Although most fields of science are constantly struggling with which methodologies to use, the field of systematics, and especially paleontology, has adopted phylogenetic systematics (cladistic methodology) to the exclusion of other approaches. Despite a barrage of cautions and criticism, cladistics reigns. Considered a triumph of the field of paleontology and the crown jewel of cladistic methodology is the proof of the dinosaurian origin of birds. Among the most important features thought to link theropod dinosaurs and birds is a hand reduced to three fingers, a tridactyl hand. We know that the grasping raking hand of theropod dinosaurs is composed of digits 1-2-3, that is, the thumb and the next two fingers, because a variety of late Triassic forms, show digits 4 and 5 having undergone dramatic reduction and remaining only in vestigial form (Fig. 1). Yet virtually all credible developmental studies have concluded that the bird hand is composed of digits 2-3-4. Here, in a provocative theoretical paper, Wagner and Gauthier (1) accept the developmental evidence that birds have a 2-3-4 hand but propose a frame-shift hypothesis, by which the developmental properties responsible for digits (D)1–D3 are shifted onto embryonic precartilaginous condensations (C)2–C4. To put it another way, although these authors accept that the homology of the primordial condensations are correctly identified as C2, C3, and C4, they propose that subsequent anatomical differentiation reflects a frame shift in digit primoidia (anlagen) in later ontogeny such that C2 becomes D1, C3 becomes D2, and C4 becomes D3. By this proposed shift, birds can still be nested in the same clade or monophyletic grouping as maniraptoran theropod dinosaurs (the putative bird ancestors), as concluded by cladistic hypotheses, which date back to a paper by Gauthier (2), generally conceded to be the pivotal paper for this theory. As Neil Shubin (ref. 3, p. 263) notes, the phylogenetic interpretation of Gauthier (2) unambiguously supports the view of nonavian theropod digital reduction. The extension of this view to birds involves homoplasy (convergence) either in developmental patterns (in the I-II-III interpretation) or in numerous other skeletal characters (in the II-III-IV interpretation). But all recent developmental biologists have concluded that birds have a 2-3-4 hand (4–6). The developmental evidence overwhelmingly suppports the 2-3-4 theory of the wing skeleton in birds (ref. 6; Fig. 2). Too, phalangeal formulae, used to ally birds and theropods, are highly variant among amniotes. They are also developmentally plastic, as indicated by Zho and Niswander (7), who studied a bone morphogenetic protein that mediates programmed cell death (apoptosis) and showed that blockage of this factor in the avian limb results in hands missing only the most distal phalanges, thus providing a mechanism for symmetrical phalangeal reduction.
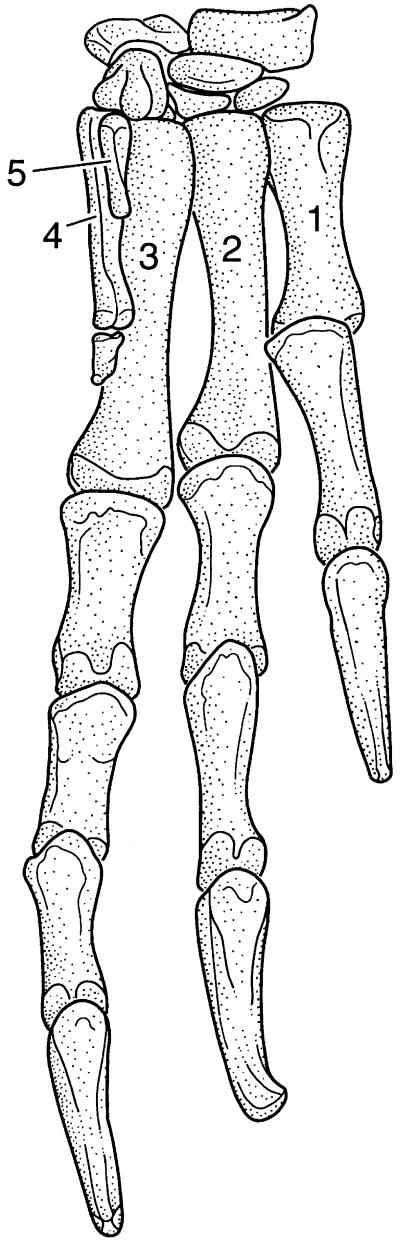
Figure 1.
The left hand of the late Triassic Herrerasaurus, typical of many late Triassic theropods, illustrating a pentadactyl hand but with digits 4 and 5 greatly reduced. Note that the longest finger in primitive dinosaurs is not the middle finger, as in Archaeopteryx (and birds), Deinonychus, and most tetrapods including man, but finger 3, which is what one would expect. Note also the primitive nature of the carpal elements, with nothing remotely resembling a semilunate carpal or any birdlike features (modified after ref. 27).
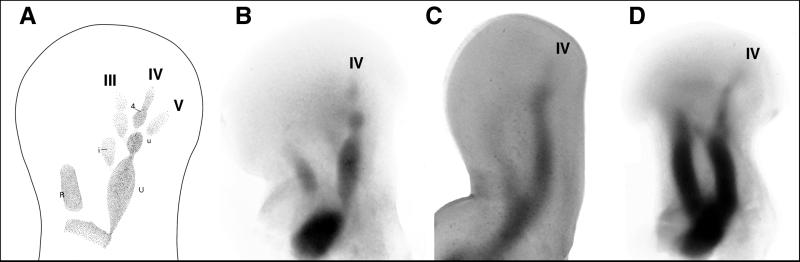
Figure 2.
Illustrations to show the developing primary axis. (A) Diagrammatic illustration of developing turtle limb bud to show general topography (precondensations of: R, radius, U, ulna, u, ulnare, I, intermedium, 4, carpal four, and digits III–V, 3–5). The primary axis therefore is highly conserved developmentally and invariably identifies digit IV, 4. (B–D) Illustrations of developing limb buds in turtle, bird, and alligator, respectively, illustrating the conserved developmental pattern of the primary axis. [A was modified from Burke and Alberch (28); C and D, photos from specimens prepared by A. C. Burke].
Some obvious objections to the frame-shift hypothesis are that (i) there is no evidence for any substantial morphological change in theropod hands that would indicate any kind of shift throughout their evolution; (ii) the similarity between the hands of theropods and Archaeopteryx (which is quite distinctive) are overemphasized in drawings, and the semilunate wrist bone, considered a definitive bird–dinosaur link, is thought by many not to be homologous (8); and (iii) in bird development, the fore- and hindlimbs exhibit the same highly conserved developmental pattern (ref. 5; Fig. 3), so if there is a frame shift, it would have to occur in the forelimb but not in the hindlimb.
Figure 3.
Early chicken forelimb bud characterized by a chondrogenic Y. The strongly staining postaxial element forms the primary axis of the developing hand, a linear array that invariably identifies, in sequence, ulna-ulnare-distal carpal 4 and ultimately digit 4 in all amniotes, including birds. The adult bird hand thus comprises digits 2, 3, and 4 (from preparation by A. C. Burke).
Although a common stem ancestor for birds and dinosaurs (and an arboreal origin of flight) was accepted for most of the 20th century, in the early 1970s Yale University’s John Ostrom resurrected Huxley’s dinosaurian origin of birds hypothesis (and the ground-up or cursorial origin of flight) based on Ostrom’s discovery of the Lower Cretaceous superficially birdlike Deinonychus and the proposal that such dinosaurs were hot-blooded or endothermic. It is interesting to note that the modern version of the dinosaurian origin of birds originally had nothing to do with cladistic methodology. It sprang from overall similarity (phenetics) and an evolutionary scenario by which hot-blooded dinosaurs became clothed with feathers for insulation and somehow sprouted wing feathers, and flight originated from the ground up—the cursorial theory for avian flight. For many of today’s paleontologists, birds are simply living dinosaurs. “The smallest dinosaur is the bee hummingbird … found only in Cuba” (ref. 9, p. 25).
Despite the popularity of the dinosaurian origin of birds, many ornithologists and physiologists, in particular, have had tremendous difficulty with the theory (8, 10, 11) because of a huge and growing body of contrary evidence and the fact that a ground-up origin of avian flight is considered a near biophysical impossibility (12). Aside from criticism concerning the cursorial origin of avian flight, there are problems related to the geologic, temporal occurrence of putative dinosaurian ancestors, which occur some 30 to 80 million years after the appearance of the earliest known bird Archaeopteryx, and these forms become more and more superficially birdlike as one approaches the latest Cretaceous. There is also the fact that virtually all of the anatomical features used to ally birds and dinosaurs have been disputed.
There have been a number of embarrassing cladistic traps, the most recent relating to pterosaur phylogenetics. A cladistic analysis performed by Kevin Padian in 1984 (13) indicated that pterosaurs were a sister group of dinosaurs and therefore must have evolved from small active bipedal terrestrial predecessors from the ground up (14). Padian’s studies relied on the basal pterosaur Dimorphodon, interpreting it as digitigrade and theropodlike, with the hindlimbs obligately held in an upright bipedal posture. These assertions have recently been shown to be erroneous. Analysis of the rhamphorhynchoid Sordes revealed the presence of a membrane that extended between the hindlimbs, negating any erect bipedal posture (15), and the hairlike fibers found on the specimens were supportive elements (rods) of the membranes, not fur. More recently, the discovery of the three-dimensionally preserved articulated foot of the basal pterosaur Dimorphodon shows a flat-footed stance and confirms obligate quadupedality and a plantigrade stance as primitive features for the group (16). Quadrupedality in numerous pterosaurs has also been confirmed by the discovery of myriad trackways, none of which show any signs of bipedality. Interestingly, H. G. Seeley, who opposed Huxley’s dinosaurian origin of birds, correctly illustrated Dimorphodon in a quadrupedal stance in his popular book Dragons of the Air published in 1901 (17). This example illustrates the inherent dangers of strict adherence to phylogenetic dogma, where, regardless of the evidence, anatomical and functional explanations must fit unerringly into a rigid cladistic framework.
In 1975 the late Jurassic bird Archaeopteryx was an earthbound, predatory, feathered dinosaur that could not fly. According to the dogma of the time, hot-blooded dinosaurs developed feathers to trap heat, and their wing feathers elongated as insect traps. Since then, however, the evidence for hot-blooded dinosaurs has been dismantled (18) and Archaeopteryx has been shown to be a bird in the modern sense (8), with fully developed elliptical wings similar to modern woodland birds, and asymmetric flight feathers that form individual airfoils, a flight scapula/coracoid arrangement, and a reserved hallux, found only in perching birds, and known in no dinosaur. Too, as new specimens emerged, the creature has been shown to be more and more birdlike (19). As cladistic evidence for a dinosaurian origin of birds increased, other feathered dinosaurs (artistic inventions) have emerged, including feathered Coelophysis, Deinonychus, and more recently Unenlagia and Velociraptor; however, there has never been any evidence for these assertions. Most recently, feathered dinosaurs began to emerge from China. First was the downy dinosaur Sinosauropteryx, a rather typical compsognathoid with a line of filaform structures on the middorsal line. Of concern, however, is that down is a secondary adaptation in modern neonate birds and would be maladaptive in a terrestrial dinosaur; downy baby ostriches, when wet, will die from hypothermia unless they seek the shelter of the mother’s wings. Today there is no doubt that these structures are not down or feathers but collagen fibers that support a frill or skin flap running along the back (20). However, after Sinosauropteryx, an article in Nature announced the discovery of two additional feathered dinosaurs from China (21), named Protarchaeopteryx and Caudipteryx. A cladistic analysis using some 90 anatomical features showed these creatures to be more primitive than Archaeopteryx and therefore feathered dinosaurs. However, the specimens are some 15 to 30 million years younger than Archaeopteryx; half of the characters are primitive and should therefore not have been used, and half of the characters are not present in the fossils, thus leaving two to three skull characters that cannot be ascertained in the crushed specimen. Interestingly, the entire cladogram is rooted in the latest Cretaceous Velociraptor, which occurs some 80 million years after the earliest known bird. Indeed, Caudipteryx shows a suite of features that show it to be a secondarily flightless bird, a Mesozoic kiwi, including a protopygostyle (fused tail vertebrae), an avian occiput, reduced fibula, wing feathers attached as in archaic birds, etc. Finally, there are now excellent specimens of theropod skin, one even showing muscle fibers, and not one shows signs of anything but typical thick tuberculated reptilian skin. Feathered dinosaurs remain a myth (20).
As Wagner and Gauthier (1) correctly point out, theropods inherited carnivorous habits, including serrated teeth. Yet, a mass of gizzard stones in Caudipteryx can best be interpreted as indicating an advanced state of herbivory, and serrated teeth claimed for Protarchaeopteryx cannot be confirmed by dozens of people who have examined the specimens. In fact, absence of serrations was listed as a feature in the original description. Theropods have recurved flattened serrated teeth that differ dramatically not only in morphology but also in their tooth replacement from the simple peglike waisted nonserrated teeth of early birds and Archaeopteryx. As with the need to explain the digital mismatch by a frame-shift hypothesis, the same phenomenon has applied to flight origin scenarios. Thus, Chiappe (22) proclaimed that “Non-avian theropods such as Velociraptor, Compsognathus, and Tyrannosaurus were clearly terrestrial cursors. Thus, the ancestral mode of life of birds was that of a cursorial biped. Inferences about the habits of Archaeopteryx should be made within this framework and not the inverse.” It follows, therefore, that the feathered dinosaurs from China are just that and not secondarily flightless birds. And Archaeopteryx was a terrestrial predator, although it has a broad suite of tree-dwelling and flight adaptations, it is not fully bipedal, it lacks the antitrochanter of the femur, and it has a claw morphology consistent with arboreal and trunk climbing birds, not predatory dinosaurs. Then, just last year the February issue of Scientific American (23) featured the Lower Cretaceous Chinese bird Confuciusornis as a terrestrial predator, although it was clearly an arboreal bird, in profile quite similar to a magpie.
In the final analysis, although there is little argument with cladistic theory, implementation of the methodology is wrought with problems, and the methodology appears incapable of dealing with massive convergence. Disparate groups, such as loons, grebes, and ancient toothed hesperornithiforms, are clustered as a clade, as are hawks and owls. The legs of the latter group are all formed differently embryologically, and the former groups are molecularly disparate. Indeed, many of these cladistic groupings take us back to the systematics of the 18th and 19th centuries, which was incapable of teasing out convergence. An example particularly apropos here is that of sea-dwelling reptiles, where the Triassic Hupehsuchus slots cladistically with the Cretaceous Mosasaurus, yet there is no temporal overlap (24). As Carroll and Dong note (ref. 24, p. 131), the principle of parsimony cannot be used directly to identify homologous characters if most of the derived characters are convergent. Could the same be true for birds and theropod dinosaurs?
Although the digital mismatch between birds and dinosaurs is anatomically the most serious problem, other versions of frame-shift hypotheses will be needed to explain such problems as the transformation of teeth and tooth replacement, the transformation of a dinosaurian septate, hepatic-piston breathing system to a bird flow-through lung, the complete abandonment of a balanced seesaw body plan to the avian model, and the reelongation of already foreshortened forelimbs, to mention a few. Perhaps the greatest form of special pleading will be necessary to explain how flight could have originated from the ground up; our present knowledge indicates that there are two requisites for flight origin: small size and high places. Also, it must be explained why these superficially birdlike theropods only occur in the fossil record 30 to 80 million years after the appearance of the earliest known bird, which is already well developed, and why Triassic theropods are devoid of birdlike features.
Commenting on the “cladistic jihad,” a Russian colleague, Evgeny Kurochkin, noted in a 1996 letter to me that, “It looks like … biology in the 1940’s and 1950’s in the former Soviet Union, which have not fundamentals of Lysenko biology.” In the final analysis, paleontologists will continue to use the methodology of phylogenetic systematics to define homology a posteriori from cladistic analysis of multiple synapomorphies and will explain discrepancies by such mechanisms as Wagner and Gauthier’s frame-shift hypothesis advocated here to accommodate the cladogram. Developmental biologists will continue to use conservation of embryonic patterning and anatomical connectivity to establish homology. Many times the devil is in the details, and when anatomical features linking birds and dinosaurs are examined in detail, major problems of homology emerge. Despite the popular appeal of the dinosaur–bird nexus, Larry Witmer (25) has appropriately warned that “Dogma is a scary thing,” and Frank Close (26) has noted of the cold fusion phenomenon, “If science does not ensure that its house is in order, who will?”
Acknowledgments
I thank Ann Burke, James Farlow, Larry Martin, John Ruben, and Zhonghe Zhou for comments on the manuscript. Susan Whitfield skillfully rendered the illustrations.
Footnotes
A commentary on this article begins on page 5111.
References
- 1.Wagner G P, Gauthier J A. Proc Natl Acad Sci USA. 1999;96:5111–5116. doi: 10.1073/pnas.96.9.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gauthier J A. Mem Calif Acad Sci. 1986;8:1–55. [Google Scholar]
- 3.Shubin N H. In: Homology, the Hierarchical Basis of Comparative Biology. Hall B K, editor. San Diego: Academic; 1994. [Google Scholar]
- 4.Hinchliffe R, Hecht M K. Evol Biol. 1984;18:21–39. [Google Scholar]
- 5.Burke A C, Feduccia A. Science. 1997;278:666–668. [Google Scholar]
- 6.Müller G B, Alberch P. J Morphol. 1990;203:151–164. doi: 10.1002/jmor.1052030204. [DOI] [PubMed] [Google Scholar]
- 7.Zho H, Niswander L. Science. 1996;272:738–741. doi: 10.1126/science.272.5262.738. [DOI] [PubMed] [Google Scholar]
- 8.Feduccia A. The Origin and Evolution of Birds. New Haven, CT: Yale Univ. Press; 1996. [Google Scholar]
- 9.Norell M A, Gaffney E S, Dingus L. Discovering Dinosaurs in the American Museum of Natural History. New York: Knopf; 1995. [Google Scholar]
- 10.Martin L D. In: Origins of the Higher Groups of Vertebrates. Schultze H-P, Treube L, editors. Ithaca, NY: Cornell Univ. Press; 1991. [Google Scholar]
- 11.Ruben J A, Jones T D, Geist N R, Hillenius W J. Science. 1997;278:1267–1270. [Google Scholar]
- 12.Norberg U M. Vertebrate Flight. Berlin: Springer; 1990. [Google Scholar]
- 13.Padian K. In: Third Symposium on Mesozoic Terrestrial Ecosystems. Reif W-E, Westphal F, editors. Tubingen, Germany: Attempto; 1984. [Google Scholar]
- 14.Padian K. In: Biomechanics in Evolution. Rayner J M V, Wooton R J, editors. Cambridge, U.K.: Cambridge Univ. Press; 1991. [Google Scholar]
- 15.Unwin D, Bakhurina N N. Nature (London) 1994;371:62–64. [Google Scholar]
- 16.Clark J M, Hopson J A, Hernandez R, Fastovsky D E, Montellano M. Nature (London) 1998;391:886–889. [Google Scholar]
- 17.Seeley H G. Dragons of the Air. London: Methuen; 1901. [Google Scholar]
- 18.Ruben J A, Jones T D, Geist N R. BioEssays. 1998;20:852–859. [Google Scholar]
- 19.Elzanowski A, Wellnhofer P. J Vert Paleontol. 1996;16:81–94. [Google Scholar]
- 20.Ruben, J. A. & Jones, T. D. (1999) Am. Zool., in press.
- 21.Ji Q, Currie P J, Norell M A, Ji S. Nature (London) 1998;393:753–761. [Google Scholar]
- 22.Chiappe L M. Archaeopteryx. 1997;15:109–112. [Google Scholar]
- 23.Padian H, Chiappe L M. Sci Am. 1998;278(2):38–47. doi: 10.1038/scientificamerican0298-38. [DOI] [PubMed] [Google Scholar]
- 24.Carroll R L, Dong Z-M. Philos Trans R Soc London. 1991;331:131–153. [Google Scholar]
- 25.Witmer L M. Science. 1997;276:1209–1210. [Google Scholar]
- 26.Close F. Am Sci. 1993;81:83–84. [Google Scholar]
- 27.Sereno P C. J Vert Paleontol. 1994;13:425–450. [Google Scholar]
- 28.Burke A C, Alberch P. J Morphol. 1985;186:119–131. doi: 10.1002/jmor.1051860111. [DOI] [PubMed] [Google Scholar]