Abstract
Introduction
Tendinitis affects a substantial number of people in several occupations involving repetitive work or direct trauma. Iontophoresis is a therapeutic alternative used in the treatment of injury during the inflammatory phase. In recent years, gold nanoparticles (GNP) have been studied due to their therapeutic anti-inflammatory capacity and as an alternative to the transport of several proteins.
Purpose
This study evaluates the therapeutic effects of iontophoresis using GNPs and diclofenac diethylammonium on inflammatory parameters in rats challenged with traumatic tendinitis.
Methods
Wistar rats were divided in three treatment groups (n = 15): (1) iontophoresis + diclofenac diethylammonium; (2) iontophoresis + GNP; and (3) iontophoresis + diclofenac diethylammonium + GNP. External control was formed by challenged tendons without treatment (n = 15). Iontophoresis was administered using 0.3 mA direct current on 1.5 cm2 electrodes.
Results
The levels of both inflammatory cytokines were significantly higher in untreated challenged rats, when compared with the control (5.398 ± 234 for interleukin 1 beta and 6.411 ± 432 for tumor necrosis factor alpha), which confirms the occurrence of an inflammatory stage in injury (P < 0.05). A significant decrease was observed in expression of cytokines interleukin 1 beta in the three treatment groups, in comparison with untreated challenged tendons, although, in the group treated with diclofenac and GNP, results were similar to the control (1.732 ± 239) (P < 0.05). Concerning tumor necrosis factor alpha, only the group treated with the association diclofenac and GNPs presented decreased levels, compared with the control (3.221 ± 369) (P < 0.05).
Conclusion
The results show the efficacy of drug administration using direct current to treat tendinitis in an animal model, and the potential anti-inflammatory, carrier, and enhancing effects of GNPs in iontophoresis.
Keywords: tendinous injury, proinflammatory cytokines, electrophoresis, iontophoresis, nanoparticles
Introduction
Tendons are specialized tissues that connect muscles to bones and transmit the motion generated by the former to the latter, producing joint movements. Apart from this, tendons are living tissues that respond to mechanical effort as changes in metabolism, as well as in structural and mechanical properties.1 Tendinitis is a painful condition that takes place as a response to repetitive use or direct trauma. A short inflammation phase has been proved to take place in tendons challenged with overuse. In chronic cases, that is, in painful conditions brought about by tendon overuse, the therapeutic approaches commonly adopted with a view to modulating this inflammatory response have enjoyed limited success. More recently, the term “tendinopathy” has been increasingly used to describe the variety of painful conditions resulting from overuse.2 Tendinopathy has not been studied in depth and, although a variety of medicinal drugs have been developed to treat the condition, there is little evidence to support the use of these substances with that end. One of the reasons behind the scarcity of effective treatments of tendinopathy lies in the meager body of knowledge gained in terms of its pathogenesis.3
Two proinflammatory cytokines expressed in tendinopathies are interleukin 1 beta (IL-1β) and tumor necrosis factor alpha (TNF-α). TNF-α is the prototypical inflammatory cytokine that initiates the signaling cascade of other cytokines and trophic factors. It may be released by different cell types and it exerts its effects by interacting with the respective receptor.3 A recent study demonstrated that TNF-α is distributed and expressed in tendinocytes in an inflammatory scenario, and that these cells undergo high apoptosis and proliferation rates in tendon inflammation. Both TNF-α and IL-1β act directly in the ascending pain pathways in an inflammatory process, being able to increase expression of nerve growth factor, cyclooxygenase 2, and nitric oxide synthase, apart from stimulating synaptic vesicle budding of the dorsal root ganglion, switching synaptic plasticity to the facilitation mode. IL-1β is possibly involved in the inhibition of the ability of glial cells to remove glutamate molecules from the synaptic cleft, adding to neuronal excitability of tumor necrosis factor receptor 1.4 A significant increase in the expression of these inflammatory cytokines in tendinitis leads to fibroplast proliferation and therefore to tendon degeneration.5
Iontophoresis has been studied for over a century due to its ability to release medicinal drugs through the transcutaneous pathway based on low-frequency electrical currents.5,6 In spite of increasing skin permeability, iontophoresis promotes the transport of electrical charges through the corneous extract. Strongly charged drugs are transported via electrophoresis, while weakly charged or electrically neutral drugs may be transported by the electroosmotic flux of water generated by the preferential movement of mobile cations, like Na+, for instance, instead of the fixed anions in the corneous extract.7
The drugs commonly administered through the transcutaneous pathway are released though a variety of mechanisms, with specific markers. In rehabilitation approaches, however, drug release markers through the transcutaneous pathway are basically local; the three most common families of drugs used are: (1) anesthetics; (2) anti-irritation agents; and (3) anti-inflammatory drugs, like steroidal or nonsteroidal drugs.8
According to Santi and Singh,9 two mechanisms are responsible for the physiological effect of iontophoresis: “ionophoresis,” where ions are repelled by an electrode with identical charge, and “electroosmosis,” defined as the local movement of fluids through skin pores. A few controlled studies have appropriately addressed the therapeutic use of iontophoresis. Nino et al10 and Grewal et al11 reported the possibility that large molecules are transported by iontophoresis. Molecular structure is the key element in iontophoretic transport. Apart from this, the sequence of chemical groups in the molecular structure is also important.
In recent years, nanomaterials, whose dimensions lie in the 1–100 nm range, have been used in the development of unique devices that afford new functional properties from the chemical and physical standpoint. The potential of nanoparticles concerning chromatographic and electrophoretic techniques has been investigated in more detail of late.12 Nanotransporters represent a new platform for the transport of therapeutic agents to specific targets. More specifically, gold nanoparticles (GNPs) have become the object of special interest due to their optical, electronic, redox, and catalytic properties,12 and are today seen as an attractive alternative concerning the transport of several proteins and their respective markers, small drug molecules, large biomolecules, DNA, or RNA. Today it is known that the efficient release of these therapeutic agents is a condition for therapy success.13
Apart from being inert and nontoxic, GNPs are easy to prepare and stable in solution in the long term.14 Also, GNPs may be prepared in varied sizes and shapes.13 GNPs are able to release large molecules, not only small-molecule medicinal drugs. Tsai et al15 evaluated the intra-articular release of GNPs in induced arthritis in a rat model and observed that the technique inhibited cell proliferation and migration, reducing histological scores, microcapillary density, macrophage density, and TNF-α and IL-1β levels. Another study assessed the anti-inflammatory effects of GNPs between 20 and 45 nm in diameter on focal brain injury. The results demonstrated that treatment reduced brain levels of TNF-α, oxidative stress to DNA, and postapoptosis markers.16 Also, Turkevich et al17 investigated the antiangiogenic properties of GNPs using an ovary tumor model, observing that nanogold selectively inhibited induced expression of human vascular endothelial growth factor in human umbilical vein endothelial cells and the induced proliferation of the basic fibroblast growth factor, respectively.
In light of these anti-inflammatory properties of GNPs, as well as their ability to bind to reactive molecules in drug delivery systems, the present study investigates the potential applications of GNPs as a drug delivery technique to shed more light on the inflammatory mechanism behind tendinopathy. The results may be useful in the development of new treatment approaches using GNPs, iontophoresis, and medicinal drugs. The present study tests the hypothesis that GNP used in iontophoresis increases drug potency, promoting the ideal anti-inflammatory therapeutic effect in Achilles tendinitis challenge in rats.
Materials and methods
Preparation of GNPs
GNPs were prepared as described by Turkevich et al.17 All chemicals used were of the highest analytical grade and purchased from Sigma Aldrich (St Louis, MO). All glass vials were washed in aqua regia and rinsed in ultrapure water, after which 50 mL aurochloric acid 2 mM were gently warmed up to 90°C, whereupon 5 mL sodium citrate 39 mM were added. Then, the reaction medium was refluxed under intense shaking for 20 minutes, left to cool at room temperature before being centrifuged at 7000 rpm for 30 minutes. After centrifugation, the GNPs present in the supernatant were collected.
Animals
In total, 60 male Wistar rats (250 and 300 g) were used. Animals were obtained from the animal center maintained by the authors’ university (Universidade do Extremo Sul Catarinense, Criciúma, Santa Catarina, Brazil) and transported in cages containing five animals each to the laboratory. Animals were kept under 12-hour dark:light cycles and fed on commercial food, with water ad libitum. Next, rats were randomly divided into three treatment groups of injury challenged tendons (n = 15): (1) iontophoresis + diclofenac diethylammonium, (2) iontophoresis + GNP gel, and (3) with iontophoresis + diclofenac diethylammonium + GNP gel. An external control formed by challenged tendons without treatment was included (n = 15). For intragroup comparison, the uninjured contralateral tendons of the respective treatment group were used as internal controls. All procedures involving animals were carried out in accordance with an experimental protocol approved by the Ethics Commission for Animal Use, UNESC, following the Principles of Animal Care.18
Tendinous injury model
The injury model was adapted for tendinous injury based on Rizzi et al19 for muscle injury. An intraperitoneal injection of ketamine purchased from Bayer (São Paulo, Brazil) and xylazine obtained from Fort Dodge (Fort Dodge, IA) (80 and 20 mg/kg, respectively) was used to anesthetize rats. Next, rats were challenged with one single impact by direct trauma on Achilles tendon using an injury press, EQ-179, developed by the Industrial Center of Teaching and Research Equipment (CIDEPE, Rio Grande do Sul, Brazil). The manufacturer’s instructions state that the energy required to produce tendinous injury is 0.540 J. Therefore, three 0.05 kg metal blocks (0.150 kg in total) were left to fall together, sliding along a 12 cm vertical ruler. This impact generated kinetic energy of 0.540 J, meeting the manufacturer’s specifications.
Treatment
Prior to iontophoresis, skin impedance was determined by trichotomy in the injury site. Using a previously calibrated iontophoresis device (Endophasys D; KLD Equipment, São Paulo, Brazil), animals were submitted to daily 12-minute iontophoresis sessions of direct electrotherapy for 5 days. According to Agne,20 the transmitted electrical current is measured by multiplying the active electrode area by the constant 0.15. The electrodes used measured 1.5 cm2 and therefore the electrical current applied was 0.3 mA. Iontophoresis started immediately after injury challenge and sessions were always conducted at the same time. The negative electrode was loaded with diclofenac diethylammonium gel (11.6 mg/g) and GNP gel as the transmission medium and placed on the injury site, while the positive electrode was loaded with basic conductive gel and placed on the gastrocnemius myotendinous joint.
Sample preparation
Animals were sacrificed by decapitation 2 hours after the last session. The Achilles tendons were surgically removed and immediately homogenized in an extraction buffer (1% Triton-X 100, 100 mM Tris, pH 7.4, containing 100 mM sodium pyrophosphate, 100 mM sodium fluoride, 10 mM ethylenediaminetetraacetic acid, 10 mM sodium vanadate, 2 mM phenylmethanesulfonylfluoride, and 0.1 mg/mL aprotinin) at 4°C with a Polytron® PT-MR 2100 (Kinematica AG, Lucerne, Switzerland) and stored at −70°C for analysis. All chemicals were purchased from Sigma Aldrich (St Louis, MO).
A preliminary assay defined the suitable number of tendons for molecular analysis, revealing that a pool of three tendons was required to form a representative sample. The initial population was formed by 60 rats, and since three iontophoresis protocols were tested, each experimental group used 15 rats (plus one external control of identical number of animals). Therefore, the net number of tendons for each molecular analysis sample was five. Aliquots of samples thus prepared were retrieved, centrifuged at 1000 g for 10 minutes at 4°C, and kept at −70°C until use. The contralateral tendon of one rat, subjected to the same treatment techniques but not challenged with injury, was used as internal control.
Protein analysis by immunoblotting
The extracts obtained, as described above, were centrifuged at 11,000 rpm and 4°C in an Eppendorf Centrifuge 5804R (Eppendorf AG, Hamburg, Germany) for 40 minutes to remove insoluble material, and the supernatants of this tissue were used for protein quantification, according to the Bradford method.21 Proteins were denaturated by boiling in Laemmli sample buffer22 containing 100 mM dithiothreitol, run on 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes. Membranes were blocked, probed, and developed. Antibodies used for immunoblotting were anti-TNF-α and anti-IL-1β (Santa Cruz Biotechnology, Santa Cruz, CA). Chemiluminescent detection was performed with horseradish peroxidase-conjugate secondary antibodies (Thermo Scientific, Rockford, IL). Autoradiographs of membranes were taken for visualization of protein bands. The results of blots are presented as direct comparisons of bands in “photographic films” and quantified by densitometry using the Scion Image software (v 4.0.3.2; Scion Corporation, Frederick, MD).
Statistical analysis
Data are expressed as mean and standard error of the mean. The data were analyzed using SPSS statistical software (v 17.0; IBM Corp, Armonk, NY) based on a one-way analysis of variance (ANOVA) followed by a post-hoc Tukey test to compare the study groups, with significance level of 95% (P < 0.05).
Results
Results presented are part of the findings of a more comprehensive study that analyzes the two inflammatory cytokines in the inflammatory stage of the injury (IL-1β and TNF-α). In these results, the expression of these cytokines was analyzed in iontophoresis as a treatment approach (in the present paper), and the therapeutic pulsed ultrasound (TPU) associated with diclofenac and GNPs. The results of TPU are the subject of another publication.
Characterization of GNPs
The electronic spectrum of the GNP solution presented one plasmon band at 520 nm, which agrees with a value previously described.23 Field emission gun microscopy analysis revealed that GNPs presented a spheroid shape with mean diameter of 35 mm. The newly prepared nanoparticle solution was centrifuged and the supernatant was collected and added to a gel base commonly used in physiotherapy (1:1 w/w).
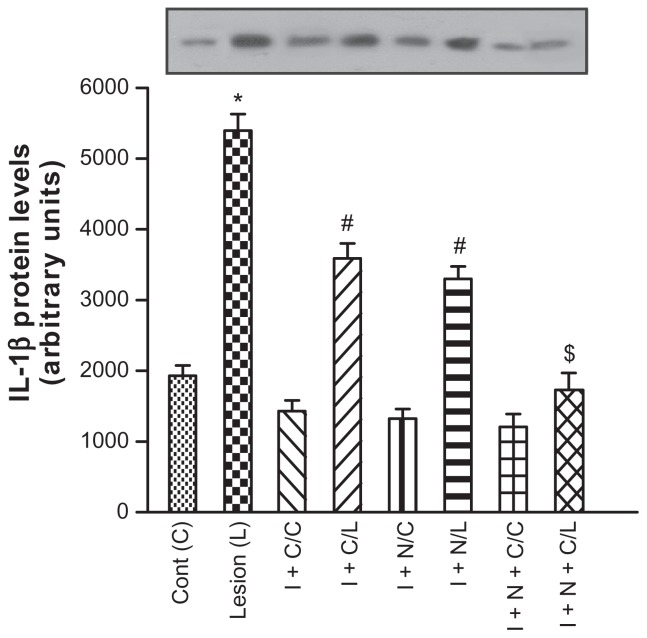
IL-1β
Figure 1 shows the levels of IL-1β determined by Western blotting. The external control group (injury challenged animals with no treatment) showed high IL-β expression (3591 ± 213). Yet, a significant reduction in IL-1β was observed in the iontophoresis + diclofenac and iontophoresis + GNPs groups, in comparison with the external control. However, the group treated with iontophoresis + diclofenac + GNPs exhibited the lowest IL-1β expression (1732 ± 239; P < 0.05), with statistical significance in comparison with the iontophoresis + diclofenac or GNPs applied individually, and even similar to the internal control value.
Figure 1.
Effect of iontophoresis with diclofenac diethylammonium, gold nanoparticle gel, and association thereof on interleukin 1 beta (IL-1β) expression after a 5-day treatment period.
Notes: Data expressed as mean ± standard deviation for 15 animals of each treatment group. *P < 0.05 in comparison with the control of the group not exposed to injury; #P < 0.05 in comparison with the group exposed to injury; $P < 0.05 with comparison to the groups treated with iontophoresis + diclofenac and iontophoresis + G NPs. Control, external control tendon, no treatment; injury, injured tendon without treatment.
Abbreviations: C, control; L, lesion; I + C/C, iontophoresis + diclofenac/internal control tendon; I + C/L, iontophoresis + diclofenac/injured tendon; I + N/C, iontophoresis + GNPs/control tendon; I + N/L, iontophoresis + GNPs/injured tendon; I + N + C/C, iontophoresis + GNPs + diclofenac/internal control tendon; I + N + C/L, iontophoresis + GNPs + diclofenac/injured tendon.
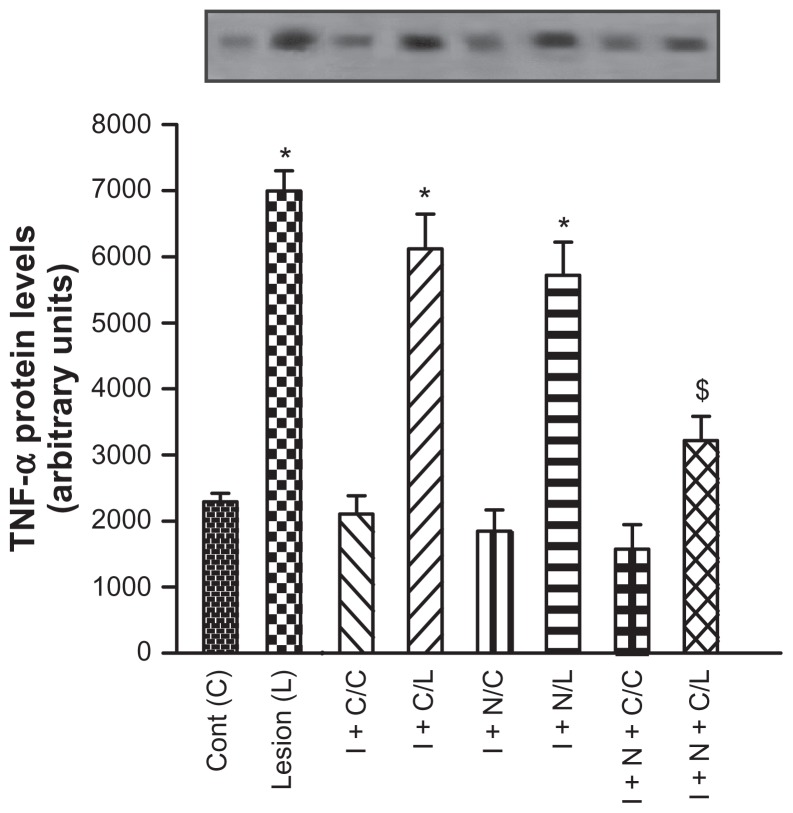
TNF-α
TNF-α levels were analyzed by Western blotting. Similarly to IL-1β, TNF-α levels were high in the external control group (6126 ± 527). Statistically significant decrease in TNF-α expression was observed only in the iontophoresis + diclofenac + GNPs group, in the treatment conditions described (3221 ± 36; P < 0.05; Figure 2).
Figure 2.
Effect of iontophoresis with diclofenac diethylammonium, gold nanoparticle gel, and association thereof on tumor necrosis factor alpha (TNF-α) expression after a 5-day treatment period.
Notes: Data expressed as mean ± standard deviation for 15 animals of each treatment group. *P < 0.05 in comparison to the control of the group not exposed to injury; $P < 0.05 in comparison to the groups treated with iontophoresis + diclofenac and iontophoresis + G NPs. Control, external control tendon, no treatment; injury, injured tendon without treatment.
Abbreviations: I + C/C, iontophoresis + diclofenac/internal control tendon; I + C/L, iontophoresis + diclofenac/injured tendon; I + N/C, iontophoresis + G NPs/control tendon; I + N/L, iontophoresis + GNPs/injured tendon; I + N + C/C, iontophoresis + GNPs + diclofenac/internal control tendon; I + N + C/L, iontophoresis + G NPs + diclofenac/injured tendon.
Discussion
The existence of an inflammatory stage in the injury was observed, with increased inflammatory cytokine expression in all groups challenged with injury. In the present study, diclofenac diethylammonium, GNPs, and an association of both were administered using direct current (iontophoresis) in Wistar rats challenged with a tendinopathy model. The expression of proinflammatory cytokines IL-1β and TNF-α. The results obtained revealed a significant decrease in IL-1β expression in the three treatment groups, when compared with the external control group (injury-challenged rats without treatment). However, the lowest IL-1β expression was observed in the iontophoresis + diclofenac + GNPs group, with statistically significant difference against the other treatment groups. Moreover, for the same combined treatment group, no statistically significant difference was observed in IL-1β expression in comparison with the internal control (unchallenged contralateral tendon). Based on the general principle of electrical transmission – that is, same-charge ions repel one another while differently charged ions are attracted to one another – a negatively charged drug has to be loaded onto the negative electrode, so that it migrates to the positive electrode. This electrical repulsive force conducts the drug inside the organism, toward the opposite electrode distally placed.23
In the present study, diclofenac diethylammonium, GNPs, and an association of both were administered using direct current (iontophoresis) in Wistar rats challenged with a tendinopathy model: the expression of proinflammatory cytokines IL-1β and TNF-α. The results obtained revealed a significant decrease in IL-1β expression in the three treatment groups, when compared with the external control group (injury-challenged rats without treatment). However, the lowest IL-1β expression was observed in the iontophoresis + diclofenac + GNPs group, with statistically significant difference against the other treatment groups. Moreover, for the same combined treatment group, no statistically significant difference was observed in IL-1β expression in comparison with the internal control (unchallenged contralateral tendon). In contrast to IL-1β expression, for which significant decreases were observed in all treatment groups, a significant drop in TNF-α expression was observed only in the iontophoresis + diclofenac + GNPs treatment group.
It appears that this is the first study to investigate the use of iontophoresis associated with drugs and metallic nanoparticles to treat tendinopathy, and to evaluate the expression of these proinflammatory cytokines in this kind of intervention. Chwalibog et al24 assessed the morphological characteristics of gold, silver, platinum, and diamond nanoparticles in terms of an interaction with bacteria and fungi. The authors reported negative zeta potential values for platinum (−9.6 mV), silver (−9.2 mV), and gold (−1.9 mV), explaining how these nanoparticles were harmful to the bacteria and fungi used. In the present study, the positive electrode was placed on the gastrocnemius myotendinous joint, while the negative electrode was placed on the injury site. The gel containing GNPs and the gel associating GNPs + diclofenac diethylammonium were loaded only on the negative electrode, while on the positive electrode gel base was used, which was also both electrodes as a transmission medium. Since GNPs have negative zeta potential, they migrated from the gastrocnemius (negative pole) to the Achilles tendon (positive pole).
Similar results were obtained in several previous studies, which reported appreciable levels of other drugs in deeper tissue layers after iontophoretic transdermal application. Glass et al25 demonstrated the penetration of dexamethasone in tissues below an injury site in monkeys. Other studies have reported the depth lidocaine reaches in human subcutaneous tissue via iontophoresis, demonstrating that the drug may reach 1.25 cm below the application site.26,27 Kigsawa et al28 used iontophoresis to transdermally transport CpG oligonucleotide in melanoma therapy. The authors observed the induction of a strong antitumor activity in a simple, noninvasive, and efficient approach to treat cancer.
Sonanave et al29 evaluated the role of GNP particle size (15, 102, and 198 nm) in penetration in rat skin. GNPs showed negative zeta potential and, the smaller the particle size, the higher the penetration rates in tissues. The authors concluded that GNPs are an attractive alternative for the transdermal transport of drugs, especially when smaller particles are used. In the present study, spherical GNPs measuring on average 30 nm were used. It is possible to hypothesize that the binding of this kind of nanoparticle to diclofenac diethylammonium afforded a more effective transport to the injury site. The therapeutic procedure using iontophoresis + diclofenac + GNPs reduced IL-1β to levels equivalent to those measured in the control group. This apparent synergism may be explained in the light of the interaction between GNPs and the groups –COOH and –NH in diclofenac, which are transported through the tissue to the injury site by iontophoresis, according to Nobuo30 and Selvakannan et al.14
Pedersen et al16 evaluated the effects of GNPs on TNF-α on a focal brain injury. The results obtained demonstrate a significant drop in brain TNF-α in the GNPs group. Tsai et al15 induced rheumatoid arthritis in an animal model and assessed inflammatory responses after intra-articular GNP administration. In that study, the authors observed significantly reduced intra-articular levels of IL-1β and TNF-α in treated rats. This was confirmed in the results of the present study, when GNPs, apart from their ability to reduce IL-1β and TNF-α expression in treated groups, were also efficient in the nanotransportation of drugs to the active inflammatory sites, as also reported by Ghosh et al.13 Further studies are being conducted by the authors of the current paper to assess oxidative stress, vascular endothelial growth factor, and metalloproteinase levels. The decrease in expression of inflammatory cytokines in tendinitis observed with the association diclofenac + GNPs and iontophoresis is an important factor in the control of the subsequent stages of tendinopathy. The inflammatory response should be strong enough to control the initial injury, which decreases the chances of higher secondary fibroblast proliferation and the resulting tendon degeneration. Iontophoresis associated with anti-inflammatory drugs has been used in the treatment of the acute stage of these injuries. The possibility to successfully treat the initial stage of tendinitis leads the authors to believe that it is possible to decrease the occurrence of tendinous rupture caused by tendon degeneration. These studies may shed new light on the mechanisms behind the interaction of iontophoresis and diclofenac diethylammonium and GNPs in tendinous injury scenarios.
Conclusion
The authors believe the results of the present paper to be the first to demonstrate the effect of GNPs associated to diclofenac diethylammonium transdermally administered using direct electric current in an animal tendinopathy model. GNPs exerted anti-inflammatory and synergistic action: enabling the transport the drug used and enhancing the therapeutic role of iontophoresis in the control of inflammatory cytokines IL-1β and TNF-α in a tendinopathy animal model.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wang JH. Mechanobiology of tendon. J Biomech. 2006;39(9):1563–1582. doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Brett M, Andres MD, George AC, et al. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466(7):1539–1554. doi: 10.1007/s11999-008-0260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Y, Murrell GA. The basic science of tendinopathy. Clin Orthop Relat Res. 2008;466(7):1528–1538. doi: 10.1007/s11999-008-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kraychete DC, Calasans MTA, Valente CML. Citocinas Pró-Inflamatórias e Dor [Pro-inflammatory cytokines and pain] Rev Bras Reumatol. 2006;46(3):199–206. [Portuguese.] [Google Scholar]
- 5.Banga AK. Electrically Assisted Transdermal and Topical Drug Delivery. London: Taylor & Francis; 1998. [Google Scholar]
- 6.Kalia YN, Naik A, Garrison J, Guy RH. Iontophoretic drug delivery. Adv Drug Deliv Rev. 2004;56(5):619–658. doi: 10.1016/j.addr.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Pikal MJ. The role of electroosmotic flow in transdermal iontophoresis. Adv Drug Deliv Rev. 2001;46(1–3):281–305. doi: 10.1016/s0169-409x(00)00138-1. [DOI] [PubMed] [Google Scholar]
- 8.Byl NN. The use of ultrasound as an enhancer for transcutaneous drug delivery: phonophoresis. Phys Ther. 1995;75(6):539–553. doi: 10.1093/ptj/75.6.539. [DOI] [PubMed] [Google Scholar]
- 9.Santi S, Singh J. Transdermal drug delivery by passive diffusion and iontophoresis: a review. Med Res Rev. 1993;13(5):569–621. doi: 10.1002/med.2610130504. [DOI] [PubMed] [Google Scholar]
- 10.Nino M, Calabrò G, Santoianni P. Topical delivery of active principles: the field of dermatological research. Dermatol Online J. 2010;16(1):4. [PubMed] [Google Scholar]
- 11.Grewal BS, Naik A, Irwin WJ, et al. Transdermal macromolecular delivery: real-time visualization of iontophoretic and chemically enhanced transport using two-photon excitation microscopy. Pharm Res. 2000;17(7):788–795. doi: 10.1023/a:1007595822786. [DOI] [PubMed] [Google Scholar]
- 12.Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104(1):293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh P, Han G, De M, et al. Gold nanoparticles in delivery applications. Adv Drug Deliv Rev. 2008;60(11):1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Selvakannan PR, Saikat M, Sumant P, et al. Capping of gold nanoparticles by the amino acid lysine renders them water-dispersible. Langmuir. 2003;19(8):3545–3549. [Google Scholar]
- 15.Tsai CY, Shiau AL, Chen SY, et al. Amelioration of collagen-induced arthritis in rats by nanogold. Arthritis Rheum. 2007;56(2):544–554. doi: 10.1002/art.22401. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen MO, Larsen A, Pedersen DS, et al. Metallic gold reduces TNFalpha expression, oxidative DNA damage and pro-apoptotic signals after experimental brain injury. Brain Res. 2009;1271:103–113. doi: 10.1016/j.brainres.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc. 1951;11:55–75. [Google Scholar]
- 18.National Institutes of Health. Guide for the Care and Use of Laboratory Animals. Bethesda, MD: National Institutes of Health Publications; 1996. [Google Scholar]
- 19.Rizzi CF, Mauriz JL, Freitas Corrêa DS, et al. Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-κB signaling pathway in traumatized muscle. Lasers Surg Med. 2006;38(7):704–713. doi: 10.1002/lsm.20371. [DOI] [PubMed] [Google Scholar]
- 20.Agne JE. Eu sei Eletroterapia [I know electrotherapy] Santa Maria: Pallotti; 2009. Portuguese. [Google Scholar]
- 21.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Uehara N. Polymer-functionalized gold nanoparticles as versatile sensing materials. Anal Sci. 2010;26(12):1219–1228. doi: 10.2116/analsci.26.1219. [DOI] [PubMed] [Google Scholar]
- 24.Chwalibog A, Sawosz E, Hotowy A, et al. Visualization of interaction between inorganic nanoparticles and bacteria or fungi. Int J Nanomedicine. 2010;5:1085–1094. doi: 10.2147/IJN.S13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glass JM, Stephen RL, Jacobson SC. The quantity and distribution of radiolabeled dexamethasone delivered to tissue by iontophoresis. Int J Dermatol. 1980;19(9):519–525. doi: 10.1111/j.1365-4362.1980.tb00380.x. [DOI] [PubMed] [Google Scholar]
- 26.Russo J, Jr, Lipman AG, Comstock TJ, et al. Lidocaine anesthesia: comparison of iontophoresis, injection, and swabbing. Am J Hosp Pharm. 1980;37(6):843–847. [PubMed] [Google Scholar]
- 27.Chien YW, Siddiqui O, Shi WM, et al. Direct current iontophoretic transdermal delivery of peptide and protein drugs. J Pharm Sci. 1989;78(5):376–383. doi: 10.1002/jps.2600780507. [DOI] [PubMed] [Google Scholar]
- 28.Kigasawa K, Kajimoto K, Nakamura T, et al. Noninvasive and efficient transdermal delivery of CpG-oligodeoxynucleotide for cancer immunotherapy. J Control Release. 2011;150(3):256–265. doi: 10.1016/j.jconrel.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Sonanave G, Tomoda K, Sano A, et al. In vitro permeation of gold nanoparticles through rat skin and rat intestine: effect of particle size. Colloids Surf B Biointerfaces. 2008;65(1):1–10. doi: 10.1016/j.colsurfb.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Nobuo U. Polymer-functionalized gold nanoparticles as versatile sensing materials. Anal Sci. 2010;26(12):1219–1228. doi: 10.2116/analsci.26.1219. [DOI] [PubMed] [Google Scholar]