Abstract
The Nicotiana attenuata LECTIN RECEPTOR KINASE 1 (LecRK1) has been recently identified as a component of the mechanism used by plants to suppress the Manduca sexta-triggered accumulation of salicylic acid (SA). The suppression of the SA burst by LecRK1 allows for the unfettered induction of jasmonic acid (JA)-mediated defense responses against M. sexta herbivory. LecRK1 contains a multi-domain extracellular region composed of a G-type Lectin domain and a PAN-AP domain separated by a variable sequence with low similarity to an EGF domain. The LecRK1 intracellular region is composed of a single domain structure with predicted Ser/Thr protein kinase activity. The multi-domain structure of the extracellular region of LecRK1 adds a level of complexity in terms of the potential ligands that this receptor protein could recognize.
Keywords: defense responses, insect elicitor, jasmonic acid, lectin receptor kinase, plant-insect interactions, salicylic acid
Insect-associated elicitors and identification of Na-LecRK1
The recognition of phytophagous insects by plants induces a set of very specific responses aimed to deter tissue consumption and to reprogram the metabolism and development of the plant to tolerate the herbivore. The recognition of insects by plants requires the plant's ability to perceive chemical cues generated by the insects and to distinguish a particular pattern of tissue disruption.1 Relatively little is known about the molecular basis of insect perception by plants and the signaling mechanisms directly associated to this perception. During insect feeding, components of the oral secretions (OS) or saliva of insects become into contact with plant cells and elicitors present in these insect-derived fluids are perceived by plant cells to initiate the activation of specific signaling cascades.1,2 Importantly, several examples have also shown that OS components can interfere with or even suppress the activation of defense responses in plants. Thus, although some OS components are perceived by plants as a signal of herbivore attack, others interfere with the induction of defense responses allowing the insect to feed in a “stealthy” manner. For example, mandibular glands of Helicoverpa zea secrete salivary glucose oxidase (GOX), an enzyme that functions as an effector to suppress the induced defenses of the host plant.3 Experimental evidence suggests that GOX contributes to the initial oxidative burst of H2O2 observed in leaves damaged by herbivores4-6 and the enhanced oxidative burst suppresses the induction of plant defense responses. GOX activity in Spodoptera exigua OS produce high levels of H2O2 that induce a strong salicylic acid (SA) burst in N. attenuata leaves.7 These high levels of SA attenuate the induction of JA-mediated defense responses.
The insect-associated elicitors that act during folivory by chewing insects are diverse in structure. They can be enzymes (e.g., glucose oxidase, β-glucosidase),4,8 fatty acid–amino acid conjugates (FACs),2,9 sulfur-containing fatty acids (caeliferins),10 fragments of cell walls (e.g., pectins and oligogalacturonides),11 or peptides released from digested plant proteins (e.g., inceptins; proteolytic fragments of the chloroplastic ATP synthase γ-subunit).12 Importantly, most of these insect-associated elicitors are not general elicitors of responses against chewing insects in all plant species but are usually restricted to particular plant–insect associations (Table 1). This selectivity probably reflects the evolutionary history of both plants and their interacting insects and, hence, it is crucial to understand the mechanisms of plant–insect interactions in the evolutionary context of the interaction.2
Table 1. Examples of chewing insect-associated elicitors that induce specific responses in plants during insect folivory.
| Elicitors | Insect species | Plant species |
|---|---|---|
| Glucose Oxidase (GOX) |
Helicoverpa zea Spodoptera exigua Helicoverpa armigera Other Lepidoptera and Hymenoptera |
Nicotiana tabacum (tobacco) Nicotiana attenuata (coyote tobacco) Medicago truncatula Solanum lycopersicum (tomato) |
| β-glucosidase |
Pieris brassicae |
Phaseolus lunatus (lima beans) Zea mays (maize) Brassica oleracea (cabbage) |
|
N-acyl-amino acids (FACs) |
Spodoptera exigua Manduca sexta Teleogryllus taiwanemma Drosophila melanogaster Several Lepidoptera |
Zea mays Glycine max (soybean) Solanum melongena (eggplant) Nicotiana attenuata Solanum nigrum |
| Caeliferins |
Schistocerca americana |
Zea mays Arabidopsis thaliana |
| Inceptin |
Produced by degradation of the plant ATP synthase γ-subunit during folivory by Spodoptera frugiperda |
Vigna unguiculata (cowpea) |
| Oligouronides | Produced by degradation of plant cell walls during insect folivory | Solanum lycopersicum |
N. attenuata is an annual fire-chasing plant native to the Great Basin desert of the southwestern USA which has evolved a large number of specific induced responses against generalist and specialist herbivore species that co-exist with the plant in the same environment. For example, N. attenuata responds very specifically to the attack by M. sexta larvae. Among the responses induced by M. sexta folivory are changes in the expression of more than 500 genes, 90 proteins, 170 metabolites and the differential production of leaf volatile organic compounds (VOCs), jasmonic acid (JA), ethylene (ET) and salicylic acid (SA).13-17 Critical defense responses against M. sexta folivory are the accumulation of the defense molecules 17-hydroxygeranyllinalool diterpene glycosides (HGL-DTGs), nicotine, phenylpropanoid-polyamine conjugates and protease inhibitors (PIs).18-21 The accumulation of these defense molecules depend on de novo biosynthesis of JA and jasmonyl-Isoleucine (JA-Ile). In addition to these jasmonates, ET and SA also play critical roles in the modulation of induced JA-mediated defense and tolerance responses.7,22,23
As mentioned above, relatively little is known about the molecular basis of insect perception by plants and the signaling mechanisms directly associated to this perception. With the aim of identifying signal transduction components of the pathways operating early during the response to M. sexta larval attack, a SuperSAGE (serial analysis of gene expression) approach combined with next generation sequencing (NGS) was recently used to quantify the early transcriptional changes elicited by the FAC N-linolenoyl-glutamic acid (18:3-Glu) in N. attenuata plants 17. The analysis targeted mRNAs encoding regulatory components: rare transcripts with very rapid FAC-elicited kinetics. Among the 547 differentially expressed transcripts, more than 25% corresponded to putative regulatory components, including 22 protein kinases 17. Among these protein kinases was LECTIN RECEPTOR KINASE 1 (LecRK1).
LecRK1 is indispensable during M. sexta herbivory to suppress the insect-mediated inhibition of defense responses
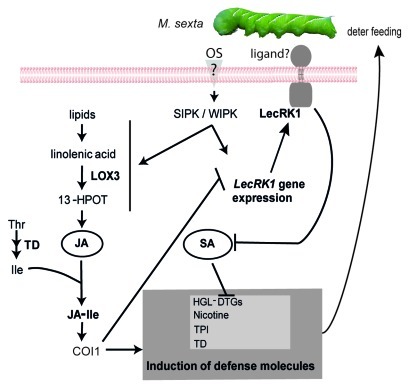
Gene function analysis performed by reducing LecRK1 expression in N. attenuata plants by both virus induced gene silencing (VIGS) and inverted repeated RNA interference (ir-RNAi) revealed that LecRK1 is essential to mount a full defense response against M. sexta folivory; larvae growing on plants with reduced expression of LecRK1 were 40 to 100% larger than larvae growing on wild type plants.24 The increased rates of larval growth were correlated with reduced levels of induction of several critical defense molecules, namely nicotine, HGL-DTGs, and trypsin protease inhibitors (TPIs). The expression of threonine deaminase (TD) was also several fold reduced in plants with reduced expression of LecRK1 compared with wild type plants. The reduced induction of these defense molecules was independent of the capacity of the plants to produce JA; the accumulation of JA and JA-Ile during M. sexta herbivory was similar in wild type plants and plants reduced in the expression of LecRK1. However, the accumulation of SA was increased by 2 fold in the latter.24 The ectopic expression of the nahG gene prevented the increased accumulation of SA during M. sexta herbivory and fully restored the defense response against this herbivore in plants with reduced expression of LecRK1. The results indicated that N. attenuata LecRK1 is indispensable to suppress the SA-mediated inhibition of defense responses and thereby to stimulate the unfettered JA-mediated induction of defense metabolites (Fig. 1).
Figure 1.
Proposed model for the role of LecRK1 in the N. attenuata defense response against M. sexta. During herbivory by M. sexta larvae, the larval OS induce the expression of the LecRK1 gene, however, LecRK1 is also constitutively expressed. The induction of LecRK1 depends on the activity of SIPK and WIPK, which in parallel activate the biosynthesis of JA.16 JA is conjugated to Ile to form JA-Ile, and TD supplies Ile for this biosynthetic process.28 JA-Ile induces the accumulation of defense metabolites or proteins (including nicotine, TPIs, HGL-DTGs and TD) via COI1-dependent mechanisms29,30 and inhibits the expression of LecRK1. LecRK1 suppresses the accumulation of SA induced by M. sexta herbivory which in turn allows for an unfettered induction of the defense metabolites nicotine, TPIs, HGL-DTGs and TD. LOX3: lipoxygenase 3; 13-HPOT: 13S-hydroperoxy-octadecatrienoic acid; SIPK, SA-induced protein kinase; WIPK, Wound-induced protein kinase; TD, threonine deaminase.
Analysis of LecRK1 mRNA expression in plants deficient in JA biosynthesis or perception showed that jasmonates inhibit the induction of the LecRK1 gene (Fig. 1). Moreover, plants with reduced expression of SIPK (SA-inducible protein kinase) and WIPK (Wound-inducible protein kinase) showed that these two regulatory components have a positive effect on the expression of LecRK1, consistent with their central role in the activation of defense responses against M. sexta herbivory25(Fig. 1). These results revealed that the induction of LecRK1 expression is under tight control; it is induced by OS elicitation but the levels of induction are checked by jasmonates (in a COI1-dependent manner). Thus, in this case, jasmonate levels would tune LecRK1 expression and thereby the accumulation of SA levels during insect herbivory (Fig. 1).
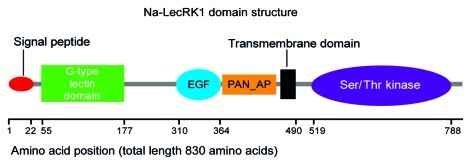
The amino acid sequence of LecRK1 contains a predicted N-terminal (Nt) extracellular region, a single transmembrane spanning α-helix and a C-terminal (Ct) cytoplasmic region (Fig. 2). The extracellular Nt region contains a predicted 22 amino acid signal sequence to the secretory pathway, a G-type Lectin domain, an Epidermal Growth Factor-like domain (EGF) domain and a PAN-AP (plasminogen/apple/ nematode) domain. The Ct cytoplasmatic region contains a predicted functional Ser/Thr kinase domain. The closest homologs to LecRK1 in other plant species that have been extensively studied are the brassica self-incompatibility determinant S receptor kinase (SRK) acting in self-recognition of pollen. SRK binds a cycteine-rich protein at a hypervariable region in the Nt extracellular region.26 LecRK1 has also close homology to the N. glutinosa RLK1 (Receptor-like Kinase 1) that interacts with elicitin (a conserved protein of ~98 amino acids) from Phytophthora capsici.27 The Lectin domains in the LecRK family of proteins usually do not carry all the conserved residues found in soluble lectins and which are responsible for carbohydrate binding. Moreover, the presence of the PAN-AP domain which has been shown to bind proteins and carbohydrates and the variable region between the lectin and the PAN-AP domains add a level of complexity in terms of the potential ligands that LecRK1 could recognize. The identification of these ligands will provide critical information about the mechanisms used by plants to perceive lepidopteran herbivory.
Figure 2.
N. attenuata LecRK1 predicted domains. Schematic representation of Na-LecRK1 domain composition and organization based on conserved domain analysis. PAN_AP, plasminogen-apple-nematode motif; EGF, epidermal growth factor-like motif; Ser/Thr kinase: serine/threonine protein kinase domain.
Acknowledgments
The DFG (BO3260/3–1) and the MPG are acknowledged for funding.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18324
References
- 1.Mithöfer A, Boland W. Recognition of herbivory-associated molecular patterns. Plant Physiol. 2008;146:825–31. doi: 10.1104/pp.107.113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonaventure G, VanDoorn A, Baldwin IT. Herbivore-associated elicitors: FAC signaling and metabolism. Trends Plant Sci. 2011;16:294–9. doi: 10.1016/j.tplants.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Eichenseer H, Mathews MC, Bi JL, Murphy JB, Felton GW. Salivary glucose oxidase: multifunctional roles for Helicoverpa zea? Arch Insect Biochem Physiol. 1999;42:99–109. doi: 10.1002/(SICI)1520-6327(199909)42:1<99::AID-ARCH10>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 4.Musser RO, Hum-Musser SM, Eichenseer H, Peiffer M, Ervin G, Murphy JB, et al. Herbivory: caterpillar saliva beats plant defences. Nature. 2002;416:599–600. doi: 10.1038/416599a. [DOI] [PubMed] [Google Scholar]
- 5.Maffei ME, Mithöfer A, Arimura GI, Uchtenhagen H, Bossi S, Bertea CM, et al. Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol. 2006;140:1022–35. doi: 10.1104/pp.105.071993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maffei ME, Mithöfer A, Boland W. Before gene expression: early events in plant-insect interaction. Trends Plant Sci. 2007;12:310–6. doi: 10.1016/j.tplants.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Diezel C, von Dahl CC, Gaquerel E, Baldwin IT. Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiol. 2009;150:1576–86. doi: 10.1104/pp.109.139550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattiacci L, Dicke M, Posthumus MA. beta-Glucosidase: an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc Natl Acad Sci USA. 1995;92:2036–40. doi: 10.1073/pnas.92.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alborn HT, Turlings TC, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH. An elicitor of plant volatiles from beet armyworm oral secretion. Science. 1997;276:945–9. doi: 10.1126/science.276.5314.945. [DOI] [Google Scholar]
- 10.Alborn HT, Hansen TV, Jones TH, Bennett DC, Tumlinson JH, Schmelz EA, et al. Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proc Natl Acad Sci USA. 2007;104:12976–81. doi: 10.1073/pnas.0705947104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bishop PD, Makus DJ, Pearce G, Ryan CA. Proteinase Inhibitor-Inducing Factor Activity in tomato leaves resides in oligosaccharides enzymically released from cell-walls. Proc Natl Acad Sci USA. 1981;78:3536–40. doi: 10.1073/pnas.78.6.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmelz EA, Carroll MJ, LeClere S, Phipps SM, Meredith J, Chourey PS, et al. Fragments of ATP synthase mediate plant perception of insect attack. Proc Natl Acad Sci USA. 2006;103:8894–9. doi: 10.1073/pnas.0602328103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermsmeier D, Schittko U, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. I. Large-scale changes in the accumulation of growth- and defense-related plant mRNAs. Plant Physiol. 2001;125:683–700. doi: 10.1104/pp.125.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giri AP, Wunsche H, Mitra S, Zavala JA, Muck A, Svatos A, et al. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VII. Changes in the plant's proteome. Plant Physiol. 2006;142:1621–41. doi: 10.1104/pp.106.088781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaquerel E, Heiling S, Schoettner M, Zurek G, Baldwin IT. Development and validation of a liquid chromatography-electrospray ionization-time-of-flight mass spectrometry method for induced changes in Nicotiana attenuata leaves during simulated herbivory. J Agric Food Chem. 2010;58:9418–27. doi: 10.1021/jf1017737. [DOI] [PubMed] [Google Scholar]
- 16.Kallenbach M, Alagna F, Baldwin IT, Bonaventure G. Nicotiana attenuata SIPK, WIPK, NPR1, and fatty acid-amino acid conjugates participate in the induction of jasmonic acid biosynthesis by affecting early enzymatic steps in the pathway. Plant Physiol. 2010;152:96–106. doi: 10.1104/pp.109.149013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilardoni PA, Schuck S, Jüngling R, Rotter B, Baldwin I, Bonaventure G. SuperSAGE analysis of the Nicotiana attenuata transcriptome after fatty acid-amino acid elicitation (FAC): identification of early mediators of insect responses. BMC Plant Biol. 2010;10:66. doi: 10.1186/1471-2229-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jassbi AR, Gase K, Hettenhausen C, Schmidt A, Baldwin IT. Silencing geranylgeranyl diphosphate synthase in Nicotiana attenuata dramatically impairs resistance to tobacco hornworm. Plant Physiol. 2008;146:974–86. doi: 10.1104/pp.107.108811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaur H, Heinzel N, Schottner M, Baldwin IT, Galis I. R2R3-NaMYB8 regulates the accumulation of phenylpropanoid-polyamine conjugates, which are essential for local and systemic defense against insect herbivores in Nicotiana attenuata. Plant Physiol. 2010;152:1731–47. doi: 10.1104/pp.109.151738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT. Nicotine's defensive function in nature. PLoS Biol. 2004;2:E217. doi: 10.1371/journal.pbio.0020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zavala JA, Patankar AG, Gase K, Hui DQ, Baldwin IT. Manipulation of endogenous trypsin proteinase inhibitor production in Nicotiana attenuata demonstrates their function as antiherbivore defenses. Plant Physiol. 2004;134:1181–90. doi: 10.1104/pp.103.035634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doares SH, Narvaez-Vasquez J, Conconi A, Ryan CA. Salicylic acid inhibits synthesis of proteinase inhibitors in tomato leaves induced by systemin and jasmonic acid. Plant Physiol. 1995;108:1741–6. doi: 10.1104/pp.108.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stotz HU, Koch T, Biedermann A, Weniger K, Boland W, Mitchell-Olds T. Evidence for regulation of resistance in Arabidopsis to Egyptian cotton worm by salicylic and jasmonic acid signaling pathways. Planta. 2002;214:648–52. doi: 10.1007/s004250100656. [DOI] [PubMed] [Google Scholar]
- 24.Gilardoni PA, Hettenhausen C, Baldwin IT, Bonaventure G. Nicotiana attenuata LECTIN RECEPTOR KINASE1 suppresses the insect-mediated inhibition of induced defense responses during Manduca sexta herbivory. Plant Cell 2011; [Epub ahead of print] PMID: 21926334. [DOI] [PMC free article] [PubMed]
- 25.Wu J, Hettenhausen C, Meldau S, Baldwin IT. Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. Plant Cell. 2007;19:1096–122. doi: 10.1105/tpc.106.049353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanabria N, Goring D, Nurnberger T, Dubery I. Self/nonself perception and recognition mechanisms in plants: a comparison of self-incompatibility and innate immunity. New Phytol. 2008;178:503–14. doi: 10.1111/j.1469-8137.2008.02403.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim YT, Oh J, Kim KH, Uhm JY, Lee BM. Isolation and characterization of NgRLK1, a receptor-like kinase of Nicotiana glutinosa that interacts with the elicitin of Phytophthora capsici. Mol Biol Rep. 2010;37:717–27. doi: 10.1007/s11033-009-9570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang JH, Wang L, Giri A, Baldwin IT. Silencing threonine deaminase and JAR4 in Nicotiana attenuata impairs jasmonic acid-isoleucine-mediated defenses against Manduca sexta. Plant Cell. 2006;18:3303–20. doi: 10.1105/tpc.106.041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paschold A, Halitschke R, Baldwin I. Co(i)-ordinating defenses: NaCOI1 mediates herbivore- induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. Plant J. 2007;51:79–91. doi: 10.1111/j.1365-313X.2007.03119.x. [DOI] [PubMed] [Google Scholar]
- 30.Heiling S, Schuman MC, Schoettner M, Mukerjee P, Berger B, Schneider B, et al. Jasmonate and ppHsystemin regulate key Malonylation steps in the biosynthesis of 17-Hydroxygeranyllinalool Diterpene Glycosides, an abundant and effective direct defense against herbivores in Nicotiana attenuata. Plant Cell. 2010;22:273–92. doi: 10.1105/tpc.109.071449. [DOI] [PMC free article] [PubMed] [Google Scholar]