-spectrometric determination of sex steroids (A,B) and corticosteroids (C,D) in the male hippocampus. LC-MS/MS chromatograms and steroid derivatives of (A) E2 and (C) CORT. CORT was extracted from the hippocampus of ADX rats. Shaded portions indicate the intensity of the fragmented ions of 17β-E2-pentafluorobenzoxy-picolinoyl [m/z = 339, (A)] and CORT [m/z = 121, (C)], respectively. The vertical axis indicates the intensity of the fragmented ions. The horizontal axis indicates the retention time of the fragmented ions. (B) No effect of castration on hippocampal E2 level as well as no correlation between plasma T and hippocampal E2. These data suggest that hippocampal E2 is mainly produced from hippocampus-synthesized T. (D) Diurnal change of the concentration of CORT in the cerebrospinal fluid (CSF) from the cisterna magna in freely moving rats. The black bar indicates a dark period during which the activity of rats is high. During a dark period, CORT concentration elevates to three- to five-fold of that during a light period. Modified from Hojo et al. (2009), Higo et al. (2011).
In the case of cultured slices or cultured neuron/glia, the endogenous E2 level may be ∼5 nM (50 fmol/mg protein) in slices determined via RIA or mass-spectrometric assay (Munetsuna et al., 2009) or 0.03-0.1 nM in the outer medium (released E2; Kretz et al., 2004; Prange-Kiel et al., 2006, 2008). Therefore, the concentration of exogenously applied E2 should be higher than the endogenous E2 level, in order to show E2 effects.
It should be noted that, however, the level of E2 reported from many labs does not fell in the same range as that of our observation that demonstrates a high level of E2. For example, only ∼35 pM E2 (10 pg/g wet weight) was observed by RIA in 60 days of male rat hippocampus, which showed age-related decrease from 0.5 nM (140 pg/g wet weight) at PD0 (Konkle and McCarthy, 2011).
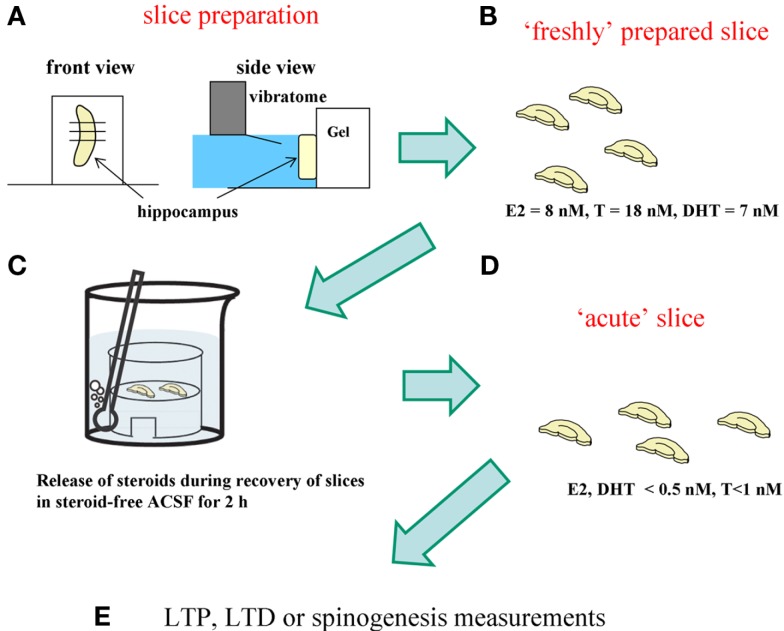
Scientists often use “acute” hippocampal slices, where hippocampal neurons are alive, in electrophysiological and spinogenesis investigations (Figure 5). “Acute” hippocampal slices are obtained after recovery incubation in steroid-free ACSF for 2 h in order to remove damaged surface cells which were damaged by vibratome slicing. During the recovery incubation with ACSF endogenous sex steroids and corticosteroids are released into the outer medium. We determined the levels of E2, T, and CORT in acute hippocampal slices to be below 0.5, 1, and 2 nM, respectively (Figure 5; Table 1). These low steroid levels in “acute” hippocampal slices support the effective action of 1–10 nM E2, T, DHT, and CORT. In vivo hippocampus, on the other hand, contains 5–10 nM E2, T, and DHT, preventing action of such low nanomolar E2, T, or DHT.
Figure 5.
Difference between “freshly isolated hippocampus” and “acute” hippocampal slices. For analysis of spinogenesis or electrophysiological experiment, “acute“ hippocampal slices are prepared according to the following procedure. (A) Hippocampus is sliced by 400 μm-thickness with a vibratome (Dosaka, Japan). (B) Hippocampal slices immediately after slice preparation contain the identical level of sex steroids and corticosteroids to that in the hippocampus in vivo. (C) During 2 h recovery in ACSF, hippocampal sex steroids, and corticosteroids diffuse into ACSF. (D) After recovery, hippocampal concentration of steroids decreases to below 0.5 nM for E2 and DHT, 1 nM for T, and 2 nM for CORT, respectively. These hippocampal slices are “acute” hippocampal slices. (E) For analysis of spinogenesis or electrophysiological experiment, “acute” slices are usually used.
Developmental and age-related change in expression of sex-steroidogenic enzymes and receptors
Exhaustive analysis of age-dependent expression for steroidogenic enzymes and receptors indicates moderate decrease of their expressions (Kimoto et al., 2010).
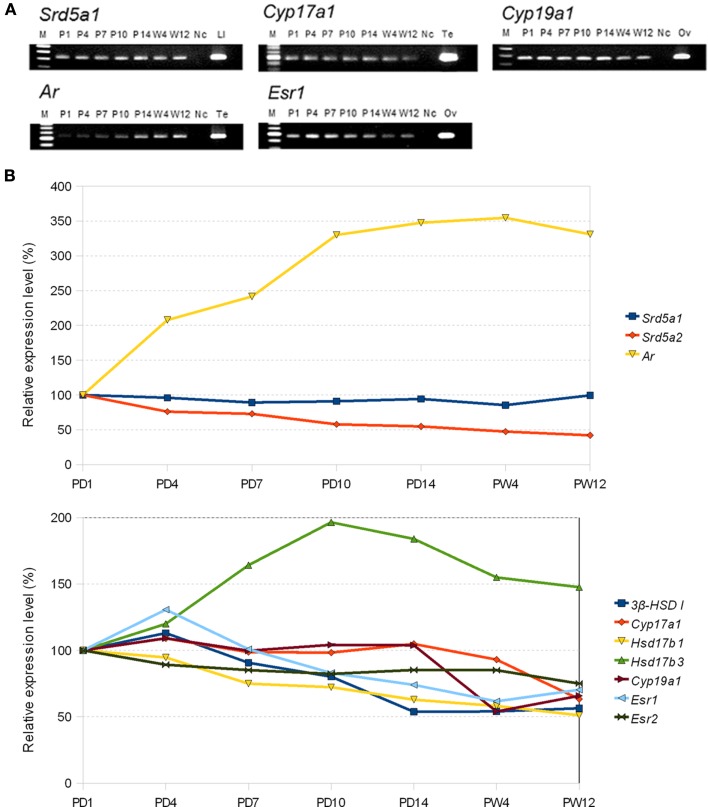
Upon development over PD1 → PD4 → PD10 → PD14 → PW4 → PW12, the expression level of steroidogenic enzymes gradually decrease to around 1/2 of PD1 at PW12, except for almost no decrease in 5α-reductase (type 1). Interestingly, a large decrease in P450scc occurs at 4–12 weeks reaching to ∼7% of PD1. Estrogen receptors ERα/β also gradually decrease to ∼70% of PD1 at PW12. On the other hand, androgen receptor (AR) increases gradually to ∼330% of PD1 at PW12. These results suggest that the effect of estrogen may be strong in PD1–PD10, and the effect of androgen may become strong in young adult days (Figure 6).
Figure 6.
(A) Typical electrophoresis gel images for the RT-PCR analysis for the mRNAs of sex-steroidogenic enzymes (Srd5a1: 5α-reductase type 1, Cyp17a1: P450 (17α), and Cyp19a1: P450arom) and sex hormone receptors (Ar: AR and Esr1: ERα). Each band of PCR product is stained with EtBr and visualized. Lanes labeled with P1 (PD1), P4, P7, P10, P14, W4 (PW4), and W12 represent the PCR products derived from the male rat hippocampus at the corresponding ages. The left-most lane (labeled with M) is the DNA ladder marker lane. The right-most lane corresponds to the positive control derived from PW12 rats. Ad, adrenal; Li, liver; Ov, ovary; Te, testis. The lane Nc represents the negative control. (B) Comparison of relative expression level of sex-steroidogenic enzymes and receptors at P1 and 12 week. PD1 is set to be 100% for each gene. Abbreviations are, for example, P1 (postnatal day 1), W4 (4 week), and W12 (12 week). Modified from Kimoto et al. (2010).
We compared not only the expression level but also the production rate of sex steroids between PW12 and PD10. The rate of metabolism for both androgens (T → DHT → androstanediol) and estrogen (T → E2) in PD10 hippocampus was estimated to be two- to seven-fold higher than that in PW12 (Higo et al., 2009).
Contrary to the widely held belief, these results indicate that only moderate decrease occur in expression level of steroidogenic enzymes as well as the production rate of sex steroids.
Interestingly, a basic steroidogenic factor sf-1/ad4bp is expressed at moderate level which is ∼150% of 3β-HSD I. Since SF-1/Ad4BP is a basic transcription factor of all the steroidogenic enzymes, genetic regulation of steroidogenic enzymes may not be very different from that in ovary or testis.
Hippocampal corticosteroid synthesis
Corticosterone (CORT), the most potent stress steroid, had been thought to be synthesized exclusively in the adrenal cortex, reaching the brain via blood circulation. De novo synthesis of CORT from progesterone (PROG) in the brain has been doubted, because cytochrome P450(c21) (deoxycorticosterone; DOC synthase), a key enzyme catalyzing the conversion of PROG to DOC, has not been detected in the hippocampus except in human hippocampus (Beyenburg et al., 2001). On the other hand, the hippocampus expresses other steroidogenic enzymes required for corticosteroid synthesis, including P450(11β1) and P450(11β2) (Gomez-Sanchez et al., 1996, 1997). Although previous studies have shown parts of the corticosteroid synthesis pathway in brain, including, PREG → PROG and DOC → CORT and DOC → aldosterone (ALDO) (Gomez-Sanchez et al., 1996, 1997; MacKenzie et al., 2000), the conversion of PROG → DOC to be demonstrated. Analysis of the pathway of corticosteroid metabolism is performed using 3H-labeled steroids as substrates. Conversion of “PROG → DOC and DOC → CORT” were clearly demonstrated (Figure 3; Higo et al., 2011). Interestingly, the conversion of CORT to other steroids was very weak, indicating that CORT is stably present once it produced.
We achieved the detection of P450(c21) mRNA by improvement of the sensitivity of PCR methods, including careful primer design by calculation of Gibbs free energy. Relative number of transcripts, expressed in the hippocampus of adult male rats, was in the order of 1/20,000 of that in the adrenal gland for P450(c21), almost the same level as that in the liver for P450(2D4), in the order of 1/5,000-1/10,000 of that in the adrenal gland for P450(11β1) and P450(11β2) (Higo et al., 2011).
The cellular localization of P450(c21), P450(2D4), and P450(11β1) was identified using in situ hybridization and immunohistochemical staining. Significant expression of both P450(2D4) and P450(11β1) was observed in pyramidal neurons (CA1, CA3) and granule neurons (DG; Figure 1). A weak expression of P450(c21) was also observed in pyramidal and granule neurons.
Immunoelectron microscopic analysis using post-embedding immunogold was performed in order to determine the localization of enzymes for corticosteroid synthesis (P450(c21), P450(2D4), P450(11β1), and 3β-HSD; Higo et al., 2011). This method is particularly useful to detect enzymes with extremely low expression level such as P450(c21). All enzymes were mainly localized in principal neurons including pyramidal neurons of CA1 and CA3 regions as well as granule neurons the DG. P450(c21) and P450(2D4) were localized not only in the endoplasmic reticulum but also in both the dendritic spines and axon terminals of principal neurons (Figure 2; Higo et al., 2011). P450(11β1) was localized in both the mitochondria and synapses of principal neurons (Figure 2). We also observed 3β-HSD in the synapses in addition to the endoplasmic reticulum. The subcellular localization of these enzymes was confirmed by Western blot with purified fractions of postsynaptic density, endoplasmic reticulum, and mitochondria.
Corticosteroid concentrations in rat hippocampus were determined by LC-MS/MS (Figure 4; Higo et al., 2011). In order to determine the net corticosteroids synthesis in the hippocampus, we used adrenalectomized (ADX) rats to eliminate adrenal-derived CORT and DOC. In ADX rats, net hippocampus-synthesized CORT and DOC were determined to ∼7 and 6 nM, respectively (Table 1). Physiological significance of the nanomolar level of CORT synthesized in the hippocampal neurons was demonstrated by enhanced spinogenesis of CA1 neurons. Even 10 nM CORT significantly increased the density of small-head spines (0.2–0.4 μm in head diameter; Higo et al., 2011).
Interestingly, the circadian rhythm of CORT level in the cerebrospinal fluid (CSF) of free moving rats was observed via LC-MS/MS in combination with the transverse microdialysis (Nakahara et al., 2003; Ishida et al., 2005; Higo et al., 2011). The concentration of CORT in the CSF elevated roughly 10-fold higher in the dark (awake) period (∼30 nM) than that in the light (sleeping) period (Figure 4; Higo et al., 2011).
Functional difference between hippocampus-synthesized steroids and circulation-derived steroids
Concerning sex steroids, one of the functional differences between E2 produced from circulating T and E2 produced from hippocampus-derived T may be the time-dependence of their levels. The brain is permeated with circulating T (male), or E2 (female), the levels of which change slowly depending on the circadian rhythm or estrous cycle. On the other hand, the endogenous synthesis of E2 (for both male and female) is a transient event depending on neural excitation such as an LTP or LTD event, because the E2 level is significantly elevated upon Ca2+ influx by NMDA stimulation (Hojo et al., 2004).
Another functional difference between hippocampal steroids and circulating steroids might be its effective concentration. The concentration of hippocampal sex steroids and corticosteroids is in the order of 10 nM, in spite of the very low expression level of steroidogenic enzymes (typically in the order of 1/100 – 1/10,000 of ovary, testis, or adrenal gland). In order to keep 1–10 nM steroids, the hippocampus does not need many steroidogenic enzymes due to its very small volume (0.14 mL for young adult, see Table 1), compared with very large blood volume (∼25 mL). Circulating steroids are diluted in the blood stream whose volume is large, upon secretion from ovary, testis, or adrenal gland within which the steroid level is very high because of many steroidogenic enzymes. Furthermore, nearly all hippocampal steroids could be free and active inside of neurons, because SHBG (sex-steroid binding globulin), CBG (corticosteroid binding globulin), or albumin is absent within neurons. In circulation, less than 5% of steroids may be free, because they bind to SHBG, CBG, or albumin in order to circulate through blood vessels (Windle et al., 1997; Breuner and Orchinik, 2002). The level of hippocampal sex steroids is higher than that of circulation-derived steroids, and therefore hippocampal sex steroids may be higher in modulation activity than circulation-derived sex steroids. However, these arguments do not rule out the possible contribution of circulation-derived sex steroids to the brain function, because peripheral E2 injection significantly increases the spine–synapse density in OVX rats (Woolley and McEwen, 1993; MacLusky et al., 2005).
Concerning corticosteroids, hippocampus-synthesized CORT does not respond to stress. Upon decapitation, the level of hippocampal CORT elevates (∼370 nM) only in adrenal intact rats whereas that in ADX rat does not (Table 1), suggesting that the hippocampus is not a stress responsive organ. Note that circulating CORT elevates transiently before awake (Windle et al., 1997) whereas CORT in CSF keeps high level during awaking period (Figure 4).
Modulation of Synaptic Plasticity by Hippocampal Sex Steroids
Spinogenesis
Hippocampus-derived E2 rapidly modulates spinogenesis. Spinogenesis includes not only spine–synapses (spines forming synapses) but also free spines (spines without forming synapses). Modulation of spinogenesis is essential action of estrogen in memory processes, involving production of new spines that creates sites for new neuronal contacts. We demonstrated that dendritic spines were rapidly modulated upon E2 application, using single spine analysis of Lucifer-Yellow injected neurons in hippocampal slices from adult male rats (3 months old; Komatsuzaki et al., 2005; Tsurugizawa et al., 2005; Mukai et al., 2006b; Murakami et al., 2006).
In adult hippocampal slices, the majority of spines (>95%) have distinct heads and necks, while the populations of stubby spines (roughly 5%, no neck) and filopodium (roughly 1%, no head) are very small. Therefore, the determination of spine head diameter distribution can be a very useful method in order to analyze the complex morphological changes in spines, instead of the conventional classification, such as mushroom/thin/stubby/filopodium.
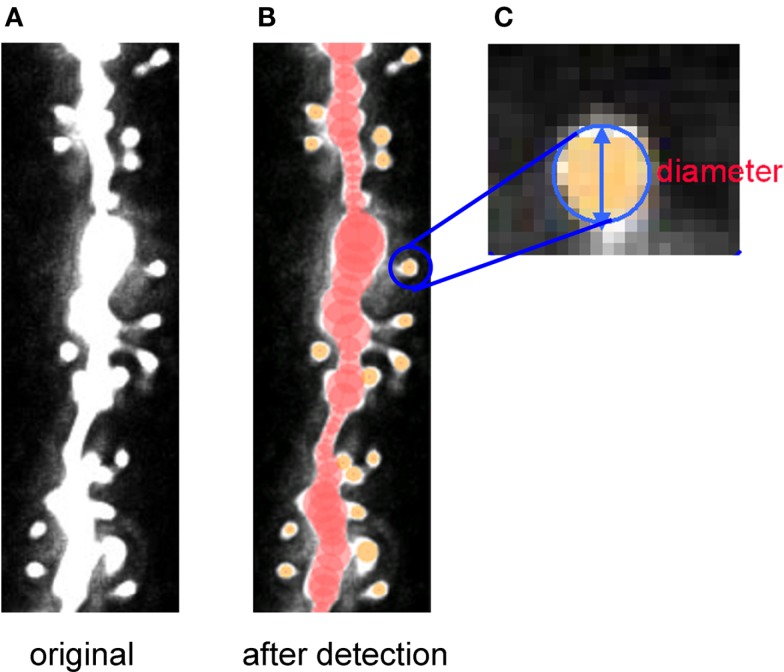
To do this, we have developed Spiso-3D software which mathematically/automatically identifies the spine head and determines the diameter of the spine (Figure 7; Mukai et al., 2011). Spiso-3D extracts spines based on geometrical features of spines, a completely different approach to other methods including the ray-bursting method (Wearne et al., 2005; Rodriguez et al., 2008) that exploits information of brightness to define boundaries of dendrites and spines. The identification of the spine head is performed by extraction of points in an isolated closed volume with a closed surface along the dendrites, from 20–30 optical slices obtained by confocal images. We use Hessian tensor that is obtained as second derivatives from Taylor expansion of the spine brightness function I(x) in each optical slice.
Figure 7.
Tracing of dendrites and detection of spines by Spiso-3D of confocal images. Spiso-3D detects spines by geometric calculation. (A) Original image of dendrite. (B) Traced dendrite (red circles) and spines (yellow circles) superimposed on the original image. (C) Calculated diameters of spines are superimposed on the original spine images. Modified from Mukai et al. (2011).
with
where u is a unit vector of direction; λ+ and λ− (λ+ > λ−) are the eigenvalues of diagonalized Hessian tensor. The spine head region points are extracted as points where both λ+ and λ− yield negative values, since the spine head is an isolated closed volume with a closed surface. Note that negative eigenvalues of Hessian tensor (λ+ and λ−) represent the negative curvature of closed spine surface.
Determination of spine head diameter is performed using the digitized “radius detection image” which is assembled from the spine head region points. The Spiso-3D determines spines using not only brightness, but also geometric features of the neuronal images, leading to the accurate identification of spines. Results obtained by Spiso-3D are almost identical to those by Neurolucida, currently used manual software, however considerably reducing human errors and experimenter labor. For quantitative analysis, we classify spines into three subclasses, i.e., small-head spines (0.2-0.4 μm), middle-head spines (0.4-0.5 μm), and large-head spines (0.5-1.0 μm).
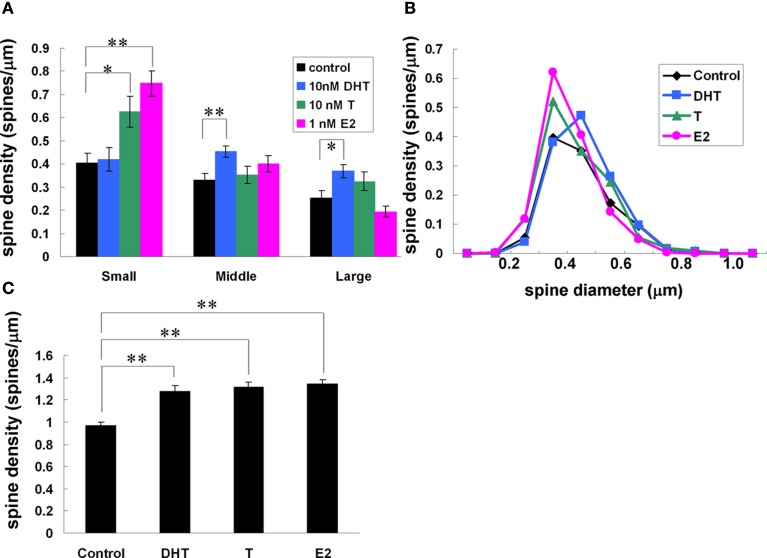
Using Spiso-3D analysis on spine head diameter, we clearly distinguished the different effects of testosterone (T, 10 nM), dihydrotestosterone (DHT, 10 nM), and 17β-E2 (E2, 1 nM) on dendritic spines of hippocampal CA1 pyramidal neurons in acute hippocampal slices (Figure 8; Mukai et al., 2011). These sex hormones rapidly (within 2 h) increased the total spine density from 0.97 spines/μm to 1.28 (T), 1.32 (DHT), and 1.34 (E2), respectively (Figure 8). While the effects of T, DHT, and E2 treatment on total spine density were indistinguishable, closer examination of spine head diameter revealed marked differences in the distribution of spine head diameter between T, DHT, and E2 treatments (Figure 8). To distinguish different responses in spine subpopulations, spines were classified into three categories according to their head diameters: (1) a small-head spine (0.2-0.4 μm), (2) a middle-head spine (0.4-0.5 μm), and (3) a large-head spine (0.5-1.0 μm). DHT treatment was found to increase large- and middle-head spines, whereas T increased large- and small-head spines. In contrast, E2 treatment increased only small-head spines (Figure 8). The observed differences in the effects of the hormones on spine subpopulations may have functional implications, for example, large-head spines may contain more AMPA receptors, since spine head size positively correlates with the density of AMPA-type glutamate receptors (Kopec et al., 2007; Shinohara et al., 2008). Since the induction of LTP is dependent on the density of AMPA receptors in spines (Kopec et al., 2007; Shinohara et al., 2008), increased density of AMPA receptors in large-head spines could facilitate LTP. Increased density of large-head spines following DHT treatment could potentially facilitate LTP induction, in contrast to T, which only moderately increased large-head spines, or E2 which had no effect on the density of large-head spines. These findings demonstrate the importance of the consideration of spine diameter to distinguish different types of neurotrophic effects.
Figure 8.
Effects of androgens and estrogens on changes in the density and morphology of spines. Spines were analyzed by Spiso-3D along the secondary dendrites in the stratum radiatum of CA1 pyramidal neurons. A 2-h treatment in ACSF without hormone (Control), with 10 nM DHT, with 10 nM T, with 1 nM E2. (A) Density of three subtypes of spines treated with DHT, T, and E2. From left to right, ACSF without hormones (black), 10 nM DHT (blue), 10 nM T (green), and 1 nM E2 (pink). (B) Histogram of spine head diameters. After a 2-h treatment in ACSF without steroids (Control, black), E2 (pink), T (green), and DHT (blue). (C) Total spine density. Vertical axis is the average number of spines per 1 μm of dendrite. Modified from Mukai et al. (2011).
We further investigated the signal cascade involving E2-induced spinogenesis. Propyl-pyrazole-trinyl-phenol (PPT, ERα agonist; Harrington et al., 2003) increased the spine density to the identical value at 1 nM E2 whereas diarylpropionitrile (DPN, ERβ agonist; Harrington et al., 2003) increased the spine density only slightly (Mukai et al., 2007). Blocking of ERα by ICI 182,780 completely suppressed the enhancing effect of E2 on the spine density. Blocking of phosphorylation of Erk MAP kinase by its inhibitors, PD98059 or U0126, completely prevented the E2-induced spinogenesis (Murakami et al., 2006). When the Ca2+ concentration in spines was further decreased by blocking NMDA receptors with its blocker, MK-801, the enhancing effect by E2 was completely suppressed. Taken together, the enhancement of the spine density is probably induced by activation of Erk MAP kinase via E2 and ERα at the basal low Ca2+ concentration of around 0.1–0.2 μM in resting neuronal synapses (Ishii et al., 2007). The morphological changes in CA1 spines occurred by 2 h E2 treatments.
The rapid effect of estrogens has also been observed in vivo. Leranth, MacLusky and co-workers have demonstrated that E2 (60 μg/kg) increases the CA1 spine–synapse density due to synaptic rearrangements in OVX adult rats as rapid as after 30 min of E2 injection using electron micrographic analysis (MacLusky et al., 2005). PPT (1.7 mg/kg) also rapidly enhances (within 40 min), but DPN (1.7 mg/kg) does not enhances, the hippocampal spine density in adult OVX female mice (Phan et al., 2011). On the other hand, the slow genomic effects (1–4 days) of E2 on spine plasticity, have been extensively investigated in vivo from the view point of estrogen replacement therapy. For example, supplement of estrogens in OVX adult female rats (Gould et al., 1990; Woolley et al., 1990; Woolley and McEwen, 1992; MacLusky et al., 2005), increases the density of spines in the stratum radiatum of CA1 pyramidal neurons, resulting in recovery of spines to the level of intact rat. These effects of enhancement in spinogenesis have also been observed as rapid as at 5 h after s.c. injection of estrogen (MacLusky et al., 2005).
It should be noted that another ERβ agonist, WAY-200070, enhanced the spine density in adult mice hippocampus (Liu et al., 2008). At the moment we cannot explain why WAY-200070 showed different results from those of DPN.
Results from in vivo investigations using whole rat may reflect not only direct but also indirect effects of E2 on glutamatergic neurons via cholinergic or serotonergic neurons, projecting to the hippocampus (Leranth et al., 2000; MacLusky et al., 2005).
In vitro investigations have also shown that spine density in CA1 increases following several days’ treatment of cultured hippocampal slices with exogenous E2 (Pozzo-Miller et al., 1999). The contribution of hippocampus-derived E2 has been reported by Rune and co-workers who demonstrated that the suppression of endogenous E2 synthesis by letrozole treatments for 4 days significantly decreases the density of spines, spine–synapses, spinophilin (spine marker), and synaptophysin (presynaptic marker) in the stratum radiatum of the CA1 region in cultured slices (Kretz et al., 2004). No increase in the density of spines, spine–synapses, and spinophilin expression is seen after exogenous application of 100 nM E2 to the medium of slice cultures that had not been treated with letrozole. Application of 100 nM E2, however, rescues the synaptophysin expression that was once decreased by letrozole. In primary cultured rat hippocampal neurons, E2 as well as WAY-200070 rapidly enhance spinogenesis with in 60 min (Srivastava et al., 2008, 2010).
In many previous works, the concentration of endogenous E2 within neurons or glia was not easy to determine accurately, therefore results were explained with the concentration of exogenously added E2. This situation leads to misunderstandings and conflicts. For in vitro experiments using slices or primary cultures of neuron/glia, the concentration of exogenously added E2 should be higher than that of endogenous E2 in order to show a significant effect. We often use “acute” hippocampal slices obtained after recovery incubation in steroid-free ACSF for 2 h for analysis of spinogenesis and electrophysiological experiments (Figure 5). During the recovery incubation with ACSF endogenous sex steroids and corticosteroids are released into the outer medium. Acute hippocampal slices used for investigations of spinogenesis and electrophysiology contain low levels of E2, T, and CORT which are below 0.5, 1.0, and 2.0 nM, respectively (Table 1). This low steroid level in “acute” hippocampal slices support the effective action of 1–5 nM E2, T, and DHT. We should also be careful about the level of exogenously added E2, T, and DHT in experiments in vivo, because the hippocampus in vivo contains 5–10 nM E2, T, and DHT (Hojo et al., 2009). However, the situation is complex in vivo, because exogenously added E2 may affect serotonin neurons projecting to the hippocampus, rather than directly affecting hippocampal glutamatergic neurons (Leranth et al., 2000).
Modulation of long-term depression and long-term potentiation
E2-induced modulation of LTD or LTP occurs only in preexistent synapses, because newly generated spines by E2 treatments do not form new synapses within 2 h, as judged from no increase in the baseline magnitude of excitatory postsynaptic potential (EPSP) signal during 2 h of E2 perfusion (Mukai et al., 2007).
Evidence is emerging that E2 exerts a rapid influence (0.5–1 h) on synaptic transmission of hippocampal slices from adult rats (3 months), as demonstrated by electrophysiology (Teyler et al., 1980; Gu and Moss, 1996; Foy et al., 1999; Ito et al., 1999; Shibuya et al., 2003).
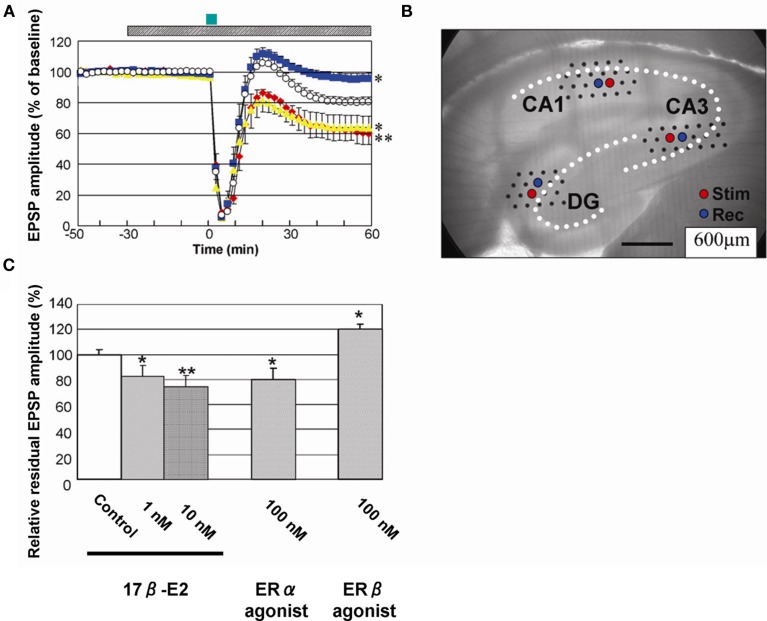
In memory processing, not only LTP (memory forming mechanism) but also LTD is essential. Mutant mice, which show enhanced LTP and suppressed LTD, have shown impaired learning of Morris water maze (Migaud et al., 1998). This suggests that LTD may be required to “correct” wrong memories formed by initial LTP processes, which store not only correct information but also wrong information. We found that LTD was very sensitive to 17β-E2 treatments in hippocampal slices from adult male rats. We demonstrated, for the first time, a significant rapid enhancement of LTD by 1–10 nM E2 perfusion in CA1, CA3, and DG (Figure 9; Mukai et al., 2007). Recordings were performed using 64 planar multielectrodes particularly arranged to stimulate the Schaffer collaterals in the stratum radiatum of CA1, the recurrent collateral fibers in the stratum radiatum of CA3, and the medial perforant pathways in the molecular layer of DG. LTD was induced pharmacologically by the transient application (3 min) of 30 μM NMDA. This LTD was induced by the activation of phosphatase due to a moderate Ca2+ influx through NMDA receptors (Lee et al., 1998). LTD is effectively induced by the transient application of NMDA to adult hippocampus, whereas low frequency stimulation cannot induce LTD in adult slices. Low frequency stimulation can induce LTD in slices from animals younger than 2 weeks. A 30-min preperfusion of 10 nM E2 significantly enhanced LTD resulting in the residual EPSP amplitude of 80–60% (CA1), 88–79% (CA3), and 95–92% (DG; Figure 9; Mukai et al., 2007). Investigations using specific estrogen agonists indicated that the contribution of estrogen receptor (ERα, but not ERβ) was essential to these E2 effects. ERα agonist (PPT) at 100 nM exhibited a significant LTD enhancement in CA1, while ERβ agonist (DPN) did induce a suppression of LTD in CA1, implying that the contribution of ERβ was opposite to that of ERα in the E2 effect on LTD. Taken collectively, E2-bound ERα may activate phosphatase at the moderate Ca2+ concentration of around 0.7–1 μM induced upon 30 μM NMDA application (Lisman, 1989), and facilitated dephosphorylation of AMPA receptors may induce enhancement of LTD. On the other hand, E2-bound ERα is not functional in LTP modulation at the transiently high Ca2+ concentration of around 5–12 μM under tetanic stimulation (Lisman, 1989; Yang et al., 1999; Mukai et al., 2006b; Hojo et al., 2008), because phosphorylation of AMPA receptors by CaM kinase II is a dominant process at the high Ca2+ concentration.
Figure 9.
Rapid modulation of LTD by 17β-E2 in hippocampal slices from adult male rats. (A) Time-dependence of maximal EPSP amplitude in CA1. E2 concentration was 0 nM (open circle), 10 nM (red closed diamond), 100 nM PPT (yellow closed triangle), and 100 nM DPN (blue closed square), respectively. Here, 100% EPSP amplitude refers to the EPSP value at t = −40 min prior to NMDA stimulation, irrespective of the test condition. LTD was induced by 30 μM NMDA perfusion at time t = 0–3 min (closed green bar above the graph). Hatched bar above the graph indicates period of time during which E2 was administered. (B) Custom-made 64 multielectrode probe (MED64, Panasonic, Japan) with the hippocampal slice. Stimulation (red circle) and recording (blue circle) electrodes are indicated. (C) Comparison of modulation effect of 17β-E2 and agonists on LTD in CA1. Vertical axis is relative EPSP amplitude at t = 60 min, where EPSP amplitude of the slice without drug application (control) is normalized as 100%. From left to right, the group applied 17β-E2, PPT (ERα agonist) and DPN (ERβ agonist) at indicated concentration. Statistical significance was calculated at 60 min by ANOVAs (*p < 0.05; **p < 0.01). Modified from Mukai et al. (2007), Hojo et al. (2008).
Concerning LTP, E2 alone does not affect baseline fEPSP (field EPSP) and/or LTP in the hippocampus of 3-month-old adult male rats (Ito et al., 1999; Mukai et al., 2007; Ogiue-Ikeda et al., 2008; Ooishi et al., 2011) and aged (3- to 5-month-old as well as 18- to 24-month-old) Sprague-Dawley rats (Vouimba et al., 2000). In contrast to the case of 3 months or older rats, several studies have shown that E2 alone rapidly increases baseline fEPSP (thereby enhances LTP) in the hippocampus of 4- to 8-week-old Sprague-Dawley rats (Foy et al., 1999; Bi et al., 2000; Kramar et al., 2009). E2 perfusion sometimes increases baseline fEPSP in the hippocampus of 4-week-old Wistar rats (∼20% of all experiments; Kawato, 2004). Therefore, these differences about E2-induced elevation of baseline fEPSP may depend on the age (more than 3 months old or less than 8 weeks old) of rats.
Interestingly, E2 has a protective effect on CORT-induced suppression of LTP, although E2 alone has no effect on LTP. A 30-min perfusion of 1 μM of CORT prior to the LTP induction (tetanic stimulation) suppresses the magnitude of LTP in the hippocampal slice. The magnitude of LTP was restored to that of control slices (without CORT perfusion) by co-perfusion of 1 μM of CORT and 1 or 10 nM of E2 (Ooishi et al., 2011). Note that 1 μM of CORT is close to the circulating level of total CORT under stressful condition, although free CORT may be around 500 nM with another 500 nM bound to CBG which has maximum binding capacity of around 500 nM.
Synaptic or extranuclear estrogen receptors
What is the receptor of 17β-E2 that mediates rapid actions (1–2 h) on synaptic plasticity in the hippocampus? Putative synaptic membrane estrogen receptors remain poorly defined. Many attempts have been made to identify membrane estrogen receptors. At the present stage, the most probable candidates for synaptic estrogen receptors may be ERα, ERβ, and GPR30.
Classical nuclear type receptors ERα and ERβ are candidates for synaptic estrogen receptors. Because ICI do not suppress E2-induced rapid modulation of electrophysiological properties such as LTD, LTP, and kinate-induced currents, classical estrogen receptors are suggested to be not involved in these modulations (Gu and Moss, 1996). However, these results do not eliminate the possibility that ERα and ERβ could drive these synaptic transmissions. ICI has been indicated to display its effect by inhibiting dimerization of ERα and ERβ. If dimerization processes are not involved in rapid modulation of electrophysiological phenomena, then ICI cannot block these phenomena. On the other hand, rapid enhancement of spinogenesis via ERα was significantly blocked by ICI (Mukai et al., 2007), therefore dimerization processes occur for synaptic ERα in spinogenesis.
We identified the membrane estrogen receptor ERα localized in the spines of hippocampal pyramidal and granule neurons by means of immunoelectron microscopic analysis as well as Western blot analysis using affinity–column-purified anti- ERα antibody RC-19 (C-terminal antibody; Figure 10; Mukai et al., 2007). Attention must be paid that non-purified ERα antisera often react significantly with unknown proteins, resulting in wrong staining different from real ERα distribution. A post-embedding immunogold electron microscopic analysis demonstrated the synaptic localization of ERα in the glutamatergic neurons in CA1, CA3, and DG (Figure 10). ERα was also localized in the nuclei. Western blot analysis demonstrated that ERα (67 kDa) and Erk MAP kinase were tightly associated with postsynaptic density fractions (PSD; Figure 10). On the other hand, ERα was not expressed at dendritic raft of adult male (Figure 10). Because the E2-induced modulation of LTD and spine density appeared so rapidly in the time range of 1–2 h, the synaptic ERα observed at PSD or postsynaptic compartments probably plays an essential role in driving rapid processes. Interestingly, a significant accumulation of ERα at PSD was observed by a 3-min stimulation with 30 μM NMDA used for the LTD-induction, implying that ERα may be dynamically movable in spines or dendrites (Figure 10; Hojo et al., 2008).
Figure 10.
Localization of ERα in hippocampal synapses. (A) – (C) Immunoelectron microscopic analysis of the distribution of ERα within axospinous synapses in the stratum radiatum of the hippocampal slices from adult male rat. (A) Gold particles (arrowheads) were localized in the pre- and postsynaptic regions. (B) In dendritic spines, gold particles were associated with PSD regions. (C) Gold particles were also localized in the nuclei. Pre, presynaptic region; post, postsynaptic region; Scale bar, 200 nm. (D–F) Immunohistochemical staining of ERα in the hippocampal slices from adult male rat [(D): whole hippocampus; (E): DG] and adult male ERαKO mouse [(F): DG]. gcl, Granule cell layer; hl, hilus. Scale bar, 500 μm for (D), and 200 μm for (E,F). (G) Western blot analysis of ERα in male rat hippocampal neurons. Blot of ERα in postsynaptic density (PSD), dendritic raft (D-Raft), and cytoplasm (CYT). From left to middle, blot of PSD fractions with RC-19 IgG (ERα), PSD-95 IgG (PSD-95), and Erk MAP kinase IgG (MAPK). From middle to right, blot of D-Raft with RC-19 (ERα) and flotillin-1 IgG (flotillin-1). At right-most lane, blot of CYT with RC-19 (ERα). The amount of protein applied was 20 μg for each lane, except for left-most PSD lane in which 60 μg protein was applied in order to improve the signal to noise ratio. Modified from Mukai et al. (2007, 2010).
Specific binding of purified RC-19 antibody to real ERα (67 kDa) in the hippocampus was verified using MALDI-TOF mass-spectrometric analysis of RC-19 reacted proteins as well as the absence of reactivity of RC-19 with ERαKO mice hippocampus (Mukai et al., 2007). These analyses are essential in the hippocampus, because we found that non-purified MC-20 antisera, frequently used in previous investigations, often reacted with 62 kDa unknown proteins in the brain (Toran-Allerand et al., 2002) and did not significantly react with real ERα (67 kDa; Mukai et al., 2007). Non-purified antisera may largely react with proteins having amino acid sequences similar to the real antigen in the hippocampus in which extremely low level of ERα is expressed as compared with that in the ovary. Surprisingly, ERα antisera are often examined for their reactivity only in endocrine organs such as the ovary in which ERα is highly expressed. Therefore, staining of interneurons and no staining of primary neurons with non-purified antisera such as MC-20 probably do not show real ERα distribution in the hippocampus. Antisera should be purified before application to the hippocampus.
ERα knock-out mice may be useful to investigate the participation of ERα in modulation of synaptic plasticity. However, so far inadequate data for true ERα knock-out mice are available. Electrophysiological investigations are performed by using knock-down mice (not knock-out mice) by Moss and co-workers (Gu et al., 1999). They have reported no essential contribution of ERα to E2-induced rapid enhancement of the kainate currents of CA1 neurons. They reach this conclusion due to the observation of very small difference in E2 effect on the kainate currents between wild-type and ERα-Neo knock-down mice which have been constructed by the method of Neomycin-insertion into exon 1 (the previously named exon 2; Lubahn et al., 1993). It should be noted that in Neomycin-insertion ERα-Neo knock-down mice, N-terminal-modified ERα (61 kDa) is expressed (Couse et al., 1995; Kos et al., 2002; Pendaries et al., 2002). Because the N-terminal-modified ERα is demonstrated to be still active on E2 binding and drives genomic processes (Couse et al., 1995; Kos et al., 2002; Pendaries et al., 2002), the participation of ERα in electrophysiological properties of the CA1 cannot be excluded from their investigations. Therefore, it is necessary to investigate real ERα knock-out mice which are, for example, deleted in the whole exon 2 of the mouse ERα gene (Dupont et al., 2000). Note that nomenclature of ERα exon changes recently, and the current exon 1 and exon 2 (Kos et al., 2002; Pendaries et al., 2002) correspond to the previous exon 2 and exon 3, respectively (Dupont et al., 2000).
Concerning extranuclear ERα, Mermelstein and colleagues have demonstrated that membrane associated ERα is coupled with metabotropic glutamate receptor 1 (mGluR1) in female primary cultured hippocampal neurons (Boulware et al., 2005, 2007; Boulware and Mermelstein, 2009). The mGluR1 coupled ERα is connected via caveolin and mediates the phosphorylation of MAP kinase and CREB very rapidly (5–20 min). Interestingly, these effects are observed only in female neurons and not in male neurons. Taken together with our finding of sex difference in hippocampal E2 level (Hojo et al., 2009; Mukai et al., 2010), these results suggest the sex difference in hippocampus-dependent memory processes, although the hippocampus does not have sex difference in the expression level of ERα and mGluR1. The absence of ERα in raft regions (caveolin-rich regions) of male hippocampus may also support the hippocampal sex difference (Mukai et al., 2010).
Accumulated results support that ERβ acts as a membrane receptor, extranuclear or synaptic receptor. ERβ associates with membranes in genetically expressed CHO cells and MCF-7 cells (Razandi et al., 1999; Pedram et al., 2006). ERβ rapidly attenuates LTD-induction (Mukai et al., 2007) and rescues CORT-induced suppression of LTP (Ooishi et al., 2011). ERβ rapidly prevents phosphorylation of CREB through mGluR2 and Gi via L-type calcium channel in primary cultured hippocampal neurons (Boulware and Mermelstein, 2005). Several investigations of immunostaining of ERβ suggest the extranuclear expression of ERβ including dendritic appearance in the hippocampal principal neurons (Milner et al., 2005). The subcellular immunostaining patterns of these reports might reflect the relatively minor expression of ERβ and the major expression of unknown proteins, due to multiple reactivity of non-purified ERβ antisera to several unknown proteins observed in the Western blot analysis of hippocampal tissues. The purity of commercially available ERβ antisera is worse than that of ERα antisera as judged from our Western blot analysis.
A Model of Synaptocrine and Neurocrine Mechanism
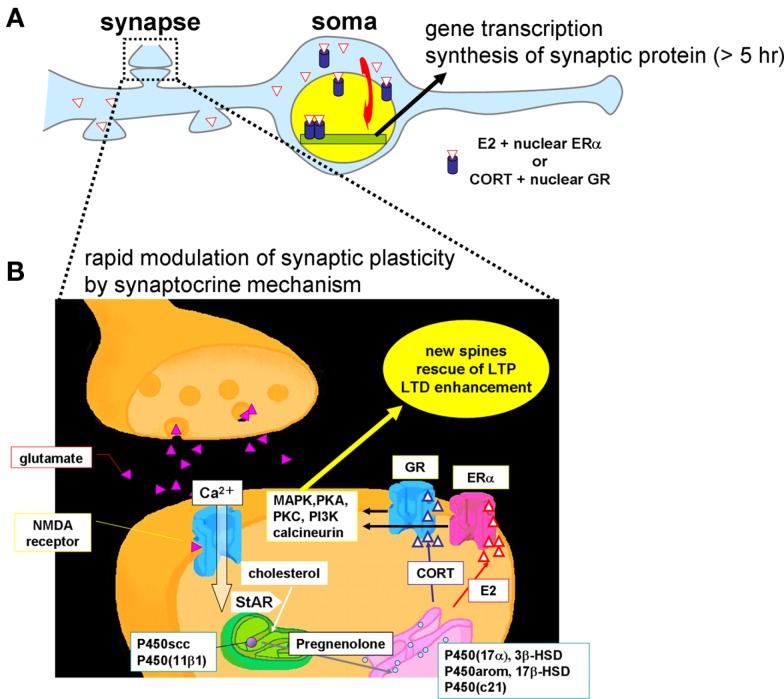
Figure 11 shows a model for the synaptic synthesis of hippocampal steroids (synaptocrine mechanism) and the modulation of the synaptic plasticity of neurons by hippocampal steroids. Hippocampal steroid synthesis proceeds in the following manner. First, glutamate release from the presynapse induces a Ca2+ influx through the NMDA receptors. The Ca2+ influx drives StAR (Kimoto et al., 2001) to transport cholesterol into the mitochondria, where P450scc converts cholesterol to PREG. The conversion of PREG has various branches: (1) sex-steroid synthesis of “PREG → DHEA → androstenediol (ADiol) → T → E2, or T → DHT → 3α, 5α-androstanediol, or PROG → androstenedione (ADione) → T,” and (2) corticosteroid synthesis of “PREG → PROG → DOC → CORT” (Figure 3). These reactions occur at spines, in addition to the endoplasmic reticulum or mitochondria in the cell body by various steroidogenic enzymes including P450(17α), P450arom, P450(c21), P450(11β1), 3β-HSD, 17β-HSD, 5α-reductase, and 3α-HSD.
Figure 11.
Schematic illustration of synaptic actions by hippocampus-synthesized steroids. (A) Slow modulation of synaptic plasticity via gene transcription and synthesis of synaptic proteins in neurons. The delayed action of E2 or CORT is mediated by ERα/ERβ or GR in cytoplasm and nuclei, respectively. New synaptic connections are formed by synthesized synaptic proteins or neurotrophic factors. (B) Rapid modulation of the synaptic plasticity via synaptic ERα and GR. Hippocampal neurons synthesize much higher level of E2 than that from circulation. The level of hippocampus-synthesized CORT is in the order of 10 nM which is sufficient to increase density of dendritic spines. Upon Ca2+ influx via NMDA receptor, transport of cholesterol to inner membrane of mitochondria by StAR is facilitated, resulting in enhancement of steroid synthesis. P450scc and P450 (11β1) are localized in mitochondria and P450 (17α), 3β-HSD, P450arom, 17β-HSD, and P450 (c21) are localized in endoplasmic reticulum. Hippocampus-synthesized sex steroids and corticosteroids bind to synaptic ERα and GR which drive signal cascades mediated via various kinases and phosphatases.
The produced E2 binds to synaptic ERα and drives a signaling pathway including MAP kinase finally resulting in the modulation of AMPA receptors (AMPA-type glutamatergic receptors) or NMDA receptors (NMDA type glutamatergic receptors). Modulation indicates, for example, phosphorylation of these receptors or AMPA receptor insertion/endocytosis. Note, of course, that hippocampal steroids are synthesized also in the endoplasmic reticulum and mitochondria in the cell bodies of neurons (intracrine mechanisms). The genomic pathway via nuclear ERα also function in delayed E2 effects, such as neuroprotection, spinogenesis, maintaining homeostasis, etc (intracrine mechanisms). Because the levels of E2, T, and DHT are much lower in the circulation (Hojo et al., 2008, 2009), hippocampus-synthesized sex steroids may play a central role in the modulation of synaptic plasticity or memory process.
Hippocampus-synthesized CORT binds to synaptic glucocorticoid receptor (GR; Ooishi et al., 2011), resulting in increase the density of spines (Higo et al., 2011). The signaling cascade mediated by hippocampus-synthesized CORT (∼10 nM) and synaptic GR is expected to be different from that mediated by high stress level of CORT (∼1 μM). Further investigations on the molecular mechanism mediated by hippocampus-synthesized CORT should be clarified.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ADiol, androstenediol; ADione, androstenedione; DHEA, dehydroepiandrosterone; DHT, dihydrotestosterone; E1, estrone; E2, estradiol; LC-MS/MS, liquid chromatography with tandem-mass-spectrometry; PFBz, pentafluorobenzoxy; PROG, Progesterone; RIA, radioimmunoassay; T, testosterone.
References
- Agis-Balboa R. C., Pinna G., Zhubi A., Maloku E., Veldic M., Costa E., Guidotti A. (2006). Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 103, 14602–14607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J., Ball G. F. (2006). Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 29, 241–249 [DOI] [PubMed] [Google Scholar]
- Baulieu E. E. (1997). Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog. Horm. Res. 52, 1–32 [PubMed] [Google Scholar]
- Baulieu E. E., Robel P. (1998). Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) as neuroactive neurosteroids. Proc. Natl. Acad. Sci. U.S.A. 95, 4089–4091 10.1073/pnas.95.8.4089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyenburg S., Watzka M., Clusmann H., Blumcke I., Bidlingmaier F., Elger C. E., Stoffel-Wagner B. (2001). Messenger RNA of steroid 21-hydroxylase (CYP21) is expressed in the human hippocampus. Neurosci. Lett. 308, 111–114 10.1016/S0304-3940(01)01991-7 [DOI] [PubMed] [Google Scholar]
- Bi R., Broutman G., Foy M. R., Thompson R. F., Baudry M. (2000). The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc. Natl. Acad. Sci. U.S.A. 97, 3602–3607 10.1073/pnas.060034497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware M. I., Kordasiewicz H., Mermelstein P. G. (2007). Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J. Neurosci. 27, 9941–9950 10.1523/JNEUROSCI.1647-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware M. I., Mermelstein P. G. (2005). The influence of estradiol on nervous system function. Drug News Perspect. 18, 631–637 10.1358/dnp.2005.18.10.959577 [DOI] [PubMed] [Google Scholar]
- Boulware M. I., Mermelstein P. G. (2009). Membrane estrogen receptors activate metabotropic glutamate receptors to influence nervous system physiology. Steroids 74, 608–613 10.1016/j.steroids.2008.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware M. I., Weick J. P., Becklund B. R., Kuo S. P., Groth R. D., Mermelstein P. G. (2005). Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J. Neurosci. 25, 5066–5078 10.1523/JNEUROSCI.1427-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner C. W., Orchinik M. (2002). Plasma binding proteins as mediators of corticosteroid action in vertebrates. J. Endocrinol. 175, 99–112 10.1677/joe.0.1750099 [DOI] [PubMed] [Google Scholar]
- Caruso D., Pesaresi M., Maschi O., Giatti S., Garcia-Segura L. M., Melcangi R. C. (2010). Effect of short-and long-term gonadectomy on neuroactive steroid levels in the central and peripheral nervous system of male and female rats. J. Neuroendocrinol. 22, 1137–1147 10.1111/j.1365-2826.2010.02064.x [DOI] [PubMed] [Google Scholar]
- Couse J. F., Curtis S. W., Washburn T. F., Lindzey J., Golding T. S., Lubahn D. B., Smithies O., Korach K. S. (1995). Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol. Endocrinol. 9, 1441–1454 10.1210/me.9.11.1441 [DOI] [PubMed] [Google Scholar]
- Dupont S., Krust A., Gansmuller A., Dierich A., Chambon P., Mark M. (2000). Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development 127, 4277–4291 [DOI] [PubMed] [Google Scholar]
- Ebner M. J., Corol D. I., Havlikova H., Honour J. W., Fry J. P. (2006). Identification of neuroactive steroids and their precursors and metabolites in adult male rat brain. Endocrinology 147, 179–190 [DOI] [PubMed] [Google Scholar]
- Foy M. R., Xu J., Xie X., Brinton R. D., Thompson R. F., Berger T. W. (1999). 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J. Neurophysiol. 81, 925–929 [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez C. E., Zhou M. Y., Cozza E. N., Morita H., Eddleman F. C., Gomez-Sanchez E. P. (1996). Corticosteroid synthesis in the central nervous system. Endocr. Res. 22, 463–470 [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez C. E., Zhou M. Y., Cozza E. N., Morita H., Foecking M. F., Gomez-Sanchez E. P. (1997). Aldosterone biosynthesis in the rat brain. Endocrinology 138, 3369–3373 10.1210/en.138.8.3369 [DOI] [PubMed] [Google Scholar]
- Gould E., Woolley C. S., Frankfurt M., McEwen B. S. (1990). Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J. Neurosci. 10, 1286–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q., Korach K. S., Moss R. L. (1999). Rapid action of 17beta-estradiol on kainate-induced currents in hippocampal neurons lacking intracellular estrogen receptors. Endocrinology 140, 660–666 10.1210/en.140.2.660 [DOI] [PubMed] [Google Scholar]
- Gu Q., Moss R. L. (1996). 17 beta-estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J. Neurosci. 16, 3620–3629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington W. R., Sheng S., Barnett D. H., Petz L. N., Katzenellenbogen J. A., Katzenellenbogen B. S. (2003). Activities of estrogen receptor alpha- and beta-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol. Cell. Endocrinol. 206, 13–22 10.1016/S0303-7207(03)00255-7 [DOI] [PubMed] [Google Scholar]
- Higashi T., Ninomiya Y., Iwaki N., Yamauchi A., Takayama N., Shimada K. (2006). Studies on neurosteroids XVIII LC-MS analysis of changes in rat brain and serum testosterone levels induced by immobilization stress and ethanol administration. Steroids 71, 609–617 10.1016/j.steroids.2006.08.003 [DOI] [PubMed] [Google Scholar]
- Higo S., Hojo Y., Ishii H., Komatsuzaki Y., Ooishi Y., Murakami G., Mukai H., Yamazaki T., Nakahara D., Barron A., Kimoto T., Kawato S. (2011). Endogenous synthesis of corticosteroids in the hippocampus. PLoS ONE 6, e21631. 10.1371/journal.pone.0021631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo S., Hojo Y., Ishii H., Kominami T., Nakajima K., Poirier D., Kimoto T., Kawato S. (2009). Comparison of sex-steroid synthesis between neonatal and adult rat hippocampus. Biochem. Biophys. Res. Commun. 385, 62–66 10.1016/j.bbrc.2009.05.005 [DOI] [PubMed] [Google Scholar]
- Hojo Y., Hattori T. A., Enami T., Furukawa A., Suzuki K., Ishii H. T., Mukai H., Morrison J. H., Janssen W. G., Kominami S., Harada N., Kimoto T., Kawato S. (2004). Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc. Natl. Acad. Sci. U.S.A. 101, 865–870 10.1073/pnas.2630225100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y., Higo S., Ishii H., Mukai H., Murakami G., Kominami T., Kimoto T., Honma S., Poirier D., Kawato S. (2009). Comparison between hippocampus-synthesized and circulation-derived sex steroids in the hippocampus. Endocrinology 150, 5106–5112 10.1210/en.2009-0305 [DOI] [PubMed] [Google Scholar]
- Hojo Y., Murakami G., Mukai H., Higo S., Hatanaka Y., Ogiue-Ikeda M., Ishii H., Kimoto T., Kawato S. (2008). Estrogen synthesis in the brain – role in synaptic plasticity and memory. Mol. Cell. Endocrinol. 290, 31–43 10.1016/j.mce.2008.04.017 [DOI] [PubMed] [Google Scholar]
- Ishida A., Mutoh T., Ueyama T., Bando H., Masubuchi S., Nakahara D., Tsujimoto G., Okamura H. (2005). Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2, 297–307 10.1016/j.cmet.2005.09.009 [DOI] [PubMed] [Google Scholar]
- Ishii H., Tsurugizawa T., Ogiue-Ikeda M., Asashima M., Mukai H., Murakami G., Hojo Y., Kimoto T., Kawato S. (2007). Local production of sex hormones and their modulation of hippocampal synaptic plasticity. Neuroscientist 13, 323–334 10.1177/10738584070130040601 [DOI] [PubMed] [Google Scholar]
- Ito K., Skinkle K. L., Hicks T. P. (1999). Age-dependent, steroid-specific effects of oestrogen on long-term potentiation in rat hippocampal slices. J. Physiol. (Lond.) 515(Pt 1), 209–220 10.1111/j.1469-7793.1999.049ad.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab R. L., Horvath T. L., Leranth C., Harada N., Naftolin F. (1993). Aromatase immunoreactivity in the rat brain: gonadectomy-sensitive hypothalamic neurons and an unresponsive “limbic ring” of the lateral septum-bed nucleus-amygdala complex. J. Steroid Biochem. Mol. Biol. 44, 481–498 [DOI] [PubMed] [Google Scholar]
- Kawato S. (2004). Endocrine disrupters as disrupters of brain function: a neurosteroid viewpoint. Environ. Sci. 11, 1–14 [PubMed] [Google Scholar]
- Kawato S., Hojo Y., Kimoto T. (2002). Histological and metabolism analysis of P450 expression in the brain. Meth. Enzymol. 357, 241–249 [DOI] [PubMed] [Google Scholar]
- Kibaly C., Patte-Mensah C., Mensah-Nyagan A. G. (2005). Molecular and neurochemical evidence for the biosynthesis of dehydroepiandrosterone in the adult rat spinal cord. J. Neurochem. 93, 1220–1230 10.1111/j.1471-4159.2005.03113.x [DOI] [PubMed] [Google Scholar]
- Kimoto T., Ishii H., Higo S., Hojo Y., Kawato S. (2010). Semicomprehensive analysis of the postnatal age-related changes in the mRNA expression of sex steroidogenic enzymes and sex steroid receptors in the male rat hippocampus. Endocrinology 151, 5795–5806 10.1210/en.2010-0581 [DOI] [PubMed] [Google Scholar]
- Kimoto T., Tsurugizawa T., Ohta Y., Makino J., Tamura H., Hojo Y., Takata N., Kawato S. (2001). Neurosteroid synthesis by cytochrome p450-containing systems localized in the rat brain hippocampal neurons: N-methyl-d-aspartate and calcium-dependent synthesis. Endocrinology 142, 3578–3589 10.1210/en.142.8.3578 [DOI] [PubMed] [Google Scholar]
- Komatsuzaki Y., Murakami G., Tsurugizawa T., Mukai H., Tanabe N., Mitsuhashi K., Kawata M., Kimoto T., Ooishi Y., Kawato S. (2005). Rapid spinogenesis of pyramidal neurons induced by activation of glucocorticoid receptors in adult male rat hippocampus. Biochem. Biophys. Res. Commun. 335, 1002–1007 10.1016/j.bbrc.2005.07.173 [DOI] [PubMed] [Google Scholar]
- Konkle A. T., McCarthy M. M. (2011). Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology 152, 223–235 10.1210/en.2010-0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec C. D., Real E., Kessels H. W., Malinow R. (2007). GluR1 links structural and functional plasticity at excitatory synapses. J. Neurosci. 27, 13706–13718 10.1523/JNEUROSCI.3503-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos M., Denger S., Reid G., Korach K. S., Gannon F. (2002). Down but not out? A novel protein isoform of the estrogen receptor alpha is expressed in the estrogen receptor alpha knockout mouse. J. Mol. Endocrinol. 29, 281–286 [DOI] [PubMed] [Google Scholar]
- Kramar E. A., Chen L. Y., Brandon N. J., Rex C. S., Liu F., Gall C. M., Lynch G. (2009). Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J. Neurosci. 29, 12982–12993 10.1523/JNEUROSCI.3059-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz O., Fester L., Wehrenberg U., Zhou L., Brauckmann S., Zhao S., Prange-Kiel J., Naumann T., Jarry H., Frotscher M., Rune G. M. (2004). Hippocampal synapses depend on hippocampal estrogen synthesis. J. Neurosci. 24, 5913–5921 10.1523/JNEUROSCI.5186-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goascogne C., Robel P., Gouezou M., Sananes N., Baulieu E. E., Waterman M. (1987). Neurosteroids: cytochrome P-450scc in rat brain. Science 237, 1212–1215 10.1126/science.3306919 [DOI] [PubMed] [Google Scholar]
- Le Goascogne C., Sananes N., Gouezou M., Takemori S., Kominami S., Baulieu E. E., Robel P. (1991). Immunoreactive cytochrome P-450(17 alpha) in rat and guinea-pig gonads, adrenal glands and brain. J. Reprod. Fertil. 93, 609–622 10.1530/jrf.0.0930609 [DOI] [PubMed] [Google Scholar]
- Lee H. K., Kameyama K., Huganir R. L., Bear M. F. (1998). NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron 21, 1151–1162 10.1016/S0896-6273(00)80632-7 [DOI] [PubMed] [Google Scholar]
- Leranth C., Shanabrough M., Horvath T. L. (2000). Hormonal regulation of hippocampal spine synapse density involves subcortical mediation. Neuroscience 101, 349–356 10.1016/S0306-4522(00)00369-9 [DOI] [PubMed] [Google Scholar]
- Liere P., Akwa Y., Weill-Engerer S., Eychenne B., Pianos A., Robel P., Sjovall J., Schumacher M., Baulieu E. E. (2000). Validation of an analytical procedure to measure trace amounts of neurosteroids in brain tissue by gas chromatography-mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 739, 301–312 10.1016/S0378-4347(99)00563-0 [DOI] [PubMed] [Google Scholar]
- Lisman J. (1989). A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc. Natl. Acad. Sci. U.S.A. 86, 9574–9578 10.1073/pnas.86.23.9574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Day M., Muniz L. C., Bitran D., Arias R., Revilla-Sanchez R., Grauer S., Zhang G., Kelley C., Pulito V., Sung A., Mervis R. F., Navarra R., Hirst W. D., Reinhart P. H., Marquis K. L., Moss S. J., Pangalos M. N., Brandon N. J. (2008). Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat. Neurosci. 11, 334–343 10.1038/nn2057 [DOI] [PubMed] [Google Scholar]
- Liu S., Sjovall J., Griffiths W. J. (2003). Neurosteroids in rat brain: extraction, isolation, and analysis by nanoscale liquid chromatography-electrospray mass spectrometry. Anal. Chem. 75, 5835–5846 [DOI] [PubMed] [Google Scholar]
- Lubahn D. B., Moyer J. S., Golding T. S., Couse J. F., Korach K. S., Smithies O. (1993). Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc. Natl. Acad. Sci. U.S.A. 90, 11162–11166 10.1073/pnas.90.23.11162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie S. M., Clark C. J., Fraser R., Gomez-Sanchez C. E., Connell J. M., Davies E. (2000). Expression of 11beta-hydroxylase and aldosterone synthase genes in the rat brain. J. Mol. Endocrinol. 24, 321–328 [DOI] [PubMed] [Google Scholar]
- MacLusky N. J., Luine V. N., Hajszan T., Leranth C. (2005). The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology 146, 287–293 [DOI] [PubMed] [Google Scholar]
- Mensah-Nyagan A. G., Do-Rego J. L., Beaujean D., Luu-The V., Pelletier G., Vaudry H. (1999). Neurosteroids: expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system. Pharmacol. Rev. 51, 63–81 [PubMed] [Google Scholar]
- Migaud M., Charlesworth P., Dempster M., Webster L. C., Watabe A. M., Makhinson M., He Y., Ramsay M. F., Morris R. G., Morrison J. H., O’Dell T. J., Grant S. G. (1998). Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature 396, 433–439 10.1038/24790 [DOI] [PubMed] [Google Scholar]
- Milner T. A., Ayoola K., Drake C. T., Herrick S. P., Tabori N. E., McEwen B. S., Warrier S., Alves S. E. (2005). Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J. Comp. Neurol. 491, 81–95 10.1002/cne.20724 [DOI] [PubMed] [Google Scholar]
- Mukai H., Hatanaka Y., Mitsuhashi K., Hojo Y., Komatsuzaki Y., Sato R., Murakami G., Kimoto T., Kawato S. (2011). Automated analysis of spines from confocal laser microscopy images: application to the discrimination of androgen and estrogen effects on spinogenesis. Cereb. Cortex. [Epub ahead of print]. 10.1093/cercor/bhr059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai H., Kimoto T., Hojo Y., Kawato S., Murakami G., Higo S., Hatanaka Y., Ogiue-Ikeda M. (2010). Modulation of synaptic plasticity by brain estrogen in the hippocampus. Biochim. Biophys. Acta 1800, 1030–1044 [DOI] [PubMed] [Google Scholar]
- Mukai H., Takata N., Ishii H. T., Tanabe N., Hojo Y., Furukawa A., Kimoto T., Kawato S. (2006a). Hippocampal synthesis of estrogens and androgens which are paracrine modulators of synaptic plasticity: synaptocrinology. Neuroscience 138, 757–764 10.1016/j.neuroscience.2005.09.010 [DOI] [PubMed] [Google Scholar]
- Mukai H., Tsurugizawa T., Ogiue-Ikeda M., Murakami G., Hojo Y., Ishii H., Kimoto T., Kawato S. (2006b). Local neurosteroid production in the hippocampus: influence on synaptic plasticity of memory. Neuroendocrinology 84, 255–263 10.1159/000097747 [DOI] [PubMed] [Google Scholar]
- Mukai H., Tsurugizawa T., Murakami G., Kominami S., Ishii H., Ogiue-Ikeda M., Takata N., Tanabe N., Furukawa A., Hojo Y., Ooishi Y., Morrison J. H., Janssen W. G., Rose J. A., Chambon P., Kato S., Izumi S., Yamazaki T., Kimoto T., Kawato S. (2007). Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. J. Neurochem. 100, 950–967 10.1111/j.1471-4159.2006.04264.x [DOI] [PubMed] [Google Scholar]
- Munetsuna E., Hojo Y., Hattori M., Ishii H., Kawato S., Ishida A., Kominami S. A., Yamazaki T. (2009). Retinoic acid stimulates 17beta-estradiol and testosterone synthesis in rat hippocampal slice cultures. Endocrinology 150, 4260–4269 10.1210/en.2008-1644 [DOI] [PubMed] [Google Scholar]
- Murakami G., Tsurugizawa T., Hatanaka Y., Komatsuzaki Y., Tanabe N., Mukai H., Hojo Y., Kominami S., Yamazaki T., Kimoto T., Kawato S. (2006). Comparison between basal and apical dendritic spines in estrogen-induced rapid spinogenesis of CA1 principal neurons in the adult hippocampus. Biochem. Biophys. Res. Commun. 351, 553–558 10.1016/j.bbrc.2006.10.066 [DOI] [PubMed] [Google Scholar]
- Naftolin F., Horvath T. L., Jakab R. L., Leranth C., Harada N., Balthazart J. (1996). Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology 63, 149–155 10.1159/000126951 [DOI] [PubMed] [Google Scholar]
- Nakahara D., Nakamura M., Iigo M., Okamura H. (2003). Bimodal circadian secretion of melatonin from the pineal gland in a living CBA mouse. Proc. Natl. Acad. Sci. U.S.A. 100, 9584–9589 10.1073/pnas.1631069100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiue-Ikeda M., Tanabe N., Mukai H., Hojo Y., Murakami G., Tsurugizawa T., Takata N., Kimoto T., Kawato S. (2008). Rapid modulation of synaptic plasticity by estrogens as well as endocrine disrupters in hippocampal neurons. Brain Res. Rev. 57, 363–375 10.1016/j.brainresrev.2007.06.010 [DOI] [PubMed] [Google Scholar]
- Ooishi Y., Mukai H., Hojo Y., Murakami G., Hasegawa Y., Shindo T., Morrison J., Kimoto T., Kawato S. (2011). Estradiol rapidly rescues synaptic transmission from corticosterone-induced suppression via synaptic/extranuclear steroid receptors in the hippocampus. Cereb. Cortex. 10.1093/cercor/bhr164 [DOI] [PubMed] [Google Scholar]
- Pedram A., Razandi M., Levin E. R. (2006). Nature of functional estrogen receptors at the plasma membrane. Mol. Endocrinol. 20, 1996–2009 [DOI] [PubMed] [Google Scholar]
- Pendaries C., Darblade B., Rochaix P., Krust A., Chambon P., Korach K. S., Bayard F., Arnal J. F. (2002). The AF-1 activation-function of ERalpha may be dispensable to mediate the effect of estradiol on endothelial NO production in mice. Proc. Natl. Acad. Sci. U.S.A. 99, 2205–2210 10.1073/pnas.042688499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan A., Lancaster K. E., Armstrong J. N., MacLusky N. J., Choleris E. (2011). Rapid effects of estrogen receptor alpha and beta selective agonists on learning and dendritic spines in female mice. Endocrinology 152, 1492–1502 10.1210/en.2010-1273 [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller L. D., Inoue T., Murphy D. D. (1999). Estradiol increases spine density and NMDA-dependent Ca2+ transients in spines of CA1 pyramidal neurons from hippocampal slices. J. Neurophysiol. 81, 1404–1411 [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J., Fester L., Zhou L., Lauke H., Carretero J., Rune G. M. (2006). Inhibition of hippocampal estrogen synthesis causes region-specific downregulation of synaptic protein expression in hippocampal neurons. Hippocampus 16, 464–471 [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J., Jarry H., Schoen M., Kohlmann P., Lohse C., Zhou L., Rune G. M. (2008). Gonadotropin-releasing hormone regulates spine density via its regulatory role in hippocampal estrogen synthesis. J. Cell Biol. 180, 417–426 10.1083/jcb.200707043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange-Kiel J., Wehrenberg U., Jarry H., Rune G. M. (2003). Para/autocrine regulation of estrogen receptors in hippocampal neurons. Hippocampus 13, 226–234 10.1002/hipo.10075 [DOI] [PubMed] [Google Scholar]
- Razandi M., Pedram A., Greene G. L., Levin E. R. (1999). Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol. Endocrinol. 13, 307–319 10.1210/me.13.2.307 [DOI] [PubMed] [Google Scholar]
- Revest J. M., Di Blasi F., Kitchener P., Rouge-Pont F., Desmedt A., Turiault M., Tronche F., Piazza P. V. (2005). The MAPK pathway and Egr-1 mediate stress-related behavioral effects of glucocorticoids. Nat. Neurosci. 8, 664–672 10.1038/nn1441 [DOI] [PubMed] [Google Scholar]
- Rodriguez A., Ehlenberger D. B., Dickstein D. L., Hof P. R., Wearne S. L. (2008). Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS ONE 3, e1997. 10.1371/journal.pone.0001997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R. M., Krey L. C., McEwen B. S. (1985). Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J. Neurosci. 5, 1222–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K., Takata N., Hojo Y., Furukawa A., Yasumatsu N., Kimoto T., Enami T., Suzuki K., Tanabe N., Ishii H., Mukai H., Takahashi T., Hattori T. A., Kawato S. (2003). Hippocampal cytochrome P450s synthesize brain neurosteroids which are paracrine neuromodulators of synaptic signal transduction. Biochim. Biophys. Acta 1619, 301–316 [DOI] [PubMed] [Google Scholar]
- Shinohara Y., Hirase H., Watanabe M., Itakura M., Takahashi M., Shigemoto R. (2008). Left-right asymmetry of the hippocampal synapses with differential subunit allocation of glutamate receptors. Proc. Natl. Acad. Sci. U.S.A. 105, 19498–19503 10.1073/pnas.0807461105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinzawa K., Ishibashi S., Murakoshi M., Watanabe K., Kominami S., Kawahara A., Takemori S. (1988). Relationship between zonal distribution of microsomal cytochrome P-450s (P-450(17)alpha,lyase and P-450C21) and steroidogenic activities in guinea-pig adrenal cortex. J. Endocrinol. 119, 191–200 10.1677/joe.0.1190191 [DOI] [PubMed] [Google Scholar]
- Srivastava D. P., Woolfrey K. M., Jones K. A., Shum C. Y., Lash L. L., Swanson G. T., Penzes P. (2008). Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc. Natl. Acad. Sci. U.S.A. 105, 14650–14655 10.1073/pnas.0803678105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava D. P., Woolfrey K. M., Liu F., Brandon N. J., Penzes P. (2010). Estrogen receptor beta activity modulates synaptic signaling and structure. J. Neurosci. 30, 13454–13460 10.1523/JNEUROSCI.3264-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyler T. J., Vardaris R. M., Lewis D., Rawitch A. B. (1980). Gonadal steroids: effects on excitability of hippocampal pyramidal cells. Science 209, 1017–1018 10.1126/science.7190730 [DOI] [PubMed] [Google Scholar]
- Toran-Allerand C. D., Guan X., MacLusky N. J., Horvath T. L., Diano S., Singh M., Connolly E. S., Jr., Nethrapalli I. S., Tinnikov A. A. (2002). ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J. Neurosci. 22, 8391–8401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurugizawa T., Mukai H., Tanabe N., Murakami G., Hojo Y., Kominami S., Mitsuhashi K., Komatsuzaki Y., Morrison J. H., Janssen W. G., Kimoto T., Kawato S. (2005). Estrogen induces rapid decrease in dendritic thorns of CA3 pyramidal neurons in adult male rat hippocampus. Biochem. Biophys. Res. Commun. 337, 1345–1352 10.1016/j.bbrc.2005.09.188 [DOI] [PubMed] [Google Scholar]
- Vouimba R. M., Foy M. R., Foy J. G., Thompson R. F. (2000). 17beta-estradiol suppresses expression of long-term depression in aged rats. Brain Res. Bull. 53, 783–787 10.1016/S0361-9230(00)00377-4 [DOI] [PubMed] [Google Scholar]
- Wang M. D., Wahlstrom G., Backstrom T. (1997). The regional brain distribution of the neurosteroids pregnenolone and pregnenolone sulfate following intravenous infusion. J. Steroid Biochem. Mol. Biol. 62, 299–306 [DOI] [PubMed] [Google Scholar]
- Wearne S. L., Rodriguez A., Ehlenberger D. B., Rocher A. B., Henderson S. C., Hof P. R. (2005). New techniques for imaging, digitization and analysis of three-dimensional neural morphology on multiple scales. Neuroscience 136, 661–680 10.1016/j.neuroscience.2005.05.053 [DOI] [PubMed] [Google Scholar]
- Wehrenberg U., Prange-Kiel J., Rune G. M. (2001). Steroidogenic factor-1 expression in marmoset and rat hippocampus: co-localization with StAR and aromatase. J. Neurochem. 76, 1879–1886 [DOI] [PubMed] [Google Scholar]
- Windle R. J., Brady M. M., Kunanandam T., Da Costa A. P., Wilson B. C., Harbuz M., Lightman S. L., Ingram C. D. (1997). Reduced response of the hypothalamo-pituitary-adrenal axis to alpha1-agonist stimulation during lactation. Endocrinology 138, 3741–3748 [DOI] [PubMed] [Google Scholar]
- Woolley C. S. (1998). Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm. Behav. 34, 140–148 10.1006/hbeh.1998.1466 [DOI] [PubMed] [Google Scholar]
- Woolley C. S., Gould E., McEwen B. S. (1990). Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 531, 225–231 10.1016/0006-8993(90)90778-A [DOI] [PubMed] [Google Scholar]
- Woolley C. S., McEwen B. S. (1992). Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J. Neurosci. 12, 2549–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley C. S., McEwen B. S. (1993). Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J. Comp. Neurol. 336, 293–306 [DOI] [PubMed] [Google Scholar]
- Woolley C. S., McEwen B. S. (1994). Estradiol regulates hippocampal dendritic spine density via an N-methyl-d-aspartate receptor-dependent mechanism. J. Neurosci. 14, 7680–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K., Kobayashi S., Tsukamoto S., Numazawa M. (2007a). Synthesis of pyridine-carboxylate derivatives of hydroxysteroids for liquid chromatography-electrospray ionization-mass spectrometry. Steroids 72, 50–59 10.1016/j.steroids.2007.07.003 [DOI] [PubMed] [Google Scholar]
- Yamashita K., Okuyama M., Watanabe Y., Honma S., Kobayashi S., Numazawa M. (2007b). Highly sensitive determination of estrone and estradiol in human serum by liquid chromatography-electrospray ionization tandem mass spectrometry. Steroids 72, 819–827 10.1016/j.steroids.2007.07.003 [DOI] [PubMed] [Google Scholar]
- Yang S. N., Tang Y. G., Zucker R. S. (1999). Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J. Neurophysiol. 81, 781–787 [DOI] [PubMed] [Google Scholar]
- Zwain I. H., Yen S. S. (1999). Dehydroepiandrosterone: biosynthesis and metabolism in the brain. Endocrinology 140, 880–887 [DOI] [PubMed] [Google Scholar]