Abstract
Background
α-thalassemia results from decreased production of α-globin chains that make up part of hemoglobin tetramers (Hb; α2β2) and affects up to 50% of individuals in some regions of sub-Saharan Africa. Heterozygous (−α/αα) and homozygous (−α/−α) genotypes are associated with reduced risk of severe Plasmodium falciparum malaria, but the mechanism of this protection remains obscure. We hypothesized that α-thalassemia impairs the adherence of parasitized red blood cells (RBCs) to microvascular endothelial cells (MVECs) and monocytes – two interactions that are centrally involved in the pathogenesis of severe disease.
Methods and Findings
We obtained P. falciparum isolates directly from Malian children with malaria and used them to infect αα/αα (normal), −α/αα and −α/−α RBCs. We also used laboratory-adapted P. falciparum clones to infect −/−α RBCs obtained from patients with HbH disease. Following a single cycle of parasite invasion and maturation to the trophozoite stage, we tested the ability of parasitized RBCs to bind MVECs and monocytes. Compared to parasitized αα/αα RBCs, we found that parasitized −α/αα, −α/−α and −/−α RBCs showed, respectively, 22%, 43% and 63% reductions in binding to MVECs and 13%, 33% and 63% reductions in binding to monocytes. α-thalassemia was associated with abnormal display of P. falciparum erythrocyte membrane protein 1 (PfEMP1), the parasite’s main cytoadherence ligand and virulence factor, on the surface of parasitized RBCs.
Conclusions
Parasitized α-thalassemic RBCs show PfEMP1 display abnormalities that are reminiscent of those on the surface of parasitized sickle HbS and HbC RBCs. Our data suggest a model of malaria protection in which α-thalassemia ameliorates the pro-inflammatory effects of cytoadherence. Our findings also raise the possibility that other unstable hemoglobins such as HbE and unpaired α-globin chains (in the case of β-thalassemia) protect against life-threatening malaria by a similar mechanism.
Introduction
α-thalassemia is an inherited disorder of hemoglobin (Hb) synthesis, in which reduced production of α-globin chains leads to decreased amounts of normal α2β2 tetramers and increased amounts of unpaired β-globin chains. In sub-Saharan Africa, α-thalassemia states are produced by a 3.7-kb deletion that leaves one functional copy of duplicated α-globin genes. Heterozygotes (−α/αα) have an essentially normal phenotype while homozygotes (−α/−α) have mild microcytic anemia. HbH disease is a chronic hemolytic disorder that may produce severe anemia requiring periodic blood transfusions. While the prevalence of α-thalassemia can exceed 50% in some malaria-endemic areas of sub-Saharan Africa, HbH disease (−/−α) is extremely rare because the mutant cis allele (−/) is very uncommon.
One study associated α-thalassemia with reduced risk of severe Plasmodium falciparum malaria in Papua New Guinea [1], but only recently have epidemiological studies confirmed this finding in sub-Saharan Africa. Case-control and cohort studies have shown that heterozygous and homozygous α-thalassemia variously protect against severe malaria syndromes, including cerebral malaria (CM; coma with or without convulsions), severe malarial anemia (SMA; Hb level ≤50 g/l), and deep acidotic breathing [2], [3], [4], [5]. While these observations provide strong evidence that α-thalassemia prevents the progression from uncomplicated to severe malaria, the mechanism of this protection has not been established.
In considering candidate mechanisms of protection by α-thalassemia, we reasoned that they should be consistent with a well-established epidemiological observation reported from numerous African settings. Specifically, α-thalassemia is not associated with reduced parasite prevalence [5], [6], [7] or densities in vivo as determined by examining blood smears from children with asymptomatic parasitemia [2], [8], uncomplicated malaria [2], [3], [4], [5], [6], [7], [9], [10], [11], [12], or severe malaria [2], [13]. As noted by other investigators [3], [5], [14], these observations indicate that α-thalassemia does not protect against severe malaria by mechanisms that impair the ability of parasites to invade or develop within RBCs [15], [16], or promote the removal of parasitized RBCs from the bloodstream by increased neoantigen expression [15], [17] or increased antibody binding [18]. Indeed, we have observed extremely high parasite burdens (up to 200,000/µl) in some −α/αα and −α/−α children with malaria (unpublished data).
A central question thus emerges from these considerations: how can marginal decreases in α-globin synthesis enable children to develop high parasite densities and symptoms of uncomplicated malaria and yet not develop severe complications of the disease? To explore this question, we hypothesized that α-thalassemia impairs the adherence of parasitized RBCs to microvascular endothelial cells (MVECs) and monocytes. These cell-cell interactions are mediated by P. falciparum erythrocyte membrane protein 1 (PfEMP1), a family of clonally-expressed, antigenically-variant proteins [23], [24], [25]. P. falciparum parasites export PfEMP1 proteins and concentrate them in knob-like protrusions on the surface of their host RBCs. This host cell modification enables a large mass of parasitized RBCs to sequester in venous microvessels [26] and avoid clearance from the bloodstream by the spleen. However, the adherence of parasitized RBCs to MVECs and monocytes also contributes to the development of life-threatening malaria by causing systemic microvascular inflammation [27], [28], [29], [30].
Using naturally-circulating P. falciparum isolates and freshly-drawn RBCs from our study site in rural Mali, where approximately 40% of children are α-thalassemic, we show that α-thalassemia impairs the cytoadherence of parasitized RBCs and is associated with abnormal PfEMP1 display.
Methods
Red Blood Cells
All blood samples were obtained from healthy Malian children who were confirmed to be aparasitemic by examination of thick blood smears. Blood samples from two patients with HbH disease were obtained in the United States. Written, informed consent was obtained from all blood donors or their parents. Blood collection was approved by the Institutional Review Boards of the National Institute of Allergy and Infectious Diseases, the University of Bamako, and the Johns Hopkins School of Medicine. Whole blood samples were drawn into Vacutainers® (Becton-Dickinson, Franklin Lakes, NJ) containing acid-citrate-dextrose (ACD) anticoagulant. After removing buffy coat leukocytes, RBCs were washed three times with RPMI-1640 (Invitrogen, Carlsbad, CA) and stored at 50% hematocrit at 4°C prior to use (within 4–36 h of blood draw). In some flow cytometry experiments, whole blood samples were stored in ACD Vacutainers® on ice during a 36-hour transport from Mali to the United States and then used within 4–24 h of arrival. Hemoglobin types were determined by HPLC (D-10 Instrument, Bio-Rad, Hercules, CA). The presence of the G6PD*A− allele was determined as described [19]. The presence of the 3.7-kb deletional determinant of α-thalassemia was determined as described [20]. In all experiments, normal and α-thalassemic RBCs were obtained simultaneously and processed in parallel. Normal and α-thalassemic RBC donors from Mali had normal HbA and did not carry the G6PD*A− allele.
Parasite Culture
P. falciparum clones (3D7, FCR-3, A4tres and FVO) were cultured in O+ RBCs at 5% hematocrit in complete medium (CM; RPMI-1640 supplemented with 25 mg/ml HEPES, 2 mg/mL sodium bicarbonate, 50 µg/ml gentamicin, and 0.5% Albumax II (Gibco-BRL, Grand Island, NY) in 0.2 µm-vented, 75-cm2 flasks (Corning Inc., Corning, NY). Knobby parasite lines were maintained by periodic gelatin flotation [21]. Cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 and media was changed every 8–12 h. Naturally-circulating P. falciparum isolates (ring stage) were obtained directly from Malian children with malaria and cultured for 12–24 hours to the trophozoite stage, as above. Trophozoite-infected RBCs containing paramagnetic hemozoin were enriched to >95% purity by magnetic separation (Miltenyi Biotec, Auburn, CA), inoculated into normal and α-thalassemic RBC samples, and cultured at 1–2% hematocrit as above. In all experiments, parasitized RBCs were assayed after one cycle of invasion and development to the trophozoite stage (∼36 h).
Endothelial Cell Adherence Assay
Endothelial cell adherence assays were conducted in Mali, except those using HbH RBCs obtained in the United States. Human adult dermal microvascular endothelial cells (MVECs; Lonza, Walkersville, MD) were maintained in the manufacturer’s EGM2-MV medium and grown on LabTek CC-2 coated 8-well chamber slides (Nalge Nunc International, Rochester, NY) to ∼50% confluency at 37°C in a humidified atmosphere of 5% CO2. Trophozoite-infected RBCs were adjusted to 5–20% parasitemia and 1% hematocrit by the addition of noninfected RBCs in binding media (BM; RPMI-1640, 0.5% BSA, pH 6.7). Adherent MVECs were washed with BM and then incubated with 150 µl of the parasitized RBC suspension for 1 h at 37°C with constant horizontal agitation (100 rpm). After parasite suspensions were removed from each well, slides were washed by dipping twice in BM at 37°C, fixed in 2% glutaraldehyde at ambient temperature for 2 h, and stained in 1 or 2.5% Giemsa for 30–60 min. In each experiment, the number of parasitized RBCs bound to ∼700 MVECs was counted from duplicate wells. For each slide, the number of parasitized α-thalassemic RBCs per MVEC was normalized to counts from parasitized normal RBCs.
Monocyte Adherence Assay
Monocyte adherence assays were conducted in Mali, except those using HbH RBCs obtained in the United States. CD14+ monocytes (Lonza) were plated onto CC2 Lab-Tek chamber slides (Nalge Nunc International) at 4×105 cells per well and cultured for 48 h in RPMI 1640 containing 25 mM HEPES, 50 µg/ml gentamicin, and 10% fetal bovine serum at 37°C in an atmosphere of 5% CO2. Normal and α-thalassemic RBCs infected with P. falciparum trophozoites (3D7, A4tres, FCR-3, naturally-circulating isolates) were purified by magnetic separation, adjusted to 3–15% parasitemia and 1% hematocrit as previously described. Adherent monocytes were washed with binding media and incubated with 150 µl of the parasitized RBC suspension for 1 h at 37°C with gentle horizontal rotation. The parasite suspension was removed from each well and the slides were gently washed by dipping four times in binding media. Slides were dried and stained using Hema 3 (Fischer Scientific). Adherence was measured by counting the number of infected RBCs bound to a minimum of 700 monocytes from duplicate wells. For each slide, the number of parasitized α-thalassemic RBCs per monocyte was normalized to the counts from parasitized normal RBCs.
Flow Cytometry
Flow cytometry was conducted in the United States using α-thalassemic RBCs obtained in Mali and HbH RBCs obtained in the United States. Rat or rabbit polyclonal antisera raised against PfEMP-1 variants expressed by the P. falciparum clones FVO and A4tres were kindly provided by Morris Makobongo and Dror Baruch (NIAID). Rabbit polyclonal antisera specific for a recombinant PfEMP1 variant subdomain (VAR2CSA, DBL3X) was kindly provided by Kavita Singh (NIAID). Samples of trophozoite-infected RBCs (1.5×106 cells; 1% parasitemia) were stained with various dilutions of antiserum in FACS staining buffer (FSB; PBS, 2% FBS, 0.1% sodium azide) for 45 min at ambient temperature and washed twice with FSB. Samples were then incubated with Alexa 488-conjugated anti-rat or anti-rabbit IgG (Molecular Probes, Inc., Eugene, OR) and ethidium bromide (2 µg/ml) at ambient temperature for 30 min and washed twice with FSB. A FACSort instrument (Becton-Dickinson, San Jose, CA) and FlowJo software (Tree Star, Inc., Ashland, OR) were used to acquire and analyze 250,000 to 500,000 events from each sample.
Atomic Force Microscopy
Parasitized α-thalassemic RBCs were obtained directly from Malian children with malaria, cultured to the trophozoite stage, and prepared for atomic force microscopy (AFM) imaging as described [22]. We also inoculated HbH RBCs with P. falciparum clone FCR-3 and cultured them through one cycle of invasion and development to the trophozoite stage (∼36 h). A Bioscope AFM (Veeco Instruments, Santa Barbara, CA) on a wide-field Axiovert 200 fluorescence microscope (Carl Zeiss, Inc., Thornwood, NY) was optimized to image the surface topography of RBCs and to identify the parasite stage within an individual AFM-imaged RBC. The X and Y piezoelectric scanners of the Bioscope AFM were disconnected. A custom built closed-loop XY scanner stage (nPoint, Inc., Madison, WI) was used to minimize scanning artifacts and thermal drift of the scanner for improved image accuracy. AFM was performed in tapping mode in air using Nanosensors pointprobe tips (Nanosensors, Switzerland) with a cantilever resonant frequency of 327–397 kHz. Topographic and error signal (amplitude) images were collected simultaneously. Parasites were stained with YOYO-1 fluorescent nucleic acid staining reagent (Molecular Probes, Inc.). Bright field and fluorescent images were collected with a chilled CCD video camera (Model C5985, Hamamatsu Photonic Systems, Bridgewater, NJ). Image-Pro Plus version 5.0 software (Media Cybernetics, Silver Spring, MD) was used to merge these images to allow the approximate identification of the parasite stage.
Statistical Analysis
In assays of cytoadherence and PfEMP1 expression, results from parasitized α-thalassemic RBCs were normalized to results from parasitized normal RBCs tested in parallel. 2-tailed P values were calculated by one-sample t test of the mean using GraphPad software version 5.01 (Graphpad Software, La Jolla, CA).
Results
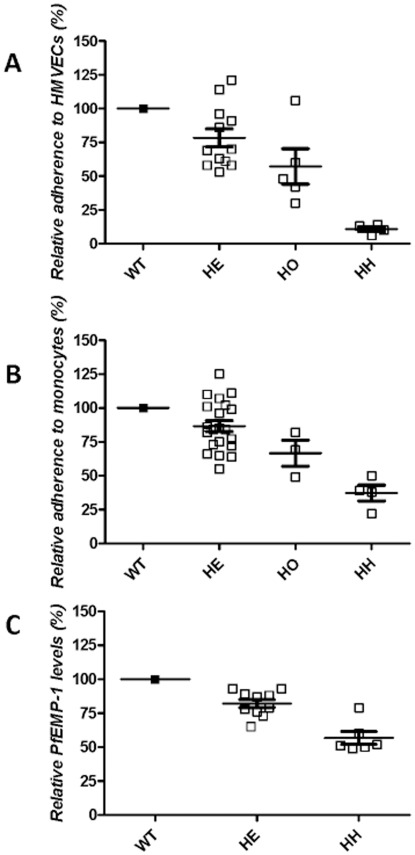
Because cytoadherence is centrally involved in the pathogenesis of severe malaria, we tested P. falciparum-infected normal and α-thalassemic RBCs for their adherence to MVECs. In these and other cytoadherence comparisons, we used naturally-circulating P. falciparum isolates obtained directly from Malian children with malaria, and freshly-drawn nonparasitized RBCs from healthy Malian children. In experiments using −/−α RBCs, we used laboratory-adapted P. falciparum clones, and freshly-drawn RBCs from two patients with HbH disease. Relative to parasitized αα/αα RBCs, parasitized −α/αα and −α/−α RBCs showed 22% and 43% reductions in adherence to MVECs, respectively (mean ± SEM; 78%±6.6%, P = 0.007, N = 12 for −α/αα; 57%±13.0%, P = 0.03, N = 5 for −α/−α) ( Fig. 1a ). Although the −/−α phenotype is not present in sub-Saharan Africa, we chose to use −/−α RBCs to test the effects of further reductions in α-globin expression on cytoadherence. Parasitized −/−α RBCs showed an 89% reduction in adherence to MVECs (11%±1.8%, P<0.0001, N = 4) ( Fig. 1a ).
Figure 1. Relative cytoadherence and surface PfEMP1 levels of parasitized RBCs. a,
Adherence of parasitized RBCs to MVECs. The numbers of parasitized −α/αα (HE), −α/−α (HO) and −/−α (HH) RBCs adhering to MVECs were normalized to those of parasitized αα/αα RBCs tested in parallel. The mean (± SEM) number of parasitized αα/αα RBCs per 100 MVECs was 260±40, N = 19. Results were obtained from 19 naturally-circulating parasite isolates and 2 laboratory-adapted parasite clones (A4tres and FCR-3), multiple blood donors (5 αα/αα, 2−α/αα, 2 −α/−α and 2−/−α), and 4 MVEC donors (not all combinations tested). This resulted in −α/αα, −α/−α and −/−α samples being compared to αα/αα samples 12, 5 and 4 times. b, Adherence of parasitized RBCs to monocytes. The numbers of parasitized −α/αα, −α/−α and −/−α RBCs adhering to monocytes were normalized to those of αα/αα RBCs tested in parallel. The mean (± SEM) number of parasitized αα/αα RBCs per 100 monocytes was 136±10, N = 12. Results were obtained from 3 naturally-circulating parasite isolates and 3 laboratory-adapted parasite clones (3D7, A4tres and FCR-3), multiple blood donors (5 αα/αα, 3 −α/αα, 2 −α/−α and 2−/−α) and 4 monocyte donors (not all combinations tested). This resulted in −α/αα, −α/−α and −/−α samples being compared to αα/αα samples 20, 3 and 4 times. The αα/αα and −α/αα RBCs were different from those used in endothelial cell adherence assays. c, PfEMP1 expression levels (median fluorescence intensities, MFI) on the surface of parasitized RBCs. The mean (± SEM) MFI of parasitized αα/αα RBCs was 556±153, N = 6. Results were obtained from 2 laboratory-adapted parasite clones (A4tres, FVO and FCR3CSA), multiple blood donors (4 αα/αα, 6 −α/αα and 2−/−α), and various concentrations of 2 antisera (not all combinations tested). This resulted in −α/αα, and −/−α samples being compared to αα/αα samples 10 and 6 times. The αα/αα and −α/αα RBCs were different from those used in endothelial cell and monocyte adherence assays.
P. falciparum infection is associated with elevated levels of TNF and other monocyte-derived cytokines that cause fever and other symptoms of malaria. Excessive levels of these cytokines correlate with the development of severe malaria syndromes [31]; for example, TNF causes dyserythropoiesis that contributes to the development of severe malarial anemia. The interaction between PfEMP1 on parasitized RBCs and CD36 on monocytes leads to monocyte activation and cytokine production in vitro [32], [33]. To measure the effects of α-thalassemia on this interaction, we tested the adherence of parasitized RBCs to monocytes. Relative to parasitized αα/αα RBCs, parasitized −α/αα, −α/−α and −/−α RBCs showed 13%, 33% and 63% reductions in adherence to monocytes, respectively (mean ± SEM; 87%±4.0%, P = 0.003, N = 21 for −α/αα; 67%±9.6%, P = 0.07, N = 3 for −α/−α; 37%±5.8%, P = 0.002, N = 4 for −/−α) ( Fig. 1b ).
Reduced expression of PfEMP1 on the surface of parasitized RBCs containing the malaria-protective HbC or HbS variants is associated with reductions in cytoadherence. Using specific antisera in a flow cytometric assay, we found that parasitized –α/αα and −/−α BCs showed 18% and 43% reductions in surface PfEMP1 levels, respectively, relative to parasitized αα/αα RBCs (mean ± SEM; 82%±3.0%, P = 0.0002, N = 10 for −α/αα; 57%±4.7%, P = 0.0003, N = 6 for −/−α) ( Fig. 1c ). We were unable to quantify PfEMP1 levels on the surface of parasitized −α/−α RBCs. This is because the homozygous α-thalassemic children in our cohort had confounding polymorphisms such as HbC, HbS and G6PD deficiency, were parasitized at the time of screening, or refused to donate a blood sample.
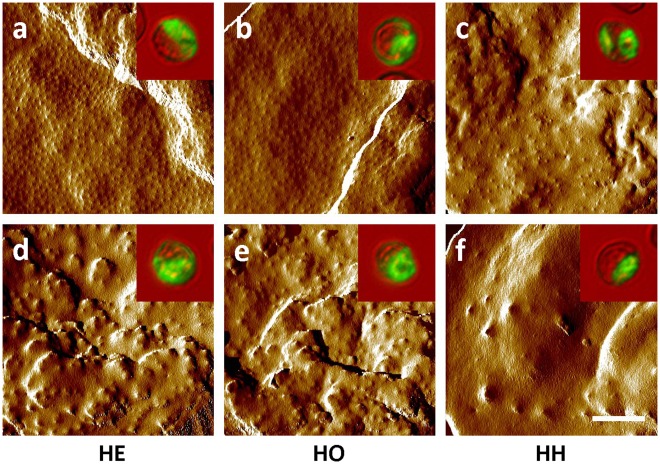
On the surface of parasitized RBCs, PfEMP1 proteins are concentrated on knob-like protrusions that make contact with host cells [32], [34], [35]. Alterations in knob assembly can therefore affect the amount and distribution of PfEMP1 [34], [36]. To determine whether reduced surface levels of PfEMP1 are associated with aberrant knob assembly on parasitized α-thalassemic RBCs, we examined the surface of these cells. Atomic force micrographs of naturally-parasitized −α/αα and −α/−α RBCs (cultured ex vivo from ring forms to mature trophozoites) showed populations expressing fine, regularly distributed knobs ( Fig. 2a,b ) characteristic of parasitized normal RBCs as well as cell populations expressing large, widely separated knobs ( Fig. 2d,e ) reminiscent of parasitized HbC and HbS RBCs [22], [37]. Knobs on the surface of trophozoite-infected −/−α RBCs were found to be variously abnormal in size and distribution ( Fig. 2c ) and in some cases essentially absent ( Fig. 2f ).
Figure 2. Distribution and morphology of knobs on the surface of parasitized RBCs.
Atomic force micrographs (AFMs) of parasitized −α/αα (HE) (a,d) and −α/−α (HO) (b,e) RBCs obtained from naturally-parasitized Malian children with malaria and −/−α (HH) (c,f) RBCs infected with a laboratory-adapted P. falciparum clone showing normal (a,b) or abnormal (c–f) knob distributions and morphologies. AFM images are representative of 32, 10 and 18 images of parasites in −α/αα, −/−αα and −/−α RBCs. Inlays show YOYO-1-stained parasites that correspond to those imaged by AFM. Comparison AFMs of parasitized HbA, HbC and HbS RBCs have been reported previously [22], [37].
Discussion
α-thalassemia protects against different clinical forms of severe malaria, especially SMA but also CM and deep acidotic breathing – syndromes associated with fatal outcome. The mechanism by which α-thalassemia protects against these severe malaria syndromes has remained obscure, although it does not seem to affect parasite density in vivo. Microcytosis has recently been implicated as a mechanism of protection against SMA by homozygous, but not heterozygous, α-thalassemia in Papua New Guinea [14]. According to this model, increased RBC counts protect homozygotes against SMA by reducing the amount of Hb loss at any given parasite density. While microcytosis might prevent SMA, as defined as Hb concentration ≤50 g/l [14], the role of α-thalassemia homozygosity in protection against death from SMA is less clear. This is because SMA can be associated with very low fatality rates (∼1%) [38] unless it is complicated by other signs of severe malaria, including coma and deep acidotic breathing [4], [38].
To explore how heterozygous and homozygous forms of α-thalassemia protect against CM and complicated SMA, we hypothesized that these traits weaken the adherence of parasitized RBCs to host cells within microvessels – a pro-inflammatory process that is essential in the pathogenesis of severe disease syndromes. We found that progressive reductions in α-globin production produce commensurate reductions in the adherence of parasitized RBCs to MVECs and monocytes. Decreased levels and abnormal distribution of PfEMP1 on the surface of parasitized α-thalassemic RBCs correlate with these findings. Based on these data, we propose the following model to explain how α-thalassemia protects against severe malaria syndromes without affecting parasite densities in vivo.
P. falciparum merozoites readily invade α-thalassemic RBCs and develop into viable trophozoites expressing PfEMP1. The amount and distribution of PfEMP1 is sufficiently normal to mediate effective sequestration of trophozoite-infected RBCs. This normal cycle of invasion, development, and sequestration enables parasites to avoid clearance from the bloodstream by the spleen and to multiply to densities that are equivalent to those observed in non-thalassemic children with malaria. The avidity of parasitized RBCs for MVECs and monocytes is sufficiently weakened by α-thalassemia, however, so that these host cell populations are not maximally activated. The resulting levels of inflammation produce symptoms of uncomplicated malaria, but are not high enough to cause severe disease and death. This model of protection is consistent with recent data showing that the degree of endothelial cell activation correlates with avidity of cytoadherence [39], [40]. It is also consistent with the finding that parasitized α-thalassemic RBCs show impaired adherence to human umbilical vein endothelial cells and rosetting [41], [42] (the binding of a parasitized RBC to several other noninfected RBCs), a different cytoadherence interaction also implicated in the development of severe disease.
Our model suggests that α-thalassemia heterozygous and homozygous states confer protection against CM and complicated SMA by impairing PfEMP1-mediated activation of host cells. By impairing endothelial cell activation, for example, α-thalassemia could dampen the degree of endothelial dysfunction that contributes to CM. By impairing monocyte activation, α-thalassemia could produce lower levels of cytokines and other inflammatory mediators that suppress erythropoiesis [43]. Some evidence for this possibility was recently provided by Veenemans et al. who showed that in Tanzanian and Kenyan children, α-thalassemia limits the decline in Hb levels associated with episodes of symptomless parasitemia – particularly those accompanied by inflammation [8]. The lower mean corpuscular Hb concentrations associated with α-thalassemia may contribute to this protective mechanism by decreasing the amount of free Hb released upon the rupture of each schizont-infected RBC. This mechanism would have the effect of lessening the severity of free Hb-induced endothelial cell dysfunction, as proposed by May et al. [4].
Our model also provides a plausible explanation for how heterozygous α-thalassemia confers less protection against severe disease than do HbS and HbC. Indeed, large numbers of α-thalassemic children do develop severe disease despite their abnormal RBC phenotype. Multiple studies have reported significant numbers of α-thalassemic children who develop life-threatening manifestations of disease [(e.g., 31% (94/301) of Ghanaian children in [2]; 60% (391/655) of Kenyan children in [3]; and 51% (70/137) of Kenyan children in [13])]. Relatively mild reductions in surface levels of particular PfEMP1 variants may not suffice to significantly impair the avidity of parasitized RBCs for host cells, resulting in relatively significant proportions of children with severe disease carrying the heterozygous α-thalassemia phenotype. A major limitation of our study is that it used −α/−α RBCs from relatively few donors. While data from −α/−α (and HbH) RBC samples were useful to explore the effect of α-thalassemia dose on PfEMP1 display, additional laboratory and epidemiological studies are needed to determine the relative contribution of impaired cytoadherence to the malaria-protective effects of homozygous α-thalassemia.
Parasitized α-thalassemic RBCs show PfEMP1 display abnormalities that are reminiscent of those on the surface of parasitized HbS and HbC RBCs. Shared characteristics between HbS, HbC, and α-thalassemic RBCs suggest possible mechanisms by which these diverse hemoglobinopathies impair the ability of P. falciparum parasites to remodel their host RBC membrane. HbS, HbC, and unpaired β-globin chains (in the case of α-thalassemia) undergo accelerated degradation to hemichromes [44], [45]. These hemichromes bind the inner leaflet of the RBC membrane where they promote the heme iron-mediated oxidation of membrane proteins and lipids [46], [47] – a process that consumes antioxidants as well. Since parasites induce hemichrome formation as they mature from ring to trophozoite stages [48], the amount of hemichromes produced by parasites in HbS, HbC, and α-thalassemic RBCs would be greater than in normal RBCs [49]. Excessive hemichrome-induced damage to the parasitized RBC membrane could interfere with the trafficking and knob incorporation of PfEMP1.
Finally, our data suggest that α-thalassemia protects against severe P. falciparum malaria by the same mechanism as HbS and HbC: ameliorating the pro-inflammatory effects of cytoadherence. This model also raises the possibility that other unstable hemoglobins such as HbE and unpaired α globin chains (in the case of β-thalassemia) protect against severe malaria by a similar mechanism. While appropriate case-control and cohort studies have not yet been conducted to determine whether HbE or β-thalassemia confer protection against fatal malaria syndromes, neither hemoglobinopathy has been associated with reduced parasite prevalence or densities in vivo [50], [51], [52]. We speculate that diverse hemoglobinopathies have been naturally selected worldwide for the common phenotype of abnormal PfEMP1 display. If true, these findings would suggest that PfEMP1-mediated phenomena are centrally responsible for malaria-related deaths and that therapeutics and vaccines that interfere with cytoadherence may reduce malaria mortality.
Acknowledgments
This research was supported by the Intramural Research Program of the NIAID, NIH. The authors wish to acknowledge Jennifer M. Anderson, Steve Beaudry, Mory Doumbia, Seydou Doumbia, Robert W. Gwadz, Carole A. Long, Sam Moretz, Dick Sakai, Cheick Traore, and Thomas E. Wellems for their efforts in support of this work.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Allen SJ, O’Donnell A, Alexander ND, Alpers MP, Peto TE, et al. alpha+-Thalassemia protects children against disease caused by other infections as well as malaria. Proc Natl Acad Sci U S A. 1997;94:14736–14741. doi: 10.1073/pnas.94.26.14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mockenhaupt FP, Ehrhardt S, Gellert S, Otchwemah RN, Dietz E, et al. Alpha(+)-thalassemia protects African children from severe malaria. Blood. 2004;104:2003–2006. doi: 10.1182/blood-2003-11-4090. [DOI] [PubMed] [Google Scholar]
- 3.Williams TN, Wambua S, Uyoga S, Macharia A, Mwacharo JK, et al. Both heterozygous and homozygous alpha+ thalassemias protect against severe and fatal Plasmodium falciparum malaria on the coast of Kenya. Blood. 2005;106:368–371. doi: 10.1182/blood-2005-01-0313. [DOI] [PubMed] [Google Scholar]
- 4.May J, Evans JA, Timmann C, Ehmen C, Busch W, et al. Hemoglobin variants and disease manifestations in severe falciparum malaria. JAMA. 2007;297:2220–2226. doi: 10.1001/jama.297.20.2220. [DOI] [PubMed] [Google Scholar]
- 5.Wambua S, Mwangi TW, Kortok M, Uyoga SM, Macharia AW, et al. The effect of alpha+-thalassaemia on the incidence of malaria and other diseases in children living on the coast of Kenya. PLoS Med. 2006;3:e158. doi: 10.1371/journal.pmed.0030158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen SJ, Rowe P, Allsopp CE, Riley EM, Jakobsen PH, et al. A prospective study of the influence of alpha thalassaemia on morbidity from malaria and immune responses to defined Plasmodium falciparum antigens in Gambian children. Trans R Soc Trop Med Hyg. 1993;87:282–285. doi: 10.1016/0035-9203(93)90129-e. [DOI] [PubMed] [Google Scholar]
- 7.Enevold A, Lusingu JP, Mmbando B, Alifrangis M, Lemnge MM, et al. Reduced risk of uncomplicated malaria episodes in children with alpha+-thalassemia in northeastern Tanzania. Am J Trop Med Hyg. 2008;78:714–720. [PubMed] [Google Scholar]
- 8.Veenemans J, Andang’o PE, Mbugi EV, Kraaijenhagen RJ, Mwaniki DL, et al. Alpha+ -thalassemia protects against anemia associated with asymptomatic malaria: evidence from community-based surveys in Tanzania and Kenya. J Infect Dis. 2008;198:401–408. doi: 10.1086/589884. [DOI] [PubMed] [Google Scholar]
- 9.Mockenhaupt FP, Falusi AG, May J, Ademowo OG, Olumese PE, et al. The contribution of alpha+-thalassaemia to anaemia in a Nigerian population exposed to intense malaria transmission. Trop Med Int Health. 1999;4:302–307. doi: 10.1046/j.1365-3156.1999.00386.x. [DOI] [PubMed] [Google Scholar]
- 10.Mockenhaupt FP, Bienzle U, May J, Falusi AG, Ademowo OG, et al. Plasmodium falciparum infection: influence on hemoglobin levels in alpha-thalassemia and microcytosis. J Infect Dis. 1999;180:925–928. doi: 10.1086/314959. [DOI] [PubMed] [Google Scholar]
- 11.Mockenhaupt FP, May J, Bergqvist Y, Meyer CG, Falusi AG, et al. Evidence for a reduced effect of chloroquine against Plasmodium falciparum in alpha-thalassaemic children. Trop Med Int Health. 2001;6:102–107. doi: 10.1046/j.1365-3156.2001.00688.x. [DOI] [PubMed] [Google Scholar]
- 12.Imrie H, Fowkes FJ, Michon P, Tavul L, Hume JC, et al. Haptoglobin levels are associated with haptoglobin genotype and alpha+ -Thalassemia in a malaria-endemic area. Am J Trop Med Hyg. 2006;74:965–971. [PubMed] [Google Scholar]
- 13.Williams TN, Mwangi TW, Wambua S, Peto TE, Weatherall DJ, et al. Negative epistasis between the malaria-protective effects of alpha+-thalassemia and the sickle cell trait. Nat Genet. 2005;37:1253–1257. doi: 10.1038/ng1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fowkes FJ, Allen SJ, Allen A, Alpers MP, Weatherall DJ, et al. Increased microerythrocyte count in homozygous alpha(+)-thalassaemia contributes to protection against severe malarial anaemia. PLoS Med. 2008;5:e56. doi: 10.1371/journal.pmed.0050056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuthavong Y, Butthep P, Bunyaratvej A, Fucharoen S, Khusmith S. Impaired parasite growth and increased susceptibility to phagocytosis of Plasmodium falciparum infected alpha-thalassemia or hemoglobin Constant Spring red blood cells. Am J Clin Pathol. 1988;89:521–525. doi: 10.1093/ajcp/89.4.521. [DOI] [PubMed] [Google Scholar]
- 16.Pattanapanyasat K, Yongvanitchit K, Tongtawe P, Tachavanich K, Wanachiwanawin W, et al. Impairment of Plasmodium falciparum growth in thalassemic red blood cells: further evidence by using biotin labeling and flow cytometry. Blood. 1999;93:3116–3119. [PubMed] [Google Scholar]
- 17.Williams TN, Weatherall DJ, Newbold CI. The membrane characteristics of Plasmodium falciparum-infected and -uninfected heterozygous alpha(0)thalassaemic erythrocytes. Br J Haematol. 2002;118:663–670. doi: 10.1046/j.1365-2141.2002.03610.x. [DOI] [PubMed] [Google Scholar]
- 18.Luzzi GA, Merry AH, Newbold CI, Marsh K, Pasvol G, et al. Surface antigen expression on Plasmodium falciparum-infected erythrocytes is modified in alpha- and beta-thalassemia. J Exp Med. 1991;173:785–791. doi: 10.1084/jem.173.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guindo A, Fairhurst RM, Doumbo OK, Wellems TE, Diallo DA. X-linked G6PD deficiency protects hemizygous males but not heterozygous females against severe malaria. PLoS Med. 2007;4:e66. doi: 10.1371/journal.pmed.0040066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crompton PD, Traore B, Kayentao K, Doumbo S, Ongoiba A, et al. Sickle cell trait is associated with a delayed onset of malaria: implications for time-to-event analysis in clinical studies of malaria. J Infect Dis. 2008;198:1265–1275. doi: 10.1086/592224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasvol G, Wilson RJ, Smalley ME, Brown J. Separation of viable schizont-infected red cells of Plasmodium falciparum from human blood. Ann Trop Med Parasitol. 1978;72:87–88. doi: 10.1080/00034983.1978.11719283. [DOI] [PubMed] [Google Scholar]
- 22.Arie T, Fairhurst RM, Brittain NJ, Wellems TE, Dvorak JA. Hemoglobin C modulates the surface topography of Plasmodium falciparum-infected erythrocytes. J Struct Biol. 2005;150:163–169. doi: 10.1016/j.jsb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Baruch DI, Pasloske BL, Singh HB, Bi X, Ma XC, et al. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 24.Smith JD, Chitnis CE, Craig AG, Roberts DJ, Hudson-Taylor DE, et al. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su XZ, Heatwole VM, Wertheimer SP, Guinet F, Herrfeldt JA, et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 26.Silamut K, Phu NH, Whitty C, Turner GD, Louwrier K, et al. A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am J Pathol. 1999;155:395–410. doi: 10.1016/S0002-9440(10)65136-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner GD, Morrison H, Jones M, Davis TM, Looareesuwan S, et al. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol. 1994;145:1057–1069. [PMC free article] [PubMed] [Google Scholar]
- 28.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 29.Rowe A, Obeiro J, Newbold CI, Marsh K. Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infect Immun. 1995;63:2323–2326. doi: 10.1128/iai.63.6.2323-2326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlson J, Helmby H, Hill AV, Brewster D, Greenwood BM, et al. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990;336:1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- 31.Grau GE, Taylor TE, Molyneux ME, Wirima JJ, Vassalli P, et al. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989;320:1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 32.Ockenhouse CF, Magowan C, Chulay JD. Activation of monocytes and platelets by monoclonal antibodies or malaria-infected erythrocytes binding to the CD36 surface receptor in vitro. J Clin Invest. 1989;84:468–475. doi: 10.1172/JCI114188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGilvray ID, Serghides L, Kapus A, Rotstein OD, Kain KC. Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: a role for CD36 in malarial clearance. Blood. 2000;96:3231–3240. [PubMed] [Google Scholar]
- 34.Crabb BS, Cooke BM, Reeder JC, Waller RF, Caruana SR, et al. Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell. 1997;89:287–296. doi: 10.1016/s0092-8674(00)80207-x. [DOI] [PubMed] [Google Scholar]
- 35.MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 36.Horrocks P, Pinches RA, Chakravorty SJ, Papakrivos J, Christodoulou Z, et al. PfEMP1 expression is reduced on the surface of knobless Plasmodium falciparum infected erythrocytes. J Cell Sci. 2005;118:2507–2518. doi: 10.1242/jcs.02381. [DOI] [PubMed] [Google Scholar]
- 37.Cholera R, Brittain NJ, Gillrie MR, Lopera-Mesa TM, Diakite SA, et al. Impaired cytoadherence of Plasmodium falciparum-infected erythrocytes containing sickle hemoglobin. Proc Natl Acad Sci U S A. 2008;105:991–996. doi: 10.1073/pnas.0711401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 39.Jenkins N, Wu Y, Chakravorty S, Kai O, Marsh K, et al. Plasmodium falciparum intercellular adhesion molecule-1-based cytoadherence-related signaling in human endothelial cells. J Infect Dis. 2007;196:321–327. doi: 10.1086/518795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakravorty SJ, Hughes KR, Craig AG. Host response to cytoadherence in Plasmodium falciparum. Biochem Soc Trans. 2008;36:221–228. doi: 10.1042/BST0360221. [DOI] [PubMed] [Google Scholar]
- 41.Carlson J, Nash GB, Gabutti V, al-Yaman F, Wahlgren M. Natural protection against severe Plasmodium falciparum malaria due to impaired rosette formation. Blood. 1994;84:3909–3914. [PubMed] [Google Scholar]
- 42.Udomsangpetch R, Sueblinvong T, Pattanapanyasat K, Dharmkrong-at A, Kittikalayawong A, et al. Alteration in cytoadherence and rosetting of Plasmodium falciparum-infected thalassemic red blood cells. Blood. 1993;82:3752–3759. [PubMed] [Google Scholar]
- 43.Ekvall H. Malaria and anemia. Curr Opin Hematol. 2003;10:108–114. doi: 10.1097/00062752-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Campwala HQ, Desforges JF. Membrane-bound hemichrome in density-separated cohorts of normal (AA) and sickled (SS) cells. J Lab Clin Med. 1982;99:25–28. [PubMed] [Google Scholar]
- 45.MacDonald VW, Charache S. Drug-induced oxidation and precipitation of hemoglobins A, S and C. Biochim Biophys Acta. 1982;701:39–44. doi: 10.1016/0167-4838(82)90309-0. [DOI] [PubMed] [Google Scholar]
- 46.Hebbel RP, Eaton JW, Balasingam M, Steinberg MH. Spontaneous oxygen radical generation by sickle erythrocytes. J Clin Invest. 1982;70:1253–1259. doi: 10.1172/JCI110724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hebbel RP. Auto-oxidation and a membrane-associated ‘Fenton reagent’: a possible explanation for development of membrane lesions in sickle erythrocytes. Clin Haematol. 1985;14:129–140. [PubMed] [Google Scholar]
- 48.Giribaldi G, Ulliers D, Mannu F, Arese P, Turrini F. Growth of Plasmodium falciparum induces stage-dependent haemichrome formation, oxidative aggregation of band 3, membrane deposition of complement and antibodies, and phagocytosis of parasitized erythrocytes. Br J Haematol. 2001;113:492–499. doi: 10.1046/j.1365-2141.2001.02707.x. [DOI] [PubMed] [Google Scholar]
- 49.Ayi K, Turrini F, Piga A, Arese P. Enhanced phagocytosis of ring-parasitized mutant erythrocytes: a common mechanism that may explain protection against falciparum malaria in sickle trait and beta-thalassemia trait. Blood. 2004;104:3364–3371. doi: 10.1182/blood-2003-11-3820. [DOI] [PubMed] [Google Scholar]
- 50.Brown AE, Webster HK, Fucharoen S, Bunyaratvej A. Haemoglobin-E trait and the clinical course of malaria in Thai soldiers. Eur J Haematol. 1990;45:120–121. doi: 10.1111/j.1600-0609.1990.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 51.Kitayaporn D, Nelson KE, Charoenlarp P, Pholpothi T. Haemoglobin-E in the presence of oxidative substances from fava bean may be protective against Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 1992;86:240–244. doi: 10.1016/0035-9203(92)90292-k. [DOI] [PubMed] [Google Scholar]
- 52.Oo M, Tin S, Marlar T, O’Sullivan WJ. Genetic red cell disorders and severity of falciparum malaria in Myanmar. Bull World Health Organ. 1995;73:659–665. [PMC free article] [PubMed] [Google Scholar]