Abstract
Background
In this paper, a series of amphiphilic triblock copolymers based on polyethylene glycol–poly ɛ-caprolactone–polyethylenimine (mPEG–PCL-g–PEI) were successfully synthesized, and their application for codelivery of chemotherapeutic drugs and DNA simultaneously was investigated.
Methods and results
These copolymers could self-assemble into micelles with positive charges. The size and zeta potential of the micelles was measured, and the results indicate that temperature had a large effect on the micelles obtained. In vitro gene transfection evaluation in cancer cells indicated that the self-assembled micelles could serve as potential gene delivery vectors. In addition, hydrophobic drug entrapment efficiency and codelivery with the gene was also studied in vitro. The self-assembled micelles could load doxorubicin efficiently and increase cellular uptake in vitro, while maintaining high gene transfection efficiency.
Conclusion
The triblock copolymer mPEG–PCL-g–PEI could be a novel vector for codelivery of drug and gene therapy.
Keywords: self-assembly, triblock copolymer, DNA, drug codelivery, gene transfection
Introduction
Chemotherapy and gene therapy have played important roles in cancer research over the past 2 decades. Gene therapy, as a novel treatment for various genetic diseases and cancers, needs high transfection efficiency to achieve its therapeutic efficacy.1–4 Development of nonviral gene carriers that deliver genes into cells effectively has attracted attention worldwide in recent years. However, because of limitations regarding safety and low therapeutic efficacy, few nonviral gene vectors have been used in clinical applications. For these reasons, codelivery of low-molecular-weight drugs and functional genes is a promising approach to improve therapeutic efficacy in a number of diseases, especially cancer.5–9 Many pharmaceutical carriers, including polymeric nano-capsules, liposomes, dendrimers, cell ghosts, and micelles, are widely used to deliver both chemotherapeutics and biotherapeutics, ie, proteins and functionally modified plasmids.10–15 Among these carriers, micellar drug carriers are becoming one of the most versatile vehicles for delivery of hydrophobic drugs. To improve their therapeutic effect, advanced micellar carriers based on amphiphilic polymers have significant promise for enhancing the efficiency of drug delivery both in vitro and in vivo.16
Micelles self-assembled from amphiphilic block copolymers are usually engineered to have more than one useful function, eg, a prolonged circulation time, targeting to a local pathological site, and enriched delivery to tumor tissue via the enhanced permeability and retention effect. These functions improve bioavailability and decrease the risk of side effects.17
In the past decade, polyethylene glycol–poly ɛ-caprolactone (PEG–PCL) micelles have been reported to feature a self-assembled core-shell structure in nanoscale. PEG is a biocompatible water-soluble polymer without immunogenicity, and is widely used in designing amphiphilic block copolymers.18,19 As a promising polymer, it has specific advantages in that it is nontoxic and can be cleared from the body by renal filtration. As a hydrophobic block, PCL is approved by the US Food and Drug Administration for use in humans due to the biocompatibility of its degradation products.20 The hydrophobic core provides a nano depot for hydrophobic drug loading, while the hydrophilic shell improves drug solubility.
Gene therapy using nonviral vectors is expected to be able to cure various inherited disorders and cancers. Polyethylenimine (PEI), a cationic polymer, is well known to be one of the most successful and efficient gene-delivery vectors. The most prominent feature of PEI is its high cationic charge density. Its transfection efficiency and cytotoxic effects are influenced by its molecular weight and the degree of branching of PEI. Many researchers have suggested that branched PEI provides the highest transfection efficiency at 25 kD. However, an increasing degree of branching and molecular weight are confirmed to increase its cytotoxicity both in vitro and in vivo. In order to increase transfection efficiency and decrease toxicity, lowmolecular- weight 800 or 2000 Da PEIs can be connected together using short biodegradable linkages.21
In this study, because triblock copolymer mPEG–PCL-g– PEI could self-assemble into nanomicelles with positive potential, a further study of different compositions of this copolymer was investigated to determine their potential effects regarding codelivery of drugs and genes.
Materials and methods
Materials
mPEG (molecular weight 2000, 5000) was purchased from Sigma-Aldrich (St Louis, MO) and dried under vacuum at 80°C before chemical synthesis. Stannous octoate [Sn(Oct)2], Hoechst 33342, acryloyl chloride, and 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide (MTT) were also purchased from Sigma-Aldrich. Triethylamine, chloroform, dichloromethane, and petroleum ether were purchased from Chengdu KeLong Chemicals (Chengdu, China). All cell lines were obtained from the American Type Culture Collection (Manassas, VA).
Synthesis of mPEG–PCL-g–PEI copolymer
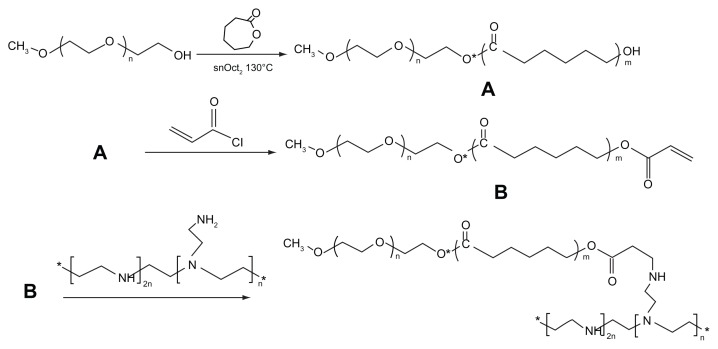
First of all, mPEG–PCL was synthesized by ring-opening polymerization of ɛ-CL and mPEG, using SnOct2 as the catalyst. The crude polymer, mPEG–PCL, was dissolved in dichloromethane and precipitated with petroleum ether. After eliminating the residual petroleum ether by vacuum at 45°C, a corresponding amount of acryloyl chloride was added to the anhydrous mPEG–PCL dichloromethane solution drop by drop; after stirring at 40°C for 6 hours, the copolymer mPEG–PCL containing –C=C was obtained. Finally, mPEG– PCL-g–PEI was synthesized according to methods described elsewhere.22,23 A specified amount of PEI was dissolved in chloroform, then the above copolymer chloroform solution was added drop by drop and stirred for 24 hours at 50°C to complete the reaction. All the final products were collected by petroleum ether coprecipitation and purification, dialyzed and lyophilized, after which the products obtained were stored in a tower dryer until further use.
Characterization
To characterize the chemical composition and confirm that the products were successfully synthesized, 1H-NMR spectra were recorded using a Varian 400 spectrometer (Varian, Palo Alto, CA) at 400 mHz in CDCl3 with tetramethyl silane as an internal reference. The particle size and zeta potential of the mPEG–PCL-g–PEI was determined using a Malvern Nano- ZAS 90 laser particle size analyzer (Malvern Instruments, Malvern, UK). The prepared nanoparticles were dispersed in deionized water at a concentration of 5 mg/mL and examined at various temperatures. All experimental groups were tested three times. The critical micelle concentration (CMC) was determined by fluorescence measurement using pyrene as the fluorescent probe at 25°C.24,25 The concentration of block copolymer was varied from 1 × 10−6 to 0.1 mg/mL. Briefly, aliquots of pyrene solution (10 μM) were added into containers and dried with nitrogen. Next, 10 mL of copolymer solution at different concentrations was added to the containers and the solutions were treated using ultrasound for 2 hours. The fluorescent spectra were recorded using fluorescence spectrometry (LS55; PerkinElmer, Shelton, CT). The excitation wavelength was 333 nm, and the emission fluorescence was monitored at 373 nm and 384 nm. The CMC was estimated by analysis of the ratio of intensities at 373 nm to 384 nm (I373/I384), and the cross-point of the ratio was the result.
Micelle formation and drug encapsulation
Blank and doxorubicin-loaded mPEG–PCL-g–PEI nanoparticles were prepared using the self-assembly method.26 mPEG–PCL-g–PEI copolymers with predetermined molecular weights were directly added to deionized water followed by increasing the temperature to 55°C. Being amphiphilic, the mPEG–PCL-g–PEI copolymer could self-assemble into nanoscale micelles in aqueous solution.
Doxorubicin-loaded mPEG–PCL-g–PEI nanoparticles were prepared using a pH-induced self-assembly method. 27 Briefly, 0.2 mL of phosphate-buffered saline (10×, pH 7.4) was added to 1 mL of aqueous solution containing the nanoparticles (20 mg/mL), after which 0.8 mL of aqueous doxorubicin solution (2 mg/mL) was dropped into the nanoparticle solution under moderate stirring. After ultrafiltration, the doxorubicin-loaded mEPG–PEC-g–PEI nanoparticles were lyophilized for further examination. Drug-loading content and drug-loading efficiency were calculated as follows:
where DLC is the drug-loading content and DLE is the drug-loading efficiency.
Cytotoxicity of mPEG–PCL-g–PEI nanoparticles
The cytotoxicity of the mPEG–PCL-g–PEI nanoparticles was evaluated by reduction of the MTT assay. Each cell line (L929 and HEK293) was seeded in 96-well plates at a density of 2 × 104 cells/well in 100 μL of growth medium and incubated overnight, after which the cells were exposed to a series of mPEG–PCL-g–PEI nanoparticles at different concentrations. After incubating the cells for a further 24 hours, the MTT assay was carried out. All absorbances were measured at 570 nm using a Spectramax microplate reader (Molecular Devices, Sunnyvale, CA), and untreated cells were used as controls.
Gel retardation assay
The DNA adsorption capability of the mPEG–PCL-g–PEI cationic micelles was determined using a gel retardation assay. For preparation of the complexes, the plasmid and micelles were diluted in double-distilled H2O to equal volumes and mixed by pipetting. After incubation for 30 minutes, the pDNA/mPEG–PCL-g–PEI complexes were electrophoresed on 1% agarose gel (100 V, 30 minutes).
Morphology study
The morphology of all the blank micelles, drug-loaded micelles, and DNA/micelle complexes was observed under transmission electron microscopy (TEM; H-6009IV; Hitachi, Tokyo, Japan) or atomic force microscopy (SPA-400; Seiko Instruments Inc, Chiba, Japan). Briefly, a sample of the nanoparticle solution was diluted and negatively stained with phosphotungstic acid, then placed on a copper grid covered with nitrocellulose, after which it was dried at room temperature and could be imaged under TEM. Atomic force microscopy was also used to analyze the morphology and size of the mPEG–PCL-g–PEI/DNA complexes and blank micelles. The complex solution was prepared by mixing 3 μg of plasmid DNA with polymer solution at a w/w ratio of 20. After the micelles were incubated with the plasmid DNA at room temperature for 30 minutes, the micelle–DNA complex solution was diluted and dried on a mica surface. Imaging was carried out in noncontact mode.
In vitro transfection
B16-F10 and 293T cells were plated onto six-well plates at a density of 2 × 105 cells/well. After incubation for 24 hours, the medium was removed from each well. The cells were washed once with Dulbecco’s modified Eagle’s medium without serum and antibiotic, and then 900 μL of serum-free medium and 100 μL of pDNA/micelle complex solution containing 2 μg green fluorescence protein reporter plasmid were added. After coculture with mPEG–PCL-g–PEI/plasmid DNA complexes for 6 hours at 37°C, the serum-free medium was discarded, and fresh medium was added for further culture. Green fluorescence protein expression levels were recorded and the transfection efficiency of each well was examined using flow cytometry.
Cellular uptake of drug-loaded micelles and gene expression
Laser scanning confocal microscopy and fluorescence microscopy were used to visualize and examine cellular uptake and gene expression.23 In short, B16-F10 cells were seeded into six-well plates with a cover slip on each well. After the cells had grown to a suitable density, the growth medium was replaced with fresh RPMI-1640 medium containing doxorubicin- loaded micelles and coincubated at 37°C for a predetermined time. Cellular uptake was terminated by washing cells three times with ice-cold phosphate-buffered saline (pH 7.4). The fixed cells were observed using an LSM 510 confocal laser scanning microscope (Leica, Germany), and objective lenses at 40× and 100× magnifications, respectively.
In the experiment involving codelivery of the drug and gene, Nile red was used as a model agent for the hydrophobic drug. In brief, Nile red dissolved in acetone was added into a bottle, then the solution was dried with nitrogen; 1 mL of mPEG–PCL-g–PEI micelles (5 mg/mL) was then added to load the Nile red through ultrasonication. After loading with the red dye, the micelles were used to deliver green fluorescence protein in vitro with a ratio of mPEG–PCL-g–PEI/ DNA at 40. Images of gene expression and cellular uptake of Nile red were taken by Cellomics ArrayScan VTI 600 (Thermo Fisher Scientific, Waltham, MA).
Results
mPEG–PCL-g–PEI could be self-assembled into nanoparticles. The uptake and distribution of mPEG–PCL-g–PEI triblock copolymer micelles was investigated in vitro and in vivo in a prior study.23 The self-assembled amphipathic micelles were positively charged, which encouraged us to study their gene transfection capability and also drug delivery efficiency in detail. In this study, triblock mPEG–PCL-g–PEI copolymers were synthesized, and the cytotoxicity, size, and zeta potential of the micelles that self-assembled from these mPEG–PCL-g– PEI copolymers was examined. Moreover, their gene transfection efficiency and drug delivery capability were investigated in a B16-F10 cell line.
Synthesis of triblock mPEG–PCL-g–PEI copolymer
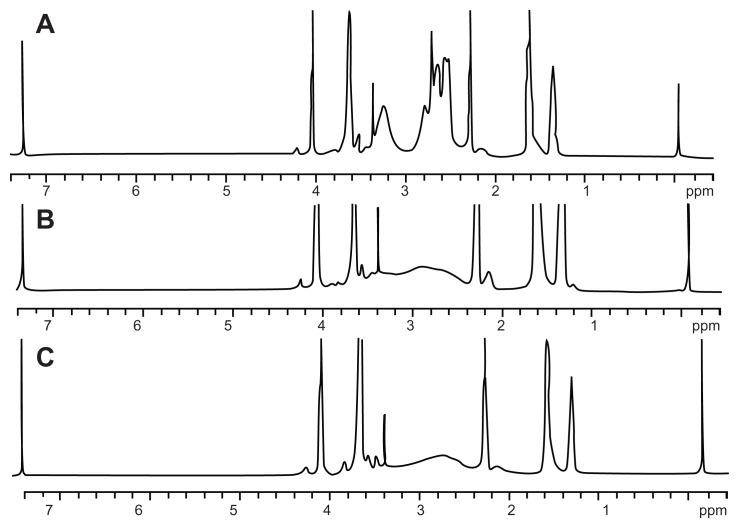
The synthesis method used is shown in Figure 1. In order to improve the efficiency of synthesis, we used acryloyl chloride instead of the glycidyl methacrylate reported previously.22,23 The 1H-NMR spectra for the various formulations of mPEG–PCL-g–PEI in CDCl3 are shown in Figure 2. The characteristic resonance signals from mPEG, PCL, and PEI were observed. The sharp peaks appearing at 3.64 and 3.36 ppm were attributed to the methylene protons of the –CH2CH2O– and –OCH3 end groups in the PEG blocks. Typical signals of PCL appeared at 1.32, 1.56, 2.31, and 4.06 ppm, which were assigned to the methylene protons of –(CH2)3–, –OCCH2–, and –CH2OOC– in the PCL blocks. The three peaks observed at 2.5–2.8 were assigned to the methane protons of –CH2 NH– contained in the PEI.21,22 The molecular weight of a copolymer was calculated using the formula below:
Figure 1.
Synthesis of mPEG–PCL-g–PEI amphiphilic triblock copolymer. (A) indicates the block copolymer mPEG–PCL; (B) indicates a copolymer mPEG–PCL with -C=C- in its end.
Abbreviations: PEG, polyethylene glycol; PCL, poly ɛ-caprolactone; PEI, polyethylenimine.
Figure 2.
1H-NMR spectra of mPEG–PCL-g–PEI with three different compositions. (A) mPEG–PCL-g–PEI with composition 2000-2000-2000; (B) mPEG–PCL-g–PEI with composition 2000-6000-2000; (C) mPEG–PCL-g–PEI with composition 5000-2000-2000.
Abbreviations: 1H-NMR, proton nuclear magnetic resonance; PEG, polyethylene glycol; PCL, poly ɛ-caprolactone; PEI, polyethylenimine.
where IA and IB are integral intensities of the peaks at about 4.06 and 3.64 ppm, respectively, and x and y represent the degree of polymerization in the PCL and PEG blocks. The results indicate that three copolymers with molecular weights of 5339.6, 8786.7, and 11703 were formed, consistent with the requirements of the original design (Table 1). All the 1H-NMR results showed that the triblock mPEG–PCL-g–PEI copolymers were successfully synthesized in various formulations.
Table 1.
Physical chemistry parameters of triblock copolymers
| Polymer | Theoretical molecular weight | Mn/kDa (1H-NMR)a | n (%) | CMC (mg/mL)b | Encapsulation efficiency (%) | Drug-loading content (wt%) |
|---|---|---|---|---|---|---|
| 1 | 5000-2000-2000 | 8786.7 | 4.0 | 0.03 | 96.0 ± 0.49 | 8.76 ± 0.04 |
| 2 | 2000-2000-2000 | 5339.6 | 12.4 | 0.05 | 98.2 ± 0.01 | 8.94 ± 0.0006 |
| 3 | 2000-6000-2000 | 11703 | 4.0 | 0.005 | 91.8 ± 0.23 | 8.41 ± 0.02 |
Notes:
Determined from 1H-NMR by comparing the integrals of signals at δ = 4.06 and 3.65;
Determined using pyrene as a fluorescence probe.
Abbreviations: 1H-NMR, proton nuclear magnetic resonance; CMC, critical micelle concentration.
Characterization of mPEG–PCL-g–PEI micelles
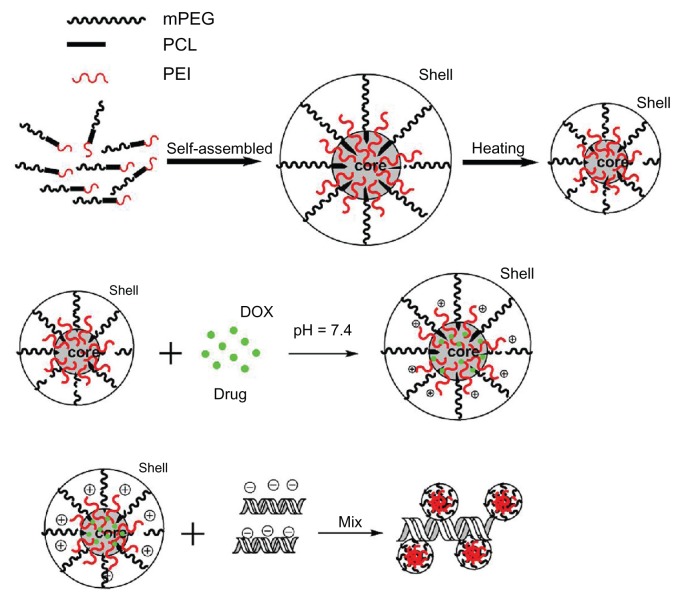
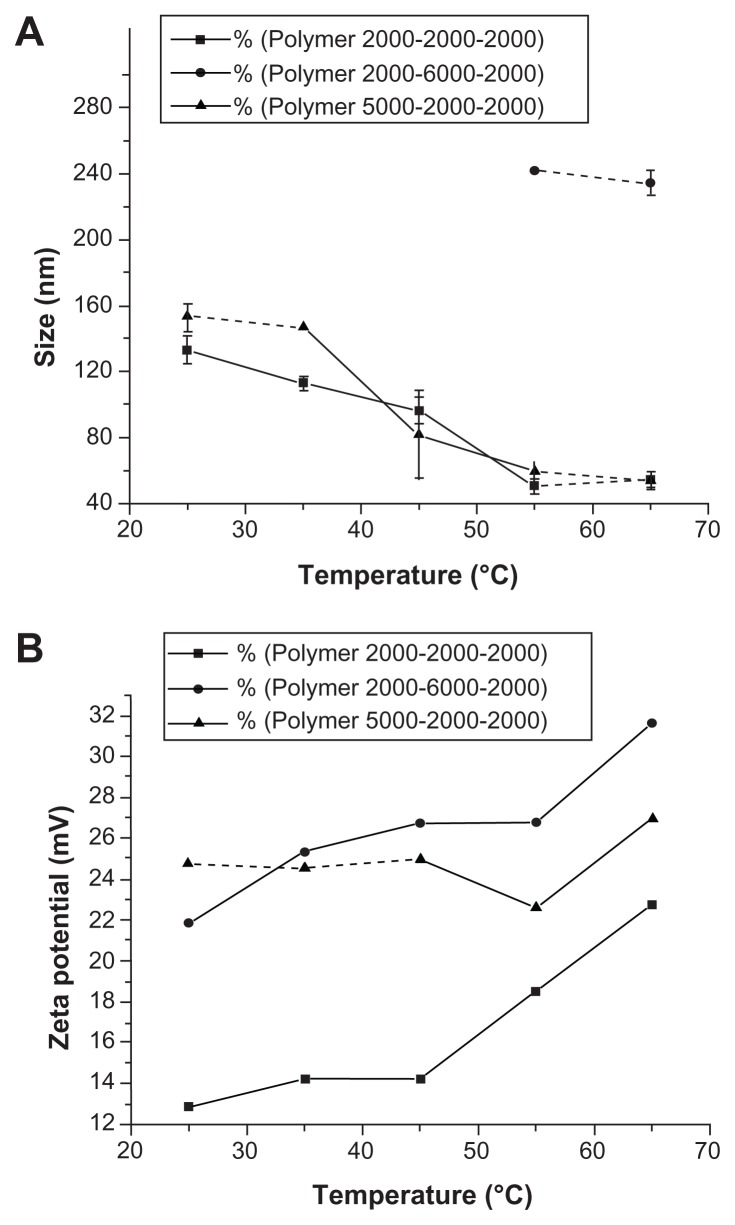
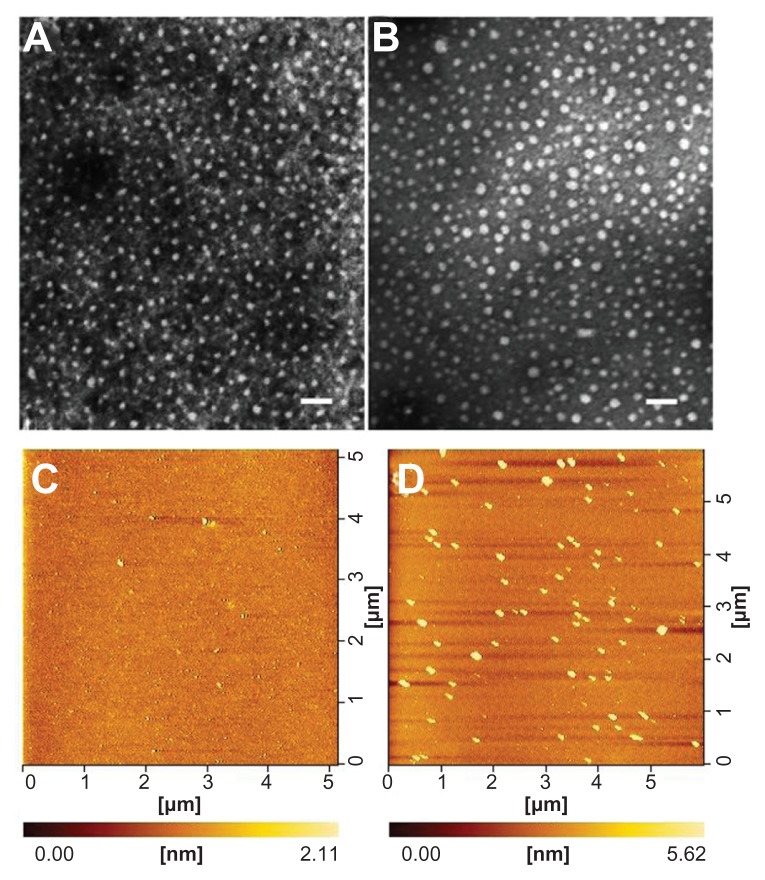
As a triblock copolymer, mPEG–PCL-g–PEI could self-assemble into nanoparticles with a core-shell structure and a positive charge on their surface. These cationic nanoparticles could also load hydrophobic chemotherapeutics and plasmid DNA, and the schematic illustration for this is shown in Figure 3. The size and zeta potential of these self-assembled mPEG–PCL-g–PEI micelles were measured, and the results are shown in Figure 4. As previously reported, the mPEG–PCL-g–PEI copolymer could be self-assembled into nanoscale particles with a positive charge.23 In this further study, different formulations of mPEG–PCL-g–PEI were prepared, and the size and zeta potential of these self-assembled nanomicelles was also measured at different temperatures. The results indicate that the copolymer could self-assemble into nanoparticles at over a wide range of temperatures. In addition, their positive charge varied at different temperatures of self-assembly. As the temperature increased, the size of the nanomicelles decreased, while the positive charge on all the micelles increased (Figure 4B). The morphology of the mPEG–PCL-g–PEI micelle and drug-loaded nanoparticles were determined by TEM (Figure 5A and B), while the DNA-binding complexes were investigated using atomic force microscopy (Figure 5C and D).
Figure 3.
Schematic illustration of self-assembled mPEG–PCL-g–PEI micelles and the capability of drug loading and gene adsorption.
Abbreviations: PEG, polyethylene glycol; PCL, poly ɛ-caprolactone; PEI, polyethylenimine.
Figure 4.
Sizes and zeta potential of the various composition copolymers mPEG– PCL-g–PEI self-assembled at different temperatures.
Abbreviations: PEG, polyethylene glycol; PCL, poly ɛ-caprolactone; PEI, polyethylenimine.
Figure 5.
Morphology of mPEG–PCL-g–PEI (5000-2000-2000) nanoparticles determined by transmission electron microscopy (A and B) and atomic force microscopy (C and D). (A) mPEG–PCL-g–PEI blank micelles; (B) micelles containing doxorubicin at a copolymer concentration of 0.5 mg/mL; (C) mPEG–PCL-g–PEI blank micelles; and (D) mPEG–PCL-g–PEI/DNA complexes.
Note: The scale bar is 100 nm.
Abbreviations: PEG, polyethylene glycol; PCL, poly ɛ-caprolactone; PEI, polyethylenimine.
Cytotoxicity
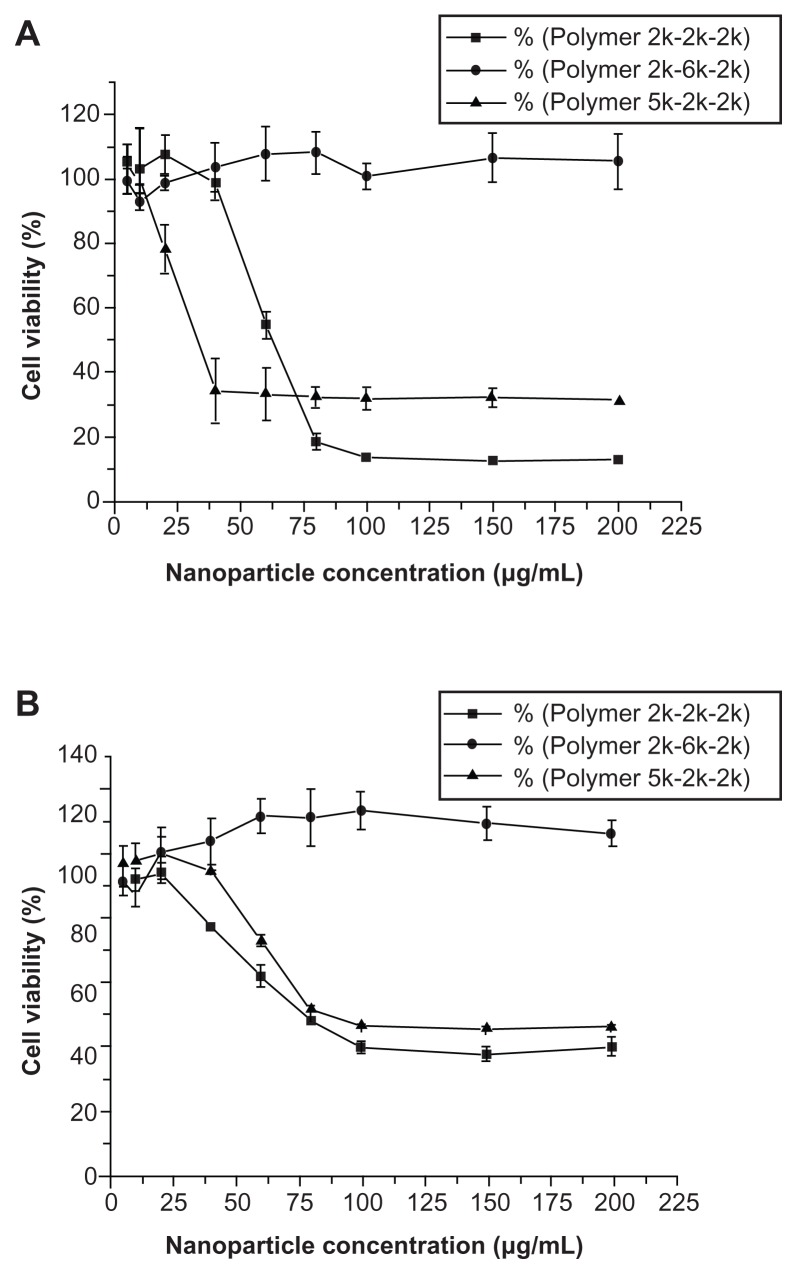
The cytotoxicity of all the mPEG–PCL-g–PEI copolymers was evaluated by MTT assay (Figure 6). Two cell lines, ie, L929 and HEK293, were used. Low cytotoxicity was observed for the L929 cell line at all concentrations, while higher toxicity was seen only at higher concentrations in the HEK293 cell line. As a cationic polymer, three copolymers with different compositions could be self-assembled into positively charged micelles, with cytotoxicity at higher concentrations.
Figure 6.
Cytotoxicity of mPEG–PCL-g–PEI nanoparticles of various compositions on (A) L929 cells and (B) HEK293 cells, and cell viability were determined by MTT assay
Notes: Mean ± SD, n = 3. The SD is shown by error bars.
Abbreviations: SD, standard deviation; PEG, polyethylene glycol; PCL, poly ɛ-caprolactone; PEI, polyethylenimine.
Gene transfection
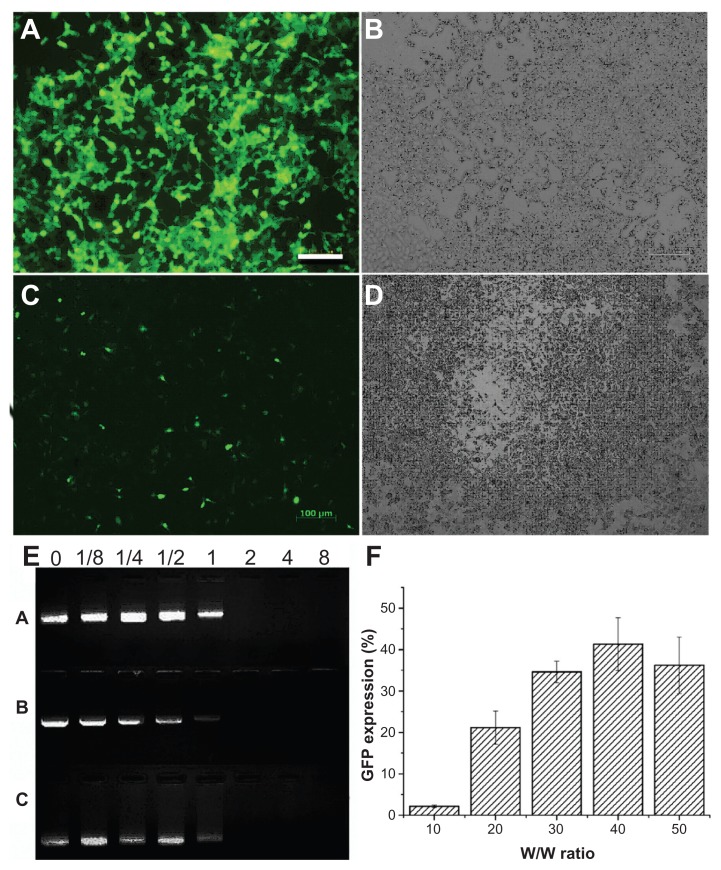
Triblock polymers were synthesized in three proportions, and because these copolymers contained the PEI chain, their potential capacity for gene transfection was investigated in both 293T and B16-F10 cell lines (Figure 7A–D). The copolymer could form a polycomplex with plasmid green fluorescence protein at various w/w ratios of polymer to DNA (ie, 10:1, 20:1, 30:1, 40:1, and 50:1). Firstly, the 293T cell line was chosen to find the ratio suitable for gene transfection, and then the B16-F10 cell line was chosen to test the gene delivery efficiency in cancer cells. A gene transfection efficiency of 60% ± 5% was achieved in the 293T cell line, and of 40% ± 5% in the B16-F10 cell line (Figure 7F). The DNA-condensing ability of mPEG–PCL-g–PEI was determined using a gel retardation assay, and the results are shown in Figure 7E. All of the synthesized polymers could bind DNA efficiently at ratios above 1 (M/M). The mPEG–PCL-g–PEI copolymers with the composition of 2000-2000-2000 and 2000-6000-2000 had little transfection efficiency in either the 293T or B16-F10 cell line. The mPEG-PCL-g-PEI copolymer with the 5000-2000-2000 composition had the highest transfection efficiency, so we chose this one for study of cellular uptake and codelivery of Nile red with DNA.
Figure 7.
The gene delivery capability of the triblock copolymer were investigated in both B16-F10 and 293T cell lines. The copolymer used was mPEG–PCL-g–PEI with the highest transfection efficiency at a composition of 5000-2000-2000 (A–D and F). (A) Fluorescence microscopy image of 293T cells; (B) white light image in 293T; (C) fluorescence microscopy image in B16-F10 cells; (D) white light image in B16-F10 cells; (E) agarose gel electrophoresis of mPEG–PCL-g–PEI/DNA complexes using three types of composition at various M/M ratios (A is the triblock copolymer with a composition of 2000-2000-2000, while B is 2000-6000-2000, and C is 5000-2000-2000); and (F) percentage of green fluorescence protein expression in B16-F10 cells.
Abbreviations: PEG, polyethylene glycol; PCL, poly ɛ-caprolactone; PEI, polyethylenimine.
Drug loading and cellular uptake
As a model drug, doxorubicin is considered to be hydrophobic in alkaline conditions. In addition, because the spontaneous fluorescence test is easy to perform, it is widely used in developing drug delivery systems, both in vitro and in vivo. To test the drug-loading capacity of the triblock mPEG– PCL-g–PEI copolymer, doxorubicin was chosen to test drug encapsulation efficiency. To obtain a credible result, the method used was derived from that in a previous report.27 The amphipathic mPEG–PCL-g–PEI copolymer could be self-assembled into micelles with a hydrophobic core and hydrophilic shell in aqueous solution. During the process of the pH change from acid to alkaline, the insolubility of doxorubicin decreased. After stirring, the doxorubicin was wrapped into a hydrophobic core, forming drug-loaded micelles. Drug encapsulation efficiency was easily measured by detecting the concentration of free drug. In this experiment, the encapsulation efficiency of doxorubicin was over 91%, while the maximum drug-loading content was 8.4%.
Images of the cellular uptake of doxorubicin-loaded micelles were captured using laser confocal microscopy. In this section, the mPEG–PCL-g–PEI copolymer with a composition of 5000-2000-2000 was chosen, and the result showed that the uptake of drug-loaded micelles was significantly higher than that of the free drug at various time points in the B16-F10 cell line (Figure 8).
Figure 8.
Confocal microscopic images of cellular uptake cocultured with doxorubicin-loaded micelles. The composition of mPEG–PCL-g–PEI copolymer was 5000-2000-2000. (A) Free doxorubicin, 1 hour, (B) free doxorubicin, 4 hours, (C) doxorubicin-loaded micelles, 1 hour, and (D) doxorubicin-loaded micelles, 4 hours.
Note: The scale bar in images is 47.62 μm.
Abbreviations: PEG, polyethylene glycol; PCL, poly ɛ-caprolactone; PEI, polyethylenimine.
Codelivery for drug and gene
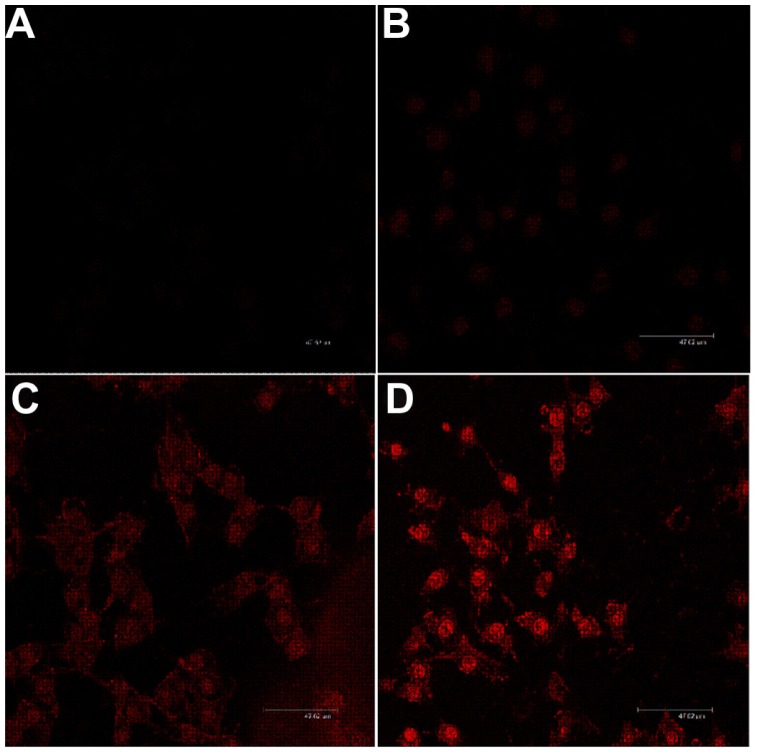
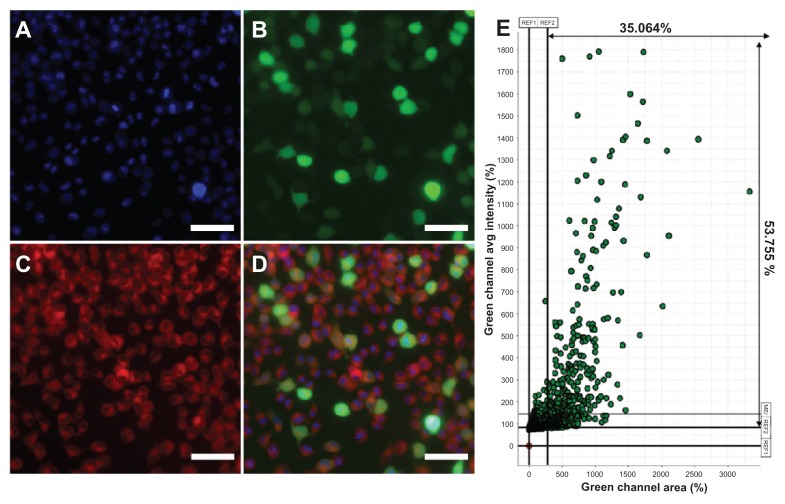
To test the hypothesis that the gene could be delivered into cells after the hydrophobic drug was loaded into mPEG–PCL-g– PEI micelles, Nile red and plasmid green fluorescence protein were codelivered into B16-F10 cells. As a hydrophobic dye, Nile red were chosen to model the hydrophobic drug, and plasmid green fluorescence protein was used as the reporter gene. The result of high-content analysis was 35.064%, which was consistent with flow cytometry. The results shown in Figure 9 indicate that the self-assembled micelles based on the mPEG–PCL-g–PEI copolymer could codeliver hydrophobic fluorescent dye and genes to the same cells, without significant difference in transfection efficiency.
Figure 9.
Fluorescence microscopy images of B16-F10 mouse melanoma cancer cells treated with Nile red-loaded micelles/plasmid green fluorescence protein complexes at 37°C. (A) Blue fluorescence (Hoechst 33342) filed, (B) green fluorescence field, (C) red fluorescence (Nile red) field, and (D) merged image. (E) The results were analyzed and indicated more than 35% cells were green fluorescence protein expression.
Note: The scale bar was 20 μm.
Discussion
Use of chemotherapeutic agents is often limited by systemic drug toxicity and poor aqueous solubility. Drug-delivery systems based on block copolymer micelles are considered to be a promising approach for improving the therapeutic index and reducing systemic toxicity.28–30 As an amphiphilic diblock copolymer, the biodegradable PCL–PEG copolymer is widely studied for its self-assembling properties and its potential use as a drug carrier.31,32 PEG chains on nanoparticle surfaces have been described as efficient drug carriers in the bloodstream with a range of applications, including prolonging the systemic cycle time of colloidal drug delivery systems and evading recognition by cells of the mononuclear phagocyte system. In theory, tumors have vessels with poorly aligned endothelial cells and wide fenestrations, so the long circulation time of block copolymer micelles can increase site-specific tumor accumulation. Meanwhile, PEI, reported to be a “proton sponge”, has been extensively studied in recent years as a nonviral gene vector, because it can absorb DNA by positive and negative electrical interactions. The formation of nanometer-scale complexes showed enhanced potentiation of transfection in vitro and in vivo. In a previous study, a PEI (molecular weight 800) chain was connected to classic PEG-PCL amphiphilic compounds to track the metabolism and distribution of the self-assembled micelles in tumor tissue. Self-assembled micelles were constructed with a positive charge, with the potential for DNA binding.23 To investigate the feasibility of codelivery of both drug and gene in vitro, mPEG–PCL-g–PEI of various compositions was synthesized. As a three-block amphiphilic polymer, mPEG–PCL-g–PEI contained mPEG, PEI as a hydrophilic segment and PCL as a hydrophobic segment. When the copolymer was dissolved in aqueous solution, hydrophobic molecules spontaneously formed a hydrophobic region by molecular interaction; at the same time, the hydrophilic mPEG chain formed a hydrophilic shell. Following a temperature increase, the molecular movement became more active, and micelles were formed with hydrophilic shells and hydrophobic cores.
In theory, gene transfection efficiency is dependent on particle size and zeta potential. In this study, the size and zeta potential of the self-assembled micelles were obtained by electron microscopy or Malvern particle analysis, and are shown in Figure 4. With increasing temperature, the motion of a single mPEG–PCL-g–PEI molecule became more active, making interaction between single molecules easier, which led to formation of smaller micelles. As a hydrophilic segment, PEI could be exposed more easily, thus resulting in micelles with a higher positive charge. With regard to in vitro application, 25 kDa PEI has the highest transfection efficiency at the optimal nitrogen/phosphorus ratio, and therefore was chosen as a positive control. However, it has many limitations to further application in vivo because of its high toxicity and nonbiodegradability. Within a certain range, increased transfection efficiency is always associated with higher toxicity, so the balance between transfection efficiency and cytotoxicity is of importance when developing cationic gene carriers. In recent years, in an effort to develop PEI-based nonviral gene delivery systems with high transfection efficiency and low cytotoxicity, many researchers have focused on low molecular weight PEI. In the present study, to provide DNA adsorption capability, PEI ( molecular weight 2000) was linked onto mPEG–PCL copolymers of variable composition. The designed mPEG–PCL-g–PEI copolymer (2000-2000-2000 and 5000-2000-2000) had the same capability for self-assembly and formation of micelles, while the designed mPEG–PCL-g–PEI (2000-6000-2000) copolymer was not dissolvable and could not form micelles by self-assembly in heated water, in contrast with the other two predesigned copolymers. Further, the mPEG–PCL-g–PEI (5000-2000-2000) copolymer had the highest transfection efficiency in cancer cell lines among the synthesized copolymers with various compositions, ie, although containing the same segments, the copolymer composition was confirmed to have a significant influence on gene transfection efficiency.21
The ratio between the hydrophilic and hydrophobic segments had a significant effect on the properties of the micelles, including dissolvability, stability, CMC, and drug encapsulation efficiency. Increasing the proportion of the hydrophobic segment decreased the water solubility of the block copolymer. Meanwhile, for the triblock copolymers, the ratio of the two hydrophilic segments also affected the stability, cytotoxicity, and gene transfection efficiency of the self-assembled micelles. In this study, mPEG–PCL-g– PEI with a PCL chain of molecular weight 6000 did not dissolve in heated water, while the other two compositions could self-assemble into nanomicelles. In addition, because most chemotherapeutic drugs are sensitive to temperature, temperature control is necessary during the process of drug loading. The drug-loading content of the mPEG–PCL-g–PEI copolymers with variable compositions was not significantly different at room temperature.
The result of laser confocal microscopy showed cellular uptake of the doxorubicin-loaded micelles and free doxorubicin in vitro, and the amount of enveloped doxorubicin taken up was much higher than that of free doxorubicin (Figure 8). The results indicate that the positive charge on the surface of the nanoparticles might enhance cellular uptake, as suggested in earlier literature.33 The reason for this might be related to the nature of the positively charged micelles. On the one hand, nanotechnology for drug delivery has the advantage of increasing the uptake efficiency of a chemotherapeutic agent. However, on the other hand, for this mPEG–PCL-g–PEI copolymer, the self-assembled micelles coated with positive charges could also benefit from bonding between micelles and the cell membrane through electrostatic adsorption.
To demonstrate the codelivery of a drug and a gene, Nile red was first loaded into the micelles, after which the loaded micelles were used to deliver the green fluorescence protein reporter gene into cancer cells. It was reported that the presence of chemotherapeutic agents would affect gene expression, so in the course of the in vitro codelivery study, doxorubicin was replaced instead by Nile red.34,35 The chemotherapeutic drug might affect gene expression, but it would be more advantageous to deliver the drug with DNA in the same carrier so that the delivered drug and gene could exert a combined action in the same cells simultaneously.7 The result of codelivery of Nile red and plasmid green fluorescence protein is shown in Figure 9. The cellular uptake of micelles loaded with Nile red did not affect green fluorescence protein expression in the same cell. The transfection efficiency in the codelivery groups were more than 35%. In other words, the micelles loaded with Nile red could also deliver DNA to the same cells, and the transfection efficiency was not significantly different from that obtained for the blank micelle groups.
Conclusion
In this study, we synthesized and characterized a triblock mPEG–PCL-g–PEI copolymer of variable composition which could self-assemble into nanomicelles. In addition, when linked to PEI, the self-assembled nanomicelles became positively charged. The aim of this study was to investigate mPEG–PCL-g–PEI, an amphipathic biodegradable material, which could codeliver chemicals and genes in vitro. The result of cell uptake for doxorubicin-labeled nanomicelles in vitro showed that the drug-loaded micelles could markedly enhance drug uptake efficiency, and the drug-loaded micelles could deliver the reporter gene into cells without decreasing the transfection efficiency. The mPEG–PCL-g–PEI copolymer with the component 5000-2000-2000 had the highest transfection efficiency, while the copolymer with the component 2000-2000-2000 had the highest drug-loading efficiency and drug-loading content. In summary, as an amphipathic copolymer, mPEG–PCL-g–PEI would be a simple delivery system for highly efficient codelivery of drugs and genes.
Acknowledgment
This work was financially supported by National Science and Technology Major Project (2011ZX09102-001-10), Sichuan Key Technology R&D Program, (2011SZ0219), New Century Excellent Talents in University (NCET-08-0371), National Natural Science Foundation (NSFC81071864), and Chinese Key Basic Research Program (2010CB529906).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen L, Waxman DJ. Intratumoral activation and enhanced chemotherapeutic effect of oxazaphosphorines following cytochrome P-450 gene transfer: development of a combined chemotherapy/cancer gene therapy strategy. Cancer Res. 1995;55(3):581–589. [PubMed] [Google Scholar]
- 2.Zhang Z, Li M, Wang H, Agrawal S, Zhang R. Antisense therapy targeting MDM2 oncogene in prostate cancer: Effects on proliferation, apoptosis, multiple gene expression, and chemotherapy. Proc Natl Acad Sci U S A. 2003;100(20):11636–11641. doi: 10.1073/pnas.1934692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cross D, Burmester JK. Gene therapy for cancer treatment: past, present and future. Clin Med Res. 2006;4(3):218–227. doi: 10.3121/cmr.4.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lumniczky K, Sáfrány G. Cancer gene therapy: combination with radiation therapy and the role of bystander cell killing in the anti-tumor effect. Pathol Oncol Res. 2006;12(2):118–124. doi: 10.1007/BF02893457. [DOI] [PubMed] [Google Scholar]
- 5.Cotten M, Wagner E, Zatloukal K, Phillips S, Curiel DT, Birnstiel ML. High-efficiency receptor-mediated delivery of small and large (48 kilobase gene constructs using the endosome-disruption activity of defective or chemically inactivated adenovirus particles. Proc Natl Acad Sci U S A. 1992;89(13):6094–6098. doi: 10.1073/pnas.89.13.6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malone RW, Hickman MA, Lehmann-Bruinsma K, et al. Dexamethasone enhancement of gene expression after direct hepatic DNA injection. J Biol Chem. 1994;269(47):29903–29907. [PubMed] [Google Scholar]
- 7.Wang Y, Gao S, Ye WH, Yoon HS, Yang YY. Co-delivery of drugs and DNA from cationic core C shell nanoparticles self-assembled from a biodegradable copolymer. Nat Mater. 2006;5(10):791–796. doi: 10.1038/nmat1737. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Sun H, Ma PX. Host-guest interaction mediated polymeric assemblies: multifunctional nanoparticles for drug and gene delivery. ACS Nano. 2010;4(2):1049–1059. doi: 10.1021/nn901213a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu LY, Bae YH. Self-assembled polyethylenimine-graft-poly ([epsilon]-caprolactone) micelles as potential dual carriers of genes and anticancer drugs. Biomaterials. 2007;28(28):4132–4142. doi: 10.1016/j.biomaterials.2007.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putnam D, Kopeek J. Polymer conjugates with anticancer activity. Biopolymers II. 1995;122:55–123. [Google Scholar]
- 11.Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47(1):113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 12.Lian T, Ho RJY. Trends and developments in liposome drug delivery systems. J Pharm Sci. 2001;90(6):667–680. doi: 10.1002/jps.1023. [DOI] [PubMed] [Google Scholar]
- 13.Thassu D, Deleers M, Pathak Y, editors. Nanoparticulate Drug Delivery Systems. New York, NY: Informa Healthcare USA Inc; 2007. [Google Scholar]
- 14.Torchilin V. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur J Pharm Biopharm. 2009;71(3):431–444. doi: 10.1016/j.ejpb.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pridgen EM, Langer R, Farokhzad OC. Biodegradable, polymeric nanoparticle delivery systems for cancer therapy. Nanomedicine. 2007;2(5):669–680. doi: 10.2217/17435889.2.5.669. [DOI] [PubMed] [Google Scholar]
- 16.Branco MC, Schneider JP. Self-assembling materials for therapeutic delivery. Acta Biomater. 2009;5(3):817–831. doi: 10.1016/j.actbio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun TM, Du JZ, Yan LF, Mao HQ, Wang J. Self-assembled biodegradable micellar nanoparticles of amphiphilic and cationic block copolymer for siRNA delivery. Biomaterials. 2008;29(32):4348–4355. doi: 10.1016/j.biomaterials.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 18.Adams ML, Lavasanifar A, Kwon GS. Amphiphilic block copolymers for drug delivery. J Pharm Sci. 2003;92(7):1343–1355. doi: 10.1002/jps.10397. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto Y, Nagasaki Y, Kato Y, Sugiyama Y, Kataoka K. Long-circulating poly (ethylene glycol)-poly (-lactide) block copolymer micelles with modulated surface charge. J Control Release. 2001;77(1–2):27–38. doi: 10.1016/s0168-3659(01)00451-5. [DOI] [PubMed] [Google Scholar]
- 20.Woodward SC, Brewer P, Moatamed F, Schindler A, Pitt C. The intracellular degradation of poly (epsilon-caprolactone) J Biomed Mater Res. 1985;19(4):437–444. doi: 10.1002/jbm.820190408. [DOI] [PubMed] [Google Scholar]
- 21.Shuai X, Merdan T, Unger F, Wittmar M, Kissel T. Novel biodegradable ternary copolymers hy-PEI-g-PCL-b-PEG: synthesis, characterization, and potential as efficient nonviral gene delivery vectors. Macromolecules. 2003;36(15):5751–5759. [Google Scholar]
- 22.Liu Y, Nguyen J, Steele T, Merkel O, Kissel T. A new synthesis method and degradation of hyper-branched polyethylenimine grafted poly-caprolactone block mono-methoxyl poly (ethylene glycol) copolymers (hy-PEI-g-PCL-b-mPEG) as potential DNA delivery vectors. Polymer. 2009;50(16):3895–3904. [Google Scholar]
- 23.Shi S, Fan M, Wang X, et al. Synthesis and characterization of mPEG– PCL-g–PEI and self-assembled nanoparticle uptake in vitro and in vivo. J Phys Chem C. 2010;114(49):21315–21321. [Google Scholar]
- 24.Sun P, Zhou D, Gan Z. Novel reduction-sensitive micelles for triggered intracellular drug release. J Control Release. 2011;155(1):96–103. doi: 10.1016/j.jconrel.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Sun H, Guo B, Cheng R, Meng F, Liu H, Zhong Z. Biodegradable micelles with sheddable poly (ethylene glycol) shells for triggered intracellular release of doxorubicin. Biomaterials. 2009;30(31):6358–6366. doi: 10.1016/j.biomaterials.2009.07.051. [DOI] [PubMed] [Google Scholar]
- 26.Wei XW, Gong CY, Shi S, et al. Self-assembled honokiol-loaded micelles based on poly (-caprolactone)-poly (ethylene glycol)-poly (-caprolactone) copolymer. Int J Pharm. 2009;369(1–2):170–175. doi: 10.1016/j.ijpharm.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 27.Gou ML, Zheng XL, Men K, et al. Poly (epsilon-caprolactone)/poly (ethylene glycol)/poly (epsilon-caprolactone) nanoparticles: preparation, characterization, and application in doxorubicin delivery. J Phys Chem B. 2009;113(39):12928–12933. doi: 10.1021/jp905781g. [DOI] [PubMed] [Google Scholar]
- 28.Mikhail AS, Allen C. Block copolymer micelles for delivery of cancer therapy: transport at the whole body, tissue and cellular levels. J Control Release. 2009;138(3):214–223. doi: 10.1016/j.jconrel.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Nishiyama N, Kataoka K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol Ther. 2006;112(3):630–648. doi: 10.1016/j.pharmthera.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Lee H, Allen C. Formulation of drugs in block copolymer micelles: drug loading and release. Curr Pharm Des. 2006;12(36):4685–4701. doi: 10.2174/138161206779026263. [DOI] [PubMed] [Google Scholar]
- 31.Gou ML, Zheng XL, Men K, et al. Self-assembled hydrophobic honokiol loaded MPEG-PCL diblock copolymer micelles. Pharm Res. 2009;26(9):2164–2173. doi: 10.1007/s11095-009-9929-8. [DOI] [PubMed] [Google Scholar]
- 32.Wei XW, Gong CY, Gou ML, et al. Biodegradable poly (-caprolactone)- poly (ethylene glycol) copolymers as drug delivery system. Int J Pharm. 2009;381(1):1–18. doi: 10.1016/j.ijpharm.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 33.Xia T, Kovochich M, Liong M, et al. Polyethyleneimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNA constructs. ACS Nano. 2009;3(10):3273–3286. doi: 10.1021/nn900918w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Son K, Huang L. Factors influencing the drug sensitization of human tumor cells for in situ lipofection. Gene Ther. 1996;3(7):630–634. [PubMed] [Google Scholar]
- 35.Nair RR, Rodgers JR, Schwarz LA. Enhancement of transgene expression by combining glucocorticoids and anti-mitotic agents during transient transfection using DNA-cationic liposomes. Mol Ther. 2002;5(4):455–462. doi: 10.1006/mthe.2002.0567. [DOI] [PubMed] [Google Scholar]