Abstract
Background
Robust species delimitations are fundamental for conservation, evolutionary, and systematic studies, but they can be difficult to estimate, particularly in rapid and recent radiations. The consensus that species concepts aim to identify evolutionarily distinct lineages is clear, but the criteria used to distinguish evolutionary lineages differ based on the perceived importance of the various characteristics of evolving populations. We examined three different species-delimitation criteria (monophyly, absence of genetic intermediates, and diagnosability) to determine whether currently recognized species of Hawaiian Pritchardia are distinct lineages.
Results
Data from plastid and nuclear genes, microsatellite loci, and morphological characters resulted in various levels of lineage subdivision that were likely caused by differing evolutionary rates between data sources. Additionally, taxonomic entities may be confounded because of the effects of incomplete lineage sorting and/or gene flow. A coalescent species tree was largely congruent with the simultaneous analysis, consistent with the idea that incomplete lineage sorting did not mislead our results. Furthermore, gene flow among populations of sympatric lineages likely explains the admixture and lack of resolution between those groups.
Conclusions
Delimiting Hawaiian Pritchardia species remains difficult but the ability to understand the influence of the evolutionary processes of incomplete lineage sorting and hybridization allow for mechanisms driving species diversity to be inferred. These processes likely extend to speciation in other Hawaiian angiosperm groups and the biota in general and must be explicitly accounted for in species delimitation.
Keywords: Hawaii, Hybridization, Lineage sorting, Microsatellite, Pritchardia, Radiation
Background
Species are a fundamental unit in biological studies and their robust delimitation is essential to many fields of evolutionary biology, particularly systematics, biogeography, and conservation biology. Lineage separation and divergence form a temporal process that may render populations monophyletic, reproductively isolated, ecologically divergent, and/or morphologically distinctive. These properties serve as operational criteria for systematists to delimit species and they can occur at different times or orders during speciation. De Queiroz [1,2] proposed that at the root of all modern species concepts is the general agreement on the fundamental nature of species: species are separately evolving metapopulation lineages. The perspective that species are lineages, and that multiple criteria should be used to identify them, has been termed the general lineage species concept [1]. Applying this lineage-based framework to species delimitation shifts the focus from a single operational criterion and increases the importance of sampling multiple lines of evidence. Species delimitation is notoriously difficult when alternative criteria delimit incongruent species boundaries, but this is to be expected in recent radiations (e.g. [3-5]). Evaluating multiple criteria not only increases our ability to detect recently separated lineages, but also can provide stronger support for lineage separation when they are in agreement [2,6,7].
The difficulty in recognizing species and their limits (the "species problem" [8]) is particularly compounded on islands. Because most islands are considerably younger terrestrial systems than continental areas [9], there has generally been less time for the completion of speciation processes. Time is an important factor for incomplete lineage sorting because the existence of ancestral polymorphism and differential extinction thereof can cause bias in phylogenetic inference (e.g. [10]) and the identification of distinct lineages (e.g. [11]). Furthermore, the tendency for island colonizers to quickly fill available habitat often leads to species that are ecologically isolated but not considerably diverged genetically, potentially leading to hybridization if mating barriers are broken down due to secondary contact (e.g. [12,13]). The evolutionary processes of incomplete lineage sorting and hybridization cause the "species problem" to be compounded on young, volcanic islands. Hawai'i is the longest archipelago on earth and has developed linearly in a sequential fashion from a volcanic hotspot [14]. Recent study of the extant high islands has shown that the terrestrial biota evolved over the last 29-23 Ma [15] and that they harbor the highest degree of endemism of any known flora [16,17]. The species richness of the Hawaiian Islands also contributes to the Polynesian/Micronesia biodiversity hotspot [18]. Difficulties in delimiting species is not restricted to angiosperms on the Hawaiian Islands (e.g. [19-24]), but has also been highlighted in Hylaeus bee [25] and spoon tarsus Drosophila [26] studies.
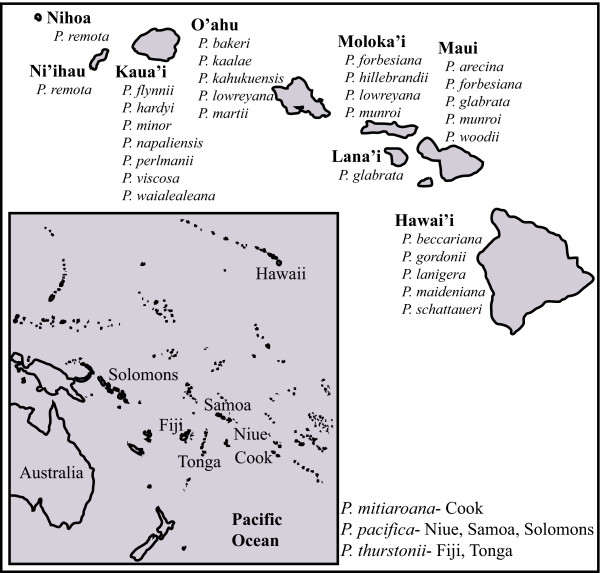
An excellent group within which to address the evaluation of species boundaries based on various delimitation criteria is the Hawaiian Pritchardia (Arecaceae/Palmae) radiation. Pritchardia is economically important as a widely cultivated ornamental palm [27], displays high endemism, and is a conservation priority for the State of Hawaii (15 threatened or endangered species [28]). Pritchardia is one of the most species-rich plant genera in Hawaii [29] and contains 27 currently recognized, primarily single-island endemic species (Figure 1, [29,30]). The genus also occurs on small islands in the eastern Pacific (Cook, Fiji, Niue, Samoa, Solomon, Tonga). Based on the most recent phylogenetic results Pritchardia is monophyletic and sister to Copernicia, although definitive generic relationships among Copernicia, Pritchardia, and Washingtonia were uncertain due to gene-tree incongruence [31]. Previous work has also shown that the North American and Caribbean lineage leading to Pritchardia colonized the eastern Pacific and then dispersed to Hawaii between 3.5-8 million years ago (MA; mean stem-crown ages [31]). Although no explicit species concept was applied, Hodel [29] recently revised Pritchardia using morphological data. Hodel [29] noted that character states were often difficult to define because Pritchardia morphology is highly labile based on environmental conditions (see also [32,33]). Accurate estimation of species limits is important to understanding the evolution and radiation of Pritchardia species and is essential to conservation efforts on the Hawaiian Islands.
Figure 1.
The geographic distribution of Hawaiian and eastern Pacific Pritchardia species according to the most recent morphological classification [29,30].
Species concepts can address both the evolutionary patterns consistent with evolution along lineages and the evolutionary processes that are fundamental in maintaining distinct lineages (e.g. [7]). Under the phylogenetic species concept I (PSCI [34]), species are defined as "the smallest aggregation of populations (sexual) or lineages (asexual) diagnosable by a unique combination of character states in comparable individuals" (p. 211 [35]). To apply PSCI, fixed (or mutually exclusive) character-state differences are used as evidence to infer that gene flow has ceased between the sampled populations in population aggregation analysis (PAA [36]). An alternate version of the phylogenetic species concept (PSCII) requires exclusivity to recognize a species and differs from PSCI by basing species recognition strictly on monophyletic groups ([37]; properly exclusive lineages [38]). A third alternative is the genotypic cluster species concept (GSC [39]), which defines species as genetic groups with few or no intermediates between them. The GSC can be implemented using a variety of clustering algorithms or assignment tests. Looking across species delimitation criteria allows for the implementation of the general-lineage species concept where the greater the number of criteria satisfied by a putative lineage, the more likely it is to represent an independent evolutionary trajectory [2].
Adaptive radiations are difficult evolutionary scenarios to evaluate because phylogenetic lineages may be so recently separated that each species' alleles have not coalesced since the time of speciation [40]. Among recently diverged species, genealogies inferred from independent genomic regions are likely to disagree due to the differential sorting of ancestral polymorphism into daughter lineages such that each inferred gene tree might differ from the species tree (e.g. [41]). Because estimation of a coalescent species tree explicitly models incomplete lineage sorting, its comparison with the simultaneous-analysis [42,43] allows for the inference of hybridization from any incongruence between the two topologies when only orthologous alleles are sampled.
In this study we aim to provide a comprehensive assessment of species diversity in Pritchardia using a multifaceted approach and independent sources of plastid, nuclear, and morphological data to assess three species-delimitation criteria - monophyly, the absence of genotypic intermediates, and diagnosability using mutually exclusive character states. We test whether currently recognized Pritchardia species merit taxonomic recognition as distinct evolutionary lineages, particularly with respect to the accumulation of evidence in favor of their delimitation. We also take advantage of the power of the coalescent to infer the species tree to understand potential conflicts in our results that can be introduced by incomplete lineage sorting and/or hybridization.
Results
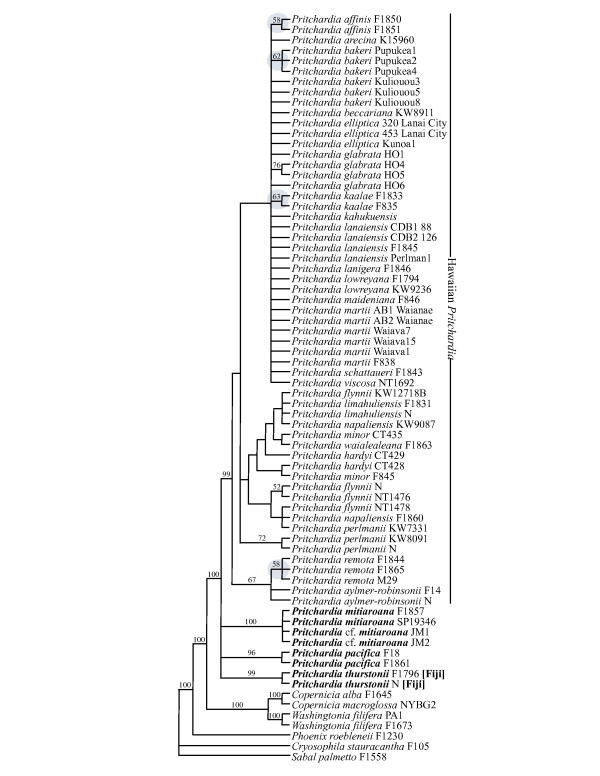
Gene-tree incongruence was detected among five of the seven loci for the resolution of the sister group of Pritchardia and among two of the seven loci for the sister group of Hawaiian Pritchardia (Additional file 1). The analysis 1 (A1) dataset comprised seven genes and five microsatellite loci for 72 individuals; 134 characters are variable and 81 are parsimony-informative within Pritchardia (Figure 2). Application of PSCII to the Pritchardia relationships in our A1 matrix indicated that the three currently recognized species of eastern Pacific Pritchardia (P. thurstonii, P. pacifica, and P. mitiaroana; Figure 2) are each distinct evolutionary lineages. Despite low branch support, Hawaiian P. affinis, P. kaalae, and P. remota were resolved as unique monophyletic groups and satisfy the PSCII criterion. A monophyletic group of P. bakeri from Pupukea, O'ahu was also resolved and likely represents population structure within the Ko'olau mountain range. A clade that included a subset of P. glabrata individuals and another clade that included a subset of P. perlmanii individuals were resolved, consistent with each of these being distinct evolutionary lineages according to the PSCII criterion.
Figure 2.
Analysis 1 (A1) parsimony tree inferred from DNA sequence and nuclear microsatellite data with which the phylogenetic species concept II was applied. Currently recognized species that are supported in this analysis are indicated with a grey circle; species from the eastern Pacific are in bold font; and the Fijian species is also indicated.
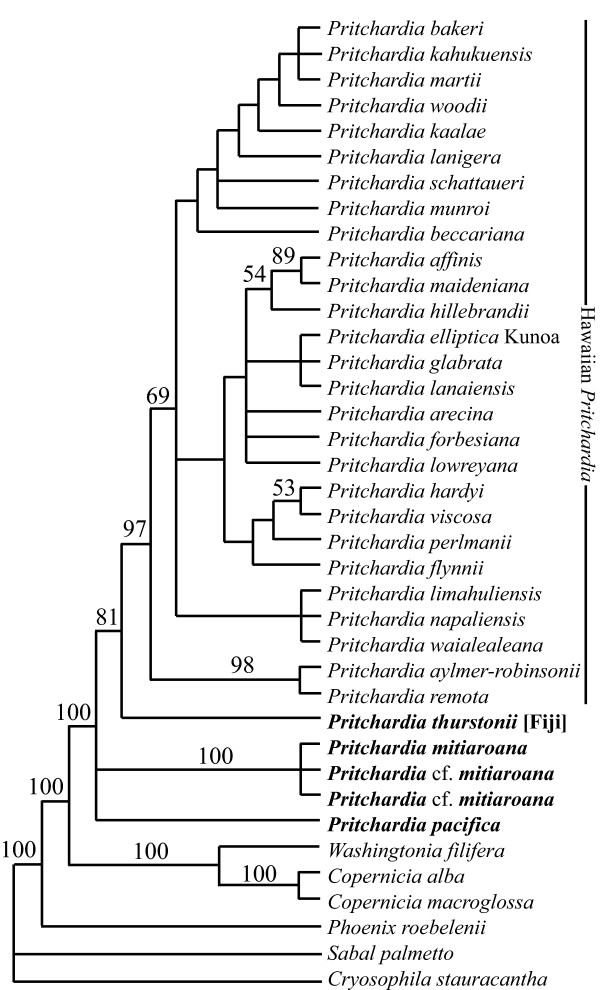
The 35 Pritchardia species sampled in the analysis 2 (A2) matrix was reduced to 32 by deleting wildcard taxa identified from comparisons of the Adams and strict consensus trees (Figure 3). The reduced A2 matrix has 79 parsimony informative characters. The eastern Pacific Pritchardia species P. pacifica and P. mitiaroana were resolved as part of a basal polytomy within Pritchardia, but there was strong support for monophyly of P. mitiaroana [100% jackknife support (JK)]. Pritchardia thurstonii was well supported (81% JK) as the sister species to the Hawaiian clade, which was strongly supported (97% JK) as a monophyletic group. Pritchardia aylmer-robinsonii and P. remota were strongly supported as sister species (98% JK), consistent with their synonymy. Pritchardia affinis and P. maideniana were well supported (89% JK) as sister taxa, also consistent with recent synonymy, and P. hillebrandii was weakly supported (54% JK) as its sister species. Pritchardia hardyi and P. viscosa were also weakly supported (53% JK) as sister species.
Figure 3.
Analysis 2 (A2) parsimony tree inferred from composite taxa constructed from the data in A1 together with isozyme and morphological data and showing inter-specific relationships where eastern Pacific species are indicated with bold font and the Fijian species is also indicated.
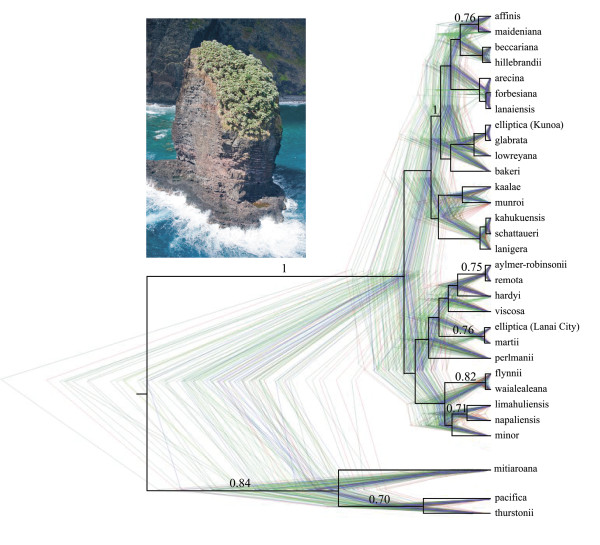
We explicitly modeled incomplete lineage sorting through the use of a multispecies coalescent tree for the sequence data (Figure 4). The topology did not have any mutually well-supported (≥75% branch support) conflicts with the A1 or A2 trees. The congruence between methods indicates that the trees used for species delimitation (A1) and for inference of inter-specific relationships (A2) is not biased by patterns of lineage sorting. The *BEAST species tree resolved four moderately supported groupings of Hawaiian individuals not seen in the comparable A2 analysis (Figure 3), although this may be due to inherent differences between parsimony and Bayesian tree reconstruction and JK support vs. posterior probabilities [44,45]. The recently synonymized P. affinis into P. maideniana, P. aylmer-robinsonii into P. remota, and P. limahuliensis into P. napaliensis [29] were each resolved as monophyletic groups. Although the posterior probabilities for these cases of synonymy were modest [between 0.71 and 0.76 posterior probability (PP)], these three taxonomic changes based on morphology [29] are consistent with our molecular results.
Figure 4.
Relationships amongst predefined Pritchardia lineages where resampled posterior species trees as inferred from *BEAST are in color and posterior probabilities ≥0.5 based on the single combined tree are overlaid in black. Pritchardia hillebrandii, which has one of the most restricted distributions in the genus, is pictured on Huelo Islet (photo and copyright D.R. Hodel).
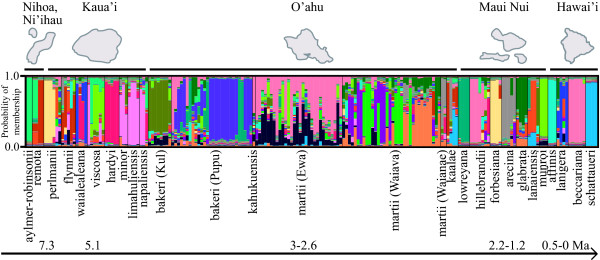
Significant p-values indicating disequilibrium were detected in the '90' microsatellite locus for two populations (P. martii Waianae and P. lanigera 2) and no evidence for stutter, large allele dropout, or null alleles was detected at any of the loci based on 99% confidence intervals. Structure analyses resulted in mean LnP(K) values that appeared to plateau when graphed in Structure Harvester, making it difficult to identify the most likely K value for the number of genetic groups present in the data. Therefore the ΔK method was applied and the highest probability for the number of groups that individuals were assigned to (K) was 21 (Mean LnP(K) = -2802, ΔK = 3.84). Upon visualization of population assignments from across the Structure iterations, the presence of genetic intermediates between Pritchardia species was evident (Figure 5). Levels of admixture were particularly high in areas of sympatry such as in the Makaleha and Namolokama ranges in Kaua'i where up to five species overlap in geographic distribution [P. flynii, P. hardyi, P. perlmanii (albeit to a lesser extent), P. viscosa, and P. waialealeana] and in the Ko'olau Mountains of O'ahu where three species are sympatric (P. bakeri, P. kahukuensis, and P. martii). Genetic subdivision and little admixture between species were detected among P. affinis, P. aylmer-robinsonii, P. beccariana, P. forbesiana, P. hardyi, P. lowreyana, P. munroi, and P. schattaueri and these eight groups meet the necessary criterion for species delimitation according to the GSC of high probability of assignment to their respective genetic groups (> 0.8 membership coefficient). However, the individual Q matrix of assignment to groups shows that P. beccariana, P. forbesiana, and P. lowreyana do not represent distinct evolutionary lineages according to the GSC because they do not group as unique clusters; with other individuals in the Q matrix having > 0.8 PP of falling within those groups. Although the 0.8 cut-off is arbitrarily defined, the maximum values from the Q matrices show a discontinuous distribution where individuals have a membership coefficient of > 0.8, while the remaining have < 0.5 with few in between. Therefore, based on our data, only P. affinis, P. aylmer-robinsonii, P. hardyi, P. munroi, and P. schattaueri meet the necessary and sufficient criteria as distinct evolutionary lineages without intermediates according to the GSC (Table 1).
Figure 5.
Putative species are labeled below and Hawaiian distributions from the oldest to the youngest island are indicated above the bar plot. Pritchardia bakeri populations from Kuliouou and Pupukea are abbreviated as Kul and Pupu respectively. Evidence for distinct evolutionary lineages without significant admixture or the presence of intermediates supports Pritchardia affinis, P. aylmer-robinsonii, P. hardyi, P. munroi, and P. schattaueri as independent lineages. Putative species are labeled below and Hawaiian distributions from the oldest to the youngest island are indicated above the bar plot.
Table 1.
Conformance of currently recognized Hawaiian Pritchardia with three distinct criteria for species delimitation
| Hawaiian Pritchardia | Monophyletic | Genotypic cluster | Diagnosable |
|---|---|---|---|
| affinis | 58% | 12; 0.91 | No |
| arecina | No | 19; 0.71 | Yes |
| aylmer-robinsonii | No | 18; 0.91 | Yes |
| bakeri Kuliouou | No | 13; 0.67 | No |
| bakeri Pupukea | 62% | 17; 0.51 | Yes |
| beccariana | No | 14; 0.90 | No |
| elliptica Kunoa | No | 4; 0.48 | No |
| elliptica Lanai City | No | - | No |
| flynii | No | 11; 0.40 | No |
| forbesiana | No | 10; 0.83 | Yes |
| glabrata | 76% | 20; 0.58 | Yes |
| gordonii | - | - | Yes |
| hardyi | No | 7; 0.86 | Yes |
| hillebrandii | No | 14; 0.59 | Yes |
| kaalae | 63% | 11; 0.31 | Yes |
| kahukuensis | No | 21; 0.52 | No |
| lanaiensis | No | 8; 0.46 | No |
| lanigera | No | 9; 0.55 | No |
| limahuliensis | No | 4; 0.78 | No |
| lowreyana | No | 15; 0.89 | Yes |
| maideniana | No | - | Yes |
| martii Ewa | No | 21; 0.44 | No |
| martii Waiawa | No | 19; 0.54 | Yes |
| martii Waianae | No | 11; 0.31 | No |
| minor | No | 4; 0.62 | No |
| munroi | No | 16; 0.83 | Yes |
| napaliensis | No | 4; 0.52 | No |
| perlmanii | 72% | 10; 0.57 | No |
| remota | 58% | 11; 0.51 | Yes |
| schattaueri | No | 2; 0.91 | Yes |
| viscosa | No | 16; 0.40 | Yes |
| waialealeana | No | 9; 0.50 | Yes |
| woodii | - | - | Yes |
Monophyly, as required by the phylogenetic species concept II, is shown as parsimony jackknife branch support. Genotypic clusters are labeled with their inferred genetic group and their estimated membership coefficient. Diagnosability to satisfy the phylogenetic species concept I was determined by PAA
Four distinct lineages were identified within Pritchardia microsatellite data using PAA, 33 in the sequence data and 12 in morphology, although individuals with missing data for diagnostic characters were left out of aggregations to avoid collapsing otherwise distinct groups (Additional file 2). For example, in the sequence data seven terminals had missing data for diagnostic characters and were arbitrarily assigned to a single group rather than collapsing the otherwise diagnosable groups. Due to differential sampling only the individuals sampled for the sequence dataset were used to perform PAA across the microsatellite and morphological data. In the three datasets we generated for this study, 43 lineages were indentified that are diagnosable and satisfy the PSCI. Of the 43 PSCI species, unique combinations of character states support 18 currently recognized Pritchardia species (Table 1).
Discussion
The Hawaiian Islands have an unparalleled number of well-studied examples of adaptive evolution because of their high ecological heterogeneity, volcanic origin, and isolation from the nearest continental land mass [46]. Despite the limited time available for diversification in comparison to ancient landmasses [15,47], the Hawaiian Islands have the highest degree of endemism of any known flora [16,32]. Within the Hawaiian Islands, many angiosperms show evidence for recent and rapid radiations, which frequently make species delimitation difficult (e.g. [21-26]). We applied three species-delimitation criteria to identify evolutionary lineages in Hawaiian Pritchardia. Robust species delimitations are important for Pritchardia because many of the currently recognized species are of conservation concern and threats continue to increase due habitat degradation and invasive herbivores and competitors [48].
We applied the criterion of monophyly to test whether currently recognized Pritchardia species are distinct evolutionary lineages using PSCII. Maximum parsimony (MP) analysis of the A1 matrix revealed support for P. affinis, P. glabrata, P. kaalae, P. perlmanii and P. remota as clades (Figure 2). Although these are weakly supported lineages, they satisfy the monophyly requirement of PSCII [37]. Despite its popularity, monophyly as inferred from a phylogenetic tree may be a poor indicator of whether evolutionary lineages are distinct in the presence of gene flow [49,50] or due to the error associated with randomly sampling few individuals from a complex underlying genealogy [51]. Furthermore, decoupling hybridization from incomplete lineage sorting on a phylogeny is difficult in recently diverged species because both produce the same pattern of few to no polymorphisms between morphologically identifiable species [52-54].
The genotypic cluster criterion defines species as "distinguishable groups of individuals that have few or no intermediates when in contact" (p. 296 [39]). A Bayesian assignment test was used to quantify the degree of admixture (essentially the absence of intermediates) between species. Although issues can arise with imperfect geographical sampling, especially in cases of isolation by distance or environmental gradients (e.g. [55]), strong signal for the delimitation of P. affinis, P. aylmer-robinsonii, P. hardyi, P. munroi, and P. schattaueri was detected with high probability of assignment to unique populations. A lack of intermediates satisfies this species criterion and these five groups are distinct evolutionary lineages according to the GSC. On the other end of the speciation spectrum, sympatric species appear to have ongoing gene flow among lineages where the probabilities of membership among some heterogeneous individuals and populations were shared (Figure 5), particularly in the mountains of Kaua'i and O'ahu.
Under the criterion of diagnosability, species are identified as the smallest aggregation of populations diagnosable by a unique combination of character states [35]. Using PAA, 43 lineages were identified as diagnosable and although they conform to PSCI as independent lineages, we do not advocate their formal recognition as species. Rather, our goal was to implement the general lineage species concept using multiple species-delimitation criteria to reach a more stable taxonomic solution for the Hawaiian Pritchardia. Furthermore, PAA can be highly sensitive where incomplete sampling of characters, individuals within populations, or populations can each lead to incorrect assessment of species [36]. In our Hawaiian Pritchardia data, the nucleotide sequence matrix had 24% percent missing or ambiguous data, which was mostly due to a lack of sampling in the Malate synthase (MS) gene (Additional file 1). The microsatellite and morphological matrices had only 0.05% missing data. Between one and six individuals were sampled per population with an average of 1.5 individuals and 1.7 populations per species in the nucleotide sequences of the A1 matrix. Between one and 34 individuals were sampled per population with an average of seven individuals and two populations per species for the microsatellite matrix. The morphological matrix comprised character states that were fixed within currently recognized species and were not typically scored from the actual specimens used in the sequence-based and microsatellite analyses. Additionally, ten of the morphological characters were derived from species descriptions [29,30] rather than herbarium material. Certainly no study is immune to these types of weakness, but we recognize that undersampling of individuals within populations and populations within species have affected the PAA results in this study by over-splitting and thus increasing the number of apparent species.
Distinct evolutionary lineages of Pritchardia
As currently defined, Pritchardia species are primarily recognized by their geographic distributions and a suite of morphological characters [29,30]. Yet when considering distinct evolutionary lineages identified in this study, none of the Pritchardia species satisfy all the species-delimitation criteria that we applied. Some species-delimitation criteria recognize more lineages than others in part because criteria are met at different times during cladogenesis [2]. Furthermore, when considering the amount of data used in the application of each criterion to infer species delimitations in Hawaiian Pritchardia we found the method that uses the most data, PAA, was the most powerful because it recognized the greatest number of splits.
Seven Pritchardia lineages satisfy two species-delimitation criteria (P. affinis, P. glabrata, P. hardyi, P. kaalae, P. munroi, P. remota, and P. schattaueri). The taxonomic status of P. affinis and P. remota are discussed in the interpretation of the A2 and coalescent species trees (see below). Pritchardia lanaiensis and P. elliptica were recently synonymized into P. glabrata [29], yet our results are inconsistent with this designation because of the diagnostic grouping of all P. glabrata sensu stricto individuals in PAA (Additional file 2).
Pritchardia hardyi, P. munroi, and P. schattaueri are all distinct lineages based on the species-delimitation criteria of a lack of intermediates and the presence of diagnostic character states. These results are consistent with Hodel's [29] description of morphological autapomorphies that define each of these three independent lineages. Pritchardia kaalae is identified as an independent lineage based on the formation of a monophyletic group and the presence of diagnostic character states. Despite its distinction as an independent lineage, P. kaalae appears to have significant levels of admixture based on the Structure results, particularly with Waianae and central Ko'olau (Waiava) populations of P. martii (Figure 5). Admixture may be indicative of the Pritchardia-dominated ancestral forest of the extensive O'ahu plain that spanned the Waianae and Ko'olau mountains and facilitated gene flow between ranges [46,56]. The once-contiguous palm forest likely formed an isolation-by-distance-based cline of gene flow, and extinction of the intervening lowland populations may have subsequently formed reproductively isolated lineages.
Eleven Pritchardia lineages satisfy only one species-delimitation criterion (P. arecina, P. forbesiana, P. gordonii, P. hillebrandii, P. lowreyana, P. maideniana, P. perlmanii, P. viscosa, P. waialealeana, and P woodii). Some of these Pritchardia lineages may be recognized as independent due to the sampling artifacts described above. This is particularly a concern with P. gordonii and P. woodii, which were only sampled for morphology, and P. hillebrandii, which was not sampled for the sequence data. Future efforts to tease apart distinct evolutionary lineages in Pritchardia should focus on these particular groups, as well as areas of sympatry, with increased sampling of both individuals within populations and of populations within species.
Sister-group and inter-specific relationships of Pritchardia
In previous studies the sister group of Pritchardia has been inferred to be either Copernicia (53% bootstrap, BS, in maximum representation with parsimony analysis [57]; < 50% JK/BS and 0.89 PP [31]) or Washingtonia (52% BS [58]). Our study is consistent with previous work showing the close relationships among the three genera (Copernicia, Pritchardia, and Washingtonia). In the A1 and A2 matrices, Copernicia and Washingtonia together are inferred to be the sister group to Pritchardia with strong support (100% JK; Figures 2 and 3).
We formed composite terminals [59] from the A1 matrix where taxa were combined at a level for which monophyly is assumed a prior thereby reducing missing data. Sequence data was augmented with allozyme and morphological data to construct the A2 matrix for simultaneous analysis of inter-specific relationships (Figure 3). The A2 matrix did not incorporate potential hybrid lineages because of the terminal omission iterations and was compared to the coalescent species tree to assess effects of incomplete lineage sorting. In the A2 tree, P. thurstonii is sister to the Hawaiian clade, which is well supported as monophyletic (97% JK) and consistent with Bacon et al. (64% BS/65% JK [31]). Zielger [60] proposed the sister relationship between Fijian and Hawaiian Pritchardia based on his hypothesis of an adaptive shift in fruit size upon colonization of the Hawaiian Islands. The sister relationship between Fijian and Hawaiian angiosperms has also been noted in Cyrtandra [23] and Pittosporum [21,26], but not in taxa that ultimately descended from American ancestors [61], such as Pritchardia.
The strongly supported sister relationship P. aylmer-robinsonii and P. remota (98% JK, Figure 3) is consistent with their synonymy [29]. Excluding P. remota from Nihoa and Ni'ihau, the backbone of the Hawaiian clade is a trichotomy. Weak support was provided for a sister relationship between P. hardyi and P. viscosa (53% JK), which had been previously suggested based on their flat leaf blades, the density of lepidia on the abaxial surface of the leaf, and their stiff leaf tips [29]. Hodel [29] also identified a close relationship between P. maideniana (including P. affinis) and P. hillebrandii based on morphological aspects of the lepidia and inflorescences, for which we inferred a well-supported P. maideniana sensu lato (89% JK) that was weakly supported as sister to P. hillebrandii (54% JK).
The coalescent-species-tree approach has been suggested to be a more accurate estimation of lineage splitting than concatenation because it can model the stochastic forces that drive population divergence [40,62-64]. Yet missing data and other issues with species-tree estimation such as mutational and coalescent variance can have detrimental effects on modeling incomplete lineage sorting (e.g. [65]). Another important consideration with species-tree estimation is that species are defined a priori and the coalescent model assumes species are monophyletic. This can be highly unlikely in recent radiations where ancestral species are still extant. Despite these issues, the advantage of directly modeling intraspecies polymorphism and incomplete lineage sorting makes species-tree estimation an important approach to data exploration in the identification of evolutionary lineages, especially in rapid species radiations [62].
The coalescent-species-tree topology provided moderate branch support for three clades that are consistent with recent synonymy [P. aylmer-robinsonii (0.75 PP), P. maideniana (0.76 PP), and P. napaliensis (0.71 PP); Figure 4]. The species tree identified P. flynnii and P. waialealeana as sister taxa, which together are sister to P. minor (Figure 4). Lastly, individuals planted by early Hawaiian naturalist George Munro in Lana'i City, Lana'i had been hypothesized to represent the extinct P. elliptica lineage (R.W. Hobdy, pers. comm. 2008), but are here shown to be consistent with a P. marti source from O'ahu (0.76 PP; see also Additional file 3) and separated from P. elliptica individuals collected from natural populations in Kunoa Valley by eight branches, one of which is highly supported (1.0 PP; Figure 4). Secondly, the species tree was used to test for congruence with the simultaneous-analysis A2 topology. Because the two distinct methods generally resolved the same well-supported clades, we can infer that the extrapolation from the gene trees to the phylogenetic tree is likely accurate in the simultaneous analysis. This is not to say that the process of lineage sorting has not occurred, but rather we have no evidence that it has confounded the species-level relationships inferred from the simultaneous-analysis tree.
Incomplete lineage sorting, the tempo of radiation, and hybridization in Pritchardia
The identification of distinct evolutionary lineages is a necessary precursor to the delimitation of species [2]. Satisfaction of multiple species criteria can ensure accurate, stable, and uncontroversial species delimitations (e.g., [4,7]). For taxa of conservation concern, accurate identification of lineages may facilitate management efforts by focusing on distinct species, rather than ambiguous groups. Our results, which are based on data from both the plastid and nuclear genomes, show little sequence differentiation among most Pritchardia species. The lack of differentiation may be due to incomplete lineage sorting, the tempo of the Pritchardia radiation, and/or hybridization between sympatric species, and distinguishing between these factors can be difficult.
Incomplete lineage sorting is one hypothesis for gene-tree incongruence and a lack of resolution within island radiations. Differential lineage sorting can bias species inference and may be further compounded by the estimated long generation time for other tropical understory palms that have undergone island colonization (e.g., 68-year mean in the Fijian endemic Balaka microcarpa [66]). Large ancestral effective population sizes have been hypothesized from fossil evidence and Pritchardia has been shown to be the dominant component of pre-human Quaternary forests on the Hawaiian Islands (2.6 Ma-822 year before present [67,68]). Despite this, coalescence times for Hawaiian Pritchardia species are likely to be shorter than their continental tribal counterparts. Congruence between the simultaneous and species-tree analyses together with information on coalescence times suggests that differential lineage sorting did not drive current diversity patterns within Pritchardia.
A general trend emerging from this and other phylogenetic studies on the Hawaiian flora is the difficulty in estimating relationships among woody and long-lived groups [e.g., Cyrtandra [19], lobeliads (e.g. [69]), Melicope [20]; Metrosideros [21,70]; Pittosporum [22,23]; Pyschotria [71]; Santalum [24], Schiedea [5], and the silversword alliance (e.g. [72])]. Another example is Hawaiian Pritchardia. Aside from the sympatric species, the lack of resolution may be caused by the insufficient time for divergence between lineages. Because of the age of the oldest extant Hawaiian Island (Nihoa; 7.3 Ma [14,15,47]) and because the Pritchardia colonization of the Hawaiian Islands was estimated to occur between 3.5-8 Ma (mean stem to crown-stem ages [31]), an average of three new species would have had to form every million years to account for the 24 currently recognized species in the radiation. Clearly this rapid rate of cladogenesis has not allowed for much divergence within the Hawaiian Pritchardia radiation.
We also suggest that hybridization has played a key role in the diversification of Hawaiian Pritchardia lineages from geographic regions of sympatry of Kaua'i (P. flynnii, P. limahuliensis, P. minor, P. napaliensis, P. waialealeana, and P. viscosa) and O'ahu (P. bakeri, P. kahukuensis, and P. martii). Removing wildcard terminals through the use of Adams consensus trees may be biased towards deletion of hybrids given that they are expected to be resolved as basal lineages [73] and our iterative exclusion process is consistent with the exclusion of hybrids because 66% (22 of the 33) of the excluded terminals were from areas of high sympatry such as in the Makaleha and Namolokamain ranges in Kaua'i and in the Ko'olau Mountains of O'ahu. Examination of the character conflict present was hampered by a general lack of resolution in the gene trees (each nDNA versus the single cpDNA tree; Additional files 4 and 5). Despite this, review of the parsimony-informative sequence characters revealed six polymorphisms found on both forward and reverse sequence reads that suggest introgression in two genes given how different the alleles are (nuclear MS amongst P. perlmanii individuals and plastid trnD-trnT amongst P. hardyi individuals). Although widespread hybridization has been observed in cultivation [74,75], it has been difficult to detect in the field due to the high phenotypic plasticity that characterizes Pritchardia [29,32].
Conclusions
The ability to hybridize is common among island species (e.g. [9]) and has likely been a major force in shaping other Hawaiian angiosperm lineages such as Metrosideros [21,70], Pittosporum [22,23], and silverswords (e.g. [76]). Outside of the lack of reproductive barriers or incompatibility mechanisms, anthropogenic change on the archipelago may have caused a breakdown of species boundaries. For example, native Hawaiians cultivated Pritchardia species in coastal settlements and although they had a variety of ethnobotanical uses (reviewed in [77]), the leaves and fibers were primarily used for thatching. The movement of plants by humans could have introduced new genotypes into existing coastal native species and admixed with other cultivated species. Also, the likely extinction of natural pollinators and dispersers and the introduction of invasive species that generally have higher mobility and efficiency [78] may also facilitate gene flow between populations and species.
Research at the interface of population genetics and phylogenetics is greatly expanding, as seen in the increasing numbers of publications on coalescent methods to infer species trees (e.g. [40,62-64]). A limitation to the current implementations of species-tree methods is the assumption of lack of gene flow among lineages, yet in empirical studies this assumption is often violated, especially at the taxonomic level these methods are designed for. Although there are methods that model gene flow as well as the coalescent (i.e., the isolation-with-migration model of Hey & Nielsen [79] or the hierarchical approximate Bayesian computation approach of Huang et al. [80]), these approaches do not provide an estimate of a species tree under a model of divergence with gene flow and may be less powerful than species-tree estimates because they require such strong priors (e.g., on migration rates [62]). To best address species delimitation in rapid radiations, especially in island groups like Pritchardia palms, methods that allow for simultaneously capturing vertical and horizontal inheritance of genetic information are needed, but are not yet available ([81] but see [82]).
Methods
Phylogenetic analyses
Total genomic DNA was extracted from silica-gel dried leaves following Alexander et al. [83]. Sequences for three plastid (matK, ndhF, and trnD-trnT) and four nuclear loci (CISPs 4 and 5, MS, and RPB2) were generated ([84-88] respectively). Amplified products were purified using Qiagen PCR purification kits and sequenced by the Cancer Research Center DNA Sequencing Facility at the University of Chicago or at Macrogen. All 502 new sequences generated in this study have been deposited in GenBank under accession numbers JF904936 to JF905438 (Appendix A).
Two phylogenetic analyses were conducted within Pritchardia. A1 included sequence data generated from seven loci and microsatellite data coded as multistate characters with heterozygous individuals coded as subset polymorphisms. Sampling for A1 included all previously recognized Pritchardia species except for P. gordonii and P. woodii, which are both recently described species with highly restricted distributions and are considered endangered [29]. Based on a recent tribal-level analysis [31] two species of each of the most closely related genera (Copernicia and Washingtonia) and three other Coryphoideae (Cryosophila, Phoenix, and Sabal) were sampled as outgroups. The initial simultaneous analysis included 105 terminals.
Preliminary nucleotide alignments were obtained independently for each of the seven loci using default parameters in MUSCLE v3.6 [89] and manual adjustments were performed in MacClade v4.03 [90] following Simmons [91]. Each parsimony-informative character was confirmed by rechecking chromatograms in Aligner (CodonCode Corp., MA). MP tree searches were conducted using 1,000 random addition tree-bisection-reconnection (TBR) searches in PAUP* v4.0b10 [92] with a maximum of ten trees held per replicate. MP JK analyses [93] were conducted using PAUP* and 1,000 replicates were performed with 100 random addition TBR searches per replicate. Maximum likelihood (ML [94]) analyses of nucleotide and microsatellite characters from each of the molecular data matrices were performed. jModeltest v0.1.1 [95] was used to select the best-fit likelihood model for each data matrix using the Akaike Information Criterion [96] without considering invariant-site models following Yang [97]. Searches for optimal ML trees and 1,000 BS replicates [98] in the CIPRES Portal v2.2 used the RAxML-HPC2 algorithm [99,100]. Adams consensus trees [101] from parsimony analyses were examined using the A1 dataset to identify wildcard terminals [102] of uncertain phylogenetic position that were then omitted. Iterations were conducted until a trade-off was reached between sacrificing taxonomically important terminals and gaining resolution in the strict consensus tree. A total of 72 of the original 105 terminals were included in the final A1 matrix.
A2 incorporated the A1, morphological, and isozyme data and was reduced to 35 composite terminals representing all putative Pritchardia species. Nine discrete morphological characters of flower and fruit morphology were measured from specimens at BISH, NY, PTBG, and US and ten morphological characters were derived from species descriptions ([29,30] Table 2). To include lineages that are not currently recognized as species [29,30] morphological character states were extrapolated from recognized species to now synonymous entities. We did not incorporate the preliminary morphological matrix from Gemmill [77] because of scoring inconsistencies. A matrix of seven variable isozymes was derived from Gemmill [77]. Three terminals (Pritchardia gordonii, cultivated 'elliptica' from Lana'i City, Lana'i, and P. minor) were omitted from the A2 matrix following the iterative procedure outlined above. The two simultaneous analyses (A1 and A2; TreeBase study accession 11604) were performed and the trees subsequently examined to determine the degree of support for monophyletic species (A1; PSCII) and for inferring robust inter-specific relationships due to decreased missing data and the use of all available characters (A2).
Table 2.
List of the Pritchardia morphological characters that were included in analysis 2
| Character | Character State |
|---|---|
| 1. Hastula shape | 0 = rounded |
| 1 = triangular, apiculate | |
| 2. Degree of panicle branching | 0 = two orders |
| 1 = three orders | |
| 3. Inflorescence length | 0 = shorter than petioles |
| 1 = equal | |
| 2 = longer than petioles | |
| 4. Petiole fiber density | 0 = scare to moderate |
| 1 = abundant | |
| 5. Abaxial leaf blade folds | 0 = glaucous |
| 1 = cottony, mealy indumentum | |
| 6. Abaxial leaf blade cover | 0 = green |
| 1 = silvery-gray | |
| 7. Leaf blade shape | 0 = nearly circular |
| 1 = diamond | |
| 8. Leaf blade with waxy, glaucous bloom | 0 = absent |
| 1 = present | |
| 9. Leaf blade surface | 0 = flat |
| 1 = nearly flat, undulate | |
| 10. Leaf tips | 0 = drooping |
| 1 = stiff | |
| 11. Lepidia density | 0 = absent |
| 1 = incompletely covered | |
| 2 = completely covered | |
| 12. Rachillae tomentum | 0 = glabrous |
| 1 = velutinous | |
| 2 = floccose, lanate | |
| 13. Rachillae viscosity | 0 = absent |
| 1 = present | |
| 14. Style - ovary ratio | 0 = equal |
| 1 = style longer | |
| 2 = style shorter | |
| 15. Outer calyx venation | 0 = absent |
| 1 = conspicuous | |
| 2 = present near opening with finer lines | |
| 16. Calyx indumentum | 0 = glabrous |
| 1 = tomentose | |
| 2 = viscous | |
| 17. Fruit ridges | 0 = absent |
| 1 = present | |
| 18. Fruit shape | 0 = globose |
| 1 = ellipsoid | |
| 2 = ovoid | |
| 3 = obovoid | |
| 4 = oblate | |
| 19. Fruit length | 0 = < 3 cm |
| 1 = > 3 cm |
Characters states were identified from herbarium specimens at BISH, NY, PTBG, and US and derived from the most recent review of the genus [30].
Coalescent-species-tree analysis
The coalescent species tree was inferred using *BEAST in BEAST v1.6.1 [62,103]. *BEAST infers coalescent species trees from multilocus data and has been shown to have advantages in computational speed and accuracy over similar methods when applied to rapid radiations [62] Coalescent-species-tree methods estimate each gene genealogy independently and assume that conflict between gene trees is due exclusively to incomplete lineage sorting. The sequence data from the A1 matrix was analyzed to avoid the inclusion of any potential hybrids. Each of the seven sequenced loci was unlinked to allow for variation in substitution models and the clock models for the chloroplast loci were linked to account for its presumed single hierarchical history. The analysis was run using a Yule species tree prior and the GTR+Γ model of nucleotide substitution with four rate categories. The Markov chains were run for 50 million generations and repeated 10 times to test for Markov chain Monte Carlo chain convergence and to ensure effective sample sizes (ESS) exceeded 200. Burn-in was determined in Tracer v1.5 based on ESS and parameter trajectories and was then removed in LogCombiner v1.6.1. Tree files were summarized in biopy v0.1.2 [104], the posterior was resampled, and the variance among 100 random resampled species trees was visualized in DensiTree [105]. We also estimated a single coalescent species tree in FigTree v1.3.1 by combining all tree files in LogCombiner v.1.6.1 [102]. We compared the coalescent species tree with the simultaneous analysis to determine whether accounting for incomplete lineage sorting resulted in a different topology. The coalescent species tree and the A1 and A2 topologies also allowed for testing of recent synonymy of species ([29]; Pritchardia affinis into P. maideniana, P. aylmer-robinsonii into P. remota, P. elliptica and P. lanaiensis into P. glabrata, and P. limahuliensis into P. napaliensis).
Population structure analyses
To test for the presence of intermediates between Hawaiian Pritchardia species, five microsatellite markers [106] were amplified in 197 individuals representing all 28 of the previously recognized species. PeakScanner software was used for allele calling and FlexiBin v2 was used to bin alleles [107]. GenoDive v20b19 [108] was used to test for Hardy-Weinberg equilibrium within populations with the default settings. Using the default settings, Microchecker v.2.2.3 [109] was used to check for stutter, large-allele dropout, or evidence for null alleles based on a 99% confidence interval. A Bayesian procedure (Structure v2.3.2 [110]) was used that minimizes the deviation from Hardy-Weinberg and linkage equilibrium within each putative cluster by the fractional assignment of individual genomes to K populations. The admixture model was implemented with correlated allele frequencies and without the use of a priori information from populations of origin. Simulations included 10 iterations for each K value from K = 1 to 30, with a 100,000-generation burn-in and 100,000 chain length. The most probable number of genetically homogeneous groups (K) was determined by the ΔK statistical procedure [111] as implemented in Structure Harvester v0.6 [112]. Multimodality across the 10 replicate iterations of the Structure analysis was addressed by permuting 1,000 times using the greedy algorithm and averaging across membership coefficients in CLUMPP [113]; the results were graphically displayed using Distruct v1.1 [114].
Population aggregation analysis
Mutually exclusive character states were used to test if gene flow had ceased between the sampled populations [35]. To examine whether previously recognized species were diagnosable and satisfy the PSCI, character-state differences were identified using PAA. As more populations are incorporated into PAA, each is compared to all species previously delimited. Each time a species profile is aggregated due to the inclusion of another population, the new profile is compared to all other species profiles to check if further aggregation is needed. We used PAA for the microsatellite, morphological, and sequence data independently of each other (because of differences in which terminals were sampled), and then performed PAA across all three data types to detect diagnosable groups. Missing and ambiguous data were treated as polymorphic for all states present, but these entries were not used to collapse otherwise diagnosable groups in PAA (J. I. Davis, pers. comm. 2011).
Authors' contributions
CDB, MPS, and WLW designed the study; CDB and MJM generated the data; CDB, MPS, and WLW analyzed the data and wrote the paper. All authors read and approved the final manuscript.
Appendix A
List of taxa sampled with taxonomic authorities, voucher information, and GenBank accession numbers for new sequences generated for this study. Fairchild Tropical Botanical Garden and National Tropical Botanic Garden are abbreviated as FTBG and NTBG respectively.
Pritchardia affinis Becc.- C. Gemmil 83 (PTBG), FTBG DNA Bank 1850, Hawai'i; CISP4 JF904936, CISP5 JF905062, matK JF905351, ndhF JF905121, RPB2 JF905197, trnDT JF905269. P. affinis Becc.- S. Perlman 13745 (PTBG), FTBG DNA Bank 1851, Hawai'i; CISP4 JF904937, CISP5 JF905023, matK JF905352, ndhF JF905122, RPB2 JF905198, trnDT JF905270. P. arecina Becc.- K. Wood 7991 (PTBG), FTBG DNA Bank 1853, Maui; CISP4 JF904938, matK JF905353, ndhF JF905123, RPB2 JF905199, trnDT JF905271. P. arecina Becc.-Baker 1183 (K), Royal Botanic Gardens, Kew DNA Bank 15960, Maui; CISP4 JF904939, CISP5 JF905024, matK JF905354, ndhF JF905124, RPB2 JF905200, trnDT JF905272. P. aylmer-robinsonii H.St.John- FTBG Live Collection 85184C, FTBG DNA Bank 14, Ni'ihau; CISP4 JF904940, CISP5 JF905025, matK JF905355, ndhF JF905125, RPB2 JF905201, trnDT JF905273. P. aylmer-robinsonii H.St.John-NTBG Live Collection, Ni'ihau; CISP4 JF904941, CISP5 JF905026, matK JF905356, ndhF JF905126, RPB2 JF905202, trnDT JF905274. P. bakeri Hodel-Bacon Pupukea1 SN, O'ahu; CISP4 JF904942, RPB2 JF905203. P. bakeri Hodel-Bacon Pupukea2 SN, O'ahu; CISP4 JF904943, CISP5 JF905027, RPB2 JF905204. P. bakeri Hodel-Bacon Pupukea3 SN, O'ahu; CISP4 JF904944. P. bakeri Hodel-Bacon Pupukea4 SN, O'ahu; CISP4 JF904945. P. bakeri Hodel-Bacon Kuliouou3 SN, O'ahu; CISP4 JF904989, matK JF905402, MS JF905094, ndhF JF905164, trnDT JF905316. P. bakeri Hodel-Bacon Kuliouou5 SN, O'ahu; CISP4 JF904990, matK JF905403, MS JF905095, ndhF JF905165, trnDT JF905317. P. bakeri Hodel-Bacon Kuliouou8 SN, O'ahu; CISP4 JF904991, matK JF905404, MS JF905096, ndhF JF905166, RPB2 JF905244, trnDT JF905318. P. beccariana Rock- J. Horn 4953 (PTBG), FTBG DNA Bank 1863, Hawai'i; CISP4 JF904946, CISP5 JF905063, matK JF905357, ndhF JF905127, RPB2 JF905205, trnDT JF905275. P. beccariana Rock-Wood 8911 (PTBG), Hawai'i; CISP4 JF904947, CISP5 JF905028, matK JF905358, RPB2 JF905206, trnDT JF905276. P. elliptica Rock & Caum-cultivated 320 Mahana St. Lana'i City, Lana'i; CISP4 JF904948, matK JF905361. P. elliptica Rock & Caum-cultivated 452 Lana'i St. Lana'i City, Lana'i; CISP4 JF904949, CISP5 JF905029, matK JF905362, ndhF JF905128, RPB2 JF905207, trnDT JF905277. P. elliptica Rock & Caum-cultivated 712 Puulani St. Lana'i City, Lana'i; CISP4 JF904950. P. elliptica Rock & Caum-Oppenheimer SN1, Kunoa Valley, Lana'i; CISP4 JF904951, CISP5 JF905030, matK JF905363, ndhF JF905129, RPB2 JF905208, trnDT JF905278. P. elliptica Rock & Caum-Oppenheimer SN6, Kunoa Valley, Lana'i; CISP5 JF905031, matK JF905359, ndhF JF905130, trnDT JF905279. P. elliptica Rock & Caum-Oppenheimer SN7, Kunoa Valley, Lana'i; CISP4 JF904952, CISP5 JF905032, matK JF905364, ndhF JF905131, trnDT JF905280. P. elliptica Rock & Caum-Oppenheimer SN8, Kunoa Valley, Lana'i; CISP4 JF904953, CISP5 JF905033, matK JF905360, RPB2 JF905209. P. flynnii Lorence & Gemmill-Wood 12718B (PTBG), Kaua'i; CISP4 JF904954, matK JF905366, MS JF905087, RPB2 JF905210. P. flynnii Lorence & Gemmill-Wood 12718C (PTBG), Kaua'i; CISP4 JF904955, matK JF905365. P. flynnii Lorence & Gemmill-NTBG Live Collection, Kaua'i; CISP4 JF904956, CISP5 JF905034, matK JF905367, ndhF JF905132, RPB2 JF905211, trnDT JF905281. P. flynnii Lorence & Gemmill-Tangalin 1476 (PTBG), Kaua'i; CISP4 JF904957, matK JF905368, MS JF905097, RPB2 JF905212, trnDT JF905282. P. flynnii Lorence & Gemmill-Tangalin 1478 (PTBG), Kaua'i; CISP4 JF904958, CISP5 JF905035, matK JF905369, ndhF JF905133, RPB2 JF905213, trnDT JF905283. P. flynnii Lorence & Gemmill-Tangalin 1480 (PTBG), Kaua'i; trnDT JF905284. P. forbesiana Rock- J. Horn 4948 (FTBG), FTBG DNA Bank 1798, Maui; matK JF905370, ndhF JF905134, RPB2 JF905214, trnDT JF905285. P. forbesiana Rock-NTBG Live Collection, Maui; CISP4 JF904959, CISP5 JF905036, matK JF905371, ndhF JF905135, RPB2 JF905215, trnDT JF905286. P. glabrata Becc. & Rock-FTBG DNA Bank 824, Maui; CISP4 JF904960, CISP5 JF905037, matK JF905372, ndhF JF905136, RPB2 JF905216. P. glabrata Becc. & Rock-Oppenheimer SN1, Maui; CISP4 JF904961, CISP5 JF905038, matK JF905373, RPB2 JF905217, trnDT JF905287. P. glabrata Becc. & Rock-Oppenheimer SN4, Maui; CISP4 JF904962, CISP5 JF905039, matK JF905374, MS JF905098, ndhF JF905137, RPB2 JF905218, trnDT JF905288. P. glabrata Becc. & Rock-Oppenheimer SN5, Maui; CISP4 JF904963, CISP5 JF905040, matK JF905375, MS JF905099, ndhF JF905138, RPB2 JF905219, trnDT JF905289. P glabrata Becc. & Rock-Oppenheimer SN6, Maui; CISP4 JF904964, matK JF905376, ndhF JF905139, RPB2 JF905220, trnDT JF905290. P. hardyi Rock-Trauernicht 428 (PTBG), Kaua'i; CISP4 JF904965, matK JF905377, RPB2 JF905221, trnDT JF905291. P. hardyi Rock-Trauernicht 429 (PTBG), Kaua'i; matK JF905378, ndhF JF905140. P. hardyi Rock-Trauernicht 430 (PTBG), Kaua'i; CISP4 JF904966, matK JF905379, ndhF JF905141. P. hardyi Rock- J. Horn 4938 (PTBG), Kaua'i, FTBG DNA Bank 1848; CISP4 JF904967, CISP5 JF905064, matK JF905380, MS JF905088, ndhF JF905142, RPB2 JF905222, trnDT JF905292. P. hardyi Rock-J. Horn 4951 (PTBG), FTBG DNA Bank 1858, Kaua'i; CISP4 JF904968, CISP5 JF905065, matK JF905381, MS JF905089, ndhF JF905143, RPB2 JF905223, trnDT JF905293. P. hardyi Rock-Tangalin 1705 (PTBG), Kaua'i; trnDT JF905294. P. hillebrandii Becc.-FTBG Live Collection 2000301A, FTBG DNA Bank 646, Moloka'i; CISP4 JF904969, CISP5 JF905041, matK JF905382, ndhF JF905144, RPB2 JF905224, trnDT JF905295. P. hillebrandii Becc.-S. Zona 1006 (FTG), FTBG DNA Bank 834, Moloka'i; CISP4 JF904970, CISP5 JF905042, matK JF905383, ndhF JF905145, RPB2 JF905225, trnDT JF905296. P. kaalae Rock-S. Zona 1008 (FTG), FTBG DNA Bank 835, O'ahu; CISP4 JF904973, CISP5 JF905043, matK JF905386, ndhF JF905148, RPB2 JF905228, trnDT JF905299. P. kaalae Rock-K. Wood 300 (PTBG), FTBG DNA Bank 1833, O'ahu; CISP4 JF904971, CISP5 JF905066, matK JF905384, ndhF JF905146, RPB2 JF905226, trnDT JF905297. P. kaalae Rock-S. Perlman 16710 (PTBG), FTBG DNA Bank 1847, O'ahu; CISP4 JF904972, CISP5 JF905067, matK JF905385, ndhF JF905147, RPB2 JF905227, trnDT JF905298. P. kahukuensis Caum-Kawelo SN (BISH), O'ahu; CISP4 JF904974, CISP5 JF905044, matK JF905387, ndhF JF905149, RPB2 JF905229, trnDT JF905300. P. lanaiensis Becc. & Rock-Bacon 88, Lana'i; CISP4 JF904975, CISP5 JF905045, matK JF905388, ndhF JF905150, RPB2 JF905230, trnDT JF905301. P. lanaiensis Becc. & Rock-Bacon 126, Lana'i; CISP4 JF904976, CISP5 JF905068, matK JF905389, ndhF JF905151, RPB2 JF905231, trnDT JF905302. P. lanaiensis Becc. & Rock-S. Perlman 16385 (PTBG), FTBG DNA Bank 1845, Lana'i; CISP4 JF904977, CISP5 JF905069, matK JF905390, MS JF905100, ndhF JF905152, RPB2 JF905232, trnDT JF905303. P. lanaiensis Becc. & Rock-Perlman 19968 (PTBG), Lana'i; CISP4 JF904978, CISP5 JF905046, matK JF905391, ndhF JF905153, RPB2 JF905233, trnDT JF905304. P. lanigera Becc.-K. Wood 7611 (PTBG), FTBG DNA Bank 1846, Hawai'i; CISP4 JF904979, CISP5 JF905070, matK JF905392, MS JF905101, ndhF JF905154, RPB2 JF905234, trnDT JF905305. P. limahuliensis H.St.John- J. Horn 4947 (PTBG), FTBG DNA Bank 1831, Kaua'i; CISP4 JF904980, matK JF905393, MS JF905102, ndhF JF905155, RPB2 JF905236, trnDT JF905307. P. limahuliensis H.St.John-NTBG Live Collection, Kaua'i; CISP4 JF904981, CISP5 JF905071, matK JF905394, MS JF905103, ndhF JF905156, RPB2 JF905235, trnDT JF905308. P. lowreyana Rock ex Becc.- J. Horn 4943 (PTBG), FTBG DNA Bank 1794, Moloka'i; CISP4 JF904982, CISP5 JF905072, matK JF905395, ndhF JF905157, RPB2 JF905237, trnDT JF905309. P. lowreyana Rock ex Becc.-Wood 9236 (PTBG), Moloka'i; CISP4 JF904983, CISP5 JF905047, matK JF905396, ndhF JF905158, RPB2 JF905238, trnDT JF905310. P. martii (Gaudich.) H.Wendl.- Bakutis Waianae SN1, O'ahu; CISP4 JF904984, CISP5 JF905048, matK JF905397, ndhF JF905159, RPB2 JF905239, trnDT JF905311. P. martii (Gaudich.) H.Wendl.- Bakutis Waianae SN2, O'ahu; CISP4 JF904985, CISP5 JF905049, matK JF905398, MS JF905090, ndhF JF905160, RPB2 JF905240, trnDT JF905312. P. martii (Gaudich.) H.Wendl.- Bacon Waiava1, O'ahu; CISP4 JF904988, CISP5 JF905052, matK JF905401, ndhF JF905163, RPB2 JF905243, trnDT JF905315. P. martii (Gaudich.) H.Wendl.- Bacon Waiava7, O'ahu; CISP4 JF904986, CISP5 JF905050, matK JF905399, MS JF905104, ndhF JF905161, RPB2 JF905241, trnDT JF905313. P. martii (Gaudich.) H.Wendl.- Bacon Waiava15, O'ahu; CISP4 JF904987, CISP5 JF905051, matK JF905400, ndhF JF905162, RPB2 JF905242, trnDT JF905314. P. martii (Gaudich.) H.Wendl.- J. Horn 4937 (PTBG), FTBG DNA Bank 1855, O'ahu; CISP4 JF904992, CISP5 JF905073, matK JF905405, ndhF JF905167, RPB2 JF905245, trnDT JF905319. P. martii (Gaudich.) H.Wendl.- J. Horn 4954 (PTBG), FTBG DNA Bank 1859, O'ahu; CISP4 JF904993, CISP5 JF905074, matK JF905406, ndhF JF905168, RPB2 JF905246, trnDT JF905320. P. martii (Gaudich.) H.Wendl.-NTBG live collection, O'ahu; CISP4 JF904994, CISP5 JF905053, matK JF905406, ndhF JF905169. P. minor Becc.-Trauernicht 432 (PTBG), Kaua'i; matK JF905408. P. minor Becc.-Trauernicht 434 (PTBG), Kaua'i; CISP4 JF904995, matK JF905409, ndhF JF905170. P. minor Becc.-Trauernicht 435 (PTBG), Kaua'i; CISP4 JF904996, matK JF905410, MS JF905105, ndhF JF905171, trnDT JF905321. P. minor Becc.-J. Horn 4946 (PTBG), FTBG DNA Bank 1797, Kaua'i; CISP4 JF904997, CISP5 JF905075, matK JF905411, ndhF JF905172, RPB2 JF905247, trnDT JF905322. P. minor Becc.- S. Zona 1033 (FTG), FTBG DNA Bank 845, Kaua'i; CISP4 JF904998, CISP5 JF905054, matK JF905412, ndhF JF905173, RPB2 JF905248, trnDT JF905323. P. minor Becc.- -Tangalin 1708 (PTBG), Kaua'i; trnDT JF905324. P. mitiaroana J.Drans. & Y.Ehrh.- S. Perlman 19346 (PTBG), FTBG DNA Bank 1857, Cook Islands; CISP4 JF904999, CISP5 JF905076, matK JF905413, MS JF905091, ndhF JF905174, RPB2 JF905249, trnDT JF905325. P. mitiaroana J.Drans. & Y.Ehrh.-Perlman 19346 (PTBG), Cook Islands; CISP4 JF905000. P. cf. mitiaroana J.Drans. & Y.Ehrh.-Meyer SN 'pericularum', French Polynesia; CISP4 JF905006, CISP5 JF905057, matK JF905419, MS JF905108, ndhF JF905181, RPB2 JF905254, trnDT JF905332. P. cf. mitiaroana J.Drans. & Y.Ehrh.-Meyer SN 'vuylstekeana', French Polynesia; CISP4 JF905019, CISP5 JF905085, matK JF905434, MS JF905118, ndhF JF905193, RPB2 JF905265, trnDT JF905346. P. munroi Rock- J. Horn 4942 (PTBG), FTBG DNA Bank 1832, Moloka'i; CISP4 JF905001, CISP5 JF905077, matK JF905414, ndhF JF905175, RPB2 JF905250, trnDT JF905326. P. munroi Rock- S. Zona 1036 (FTG), FTBG DNA Bank 841, Moloka'i; CISP4 JF905002, CISP5 JF905055, matK JF905415, ndhF JF905176, RPB2 JF905251, trnDT JF905327. P. napaliensis H.St.John- S. Perlman 11297 (PTBG), FTBG DNA Bank 1860, Kaua'i; CISP4 JF905003, CISP5 JF905078, matK JF905416, MS JF905106, ndhF JF905177, RPB2 JF905268, trnDT JF905328. P. napaliensis H.St.John-Wood 9087 (PTBG), Kaua'i; CISP4 JF905004, CISP5 JF905056, matK JF905417, MS JF905092, ndhF JF905178, trnDT JF905329. P. pacifica Seem. & H.Wendl.- FTBG Live Collection 93691D, FTBG DNA Bank 18, Fiji; CISP4 JF905005, CISP5 JF905079, ndhF JF905179, RPB2 JF905252, trnDT JF905330. P. pacifica Seem. & H.Wendl.-J. Horn 4952 (PTBG), FTBG DNA Bank 1861, Fiji; CISP5 JF905080, matK JF905418, MS JF905107, ndhF JF905180, RPB2 JF905253, trnDT JF905331. P. perlmanii Gemmill-Wood 7331 (PTBG), Kaua'i; CISP4 JF905007, matK JF905421, MS JF905109, ndhF JF905183, trnDT JF905333. P. perlmanii Gemmill-Wood 8091 (PTBG), Kaua'i; CISP4 JF905008, CISP5 JF905058, matK JF905422, MS JF905110, ndhF JF905184, RPB2 JF905255, trnDT JF905334. P. perlmanii Gemmill-NTBG Live Collection, Kaua'i; matK JF905420, MS JF905111, ndhF JF905182, trnDT JF905335. P. remota (Kuntze) Becc.- J. Horn 4955 (PTBG), FTBG DNA Bank 1844, Nihoa; CISP4 JF905009, CISP5 JF905081, matK JF905423, ndhF JF905185, RPB2 JF905256, trnDT JF905336. P. remota (Kuntze) Becc.-J. Horn 4936 (PTBG), FTBG DNA Bank 1865, Nihoa; CISP4 JF905010, CISP5 JF905082, matK JF905424, ndhF JF905186, RPB2 JF905257, trnDT JF905337. P. remota (Kuntze) Becc.-Montgomery Botanical Center Live Collection 29, Nihoa; CISP4 JF905011, matK JF905425, MS JF905112, RPB2 JF905258, trnDT JF905338. P. schattaueri Hodel-J. Horn 4939 (PTBG), FTBG DNA Bank 1843, Hawai'i; CISP4 JF905012, CISP5 JF905083, matK JF905426, MS JF905113, ndhF JF905187, RPB2 JF905259, trnDT JF905339. P. schattaueri Hodel- S. Zona 1001 (FTG), FTBG DNA Bank 839, Hawai'i; CISP4 JF905013, CISP5 JF905059, matK JF905427, MS JF905114, ndhF JF905188, RPB2 JF905260, trnDT JF905340. P. thurstonii F.Muell. & Drude-NTBG Live Collection, Fiji; CISP4 JF905014, CISP5 JF905060, matK JF905428, MS JF905115, ndhF JF905189, RPB2 JF905261, trnDT JF905341. P. viscosa Rock- J. Horn 4943 (PTBG), FTBG DNA Bank 1795, Kaua'i; CISP4 JF905015, CISP5 JF905084, matK JF905429, ndhF JF905190, RPB2 JF905262, trnDT JF905342. matK JF905430, ndhF JF905191. P. viscosa Rock-Tangalin 1693 (PTBG), Kaua'i; CISP4 JF905016, matK JF905431, MS JF905116, RPB2 JF905263, trnDT JF905343. P. viscosa Rock-Tangalin 1694 (PTBG), Kaua'i; CISP4 JF905017, matK JF905432, MS JF905117, RPB2 JF905264, trnDT JF905344. P. viscosa Rock-Perlman 16679A (PTBG), Kaua'i; CISP4 JF905018, matK JF905433, MS JF905093, ndhF JF905192, trnDT JF905345. P. waialealeana Read-Trauernicht 423 (PTBG), Kaua'i; matK JF905436, trnDT JF905347. P. waialealeana Read-Lorence 8446 (PTBG), Kaua'i; CISP4 JF905021, matK JF905435, ndhF JF905194, trnDT JF905348. P. waialealeana Read- J. Horn 4950 (PTBG), FTBG DNA Bank 1863, Kaua'i; CISP4 JF905020, CISP5 JF905086, matK JF905437, MS JF905119, ndhF JF905195, RPB2 JF905266, trnDT JF905349. P. waialealeana Read-NTBG Live Collection, Kaua'i; CISP4 JF905022, CISP5 JF905061, matK JF905438, MS JF905120, ndhF JF905196, RPB2 JF905267, trnDT JF905350.
Appendix B
Supplementary Material
Figure S1. Parsimony strict consensus trees of all the sequence data summarized to show only the inter-generic relationships and Pritchardia from different island chains. Parsimony jackknife support values above, and likelihood bootstrap values below each branch of each gene individually, the plastid partition, and the simultaneous analysis.
Table S1. Mutually exclusive character states were used to test if gene flow had ceased between the sampled populations using population aggregation analysis for each of the three datasets listed in columns with spaces between each of the independent lineages. In the sequence dataset, terminals with missing data for diagnostic characters were arbitrarily assigned to a single group rather than collapsing the otherwise diagnosable groups and are indicated with *.
Figure S2. Parsimony simultaneous analysis and strict consensus tree of all the 105 terminals sampled for nucleotide data with parsimony jackknife values shown.
Figure S3. The individual nuclear gene trees estimated for Pritchardia species delimitation as shown in the parsimony strict consensus with parsimony jackknife values above and likelihood bootstrap values below each branch.
Figure S4. The individual plastid gene trees and the plastid simultaneous-analysis estimated for Pritchardia species delimitation with jackknife branch support values above and bootstrap values below each branch.
Contributor Information
Christine D Bacon, Email: christinedbacon@gmail.edu.
Miles J McKenna, Email: miles.mckenna@gmail.com.
Mark P Simmons, Email: psimmons@lamar.colostate.edu.
Warren L Wagner, Email: wagnerw@si.edu.
Acknowledgements
The authors thank Ane Bakutis, Susan Ching, Don Hodel, Leland Miyano, Hank Oppenheimer, Steve Perlman, Geovanni Romero, Natalia Tangalin, Clay Trauernicht, Dick Watling, and Ken Wood for assistance with fieldwork; Jean-Yves Meyer and Lauren Weisenberger for DNA samples; Don Hodel and David Lorence for insightful feedback and discussions; Don Hodel for the use of the Pritchardia hillebrandii image; and Gabriel Johnson (Smithsonian) and Arwen Milroy (Colorado State University) for lab assistance. This research was supported by a National Tropical Botanical Garden McBryde Graduate Student Fellowship, an NSF-DDIG (DEB-1010731) award, a Smithsonian Pre-Doctoral Fellowship, a Montgomery Botanical Center Research Associateship, the Hunt Institute Lawrence Memorial Award, and the Bruno Klinger Memorial Scholarship to C.D.B.
References
- de Queiroz A. In: Endless forms: species and speciation. Howard DJ, Berlocher SH, editor. New York: Oxford University Press; 1998. The general lineage concept of species, species criteria, and the process of speciation: a conceptual unification and terminological recommendations; pp. 57–75. [Google Scholar]
- de Queiroz A. Species concepts and species delimitation. Syst Biol. 2007;56:879–886. doi: 10.1080/10635150701701083. [DOI] [PubMed] [Google Scholar]
- Belfiore NM, Liu L, Moritz C. Multilocus phylogenetics of a rapid radiation in the genus Thomomys(Rodentia: Geomyidae) Syst Biol. 2008;57:294–310. doi: 10.1080/10635150802044011. [DOI] [PubMed] [Google Scholar]
- Leaché AD, Koo MS, Spencer CL, Papenfuss TJ, Fisher RN, McGuire JA. Quantifying ecological, morphological, and genetic variation to delimit species in the coast horned lizard species complex (Phyrnosoma) Proc Nat Acad Sci USA. 2009;106:12418–12423. doi: 10.1073/pnas.0906380106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willyard A, Wallace LE, Wagner WL, Weller SG, Sakai AK, Nepokroeff M. Estimating the species tree for Hawaiian Schiedea (Caryophyllaceae) from multiple loci in the presence of reticulate evolution. Molec Phylogenet Evol. 2011;60:29–48. doi: 10.1016/j.ympev.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Barrett CF, Freudenstein JV. An integrative approach to delimiting species in a rare but widespread mycoheterotrophic orchid. Molec Ecol. 2011;20:2771–2786. doi: 10.1111/j.1365-294X.2011.05124.x. [DOI] [PubMed] [Google Scholar]
- Reeves PA, Richards CM. Species delimitation under the general lineage concept: an empirical example using wild North American Hops (Cannabaceae: Humulus lupulus) Syst Biol. 2011;60:45–59. doi: 10.1093/sysbio/syq056. [DOI] [PubMed] [Google Scholar]
- de Queiroz A. Different species problems and their resolution. BioEssays. 2005;27:1263–1269. doi: 10.1002/bies.20325. [DOI] [PubMed] [Google Scholar]
- Carlquist S. Island Biology. New York: Columbia University Press; 1974. [Google Scholar]
- Doyle JJ. Gene trees and species trees: molecular systematics as one-character taxonomy. Syst Bot. 1992;17:144–163. doi: 10.2307/2419070. [DOI] [Google Scholar]
- Knowles LL, Carstens BC. Delimiting species without monophyletic gene trees. Syst Biol. 2007;56:887–895. doi: 10.1080/10635150701701091. [DOI] [PubMed] [Google Scholar]
- Barrier M, Baldwin BG, Robichaux RH, Purugganan MD. Interspecific hybrid ancestry of a plant radiation: allopolyploidy of the Hawaiian Silversword Alliance (Asteraceae) inferred from floral homeotic gene duplications. Mol Biol Evol. 1999;16:1105–1113. doi: 10.1093/oxfordjournals.molbev.a026200. [DOI] [PubMed] [Google Scholar]
- Givnish TJ. Ecology of plant speciation. Taxon. 2010;59:1326–1366. [Google Scholar]
- Carson HL, Clague DA. In: Hawaiian biogeography: evolution on a hot spot archipelago. Wagner WL, Funk VA, editor. Washington: Smithsonian Institution Press; 1995. Geology and biogeography; pp. 14–29. [Google Scholar]
- Clague DA, Braga JC, Bassi D, Fullagar PD, Renema W, Webster JM. The maximum age of Hawaiian terrestrial lineages: geological constraints from Kōko Seamount. J Biogeography. 2010;37:1022–1033. [Google Scholar]
- Sakai AK, Wagner WL, Ferguson DM, Herbst DR. Biogeographical and ecological correlates of dioecy in the Hawaiian flora. Ecology. 1995;76:2530–2543. doi: 10.2307/2265826. [DOI] [Google Scholar]
- Sakai AK, Wagner WL, Ferguson DM, Herbst DR. Origins of dioecy in the Hawaiian flora. Ecology. 1995;76:2517–2529. doi: 10.2307/2265825. [DOI] [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2004;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Clark JR, Wagner WL, Roalson EH. Patterns of diversification and ancestral range reconstruction in the southeast Asian-Pacific angiosperm lineage Cyrtandra (Gesneriaceae) Molec Phylogenet Evol. 2009;53:982–994. doi: 10.1016/j.ympev.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Harbaugh DT, Wagner WL, Allen GJ, Zimmer EA. The Hawaiian Archipelago is a stepping stone for dispersal in the Pacific: an example from the plant genus Melicope (Rutaceae) J Biogeography. 2009;36:230–214. doi: 10.1111/j.1365-2699.2008.02008.x. [DOI] [Google Scholar]
- Harbaugh DT, Wagner WL, Percy DM, James HF, Fleisher RC. Genetic structure of the polymorphic Metrosideros (Myrtaceae) complex in the Hawaiian Islands using nuclear microsatellite data. PLoS ONE. 2009;4:4698. doi: 10.1371/journal.pone.0004698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmill CEC, Allen GJ, Wagner WL, Zimmer EA. Evolution of insular Pittosporum (Pittosporaceae): origin of the Hawaiian radiation. Molec Phylogenet Evol. 2002;22:31–42. doi: 10.1006/mpev.2001.1019. [DOI] [PubMed] [Google Scholar]
- Bacon CD, Allen GJ, Zimmer EA, Wagner WL. Genome scans reveal high levels of gene flow in Hawaiian Pittosporum. Taxon. 2011;60:733–741. [Google Scholar]
- Harbaugh DT, Baldwin BG. Phylogeny and biogeography of the sandalwoods (Santalum, Santalaceae): repeated dispersals throughout the Pacific. Am J Bot. 2007;94:1028–1040. doi: 10.3732/ajb.94.6.1028. [DOI] [PubMed] [Google Scholar]
- Magnacca KN, Danforth BN. Low nuclear DNA variation supports a recent origin of Hawaiian Hylaeus bees (Hymenoptera: Colletidae) Molec Phylogenet Evol. 2007;43:908–915. doi: 10.1016/j.ympev.2006.09.004. [DOI] [PubMed] [Google Scholar]
- LaPoint RT, Gidaya A, O'Grady PM. Phylogenetic relationships in the spoon tarsus subgroup of Hawaiian Drosophila: conflict and discordance between gene trees. Molec Phylogenet Evol. 2011;58:492–501. doi: 10.1016/j.ympev.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Maunder M, Lyte B, Dransfield J, Baker W. The conservation value of botanic garden palm collections. Biol Cons. 2001;98:259–271. doi: 10.1016/S0006-3207(00)00160-9. [DOI] [Google Scholar]
- IUCN Red List of Threatened Species. http://www.iucnredlist.org
- Hodel DR. A review of the genus Pritchardia. Palms. 2007;51:S1–S53. [Google Scholar]
- Hodel DR. A new species of Pritchardia and the rediscovery of P. lowreyana on Oahu, Hawaii. Palms. 2009;53:173–179. [Google Scholar]
- Bacon CD, Baker WJ, Simmons MP. Miocene dispersal drives island radiations in Trachycarpeae (Arecaceae) Syst Biol. 2012. [DOI] [PubMed]
- Wagner WL, Herbst DR, Sohmer SH. Manual of the Flowering Plants of Hawai'i. Honolulu: University of Hawai'i Press and Bishop Museum Press; 1999. [Google Scholar]
- St John H. Notes on Pritchardia. Occas Pap Bernice P Bishop Mus. 1932;9:3–5. [Google Scholar]
- Cracraft J. Species concepts and speciation analysis. Curr Ornithol. 1983;1:159–187. [Google Scholar]
- Nixon KC, Wheeler QD. An amplification of the phylogenetic species concept. Cladistics. 1990;6:211–223. doi: 10.1111/j.1096-0031.1990.tb00541.x. [DOI] [Google Scholar]
- Davis JI, Nixon KC. Populations, genetic variation, and the delimitation of phylogenetic species. Syst Biol. 1992;41:421–435. [Google Scholar]
- de Queiroz A, Donoghue MJ. Phylogenetic systematics and the species problem. Cladistics. 1988;4:317–338. doi: 10.1111/j.1096-0031.1988.tb00518.x. [DOI] [PubMed] [Google Scholar]
- Freudenstein JV. Ancestors, and classification: a response to Sosef and Brummitt. Taxon. 1998;47:95–104. doi: 10.2307/1224023. [DOI] [Google Scholar]
- Mallet J. A species definition for the modern synthesis. Trends Ecol Evol. 1995;10:284–298. doi: 10.1016/0169-5347(95)90031-4. [DOI] [PubMed] [Google Scholar]
- Edwards SV, Liu L, Pearl DK. High-resolution species trees without concatenation. Proc Nat Acad Sci USA. 2007;104:5936–5941. doi: 10.1073/pnas.0607004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan JH, Rosenberg NA. Discordance of species trees with their most likely gene trees. PLoS Genetics. 2006;2:762–768. doi: 10.1371/journal.pgen.0020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluge AG. A concern for evidence and a phylogenetic hypothesis for relationships among Epicrate (Boideae, Serpentes) Syst Zool. 1989;38:7–25. doi: 10.2307/2992432. [DOI] [Google Scholar]
- Nixon KC, Carpenter JM. On simultaneous analysis. Cladistics. 1996;12:221–242. doi: 10.1111/j.1096-0031.1996.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Simmons MP, Pickett KM, Miya M. How meaningful are Bayesian support values? Mol Biol Evol. 2004;21:188–189. doi: 10.1093/molbev/msh014. [DOI] [PubMed] [Google Scholar]
- Randle CP, Pickett KM. The conflation of ignorance and knowledge in the inference of clade posteriors. Cladistics. 2010;26:550–559. doi: 10.1111/j.1096-0031.2009.00301.x. [DOI] [PubMed] [Google Scholar]
- Carlquist S. Hawaii: a natural history. Lawai: Pacific Tropical Botanical Garden Press; 1980. [Google Scholar]
- Price JP, Clague DA. How old is the Hawaiian biota? Geology and phylogeny suggest recent divergence. Proc Royal Soc B. 2002;269:2429–2435. doi: 10.1098/rspb.2002.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin MH, Wood KR, Perlman SP, Maunder M. A review of the conservation status of the endemic Pritchardia palms of Hawaii. Oryx. 2004;38:273–281. [Google Scholar]
- Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Reeves PA, Richards CM. Distinguishing terminal monophyletic groups from reticulate taxa: performance of phenetic, tree-based, and network procedures. Syst Biol. 2007;56:302–320. doi: 10.1080/10635150701324225. [DOI] [PubMed] [Google Scholar]
- Rosenberg NA. Statistical tests for taxonomic distinctiveness from observations of monophyly. Evolution. 2007;61:317–323. doi: 10.1111/j.1558-5646.2007.00023.x. [DOI] [PubMed] [Google Scholar]
- Wendel JF, Doyle JJ. In: Molecular Systematic of Plants II. Soltis P, Doyle JJ, editor. Kluwer Academic Publishers: Norwell; 1998. Phylogenetic incongruence: window into genome history and molecular evolution; pp. 265–296. [Google Scholar]
- Holder MT, Anderson JA, Holloway AK. Difficulties in detecting hybridization. Syst Biol. 2001;50:978–982. doi: 10.1080/106351501753462911. [DOI] [PubMed] [Google Scholar]
- Mallet J. Hybridization as an invasion of the genome. Trends Ecol Evol. 2005;20:229–237. doi: 10.1016/j.tree.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Schwartz MK, McKelvey KS. Why sampling scheme matters: the effect of sampling scheme on landscape genetics results. Cons Genetics. 2009;10:441–452. doi: 10.1007/s10592-008-9622-1. [DOI] [Google Scholar]
- Cuddihy LW, Stone CP. Alteration of Native Hawaiian Vegetation. Honolulu: University of Hawaii Press; 1990. [Google Scholar]
- Baker WJ, Savolainen V, Asmussen-Lange CB, Chase MW, Dransfield J, Forest F, Harley MM, Uhl NW, Wilkinson M. Complete generic-level phylogenetic analyses of palms (Arecaceae) with comparison of supertree and supermatrix approaches. Syst Biol. 2009;58:240–256. doi: 10.1093/sysbio/syp021. [DOI] [PubMed] [Google Scholar]
- Asmussen CB, Dransfield J, Deickmann V, Barford AS, Pintaud J-C, Baker WJ. A new subfamily classification of the palm family (Arecaceae): evidence from plastid DNA. Bot J Linn Soc. 2006;151:15–38. doi: 10.1111/j.1095-8339.2006.00521.x. [DOI] [Google Scholar]
- Nixon KC, Davis JI. Polymorphic taxa, missing values and cladistic analysis. Cladistics. 1991;7:233–241. doi: 10.1111/j.1096-0031.1991.tb00036.x. [DOI] [PubMed] [Google Scholar]
- Ziegler AC. Hawaiian Natural History, ecology, and evolution. Honolulu: University of Hawaii Press; 2002. [Google Scholar]
- Baldwin BG, Wagner WL. Hawaiian angiosperm radiations of North American origin. Ann Bot. 2010;105:849–879. doi: 10.1093/aob/mcq052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heled J, Drummond A. Bayesian inference of species trees from multilocus data. Mol Biol Evol. 2010;27:570–276. doi: 10.1093/molbev/msp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison WP, Knowles LL. Inferring phylogeny despite incomplete lineage sorting. Syst Biol. 2006;55:21–30. doi: 10.1080/10635150500354928. [DOI] [PubMed] [Google Scholar]
- Kubatko LS, Degnan JH. Inconsistency of phylogenetic estimates from concatenated data under coalescence. Syst Biol. 2007;56:17–24. doi: 10.1080/10635150601146041. [DOI] [PubMed] [Google Scholar]
- Huang H, He Q, Kubatko LS, Knowles LL. Sources of error inherent in species-tree estimation: impact of mutational and coalescent effects on accuracy and implications for choosing among different methods. Syst Biol. 2010;59:573–583. doi: 10.1093/sysbio/syq047. [DOI] [PubMed] [Google Scholar]
- Ash J. Demography and production of Balaka microcarpa Burret (Arecaceae), a tropical understory palm in Fiji. Australian J Bot. 1998;36:67–80. [Google Scholar]
- Burney DA, James HF, Burney LP, Olson SL, Kikuchi W, Wagner WL, Burney M, McCloskey D, Kikuchi D, Grady FV, Gage R II, Nishek R. Fossil evidence for a diverse biota from Kaua'i and its transformation since human arrival. Ecol Monographs. 2001;71:615–641. [Google Scholar]
- Burney DA, Kikuchi WKP. A millennium of human activity at Makauwahi Cave, Maha'ulepu, Kaua'i. Human Ecology. 2006;34:219–247. doi: 10.1007/s10745-006-9015-3. [DOI] [Google Scholar]
- Givnish TJ, Millam KC, Mast AR, Paterson TB, Theim TJ, Hipp AL, Henss JM, Smith JF, Wood KR, Sytsma KJ. Origin, adaptive radiation and diversification of the Hawaiian lobeliads (Asterales: Campanulaceae) Proc Royal Soc B. 2009;276:407–416. doi: 10.1098/rspb.2008.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy DM, Garver AM, Wagner WL, James HF, Cunningham CW, Miller SE, Fleischer RC. Progressive island colonization and ancient origin of Hawaiian Metrosideros (Myrtaceae) Proc Royal Soc B. 2008;275:1479–1490. doi: 10.1098/rspb.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepokroeff M, Sytsma KJ, Wagner WL, Zimmer EA. Reconstructing ancestral patterns of colonization and dispersal in the Hawaiian understory tree genusPsychotria (Rubiaceae): a comparison of parsimony and likelihood approaches. Syst Biol. 2003;52:820–838. [PubMed] [Google Scholar]
- Baldwin BG, Sanderson MJ. Age and rate of diversification of the Hawaiian silversword alliance (Compositae) Proc Nat Acad Sci USA. 1998;95:8402–9406. doi: 10.1073/pnas.95.16.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade LA. Hybrids and phylogenetic systematics II. The impact of hybrids on cladistic analysis. Evolution. 1992;46:1392–1946. doi: 10.1111/j.1558-5646.1992.tb01127.x. [DOI] [PubMed] [Google Scholar]
- Hodel DR. Notes on Pritchardia. Principes. 1980;24:65–81. [Google Scholar]
- Ellison D, Ellison A. Cultivated Palms of the World. Sydney: University of New South Wales Press Ltd; 2001. [Google Scholar]
- Friar EA, Prince LM, Cruse-Sanders JM, McGlaughlin ME, Butterworth CA, Baldwin BG. Hybrid origin and genomic mosaicism of Dubautia scabra(Hawaiian Silversword Alliance; Asteraceae, Madiinae) Syst Bot. 2008;33:589–597. doi: 10.1600/036364408785679815. [DOI] [Google Scholar]
- Gemmill CEC. PhD thesis. University of Colorado, Department of Environmental, Population, and Organismic Biology; 1996. Population genetics and systematics of the Hawaiian taxa Pritchardia(Arecaceae) and Brighamia (Campanulaceae) [Google Scholar]
- Aizen MA, Morales CL, Morales JM. Invasive mutualists erode native pollination webs. PLoS Biol. 2008;6:31. doi: 10.1371/journal.pbio.0060031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hey J, Nielsen R. Multilocus methods for estimating population sizes, migration rates and divergence time, with application to the divergence of Drosophila pseudoocscura and D. persimilis. Genetics. 2004;167:747–760. doi: 10.1534/genetics.103.024182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Takebayashi N, Qi Y, Hickerson MJ. MTML-msBayes: approximate Bayesian comparative phylogeographic inference from multiple taxa and multiple loci with rate heterogeneity. BMC Bioinform. 2011;12:1. doi: 10.1186/1471-2105-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Than C, Degnan JH, Nakhleh L. Coalescent histories on phylogenetic networks and detection of hybridization despite incomplete lineage sorting. Syst Biol. 2011;60:138–149. doi: 10.1093/sysbio/syq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Ané C. Comparing two Bayesian methods for gene tree/species tree reconstruction: simulations with incomplete lineage sorting and horizontal gene transfer. Syst Biol. 2011;60:261–275. doi: 10.1093/sysbio/syr003. [DOI] [PubMed] [Google Scholar]
- Alexander PA, Govindarajalu R, Bacon CD, Bailey CD. Recovery of plant DNA using a reciprocating saw and silica-based columns. Mol Ecol Notes. 2007;7:5–9. [Google Scholar]
- Cuenca A, Asmussen-Lange CB. Phylogeny of the palm tribe Chamaedoreeae (Arecaceae) based on plastid DNA sequences. Molec Phylogenet Evol. 2007;32:250–563. doi: 10.1016/j.ympev.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Hahn WJ. A phylogenetic analysis of the Arecoid line of palms based on plastid DNA sequence data. Mol Phylogenet Evol. 2002;23:189–204. doi: 10.1016/S1055-7903(02)00022-2. [DOI] [PubMed] [Google Scholar]
- Bacon CD, Feltus FA, Paterson AH, Bailey CD. Novel nuclear intron-spanning primers for Arecaceae evolutionary biology. Mol Ecol Notes. 2008;8:211–214. doi: 10.1111/j.1471-8286.2007.01928.x. [DOI] [PubMed] [Google Scholar]
- Crisp MD, Isagi Y, Kato Y, Cook LG, Bowman DMJS. Livistona palms in Australia: ancient relics or opportunistic immigrants? Molec Phylogenet Evol. 2010;54:512–523. doi: 10.1016/j.ympev.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Roncal J, Fransisco-Ortega J, Asmussen CB, Lewis CE. Molecular phylogenetics of tribe Geonomeae (Arecaceae) using nuclear DNA sequences of phosphoribulokinase and RNA polymerase. Syst Bot. 2005;30:275–283. doi: 10.1600/0363644054223620. [DOI] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison DR, Maddison WP. MacClade: Analysis of Phylogeny and Character Evolution version 4.03. Sunderland: Sinauer Associates; 2001. [Google Scholar]
- Simmons MP. Independence of alignment and tree search. Molec Phylogenet Evol. 2004;31:874–879. doi: 10.1016/j.ympev.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Swofford DL. PAUP* 4.0: Phylogenetic Analysis Using Parsimony (*and Other Methods) Sunderland: Sinauer Associates; 2001. [Google Scholar]
- Farris JS, Albert VA, Källersjö M, Lipscomb D, Kluge AG. Parsimony jackknifing outperforms neighbor-joining. Cladistics. 1996;12:99–124. doi: 10.1111/j.1096-0031.1996.tb00196.x. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Maximum likelihood and minimum-step methods for estimating evolutionary trees from data on discrete characters. Syst Zool. 1973;22:240–249. doi: 10.2307/2412304. [DOI] [Google Scholar]
- Posada D. jModelTest: phylogenetic model averaging. Mol Biol Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Akaike H. A new look at the statistical model identification. IEEE Trans Aut Control. 1974;19:716–723. doi: 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- Yang Z. Computational Molecular Evolution. Oxford: Oxford University Press; 2006. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Ludwig T, Meier H. RAxML-III: a fast program for maximum likelihood-based inference of large phylogenetic trees. Bioinformatics. 2005;21:456–463. doi: 10.1093/bioinformatics/bti191. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web-servers. Syst Biol. 2008;75:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Adams EN. Consensus techniques and the comparison of taxonomic trees. Syst Zool. 1972;21:390–397. doi: 10.2307/2412432. [DOI] [Google Scholar]
- Nixon KC, Wheeler QD. In: Extinction and Phylogeny. Wheeler QD, Novacek M, editor. New York: Colombia University Press; 1991. Extinction and the origin of species; pp. 119–143. [Google Scholar]
- Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaché AD, Rannala B. The accuracy of species tree estimation under simulation: a comparison of methods. Syst Biol. 2011;60:126–137. doi: 10.1093/sysbio/syq073. [DOI] [PubMed] [Google Scholar]
- Heled J. biopy: a small collection of bioinformatic scripts. 2011. Python package 0.1.2.
- Bouckaert RR. DensiTree: making sense of sets of phylogenetic trees. Bioinformatics. 2010;26:1372–1373. doi: 10.1093/bioinformatics/btq110. [DOI] [PubMed] [Google Scholar]
- Bacon CD, Johnson GP, Meimberg H, Puppo P, Simmons MP, Wagner WL. Development of microsatellites in the Hawaiian endemic palm Pritchardia martii and their utility in congeners. Am J Bot PNP. 2011;6:139–140. doi: 10.3732/ajb.1100073. [DOI] [PubMed] [Google Scholar]
- Amos W, Hoffman JI, Frodsham A, Zhang L, Best S, Hill AVS. Automated binning of microsatellite alleles: problems and solutions. Mol Ecol Notes. 2007;7:10–14. [Google Scholar]
- Meirmans PG, Van Tienderen PH. GENOTYPE and GENODIVE: two programs for the analysis of genetic diversity of asexual organisms. Mol Ecol Notes. 2004;4:792–794. doi: 10.1111/j.1471-8286.2004.00770.x. [DOI] [Google Scholar]
- Oosterhout CV, Hutchinson WF, Wills DPM, Shipley P. Micro-checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes. 2004;4:535–538. doi: 10.1111/j.1471-8286.2004.00684.x. [DOI] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molec Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Earl DA. Structure Harvester v0.6. http://users.soe.ucsc.edu/%7Edearl/software/struct_harvest/
- Jakobbson M, Rosenberg NA. CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics. 2007;23:1801–1806. doi: 10.1093/bioinformatics/btm233. [DOI] [PubMed] [Google Scholar]
- Rosenberg NA. Distruct: a program for the graphical display of population structure. Mol Ecol Notes. 2004;4:137–138. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Parsimony strict consensus trees of all the sequence data summarized to show only the inter-generic relationships and Pritchardia from different island chains. Parsimony jackknife support values above, and likelihood bootstrap values below each branch of each gene individually, the plastid partition, and the simultaneous analysis.
Table S1. Mutually exclusive character states were used to test if gene flow had ceased between the sampled populations using population aggregation analysis for each of the three datasets listed in columns with spaces between each of the independent lineages. In the sequence dataset, terminals with missing data for diagnostic characters were arbitrarily assigned to a single group rather than collapsing the otherwise diagnosable groups and are indicated with *.
Figure S2. Parsimony simultaneous analysis and strict consensus tree of all the 105 terminals sampled for nucleotide data with parsimony jackknife values shown.
Figure S3. The individual nuclear gene trees estimated for Pritchardia species delimitation as shown in the parsimony strict consensus with parsimony jackknife values above and likelihood bootstrap values below each branch.
Figure S4. The individual plastid gene trees and the plastid simultaneous-analysis estimated for Pritchardia species delimitation with jackknife branch support values above and bootstrap values below each branch.