Abstract
OBJECTIVE:
To contrast the behavioral and social phenotypes including a screen for autistic behaviors in boys with 47,XYY syndrome (XYY) or 47,XXY Klinefelter syndrome (KS) and controls and investigate the effect of prenatal diagnosis on the phenotype.
METHODS:
Patients included 26 boys with 47,XYY, 82 boys with KS, and 50 control boys (ages 4–15 years). Participants and parents completed a physical examination, behavioral questionnaires, and intellectual assessments.
RESULTS:
Most boys with XYY or KS had Child Behavior Checklist parental ratings within the normal range. On the Child Behavior Checklist, mean problem behaviors t scores were higher in the XYY versus KS groups for the Problem Behavior, Externalizing, Withdrawn, Thought Problems, and Attention Problems subscales. On the Conners’ Parent Rating Scale–Revised, the XYY versus KS group had increased frequency of hyperactive/impulsive symptoms (P < .006). In addition, 50% and 12% of the XYY and KS groups, respectively, had scores >15 for autism screening from the Social Communication Questionnaire. For the boys with KS, prenatal diagnosis was associated with fewer problem behaviors.
CONCLUSIONS:
A subset of the XYY and KS groups had behavioral difficulties that were more severe in the XYY group. These findings could guide clinical practice and inform patients and parents. Boys diagnosed with XYY or KS should receive a comprehensive psychoeducational evaluation and be screened for learning disabilities, attention-deficit/hyperactivity disorder, and autism spectrum disorders.
KEY WORDS: XXY, XYY, Klinefelter syndrome, autism, ADHD
Two sex chromosome aneuploidy disorders affecting male individuals, 47,XYY syndrome (XYY) and 47,XXY Klinefelter syndrome (KS), are relatively common and underdiagnosed, and their distinguishing features are not well known. XYY syndrome occurs in 1 of 1000 male individuals and KS, the most common human sex chromosome disorder,1–3 occurs in 1 of 426 to 1 of 1000 male individuals.4 Some physical, cognitive, and behavioral characteristics of boys with XYY resemble those observed in KS, including tall stature, verbal learning disabilities, and attentional deficits1–8; however, boys with XYY have normal pubertal development and testosterone levels, whereas boys with KS experience childhood-onset testicular failure.5,8–14
The cognitive phenotypes are similar in XYY and KS, including language-based learning disabilities and mild deficits in general cognitive ability as measured by full-scale IQ, academic achievement, verbal memory, and attention.15–20 Delayed speech development requiring speech therapy6,11,18,19,21,22 has also been observed in both boys with XYY and boys with KS. Thus, there appears to be considerable overlap in the cognitive phenotypes in these 2 disorders,15 but the similarities and differences of the behavioral phenotypes are not as well known. Behavioral features described in XYY include increased risk of impulsivity5,23,24 and difficulties related to behavioral dysregulation.16,17,24–31 In addition, boys with XYY have an increased risk for features consistent with autism spectrum disorders (ASD),11,32–34 which has also been described to a lesser extent in KS.34,35 In contrast, the behavioral phenotype of KS includes an increased tendency toward shyness, diminished self-esteem,36,37 anxiety, and social isolation.29,38,39
Most behavioral research on these syndromes was conducted 10 to 20 years ago, with varied ascertainment (population-based versus clinic-based), small sample sizes, and wide age ranges (children versus adults).5,16–18,25,29,30,40 The goal of this study was to compare and contrast the behavioral and social phenotypes (including a screen for autistic behaviors) in boys with XYY, boys with KS, and age-matched control boys, and to investigate the effect of prenatal versus postnatal ascertainment on the observed behavioral phenotypes.
Methods
Participants
Participants were recruited for research participation from the pediatric endocrinology clinic at Thomas Jefferson University, Internet postings, or self-referral. The study was approved by the Human Studies Committee at Thomas Jefferson University and University of Texas Southwestern Medical School. All participants and their parents gave informed consent and assent (age-appropriate). The clinical evaluation was performed at Thomas Jefferson University, and confirmatory karyotyping was performed by the clinical cytogenetics laboratory at University of Texas Southwestern Medical School.
Assessment Procedures
Participants and parents completed a physical examination, behavioral questionnaires, and intellectual assessments during a 2-day testing session. Parents were asked about previous diagnoses of attention and autism disorders. The results of cognitive testing were reported previously.15
Anthropometric Measurements
The clinical assessment included measurement of height, weight, and head circumference that were converted to SD scores by using age- and gender-specific norms.41,42 Pubertal development was assessed by an experienced pediatric endocrinologist (J.L.R.) and included evaluation of testicular volume (Prader orchidometer43) and pubic hair development (Tanner method44).
Cognitive Evaluation
Participants were individually administered the Differential Ability Scales.45 General Conceptual Ability is a general index score.
Socioeconomic Status
Socioeconomic status (SES) estimate was calculated for children by using the Hollingshead 2-Factor Index of Social Status based on education and occupation of parents.46 Higher SES is associated with higher levels of parental education.
Behavioral Questionnaires
The primary caregiving parent of each child completed the parent questionnaires and the child completed the child questionnaires under the supervision of the examiner. (See Supplemental Information for additional detail)
Parent Questionnaires
The Child Behavior Checklist (CBCL)47 is a standardized measure of behavior problems and social competency in children ages 2 to 18 years and includes t scores for 10 problem behavior areas and 3 social competency areas (activities, social, and school). The behavior problems scales include internalizing, externalizing, and total behavior domain scores.
Conners’ Parent Rating Scale–Revised-Long Version (CPRS-R)48 is a standardized measure assessing parental report of attention problems, hyperactivity, impulsivity, and other behavioral symptoms associated with attention-deficit/hyperactivity disorder (ADHD) in children ages 3 to 17 years. Subscales include Oppositional, Cognitive Problems/Inattention, Hyperactivity, Anxious-Shy, Perfectionism, Social Problems, and Psychosomatic. Index scales include Restless-Impulsive Global index, Emotional Lability Global index, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV): Inattentive index, and DSM-IV: Hyperactive-Impulsive index.
The Social Communication Questionnaire (SCQ)49 is a screen for autistic behaviors and includes 40 items that were determined to be the most predictive for autism diagnoses from the Autism Diagnostic Interview. The Current version is used for children 4 to 5 years of age, and the Lifetime version is for children 6 years and older.
Child Self-Report Questionnaires
Genetic Testing
A peripheral blood karyotype was obtained for all XYY and KS participants. Controls were not karyotyped but are presumed to have a normal karyotype (46,XY), as the prevalence of chromosome abnormalities in unselected male individuals is <1%.52
Statistics
Raw scores were converted to t scores (mean of 50, SD of 10), based on the test-specific norms. Results are presented as the mean ± SD. We used analysis of variance to test for statistically significant differences between the XYY, KS, and control groups. We also performed post hoc analysis of covariance for the XYY and KS groups separately, comparing behavioral features in those diagnosed in utero versus those diagnosed after birth. Pearson correlations were performed for continuous variables and Fisher’s exact test was performed for the comparison of dichotomous variables. All P values are provided without adjustment for multiple comparisons, and P values ≤.05 were considered statistically significant.
Results
Genetic Results
Karyotype results showed 26 boys with XYY and 82 boys with 47,XXY; no mosaicism was detected.
Demographics and Auxologic Results
Our study included 26 boys with XYY, 82 boys with KS, and 50 control boys, ages 4 to 15 years (Table 1). The 3 groups had similar age, SES, race, and pubic hair Tanner stage development (Table 1) and came from a broad US geographic distribution. Most participants were white. The boys with XYY or XXY were, on average, taller than the control boys (P < .0001), but had similar weight SD score. Overall, testicular volume SD score was the lowest in the boys with KS (P < .0001), consistent with testicular failure in this group. Testicular volume was increased in the boys with XYY, compared with controls (>1 SD in 13/25 boys with XYY [ages 4.3–13.6 years]), perhaps reflecting early pubertal development. Most of these 13 boys with testicular enlargement had Tanner 1 pubic hair development. Testicular volume SD scores were significantly related to height SD scores (r = 0.48, P = .01) but not head circumference SD scores. Head circumference differed in the 3 groups (P < .03) and was highest in the boys with XYY (Table 1).
TABLE 1.
Demographics and Auxologic Measurements (Mean ± SD)
| XXY | XYY | Controls | P valuea | |
|---|---|---|---|---|
| n | 82 | 26 | 50 | |
| Age | 9.2 ± 2.5 | 9.5 ± 2.8 | 9.5 ± 2.9 | .87 |
| SES | 50 ± 11b | 52 ± 10 | 54 ± 9 | .05 |
| Height SDS | 0.8 ± 1.1 | 1.0 ± 1.2 | 0.1 ± 0.9 | .0001a |
| Weight SDS | 0.6 ± 1.2 | 0.7 ± 1.2 | 0.5 ± 1.1 | .57 |
| Head circumference SDS | 0.3 ± 1.5b | 1.2 ± 2.1c | 0.9 ± 1.4 | .03 |
| Tanner stage-pubic hair | 1.4 ± 0.8 | 1.4 ± 1.1 | 1.3 ± 0.8 | .91 |
| Testicular volume SDS (mean of 2) | −1.0 ± 1.6b | 2.8 ± 4.1c | 1.1 ± 2.5 | .0001a |
| Race (% Caucasian) | 81% | 88% | 78% | .53 |
| General conceptual ability standard score | 88 ± 14b | 91 ± 17d | 110 ± 16 | < .0001a |
ANOVA, comparison of 3 groups.
P < .05, XXY versus controls, ANOVA (post hoc).
P < .05, XYY versus XXY, ANOVA (post hoc).
P < .05, XYY versus controls, ANOVA (post hoc).
Diagnosis of XYY was made prenatally in 6 of 26 boys (routine prenatal screening), in infancy in 4 (2 for hypotonia, 1 for genitalia, and 1 for other), in childhood (ages 2–12 years) in 15 (2 for language issues, 3 for behavior issues, and 10 for other developmental reasons), and after age 12 in 1 (behavior issues). Of the 26 boys with XYY, 12 (46%) received special education services in school, 24 (92%) had received speech and/or reading therapy, and 20 (77%) received occupational and/or physical therapy. No boys with XYY were diagnosed with testicular failure or had received previous testosterone treatment.
Diagnosis of KS was made prenatally in 44 of 82 boys (routine prenatal screening), in infancy in 6 (1 for hypotonia, and 5 for other developmental reasons), in childhood (ages 2–12 years) in 28 (8 for language issues, 6 for behavior issues, 1 for tall stature, and 13 for other developmental reasons), and after age 12 in 4 (1 for language issues and 3 for puberty issues). Of the 82 boys with KS, 16 (20%) had received special education services in school, 60 (73%) received speech and/or reading therapy, and 49 (59%) received occupational and/or physical therapy. No boys with KS had received testosterone treatment before or at the time of the evaluation.
The control boys had heights and weights between the 5th and the 95th percentiles. None had a prior diagnosis of learning disability or ADHD.
Cognitive Results
Results from the Differential Abilities Scale revealed, on average, higher General Conceptual Ability t scores in the control group, compared with the XYY and KS groups (Table 1, P < .0001). Performance in the XYY and KS groups was similar and was published previously.15
Parent Behavioral Questionnaire Results
1. CBCL: Behavioral Problems and Social Competence:
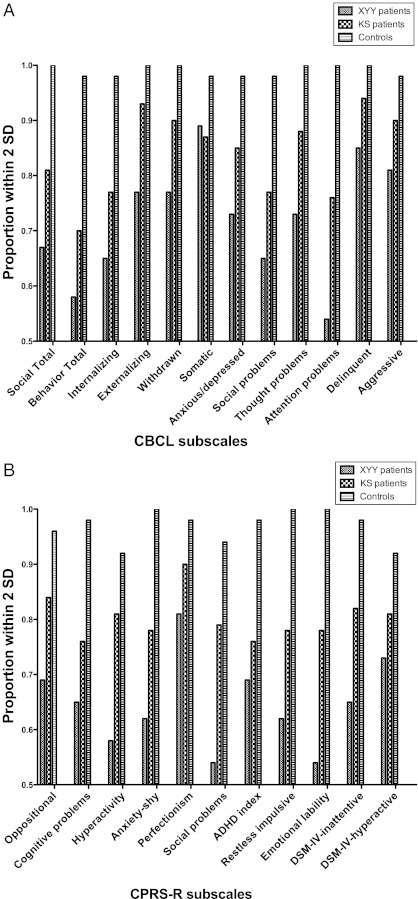
In general, most of the boys with XYY or KS had scores within 2 SDs of the population mean across all Behavioral and Social Competence domains (Fig 1A). When behaviors were compared for the 3 groups, the higher scores in a subset of boys with XYY and KS led to significant differences, compared with the control group, on all CBCL scales (Table 2). The mean t scores for the XYY group were significantly higher compared with the KS group for Externalizing Total, Withdrawn, Thought Problems, Attention Problems, and Aggressive Behavior (P < .05).
FIGURE 1.
a, The proportion of participants with XYY, KS, and controls with t scores within ±2 SD for the CBCL subscales. B, The proportion of participants with XYY, KS, and controls with t scores within ±2 SD for the CPRS-R subscales.
TABLE 2.
Analysis of Variance Parent Behavioral Questionnaires (Mean t Score ± SD)
| XXY | XYY | Controls | P valuea | |
|---|---|---|---|---|
| CBCL-Problem behaviors | ||||
| n | 82 | 26 | 50 | |
| Behavior summary scores (Lower is better) | ||||
| Behavior total | 61.3 ± 12.0b | 67.3 ± 10.0c | 46.7 ± 10.0 | .0001a |
| Internalizing total | 59.3 ± 11.3b | 62.7 ± 12.0c | 46.0 ± 9.3 | .0001a |
| Externalizing total | 54.7 ± 12.7 | 61.3 ± 11.3c,d | 46.0 ± 11.3 | .0001a |
| Problem Behaviors | ||||
| Withdrawn | 58.7 ± 9.30b | 64.0 ± 11.3c,d | 51.3 ± 3.3 | .0001a |
| Somatic complaints | 61.3 ± 8.70b | 60.0 ± 9.30c | 54.7 ± 6.0 | .0001a |
| Anxious/depressed | 59.7 ± 9.30b | 62.7 ± 12.0c | 52.0 ± 4.7 | .0001a |
| Social problems | 64.7 ± 10.7b | 67.3 ± 11.3c | 52.0 ± 4.7 | .0001a |
| Thought problems | 58.7 ± 8.70b | 66.7 ± 10.7c,d | 52.0 ± 4.0 | .0001a |
| Attention problems | 64.0 ± 11.3b | 70.0 ± 10.0c,d | 52.7 ± 4.7 | .0001a |
| Delinquent behavior | 57.3 ± 8.0b | 60.0 ± 9.30c | 52.0 ± 3.3 | .0001a |
| Aggressive behavior | 58.0 ± 10.0b | 63.3 ± 10.7c,d | 53.3 ± 5.3 | .0001a |
| Sex problems | 56.7 ± 10.0b | 58.0 ± 12.0 | 52.0 ± 5.3 | .01a |
| CBCL Social competence scales (Higher is better) | ||||
| Activities total | 45.0 ± 7.30b | 43.0 ± 8.0c | 50.0 ± 6.0 | .0001a |
| Social total | 40.0 ± 10.0b | 37.0 ± 10.7c | 49.0 ± 6.0 | .0001a |
| School total | 33.0 ± 7.30b | 31.0 ± 6.7c | 50.0 ± 7.3 | .0001a |
| CPRS (Lower is better) | ||||
| Oppositional | 58.0 ± 12.0b | 66.0 ± 12.0c,d | 49.3 ± 8.7 | .0001a |
| Cognitive problems/Inattention | 62.0 ± 10.7b | 66.7 ± 10.0c | 47.3 ± 7.3 | .0001a |
| Hyperactivity | 57.3 ± 12.7 | 66.0 ± 12.7c,d | 52.7 ± 10 | .0001a |
| Anxiety-shy | 59.3 ± 12.0b | 62.7 ± 14.0c | 49.3 ± 8.0 | .0001a |
| Perfectionism | 52.7 ± 10.7b | 58.0 ± 12.7c | 47.3 ± 8.0 | .0001a |
| Social problems | 60.0 ± 14.0b | 69.3 ± 14.7c,d | 48.7 ± 8.0 | .0001a |
| Psychosomatic | 60.7 ± 14.7b | 58.0 ± 14.0c | 49.3 ± 8.7 | .003a |
| Global index: ADHD index | 61.3 ± 11.3b | 66.0 ± 9.3c | 48.0 ± 8.0 | .0001a |
| Global index: Restless/impulsive | 59.3 ± 11.3b | 66.0 ± 10.0c,d | 50.0 ± 9.3 | .0001a |
| Global index: Emotional lability | 61.3 ± 12.0b | 69.3 ± 12.7c,d | 47.3 ± 8.7 | .0001a |
| DSM-IV-Inattentive | 60.7 ± 10.7b | 66.0 ± 10.7c,d | 48.0 ± 8.0 | .0001a |
| DSM-IV Hyperactive-impulsive | 58.7 ± 12.7b | 66.0 ± 11.3c,d | 52.0 ± 10.0 | .0003a |
| DSM-IV total | 60.0 ± 11.3b | 66.7 ± 10.0c | 50.0 ± 8.7 | .0001a |
| SCQ (raw scores), (n) | 7.6 ± 6.3 (34) | 15.1 ± 9.9c,d (22) | 2.9 ± 2.4 (31) | .0001a |
| % > cutoff = 15 | 4/34 (12%) | 11/22 (50%) | 0/31 | .0001a |
ANOVA, comparison of 3 groups.
P < .05, XXY versus controls, ANOVA (post hoc).
P < .05, XYY versus controls, ANOVA (post hoc).
P < .05, XYY versus XXY, ANOVA (post hoc).
Prenatal diagnosis was not a significant covariate for the boys with XYY. For the boys with KS, diagnosis in utero was associated with fewer problem behaviors on the Somatic (P < .003) and Thought Problems subscales (P < .02).
2. CPRS-R:
In general, most of the boys in both the XYY and KS groups had scores within 2 SDs of the population mean (Fig 1B) for all domains of the CPRS-R. Comparison of the 3 groups revealed that both the XYY and KS groups had significantly higher mean t scores (more behavioral difficulties) compared with the control group for all CPRS-R scales (P < .003), except in the area of hyperactivity for the KS group, where there was no significant difference from the control group (Table 2). Compared with the KS group, the XYY group showed more behavioral difficulties in the domains of externalizing behaviors, withdrawal, thought problems, attention problems, and aggressive behaviors.
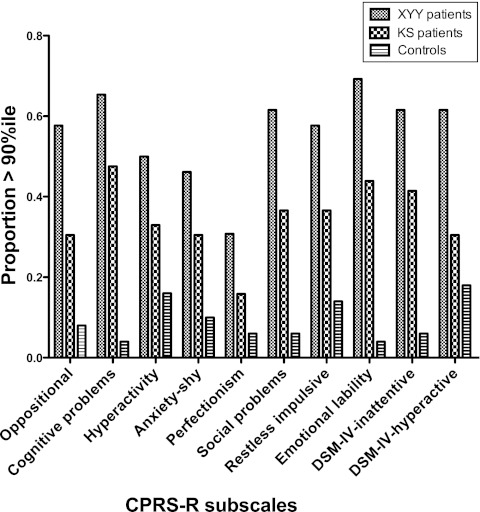
We next compared the proportion of each group with scores >90th percentile for the DSM-IV Inattentive scale, the DSM-IV Hyperactive/Impulsive scale, and the other CRPS-R subscales (Fig 2). The frequency of reaching or exceeding the 90th percentile differed significantly among the 3 groups (P < .007 for all CPRS subscales except Perfectionism, P < .02, Fisher’s exact test). In the XYY group, 62% vs 41% of the KS group had t scores greater than the 90th percentile for DSM-IV Inattentive symptoms (P < .006), and 62% vs 30% of the KS group had t scores >90th percentile for DSM-IV Hyperactive/Impulsive symptoms. Of note, these results are similar to 12 (46%) of 26 XYY boys and 28 (34%) of 82 KS boys, who had been previously diagnosed with ADHD.
FIGURE 2.
The proportion of participants with XYY, KS, and controls with t scores >90th percentile for the CPRS-R subscales.
Prenatal diagnosis in utero was not a significant covariate for the boys with XYY. For the boys with KS, prenatal diagnosis was associated with better outcome in the Cognitive problems/Inattention subscale (P < .04).
3. SCQ:
Mean raw scores on the SCQ and the proportion of boys with scores exceeding the cutoff score of 15 were compared among the 3 groups to screen for possible autistic characteristics (Table 2). The 3 groups differed significantly (P < .0001); the KS and XYY groups had mean SCQ levels that were significantly higher than controls, and the XYY group was significantly higher than KS group (P < .05, Table 2). A total of 50% of the XYY group, 12% of the KS group, and none of the controls had SCQ scores above the cutoff of 15 (P < .0001, Fisher’s exact test). A total of 8 (31%) of 26 boys with XYY versus 5 (6%) of 82 boys with KS had previously been diagnosed with ASD. Prenatal diagnosis was not a significant covariate for the XYY or KS groups.
Child Self-Report Anxiety and Depression Behavioral Questionnaire Results
There were no significant differences among the 3 groups for responses from self-report questionnaires for depression (Children’s Depression Inventory) and anxiety (Revised Child's Manifest Anxiety Scale) (Table 3). Prenatal diagnosis was not a significant covariate for the XYY or KS groups.
TABLE 3.
Child Anxiety and Depression Questionnaires (Mean t Score ± SD)
| XXY | XYY | Controls | P Valuea | |
|---|---|---|---|---|
| CDI (Lower is better) | ||||
| n | 67 | 16 | 38 | |
| Total | 47.3 ± 8.7 | 48.7 ± 10.0 | 44.7 ± 7.3 | .20 |
| Negative mood | 47.3 ± 9.3 | 48.0 ± 10.0 | 46.0 ± 7.3 | .60 |
| Interpersonal problem | 49.3 ± 9.3 | 50.0 ± 10.0 | 47.3 ± 6.7 | .38 |
| Ineffectiveness | 47.3 ± 9.3 | 50.0 ± 10.7 | 44.7 ± 6.7 | .12 |
| Anhedonia | 50.0 ± 10.7 | 50.7 ± 10.0 | 46.7 ± 8.0 | .17 |
| Negative self esteem | 46.0 ± 6.7 | 45.3 ± 8.7 | 45.3 ± 7.3 | .87 |
| RCMAS (Lower is better) | ||||
| n | 67 | 17 | 38 | |
| Total anxiety | 50.7 ± 12.7 | 48.7 ± 8.7 | 46.7 ± 10.0 | .21 |
| Physiologic | 49.3 ± 12.7 | 49.3 ± 9.3 | 44.0 ± 10.7 | .09 |
| Worry/Oversensitivity | 50.0 ± 11.3 | 47.3 ± 9.3 | 46.7 ± 8.7 | .29 |
| Social concerns | 51.3 ± 10.0 | 47.3 ± 8.7 | 46.0 ± 8.7 | .25 |
| Lie scale | 48.7 ± 12.0 | 53.3 ± 9.3 | 51.3 ± 10.7 | .74 |
CDI, Children’s Depression Inventory; RCMAS, Revised Child's Manifest Anxiety Scale.
ANOVA, comparison of 3 groups.
Discussion
The goal of this study was to compare and contrast behavioral and social phenotypes in boys with the sex chromosome disorders, 47,XYY and 47,XXY (KS) versus age-matched controls. The current study extends previous findings by providing data on a larger sample of boys with XYY or KS who were recruited from a wide geographic region on the basis of karyotype rather than psychological diagnoses. An advantage to comparing the XYY and KS groups in this study is that they have similar general cognitive abilities.15
Importantly, in previously published newborn screening studies and in the current study, there is significant variability within the groups, and many of the boys with XYY or KS did not show significant behavioral problems. Our results do indicate, however, that there is increased risk for significant behavioral problems in a subset of boys from the XYY and KS groups, in agreement with previous reports.5,23,24,53 The behavioral phenotypes in the XYY and KS groups differed somewhat in that problem behaviors were more significant in the XYY versus the KS group. There did not seem to be increased anxiety or depressive symptoms in either group.
The behavioral phenotype previously described in XYY syndrome includes an increased risk of impulsivity,5,23,24 poor adaptation to social situations, and behavioral problems related to externalizing behaviors.16,17,24–30 Although our results support these increased risks, it is important to underscore that past research linking XYY to increased risk for criminality must be viewed with extreme caution, given their reliance on small sample sizes and selected rather than broader-based sampling approaches.
In our study, 62% of boys with XYY and 42% of boys with KS had significantly elevated symptoms of ADHD, based on the CPRS-R, compared with a 4% to 5% prevalence rate of ADHD in the general population.54,55 Previous studies also report an increased risk for ADHD, including 11% of a cohort of 26 males with XYY,11 and 63% in a group of 51 males with KS based on standardized DSM-IV interview.56
Half of the XYY group in the current study scored above the cutoff score on the SCQ for screening for ASD, versus 12% of the KS and none of the control group. These frequencies differ from the overall prevalence of ASD in the general population of ∼1 of 125,57 and are consistent with previous reports,11,32–34 suggesting there may be an increased risk for ASD features, particularly in the XYY group. The behavioral phenotype of both XYY and KS includes features that overlap considerably with ASD, such as language disorders, other social deficits, and anxiety/withdrawal symptoms. Additional diagnostic studies are needed to determine whether male subjects with XYY or KS indeed meet criteria for ASD by using standardized autism assessments.
Interestingly, the XYY but not the KS group tended to have increased head circumference relative to the control group, in agreement with most,11,17,33 but not all58 previous studies. Brain imaging studies have demonstrated relatively reduced brain volumes in boys with KS.59 In contrast, increased head circumference and increased brain volumes have also been reported in a subset of children with autism.60–62 Future imaging studies will define the underlying brain structure related to these findings in XYY.
Potential factors related to the observed behavioral differences in the XYY and KS groups are their age at diagnosis and the reason for diagnosis. Ascertainment from clinic samples referred for developmental and behavioral issues63 could affect the description of the XYY phenotype. Previous studies have supported better neurodevelopmental outcomes in prenatally versus postnatally diagnosed KS cohorts,25 likely reflecting differences in SES, genetic, and environmental factors in the 2 groups and less bias toward behavioral/developmental findings in boys diagnosed prenatally. In agreement, we also noted relatively better outcomes in prenatally versus postnatally diagnosed boys with KS. We did not find a significant impact of prenatal diagnosis in the XYY group, most likely reflecting the smaller sample size and fewer prenatally diagnosed subjects in this group. Most (59%) of our KS cohort but only 23% of the boys with XYY were diagnosed prenatally. Most (77%) of the boys with XYY were diagnosed postnatally on the basis of developmental or behavioral issues, which would create a sampling bias for more severe behavioral features in our sample. Also, in contrast to KS, the diagnosis of XYY is often delayed.11,30,64 Milder behavioral findings would perhaps also have been found in a larger, prenatally diagnosed XYY cohort; however, subsets of boys with XYY ascertained without bias from newborn screening studies also have behavior findings,18,40,65 suggesting that the association with the karyotype is genuine.
We previously noted considerable overlap in the XYY and KS groups for cognitive function.15 The similarity of findings in these 2 genetic disorders may be related to overlapping gene dosage abnormalities in the pseudoautosomal region (PAR1), a 2.6-Mb interval at the tips of Xp and Yp, where genes are equally expressed.66,67 Similarly, tall stature in both of these populations is thought to be attributable to increased expression from 3 instead of 2 copies of the height-determining SHOX gene.68
The distinctions in behavioral phenotypes of KS and XYY may be related to hormonal and/or genetic factors that differ between the 2 groups. The clearest hormonal difference is normal testosterone in XYY versus testosterone deficiency in KS; behavioral effects of testosterone are well known.69 Genetic factors that differ in XYY versus KS are related to the extra X chromosome in KS versus the extra Y chromosome in XYY. Because a substantial fraction of genes on the X escape X-inactivation to some degree,70 these genes would be overexpressed in the KS group only. The parental origin of the extra chromosome may differentially affect KS and XYY because in KS, the supernumerary X chromosome may be maternal or paternal, whereas in males with XYY, the extra Y chromosome is always paternal in origin. Notably, no parent of origin difference in the KS phenotype has been conclusively demonstrated.71–74
The extra Y chromosome in XYY remains active, and expression of all Y-linked genes is increased in the XYY group only. Previously, the Y chromosome was thought to have a relatively small number of sex-determining and testicular function genes, but is now known to contain additional genes.75,76 Given the increased proportion of boys with XYY versus KS with elevated screening SCQ scores, we hypothesize that the ASD-like behavioral features in XYY are based on an abnormal dosage of 1 or more of these Y-specific genes. Y chromosome candidate genes with potential neural impact include PCDH11Y (protocadherin 11Y),77 TBL1Y (transducin β-like 1, Y-linked),78,79 and NLGN4Y (neuroligin 4 Y).78,79 Mutations of the closely related, X-linked gene NLGN4× (neuroligin 4 X) have been firmly implicated in autism/ASD and mental retardation (reviewed in ref 80).81,82
Conclusions
These behavioral phenotype results provide support for clinical care recommendations and counseling in XYY and KS. Given the increased risk for developmental and behavioral findings, boys diagnosed with either XYY or KS should receive a comprehensive psychoeducational evaluation and be screened for learning disabilities, ADHD, and ASDs. Educational and behavioral interventions can help address these issues in school and home settings and should be provided to the subset identified as having learning disabilities or psychological/behavioral difficulties to reduce the risk of long-term sequelae. Attention deficits are too common in boys to justify widespread screening for sex chromosomal abnormalities on the basis of this behavioral indication; however, genetic evaluation should be considered when ADHD is coupled with significant language delays and physical findings, such as tall stature or signs of atypical testicular development. Helpful resources and contacts through advocacy groups include KS&A (Knowledge, Support and Action, www.genetic.org) and UNIQUE: Rare Chromosomal Disorder Support Group (www.rarechromo.org). Next steps will need to focus on identifying underlying genetic, neurobiological, and environmental factors contributing to the variability and severity of behavioral and psychological symptoms in XYY and KS and on the development of evidence-based treatments.
Supplementary Material
Acknowledgments
We thank the families who participated in these studies.
Glossary
- ADHD
attention-deficit/hyperactivity disorder
- ASD
autism spectrum disorder
- CBCL
The Child Behavior Checklist
- CPRS-R
Conners’ Parent Rating Scale–Revised
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- KS
47,XXY Klinefelter syndrome
- SCQ
Social Communication Questionnaire
- SES
socioeconomic status
- XYY
47,XYY syndrome
Footnotes
Dr Tartaglia is an expert witness regarding behavior in Klinefelter syndrome. She also serves as the Medical Director of The XXYY Project (a volunteer position).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by National Institutes of Health grant RO1NS 050597 Delaware Health Science Alliance Pilot Award (J.L.R.). Dr Tartaglia acknowledges support from the University of Colorado Intellectual and Developmental Disabilities Research Center. Funded by the National Institutes of Health.
References
- 1.Rovet J, Netley C, Bailey J, Keenan M, Stewart D. Intelligence and achievement in children with extra X aneuploidy: a longitudinal perspective. Am J Med Genet. 1995;60(5):356–363 [DOI] [PubMed] [Google Scholar]
- 2.MacLean N, Harnden DG, Court Brown WM. Abnormalities of sex chromosome constitution in newborn babies. Lancet. 1961;2(7199):406–408 [DOI] [PubMed] [Google Scholar]
- 3.Robinson A, Bender BG, Linden MG, Salbenblatt JA. Sex chromosome aneuploidy: the Denver Prospective Study. Birth Defects Orig Artic Ser. 1990;26(4):59–115 [PubMed] [Google Scholar]
- 4.Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab. 2003;88(2):622–626 [DOI] [PubMed] [Google Scholar]
- 5.Welch J. Clinical Aspects of the XYY Syndrome. New York, NY: Alan R. Liss, Inc; 1985 [Google Scholar]
- 6.Ratcliffe SG. Speech and learning disorders in children with sex chromosome abnormalities. Dev Med Child Neurol. 1982;24(1):80–84 [DOI] [PubMed] [Google Scholar]
- 7.Ratcliffe SG, Pan H, McKie M. Growth during puberty in the XYY boy. Ann Hum Biol. 1992;19(6):579–587 [DOI] [PubMed] [Google Scholar]
- 8.Aksglaede L, Skakkebaek NE, Juul A. Abnormal sex chromosome constitution and longitudinal growth: serum levels of insulin-like growth factor (IGF)-I, IGF binding protein-3, luteinizing hormone, and testosterone in 109 males with 47,XXY, 47,XYY, or sex-determining region of the Y chromosome (SRY)-positive 46,XX karyotypes. J Clin Endocrinol Metab. 2008;93(1):169–176 [DOI] [PubMed] [Google Scholar]
- 9.Baghdassarian A, Bayard F, Borgaonkar DS, Arnold EA, Solez K, Migeon CJ. Testicular function in XYY men. Johns Hopkins Med J. 1975;136(1):15–24 [PubMed] [Google Scholar]
- 10. Benezech, M and Noel, B Clinical Aspects of the XYY Syndrome. New York, NY: Alan R. Liss, Inc; 1985 [Google Scholar]
- 11.Geerts M, Steyaert J, Fryns JP. The XYY syndrome: a follow-up study on 38 boys. Genet Couns. 2003;14(3):267–279 [PubMed] [Google Scholar]
- 12.Ratcliffe SG, Read G, Pan H, Fear C, Lindenbaum R, Crossley J. Prenatal testosterone levels in XXY and XYY males. Horm Res. 1994;42(3):106–109 [DOI] [PubMed] [Google Scholar]
- 13.Rudd BT, Galal OM, Casey MD. Testosterone excretion rates in normal males and males with an XYY complement. J Med Genet. 1968;5(4):286–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price WH, van der Molen HJ. Plasma testosterone levels in males with the 47,XYY karyotype. J Endocrinol. 1970;47(1):117–122 [DOI] [PubMed] [Google Scholar]
- 15.Ross JL, Zeger MP, Kushner H, Zinn AR, Roeltgen DP. An extra X or Y chromosome: contrasting the cognitive and motor phenotypes in childhood in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. Dev Disabil Res Rev. 2009;15(4):309–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Netley CT. Summary overview of behavioural development in individuals with neonatally identified X and Y aneuploidy. Birth Defects Orig Artic Ser. 1986;22(3):293–306 [PubMed] [Google Scholar]
- 17.Evans JA, de von Flindt R, Greenberg C, Ramsay S, Hamerton JL. Physical and psychologic parameters in children with sex chromosome anomalies: further follow-up from the Winnipeg Cytogenetic Study of 14,069 newborn infants. Birth Defects Orig Artic Ser. 1986;22(3):183–207 [PubMed] [Google Scholar]
- 18.Bender BG, Puck MH, Salbenblatt JA, Robinson A. The development of four unselected 47,XYY boys. Clin Genet. 1984;25(5):435–445 [DOI] [PubMed] [Google Scholar]
- 19.Robinson A, Bender BG, Puck MH, Salbenblatt JA. Growth and Development of Children With a 47,XYY Karyotype. New York, NY: Alan R. Liss, Inc; 1985 [Google Scholar]
- 20.Leggett V, Jacobs P, Nation K, Scerif G, Bishop DV. Neurocognitive outcomes of individuals with a sex chromosome trisomy: XXX, XYY, or XXY: a systematic review. Dev Med Child Neurol. 2010;52(2):119–129 [DOI] [PMC free article] [PubMed]
- 21.Valentine GH. The growth and development of six XYY children. Birth Defects Orig Artic Ser. 1979;15(1):175–190 [PubMed] [Google Scholar]
- 22.Daly RF. The XYY condition in childhood: clinical observation. Pediatrics. 1969;44(4):621. [PubMed] [Google Scholar]
- 23.Theilgaard A. A psychological study of the personalities of XYY- and XXY-men. Acta Psychiatr Scand Suppl. 1984;315:1–133 [PubMed] [Google Scholar]
- 24.Money J, Annecillo C, Van Orman B, Borgaonkar DS. Cytogenetics, hormones and behavior disability: comparison of XYY and XXY syndromes. Clin Genet. 1974;6(5):370–382 [DOI] [PubMed] [Google Scholar]
- 25.Linden MG, Bender BG. Fifty-one prenatally diagnosed children and adolescents with sex chromosome abnormalities. Am J Med Genet. 2002;110(1):11–18 [DOI] [PubMed] [Google Scholar]
- 26.Ratcliffe SG, Tierney I, Nshaho J, Smith L, Springbett A, Callan S. The Edinburgh study of growth and development of children with sex chromosome abnormalities. Birth Defects Orig Artic Ser. 1982;18(4):41–60 [PubMed] [Google Scholar]
- 27.Lettiero T, Del Giudice E, Imperati F, Ciao A, Capalbo D, Salerno M. Hormonal and neuropsychological evaluation of two 47,XYY patients with pituitary abnormalities. Am J Med Genet A. 2008;146(3):397–400 [DOI] [PubMed] [Google Scholar]
- 28.Götz MJ, Johnstone EC, Ratcliffe SG. Criminality and antisocial behaviour in unselected men with sex chromosome abnormalities. Psychol Med. 1999;29(4):953–962 [DOI] [PubMed] [Google Scholar]
- 29.Ratcliffe S. Long-term outcome in children of sex chromosome abnormalities. Arch Dis Child. 1999;80(2):192–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fryns JP, Kleczkowska A, Kubień E, Van den Berghe H. XYY syndrome and other Y chromosome polysomies. Mental status and psychosocial functioning. Genet Couns. 1995;6(3):197–206 [PubMed] [Google Scholar]
- 31.Schiavi RC, Theilgaard A, Owen DR, White D. Sex chromosome anomalies, hormones, and aggressivity. Arch Gen Psychiatry. 1984;41(1):93–99 [DOI] [PubMed] [Google Scholar]
- 32.Kielinen M, Rantala H, Timonen E, Linna SL, Moilanen I. Associated medical disorders and disabilities in children with autistic disorder: a population-based study. Autism. 2004;8(1):49–60 [DOI] [PubMed] [Google Scholar]
- 33.Nicolson R, Bhalerao S, Sloman L. 47,XYY karyotypes and pervasive developmental disorders. Can J Psychiatry. 1998;43(6):619–622 [DOI] [PubMed] [Google Scholar]
- 34.Bishop DV, Jacobs PA, Lachlan K, et al. Autism, language and communication in children with sex chromosome trisomies. Arch Dis Child. 2011;96(10):954–959 [DOI] [PMC free article] [PubMed]
- 35.van Rijn S, Swaab H, Aleman A, Kahn RS. Social behavior and autism traits in a sex chromosomal disorder: Klinefelter (47XXY) syndrome. J Autism Dev Disord. 2008;38(9):1634–1641 [DOI] [PubMed] [Google Scholar]
- 36.Bancroft J, Axworthy D, Ratcliffe S. The personality and psycho-sexual development of boys with 47 XXY chromosome constitution. J Child Psychol Psychiatry. 1982;23(2):169–180 [DOI] [PubMed] [Google Scholar]
- 37.Stewart DA, Bailey JD, Netley CT, Rovet J, Park E. Growth and development from early to midadolescence of children with X and Y chromosome aneuploidy: the Toronto Study. Birth Defects Orig Artic Ser. 1986;22(3):119–182 [PubMed] [Google Scholar]
- 38.Mandoki MW, Sumner GS, Hoffman RP, Riconda DL. A review of Klinefelter’s syndrome in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1991;30(2):167–172 [DOI] [PubMed] [Google Scholar]
- 39.Nielsen J, Wohlert M. Chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Hum Genet. 1991;87(1):81–83 [DOI] [PubMed] [Google Scholar]
- 40.Bender BG, Linden MG, Robinson A. Neuropsychological impairment in 42 adolescents with sex chromosome abnormalities. Am J Med Genet. 1993;48(3):169–173 [DOI] [PubMed] [Google Scholar]
- 41.Hamill PV, Drizd TA, Johnson CL, Reed RB, Roche AF, Moore WM. Physical growth: National Center for Health Statistics percentiles. Am J Clin Nutr. 1979;32(3):607–629 [DOI] [PubMed] [Google Scholar]
- 42.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall JG,, Froster-Iskenius UG, Allanson JE. Handbook of Normal Physical Measurements. Oxford, United Kingdom: Oxford University Press; 1995 [Google Scholar]
- 44.Marshall WATJ, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elliott CD. Differential Ability Scales—Introductory and Technical Handbook. San Diego, CA: Harcourt, Brace, Jovanovich; 1983 [Google Scholar]
- 46.Hollingshead AB, Redlich F. Social Class and Mental Illness. New York, NY: John Wiley; 1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Achenbach TM, Edelbrock CS. The Child Behavior Profile: II. Boys aged 12–16 and girls aged 6–11 and 12–16. J Consult Clin Psychol. 1979;47(2):223–233 [DOI] [PubMed] [Google Scholar]
- 48.Conners C. Conners' Rating System—Revised Parent and Teacher Technical Manual. Tonawanda, NY: Multi-Health Systems, Inc; 1997 [Google Scholar]
- 49.Rutter M, Bailey A, Lord C. The Social Communication Questionnaire Manual. Los Angeles, CA: Western Psychological Services; 2003 [Google Scholar]
- 50.Kovacs MaMS CDI Children's Depression Inventory Technical Manual Update. North Tonawanda, NY: Multi-Health Systems, Inc; 2003 [Google Scholar]
- 51.Reynolds CR, Richmond BO. What I think and feel: a revised measure of children’s manifest anxiety. J Abnorm Child Psychol. 1978;6(2):271–280 [DOI] [PubMed] [Google Scholar]
- 52.Hook, EB and Hamerton, JL. The Frequency of Chromosome Abnormalities Detected in Consecutive Newborn Studies—Results by Sex and by Severity of Phenotypic Involvement in Population Cytogenetics: Studies in Humans. New York, NY: Academic Press, Inc; 1977 [Google Scholar]
- 53.Salbenblatt JA, Bender BG, Puck MH, Robinson A, Webber ML. Development of eight pubertal males with 47,XXY karyotype. Clin Genet. 1981;20(2):141–146 [DOI] [PubMed] [Google Scholar]
- 54.Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104(2). Available at: www.pediatrics.org/cgi/content/full/104/2/e20 [DOI] [PubMed] [Google Scholar]
- 55.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Press; 1994 [Google Scholar]
- 56.Bruining H, de Sonneville L, Swaab H, et al. Dissecting the clinical heterogeneity of autism spectrum disorders through defined genotypes. PLoS ONE. 2010;5(5):e10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Autism and Developmental Disabilities Monitoring Network Surveillance Year 2006 Principal Investigators. Centers for Disease Control and Prevention (CDC) . Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ. 2009;58(10):1–20 [PubMed] [Google Scholar]
- 58.Ratcliffe SG, Masera N, Pan H, McKie M. Head circumference and IQ of children with sex chromosome abnormalities. Dev Med Child Neurol. 1994;36(6):533–544 [DOI] [PubMed] [Google Scholar]
- 59.Giedd JN, Clasen LS, Wallace GL, et al. XXY (Klinefelter syndrome): a pediatric quantitative brain magnetic resonance imaging case-control study. Pediatrics. 2007;119(1). Available at: www.pediatrics.org/cgi/content/full/119/1/e232 [DOI] [PubMed] [Google Scholar]
- 60.Lainhart JE, Bigler ED, Bocian M, et al. Head circumference and height in autism: a study by the Collaborative Program of Excellence in Autism. Am J Med Genet A. 2006;140(21):2257–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Courchesne E, Karns CM, Davis HR, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57(2):245–254 [DOI] [PubMed] [Google Scholar]
- 62.Hazlett HC, Poe M, Gerig G, et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62(12):1366–1376 [DOI] [PubMed] [Google Scholar]
- 63.Tartaglia N, Bacalman S, Hansen R, et al. Autism spectrum disorders in XXY, XYY, and XXYY syndromes [Abstract] . J Dev Behav Pediatr. 2007;28(4):S8 [Google Scholar]
- 64.Abramsky L, Chapple J. 47,XXY (Klinefelter syndrome) and 47,XYY: estimated rates of and indication for postnatal diagnosis with implications for prenatal counselling. Prenat Diagn. 1997;17(4):363–368 [DOI] [PubMed] [Google Scholar]
- 65.Salbenblatt JA, Meyers DC, Bender BG, Linden MG, Robinson A. Gross and fine motor development in 47,XXY and 47,XYY males. Pediatrics. 1987;80(2):240–244 [PubMed] [Google Scholar]
- 66.Rappold GA. The pseudoautosomal regions of the human sex chromosomes. Hum Genet. 1993;92(4):315–324 [DOI] [PubMed] [Google Scholar]
- 67.Vaknin Z, Reish O, Ben-Ami I, Heyman E, Herman A, Maymon R. Prenatal diagnosis of sex chromosome abnormalities: the 8-year experience of a single medical center. Fetal Diagn Ther. 2008;23(1):76–81 [DOI] [PubMed] [Google Scholar]
- 68.Ottesen AM, Aksglaede L, Garn I, et al. Increased number of sex chromosomes affects height in a nonlinear fashion: a study of 305 patients with sex chromosome aneuploidy. Am J Med Genet A. 2010;152A(5):1206–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nielsen J, Pelsen B. Follow-up 20 years later of 34 Klinefelter males with karyotype 47,XXY and 16 hypogonadal males with karyotype 46,XY. Hum Genet. 1987;77(2):188–192 [DOI] [PubMed] [Google Scholar]
- 70.Carrel L, Cottle AA, Goglin KC, Willard HF. A first-generation X-inactivation profile of the human X chromosome. Proc Natl Acad Sci U S A. 1999;96(25):14440–14444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stemkens D, Roza T, Verrij L, et al. Is there an influence of X-chromosomal imprinting on the phenotype in Klinefelter syndrome? A clinical and molecular genetic study of 61 cases. Clin Genet. 2006;70(1):43–48 [DOI] [PubMed] [Google Scholar]
- 72.Wikström AM, Painter JN, Raivio T, Aittomäki K, Dunkel L. Genetic features of the X chromosome affect pubertal development and testicular degeneration in adolescent boys with Klinefelter syndrome. Clin Endocrinol (Oxf). 2006;65(1):92–97 [DOI] [PubMed] [Google Scholar]
- 73.Boks MP, de Vette MH, Sommer IE, et al. Psychiatric morbidity and X-chromosomal origin in a Klinefelter sample. Schizophr Res. 2007;93(1-3):399–402 [DOI] [PubMed] [Google Scholar]
- 74.Ross JL, Roeltgen DP, Stefanatos G, et al. Cognitive and motor development during childhood in boys with Klinefelter syndrome. Am J Med Genet A. 2008;146A(6):708–719 [DOI] [PubMed] [Google Scholar]
- 75.Bellott DW, Page DC. Reconstructing the evolution of vertebrate sex chromosomes. Cold Spring Harb Symp Quant Biol. 2009;74:345–353 [DOI] [PubMed] [Google Scholar]
- 76.Fukuda T, Akiyama N, Ikegami M, et al. Expression of hydroxyindole-O-methyltransferase enzyme in the human central nervous system and in pineal parenchymal cell tumors. J Neuropathol Exp Neurol. 2010;69(5):498–510 [DOI] [PubMed] [Google Scholar]
- 77.Carrasquillo MM, Zou F, Pankratz VS, et al. Genetic variation in PCDH11X is associated with susceptibility to late-onset Alzheimer’s disease. Nat Genet. 2009;41(2):192–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423(6942):825–837 [DOI] [PubMed] [Google Scholar]
- 79.Caglayan AO. Genetic causes of syndromic and non-syndromic autism. Dev Med Child Neurol. 2010;52(2):130–138 [DOI] [PubMed] [Google Scholar]
- 80.Powell CM. Neuroligins and Neurexins: Synaptic Bridges Implicated in Autism. Heidelberg, Germany: Springer Science; 2011 [Google Scholar]
- 81.Laumonnier F, Bonnet-Brilhault F, Gomot M, et al. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004;74(3):552–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yan J, Feng J, Schroer R, et al. Analysis of the neuroligin 4Y gene in patients with autism. Psychiatr Genet. 2008;18(4):204–207 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.