Abstract
The use of activable cell-penetrating peptides (ACPPs) as molecular imaging probes is a promising new approach for the visualization of enzymes. The cell-penetrating function of a polycationic cell-penetrating peptide (CPP) is efficiently blocked by intramolecular electrostatic interactions with a polyanionic peptide. Proteolysis of a proteinase-sensitive substrate present between the CPP and polyanionic peptide affords dissociation of both domains and enables the activated CPP to enter cells. This ACPP strategy could also be used to modify antitumor agents for tumor-targeting therapy. Here, we aimed to develop a conjugate of ACPP with antitumor drug doxorubicin (DOX) sensitive to matrix metalloproteinase-2 and -9 (MMP-2/9) for tumor-targeting therapy purposes. The ACPP-DOX conjugate was successfully synthesized. Enzymatic cleavage of ACPP-DOX conjugate by matrix metalloproteinase (MMP)-2/9 indicated that the activation of ACPP-DOX occurred in an enzyme concentration–dependent manner. Flow cytometry and laser confocal microscope studies revealed that the cellular uptake of ACPP-DOX was enhanced after enzymatic-triggered activation and was higher in HT-1080 cells (overexpressed MMPs) than in MCF-7 cells (under-expressed MMPs). The antiproliferative assay showed that ACPP had little toxicity and that ACPP-DOX effectively inhibited HT-1080 cell proliferation. These experiments revealed that the ACPP-DOX conjugate could be triggered by MMP-2/9, which enabled the activated CPP-DOX to enter cells. ACPP-DOX conjugate may be a potential prodrug delivery system used to carry antitumor drugs for MMP-related tumor therapy.
Keywords: activable cell-penetrating peptide, matrix metalloproteinase, proteinase-sensitive substrate, cellular uptake, antiproliferative, enzymatic cleavage, tumor extracellular environment
Introduction
The use of cell-penetrating peptides (CPPs) has become one of the most popular and efficient techniques for achieving intracellular access. Commonly used CPPs include TAT, polyarginine, penetratin, and transportan. These CPPs have successfully delivered proteins,1 nucleic acids,2 small molecule therapeutics,3 quantum dots,4 and magnetic resonance imaging contrast agents5 into cells. With the increased interest in CPP-mediated cargo delivery, CPPs have been applied in antitumor agent delivery. Doxorubicin (DOX) is a potent anthracycline used for the treatment of various types of cancer. It causes cytotoxicity through topoisomerase II–mediated DNA breaks. Some publications6,7 have reported that CPP-DOX conjugates displayed excellent therapeutic efficacy for related tumor therapy. Efficient induction of apoptosis by CPP-DOX was found in the human breast cancer cell line MDA-MB-231.8,9 In addition, various CPP-drug conjugates have also been developed to improve antitumor efficacy.10,11 However, the majority of known CPPs are not cell or tissue specific; CPPs and their attached therapeutic molecules are dispersed throughout almost all of the body irrespective of the method of administration. This property remains the major drawback for CPP application as drug delivery systems.12–14
The enzyme-sensitive substrate strategy is explorative and utilizes the stimulation of the unique tumor extracellular environment based on the principle of proteolysis. Proteolysis is a simple hydrolytic process that separates two adjacent amino acid residues at the amide bond and is triggered by proteases.15 Enzyme cleavable conjugates, containing enzyme-sensitive substrate, mainly take advantage of the selectivity and specificity of enzymes that are differentially active in the immediate environment of tumor cells.
Activable cell-penetrating peptides (ACPPs) containing polycationic CPPs, enzyme-sensitive substrate, and polyanionic inhibitory domains have been developed by Tsien’s group as molecular imaging probes in tumor detection and intraoperative guidance.16–20 The ACPPs are inactive in the circulation since the cell-penetrating function of CPP is efficiently blocked by intramolecular electrostatic interactions with a polyanionic peptide. Proteolysis of a cleavable enzyme-sensitive substrate present between the CPP and polyanionic peptide by specific enzymes overexpressed on the disease site affords dissociation of both domains and enables the activated CPP to enter cells. This ACPP approach offers ways to address the major issue that CPPs lack cell specificity by means of the selective trigger of proteases in diseased tissue, and can be used for tumor-targeting therapy. It is interesting and meaningful to explore whether ACPP-drug conjugates can be exploited for targeted drug delivery via enhanced targeting ability.
Here, we aimed to develop an ACPP-DOX conjugate sensitive to matrix metalloproteinases (MMPs) for tumor-targeting therapy purposes. MMPs are one type of frequently used cleavable enzyme that are associated with tumor disease. The ACPP-DOX conjugate delivery system involves the polycationic domain (CPP), the cleavable MMP-2/9-sensitive substrate, the polyanionic domain (inhibitory or attenuating sequence), and DOX. The proposed mechanism of intracellular access enhancement is exhibited in Figure 1A. This ACPP-DOX delivery system was temporarily inactive in circulation and in non- or under-expressed MMP tissue and subsequently activated by overexpressed MMP-2/9 in targets where cargo could be released. The design, synthesis, and validation of the selectively triggered characterizations of this ACPP-DOX conjugate by extra MMPs and endogenous MMPs were determined by high-performance liquid chromatography (HPLC), flow cytometry, and confocal microscopy. To examine the effect of ACPP-DOX, DOX, and ACPP on cell growth and viability, the antiproliferative assay was measured on HT-1080 and MCF-7 cells. The novel ACPP-DOX delivery system provides a new choice for MMP-related tumor therapy.
Figure 1.
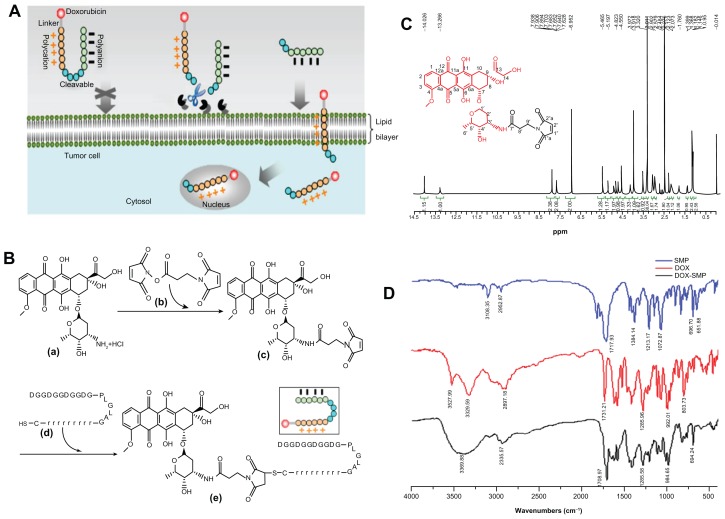
(A) Proposed schematic diagram of ACPP-DOX conjugate for antitumor drug delivery. ACPP-DOX conjugate is inactivated initially and circulates freely in vivo. When the ACPP-DOX conjugate accesses the tumor tissue, it is triggered and activated by overexpressed extracellular MMPs (MMP-2/9). The CPP-DOX releases and translocates into cells. The ACPP-DOX conjugate includes a cargo of doxorubicin, polycational CPP, polyanional attenuating peptides, and MMP-specific substrates. (B) Synthesis scheme of ACPP-DOX conjugates: (a) doxorubicin (DOX), (b) N-succinimidyl-3-maleimidopropionate (SMP), (c) DOX-SMP, (d) ACPP, an activatable cell-penetrating peptide. (e) ACPP-DOX, a derivative of DOX linked with the ACPP. (C) 1H-NMR spectrum of DOX-SMP. (D) FT-IR spectra of SMP, DOX, and DOX-SMP.
Abbreviations: ACPP-DOX, activable cell-penetrating peptide–doxorubicin; CPP, cell-penetrating peptide; MMP, matrix metalloproteinase.
Materials and methods
Materials
DOX (>99%) was kindly donated by Hisun Pharmaceutical Co, Ltd, (Zhejiang, People’s Republic of China). N-succinimidyl 3-maleimidopropionate (SMP; >98%) was purchased from Jiaxing Biomatrix Co, Ltd, (Zhejiang, People’s Republic of China). Trypsin was obtained from Amresco Inc (Solon, OH). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and collagenase IV were purchased from Sigma-Aldrich (St Louis, MO). Penicillin, streptomycin, and Hoechst 33258 were provided by Macgene Co, Ltd, (Beijing, People’s Republic of China), while 4-aminophenylmercuric acetate (APMA) was purchased from Merck Co, Ltd, (Darmstadt, Germany). MMP-2 was procured from PeproTech EC Ltd (London, UK). All other chemicals were of analytic or HPLC grade.
The ACPP (H2 N-DGGDGGDGGDGPLGLAGrrrrrrrrrC-COOH [the uppercase letters represent L-amino acids, while the lowercase letters represent D-amino acids]) was custom synthesized via a standard Fmoc solid-phase peptide synthesis method by Shanghai GL Biochem Co, Ltd, (Shanghai, People’s Republic of China). The purity of ACPP was 97.9%. The correct product was confirmed by electrospray ionization mass spectroscopy (ESI-MS): m/z, 2895.2 (calculated), 2895.57 (observed, [M+3H]3+: 966.19), 2896.18 (observed, [M+2H]2+: 1449.09).
Cell lines
HT-1080 (human fibrosarcoma) cells were purchased from the Cell Culture Centre, Peking Union Medical College ( Beijing, People’s Republic of China). The cells were cultured in Minimum Essential Medium with Earle’s salts, L-glutamine, nonessential amino acid (Macgene, Beijing, People’s Republic of China), 10% fetal bovine serum (FBS), 100 units/mL penicillin, and 100 μg/mL streptomycin.
MCF-7 (human breast adenocarcinoma) cells were obtained from the Institute of Hematology and Blood Diseases Hospital (Tianjin, China). The cells were maintained in Dulbecco’s Modified Eagle’s Medium with glucose, sodium pyruvate, L-glutamine, and 4-(2-hydroxyethyl)-1-piperazineethane-sulfonic acid ( Macgene, Beijing, People’s Republic of China) supplemented with 10% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin.
All the cells were cultured in an incubator maintained at 37°C with 5% CO2 under fully humidified conditions. All experiments were performed on cells in the exponential growth phase.
Synthesis of ACPP-DOX conjugate
ACPP-DOX conjugate was synthesized by coupling ACPP with DOX using a linker of SMP. Thus, DOX-SMP was prepared first. The general synthetic scheme is depicted in Figure 1B. Briefly, DOX (43.5 mg, 1 Eq), SMP (22.0 mg, 1.1 Eq), and triethylamine (TEA; 2 Eq) were dissolved in 8 mL of dimethylformamide (DMF) and stirred at room temperature for 2 hours, while the progress of the reaction was monitored by thin-layer chromatography (TLC; chloroform:methanol: ammonia = 70:30:3). The reaction solution was treated with 50 mL of cold anhydrous diethyl ether and the resulting precipitate was washed twice with 5 mL of anhydrous diethyl ether. The red solid that was obtained was separated from the solvent by centrifugation. The product was dried to give a 64.3% yield in red crystals.
Secondly, the ACPP-DOX was synthesized. DOX-SMP (2.6 mg, 1 Eq), ACPP (10 mg, 1 Eq), TEA (10 μL, 21 Eq), and 1 mL of DMF were mixed and stirred at room temperature for 2 hours, while the progress of the reaction was monitored using TLC and HPLC. The reaction solution was treated with 10 mL of cold anhydrous diethyl ether and the resulting precipitate was washed twice with 5 mL of anhydrous diethyl ether. The resulting red solid was separated from the solvent by centrifugation, and then the product was dried to give a 78.8% yield in red crystals.
Enzymatic-triggered cleavage of ACPP-DOX by MMPs
To analyze the enzymatic cleavage of the matrix metalloproteinase (MMP)-sensitive conjugates, ACPP-DOX cleavage was studied in the presence of MMP-2/9. Collagenase IV (containing MMP-2/9) was activated with the 2.5 mM APMA solution for 1 hour at 37°C. ACPP-DOX stock solution was mixed with activated collagenase IV and incubated at 37°C. The aliquots were removed between 1 hour and 4 hours and analyzed by HPLC. The HPLC system was equipped with a Waters 2487 Dual Wavelength Absorbance Detector and 1525 Pump (Milford, MA), and the wavelength was set at 220 nm. To determine ACPP-DOX conjugate and hydrolysate simultaneously, a gradient was used as the eluent. The flow was 1 mL/min with a mixed mobile phase that included solvent A (water with 0.1% trifluoroaceticacid [TFA]) and solvent B (acetonitrile with 0.1% TFA). The gradient was as follows: 0–15 minutes, from 95% solvent A to 70% solvent A; 15–25 minutes, from 70% solvent A to 100% solvent B. This system was sustained at 100% solvent B for 5 minutes.
Cellular uptake
Based on confirmed reports, MCF-7 cells are known to under-secrete MMP-2/9.21,22 MCF-7 cells were selected as low MMP-secreting tumor cell types to investigate the uptake of activable conjugates after extra MMPs trigger cleavage. HT-1080 cells were used as overexpressed MMP-2/916,23 tumor cell types to study the cellular uptake of activable ACPP-DOX after cell-secreted MMPs trigger cleavage. Innate fluorescence of DOX allowed us to use flow cytometry and confocal microscopy to study penetration and localization of free or conjugated DOX.
Collagenase IV or MMP-2 was activated at 37°C with the 2.5 mM APMA solution. MCF-7 cells were seeded into six-well plates (2 × 105 cells per well) and allowed to grow for 24 hours. These cells were washed with phosphate-buffered saline (PBS; 0.1 M, pH 7.4) and preincubated with different concentrations of activated enzyme solutions for 1 hour. Then the supernatants were removed and discarded, and the cells were incubated with 5 μM of ACPP-DOX dissolved in 1 mL of blank culture for 12 hours. After the incubation, the cells were washed three times with PBS (0.1 M, pH 7.4). A total of 1 mL of 0.25% trypsin solution was added, and the cells were detached from cell culture by incubation for 3 minutes at 37°C. The cell suspensions were centrifuged for 3 minutes and resuspended in 0.5 mL PBS. Single-cell suspensions were prepared by filtration through a 300-mesh filter. Finally, the cells suspended in PBS were subjected to flow cytometry analysis using a FACScan (Becton Dickinson, San Jose, CA). The cells associated with DOX or ACPP-DOX were excited with an argon laser at 488 nm and fluorescence was detected at 588 nm. Files of 10,000 gated events were collected and analyzed with the FACStation software (Cellquest 3.3) program.
MCF-7 and HT-1080 cells were seeded into six-well plates (2 × 105 cells per well) and allowed to grow for 24 hours. The cells were incubated with free DOX or ACPP-DOX at an equal concentration of 10 μM for different periods. After the incubation, the drug solutions were removed and the cells were washed three times with PBS (0.1 M, pH 7.4). Subsequently, the same flow cytometry procedure described above was performed for determining cellular uptake.
Following the culture of MCF-7 and HT-1080 cells for 24 hours on a petri dish, free DOX or ACPP-DOX at a concentration of 5 μM was added to each dish and incubated at 37°C. The medium was removed and discarded, and the cells were washed with PBS (0.1 M, pH 7.4), followed by fixing with 4% paraformaldehyde for 10 minutes. Nuclear staining was performed by Hoechst 33258 for 15 minutes, and the fluorescent images of cells were analyzed using a laser scanning confocal microscope (TCS SP5; Leica Microsystems GmbH, Wetzlar, Germany).
Antiproliferative activity against MCF-7 and HT-1080 cells
The antiproliferative activity of DOX, ACPP-DOX, and ACPP was analyzed by the MTT method.24 MCF-7 and HT-1080 cells were seeded at a density of 2 × 104 cells/well in 96-well plates. After culturing for 24 hours in an incubator (37°C, 5% CO2), 20 μL of various sample solutions in a concentration range of 1 μM to 100 μM were added to each well, and the plates were incubated for 24 hours. Next, 20 μL of MTT solution (5.0 mg/mL) was added to each well, and the plates were incubated for another 4 hours. After that, the MTT medium was replaced by 200 μL of dimethyl sulfoxide (DMSO) in each well, and the mixture was shaken at room temperature to dissolve the reacted dye. The optical density was measured at 570 nm using a microplate reader (Bio-Rad, Hercules, CA). Cell viability = (A−B)/A × 100%, where A was the absorbance of the cells incubated with the culture medium and B was the absorbance of the cells incubated with the DOX, ACPP-DOX, or ACPP, respectively. All samples were evaluated in sextuplicate.
Data analysis
Data were expressed as the mean ± standard deviation, and statistical analysis was performed using an unpaired Student’s t-test. Data were considered significantly different at P < 0.05.
Results and discussion
Design and synthesis of ACPP-DOX
For tumor cells to invade and metastasize, the first barrier to overcome is the basement membrane, which is composed of type IV collagen. Type IV collagen is susceptible to degradation by MMP-2 and MMP-9.25
H2 N-DGGDGGDGGDGPLGLAG-rrrrrrrrrC-COOH was designed as the ACPP. The ACPP includes three units: the cell-penetrating domain (polyarginine, R9), the cleavable enzyme-specific substrate domain of MMP-2/9 (PLGLAG), and the attenuating peptides domain ( DGGDGGDGGDG). Some research indicates that L-polyarginine (R9) was 20-fold more efficient than Tat(49-57) at cellular uptake as determined by Michaelis–Menten kinetic analysis, and the D-polyarginine (R9) exhibited an even greater enhancement of uptake rate (>100-fold) than the R9.26 PLGLAG was considered a remarkably sensitive or cleavable sequence by MMP-2 and MMP-9.19,27 To prevent CPP distribution toward normal cells, polyanional DGGDGGDGGDG is added to the molecule as an attenuating or shielding motif. The C-terminal cysteine is a linker designed to couple easily with the maleimide group. Thus, the ACPP peptide is temporarily inactivated, and subsequent activation is triggered by overexpressed MMPs in tumor targets where the CPP will be released.
DOX-SMP was synthesized by a reaction between the 3′ amino group of the daunosamine sugar of DOX and an active ester group of SMP. TEA was added to remove the hydrochloride salt and also to maintain basic conditions to favorably generate DOX-SMP. DOX-SMP HPLC analysis: 93.5%; yield: 64.3%; matrix-assisted laser desorption ionization orthogonal time of flight mass spectrometry (MALDI-TOF-MS) (m/z, [M+Na]+): 717.6 (calculated), 715.95 (observed). MALDI-TOF-MS (m/z, [M+K]+): 733.6 (calculated), 732.03 (observed). ESI-MS (m/z, [M+Na]+): 717.6 (calculated), 717.1 (observed).
The 1H-NMR spectrum of DOX-SMP in DMSO-D6 (Bruker, 400M; Zurich, Switzerland) is shown in Figure 1C. O-methylation of the 4-position and 14-CH2 in the DOX-SMP molecule were seen at 3.972 ppm and 4.55 ppm (single peak), respectively. The 6-OH and 11-OH of DOX-SMP were also observed at 14.026 ppm and 13.266 ppm, respectively. Chemical shifts between 7.5 and 8.0 ppm were assigned as 1H-3H of the benzene. Furthermore, an obvious single peak at 6.952 ppm was a characteristic shift representing the 1′-H and 2′-H of the maleimide group. The 1H-NMR results indicated that the compound was in agreement with the predicted structure of DOX- SMP. In addition, the Fourier transform infrared (FTIR) spectrum of DOX-SMP (Figure 1D) showed that a single peak at 3369.88 cm−1 was attributed to a hydrogen skeletal vibration of a secondary amide group. The appearance of a secondary amide showed that DOX and SMP were coupled by an amide linkage. All of the above characteristics demonstrated that DOX-SMP was synthesized successfully.
The C-terminal amino acid of the ACPP peptide is a cysteine, which provides a chemically reactive thiol group for drug conjugation. DOX-SMP can be covalently attached to the cysteine sulfur of ACPP with maleimide by way of a Michael addition (nucleophilic addition) to produce ACPP-DOX. HPLC analysis: 95.3%; yield: 78.8%; MALDI- TOF-MS (m/z, [M-H]−): 3588.8 (calculated), 3587.26 (observed). Thus, according to the well-designed synthetic route and proven by the MALDI-TOF-MS result, a derivative of DOX linked with ACPP (ACPP-DOX) was obtained successfully.
Enzymatic-triggered cleavage of ACPP-DOX by MMPs
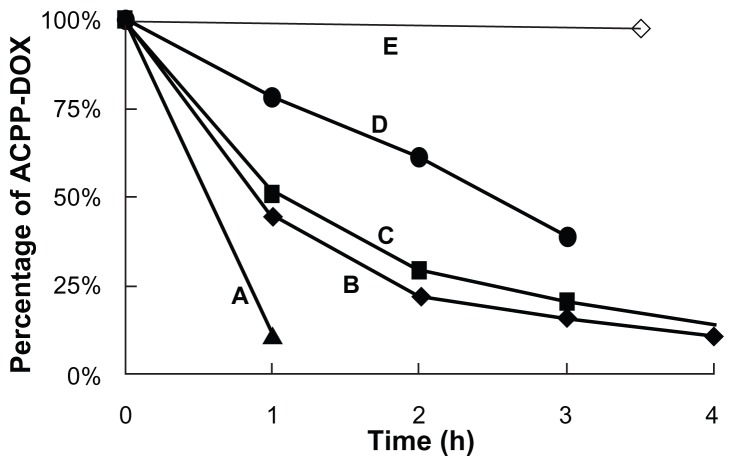
ACPP-DOX incorporates a MMP-2/9-sensitive substrate; subsequently, the triggered cleavage study was performed through an extra enzymatic digestion experiment on different [MMPs]/[ACPP-DOX] ratios for 4 hours. As MMP concentration decreased, ACPP-DOX cleavage increased proportionally (Figure 2). The half-lives of ACPP-DOX cleavage were approximately 0.6 hour, 0.9 hour, 1.1 hours, and 2.5 hours with a series of (MMPs)/(ACPP-DOX) ratios of 0.463:1, 0.288:1, 0.173:1, and 0.114:1, respectively. In contrast, almost no cleavage was observed for ACPP-DOX in the absence of MMPs after 3.5 hours of incubation. The experiment results proved that MMP-2/9 can cleave the ACPP and the scissor rate of ACPP-DOX was related to the concentration of MMP-2/9. MMP concentration was obviously related to the degree of cleavage; more cleavage was also found in MMP-activated imaging nanoparticles with the increase of extra MMPs.28 In our previous experiments, the hydrolysis fragments of ACPP have been detected by MALDI-TOF-MS and it was proved that the ACPP could be cleaved by MMP-2/9 at target site.29
Figure 2.
Enzymatic-triggered cleavage of ACPP-DOX conjugate by MMPs in different ratios.
Note: (MMPs)/(ACPP-DOX) (w/w) of (A) 0.463:1 (B) 0.288:1 (C) 0.173:1 (D) 0.114:1, and (E) absence of MMP treatment.
Abbreviations: ACPP-DOX, activable cell-penetrating peptide–doxorubicin; MMP, matrix metalloproteinase.
Enhancing cellular uptake after enzymatic-triggered cleavage of ACPP-DOX
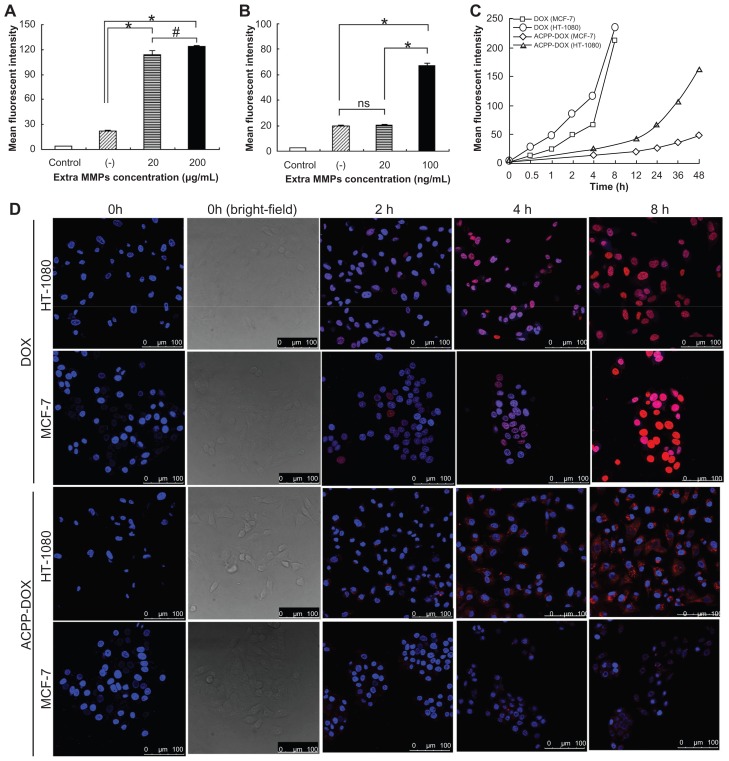
When ACPP-DOX is scissored and activated to CPP-DOX by MMP-2/9, cellular uptake of CPP-DOX is expected to be enhanced due to the effect of CPP penetration. MCF-7 cells were used as a model system of under-expressed MMP-2/9 to study the effect of extra MMP-triggered cleavage of ACPP-DOX on cellular uptake. As shown in Figure 3A, MCF-7 cell–associated fluorescence was significantly higher (5.1- or 5.6-fold) in either group after ACPP-DOX activation by MMP-2/9 than that of the group with absence of collagenase (P < 0.001). It was obvious that the increase in MMP-2/9 concentration from 20 to 200 μg/mL resulted in stronger uptake of DOX (P < 0.05). MMP-2 pretreatment was performed in MCF-7 cells (Figure 3B). ACPP-DOX showed an approximately 3.4-fold higher uptake when the concentration of MMP-2 was 100 ng/mL compared with the group without MMP-2 (P < 0.001). In contrast, 20 ng/mL of MMP-2 treatment had a lower uptake of ACPP-DOX compared to the group treated with 100 ng/mL of MMP-2 (P < 0.001).
Figure 3.
(A) The uptake intensity of MCF-7 cells after treatment with ACPP-DOX conjugate in the absence (−) or presence of extra collagenase IV at concentrations of 20 μg/mL and 100 μg/mL. (B) The uptake intensity of MCF-7 cells after treatment with ACPP-DOX conjugate in the absence (−) or presence of extra MMP-2 at concentrations of 20 ng/mL and 200 ng/mL. (C) Cellular uptake intensity–time curve of DOX and ACPP-DOX in MCF-7 and HT-1080 cells at different incubation times. (D) Confocal microscopy images of ACPP-DOX or DOX incubated with HT-1080 and MCF-7 cells at 37°C for 0 hour–8 hours.
Notes: Red represents fluorescence of DOX. Blue represents fluorescence of Hoechst 33258 for nuclei staining. Scale bars: 100 μm. *P < 0.001; #P < 0.05; ns = no significance.
Abbreviations: DOX, doxorubicin; ACPP-DOX, activable cell-penetrating peptide–doxorubicin; MMP-2, matrix metalloproteinase-2.
HT-1080 cells were used as a model system of overexpressed MMP-2/9 to study the effect of triggered cleavage of ACPP-DOX on cellular uptake. As shown in Figure 3C, fluorescence intensity of free DOX in both MCF-7 and HT-1080 cells was similar after 8 hours of incubation. Cellular uptake properties of free DOX were in accordance with the reported results and this might be responsible for transportation into cells by the passive diffusion mechanism. 30,31 Compared with free DOX, ACPP-DOX displayed lower uptake into both cells. However, it was noteworthy that the uptake intensity of ACPP-DOX was significantly higher in HT-1080 cells than in MCF-7 cells after 24 hours of incubation. After 48 hours of incubation, the uptake intensity of ACPP-DOX in HT-1080 cells was 3.3-fold higher than that in MCF-7 cells. This implied that ACPP-DOX was more sensitive to HT-1080 than to MCF-7 cells, and the sensitivity corresponded with the HT-1080 cell over-secreted MMPs.
The intracellular trafficking of DOX and ACPP-DOX was studied in HT-1080 and MCF-7 cells using confocal laser scanning microscopy. Different incubation times were employed from 2 to 8 hours (Figure 3D). The uptake was weak in both HT-1080 and MCF-7 cells treated with ACPP- DOX for 2 hours. The fluorescence emerged at 4 hours and gradually increased with longer incubation. The fluorescence intensity was significantly stronger in HT-1080 cells than in MCF-7 cells over time, which meant that more DOX entered HT-1080 cells. For DOX, its fluorescence was clearly observed in HT-1080 and MCF-7 cells after incubation for only 2 hours, a longer incubation time (4 or 8 hours) resulted in stronger fluorescence.
It was noteworthy that the degree of uptake enhancement was obviously related to the extra MMP-2/9 trigger on MCF-7 cells and the endogenous MMP trigger on HT-1080 cells. In addition, the higher uptake of nanostructured lipid carriers incorporating ACPP on HT-1080 cells was inhibited in the presence of GM6001 (Enzo Life Sciences, Inc, Farmingdale, NY), a known MMP inhibitor (data not shown). These phenomena were in accordance with extra enzymatic cleavage analysis as determined by HPLC ( Figure 2) and suggested that the cleavage was specifically by MMPs.16,32–34 Enhanced cellular uptake is important after triggered activation especially for some poor membrane-penetrating candidates.
Antiproliferative activity
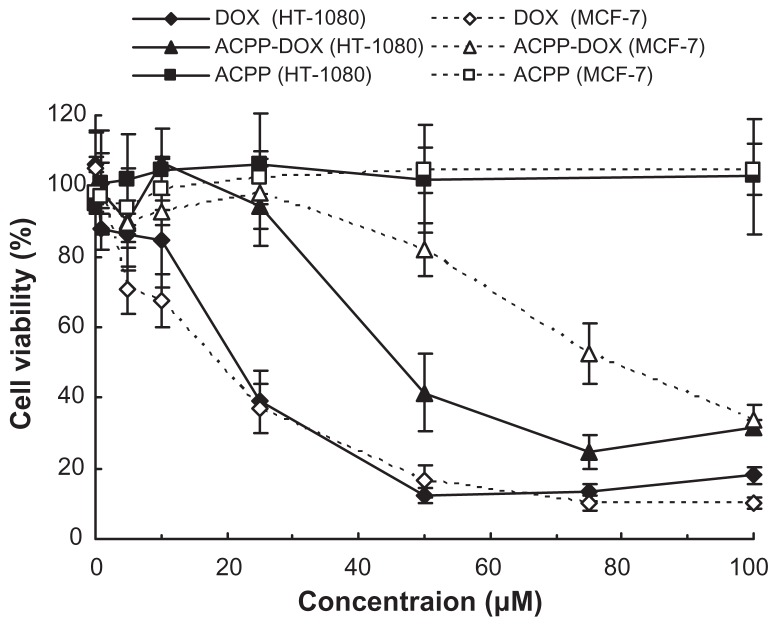
To examine the effect of ACPP-DOX, DOX, and ACPP on cell growth and viability, the potential antiproliferative activities were measured by the MTT method on HT-1080 and MCF-7 cells. For parallel comparison, cells were incubated with ACPP-DOX, DOX, and ACPP at a series of equivalent concentrations from 1 to 100 μM. As shown in Figure 4, the ACPP did not show any obvious antiproliferative activities in HT-1080 and MCF-7 cells after 24 hours of incubation, which revealed that the ACPP was relatively safe. With increased concentration of DOX, the antiproliferative activities of free DOX increased significantly on both MCF-7 and HT-1080 cells, but the ACPP-DOX conjugate displayed stronger antiproliferative activities on HT-1080 than on MCF-7 cells. Namely, the ACPP-DOX conjugate was more effective against HT-1080 than MCF-7 cells correlating to the level of MMPs, despite the fact that DOX had almost equal cytotoxicity both on HT-1080 and MCF-7 cells with no correlation to the proteases.
Figure 4.
Antiproliferative activity of DOX, ACPP-DOX, and ACPP against MCF-7 and HT-1080 cells.
Notes: Antiproliferative activity was determined using the MTT method after incubation for 24 hours in vitro. Data are represented as the mean ± standard deviation (n = 6).
Abbreviations: DOX, doxorubicin; ACPP-DOX, activable cell-penetrating peptide–doxorubicin; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
The increased cytotoxicity of ACPP-DOX conjugate toward HT-1080 cells was probably due to released CPP- DOX and enhanced cell uptake by the activation of overexpressed MMP-2/9 in this cell type. Our previous cell-uptake studies of CPP-cargos have demonstrated that polyarginine peptides as well as CPP-avidin can easily enter cells and tissues.12 Abundant research proves that CPPs could transport cargos into cells.1–5,35–37 Numerous small molecule chemotherapeutics, such as taxol,38 methotrexate,10 and DOX8,11 have shown improved activity when conjugated to a CPP. Remarkably, even though a CPP-drug conjugate may show less activity in a purified biochemical system, highly efficient cellular uptake can overcome this shortfall, because CPP-drug conjugates can effectively increase the intracellular concentrations of drug molecules and this can counteract decreases in drug activity that result from conjugation.10,14 These results indicate that ACPP-drug conjugate delivery strategy provides potential for tumor-targeted delivery of antitumor therapeutics by triggered activation.
Conclusion
The ACPP approach could potentially address the main issue that CPPs lack cell specificity by means of the selective trigger of proteases at the tumor targets. To our knowledge, reported ACPPs were mostly designed as imaging agents. We synthesized ACPP-DOX conjugate in good purity and yields. The proteolytic activation of the ACPP-DOX conjugate occurs in an enzyme concentration–dependent manner. The enhanced cellular uptake and antiproliferative activity of ACPP-DOX conjugate was observed after MMP-senstive activation and revealed that ACPP-DOX conjugate has targeting ability for tumor cells rich in MMP-2/9. The ACPP-drug conjugate delivery system triggered by protease is a promising approach for the treatment of some tumors with overexpression of MMP-2 or MMP-9.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30772665 and 30970785), National Basic Research Program of China (2007CB935801 and 2009CB930300), and the Doctoral Foundation of the Ministry of Education of China (20100001110056).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Fawell S, Seery J, Daikh Y, et al. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci U S A. 1994;91(2):664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiu YL, Ali A, Chu CY, Cao H, Rana TM. Visualizing a correlation between siRNA localization, cellular uptake, and RNAi in living cells. Chem Biol. 2004;11(8):1165–1175. doi: 10.1016/j.chembiol.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Rothbard JB, Garlington S, Lin Q, et al. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat Med. 2000;6(11):1253–1257. doi: 10.1038/81359. [DOI] [PubMed] [Google Scholar]
- 4.Santra S, Yang H, Stanley JT, et al. Rapid and effective labeling of brain tissue using TAT-conjugated CdS:Mn/ZnS quantum dots. Chem Commun (Camb) 2005;7(25):3144–3146. doi: 10.1039/b503234b. [DOI] [PubMed] [Google Scholar]
- 5.Lewin M, Carlesso N, Tung CH, et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000;18(4):410–414. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 6.Mazel M, Clair P, Rousselle C, et al. Doxorubicin-peptide conjugates overcome multidrug resistance. Anticancer Drugs. 2001;12(2):107–116. doi: 10.1097/00001813-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Liang JF, Yang VC. Synthesis of doxorubicin-peptide conjugate with multidrug resistant tumor cell killing activity. Bioorg Med Chem Lett. 2005;15(22):5071–5075. doi: 10.1016/j.bmcl.2005.07.087. [DOI] [PubMed] [Google Scholar]
- 8.Aroui S, Brahim S, De Waard M, Bréard J, Kenani A. Efficient induction of apoptosis by doxorubicin coupled to cell-penetrating peptides compared to unconjugated doxorubicin in the human breast cancer cell line MDA-MB 231. Cancer Lett. 2009;285(1):28–38. doi: 10.1016/j.canlet.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 9.Aroui S, Brahim S, Hamelin J, De Waard M, Bréard J, Kenani A. Conjugation of doxorubicin to cell penetrating peptides sensitizes human breast MDA-MB 231 cancer cells to endogenous TRAIL-induced apoptosis. Apoptosis. 2009;14(11):1352–1365. doi: 10.1007/s10495-009-0397-8. [DOI] [PubMed] [Google Scholar]
- 10.Lindgren M, Rosenthal-Aizman K, Saar K, et al. Overcoming methotrexate resistance in breast cancer tumour cells by the use of a new cell-penetrating peptide. Biochem Pharmacol. 2006;71(4):416–425. doi: 10.1016/j.bcp.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 11.Aroui S, Brahim S, Waard MD, Kenani A. Cytotoxicity, intracellular distribution and uptake of doxorubicin and doxorubicin coupled to cell-penetrating peptides in different cell lines: a comparative study. Biochem Biophys Res Commun. 2010;391(1):419–425. doi: 10.1016/j.bbrc.2009.11.073. [DOI] [PubMed] [Google Scholar]
- 12.Ma DX, Shi NQ, Qi XR. Distinct transduction modes of arginine-rich cell-penetrating peptides for cargo delivery into tumor cells. Int J Pharm. 2011;419(1–2):200–208. doi: 10.1016/j.ijpharm.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Vivès E, Schmidt J, Pèlegrin A. Cell-penetrating and cell-targeting peptides in drug delivery. Biochim Biophys Acta. 2008;1786(2):126–138. doi: 10.1016/j.bbcan.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Fonseca SB, Pereira MP, Kelley SO. Recent advances in the use of cell-penetrating peptides for medical and biological applications. Adv Drug Deliv Rev. 2009;61(11):953–964. doi: 10.1016/j.addr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Law B, Tung CH. Proteolysis: a biological process adapted in drug delivery, therapy, and imaging. Bioconjug Chem. 2009;20(9):1683–1695. doi: 10.1021/bc800500a. [DOI] [PubMed] [Google Scholar]
- 16.Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc Natl Acad Sci U S A. 2004;101(51):17867–17872. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aguilera TA, Olson ES, Timmers MM, Jiang T, Tsien RY. Systemic in vivo distribution of activatable cell penetrating peptides is superior to that of cell penetrating peptides. Integr Biol (Camb) 2009;1(5–6):371–381. doi: 10.1039/b904878b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson ES, Aguilera TA, Jiang T, et al. In vivo characterization of activatable cell penetrating peptides for targeting protease activity in cancer. Integr Biol (Camb) 2009;1(5–6):382–393. doi: 10.1039/b904890a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson ES, Jiang T, Aguilera TA, et al. Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases. Proc Natl Acad Sci U S A. 2010;107(9):4311–4316. doi: 10.1073/pnas.0910283107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen QT, Olson ES, Aguilera TA, et al. Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc Natl Acad Sci U S A. 2010;107(9):4317–4322. doi: 10.1073/pnas.0910261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giambernardi TA, Grant GM, Taylor GP, et al. Overview of matrix metalloproteinase expression in cultured human cells. Matrix Biol. 1998;16(8):483–496. doi: 10.1016/s0945-053x(98)90019-1. [DOI] [PubMed] [Google Scholar]
- 22.Das S, Banerji A, Frei E, Chatterjee A. Rapid expression and activation of MMP-2 and MMP-9 upon exposure of human breast cancer cells (MCF-7) to fibronectin in serum free medium. Life Sci. 2008;82(9–10):467–476. doi: 10.1016/j.lfs.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Albright CF, Graciani N, Han W, et al. Matrix metalloproteinase-activated doxorubicin prodrugs inhibit HT1080 xenograft growth better than doxorubicin with less toxicity. Mol Cancer Ther. 2005;4(5):751–760. doi: 10.1158/1535-7163.MCT-05-0006. [DOI] [PubMed] [Google Scholar]
- 24.Tian Q, Zhang CN, Wang XH, et al. Glycyrrhetinic acid-modified chitosan/poly(ethylene glycol) nanoparticles for liver-targeted delivery. Biomaterials. 2010;31(17):4748–4756. doi: 10.1016/j.biomaterials.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 25.Murphy G, Nagase H. Mol Aspects Med. 5. Vol. 29. Progress; 2008. in matrix metalloproteinase research; pp. 290–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc Natl Acad Sci U S A. 2000;97(24):13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kline T, Torgov MY, Mendelsohn BA, Cerveny CG, Senter PD. Novel antitumor prodrugs designed for activation by matrix metalloproteinases- 2 and -9. Mol Pharm. 2004;1(1):9–22. doi: 10.1021/mp0340183. [DOI] [PubMed] [Google Scholar]
- 28.Smith R, Sewell SL, Giorgio TD. Proximity-activated nanoparticles: in vitro performance of specific structural modification by enzymatic cleavage. Int J Nanomedicine. 2008;3(1):95–103. doi: 10.2147/ijn.s2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang PC, Qi XR. The design and enzymatic hydrolysis of activatable cell-penetrating peptide. Yao Xue Xue Bao. 2010;45(8):1048–1051. [PubMed] [Google Scholar]
- 30.Sun Y, Yan X, Yuan T, et al. Disassemblable micelles based on reduction-degradable amphiphilic graft copolymers for intracellular delivery of doxorubicin. Biomaterials. 2010;31(27):7124–7131. doi: 10.1016/j.biomaterials.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Li YL, Zhu L, Liu Z, et al. Reversibly stabilized multifunctional dextran nanoparticles efficiently deliver doxorubicin into the nuclei of cancer cells. Angew Chem Int Ed Engl. 2009;48(52):9914–9918. doi: 10.1002/anie.200904260. [DOI] [PubMed] [Google Scholar]
- 32.Terada T, Iwai M, Kawakami S, Yamashita F, Hashida M. Novel PEG-matrix metalloproteinase-2 cleavable peptide-lipid containing galactosylated liposomes for hepatocellular carcinoma-selective targeting. J Control Release. 2006;111(3):333–342. doi: 10.1016/j.jconrel.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 33.Neumann U, Kubota H, Frei K, Ganu V, Leppert D. Characterization of Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2, a fluorogenic substrate with increased specificity constants for collagenases and tumor necrosis factor converting enzyme. Anal Biochem. 2004;328(2):166–173. doi: 10.1016/j.ab.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 34.Liu S, Netzel-Arnett S, Birkedal-Hansen H, Leppla SH. Tumor cell-selective cytotoxicity of matrix metalloproteinase-activated anthrax toxin. Cancer Res. 2000;60(21):6061–6067. [PubMed] [Google Scholar]
- 35.Bullok KE, Dyszlewski M, Prior JL, Pica CM, Sharma V, Piwnica-Worms D. Characterization of novel histidine-tagged Tat-peptide complexes dual-labeled with (99 m)Tc-tricarbonyl and fluorescein for scintigraphy and fluorescence microscopy. Bioconjug Chem. 2002;13(6):1226–1237. doi: 10.1021/bc025573a. [DOI] [PubMed] [Google Scholar]
- 36.Robbins PB, Oliver SF, Sheu SM, Goodnough JB, Wender P, Khavari PA. Peptide delivery to tissues via reversibly linked protein transduction sequences. Biotechniques. 2002;33(1):190–192. 194. doi: 10.2144/02331dd02. [DOI] [PubMed] [Google Scholar]
- 37.Torchilin VP, Levchenko TS. TAT-liposomes: a novel intracellular drug carrier. Curr Protein Pept Sci. 2003;4(2):133–140. doi: 10.2174/1389203033487298. [DOI] [PubMed] [Google Scholar]
- 38.Dubikovskaya EA, Thorne SH, Pillow TH, Contag CH, Wender PA. Overcoming multidrug resistance of small-molecule therapeutics through conjugation with releasable octaarginine transporters. Proc Natl Acad Sci U S A. 2008;105(34):12128–12133. doi: 10.1073/pnas.0805374105. [DOI] [PMC free article] [PubMed] [Google Scholar]