Abstract
Background
Multidrug resistance in cancer is a major obstacle for clinical therapeutics, and is the reason for 90% of treatment failures. This study investigated the efficiency of novel multifunctional Fe3O4 magnetic nanoparticles (Fe3O4-MNP) combined with chemotherapy and hyperthermia for overcoming multidrug resistance in an in vivo model of leukemia.
Methods
Nude mice with tumor xenografts were randomly divided into a control group, and the treatment groups were allocated to receive daunorubicin, 5-bromotetrandrine (5-BrTet) and daunorubicin, Fe3O4-MNP, and Fe3O4-MNP coloaded with daunorubicin and 5-bromotetrandrine (Fe3O4-MNP-DNR-5-BrTet), with hyperthermia in an alternating magnetic field. We investigated tumor volume and pathology, as well as P-glycoprotein, Bcl-2, Bax, and caspase-3 protein expression to elucidate the effect of multimodal treatment on overcoming multidrug resistance.
Results
Fe3O4-MNP played a role in increasing tumor temperature during hyperthermia. Tumors became significantly smaller, and apoptosis of cells was observed in both the Fe3O4-MNP and Fe3O4-MNP-DNR-5-BrTet groups, especially in the Fe3O4-MNP-DNR-5-BrTet group, while tumor volumes in the other groups had increased after treatment for 12 days. Furthermore, Fe3O4-MNP-DNR-5-BrTet with hyperthermia noticeably decreased P-glycoprotein and Bcl-2 expression, and markedly increased Bax and caspase-3 expression.
Conclusion
Fe3O4-MNP-DNR-5-BrTet with hyperthermia may be a potential approach for reversal of multidrug resistance in the treatment of leukemia.
Keywords: magnetic nanoparticles, daunorubicin, 5-bromotetrandrine, multidrug resistance, hyperthermia
Introduction
Multidrug resistance is the phenomenon of cross-resistance of mammalian cells to a number of anticancer agents following exposure to one type of drug, and is a major obstacle in cancer chemotherapy,1 especially in the treatment of leukemia. Although the mechanism of multidrug resistance is complex, it is mainly due to the activation of a family of energy-dependent drug efflux pumps, such as P-glycoprotein, which can transport drugs out of the cell.2 On the other hand, alternating expression of apoptosisrelated proteins, such as Bcl-2, caspase-3, and Bax, in multidrug-resistant cancer cells causes a lack of response to many front-line chemotherapeutic agents.3 Therefore, regulation of P-glycoprotein and apoptosis-related protein has attracted research interest in an effort to overcome multidrug resistance in the treatment of cancer.
In recent years, the need to overcome multidrug resistance using a rational approach has been emphasized.4 Chinese herbal medicine, nanoparticles, and hyperthermia have contributed a lot to this line of research.5–7 It is well known that hyperthermia adjuvant to chemotherapy is playing an increasing role in the treatment of multidrug-resistant tumors in vivo, but temperature has been difficult to control.7 Fortunately, recent evidence in the literature suggests that magnetic nanoparticles can be used to improve the efficiency of hyperthermia and to regulate temperature at a stable level.8 The magnetic nanoparticles used for hyperthermia have a maximum temperature known as the Curie temperature, which is related to both magnetism and composition of the nanoparticles, but is not related to their concentration.9,10 Furthermore, the nanoparticles can be used to deliver anticancer drugs and reversal agents to improve the efficiency of treatment as well as to overcome drug resistance.11,12
Nowadays, some Chinese herbal medicines are considered to be able to reverse multidrug resistance in the treatment of cancer.5 Previous research has demonstrated that 5-bromotetrandrine (5-BrTet) can partly inhibit the function of P-glycoprotein, suppress antiapoptosis, and accumulate anticancer drugs in vivo and in vitro.5,13 Our own research has also demonstrated that Fe3O4 magnetic nanoparticles (MNP) enhance the efficiency of 5-BrTet and daunorubicin (DNR) in overcoming multidrug resistance in in vivo and in vitro models of cancer.14,15 Thus, we suspected that Fe3O4-MNP-DNR-5-BrTet with hyperthermia in an alternating magnetic field, as a multifunctional strategy, may have improved therapeutic efficiency. In the present study, we investigated tumor volume, pathology, and expression of P-glycoprotein, Bcl-2, Bax, and caspase-3 proteins to elucidate the effect of multimodal treatment in overcoming multidrug resistance after nude mice with tumor xenografts were treated with Fe3O4-MNP- DNR-5- BrTet and hyperthermia.
Materials and methods
Main agents
Adriamycin was purchased from Hisun Pharmaceutical Co, Ltd (Zhejiang, China); DNR from Main Luck Pharmaceuticals Inc (Shenzhen, China); 5-bromotetrandrin from Kang Hong Pharmaceuticals Inc (Chengdu, China); RPMI-1640 from Gibco Chemical Co (Carlsbad, CA); trichloride ferric, ferrous sulfate, oleic acid, and ammonium hydroxide from Shanghai Hanguang Chemical Reagent Co, Ltd (Shanghai, China); Pluronic F-127 from Sigma-Aldrich (St Louis, MO); hematoxylin-eosin staining kit from Beyotime Institute of Biotechnology (Shanghai, China); fetal bovine serum from Hangzhou Sijiqing Biological Engineering Materials Co, Ltd (Hangzhou, China); and monoclonal antibodies to Bax, Bcl-2, caspase-3, and P-glycoprotein from Santa Cruz Biotechnology Inc (Santa Cruz, CA).
Cell line and cell culture
A resistant human chronic myeloid leukemia cell line, K562/A02, was received as a gift from the Institute of Hematology, Chinese Academy of Medical Sciences. It was established by stepwise selection with an increasing concentration of adriamycin. The cells were maintained in RPMI-1640 medium with 10% fetal bovine serum, 100 μg/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified 5% CO2 atmosphere. To maintain a multidrug-resistant phenotype, the cell line was cultured in a medium containing 1 g/mL adriamycin for at least 1 week before use.
Experimental animals
Athymic nude BALB/c mice (4 weeks of age, 16–20 g in weight) were purchased from the Shanghai National Center for Laboratory Animals. They were maintained in a specific pathogen-free facility on a 12-hour light/dark cycle and fed with sterile food. The indoor temperature was maintained at 22°C and humidity ranged from 40% to 50%. All animal experiments were performed in adherence with the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health.
Preparation of Fe3O4-MNP and Fe3O4-MNP-DNR-5-BrTet
As described earlier, 26 g of ferric chloride hexahydrate, 18 g of ferrous sulfate heptahydrate, 5 g of oleic acid, and 50 mL of ammonium hydroxide (25%) were prepared for chemical reaction in nitrogen. The iron oxide nanoparticles were then prepared by coprecipitation of Fe3+ and Fe2+ with ammonium hydroxide. The prepared oleic acid-coated iron oxide nanoparticles were maintained at a high temperature and further modified with Pluronic F-127. Finally, the solution was gradually cooled to room temperature and extracted using a magnet, as previously described.17
After the aqueous dispersion of oleic acid-coated and Pluronic-modified iron oxide nanoparticles were synthesized, the already prepared hydrophobic DNR and 5-Br-Tet were added dropwise into the aqueous dispersion by vigorous magnetic force and stirring overnight to force the DNR and 5-Br-Tet to enter in the oleic acid layer.16 The effective drug-loading rate of DNR and 5-Br-Tet was approximately 8.25% (DNR:5-Br-Tet = 1:1 [w/w]), determined by high- performance liquid chromatography with a fluorescence detector.17
Multidrug-resistant leukemia xenograft model in nude mice
The nude mice were maintained in a specific pathogen-free facility for 7 days, and then injected with K562/A02 cells to establish a human multidrug-resistant leukemia xenograft model. Briefly, 1 × 107 K562/A02 cells suspended in 0.2 mL of RPMI-1640 medium were injected subcutaneously into the left posterior axilla of the nude mice. After the nude mice were injected with K562/A02 cells for about a week, a lump with skin-colored peplos was observed in the left posterior axilla of each mouse, which continued to grow larger until intervention, indicating that this lump was the multidrug resistant leukemia xenograft.
Treatment protocol
When the tumor size was 950 ± 150 mm3, 24 tumor-bearing nude mice were randomly divided into five groups. The treatment protocol included Group A, a negative control group in which each mouse was treated with 0.9% normal saline 0.2 mL; Group B, treated with DNR 1 mg/kg; Group C, treated with 5-BrTet 1 mg/kg and DNR 1 mg/kg; Group D, treated with Fe3O4-MNP 22 mg/kg; and Group E, treated with Fe3O4-MNP-DNR- 5-BrTet 24 mg/kg, in which DNR was 1 mg/kg and 5-BrTet was 1 mg/kg. After administration of the study treatment, all mice were kept in an alternating magnetic field at a frequency of 219 kHz and a variable field strength of 10.5–310.1 kA/m for 40 minutes. Thereafter, the tumor inhibition rate in the mice was calculated and tumor volume growth characteristics were recorded for 12 days before sacrifice. The xenograft tumor was then harvested and processed for histopathologic analysis and determination of protein expression.
Tumor volume and inhibition rate
To evaluate the effect of the study treatment, two diameters of tumor were measured with digital calipers, and then tumor size was calculated using the following formulae:
where v is tumor volume, a is the longest diameter, and b is the shortest diameter.
where RTV is relative tumor volume, V0 represents the first day before treatment, and Vx represents the x day after treatment.
where IR is the inhibition rate.
Histopathologic examination of tumors
After the xenograft tumor was harvested, it was immediately fixed in 4% paraformaldehyde, dehydrated in a graded series of alcohol, and then embedded in paraffin. Slices 0.5 mm thick were cut and stained with hematoxylin and eosin for microscopic examination. All sections were observed in a blinded manner, and photographed using a 400× light microscope.
Western blot assays
Samples of tumor tissue weighing 0.1 g was collected from each mouse and homogenized for detection of P-glycoprotein, Bcl-2, Bax, and caspase-3. Briefly, the tissues were extracted with Triton X-100, and the proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then electroblotted onto a polyvinylidene difluoride membrane (BioRad Laboratories, Hercules, CA). The membrane was blocked with buffer containing 10% fat- free dry milk. Thereafter, mouse anti-Bcl-2 antibody (1:400), mouse anti-Bax antibody (1:400), mouse anti- caspase- 3 antibody (1:400), mouse anti-P-glycoprotein antibody (1:200), and anti-β-actin (1:400) were used as the primary antibody overnight, and then subsequently incubated with a secondary horseradish peroxidase- conjugated antirabbit antibody (1:1000) for 1 hour at room temperature. The band was detected using an enhanced chemiluminescence system.
Statistical analysis
All experimental data were expressed as the mean ± standard deviation and analyzed using SPSS software (version 13.0; SPSS Inc, Chicago, IL). A P value of <0.05 was considered to be statistically significant.
Results
Characteristics of Fe3O4-MNP
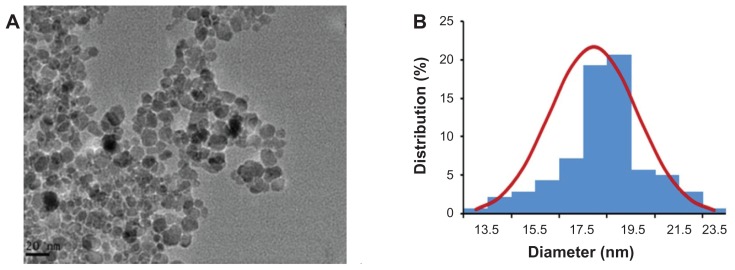
An image of the oleic acid-Pluronic-modified Fe3O4-MNP is shown in Figure 1A. As observed by transmission electronic microscopy, the nanoparticles had a spherical shape and were dispersed uniformly. The size of the Fe3O4-MNP ranged from 13.5 to 23.5 nm, and the mean size was 18.44 ± 1.84 nm (Figure 1B).
Figure 1.
(A) Image of oleic acid-Pluronic-modified iron oxide nanoparticles (Fe3O4-MNP) under transmission electronic microscopy. (B) Size distribution histogram of Fe3O4-MNP.
Abbreviation: Fe3O4-MNP, magnetic nanoparticles of Fe3O4.
Influence of extreme and moderate heat on tumors
After treatment with hyperthermia for different periods of time, the temperature change at the tumor site was determined and is shown in Table 1. It can be seen that the tumor temperature in the mice treated with Fe3O4-MNP increased to 41.71°C ± 1.52°C and in those treated with Fe3O4-MN-PDNR- 5-BrTet the temperature increased to 41.56°C ± 1.8°C after 20 minutes of hyperthermia. The tumor temperature was higher in these two groups than in the other groups, but there were no significant difference between them. Furthermore, no obvious change in temperature was observed in the mice not treated with Fe3O4-MNP throughout the study. Interestingly, except for the increased temperature at the tumor site, the mice treated with Fe3O4-MNP or Fe3O4-MNP-DNR-5-BrTet did not show any increase in temperature elsewhere. These results show that Fe3O4-MNP played an important role in the temperature changes at the tumor site in response to both extreme and moderate hyperthermia.
Table 1.
Temperature change of tumor site after treatment for different times (mean ± SD)
| Group | Temperature (°C) | ||||
|---|---|---|---|---|---|
|
|
|||||
| 0 minutes | 10 minutes | 20 minutes | 30 minutes | 40 minutes | |
| A | 30.85 ± 1.49 | 30.58 ± 1.62 | 30.38 ± 1.62 | 30.46 ± 1.96 | 29.71 ± 1.74 |
| B | 31.05 ± 1.96 | 30.71 ± 1.86 | 30.58 ± 1.71 | 30.75 ± 1.79 | 29.62 ± 1.63 |
| C | 31.02 ± 1.88 | 30.85 ± 1.49 | 30.67 ± 1.65 | 30.15 ± 1.53 | 29.67 ± 1.81 |
| D | 30.86 ± 1.96 | 35.73 ± 0.91 | 41.71 ± 1.52 | 42.91 ± 0.68 | 42.91 ± 0.63 |
| E | 30.88 ± 1.79 | 35.70 ± 1.07 | 41.56 ± 1.48 | 42.78 ± 0.46 | 42.78 ± 0.51 |
Notes: Group A, control; Group B, DNR; Group C, DNR and 5-BrTet; Group D, Fe3O4-MNP; Group E, Fe3O4-MNP-DNR-5-BrTet.
Abbreviations: DNR, daunorubicin; 5-BrTet, 5-bromotetrandrine; Fe3O4-MNP, magnetic nanoparticles of Fe3O4.
Volume and inhibitory rate in tumor tissue
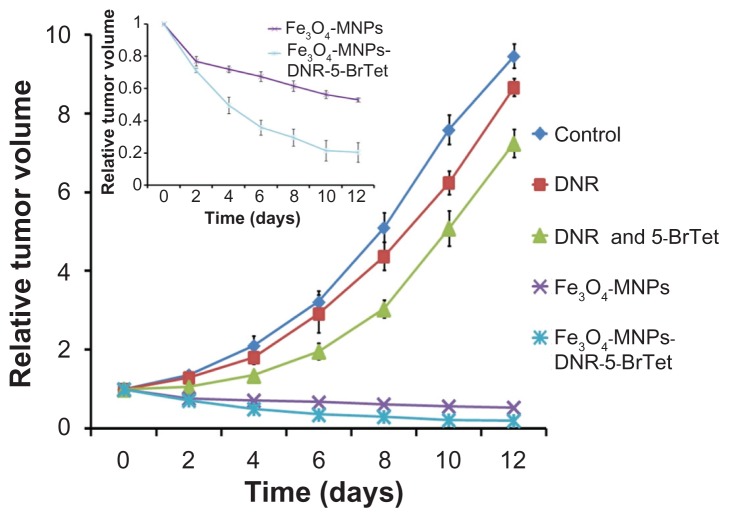
All the mice were alive and no adverse reactions were observed during the 12 days of treatment. The tumor volume was smaller and smaller in a time-dependent manner in both the Fe3O4-MNP and Fe3O4-MNP-DNR-5-BrTet groups, especially in the Fe3O4-MNP-DNR-5-BrTet group. In contrast, the tumor volume in the other groups became increasingly large, and was markedly larger in the DNR and control groups than in the DNR and 5Br-Tet groups (Figure 2). Further, the RTV in the Fe3O4-MNP and Fe3O4-MNP-DNR-5-BrTet groups was much lower than in other groups (P < 0.05) at day 12 after treatment, as shown in Figure 3. Interestingly, from day 4 onwards, the RTV decreased markedly more rapidly in the Fe3O4-MNP-DNR-5-BrTet group than in the Fe3O4-MNP group (P < 0.05, Figure 3).
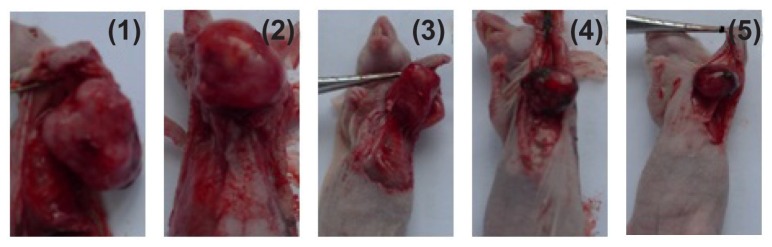
Figure 2.
Appearance of tumor body in tumor-bearing nude mice at day 12 after treatment.
Notes: (1) Controls, (2) DNR, (3) DNR and 5-BrTet, (4) Fe3O4-MNP, and (5) Fe3O4-MNP-DNR-5-BrTet.
Abbreviations: DNR, daunorubicin; 5-BrTet, 5-bromotetrandrine; Fe3O4-MNP, magnetic nanoparticles of Fe3O4.
Figure 3.
Relative tumor volume of mice after treatment for 12 days.
Abbreviations: DNR, daunorubicin; 5-BrTet, 5-bromotetrandrine; Fe3O4-MNP, magnetic nanoparticles of Fe3O4.
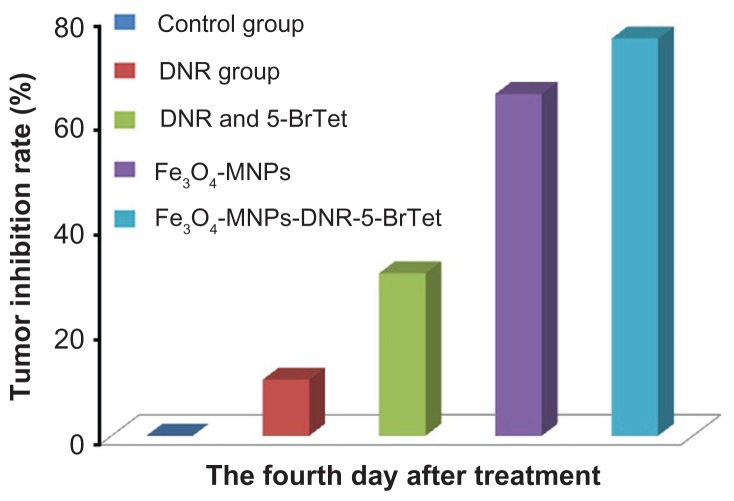
The tumor inhibition rate was higher in both the Fe3O4- MNP and Fe3O4-MNP-DNR-5-BrTet groups than in the DNR group or in the group treated with DNR combined with 5-BrTet (P < 0.05), and the change was particularly marked in the Fe3O4-MNP-DNR-5-BrTet group (Figure 5). The inhibition rate was 75.92% ± 5.77% in the Fe3O4-MNP-DNR-5- BrTet group and 65.31% ± 5.66% in the Fe3O4-MNP group, and higher than in the DNR (10.73% ± 4.58%) and DNR and 5-BrTet groups (31.04% ± 8.22%; P < 0.05), suggesting that Fe3O4-MNP-DNR-5-BrTet with hyperthermia had the strongest effect on tumor inhibition rate in a multidrug-resistant leukemia tumor model.
Figure 5.
Histopathologic examinations of K562/A02 tumors at day 12 after treatment (hematoxylin-eosin staining, 400×). (A) control, (B) DNR, (C) DNR and 5-BrTet, (D) Fe3O4-MNP, and (E) Fe3O4-MNP-DNR-5-BrTet.
Abbreviations: DNR, daunorubicin; 5-BrTet, 5-bromotetrandrine; Fe3O4,-MNP, magnetic nanoparticles of Fe3O4.
Histopathologic examination of tumor tissue
Representative histopathological images showed the neoplastic cells to have spherical heterogeneity with karyomegaly and dark blue nuclei, indicating more clear-edged mitotic figures (indicated by red arrows in Figure 5A and B). Few apoptotic cells were observed in the DNR-5-BrTet group, indicated by black arrows (Figure 5C). Notably, a reasonable quantity of apoptotic cells and an obvious apoptosis phenomenon were observed in the Fe3O4-MNP-DNR-5-BrTet and Fe3O4-MNP groups; karyopyknosis and karyorrhexis could be observed in the apoptotic cells, especially in the Fe3O4-MNP-DNR-5-BrTet group. Moreover, some brown punctiforms in localized areas could be seen in the Fe3O4- MNP and Fe3O4-MNP-DNR-5-BrTet groups, suggesting that Fe3O4-MNP can be targeted successfully to tumor tissue using additional hyperthermia.
P-glycoprotein, Bcl-2, Bax, and caspase-3 protein expression
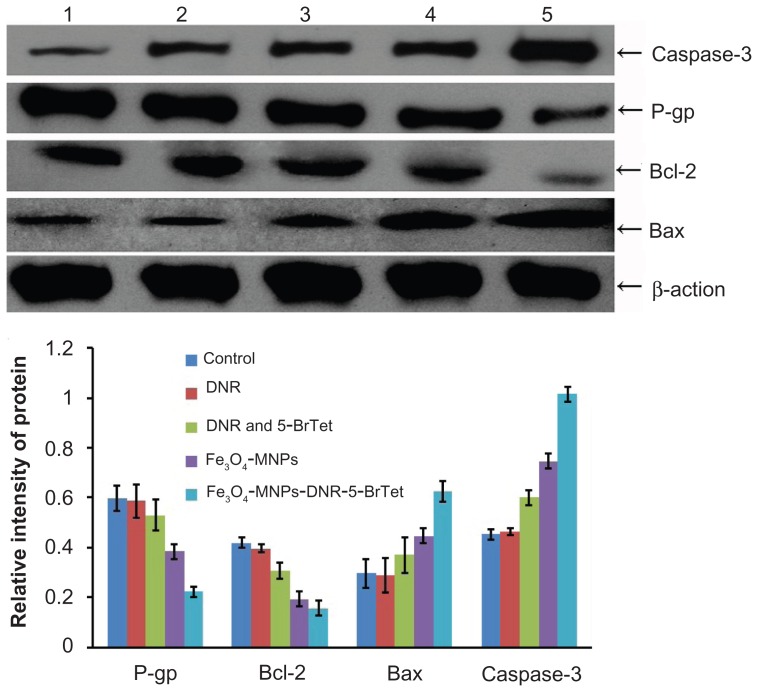
P-glycoprotein, Bcl-2, Bax and caspase-3 protein expression levels were detected by Western blotting, and are shown in Figure 6. There was no significant difference in relative intensity between any of these proteins in the control and DNR groups. However, compared with the other groups, Bcl-2 protein expression was markedly decreased and that of Bax and caspase-3 proteins was markedly increased in the Fe3O4- MNP and Fe3O4-MNP-DNR-5-BrTet groups (P < 0.05). Also, the ratio of Bax/Bcl-2 was higher in the Fe3O4-MNP-DNR- 5-BrTet and Fe3O4-MNP groups than other groups, and there was a significant difference between the Fe3O4-MNP-DNR- 5-BrTet group and the other groups (P < 0.05). Furthermore, P-glycoprotein expression was significantly lower in the Fe3O4-MNP-DNR-5-BrTet group than in the other groups (P < 0.05). Interestingly, Fe3O4-MNP-DNR-5-BrTet was more effective than Fe3O4-MNP (P < 0.05).
Figure 6.
Expression of P-glycoprotein and apoptosis-related protein after treatment.
Notes: (1) Control, (2) DNR, (3) DNR and 5-BrTet, (4) Fe3O4-MNP, and (5) Fe3O4-MNP-DNR-5-BrTet.
Abbreviations: P-gp, P-glycoprotein; DNR, daunorubicin; 5-BrTet, 5-bromotetrandrine; MNP-Fe3O4, magnetic nanoparticles of Fe3O4.
Discussion
Chemotherapy is still the main method for the majority of tumor therapeutics. However, 90% of tumor chemotherapy failure due to multidrug resistance,18 the mechanism of which is complex. P-glycoprotein-mediated multidrug resistance is the major cause of failure of chemotherapy. P-glycoprotein overexpressed in multidrug-resistant cancer cells could induce the chemotherapy drugs to pump from the cells.4,5 Unfortunately, no P-glycoprotein inhibitors have been approved for clinical use after the failure of valspodar (PSC-833).19 On the other hand, apoptosis is an important metabolic step in regulating cell numbers and their growth, and multiple antiapoptotic signals have been shown to enable cancer cells to evade destruction by chemotherapeutic drugs. Proteins of the Bcl-2 family have either proapoptotic or antiapoptotic activity and regulate the mitochondrial pathway in apoptosis.20 However, Bax and Bcl-2 expression is abnormal in hematologic malignances, increasing by up to 21%.21 Caspase-3 is the most characterized effector caspase, and its activation leads to the final stages of cell death.22 If Bcl-2 is highly expressed, the caspase-3 precursor cannot be activated and the apoptosis pathway is blocked,22 leading to disordered metabolism and tumor development.23 Therefore, there is an urgent global need to develop more effective strategies to overcome multidrug resistance.
In recent years, intensive research activity has been focused on certain Chinese herbal agents, which are safe and partly able to reverse multidrug resistance in cancer cells, and it has been found that 5-BrTet may be one such agent.5,25 5-BrTet combined with chemotherapy would not increase the toxic effects or pharmacokinetic activity of a chemotherapeutic agent.26 It is worth noting that considerable attention has been paid to nanoparticles and hyperthermia in developing rational and multifunctional compound therapeutics for cancer multidrug-resistant reversal.6,7
Many researchers have shown that chemotherapeutic agents loaded into nanoparticles have increased efficacy, solubility, and low toxicity, and that the drug is protected against chemical and biological degradation.26–28 Because of these advantages, nanoparticles can be considered to be potential chemotherapeutic drug carriers for treating multidrug-resistant cancers. Interestingly, one study of nanoparticles loaded with a chemotherapeutic agent has shown reversal of multidrug resistance.6 At present, Fe3O4 is one of the most commonly used nanoparticle materials, with low toxicity, magnetic properties, and minimal impact on body metabolism.6,26,29 In order to make the magnetic nanoparticles accumulate locally at the tumor site, an external alternating magnetic field is usually required, as well as magnetic nanoparticles with additional hyperthermia.29
Hyperthermia is an old but nowadays rapidly developing method of treating cancer, whereby a temperature of 40°C–45°C is considered optimal for hyperthermia.30 However, magnetic nanoparticles interact with each other in the magnetic field and this interaction inevitably influences the efficiency of hyperthermia. 31 Milleron and Bratton32 demonstrated that hyperthermia could increase local drug concentration and cause apoptosis of cancer cells via the mitochondrial pathway. Another study33 reported that heat generated in this way was more homogeneous than heat generated using a macroscopic implant, so it is rational to target tumor tissue using locally concentrated magnetic nanoparticles. There is evidence in the literature that hyperthermia can cause local drug accumulation in tumor tissue, leading to apoptosis of cancer cells and a change in the microenvironment of the cancer cell, which in turn affects anticancer immunity in the body.34,35 Sonvico et al36 treated tumor-bearing mice with a magnetic nanomedicine mediated hyperthermia, and 12 days after treatment, the nanomedicine in local tumor decreased to 50% of the dose of the drug at the beginning of the treatment. In our study, with the addition of hyperthermia, tumor temperature was higher in mice treated with Fe3O4-MNP or Fe3O4-MNP-DNR-5-BrTet than in the other groups, and was maintained at a stable level, with no change in temperature at other body sites in the mice. These results are in agreement with the histopathologic observation of an obvious apoptosis phenomenon in cancer cells in the mice treated with Fe3O4-MNP or Fe3O4-MNP-DNR-5-BrTet; the inhibition rate in the Fe3O4-MNP-DNR-5-BrTet group was much higher than in the other groups after 4 days of treatment. Furthermore, P-glycoprotein and Bcl-2 expression in the Fe3O4-MNP-DNR- 5-BrTet group was markedly decreased, that of caspase-3 was remarkably increased, and the proportion of Bax and Bcl-2 was significantly increased. According to our previous research, Fe3O4-MNP-DNR-5-BrTet can release a drug in a sustained manner for longer than 12 days,34 so Fe3O4-MNP-DNR-5- BrTet with hyperthermia in vivo could efficiently overcome tumor multidrug resistance for 12 days by releasing DNR and 5-Br-Tet from Fe3O4-MNP. In the present study, tumor volume continued to decrease over 12 days, and nanoparticles were observed in the tumor tissue on histopathologic examination after a single treatment with Fe3O4-MNP-DNR-5-BrTet. Meanwhile, we found that the same dose of DNR with 5-BrTet only decreased the speed of tumor growth and had much less effect on overcoming multidrug resistance than did Fe3O4- MNP-DNR-5-BrTet. Furthermore, compared with previous research, the addition of hyperthermia not only caused Fe3O4- MNP to be a more effective treatment, but also improved the efficiency of Fe3O4-MNP-DNR-5-BrTet to a greater extent than did daily administration without hyperthermia.15 Notably, all the nude mice remained alive, and no adverse reactions were observed after administration of Fe3O4-MNP-DNR-5-BrTet with hyperthermia. Thus, Fe3O4-MNP-DNR-5-BrTet with hyperthermia has decisive advantages in the treatment of local malignant tumor tissue.
Conclusion
In conclusion, Fe3O4-MNP-DNR-5-BrTet with addition of hyperthermia as a multifunctional strategy could efficiently overcome multidrug-resistant leukemia by downregulating P-glycoprotein expression and upregulating the ratio of Bax/Bcl-2. These findings demonstrate that Fe3O4-MNP-DNR- 5-BrTet with hyperthermia may be a potential approach to reverse multidrug-resistant leukemia.
Figure 4.
Tumor inhibition rate of mice at day 4 after treatment.
Abbreviations: DNR, daunorubicin; 5-BrTet, 5-bromotetrandrine; Fe3O4-MNP, magnetic nanoparticles of Fe3O4.
Acknowledgment
This work was supported by the 973 National Nature Science Foundation of People’s Republic of China (2010CB732404), the National Nature Science Project of the People’s Republic of China (81170492), and the Key Subject Foundation of Jiangsu Province.
Footnotes
Disclosure
The authors report no conflicts of interests in this work.
References
- 1.Aouali N, Eddabra L, Macadré J, Morjani H. Immunosuppressors and reversion of multidrug resistance. Crit Rev Oncol Hematol. 2005;56:61–70. doi: 10.1016/j.critrevonc.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Aller SG, Yu J, Ward A, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park MT, Kang JA, Choi JA, et al. Phytosphingosine induces apoptotic cell death via caspase 8 activation and Bax translocation in human cancer cells. Clin Cancer Res. 2003;9:878–885. [PubMed] [Google Scholar]
- 4.Nobili S, Landini I, Mazzei T, Mini E. Overcoming tumor multidrug resistance using drugs able to evade P-glycoprotein or to exploit its expression. Med Res Rev. 2011 Mar 3; doi: 10.1002/med.20239. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 5.Jin J, Wang FP, Wei H, et al. Reversal of multidrug resistance of cancer through inhibition of P-glycoprotein by 5-bromotertrandrine. Cancer Chemother Pharmacol. 2005;55:179–188. doi: 10.1007/s00280-004-0868-0. [DOI] [PubMed] [Google Scholar]
- 6.Alexiou C, Jurgons R, Schmid R, et al. Magnetic drug targeting – a new approach in locoregional tumor therapy with chemotherapeutic agents. HNO. 2005;53:618–622. doi: 10.1007/s00106-004-1146-5. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan S, Diagaradjane P, Cho SH. Nanoparticle-mediated thermal therapy: evolving strategies for prostate cancer therapy. Int J Hyperthermia. 2010;26:775–789. doi: 10.3109/02656736.2010.485593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilhelm C, Fortin JP, Gazeau F. Tumor cell toxicity of intracellular hyperthermia mediated by magnetic nanoparticles. J Nanosci Nanotechnol. 2007;7:2933–2937. doi: 10.1166/jnn.2007.668. [DOI] [PubMed] [Google Scholar]
- 9.Kotte ANTJ, van Wieringen N, Lagendijk JJW. Modelling tissue heating with ferromagnetic seeds. Phys Med Biol. 1998;43:105–120. doi: 10.1088/0031-9155/43/1/007. [DOI] [PubMed] [Google Scholar]
- 10.Salloum M, Ma R, Zhu L. Enhancement in treatment planning for magnetic nanoparticle hyperthermia: optimization of the heat absorption pattern. Int J Hyperthermia. 2009;25:309–321. doi: 10.1080/02656730902803118. [DOI] [PubMed] [Google Scholar]
- 11.Jordan A, Wust P, Scholz R, et al. Cellular uptake of magnetic fluid particles and their effects on human adenocarcinoma cells exposed to AC magnetic field in vitro. Int J Hyperthermia. 1996;12:705–722. doi: 10.3109/02656739609027678. [DOI] [PubMed] [Google Scholar]
- 12.Yan S, Zhang D, Gu N, et al. Therapeutic effect of Fe2O3 nanoparticles combined with magenetic fluid hyperthermia on cultured liver cancers. J Nanosci Nanotechnol. 2000;5:1185–1192. doi: 10.1166/jnn.2005.219. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Xu LZ, He KL, et al. Reversal effects of nomegestrol acetate on multidrug resistance in adriamycin-resistant MCF7 breast cancer cell line. Breast Cancer Res. 2001;3:253–263. doi: 10.1186/bcr303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen BA, Cheng J, Shen MF, et al. Magnetic nanoparticle of Fe3O4 and 5-bromotetrandrin interact synergistically to induce apoptosis by daunorubicin in leukemia cells. Int J Nanomedicine. 2009;4:65–71. [PMC free article] [PubMed] [Google Scholar]
- 15.Chen BA, Cheng J, Wu YN, et al. Reversal of multidrug resistance by magnetic Fe3O4 nanoparticle copolymerizating daunorubicin and 5-bromotetrandrine in xenograft nude-mice. Int J Nanomedicine. 2009;4:73–78. doi: 10.2147/ijn.s5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Chen BA, Cheng J, et al. Apoptotic mechanism of human leukemia K562/A02 cells induced by magnetic iron oxide nanoparticles coloaded with daunorubicin and 5-bromotetrandrin. Int J Nanomedicine. 2011;6:1027–1034. doi: 10.2147/IJN.S18023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Chen BA, Cheng J, et al. Synthesis and antitumor efficacy of daunorubicin-loaded magnetic nanoparticles. Int J Nanomedicine. 2011;6:203–211. doi: 10.2147/IJN.S16165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarkar FH, Li Y. Using chemopreventive agents to enhance the efficacy of cancer therapy. Cancer Res. 2006;66:3347–3350. doi: 10.1158/0008-5472.CAN-05-4526. [DOI] [PubMed] [Google Scholar]
- 19.Kolitz JE, George SL, Marcucci G, et al. P-glycoprotein inhibition using valspodar (PSC-833) does not improve outcomes for patients younger than age 60 years with newly diagnosed acute myeloid leukemia: Cancer and Leukemia Group B study 19808. Blood. 2010;116:1413–1421. doi: 10.1182/blood-2009-07-229492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasson R, Amsterdam A. Stimulation of apoptosis in human granulose cells from in vitro fertilization patients and its prevention by dexamethasone: involvement of cell contact and bcl-2 expression. Clin Endocrinol Metab. 2002;87:3441–3451. doi: 10.1210/jcem.87.7.8676. [DOI] [PubMed] [Google Scholar]
- 21.Meijerink JP, Mensink EJ, Wang K, et al. Hematopoietic malignance demonstrate loss-of-function mutation of Bax. Blood. 1998;91:2991–2997. [PubMed] [Google Scholar]
- 22.Belaud-Rotureau MA, Leducq N, Maeouilard Poulletier, de Gannes F, et al. Early transitory rise in intracellular pH leads to Bax conformation change during ceramide-induced apoptosis. Apoptosis. 2000;5:551–560. doi: 10.1023/a:1009693630664. [DOI] [PubMed] [Google Scholar]
- 23.Brauchi S, Cea C, Farias JG, Bacigalupo J, Reyes JG. Apoptosis induced by prolonged exposure to odorants in cultured cells from rat olfactory epithelium. Brain Res. 2006;1103:114–122. doi: 10.1016/j.brainres.2006.05.072. [DOI] [PubMed] [Google Scholar]
- 24.Fu L, Liang Y, Deng L, et al. Characterization of tetrandrine, a potent inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Chemother Pharmacol. 2004;53:349–356. doi: 10.1007/s00280-003-0742-5. [DOI] [PubMed] [Google Scholar]
- 25.Wang FP, Wang L, Yang JS, Nomura M, Miyamoto K. Reversal of P-glycoprotein-dependent resistance to vinblastine by newly synthesized bisbenzylisoquinoline alkaloids in mouse leukemia P388 cells. Biol Pharm Bull. 2005;28:1979–1982. doi: 10.1248/bpb.28.1979. [DOI] [PubMed] [Google Scholar]
- 26.Gindy ME, Prud’homme RK. Multifunctional nanoparticles for imaging, delivery and targeting in cancer therapy. Expert Opin Drug Deliv. 2009;6:865–878. doi: 10.1517/17425240902932908. [DOI] [PubMed] [Google Scholar]
- 27.Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol Oncol. 2008;26:57–64. doi: 10.1016/j.urolonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 29.Pridgen EM, Langer R, Farokhzad OC. Biodegradable, polymeric nanoparticle delivery systems for cancer therapy. Nanomedicine. 2007;2:669–680. doi: 10.2217/17435889.2.5.669. [DOI] [PubMed] [Google Scholar]
- 30.Giouroudi I, Kosel J. Recent progress in biomedical applications of magnetic nanoparticles. Recent Pat Nanotechnol. 2010;4:111–118. doi: 10.2174/187221010791208795. [DOI] [PubMed] [Google Scholar]
- 31.Rudolf H, Wilfried A, Carl G, et al. Physical limits of hyperthermia using magnetite fine particles. IEEE Trans Magn. 1998;34:3745–3754. [Google Scholar]
- 32.Milleron RS, Bratton SB. ‘Heated’ debates in apoptosis. Cell Mol Life Sci. 2007;64:2329–2333. doi: 10.1007/s00018-007-7135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito A, Shinkai M, Honda H, Kobayashi T. Medical application of functionalized magnetic nanoparticles. J Biosci Bioeng. 2005;100:1–11. doi: 10.1263/jbb.100.1. [DOI] [PubMed] [Google Scholar]
- 34.Hildebrandt B, Wust P, Ahlers O, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33–56. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 35.Cheng J, Wang J, Chen BA, et al. A promising strategy for overcoming MDR in tumor by magnetic iron oxide nanoparticles co-loaded with daunorubicin and 5-bromotetrandrin. Int J Nanomedicine. 2011;6:2123–2131. doi: 10.2147/IJN.S24309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonvico F, Mornet S, Vasseur S, et al. Folate-conjugated iron oxide nanoparticles for solid tumor targeting as potential specific magnetic hyperthermia mediators: synthesis physicochemical characterization, and in vitro experiments. Bioconjug Chem. 2005;16:1181–1188. doi: 10.1021/bc050050z. [DOI] [PubMed] [Google Scholar]