Abstract
Oridonin, a diterpenoid isolated from Rabdosia rubescencs, has been reported to have antitumor effects. However, low solubility has limited its clinical applications. Preparation of drugs in the form of nanosuspensions is an extensively utilized protocol. In this study, we investigated the anticancer activity of oridonin and oridonin nanosuspension on human pancreatic carcinoma PANC-1 cells. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay was performed to investigate the effect of oridonin on cell growth. Propidium iodide and Hoechst 33342 staining were used to detect morphologic changes. The percentage of apoptosis and cell cycle progression was determined by flow cytometric method staining with propidium iodide. Annexin V-fluorescein isothiocyanate (FITC)/PI staining was used to evaluate cell apoptosis by flow cytometry. Caspase-3 activity was measured by spectrophotometry. The apoptotic and cell cycle protein expression were determined by Western blot analysis. Both oridonin and oridonin nanosuspension induced apoptosis and G2/M phase cell cycle arrest, and the latter had a more significant cytotoxic effect. The ratio of Bcl-2/Bax protein expression was decreased and caspase- 3 activity was stimulated. The expression of cyclin B1 and p-cdc2 (T161) was suppressed. Our results showed that oridonin nanosuspension was more effective than free oridonin on G2/M cell cycle arrest and apoptosis in the human pancreatic cancer PANC-1 cell line.
Keywords: cyclin B1, cdc2, caspase-3, Bcl-2, Bax
Introduction
Oridonin (Figure 1), an active diterpenoid isolated from the herb Rabdosia rubescens, which is often used in Chinese traditional medicine, has been reported to have antitumor activities.1–3 Previous studies demonstrated that oridonin exhibited remarkable inhibitory effects on tumor cell proliferation and was a potent apoptosis inducer in a variety of cancer cells by regulating a series of transcription factors, protein kinases, as well as pro- and/or anti-apoptotic proteins.4–7 Accumulating evidence has shown that oridonin is able to block the progression of tumors and alleviate cancer syndrome, which may improve the survival rates of cancer patients greatly, suggesting that oridonin may be used as a potential antineoplastic drug for future cancer therapeutics. However, studies on the effects of oridonin on human pancreatic cancer are limited. Pancreatic carcinoma is one of the most common causes of cancer death. It is a fast-developing disease which is difficult to detect and diagnose and often has a poor prognosis.8,9 Therefore, adjuvant therapy, especially chemotherapy, to improve the quality of life for advanced pancreatic cancer patients is increasingly important. In this study, we investigated the anticancer activity of oridonin on human epithelial-like pancreatic cancer cell line PANC-1, which is used as an in vitro model of nonendocrine pancreatic cancer for tumorigenicity studies.
Figure 1.
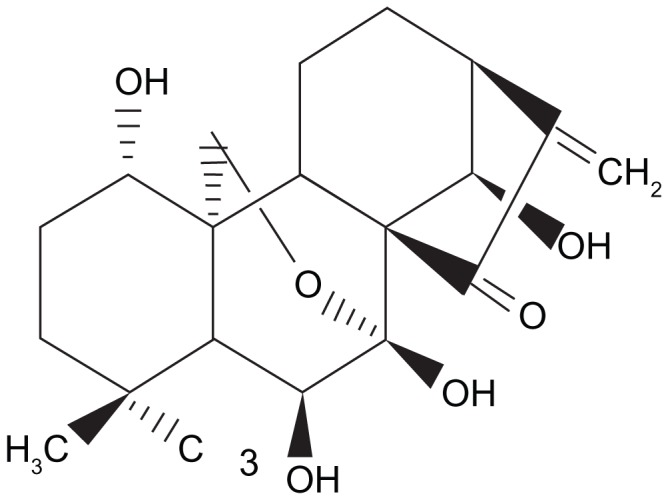
The chemical structure of oridonin.
Furthermore, low aqueous solubility and/or dissolution of oridonin and short biological half-life in vivo have limited its medical applications as a therapeutic agent. Therefore, it is critical to develop new strategies for overcoming this problem. The preparation of drugs in the form of nanosuspensions has been shown to be an extensively utilized protocol to tackle the problems of insoluble compounds.10,11 Nanosuspensions are suspensions of drug nanocrystals in a liquid stabilized by a surfactant or polymer. With small particle size and enormous particle surface, nanosuspensions increase the solubility and dissolution velocity, and consequently improve drug bioavailability. Besides, nanosuspension can also produce bioadhesion, increase stability, and enhance cytotoxicity of the agents.12 In previous studies, we prepared the stable oridonin nanosuspension by high-pressure homogenization, which resulted in increased drug saturation solubility and dissolution velocity.13 In vitro experiments showed that oridonin nanosuspension could significantly enhance cytotoxicity against K562 cells and induce a higher apoptotic rate in K562 cells compared to the oridonin solution. In vivo studies in a mouse model of sarcoma-180 solid tumors demonstrated significantly greater inhibition of tumor growth following treatment with oridonin nanosuspension than with oridonin solution at the same dosage. These results suggest that the delivery of oridonin as a nanosuspension is a promising approach for the treatment of tumors.14 Therefore, in this study, we investigated the anticancer activity of oridonin nanosuspension on human pancreatic cancer cell line PANC-1 compared with free oridonin.
Materials and methods
Materials
Oridonin (99% pure by high performance liquid chromatography) was obtained from Shanxi Huike Plants Exploitation (Xian, China). Oridonin and oridonin nanosuspension solution were prepared as described previously;15 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and dimethyl sulfoxide were purchased from Sigma-Aldrich (St Louis, MO). Fetal bovine serum was purchased from TBD Biotechnology Development (Tianjin, China). Trypsin was purchased from Gibco BRL (Gaithersburg, MD). Propidium iodide (PI), ribonuclease A, and annexin V-FITC were purchased from KeyGen Biotechnology ( Nanjing, China). Antibodies against Bax, Bcl-2, caspase- 3, and β-actin and horseradish peroxidase-conjugated secondary antibody (goat-anti-rabbit or goat-anti-mouse) were procured from Santa Cruz Biotechnology (Santa Cruz, CA); antibodies against cyclin B1, cdc2, and p-cdc2 (T161) were procured from Bioworld Technology (Minneapolis, MN). All other chemicals were of analytical grade.
Cell culture
Human pancreatic cancer cell line PANC-1 cells were cultured in DMEM supplemented with 10% newborn calf serum, 100 U/mL penicillin, and 100 μg/mL streptomycin, and were maintained at 37°C with 5% CO2 in a humidified atmosphere. All the experiments were performed on logarithmically growing cells.
Cell viability assay
The viability of cells treated with oridonin nanosuspension or free oridonin was measured by MTT assay. Cells were suspended at a final concentration of 5 × 104 cells/well and cultured in a 96-well flatbottomed microplate. After 12 hours’ incubation, the cells were treated with oridonin nanosuspension or free oridonin (1.56, 3.14, 6.25, 12.5, 25, and 50 μmol/L) for 12 hours, 24 hours, 36 hours, and 48 hours. At the end of the treatment, 20 μL MTT solution (5 mg/mL) was added to each well and the cells were then incubated for 4 hours. The culture media were then replaced with 150 μL dimethyl sulfoxide. Light absorbance of the solution was measured at 570 nm on a Bio-Rad Model 680 microplate reader (Hercules, CA). The cell inhibitory rate was calculated as follows: Inhibitory rate = (A570 control cells − A570 treated cells)/A570 control cells × 100%. All assays were done with six parallel samples.
Observation of morphologic changes
PANC-1 cells were seeded in six-well culture plates and treated with different concentrations of oridonin nanosuspension or free oridonin for 24 hours. Then the cellular morphologic changes were observed.
Hoechst 33342 and PI staining
Cells were seeded onto a 12-well plate and incubated with oridonin nanosuspension or free oridonin for 24 hours. Following the incubation period, the cells were stained with 1 μg/mL Hoechst 33342 for 30 minutes in the dark. For PI staining, after treatment cells were harvested and washed with phosphate-buffered saline (PBS). Aliquots of cell suspension (95 μL; 1 × 106 cells/mL) were mixed with 5 μL PI and incubated in the dark for 5 minutes. Stained cells were then imaged under a Nikon TE2000-U fluorescent microscope (Tokyo, Japan).
Cell cycle assay by flow cytometric analysis
PANC-1 cells treated with oridonin nanosuspension or free oridonin for different time periods were harvested, washed with PBS, and fixed in 75% ethanol overnight at 4°C. Prior to analysis, cells were washed again with PBS, resuspended and treated with ribonuclease A 0.25 mg/mL for 30 minutes at 37°C, then incubated with 20 mg/L PI in the dark for 30 minutes. Then the samples were analyzed by flow cytometer.
Evaluation of apoptosis
To quantify the apoptosis of cells treated with oridonin nanosuspension or free oridonin, annexin V-FITC/PI staining was performed, and apoptosis was evaluated by flow cytometric analysis. PANC-1 cells were exposed to different concentrations of oridonin nanosuspension or free oridonin for 24 hours. After treatment, 1 × 105 cells were collected, washed twice with PBS, and resuspended in 500 μL binding buffer. Later, 5 μL of annexin V-FITC and 5 μL of PI were added to the suspension and the cells were then incubated in the dark for 15 minutes at room temperature. Samples were then analyzed by flow cytometer.
Caspase-3 activity assay
PANC-1 cells were cultured with 10 μmol/L oridonin nanosuspension or free oridonin for different time periods (4 hours, 8 hours, 12 hours, and 24 hours). The activity of caspase-3 was detected by cleavage of chromogenic caspase-3 substrates, Ac-DEVD-pNA (acetyl-Asp-Glu-Val- Asp p-nitroanilide). Protein was extracted using ice-cold cell lysis buffer and approximately 100 μg of total protein was added to reaction buffer containing Ac-DEVD-pNA (2 mM), incubated overnight at 37°C. The free pNA cleaved from its precursor can be quantified using a spectrometer at 405 nm.
Western blot analysis
The PANC-1 cells treated with oridonin nanosuspension or free oridonin for different time periods were collected, washed with ice-cold PBS, and resuspended in lysis buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, sodium orthovanadate, sodium fluoride, ethylenediaminetetraacetic acid, and leupeptin. After 30 minutes of incubation on ice, the lysate was centrifuged at 12,000 g for 10 minutes and the supernatants were used for Western blot analysis. Protein concentration was determined by bicinchoninic acid assay. Equal amounts of total protein were separated by 12% gel electrophoresis and electrophoretically transferred to nitrocellulose membranes. Immunoblotting was performed with different antibodies and visualized using an enhanced chemiluminescence method.
Statistical analysis
The results are expressed as the mean ± standard deviation. Statistical significance of differences between groups was calculated by Student’s t-test. P values <0.05 were considered statistically significant.
Results
Cytotoxic effects of oridonin nanosuspension and free oridonin on PANC-1 cells
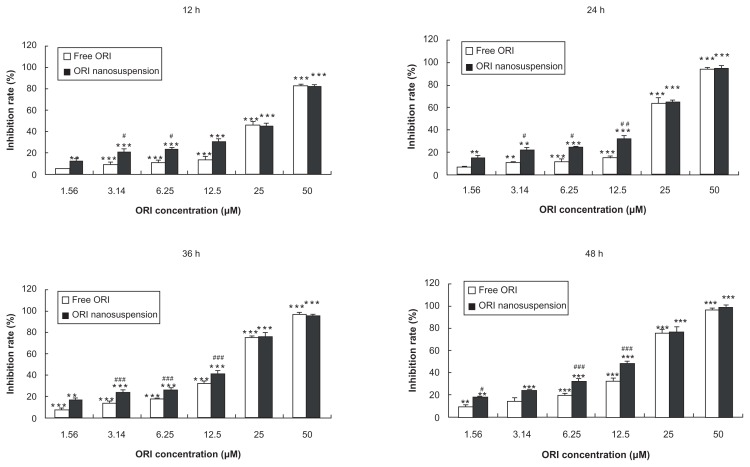
To determine the cytotoxicity effects of oridonin nanosuspension and free oridonin on PANC-1 cells, the cells were cultured with different concentrations for different time periods and the cytotoxic effect was measured by MTT assay. Oridonin nanosuspension and free oridonin induced cell death in a dose- and time-dependent manner. As shown in Figure 2, the inhibitory rate of oridonin nanosuspension is significantly higher than that of free oridonin at the concentrations of 3.14, 6.25, and 12.5 μmol/L.
Figure 2.
Cytotoxic effects of oridonin nanosuspension and free oridonin on PANC-1 cells.
Notes: Data are mean ± standard deviation (n = 3), *P < 0.05, **P < 0.01, and ***P < 0.001 vs control group, #P < 0.05, ##P < 0.01, ###P < 0.001 vs the same dose of free oridonin group.
Abbreviation: ORI, oridonin.
Oridonin nanosuspension and free oridonin induce morphologic changes and apoptotic cell death in PANC-1 cells
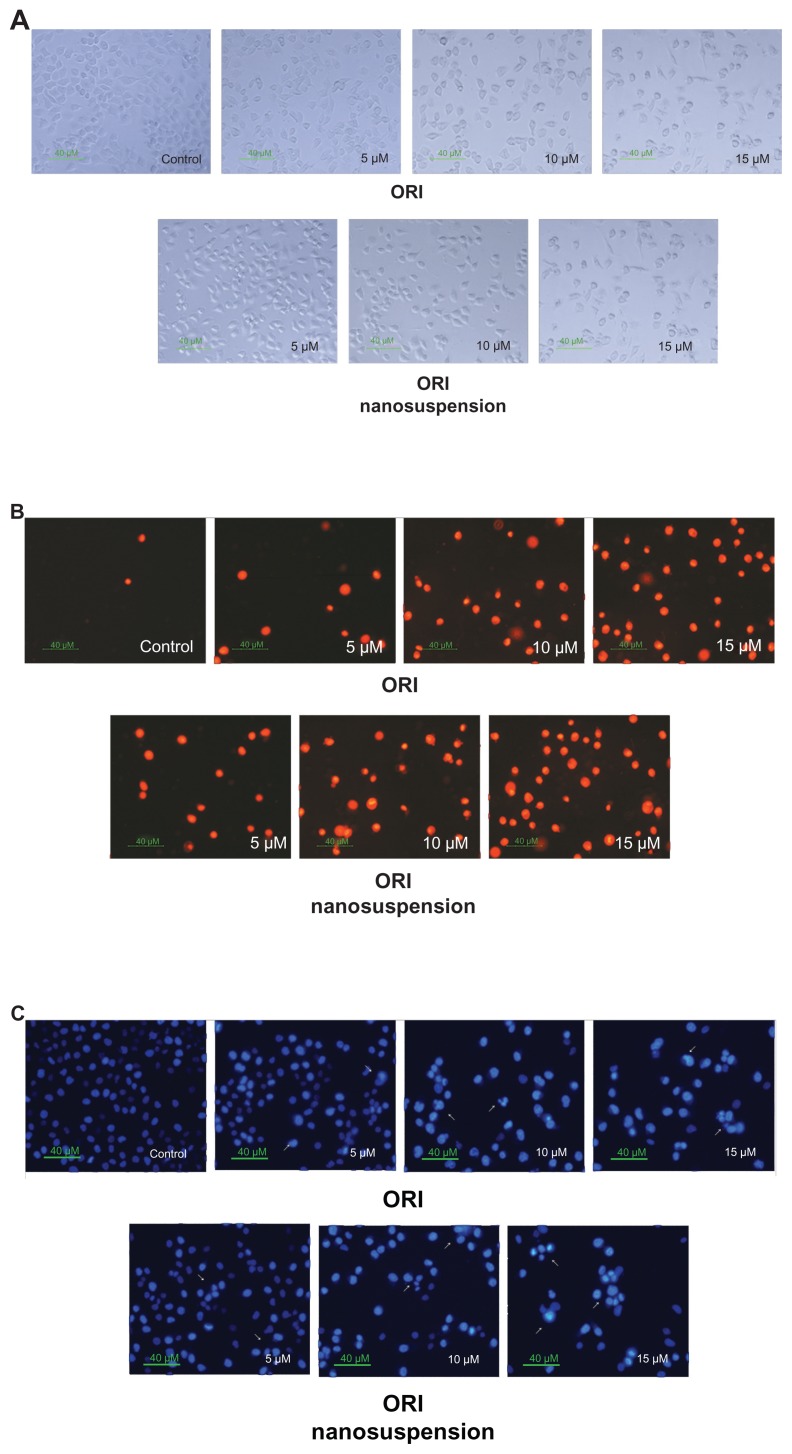
As shown in Figure 3A, after the cells were exposed to oridonin, marked morphologic changes were observed. Cells underwent contraction and became round in shape. But there were no obvious differences in morphology between free oridonin and its formulation.
Figure 3.
Oridonin nanosuspension-induced and free oridonin-induced morphologic changes of PANC-1 cells. (A) Cellular morphology was examined in the presence of different doses of oridonin nanosuspension and free oridonin. Nuclear morphology was determined using (B) propidium iodide staining and (C) Hoechst 33342 staining.
Abbreviation: ORI, oridonin.
To confirm whether oridonin-induced cell death in PANC-1 was caused by apoptosis, PI and Hoechst 33342 staining were carried out. PI is membrane impermeant, generally excluded from viable cells, and is commonly used for identifying dead cells in a population. As shown in Figure 3B, the red cell nuclei represent the middle–late apoptotic or necrotic cells. The result showed that compared with the control group, the stained cells increased in a dose-dependent manner, which means that oridonin nanosuspension and free oridonin could induce PANC-1 cell death.
Hoechst 33342 staining of the cell nuclei further confirmed that oridonin nanosuspension and free oridonin induced apoptosis in PANC-1 cells. The major findings are showed by arrows in Figure 3C. In the control group, the nuclei of the PANC-1 cells were round and homogeneously stained, but the cells treated with oridonin and its formulation showed cell shrinkage, chromatin condensation, and cell membrane blebbing.
Treatment with oridonin nanosuspension or free oridonin leads to apoptosis of PANC-1 cells
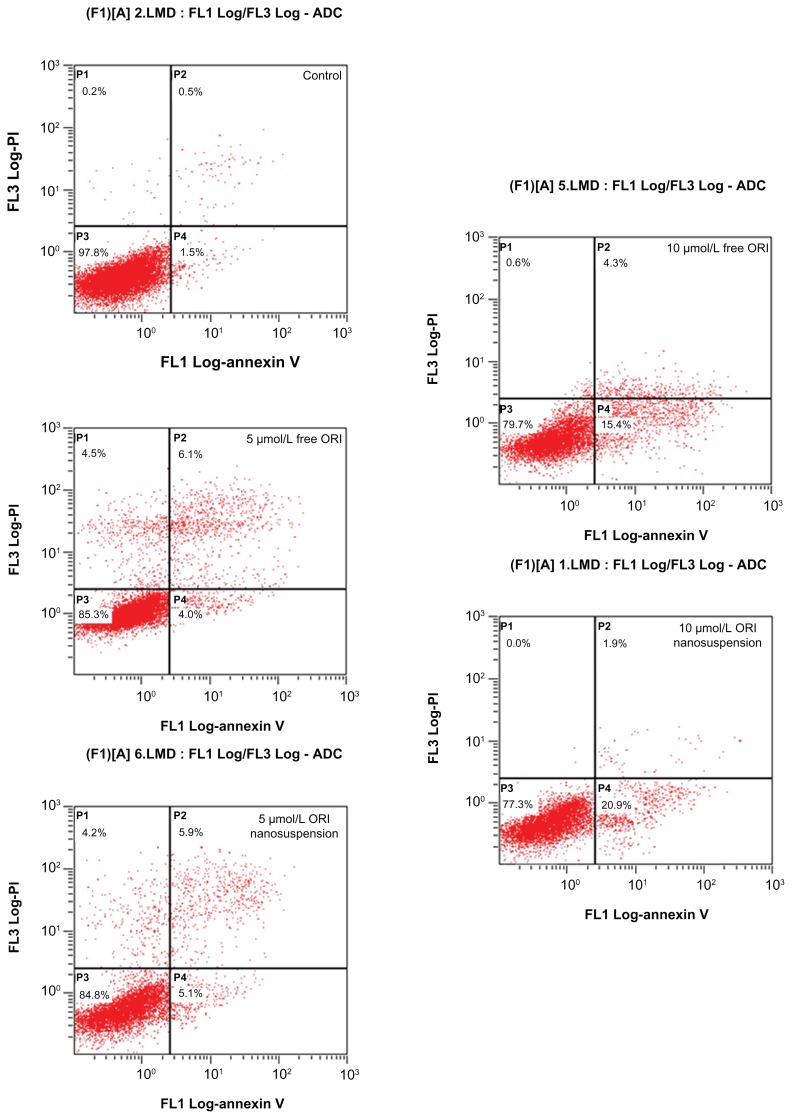
Flow cytometric analysis with annexin V-FITC and PI staining was undertaken to determine the effect of oridonin nanosuspension and free oridonin on PANC-1 apoptosis. Figure 4 shows the distribution of cell populations after 24 hours of treatment with oridonin nanosuspension or free oridonin, and the lower right quadrant represents early apoptotic cells. The results showed that the early apoptotic rates of cells were 1.5% (control), 4.0% m and 15.4% (5 μmol/L and 10 μmol/L free oridonin), 5.1%, and 20.9% (5 μmol/L and 10 μmol/L oridonin nanosuspension), respectively. These statistics indicate that oridonin nanosuspension and free oridonin both induced PANC-1 apoptosis in a dose-dependent manner. Oridonin nanosuspension at a concentration of 10 μmol/L had a more significant apoptosis-inducing effect compared with free oridonin.
Figure 4.
The effect of oridonin nanosuspension and free oridonin on PANC-1 cell apoptosis was measured by annexin V-fluorescein isothiocyanate/propidium iodide staining. The early apoptotic cells stained by annexin-V-fluorescein isothiocyanate are located in the lower right quadrant.
Abbreviation: ORI, oridonin.
Oridonin nanosuspension and free oridonin induces G2/M phase cell cycle arrest in PANC-1 cells
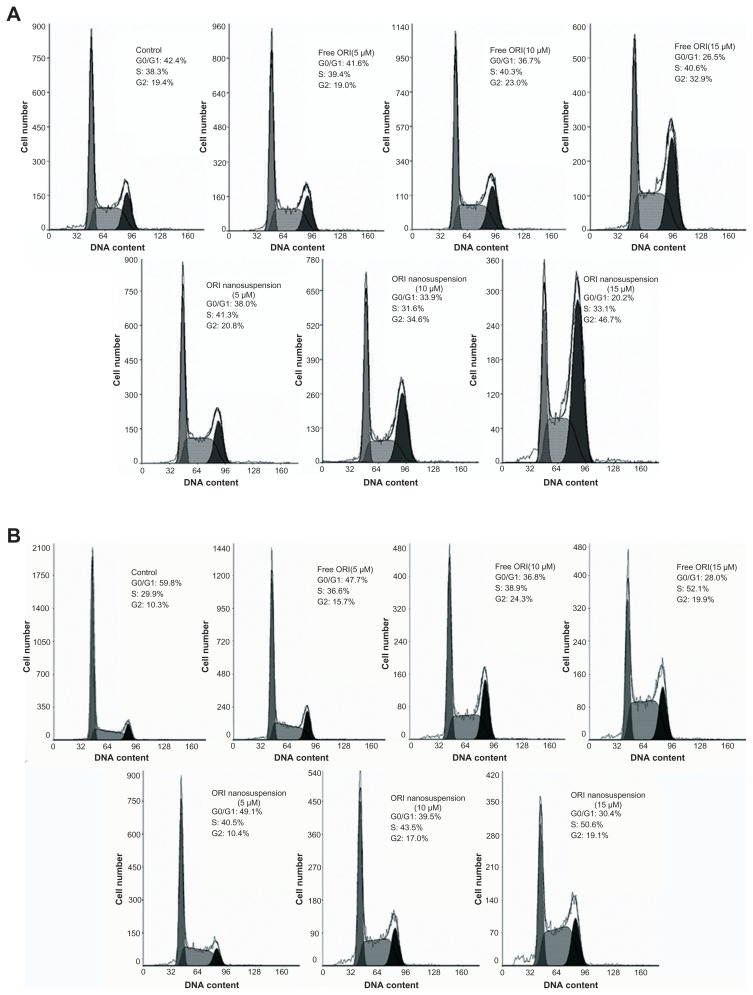
To determine whether oridonin nanosuspension and free oridonin regulate cell cycle progression in PANC-1 cells, the cells were treated for 24 hours and 48 hours with different concentrations of oridonin nanosuspension or free oridonin (5, 10, and 15 μmol/L), and the DNA was stained with PI, followed by fluorescence activated cell sorting analysis. As shown in Figure 5A, compared with the control, the percentage of cells increased in the G2/M phase in a dose-dependent manner, but did not change in the S phase. Cells treated with 10 μmol/L and 15 μmol/L oridonin nanosuspension for 24 hours had a higher fraction of G2/M phase cells (34.6% and 46.7%) compared with that of cells treated with free oridonin (23% and 32.9%). However, after 48 hours’ treatment, the fractions of cells in the S phase and the G2/M phase both increased (Figure 5B).
Figure 5.
Oridonin nanosuspension-induced and free oridonin-induced cell cycle arrest at the G2/M phase. PANC-1 cells were treated with different doses of oridonin nanosuspension and free oridonin for 24 hours (A) and 48 hours (B).
Abbreviation: ORI, oridonin.
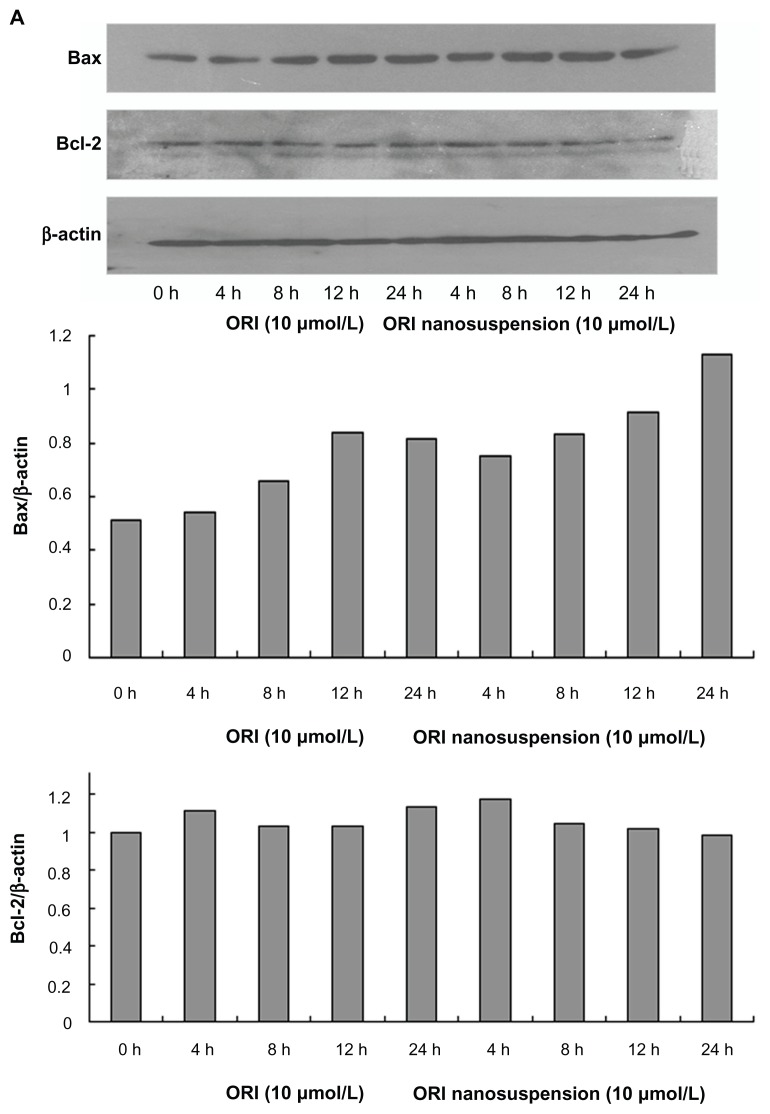
Involvement of Bax, Bcl-2, and caspase-3 in oridonin nanosuspension-induced and free oridonin-induced cell death
It is known that the Bcl-2 family of proteins play important roles in either inhibition or promotion of apoptotic cell death. To confirm whether the Bcl-2 family of proteins are involved in oridonin nanosuspension-induced or free oridonin- induced PANC-1 cell death, we examined the expression of Bcl-2 family proteins Bcl-2 and Bax by Western blot analysis. After treatment with oridonin nanosuspension or free oridonin for different time periods, the expression of Bax was upregulated, whereas there were no obvious changes in expression of Bcl-2 (Figure 6A).
Figure 6.
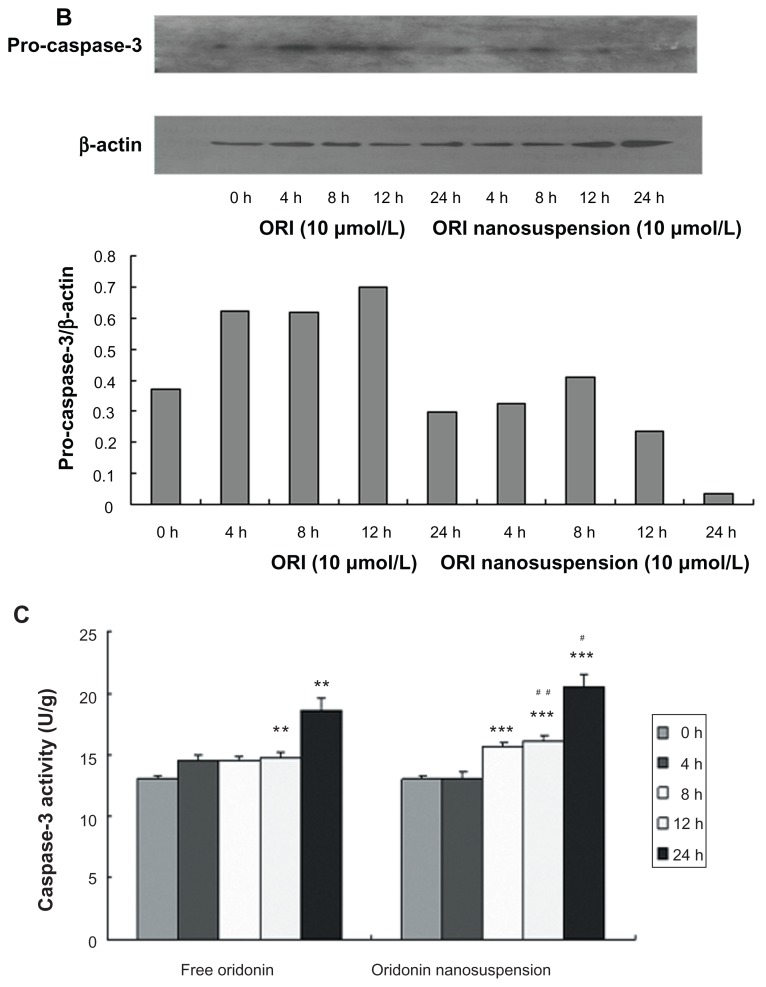
The effect of oridonin nanosuspension and free oridonin on apoptosis-related proteins in PANC-1 cells. (A) The expression of Bax, and Bcl-2. (B) The expression of pro-caspase-3. (C) The effect of free oridonin and oridonin nanosuspension on caspase-3 activity.
Notes: **P < 0.01 vs control group, ***P < 0.001 vs control group; #P < 0.05 vs oridonin group, ##P < 0.05 vs oridonin group.
Abbreviation: ORI, oridonin.
To further explore the apoptotic signaling pathway of oridonin-induced cell death, we examined the expression of pro-caspase3 (Figure 6B) and caspase-3 activity (Figure 6C). Oridonin nanosuspension and free oridonin treatment of PANC-1 cells both stimulated activity of caspase-3.
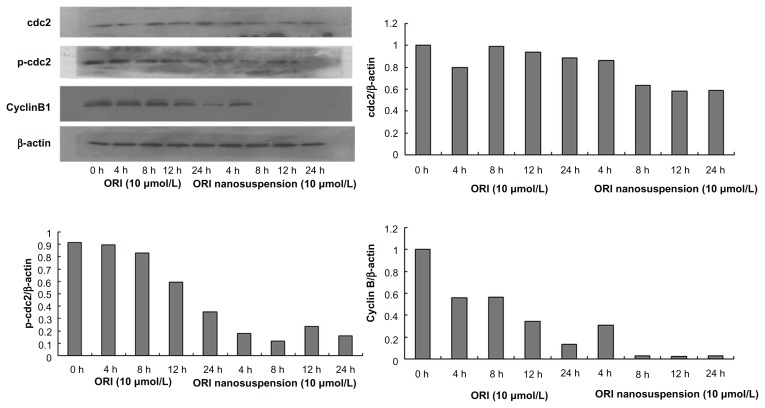
Effects of oridonin nanosuspension and free oridonin on the expression of G2/M phase cell cycle-related proteins
To examine whether the cell cycle arrest is associated with the expression of cell cycle regulatory proteins, the levels of G2/M regulatory proteins were assessed. As shown in Figure 7 the expression of cyclin B1 and p-cdc2 (T161) was suppressed in a time-dependent manner. However, neither oridonin nanosuspension nor free oridonin had an effect on the expression level of cdc2 protein.
Figure 7.
The expression of cyclin B1, cdc2, and p-cdc2 (T161) in PANC-1 cells detected by Western blot analysis.
Discussion
Many compounds extracted from Rabdosia rubescens have been proved to have antitumor activity against a number of cancer cells, and oridonin is one of the most effective derivatives. Although a previous study had shown that oridonin had considerable antiproliferation effects on some kinds of cancer, few studies of the anticancer activity of oridonin nanosuspension and free oridonin on human pancreatic cancer have been undertaken.4 In our study, we demonstrated that oridonin nanosuspension and free oridonin could both inhibit the growth of human pancreatic cancer cell line PANC-1 by inducing cell cycle arrest and apoptosis, and oridonin nanosuspension exhibited increased cytotoxic effect. Our results showed that the inhibitory rate of oridonin nanosuspension is significantly higher than that of free oridonin at the concentrations of 3.14, 6.25, and 12.5 μmol/L, while at higher concentrations, there was no statistical difference in cytoxicity. A previous study showed that oridonin nanosuspension, possessing a significantly increased solubility and dissolution rate than free oridonin, could markedly induce oridonin molecular concentration around the cells.15 Apart from this, the small size of nanosuspension may facilitate its adhesion to the cells and improve the interactions between the cells and drugs. Moreover, it is generally considered that nanoparticles can be nonspecically internalized into cells via endocytosis or phagocytosis. 16 Therefore, the oridonin nanosuspension was more effective at a lower concentration.
Observation of morphologic changes through Hoechst 33342 and PI staining and annexin V-FITC/PI staining suggested oridonin nanosuspension and free oridonin- induced cell apoptosis of PANC-1 cells, between which the former was more conspicuous. Apoptosis is regulated by a complex network of molecules, and involves the expression changes of distinct pro-apoptotic and antiapoptotic proteins. Bcl-2 family members are characterized by containing at least one of four Bcl-2 homology domains (BH1–BH4). Proteins of the Bcl-2 family such as Bax and bcl-2 have been shown to play an essential role in cell apoptosis.17 Bax can bind Bcl-2 and the heterodimer provides protection against apoptosis. Therefore, the Bcl-2/Bax ratio is important in apoptosis and its decline induces apoptosis.18 Our present results showed that after oridonin administration, the expression of Bax gradually increased with time, whereas Bcl-2 protein expression did not change.
Caspases, a family of cysteases, play an important role in regulating apoptotic signal transmission, and can be divided into two groups including upstream caspases such as caspase-2, -8, and -10, and downstream caspases such as caspase-3, -6, and -7. Caspase-3 is one of the most important “effector” caspases, and is capable of cleaving many important cellular substrates.19–21 In this study, we examined the expression of pro-caspase3 and caspase-3 activity, and the result suggested that caspase-3 was involved in oridonin nanosuspension-induced and free oridonin-induced PANC-1 cell apoptosis.
Previous studies showed that Bax/Bcl-2 acted in mitochondria to cause the cytochrome c release into cytoplasm, and then cytochrome c, apoptotic protease activating factor-1, and procaspase-9 formed a complex leading to caspase-9 activation. Activated caspase-9 cleaved caspase-3 proenzyme, and then activated caspase-3 acted on its substrates which are important for cell survival.22,23 When compared with our results, it can be deduced that this signal pathway may be involved in oridonin nanosuspension-induced and free oridonin-induced PANC-1 cell apoptosis.
As suggested by our data from cell cycle analysis, oridonin nanosuspension and free oridonin both arrested PANC-1 cells in the G2/M phase, and we also demonstrated that the expression levels of cyclin B1 and p-cdc2 (T161) decreased significantly after treatment in which oridonin nanosuspension had more significant effect due to its smaller size.24,25 It is well known that the progression through various phases of cell cycle is a tightly regulated process involving the activities of various cyclins and cyclin-dependent kinases, which are specific for different cell cycle phases.26 Cyclin B1 and cdc2, which are the master regulators in cell proliferation, play an important role in the G2/M phase transition of mitosis in cell proliferation. The inactivity of cyclin B1 and cdc2 resulted in cell cycle arrest in the G2/M phase.27,28
In summary, we demonstrated that both oridonin nanosuspension and free oridonin have significant antiproliferation effects on PANC-1 cells, and the effects were intensified by oridonin nanosuspension. However, oridonin nanosuspension did not change the antiproliferation mechanism of free oridonin. They both induced G2/M phase cell cycle arrest and apoptosis in PANC-1 cells. Our results showed that cyclin B1 and cdc2 were involved in oridonin-induced cell cycle arrest, and oridonin-induced PANC-1 cell apoptosis was mediated by a decreased Bcl-2/Bax ratio and activation of caspase-3.
Acknowledgment
This work was supported by the National Basic Research Program of China (973 Program), No 2009CB930300.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Abelson PH. Medicine from plants. Science. 1990;247:513. doi: 10.1126/science.2300807. [DOI] [PubMed] [Google Scholar]
- 2.Fuji K, Node M, Sai M, Fujita E, Takeda S, Unemi N. Terpenoids. LIII. Antitumor activity of trichorabdals and related compounds. Chem Pharm Bull (Tokyo) 1989;37:1472–1476. doi: 10.1248/cpb.37.1472. [DOI] [PubMed] [Google Scholar]
- 3.Han QB, Li ML, Li SH, Mou SH, Lin ZW, Sun HD. Ent-kaurane diterpenoids from Isodon rubescens var. lushanensis. Chem Pharm Bull (Tokyo) 2003;51:790–793. doi: 10.1248/cpb.51.790. [DOI] [PubMed] [Google Scholar]
- 4.Ikezoe T, Chen SS, Tong XJ, Heber D, Taguchi H, Koeffler HP. Oridonin induces growth inhibition and apoptosis of a variety of human cancer cells. Int J Oncol. 2003;23:1187–1193. [PubMed] [Google Scholar]
- 5.Li CY, Wang EQ, Cheng Y, Bao JK. Oridonin: an active diterpenoid targeting cell cycle arrest, apoptotic and autophagic pathways for cancer therapeutics. Int J Biochem Cell Biol. 2011;43:701–704. doi: 10.1016/j.biocel.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Gao J, Halicka HD, Huang X, Traganos F, Darzynkiewicz Z. The cytostatic and cytotoxic effects of oridonin (Rubescenin), a diterpenoid from Rabdosia rubescens, on tumor cells of different lineage. Int J Oncol. 2005;26:579–588. [PubMed] [Google Scholar]
- 7.Jin HY, Tan XZ, Liu XF, Ding YJ. Downregulation of AP-1 gene expression is an initial event in the oridonin-mediated inhibition of colorectal cancer: studies in vitro and in vivo. J Gastroenterol Hepatol. 2011;26:706–715. doi: 10.1111/j.1440-1746.2010.06500.x. [DOI] [PubMed] [Google Scholar]
- 8.Müller MW, Friess H, Köninger J, et al. Factors influencing survival after bypass procedures in patients with advanced pancreatic adenocarcinomas. Am J Surg. 2008;195:221–228. doi: 10.1016/j.amjsurg.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Wray CJ, Ahmad SA, Matthews JB, Lowy AM. Surgery for pancreatic cancer: recent controversies and current practice. Gastroenterology. 2005;128:1626–1641. doi: 10.1053/j.gastro.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 10.Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3:785–796. doi: 10.1038/nrd1494. [DOI] [PubMed] [Google Scholar]
- 11.Patravale VB, Date AA, Kulkarni RM. Nanosuspensions: a promising drug delivery strategy. J Pharm Pharmacol. 2004;56:827–840. doi: 10.1211/0022357023691. [DOI] [PubMed] [Google Scholar]
- 12.Kesisoglou F, Panmai S, Wu YH. Nanosizing-oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007;59:631–644. doi: 10.1016/j.addr.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Gao L, Zhang D, Chen M, Zheng T, Wang S. Preparation and characterization of an oridonin nanosuspension for solubility and dissolution velocity enhancement. Drug Dev Ind Pharm. 2007;33:1332–1339. doi: 10.1080/03639040701741810. [DOI] [PubMed] [Google Scholar]
- 14.Lou HY, Zhang XM, Gao L, et al. In vitro and in vivo antitumor activity of oridonin nanosuspension. Int J Pharm. 2009;379:181–186. doi: 10.1016/j.ijpharm.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Gao L, Zhang DR, Chen MH, et al. Studies on pharmacokinetics and tissue distribution of oridonin nanosuspension. Int J Pharm. 2008;355:321–327. doi: 10.1016/j.ijpharm.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Storm G, Belliot SO, Daemen T, Lasic DD. Surface medication of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv Drug Deliv Rev. 1995;17:31–48. [Google Scholar]
- 17.Kirkin V, Joos S, Zörnig M. The role of Bcl-2 family members in tumorigenesis. Biochim Biophys Acta. 2004;1644:229–249. doi: 10.1016/j.bbamcr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Yin C, Knudson CM, Korsmeyer SJ, Van Dyke T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature. 1997;385:637–640. doi: 10.1038/385637a0. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson DW, Ali A, Thornberry NA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 20.Denault JB, Salvesen GS. Caspases: keys in the ignition of cell death. Chem Rev. 2002;102:4489–4500. doi: 10.1021/cr010183n. [DOI] [PubMed] [Google Scholar]
- 21.Philchenkov AA. Caspases as regulators of apoptosis and other cell functions. Biochemistry (Mosc) 2003;68:365–376. doi: 10.1023/a:1023635510363. [DOI] [PubMed] [Google Scholar]
- 22.Zou H, Li Y, Liu X, Wang X. An APAF-1·Cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 23.Slee EA, Harte MT, Kluck RM, et al. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liversidge GG, Cundy KC. Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs. Int J Pharm. 1995;125:91–97. [Google Scholar]
- 25.Liversidge GG, Conzentino P. Drug particle size reduction for decreasing gastric irritancy and enhancing absorption of naproxen in rats. Int J Pharm. 1995;125:309–313. [Google Scholar]
- 26.Bloom J, Cross FR. Multiple levels of cyclin specificity in cell-cycle control. Nat Rev Mol Cell Biol. 2007;8:149–160. doi: 10.1038/nrm2105. [DOI] [PubMed] [Google Scholar]
- 27.Han YH, Kim SZ, Kim SH, Park WH. Arsenic trioxide inhibits the growth of Calu-6 cells via inducing a G2 arrest of the cell cycle and apoptosis accompanied with the depletion of GSH. Cancer Lett. 2008;270:40–55. doi: 10.1016/j.canlet.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Y, Qiu F, Ye YC, Tashiro S, Onodera S, Ikejima T. Oridonin induces G2/M arrest and apoptosis via activating ERK-p53 apoptotic pathway and inhibiting PTK-Ras-Raf-JNK survival pathway in murine fibrosarcoma L929 cells. Arch Biochem Biophys. 2009;490:70–75. doi: 10.1016/j.abb.2009.08.011. [DOI] [PubMed] [Google Scholar]