Abstract
Introduction:
The smoking cessation aid, varenicline, has higher affinity for the alpha4beta2-subtype of the nicotinic acetylcholine receptor (α4β2*-nAChR) than for other subtypes of nAChRs by in vitro assays. The mechanism of action of acute varenicline was studied in vivo to determine (a) subtype activation associated with physiological effects and (b) dose relationship as an antagonist of nicotine.
Methods:
Acute doses of saline, nicotine, and varenicline were given to mice, and locomotor depression and hypothermia were measured. Subunit null mutant mice as well as selective antagonists were used to study mode of action of varenicline as an agonist. Varenicline as an antagonist of nicotine was also investigated.
Results:
Varenicline evokes locomotor depression and hypothermia at higher doses than necessary for nicotine. Null mutation of the α7- or β2-nAChR subunit did not decrease the effectiveness of varenicline; however, null mutation of the β4 subunit significantly decreased the magnitude of the varenicline effect. Effects of the highest dose studied were blocked by mecamylamine (general nAChR antagonist) and partially antagonized by hexamethonium (largely peripheral nAChR antagonist). No significant block was seen with ondansetron antagonist of 5-hydroxytryptamine 3 receptor. Using a dose of nicotine selective for β2*-nAChR subtype effects with these tests, dose-dependent antagonism by varenicline was seen. Effective inhibitory doses were determined and appear to be in a range consistent with binding affinity or desensitization of β2*-nAChRs.
Conclusions:
Varenicline acts as a functional antagonist of β2*-nAChRs, blocking certain effects of nicotine. At higher doses, varenicline is an agonist of β4*-nAChRs producing physiological changes in mice.
Introduction
Varenicline, a smoking cessation aid, is marketed as a selective partial agonist for the alpha4beta2 (α4β2*)-nicotinic acetylcholine receptor (nAChR). Data on the affinity, potency, and efficacy of varenicline at various nAChR subtypes measured in vitro indicate varenicline has considerably higher affinity for the α4β2*-nAChR than for other subtypes of nAChRs in humans, rats, and mice (Anderson et al., 2008; Carroll et al., 2008; Coe et al., 2005; Grady et al., 2010; Rollema, Faessel, & Williams, 2009; Rollema et al., 2007; Smith et al., 2007). The activation potency is also selective for the α4β2*-nAChR subtype (Grady et al., 2010; Kuryatov, Berrettini, & Lindstrom, 2011; Mihalak, Carroll, & Luetje, 2006; Papke, Wecker, & Stitzel, 2010; Rollema et al., 2007). Varenicline is a partial agonist at both α4β2*- and α6β2*-nAChR subtypes and a full agonist at both the α7- and α3β4*-subtypes (Grady et al., 2010; Kuryatov et al., 2011; Mihalak et al., 2006; Papke et al., 2010; Rollema et al., 2007).
Despite the in vitro data indicating that varenicline has a higher affinity than nicotine, in animal models, a higher dose of varenicline is needed to produce an effect equivalent to nicotine for some behaviors (Carroll et al., 2008; Cunningham & McMahon, 2011; Turner, Castellano, & Blendy, 2010). In addition, a few reports have indicated that varenicline can block certain behavioral effects of nicotine (Raybuck et al., 2008; Zaniewska, McCreary, Stefanski, Przegalinski, & Filip, 2008). It is unclear whether these observations are the result of the partial agonist properties of varenicline or combinations of activity and/or desensitization at various nAChR subtypes. With widespread use in humans for smoking cessation, a number of side effects of varenicline have been reported (Ebbert, Wyatt, Hays, Klee, & Hurt, 2010). More knowledge on the relative effects of nicotine and varenicline at the various subtypes of nAChR may be helpful in designing new compounds with fewer side effects.
As a first step, we evaluated potential nicotine-like pharmacological effects of varenicline in a mouse model. We used both wild-type (WT) C57BL/6 mice as well as nAChR subunit-null mutant mice on the C57BL/6 background to assess the ability of varenicline to evoke locomotor depression and hypothermia, two effects of nicotine commonly studied in mice. In addition, we have used antagonists to block certain receptors in order to ascertain which subtypes of nAChR are mediating varenicline-induced responses. To assess only β2*-nAChR–mediated effects, we used a lower dose of nicotine (0.5 mg/kg intraperitoneal [ip]) that selectively elicits locomotor depression and hypothermia mediated by β2*-nAChRs (Tritto et al., 2004) and investigated whether a prior dose of varenicline blocked these nicotine-mediated effects. Our results show that, at low doses (below ∼0.1 mg/kg), varenicline acts as a functional antagonist of the β2*-nAChR, while at higher doses (1.0 mg/kg and above), it acts as an agonist at β4*-nAChRs possibly at peripheral locations.
Methods
Mice
C57BL/6 mice and subunit null mutant mice were bred and housed at the Institute for Behavioral Genetics, University of Colorado (Boulder, CO). All animal care and experimental procedures were in accordance with National Institutes of Health (NIH) guidelines and approved by the Animal Care and Utilization Committee of the University of Colorado. The subunit null mutant mice (β2, Picciotto et al., 1995; β4, Xu et al., 1999; and α7, Orr-Urtreger et al., 1997) have been maintained via heterozygous matings and backcrossed onto the C57BL/6 background at this facility for a minimum of 10 generations. Genotypes were determined by polymerase chain reaction from tail clippings (Salminen et al., 2004).
Mice had free access to food and water. A 12-hr light/dark cycle (lights on from 7:00 a.m. to 7:00 p.m.) was maintained, and room temperature was 22 ± 2 °C. Mice of both sexes and between 60 and 150 days were tested, and weights at time of testing were between 17 and 32 g.
Drugs
(−)-Nicotine (freebase), hexamethonium dihydrochloride, and ondansetron hydrochloride dihydrate were products of Sigma Chemical Co. (St Louis, MO). Mecamylamine hydrochloride was a gift from Merck, Sharp and Dohme Research Lab (Rahway, NJ). Varenicline tartrate was synthesized and donated by Targacept, Inc. (Winston-Salem, NC). All doses were calculated as freebase.
Behavioral Tests
Testing equipment and procedures used have been previously described (Collins, Evans, Miner, & Marks, 1986; Marks, Romm, Bealer, & Collins, 1985; McCallum, Collins, Paylor, & Marks, 2006; Tritto et al., 2004). Briefly, a symmetrical red acrylic Y-maze with three enclosed arms, each 26 cm long, 6.1 cm wide, and 10.2 cm high, was used. Crosses and rears were monitored by infrared beam breaks. A circular open-field arena (60-inch diameter) with bright-field illumination (4,500 lumens) was used, with activity monitored by infrared beam breaks set at 15 cm intervals. A Thermalert rectal probe for mice was lubricated with peanut oil. All injections were ip in isotonic saline (0.01 ml/g of body weight), and drugs were calculated as the free-base form. Mice were allowed to acclimate to the behavioral test room for at least 1 hr prior to testing. Y-maze activity (crosses and rears) was evaluated for 3 min starting 3 min after the final injection, followed by open field (distance traveled in 5 min) starting at 6 min after the final injection, and followed by rectal temperature measurement at 15 min after the final injection.
Protocol 1
Mice (C57BL/6 or various subunit null mutant mice and littermate WT controls) were injected ip and tested as described above.
Protocol 2
Mice were given saline, hexamethonium, mecamylamine, or ondansetron ip and placed in an empty cage with bedding. After 10 min, a second injection of either saline or varenicline was administered and temperature measured 15 min after the second injection (Collins et al., 1986; Zambrano, Marks, Cassels, & Maccioni, 2009). Mecamylamine, a general nAChR antagonist that is permeable to the blood-brain barrier, was administered at a dose (1 mg/kg; 0.8 mg/kg freebase) previously shown to block hypothermia mediated by nAChRs (Collins et al., 1986; Zambrano et al., 2009). Hexamethonium, an antagonist of ganglionic-type nAChR that does not cross the blood-brain barrier, was given at a dose (10 mg/kg; 7.8 mg/kg freebase) previously shown to be effective in blocking nAChR-mediated hypothermia (Zambrano et al., 2009). Ondansetron, an antagonist of the antagonist of 5-hydroxytryptamine 3 receptor (5 HT3) receptor (a receptor that has similarities to nAChRs and used to block nausea in humans, one of the reported side effects of varenicline), was given at a dose (2 mg/kg; 1.6 mg/kg freebase) previously shown to reduce sleep apnea in susceptible mice (Real, Seif, Adrien, & Escourrou, 2009).
Protocol 3
Mice were given an injection of low-dose varenicline or saline and placed in an empty cage with bedding. After 10 min, mice received a second injection of either saline or 0.5 mg/kg nicotine, and the behavioral testing procedure followed as above.
Data Analysis
Data were analyzed by one- or two-way analysis of variance (ANOVA) as indicated using Graph Pad Prism 5.0 (Graph Pad Software, La Jolla, CA). Dunnett’s multiple comparison post-hoc test was used to compare null mutant mice to WT as well as antagonist treatment to saline. To determine the dose for 50% maximum effect (ED50) and the dose for 50% inhibition (ID50), data were fit to modified Hill equations using SigmaPlot 5.0 DOS (Tritto et al., 2004). For varenicline-induced activity measures:
where Rdose is the response measured following a given dose of varenicline, Rsal is the average response following saline injection, ED50 is the dose giving 50% maximal effect, and n is the Hill coefficient. For varenicline-induced hypothermia:
where td = maximal temperature decrease. For varenicline inhibition of an effect of nicotine:
where Rdose is the response following a given dose of varenicline prior to 0.5 mg/kg nicotine, Rmax is the effect of nicotine after saline (i.e., maximum effect of 0.5 mg/kg nicotine with no prior varenicline), ID50 is the dose of varenicline giving 50% maximal effect, R0 is the residual response not affected by 0.5 mg/kg nicotine, and n is the Hill coefficient.
Results
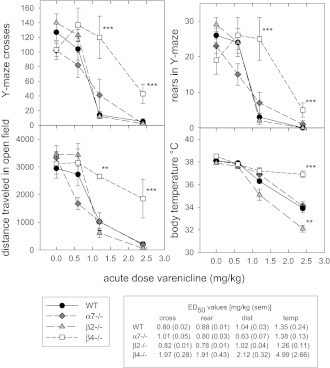
The effects of acute administration of varenicline to WT and α7-, β2-, and β4-null mutant mice are shown in Figure 1 with statistical analyses summarized in the figure legend. Like nicotine (for nicotine effects, see McCallum et al., 2006; Tritto et al., 2004), acute administration of varenicline elicits dose-dependent decreases in locomotor measures (Y-maze crosses and rears and distance traveled in the open field) and body temperature. Full pharmacological effects were observed over a relatively narrow dose range yielding steep dose–response curves. No measurable changes were observed for a dose of 0.6 mg/kg ip (2.8 μmol/kg) varenicline, while a dose of 2.4 mg/kg (11.4 μmol/kg) elicited maximal locomotor depression and a 4 °C decrease in body temperature in WT mice. The maximal response is similar to that observed following a dose of 1 mg/kg (6.2 μmol/kg) nicotine ip. The ED50 values for varenicline in WT mice, which are summarized in Figure 1, are similar for each of the tests and range from 0.80 ± 0.02 mg/kg for Y-maze crosses to 1.35 ± 0.24 mg/kg for body temperature. The effects of varenicline administration were measured on WT mice as well as α7-, β2-, and β4-null mutant mice for each of the responses (Figure 1). Statistical analyses are summarized in the legend to Figure 1. Comparison of the differences in ED50 values for each test reveals the source of the interaction term. Unlike with nicotine (Tritto et al., 2004), deletion of the β2 subunit did not decrease the effect of varenicline and even showed a significant increase from WT for the hypothermia measure. As was seen for nicotine, deletion of the α7 subunit showed no decrease from WT. However, deletion of the β4 subunit did significantly decrease the effect of varenicline as shown by decreased effectiveness of varenicline and increases in ED50 values for each of the four tests (Figure 1). Therefore, it appears that these measures of effects of varenicline are mediated largely through activation of β4*-nAChRs. In contrast, the effects of nicotine are via activation of β2*-nAChRs at doses below 0.5 mg/kg and via activation of a mixture of β2*-nAChRs and β4*-nAChRs at higher doses (McCallum et al., 2006; Tritto et al., 2004).
Figure 1.
Effects of acute varenicline in nicotinic acetylcholine receptor (nAChR) subunit-null mutant mice. Mice were injected intraperitoneally (ip) with saline or varenicline (mg/kg as freebase) and tested by Protocol 1. For each condition, 4–14 mice were tested for each genotype at each dose. Analysis by two-way analysis of variance (ANOVA) for genotype and dose indicated significant effects as follows: for Y-maze crosses, main effects of genotype, F(3, 126) = 7.2, p < .001, power = 0.97; dose F(3, 126) = 73.5, p < .001, power = 1.00; and interaction, F(9, 126) = 4.8, p < .001, power = 0.99; for Y-maze rears, main effects of genotype, F(3, 126) = 3.2, p < .05, power = 0.55; dose, F(3, 126) = 76.2, p < .001, power = 1.00; and interaction, F(9, 126) = 5.4, p < .001, power = 1.00; for distance traveled, main effects of genotype, F(3, 125) = 4.8, p < .01, power = 0.82; dose, F(3, 125) = 44.3, p < .001, power = 1.00; and interaction, F(9, 125) = 3.0, p < .01, power = 0.83; and for temperature, main effects of genotype, F(3, 126) = 20.3, p < .001, power = 1.00; dose, F(3, 126) = 116.3, p < .001, power = 1.00; and interaction, F(9, 126) = 8.0, p < .001, power = 1.00. These data were also analyzed by one-way ANOVA for effects of genotype at each dose and analyzed by Dunnett’s post-hoc test for difference from wild type (WT). Significant differences are indicates by **p < .01 and ***p < .001. ED50 values were calculated as described in Methods section.
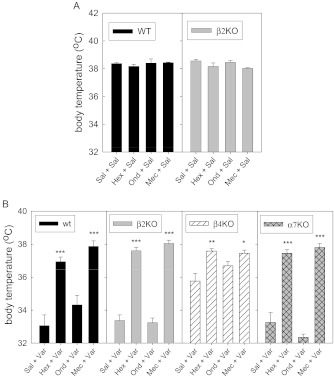
In order to further characterize potential sites of action of varenicline in vivo, various antagonists, at doses known to be effective in mice (see Methods section), were given ip before saline or varenicline administration to WT-, β2-, β4-, or α7-null mutant mice. Results are presented in Figure 2. Statistical analyses are summarized in the legend to Figure 2. No significant effects of antagonists were detected when administered prior to a saline injection in either WT- or β2-null mutant mice (Figure 2A). As seen in Figure 1, varenicline has a diminished effect in β4-null mutant mice (Figure 2B). By two-way ANOVA, varenicline effects differ from WT only in the β4-null mutant genotype, having less effect. However, in all genotypes, the broad-spectrum, central nervous system-permeable nAChR antagonist mecamylamine blocked varenicline-induced hypothermia confirming varenicline’s action as an agonist at nAChRs (Figure 2B). The ganglionic antagonist, hexamethonium, that does not easily cross the blood-brain barrier also significantly decreased varenicline-induced hypothermia, indicating that part of the hypothermia induced by varenicline is peripherally mediated. Note that hexamethonium was effective in the β4-null mutants indicative of activation of α3β2*-nAChR remaining in the periphery in these mice. The 5-HT3 antagonist, ondansetron, was without significant effect in this test. Activation of the 5-HT3 receptor has been shown to result in hypothermia in mice (Naumenko, Kondaurova, & Popova, 2009); however, varenicline does not appear to activate this receptor in mice (Lummis, Thompson, Bencherif, & Lester, 2011). The results presented above indicate that the in vivo locomotor depression and hypothermic effects elicited by varenicline in this study are at least partially mediated by β4*-nAChR (Figure 1) and that a fraction of the hypothermia resulting from varenicline administration may be mediated by peripheral nAChR (Figure 2). Based on an affinity comparison, varenicline is selective for the α4β2*-nAChR and should be somewhat more potent than nicotine at this subtype. However, varenicline is a partial, low-efficacy agonist at the α4β2*-nAChR (Grady et al., 2010; Kuryatov et al., 2011; Mihalak et al., 2006; Papke et al., 2010; Rollema et al., 2007). Being a partial agonist, perhaps, varenicline does not elicit enough receptor activation to elicit locomotor depression or hypothermia mediated by the α4β2*-nAChR subtype. Consequently, it could be acting primarily as a competitive antagonist or desensitizer of these receptors. Therefore, to evaluate the hypothesis that varenicline could inhibit α4β2-nAChR responses, we administered low doses of varenicline prior to a 0.5 mg/kg dose of nicotine that elicits effects of locomotor depression and hypothermia mediated primarily by the β2*-nAChR (McCallum et al., 2006; Tritto et al., 2004).
Figure 2.
Response to varenicline after antagonists in wild-type (WT) and null mutant mice. Mice were injected ip as indicated; protocol 2 was followed. The first intraperitoneal (ip) injection was saline or an antagonist calculated as freebase (Hex = hexamethonium, 7.8 mg/kg; Ond = ondansetron, 1.6 mg/kg; Mec = mecamylamine, 0.8 mg/kg). A second injection of saline (A) or varenicline (2.3 mg/kg freebase; B) was given 10 min later. For each condition, 3–10 mice were tested. Analysis by one-way analysis of variance (ANOVA) indicated no effect of antagonists alone for either WT mice or β2-null mutant mice. By two-way ANOVA (for antagonist and dose of varenicline), significant effects were seen for genotype, F(3, 78) = 17.4, p < .001, power = 1.00, and antagonist treatment, F(8, 78) = 18.9, p < .001, power = 1.00. The β4 genotype differs from all others, p < .001, while the other null mutants did not differ from WT. The data were also analyzed by one-way ANOVA for antagonist effects within each genotype using Dunnett’s post-hoc test for difference from saline. Significant differences are indicated by *p < .05, **p < .01, ***p < .001.
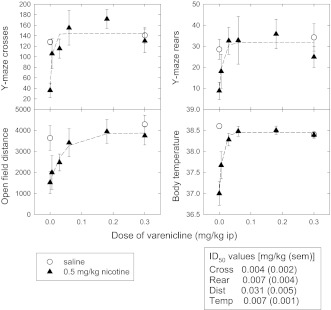
Results shown in Figure 3 demonstrate that varenicline inhibits the effects of a 0.5 mg/kg dose of nicotine. ID50 values for varenicline inhibition of nicotine at 0.5 mg/kg ip (3.1 μmol/kg) were similar for inhibition of nicotine-elicited decreases in Y-maze crosses and rears and body temperature (0.004–0.007 mg/kg or 0.02–0.03 μmol/kg), while the ID50 for blockade of nicotine-induced decreases in open-field activity was somewhat higher (0.031 mg/kg or 0.14 μmol/kg). On a mole for mole basis, these varenicline ID50 values are considerably lower than the nicotine ED50 values (Tritto et al., 2004) needed to produce effects on Y-maze rears, distance traveled in the open-field, Y-maze crosses, and hypothermia (18-, 25-, 74-, and 110-fold, respectively). It appears that varenicline at low doses acts as a functional antagonist at β2*-nAChRs and at higher doses as an agonist at β4*-nAChRs, while nicotine can affect these measures as an agonist at both of these subtypes.
Figure 3.
Effect of low-dose varenicline on nicotine-induced behaviors. C57Bl/6 mice were injected intraperitoneally (ip) with the indicated dose of varenicline (calculated as freebase), followed by an injection of saline or nicotine (0.5 mg/kg freebase) ip 10 min later followed by testing (see Protocol 3). For each condition, 4–8 mice were tested. ID50 values were calculated as described in Methods section for varenicline to block the effect of nicotine.
Discussion
By in vivo tests, measuring the well-known locomotor-depressant and hypothermia-inducing effects of nicotinic agonists, varenicline appears to have two different actions. Varenicline, in vivo, acts effectively as an antagonist at β2*-nAChRs and as an agonist at β4*-nAChRs.
Acute administration of relatively high doses of varenicline elicits locomotor depression and hypothermia. Nicotine elicits these same effects by activation of either β2*-nAChRs or β4*-nAChRs (McCallum et al., 2006; Tritto et al., 2004), but not by activation of α7-nAChRs (Tritto et al., 2004). The lack of effect of deletion of the α7 subunit indicates the same is true for varenicline (Figure 1). Nicotine effects mediated by activation of β2*-nAChRs are seen with lower doses of nicotine than those mediated by activation of β4*-nAChRs, a result predicted by their relative in vitro concentration for 50% effect (EC50) (Tritto et al., 2004). In contrast to the reduced potency of nicotine determined in β2-null mutant mice (1.4- to 4.0-fold shift to higher ED50 values; Tritto et al., 2004), deletion of the β2 subunit did not decrease the effectiveness of varenicline (Figure 1). In fact, an increase in effectiveness was seen for hypothermia in the β2-null mutant mice at the highest varenicline dose (Figure 1). Thus, varenicline appears to elicit its direct effects on locomotor depression and hypothermia largely via activation of β4*-nAChRs, as demonstrated by decreases in the effect of varenicline in β4-null mutant mice (2.0- to 3.7-fold shift to higher ED50 values; Figure 1). Furthermore, blockade by hexamethonium indicates that the locomotor-depressant and hypothermic effects of varenicline are elicited by activating, in part, peripheral, probably ganglionic, receptors (Figure 2). This result is also predicted by in vitro assays showing that varenicline is a full agonist at β4*-nAChRs (Grady et al., 2010; Mihalak et al., 2006; Rollema et al., 2010). Varenicline is also a full agonist at the α7*-nAChRs (Grady et al., 2010; Mihalak et al., 2006; Papke et al., 2010; Rollema et al., 2007, 2010). However, as seen for nicotine, this receptor subtype does not decrease these measures; no loss of effectiveness of varenicline was seen in α7-null mutant mice (Figure 1).
By in vitro assays, both nicotine and varenicline are more potent at activating β2*- than β4*-nAChRs (Grady et al., 2010; Mihalak et al., 2006; Rollema et al., 2007, 2010). With a molar comparison, varenicline is less potent than nicotine at producing in vivo locomotor depression and hypothermia in WT mice with ED50 values of 3.9–5.8 μmol/kg for varenicline (Figure 1) versus 0.6–3.2 μmol/kg for nicotine (Tritto et al., 2004). Nicotine sufficiently activates both β2*- and β4*-nAChRs, while varenicline is producing these effects solely by activation of β4*-nAChRs. The data presented here support the idea that varenicline does not activate β2*-nAChRs sufficiently to produce measurable effects on the tests administered but does produce effects by activation of β4*-nAChRs. These data are, therefore, consistent with previous in vitro data on affinity, potency, and efficacy of these drugs at various subtypes of nAChRs (Grady et al., 2010; Mihalak et al., 2006; Rollema et al., 2007, 2010).
In vitro experiments have also demonstrated that exposure of α4β2-nAChR to low concentrations of varenicline effectively blocks subsequent activation by ACh by either competing for the agonist site and/or desensitizing the receptor (Mihalak et al., 2006; Rollema et al., 2010). For mouse α4β2*-nAChRs, there is a factor of ∼100-fold in the concentrations required to bind versus activate (Marks, Robinson, & Collins, 1996). A similar factor appears to be true for varenicline (Grady et al., 2010; Mihalak et al., 2006; Rollema et al., 2007). Thus, in vivo, functional blockade of the effects of nicotine on β2*-nAChR by varenicline is consistent with in vitro data. The functional blockade is achieved following administration of relatively low doses of the drug (ID50 values of 0.004–0.030 mg/kg [0.02–0.14 μmol/kg] depending on the test; Figure 3).
The actions of varenicline at low doses, as an antagonist of β2*-nAChR, and at higher doses, as an agonist of β4*-nAChR, appear consistent with previous investigations with rodents in other laboratories. Tests where an effect of 0.6 mg/kg nicotine was seen, but that showed no effect of doses up to 1 mg/kg varenicline, include distance traveled in an elevated zero maze and homecage activity (Turner et al., 2010). Varenicline had little effect on tail flick or hot plate tests for antinociception at doses up to 10 mg/kg subcutaneous (sc) in mice, and the ED50 value for inducing hypothermia was 2.8 mg/kg (Carroll et al., 2008). These authors also reported that varenicline was very potent in antagonizing the effects of nicotine (2.5 mg/kg sc) in the tail flick (dose for 50% antagonism [AD50] of 0.0002 mg/kg) and hot plate tests (AD50 0.47 mg/kg); however, varenicline did not inhibit induction of hypothermia by a dose of nicotine that activates both β2*- and β4*-nAChRs (2.5 mg/kg). No direct effect of varenicline up to 1.0 mg/kg was found in a fear-conditioning paradigm, although at 0.1 mg/kg varenicline was able to prevent nicotine withdrawal effects in fear conditioning (Raybuck et al., 2008). In rats, acute nicotine (0.4 mg/kg sc) has a stimulatory effect on locomotor activity measured as distance traveled and varenicline at 0.3 mg/kg (0.18 mg/kg as freebase) had a smaller but significant effect on this measure also (Zaniewska et al., 2008). Perhaps, varenicline does produce enough activation via nAChRs for this stimulatory effect, which is not readily measured in mice. In this study, the effect of nicotine could be partially blocked by 0.1 mg/kg varenicline (0.06 mg/kg as freebase), again likely indicating an antagonist effect at β2*-nAChR. In a test of the ability of nicotine and varenicline to decrease responding for food in C57Bl/6 mice, nicotine (ED50 0.83 mg/kg ip) was more potent than varenicline (ED50 2.71 mg/kg ip; Cunningham & McMahon, 2011). While mecamylamine was able to block the effects of both drugs in this test, dihydro-β-erythroidine, selective for α4β2*-nAChRs, only blocked nicotine. This is consistent with agonism at α4β2-nAChRs by nicotine and agonism at β4*-nAChRs by varenicline.
In two more complex tests for anxiety, the marble-burying test and the NIH test for latency to feed in a novel environment, varenicline had effects at 0.1 mg/kg, a lower dose than for nicotine at 0.3 mg/kg (Turner et al., 2010). It has not been demonstrated whether these tests depend on activation or blockade of nAChRs. However, these data, indicating that varenicline is more potent than nicotine, would support the hypothesis that it is the blocking effect that may mediate changes in these behaviors.
Pharmacokinetics influences the concentrations of both drugs in the brain. Nicotine reaches the brain very quickly, likely somewhat faster than varenicline in mice (Reperant et al., 2010) where, after ip injection, peak effect of nicotine was seen at 15 min and varenicline not until 30 min. Nicotine is also cleared more quickly with a half-life of 6–7 min (Matta et al., 2007; Petersen, Norris, & Thompson, 1984) versus 1.4 hr for varenicline (Obach et al., 2006). It has been shown that rats and mice given the same dose of varenicline orally had similar peak concentrations even though the elimination of half-lives differ by species (1.4 and 4 hr, respectively). Varenicline is not metabolized extensively in mice; 90% is excreted unchanged (Obach et al., 2006), while nicotine is extensively metabolized (Matta et al., 2007). These pharmacokinetic factors will collectively affect brain concentrations; therefore, brain concentrations for these acute experiments are estimates.
Therapeutic doses of varenicline in humans are 0.5–2.0 mg/day or ∼0.01 to 0.03 mg/kg/day. Plasma varenicline levels effective for decreasing smoking are 7–10 ng/ml or ∼30 to 50 nM (Faessel et al., 2006), indicating that varenicline likely acts via binding to β2*-nAChRs. These pharmacologically effective concentrations would be insufficient to produce much agonist activity, even at the α4β2*-nAChR subtype (reported EC50 values for human α4β2*-nAChR range from 0.1 to 1.4 μM with efficacy values of 13%–22% of nicotine; Papke et al., 2010; Rollema et al., 2010). It appears likely that almost all β2*-nAChRs need to be occupied by varenicline to decrease smoking. Alternatively, these receptors could be desensitized by varenicline. Estimates for desensitization by varenicline for various forms of α4β2*-nAChR by expression in Xenopus oocytes are in the low nanomolar range, similar to binding Ki values (Papke, Trocme-Thibierge, Guendisch, Al Rubaiy, & Bloom, 2011; Papke et al., 2010; Rollema et al., 2010, Xiao et al., 2006). Therefore, the block by low doses of varenicline could be either by competition with nicotine for β2*-nAChR or by desensitization of β2*-nAChR.
Whether side effects of varenicline in humans are caused by binding to α4β2*-nAChRs or binding to (or activation of) other subtypes of nAChR, by interaction with other receptors, or by secondary effects such as histamine release (Rollema et al., 2009) or corticosterone release (Pauly, Grun, & Collins, 1990) is beyond the scope of this report. However, it appears that in order to be effective, concentrations of varenicline in humans need to be high enough to completely block or desensitize α4β2*-nAChRs (∼30 to 50 nM), about 100 times the binding Ki value. At doses that achieve 70 nM plasma levels, all subjects have nausea; however, this may be via interaction with receptors in the gut where concentrations could be higher (Rollema et al., 2009). Partial activation of α6β2-, α7-, or β4-nAChR or nonnicotinic receptors (such as 5-HT3; Lummis et al., 2011) may be possible at therapeutic doses leading to some side effects such as nausea. Alternatively, side effects, especially those involving complex behavior or physiology (e.g., vivid dreams or neuropsychiatric symptoms), may be inherent with this total block or desensitization of α4β2*-nAChRs (Mineur & Picciotto, 2010).
Summary
In mice, a dose of 0.5 mg/kg ip, nicotine acts as an agonist predominately at β2*-nAChRs to promote locomotor depression and hypothermia. At higher doses, nicotine can also act at β4*-nAChRs causing similar physiological effects. Varenicline, however, does not affect locomotor activity or body temperature at doses in the range where it activates β2*-nAChRs in vitro. Varenicline, a partial agonist at β2*-nAChRs, appears to have insufficient agonist activity at these receptors to affect these physiological measures. At higher doses, varenicline does decrease locomotor activity and body temperature via activation of β4*-nAChRs, many of which are in the periphery. Varenicline acts in the brain at low doses as an antagonist by its ability to block the effects of the natural agonist, ACh, or of a β2*-selective dose of nicotine.
Funding
This work was supported by National Institutes of Health grants (U19DA019375 and P30DA015663).
Declaration of Interests
None declared.
Acknowledgments
Varenicline used in this study was synthesized and provided by Targacept, Inc. We thank Bill Van Morter, Erin Meyers, and Esteban Loetz for animal husbandry and genotyping.
References
- Anderson DJ, Bunnelle W, Surber B, Du J, Surowy C, Tribollet E, et al. [3H]A-585539 [(1S,4S)-2,2-dimethyl-5-(6-phenylpyridazin-3-yl)-5-aza-2-azoniabicyclo[2.2.1]heptane], a novel high-affinity alpha7 neuronal nicotinic receptor agonist: Radioligand binding characterization to rat and human brain. Journal of Pharmacology and Experimental Therapeutics. 2008;324:179–187. doi: 10.1124/jpet.107.130062. doi:10.1124/jpet.107.130062. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: An alpha4beta2 nicotinic receptor partial agonist for smoking cessation. Journal of Medicinal Chemistry. 2005;48:3474–3477. doi: 10.1021/jm050069n. doi:10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Collins AC, Evans CB, Miner LL, Marks MJ. Mecamylamine blockade of nicotine responses: Evidence for two brain nicotinic receptors. Pharmacology, Biochemistry and Behavior. 1986;24:1767–1773. doi: 10.1016/0091-3057(86)90518-6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3737641. [DOI] [PubMed] [Google Scholar]
- Carroll F, Yokota Y, Ma W, Lee JR, Brieaddy LE, Burgess JP, et al. Synthesis, nicotinic acetylcholine receptor binding, and pharmacological properties of 3’-(substituted phenyl)deschloroepibatidine analogs. Bioorganic and Medicinal Chemistry. 2008;16:746–754. doi: 10.1016/j.bmc.2007.10.027. doi:10.1016/j.bmc.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CS, McMahon LR. The effects of nicotine, varenicline, and cytisine on schedule-controlled responding in mice: Differences in alpha4beta2 nicotinic receptor activation. European Journal of Pharmacology. 2011;654:47–52. doi: 10.1016/j.ejphar.2010.12.003. doi:10.1016/j.ejphar.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbert JO, Wyatt KD, Hays JT, Klee EW, Hurt RD. Varenicline for smoking cessation: Efficacy, safety, and treatment recommendations. Patient Preference and Adherence. 2010;4:355–362. doi: 10.2147/ppa.s10620. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21049087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faessel HM, Smith BJ, Gibbs MA, Gobey JS, Clark DJ, Burstein AH. Single-dose pharmacokinetics of varenicline, a selective nicotinic receptor partial agonist, in healthy smokers and nonsmokers. Journal of Clinical Pharmacology. 2006;46:991–998. doi: 10.1177/0091270006290669. doi:10.1177/0091270006290669. [DOI] [PubMed] [Google Scholar]
- Grady SR, Drenan RM, Breining SR, Yohannes D, Wageman CR, Fedorov NB, et al. Structural differences determine the relative selectivity of nicotinic compounds for native alpha4beta2*-, alpha6beta2*-, alpha3beta4*- and alpha7-nicotine acetylcholine receptors. Neuropharmacology. 2010;58:1054–1066. doi: 10.1016/j.neuropharm.2010.01.013. doi:10.1016/j.neuropharm.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Berrettini W, Lindstrom J. Acetylcholine receptor (AChR) alpha5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (alpha4beta2)alpha5 AChR function. Molecular Pharmacology. 2011;79:119–125. doi: 10.1124/mol.110.066357. doi:10.1124/mol.110.066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lummis SC, Thompson AJ, Bencherif M, Lester HA. Varenicline is a potent agonist of the human 5-hydroxytryptamine3 receptor. Journal of Pharmacology and Experimental Therapeutics. 2011;339:125–131. doi: 10.1124/jpet.111.185306. doi:jpet.111.185306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Robinson SF, Collins AC. Nicotinic agonists differ in activation and desensitization of 86Rb+ efflux from mouse thalamic synaptosomes. Journal of Pharmacology and Experimental Therapeutics. 1996;277:1383–1396. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8667201. [PubMed] [Google Scholar]
- Marks MJ, Romm E, Bealer SM, Collins AC. A test battery for measuring nicotine effects in mice. Pharmacology, Biochemistry and Behavior. 1985;23:325–330. doi: 10.1016/0091-3057(85)90577-5. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/4059317. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. doi:10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Collins AC, Paylor R, Marks MJ. Deletion of the beta 2 nicotinic acetylcholine receptor subunit alters development of tolerance to nicotine and eliminates receptor upregulation. Psychopharmacology (Berl) 2006;184:314–327. doi: 10.1007/s00213-005-0076-6. doi:10.1007/s00213-005-0076-6. [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Molecular Pharmacology. 2006;70:801–805. doi: 10.1124/mol.106.025130. doi:10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR. Nicotine receptors and depression: Revisiting and revising the cholinergic hypothesis. Trends in Pharmacological Sciences. 2010;31:580–586. doi: 10.1016/j.tips.2010.09.004. doi:10.1016/j.tips.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumenko VS, Kondaurova EM, Popova NK. Central 5-HT3 receptor-induced hypothermia in mice: Interstrain differences and comparison with hypothermia mediated via 5-HT1A receptor. Neuroscience Letters. 2009;465:50–54. doi: 10.1016/j.neulet.2009.09.005. doi:10.1016/j.neulet.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Obach RS, Reed-Hagen AE, Krueger SS, Obach BJ, O’Connell TN, Zandi KS, et al. Metabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metabolism and Disposition. 2006;34:121–130. doi: 10.1124/dmd.105.006767. doi:10.1124/dmd.105.006767. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Goldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M, et al. Mice deficient in the alpha7 neuronal nicotinic acetylcholine receptor lack alpha-bungarotoxin binding sites and hippocampal fast nicotinic currents. Journal of Neuroscience. 1997;17:9165–9171. doi: 10.1523/JNEUROSCI.17-23-09165.1997. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9364063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Trocme-Thibierge C, Guendisch D, Al Rubaiy SA, Bloom SA. Electrophysiological perspectives on the therapeutic use of nicotinic acetylcholine receptor partial agonists. Journal of Pharmacology and Experimental Therapeutics. 2011;337:367–379. doi: 10.1124/jpet.110.177485. doi:10.1124/jpet.110.177485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Wecker L, Stitzel JA. Activation and inhibition of mouse muscle and neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. Journal of Pharmacology and Experimental Therapeutics. 2010;333:501–518. doi: 10.1124/jpet.109.164566. doi:10.1124/jpet.109.164566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly JR, Grun EU, Collins AC. Chronic corticosterone administration modulates nicotine sensitivity and brain nicotinic receptor binding in C3H mice. Psychopharmacology (Berl) 1990;101:310–316. doi: 10.1007/BF02244047. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2362951. [DOI] [PubMed] [Google Scholar]
- Petersen DR, Norris KJ, Thompson JA. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metabolism and Disposition. 1984;12:725–731. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6150822. [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, Le Novere N, et al. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995;374:65–67. doi: 10.1038/374065a0. doi:10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- Raybuck JD, Portugal GS, Lerman C, Gould TJ. Varenicline ameliorates nicotine withdrawal-induced learning deficits in C57BL/6 mice. Behavioral Neuroscience. 2008;122:1166–1171. doi: 10.1037/a0012601. doi:10.1037/a0012601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real C, Seif I, Adrien J, Escourrou P. Ondansetron and fluoxetine reduce sleep apnea in mice lacking monoamine oxidase A. Respiratory Physiology and Neurobiology. 2009;168:230–238. doi: 10.1016/j.resp.2009.07.003. doi:10.1016/j.resp.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Reperant C, Pons S, Dufour E, Rollema H, Gardier AM, Maskos U. Effect of the alpha4beta2* nicotinic acetylcholine receptor partial agonist varenicline on dopamine release in beta2 knock-out mice with selective re-expression of the beta2 subunit in the ventral tegmental area. Neuropharmacology. 2010;58:346–350. doi: 10.1016/j.neuropharm.2009.10.007. doi:10.1016/j.neuropharm.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. doi:10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rollema H, Faessel HM, Williams KE. Varenicline overdose in a teenager—A clinical pharmacology perspective. Clinical Toxicology (Phila) 2009;47:605. doi: 10.1080/15563650902970689. doi:10.1080/15563650902970689. [DOI] [PubMed] [Google Scholar]
- Rollema H, Hajos M, Seymour PA, Kozak R, Majchrzak MJ, Guanowsky V, et al. Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochemical Pharmacology. 2009;78:813–824. doi: 10.1016/j.bcp.2009.05.033. doi:10.1016/j.bcp.2009.05.033. [DOI] [PubMed] [Google Scholar]
- Rollema H, Shrikhande A, Ward KM, Tingley FD, III, Coe JW, O’Neill BT, et al. Pre-clinical properties of the alpha4beta2 nicotinic acetylcholine receptor partial agonists varenicline, cytisine and dianicline translate to clinical efficacy for nicotine dependence. British Journal of Pharmacology. 2010;160:334–345. doi: 10.1111/j.1476-5381.2010.00682.x. doi:10.1111/j.1476-5381.2010.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, et al. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Molecular Pharmacology. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. doi:10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Smith JW, Mogg A, Tafi E, Peacey E, Pullar IA, Szekeres P, et al. Ligands selective for alpha4beta2 but not alpha3beta4 or alpha7 nicotinic receptors generalise to the nicotine discriminative stimulus in the rat. Psychopharmacology (Berl) 2007;190:157–170. doi: 10.1007/s00213-006-0596-8. doi:10.1007/s00213-006-0596-8. [DOI] [PubMed] [Google Scholar]
- Tritto T, McCallum SE, Waddle SA, Hutton SR, Paylor R, Collins AC, et al. Null mutant analysis of responses to nicotine: Deletion of beta2 nicotinic acetylcholine receptor subunit but not alpha7 subunit reduces sensitivity to nicotine-induced locomotor depression and hypothermia. Nicotine and Tobacco Research. 2004;6:145–158. doi: 10.1080/14622200310001656966. doi:10.1080/14622200310001656966. [DOI] [PubMed] [Google Scholar]
- Turner JR, Castellano LM, Blendy JA. Nicotinic partial agonists varenicline and sazetidine-A have differential effects on affective behavior. Journal of Pharmacology and Experimental Therapeutics. 2010;334:665–672. doi: 10.1124/jpet.110.166280. doi:10.1124/jpet.110.166280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, et al. Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Molecular Pharmacology. 2006;70:1454–1460. doi: 10.1124/mol.106.027318. doi:10.1124/mol.106.027318. [DOI] [PubMed] [Google Scholar]
- Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe CB, Armstrong D, et al. Multiorgan autonomic dysfunction in mice lacking the beta2 and the beta4 subunits of neuronal nicotinic acetylcholine receptors. Journal of Neuroscience. 1999;19:9298–9305. doi: 10.1523/JNEUROSCI.19-21-09298.1999. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10531434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano CA, Marks MJ, Cassels BK, Maccioni RB. In vivo effects of 3-iodocytisine: Pharmacological and genetic analysis of hypothermia and evaluation of chronic treatment on nicotinic binding sites. Neuropharmacology. 2009;57:332–342. doi: 10.1016/j.neuropharm.2009.05.004. doi:10.1016/j.neuropharm.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Stefanski R, Przegalinski E, Filip M. Effect of varenicline on the acute and repeated locomotor responses to nicotine in rats. Synapse. 2008;62:935–939. doi: 10.1002/syn.20564. doi:10.1002/syn.20564. [DOI] [PubMed] [Google Scholar]