Abstract
Delayed neutrophil apoptosis and overshooting neutrophil activity contribute to organ dysfunction and subsequent organ failure in sepsis. Here, we investigated apoptotic signaling pathways that are involved in the inhibition of spontaneous apoptosis in neutrophils isolated from major trauma patients with uneventful outcome as well as in those with sepsis development. DNA fragmentation in peripheral blood neutrophils showed an inverse correlation with the organ dysfunction at d 10 after trauma in all patients, supporting the important role of neutrophil apoptosis regulation for patient’s outcome. The expression of the antiapoptotic Bcl-2 protein members A1 and Mcl-1 were found to be diminished in the septic patients at d 5 and d 10 after trauma. This decrease was also linked to an impaired intrinsic apoptosis resistance, which has been previously shown to occur in neutrophils during systemic inflammation. In patients with sepsis development, delayed neutrophil apoptosis was found to be associated with a disturbed extrinsic pathway, as demonstrated by reduced caspase-8 activity and Bid truncation. Notably, the expression of Dad1 protein, which is involved in protein N-glycosylation, was significantly increased in septic patients at d 10 after trauma. Taken together, our data demonstrate that neutrophil apoptosis is regulated by both the intrinsic and extrinsic pathway, depending on patient’s outcome. These findings might provide a molecular basis for new strategies targeting cell death pathways in apoptosis-resistant neutrophils during systemic inflammation.
INTRODUCTION
Sepsis is a leading cause of death in intensive care unit patients, resulting in more than 200,000 deaths annually (1). Clinical and experimental observations have suggested that apoptosis may play a role in the pathogenesis of sepsis-associated multiple organ dysfunction syndrome (MODS) (2–5), but the mechanism by which MODS develops remains unclear. Neutrophils are terminally differentiated cells of the innate immune system playing an important role as a first line of defense against bacterial infections as well as in the modulation of the inflammatory response (6). Paradoxically, neutrophils also represent one of the main mediators of tissue injury in various human diseases, including sepsis (7,8). Under normal conditions neutrophil half-life in the circulation is limited to ~6–10 h, after which they undergo spontaneous apoptosis. However, during acute inflammation, neutrophil life span becomes significantly extended owing to the action of proinflammatory mediators and bacterial membrane components such as endotoxin (9). This prolonged neutrophil survival is associated with the accumulation of activated neutrophils contributing to an ongoing inflammation, and consecutive host tissue damage, with subsequent organ failure in critical ill patients. Profound inhibition of apoptosis has been already reported in neutrophils from patients with systemic inflammatory response syndrome (SIRS) (2,10), in sepsis (11–14), as well as after burn injuries (15) and acute respiratory distress syndrome (8,16,17). These observations suggest that the resolution of the inflammatory response is highly dependent from the induction of neutrophil programmed cell death.
During neutrophil spontaneous apoptosis both, mitochondria- and death receptor–mediated apoptotic signaling were shown to be activated (18). The intrinsic apoptotic pathway involves mitochondria, which release cytochrome c into the cytoplasm following outer membrane permeabilization by proapoptotic Bcl-2 family members. Cytochrome c then combines with caspase-9 and Apaf-1 to form the apoptosome, resulting in caspase-3 activation and apoptosis (19).
Human neutrophils express the proapoptotic proteins Bax, Bad, Bid, Bik and Bak and the antiapoptotic proteins Mcl-1, A1/Bfl-1 (A1) and Bcl-XL (20). Mcl-1 and A1 have very short half-lives of 2–3 h whereas the half-lives of the proapoptotic proteins are relatively long (21). The cellular levels of Mcl-1 protein have been shown to closely correlate with neutrophil survival. Hence, neutrophil half-life may be predominantly governed by the cellular levels of the antiapoptotic factors (22,23).
In the extrinsic pathway, stimulation of death receptors such as Fas, TNF-related apoptosis-inducing ligand (TRAIL) receptors 1 and 2 and TNF receptor-1 and -2 by their ligands, for example, FasL, TNF-α or TRAIL, allows the trimerization/reorganization of the death receptor on the cell surface and subsequent caspase-8 activation (24). Both, the extrinsic and intrinsic pathway may converge at the level of mitochondria.
Currently, the molecular mechanisms that underlie the delay of neutrophil apoptosis during acute inflammation and sepsis are not fully understood. Recently, we found evidence for an impaired extrinsic pathway in patients with posttraumatic sepsis development due to elevated serum levels of soluble Fas (sFas). Serum sFas has been also shown to inhibit neutrophil apoptosis in vitro. However, in this study, both trauma patients with and without sepsis development showed strong reduction of apoptosis in circulating neutrophils at least until d 10 after trauma (25).
Based on these previous findings, here we sought to characterize the impact of extrinsic and intrinsic pathways on the regulation of neutrophil apoptosis in patients with trauma-associated sepsis development, as well as in patients with uneventful outcome.
MATERIALS AND METHODS
Patients
Twenty-four patients were enrolled in this prospective study. Study approval was obtained from the Ethics Review Board of the University of Duesseldorf, Germany. Patients with blunt or penetrating multiple injuries who were admitted to our Level I Trauma Center with an Injury Severity Score (ISS) =16, intensive care unit (ICU) stay >3 d and aged 18 years or older were enrolled in this study. Written informed consent was obtained from all participants or their legal representatives if the patients lacked consciousness. Exclusion criteria were death of the patient on day of admission or within the first 2 d on ICU. In addition, patients with known preexisting immunological disorders or systemic immunosuppressive medication were excluded. The severity of injury was assessed by the ISS, based on the Abbreviated Injury Scale (AIS) (26). SIRS and sepsis were defined using the criteria outlined 2005 from the International Sepsis Forum (27). Patients were determined as septic if they fulfilled criteria for SIRS and had a proven source of infection. SIRS was defined by two or more of the following criteria: temperature >38°C or <36°C; heart rate >90 beats per min; respiratory rate >20 breaths per min or arterial carbon dioxide tension (PaCO2) <32 mmHg; and white blood cell count >12,000 cells/mm3 or <4000 cells/mm3, or with >10% immature (band) forms. To evaluate organ dysfunction/failure, the Sequential Organ Failure Assessment (SOFA) and Multiple Organ Dysfunction (MOD) score were determined prospectively on a daily basis (28). Severe sepsis referred to sepsis complicated by organ dysfunction. Organ dysfunction has been defined using the definition by the SOFA score with >2 points for at least one system (respiratory, coagulation, liver, cardiovascular, central nervous or renal system). Septic shock was defined as sepsis with acute persistent circulatory failure unexplained by other causes (>2 points in SOFA score for cardiovascular system).
Blood was collected from healthy volunteers and routinely from patients from the day of admission until d 10. Heparinized blood was immediately used after collection for neutrophil isolation. In parallel, sera were harvested by centrifugation and stored at −80°C until further processing.
Isolation and Culture Conditions of Human Neutrophils
Human neutrophils were isolated by discontinuous density-gradient centrifugation on Percoll (Biochrom) as previously described (29). After hypotonic lysis to remove contaminating erythrocytes, cells were suspended in phosphate-buffered saline (PBS). Purity and viability were routinely >95% as assessed by forward and side scatter characteristics of FACScan (BD Biosciences) and trypan blue exclusion, respectively. Subsequently, cells were frozen at −80°C or suspended in RPMI 1640 containing 2 mmol/L glutamine (Biochrom) and 1% human serum, supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin and cultured at 37°C in a humidified atmosphere containing 5% CO2.
RNA Interference and Transfection
For siRNA experiments, freshly isolated neutrophils from patients at d 1 after admission were nucleofected with mcl-1 small interfering RNA (siRNA) (Qiagen) using Amaxa Nucleofector System (Lonza), according to the manufacturer’s instructions (Human Monocyte Nucleofector Kit, Lonza) with some modifications. In brief, 5 × 106 neutrophils were suspended in 100 μL Nucleofector solution (Lonza) with 1.5 μg siRNA. The cells were electroporated and further cultured in RPMI 1640 medium containing 2 mmol/L glutamine (Bio chrom) and 10% FCS (PAA Laboratories). Alexa488-conjugated control siRNA (Qiagen), used to detect intracellular siRNA by flow cytometry and to monitor transfection efficiency, showed that the fluorescent siRNA was taken up in >90% of the cells. After 24-h culture, 0.2 μmol/L staurosporine (Alexis) was added to the cells.
Analysis of mRNA Expression by RT-PCR and Quantitative PCR
Total RNA from neutrophils was extracted using TRI Reagent (Sigma) according to the manufacturer’s instructions. Contaminating DNA was removed by digestion with DNase (DNA-free, Ambion). 500 ng RNA were reverse transcribed to complementary DNA (cDNA) using oligo(dT)15 primer, random primer, and Omniscript Reverse Transcriptase (Qiagen). Gene-specific primer pairs were designed by using the Primer Express® software (version 3.0; Applied Biosystems): Mcl-1 forward: 5′-CAAGG CATGC TTCGG AAACT-3′; Mcl-1 reverse: 5′-GATCA TCACT CGAGA CAACG ATTT-3′; A1 forward: 5′-CTCAG CACAT TGCCT CAACA G-3′; A1 reverse: 5′-GCCTG GTGGA GAGCA AAGTC-3′; Bax forward: 5′-TGGAG CTGCA GAGGA TGATT G-3′; Bax reverse: 5′-GAAGT TGCCG TCAGA AAACA T-3′; Dad1 forward: 5′-GGCGT CGGTA GTGTC TGTCA-3′; Dad1 reverse: 5′-CTGCG GAGTG GAGCT CAAG-3′; 18S forward: 5′-CATGG TGACC ACGGG TGAC-3′; 18S reverse: 5′-TTCCT TGGAT GTGGT AGCCG-3′. Relative gene expression levels were determined using SYBR Green (Applied Biosystems) incorporation following the manufacturer’s recommended protocol with the following thermal cycling conditions: 95°C, 10 min (1 cycle); 95°C, 15 s, 60°C, 60 s (40 cycles); 4°C hold. All samples were run in triplicates (ABI Prism 7300, Applied Biosystems). Expression of each target gene was normalized to the 18S RNA gene. Fold expression was calculated using the 2−ΔΔCT method (30).
Human Apoptosis Quantitative Real-Time Polymerase Chain Reaction Array
To analyze the expression of apoptosis-related genes in neutrophils after major trauma, the Human Apoptosis 96 Stell-ARay quantitative real-time polymerase chain (qPCR) array has been used according to the manufacturer’s instructions (Lonza). Data were analyzed using Global Pattern Recognition (GPR) Analysis software (Bar Harbor Biotechnology), which normalizes the data of each analyzed gene to that of every other gene without dependence of single gene normalization (31).
Western Blotting
Isolated neutrophils were suspended in radioimmunoprecipitation assay (RIPA) buffer (50 mmol/L Tris pH 8.0, 150 mmol/L NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfide), supplemented with complete protease inhibitor mixture (Roche) followed by cell sonication. Samples were separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitro-cellulose membranes (BioRad). Membranes were saturated in Tris-buffered saline supplemented with 0.1% Tween20 (TBST) and 5% w/v nonfat dry milk for 1 h and then immunolabeled with monoclonal anti-human Mcl-1 (BD Biosciences), polyclonal anti-human Bid and anti-human A1/Bfl-1 (both Cell Signaling), or anti-human Dad1 antibody (Imgenex), respectively. Blots were then washed three times with Tris-buffered saline + 0.1% Tween 20 (TBST) and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Dako) for 1 h at room temperature. Bands were visualized using SuperSignal West Pico detection kit (Thermo Fisher Scientific). Equal loading was confirmed by PonceauS staining and reincubation of membranes with a monoclonal antibody for glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Imgenex).
Quantification of Neutrophil Apoptosis
Neutrophil apoptosis was measured by using the Cell Death Detection Enzyme-Linked Immunosorbent Assay (ELISA) Plus Kit (Roche), which quantitatively detects mono- and oligonucleosomes in the cytoplasmatic fraction. Briefly, neutrophil pellets were suspended in lysis buffer and DNA fragments were detected according to the manufacturer’s instruction. Apoptosis was normalized to total double-stranded (ds) DNA, which has been quantified by PicoGreen (Invitrogen) staining. In addition, neutrophil apoptosis was measured by propidium iodide staining and flow cytometry as previously described (10). Nuclei to the left of the G1 peak (sub-G1) containing hypodiploid DNA, corresponding to fragmented DNA, were considered apoptotic.
Detection of Mitochondrial Membrane Depolarization
To measure the mitochondrial membrane potential, neutrophils were stained with the lipophilic dye 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide (JC-1; Sigma Aldrich, Taufkirchen, Germany) as previously described (10). Then, cells immediately analyzed by flow cytometry. The excitation wavelength was 488 nm and the emission wavelengths 530 nm (FL1) for green fluorescence and 590 nm (FL2) for red fluorescence. In intact cells with a high negative mitochondrial membrane potential, JC-1 forms aggregates that emit a red fluorescence (590 nm). In mitochondria with low membrane potential (depolarized), the dye forms monomers in the cytosol that emit a green fluorescence (530 nm). Results are expressed as the relative number of cells with high levels of green fluorescence (FL1), indicating mitochondrial membrane depolarization in these cells.
Determination of Caspase-8 Activity
Freshly isolated neutrophils were harvested by centrifugation and cell pellets were stored by −80°C for further processing. The activity of caspase-8 has been measured by using Caspase-Glo 8 Assay (Promega) according to manufacturer’s instructions.
Statistical Analyses
Data are presented as box plots representing the median (heavy line in boxes) and the 25th and 75th percentiles. Whiskers indicate the minimum and maximum values, respectively. To evaluate differences between several groups, a nonparametric Kruskal-Wallis test with Dunn post hoc test was performed. To compare differences between two groups, the Mann-Whitney U test has been used. For normally distributed data, the mean ± SEM is depicted. Statistical analysis was performed by using one-way analysis of variance (ANOVA), followed by the Newman-Keuls test. Correlation between numerical values was evaluated by Spearman rank correlation coefficient (ρ). Analyses were performed with GraphPad Prism Program (version 5, GraphPad Software, San Diego, CA, USA). Data were considered to be statistically significant at P < 0.05.
RESULTS
Demographics, Initial Blood Values Outcome
The 24 patients (15 male, 9 female) enrolled in this study had an ISS of 46.7 ± 3.1 (mean ± SEM, range 16–75). The mean age was 41.7 ± 3.8 years (range 19–78 years). All patients fulfilled at least two of criteria of SIRS on admission to the ICU and on d 1 after trauma. From all patients, 12 developed sepsis within 6.1 ± 0.4 d (range 4–8 d) after trauma. Among the septic patients, one patient had uncomplicated sepsis, seven patients met the criteria for severe sepsis and four patients met the criteria for septic shock. The infection site of sepsis and microbiological pathogens for each patient are given in Table 1. The most frequent focus for sepsis was a pulmonary infection. Patients with posttraumatic sepsis development were apparently older and more severely injured than those without sepsis. Further, septic patients had a longer ICU stay as well as higher SOFA and MOD scores. Three septic patients died 30.7 ± 12.3 d (range 16–55 d) after trauma as a consequence of multiple organ failure. Further patient characteristics as well as injury severity, SOFA as well as MOD score values and outcome are shown in Table 2.
Table 1.
Infection site of sepsis and microbiological pathogens.
| Subject | Infection site | Pathogen | Evidence for sepsis, d |
|---|---|---|---|
| 1 | Pneumoniae | Staphylococcus aureus | 5 |
| 2 | Pneumoniae | Escherichia coli | 7 |
| 3 | Pneumoniae | Klebsiella pneumoniae | 5 |
| 4 | Peritonitis | Enteroccocus faecium | 8 |
| 5 | Pneumoniae | Enterobacter cloacae | 4 |
| 6 | Pneumoniae | Escherichia coli | 7 |
| 7 | Pneumoniae | Staphylococcus aureus | 6 |
| 8 | Pneumoniae | Staphylococcus aureus | 7 |
| 9 | Pneumoniae | Escherichia coli | 7 |
| 10 | Pneumoniae | Klebsiella pneumoniae | 4 |
| 11 | Pneumoniae | n.d. | 8 |
| 12 | Soft tissue | Enterococcus faecalis | 5 |
n.d., Not detected; d, d after trauma.
Table 2.
Demographics, injury severity, and outcome among subsets of patients.
| All patients | No sepsis | Sepsis | |
|---|---|---|---|
| number, n | 24 | 12 | 12 |
| Age, years | 41.7 ± 3.8 | 35.8 ± 3.6 | 47.6 ± 6.4 |
| ISS | 46.7 ± 3.1 | 43.9 ± 3.8 | 49.5 ± 4.9 |
| ICU, d | 21.1 ± 3.8 | 12.0 ± 1.8 | 30.3 ± 6.5b |
| Sepsis,% (n) | 50.0 (12) | 0 (0) | 100 (12) |
| Death,% (n) | 12.5 (3) | 0 (0) | 24.0 (3) |
| SOFA d 1 | 9.0 ± 0.6 | 7.6 ± 0.8 | 10.3 ± 0.7a |
| SOFA d 5 | 6.9 ± 0.9 | 3.9 ± 1.1 | 9.5 ± 1.2b |
| SOFA d 10 | 4.5 ± 0.9 | 2.1 ± 0.7 | 6.5 ± 1.4a |
| MODS d 1 | 6.7 ± 0.6 | 5.1 ± 0.8 | 8.3 ± 0.7a |
| MODS d 5 | 5.5 ± 0.8 | 2.7 ± 0.9 | 8.3 ± 0.8c |
| MODS d 10 | 3.8 ± 0.8 | 1.3 ± 0.8 | 6.3 ± 0.9b |
Data indicated as mean ± standard error of the mean (SEM). ISS indicates injury severity score; ICU, intensive care unit length of stay; SOFA, Sequential Organ Failure Assessment score; MODS, Multiple Organ Dysfunction Score;
P < 0.05,
P < 0.01,
P < 0.001 between sepsis and no sepsis group.
Expression Profile of Apoptosis-Related Genes and Apoptosis Rate in Neutrophils from Severely Injured Patients
To identify apoptosis-relevant genes that are modified in their expression in neutrophils after major trauma, we screened the regulation of 94 genes in neutrophils isolated from multiple trauma patients. The cDNA from five healthy volunteers was pooled and used as control. Further, pooled cDNA from six patients, three with sepsis development during the first 10 d after trauma and three with uneventful recovery, was used to analyze changes in the expression of apoptosis-relevant genes at d 1, d 5, and d 10 after major trauma. We found all investigated genes to be differentially expressed in controls and patients already from d 1 until d 10 after major trauma (Table 3). Although the expression of antiapoptotic genes such as Mcl-1, A1, Dad1 was found to be strongly upregulated, a simultaneously increase in the expression of Bax, Bad, Bid and caspase-3, which are known to favor apoptosis, could be observed in trauma neutrophils. Additionally, there was an upregulation of mRNAs encoding for the death receptors tumor necrosis factor receptor superfamily member TNFRSF1A, and to a lower extent for TNFRSF1B and Fas. However, the expression of death receptor associated adapter molecules, for example, Fas-associated via death domain FADD, TNF receptor-associated factor TRAF2 and TRAF4, was markedly reduced compared with their expression found in control neutrophils.
Table 3.
Differential expression of apoptosis-related genes in neutrophils after major trauma.
| Well | Gene | Fold change, d 1 | Fold change, d 5 | Fold change, d 10 |
|---|---|---|---|---|
| A01 | SOD1 | 1,672493 | 1,688146 | 1,466493 |
| A02 | RIPK1 | −2,39164 | −3,962909 | −2,77739 |
| A03 | BNIP3 | −3,575141 | −4,78779 | −3,654895 |
| A04 | UNC5B | n.d. | −9,223153 | n.d. |
| A05 | E2F2 | 4,293041 | 1,042928 | 1,996911 |
| A06 | BBC3 | 1,37745 | 3,334892 | 4,523909 |
| A07 | AKT1 | −3,335724 | −1,928875 | 1,10089 |
| A08 | DAD1 | 6,329098 | 8,947223 | 6,318885 |
| A09 | DIABLO | 1,780151 | −1,014353 | −1,206 |
| A10 | PMAIP1 | n.d. | n.d. | n.d. |
| A11 | HIF1A | 23,785328 | 58,256212 | 81,666359 |
| A12 | TNFRSF1A | 9,862819 | 13,25661 | 10,087313 |
| B01 | STAT5B | 10,070057 | 1,162427 | −1,421713 |
| B02 | TP53INP1 | 1,043912 | −1,015127 | −1,513858 |
| B03 | HSPA1A | 1,396679 | 2,220739 | 3,227395 |
| B04 | TNF | −6,224681 | −7,248387 | −1,239648 |
| B05 | BCL2L14 | n.d. | n.d. | n.d. |
| B06 | LTBR | 2,044857 | −4,359492 | −2,763947 |
| B07 | BCL6 | 4,763427 | 5,137762 | 5,715462 |
| B08 | DFFA | −7,200015 | −18,630055 | −14,919528 |
| B09 | BAX | 2,832351 | 1,669412 | 2,290512 |
| B10 | CD40 | n.d. | −12,787479 | −2,564238 |
| B11 | BAK1 | n.d. | n.d. | n.d. |
| B12 | TRAF2 | −17,851829 | −8,492916 | −2,2984 |
| C01 | FOXO3 | 8,011098 | 4,789384 | 3,650692 |
| C02 | PAWR | −29,815694 | −21,330709 | −7,159853 |
| C03 | BAG4 | −1,077733 | −1,177609 | −1,118163 |
| C04 | FGFR3 | n.d. | n.d. | n.d. |
| C05 | SIRT1 | 1,638073 | 1,452804 | −1,540854 |
| C06 | XIAP | −2,140577 | −2,523734 | −1,435576 |
| C07 | FAS | 3,923116 | 1,981977 | −1,019054 |
| C08 | AVEN | n.d. | n.d. | n.d. |
| C09 | API5 | 1,001387 | −1,058382 | −11,060387 |
| C10 | IGF1 | −1080,88701 | −32,522359 | −123,231733 |
| C11 | BNIP3L | n.d. | n.d. | n.d. |
| C12 | HTRA2 | −1,204137 | 2,963783 | 2,925473 |
| D01 | PTEN | n.d. | n.d. | n.d. |
| D02 | BCL2L2 | n.d. | n.d. | n.d. |
| D03 | TNFAIP3 | 4,263387 | 3,366945 | 5,083316 |
| D04 | APAF1 | 1,842928 | 2,182588 | 2,243529 |
| D05 | HSGenomic | n.d. | n.d. | n.d. |
| D06 | DDIT3 | 2,030732 | 1,879098 | 6,899874 |
| D07 | BOK | −68,974921 | −14,483717 | −56,682442 |
| D08 | CASP2 | −5,16225 | −2,213087 | 1,217709 |
| D09 | GPX1 | −1,775223 | 1,40011 | 3,486719 |
| D10 | GSK3B | 5,359134 | −190,303726 | −34,314109 |
| D11 | BIRC5 | n.d. | n.d. | n.d. |
| D12 | BID | 2,316586 | 4,079918 | 2,954205 |
| E01 | FADD | −5,4189 | −1,70605 | 1,479357 |
| E02 | MCL1 | 4,03341 | 2,929471 | 1,517788 |
| E03 | PRDX2 | −47,11126 | −119,532573 | −45,630609 |
| E04 | TGFB1 | −1,825131 | −1,3051 | 1,52042 |
| E05 | BFAR | 1,358486 | 3,001409 | 3,053936 |
| E06 | CASP9 | 1,19914 | −1,27709 | −1,820999 |
| E07 | BNIP2 | 1,582275 | −1,249768 | 1,000742 |
| E08 | CASP3 | 3,923116 | 7,663163 | 3,420412 |
| E09 | BIRC2 | 8,645803 | 9,273025 | 7,237894 |
| E10 | TRADD | −5,09118 | −4,538325 | −1,180363 |
| E11 | CLU | n.d. | n.d. | n.d. |
| E12 | DAPK2 | −1,513617 | −1,374551 | 2,166506 |
| F01 | BNIP1 | n.d. | n.d. | n.d. |
| F02 | TNFRSF1B | 1,649467 | 1,448279 | 1,785181 |
| F03 | HRK | −15,221092 | −16,04954 | −7,333643 |
| F04 | TP53 | −6,267977 | −3,578512 | −1,944002 |
| F05 | HIPK2 | n.d. | n.d. | n.d. |
| F06 | STAT5A | 1,166349 | 1,740908 | 2,572855 |
| F07 | DAP | 4,35297 | 8,866348 | 4,893511 |
| F08 | CASP7 | −7,200015 | −4,333582 | −2,271004 |
| F09 | NAIP | 1,934553 | 1,783906 | 1,022053 |
| F10 | TNFRSF10A | n.d. | n.d. | n.d. |
| F11 | FASLG | n.d. | n.d. | n.d. |
| F12 | LTA | −7,663482 | −2,449114 | −2,856661 |
| G01 | TNFRSF11B | n.d. | n.d. | n.d. |
| G02 | Hs18s | −17,606058 | −18,415645 | −2,365475 |
| G03 | DPF2 | 1,961558 | 11,152846 | 7,256481 |
| G04 | CARD10 | n.d. | n.d. | n.d. |
| G05 | BAG3 | −69,45468 | −71,459801 | −16,247322 |
| G06 | CASP8 | −1,108032 | 1,036011 | 3,724044 |
| G07 | BCL2L11 | 21,141414 | 242,768933 | 352,180202 |
| G08 | BAG1 | −13,529157 | −5,131071 | −7,894949 |
| G09 | BIRC3 | 1,022429 | n.d. | n.d. |
| G10 | PERP | −6,717852 | −10,243664 | −71,374162 |
| G11 | E2F1 | n.d. | −1,66389 | −1,512285 |
| G12 | BAD | 1,285206 | 1,121353 | 3,203103 |
| H01 | DAPK1 | −3,133989 | −1,055086 | n.d. |
| H02 | BIK | 3,164549 | 1,443769 | 2,875813 |
| H03 | RAD21 | 4,293041 | −1,598213 | −1,316977 |
| H04 | BCL2L10 | −84,918089 | −12,292956 | −22,321004 |
| H05 | VEGFA | −1,308578 | 1,942669 | 5,462533 |
| H06 | BCL2 | −79,782476 | −5,322706 | −14,748844 |
| H07 | TRAF4 | −22,284984 | −11,440356 | n.d. |
| H08 | BCL2L1 | −2,883858 | 2,476049 | 2,418596 |
| H09 | GADD45G | −1,19582 | −1,385455 | 1,568592 |
| H10 | BCL10 | 4,322902 | 5,964247 | 3,440146 |
| H11 | NFKB1 | 1,150292 | −1,040127 | −1,585052 |
| H12 | BCL2A1 | 17,901394 | 3,652641 | 6,525417 |
n.d., Not detected.
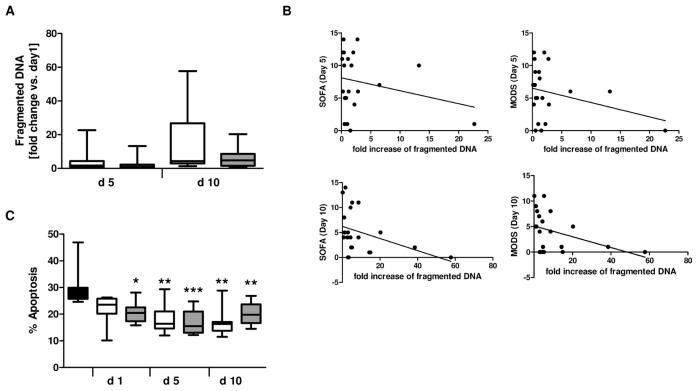
In previous studies, we have already shown that neutrophils after trauma display a prolonged life span when compared with control cells (10,25). However, as depicted in Figure 1A, there was no significant difference in apoptosis rate at d 5 and d 10 after trauma between neutrophils from patients without or with posttraumatic sepsis development, respectively. In addition, fold change in apoptosis when compared with d 1 was negatively correlated with patient’s MOD scores (ρ = −0.435, *P = 0.04 [see Figure 1 legend]) and to a lower degree with SOFA (ρ = −0.417, P = 0.059) at d 10 but not at d 5 after trauma, suggesting a link between the apoptotic status of neutrophils and patient’s clinical course (Figure 1B).
Figure 1.
Inhibition of neutrophil spontaneous apoptosis by serum factors. (A) Fold change of DNA fragmentation in neutrophils isolated from patients with (n = 10) or without (n = 10) sepsis development at d 5 and d 10 after major trauma relative to d 1. (B) Correlation of fold change of fragmented DNA with Sequential Organ Failure Assessment (SOFA) score and Multiple Organ Dysfunction Score (MODS) at d 5 and d 10 after major trauma. Spearman rho (ρ) correlation coefficient and P values are indicated. (C) Neutrophils isolated from one healthy control (1 × 106/mL) were incubated with 1% serum from healthy volunteers (control, n = 9) and with patient sera collected at d 1, d 5 and d 10 after major trauma. After 18 h culture, apoptotic neutrophils were quantified by propidium iodide staining and flow cytometry. The white bars indicate apoptosis in cells incubated with sera from patients with uneventful outcome (n = 12); the gray bars indicate apoptosis after treatment with sera from patients with sepsis development (n = 12). *P < 0.05; **P < 0.01; ***P < 0.001 versus control. A, C: □, Nonsepis;
 , sepsis; ■, control. B: SOFA d 5: ρ = −0.23, P = 0.316; SOFA d 10: ρ = −0.417, P = 0.059; MODS d 5: ρ = −0.24, P = 0.29; MODS d 10: ρ = −0.435, P = 0.04.
, sepsis; ■, control. B: SOFA d 5: ρ = −0.23, P = 0.316; SOFA d 10: ρ = −0.417, P = 0.059; MODS d 5: ρ = −0.24, P = 0.29; MODS d 10: ρ = −0.435, P = 0.04.
As previously reported, inhibition of neutrophil apoptosis after major trauma is mediated by serum factors (10,22). Indeed, treatment of control neutrophils with serum taken at d 1, d 5 and d 10 after admission from the 12 patients with and 12 patients without sepsis development led to a significant downregulation of neutrophil cell death compared with the apoptosis rate of neutrophils incubated with serum from healthy volunteers (Figure 1C). However, no differences in apoptosis could be observed between the sera from the two patient groups.
Regulation of the Neutrophil Apoptosis-Related Factors Mcl-1, A1, Dad1, and Bax during Sepsis Development after Major Trauma
To further elucidate the molecular mechanisms underlying reduced neutrophil apoptosis in patients with and without sepsis development after major trauma, we analyzed the expression of apoptotic factors, especially of those who were already shown to be important regulators of neutrophil survival (20).
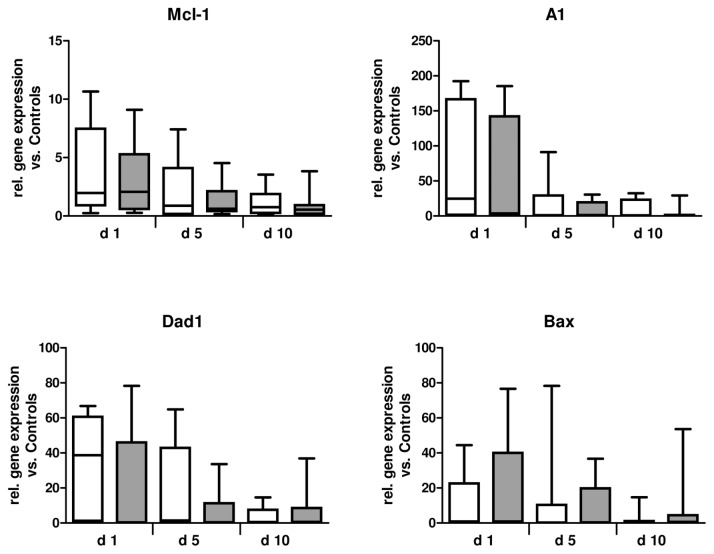
The expression levels of the antiapoptotic genes Mcl-1, A1, Dad1, and the proapoptotic gene Bax are depicted in Figure 2. These factors were found to be clearly upregulated on mRNA level in neutrophils early after major trauma (Table 3). Analysis of gene expression using real-time PCR revealed high inter-individual variation in mRNA expression levels. The expression of all genes was commonly found to be upregulated at d 1 after trauma when compared with their expression in healthy controls. The mRNA levels of Mcl-1, A1, Dad1 and Bax further declined to control levels after 10 d. No differences in mRNA expression between septic and nonseptic patients could be determined (Figure 2).
Figure 2.
mRNA expression of Bcl-2 family members and Dad1. The expression of Mcl-1, A1, DAD1 and Bax mRNA was quantified by real-time PCR in neutrophils isolated from healthy volunteers (n = 10), as well as from patients with (n = 9–10) or without (n = 10–12) sepsis development after major trauma. Gene expression was normalized to that of the 18S RNA gene. □, Nonsepis;
 , sepsis.
, sepsis.
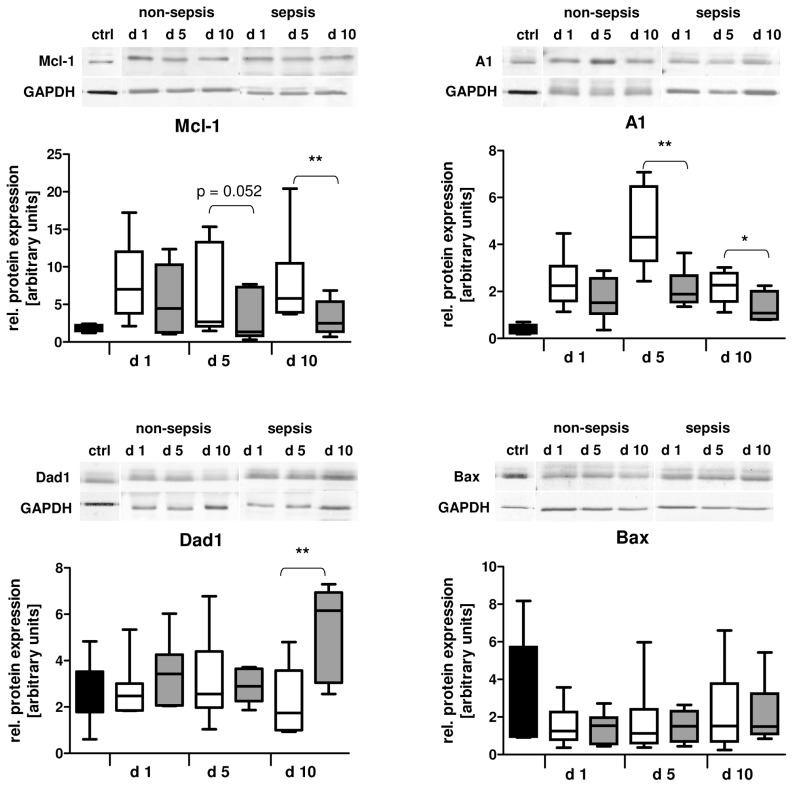
In general, the mRNA levels do not necessarily correlate to the amount of the expressed protein and many proteins have been demonstrated to be posttranslationally regulated. For instance, Mcl-1 turnover has been already shown to be modified by granulocyte-macrophage colony-stimulating factor (GM-CSF) (32). We further examined the protein expression of the apoptotic factors mentioned above using immunoblot analysis. The protein levels of Mcl-1 peaked at d 1 after trauma and were significantly reduced in the sepsis group at d 10 (P < 0.01 versus nonsepsis; Figure 3). Additionally, we found A1 protein levels to be significantly diminished in neutrophils isolated at d 5 and d 10 from patients with sepsis development (P < 0.01 and P < 0.05, respectively) when compared with neutrophils from the nonsepsis group at these times. These findings argue for a general decline of antiapoptotic Bcl-2 members in neutrophils during sepsis development. Although not being a member of the Bcl-2 family, Dad1 has been shown to interact with antiapoptotic Mcl-1 and loss of Dad1 function is associated with apoptotic cell death (33). In contrast to Mcl-1 and A1, Dad1 levels were not increased in patients when compared with control neutrophils. Whereas Dad1 protein levels in patients with uneventful recovery remained unchanged over time after major trauma, the protein was found to be significantly increased at d 10 after trauma in the sepsis group (P < 0.01 versus nonsepsis). Moreover, we found that neutrophils of all trauma patients expressed reduced levels of proapoptotic Bax over the whole observation period of 10 d without any intergroup differences. Altogether, these data suggest that neutrophils in sepsis display an obvious imbalance between pro-and antiapoptotic Bcl-2 members owing to reduced levels of Mcl-1 and A1.
Figure 3.
Protein expression of Bcl-2 family members and Dad1. The expression of Mcl-1, A1, Dad1 and Bax protein was determined by Western blot in neutrophils isolated from healthy volunteers (n = 5–13), as well as from patients with (n = 8–9) or without (n = 8–11) sepsis development after major trauma. Blots were analyzed by densitometry and normalized to GAPDH. In each case, representative blots of one healthy control, one patient with and one patient without sepsis development are depicted. *P < 0.05; **P < 0.01. □, Nonsepis;
 , sepsis; ■, control.
, sepsis; ■, control.
Reduced Mcl-1 Levels Are Associated with Impaired Intrinsic Apoptosis Resistance in Neutrophils during Sepsis
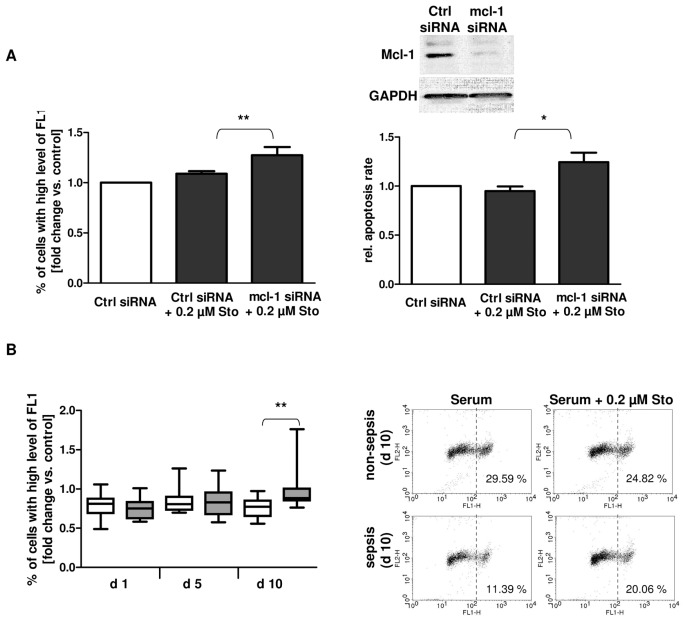
Neutrophil spontaneous apoptosis has been shown to be highly dependent on Mcl-1 protein levels. Besides Mcl-1, A1 has been identified to antagonize Bax activation and mitochondrial membrane depolarization (34). It has been already reported that neutrophils from trauma patients expressing high levels of intracellular Mcl-1 are resistant to mitochondrial membrane depolarization and thus intrinsic apoptosis in response to the proapoptotic stimulus staurosporine (10). In this study, we found neutrophils from patients with sepsis development to express reduced levels of antiapoptotic Mcl-1 and A1. We, therefore, assumed that neutrophils from septic patients might display an impaired intrinsic apoptosis resistance because of reduced Mcl-1 protein levels found in these cells. Indeed, Mcl-1 knockdown in patient neutrophils significantly enhanced sensitivity of the cells to staurosporine, resulting in increased mitochondrial membrane depolarization and apoptosis (Figure 4A). To prove our hypothesis, neutrophils from healthy donors were incubated with patient serum collected at d 1, d 5 and d 10 after severe trauma. We have previously shown that incubation of control neutrophils with patient serum promotes resistance to staurosporine-induced apoptosis by increasing the stability of Mcl-1 protein. This effect was mediated by proinflammatory cytokines, such as GM-CSF (10). Hence, reduced Mcl-1 levels in sepsis, probably because of altered cytokine levels, might be associated with a loss of mitochondrial membrane potential during intrinsic apoptosis induction. As speculated, mitochondrial disruption was significantly increased after treatment with staurosporine in cells incubated with serum from septic patients at d 10 after trauma when compared with cells incubated with serum from patients with uneventful outcome (Figure 4B).
Figure 4.
Reduced Mcl-1 protein levels are associated with impaired intrinsic apoptosis resistance during sepsis. (A) Freshly isolated neutrophils from patients (n = 8) at d 1 after trauma were nucleofected with control siRNA (Ctrl) or with mcl-1 siRNA. After 24 h culture, cells were treated with 0.2 μmol/L staurosporine (Sto) or left untreated (Ctrl). Mitochondrial membrane depolarization was quantified by JC-1 staining after 4 h. The percentage of cells with high levels of green (FL1) fluorescence was determined. Fold change versus sample without Sto treatment is depicted (left). After 18 h of culture, apoptotic neutrophils were quantified by propidium iodide staining and flow cytometry (right). Mcl-1 expression was evaluated by Western blot analysis. (B) Freshly isolated neutrophils from one healthy volunteer were preincubated with 1% serum from patients with (n = 12) or without (n = 12) sepsis development after major trauma for 1 h. Then, cells were further incubated with 0.2 μmol/L Sto for 4 h or left untreated. Mitochondrial membrane depolarization was quantified by JC-1 staining (left). Representative dot plots for cells treated with serum isolated at d 10 from one septic or nonseptic patient, respectively, are shown (right). *P < 0.05; **P < 0.01. B: □, Nonsepis;
 , sepsis.
, sepsis.
Inhibition of Caspase-8 Activity and Bid Truncation in Neutrophils during Sepsis
However, the reduced neutrophil intrinsic apoptosis resistance found in sepsis does not explain the decreased apoptosis rate of neutrophils from septic patients. We therefore investigated if other mechanisms might be of relevance for the modulation of neutrophil apoptosis. Activation of Fas receptor has been already reported in neutrophils undergoing spontaneous apoptosis. When Fas molecules cluster, they interact with the adapter proteins FADD, which in turn, recruit procaspase-8 molecules to induce their autocleavage and thus production of active caspase-8 (35). Activated caspase-8 then cleaves the proapoptotic Bcl-2 family member Bid (22 kDa) to yield truncated Bid (tBid, 15 kDa). tBid was demonstrated to promote apoptosis by linking the extrinsic to intrinsic pathway in neutrophils and different cell lines (36,37).
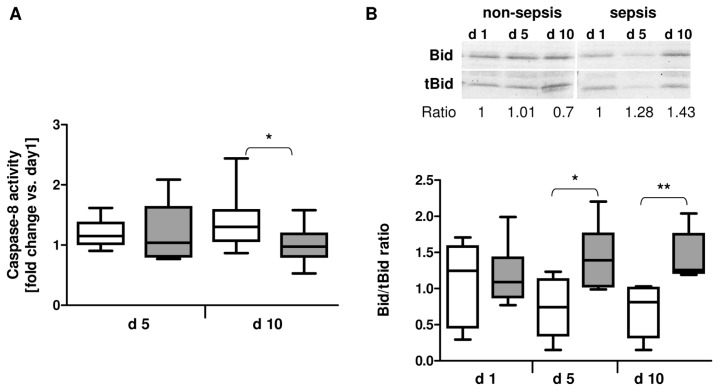
As depicted in Figure 5A, neutrophils isolated at d 10 from septic patients displayed significantly reduced caspase-8 activity when compared with neutrophils isolated from patients with uneventful outcome at the same time (P < 0.05). In addition, an increased Bid/tBid ratio has been found in neutrophils isolated at d 5 (P < 0.01) and d 10 (P < 0.01), respectively, from septic patients, indicating a reduced Bid cleavage by caspase-8 due to an impaired extrinsic apoptotic pathway in these cells (Figure 5B).
Figure 5.
Reduced caspase-8 activity and diminished Bid truncation in patients with sepsis development. (A) Fold change of caspase-8 activity in neutrophils isolated at d 5 and d 10 from patients with (n = 10) or without sepsis (n = 12) development after major trauma versus d 1. (B) Bid and truncated Bid (tBid) protein expression in neutrophils isolated at d 1, d 5 and d 10 from patients with sepsis development (n = 6) or uneventful recovery (n = 6) was analyzed by Western blot. The ratio of Bid/tBid protein expression is depicted for each patient group. *P < 0.05; **P < 0.01. □, Nonsepis;
 , sepsis.
, sepsis.
DISCUSSION
Delayed neutrophil apoptosis combined with cellular hyperactivity is widely accepted to be associated with inflammatory disorders including tissue damage and progressive organ dysfunction. In the current study, we demonstrate that apoptosis-related factors are differentially regulated in neutrophils during sepsis development after major trauma. In addition, we also found an inverse correlation between neutrophil apoptosis rate and the patients’ organ dysfunction scores suggesting an influence on sepsis-associated organ damage by delayed neutrophil apoptosis. The reduced neutrophil apoptosis rate in sepsis, which was not related to the expression of the neutrophil antiapoptotic factors Mcl-1 and A1, suggests that prolonged neutrophil life span does not solely depends on their expression levels. We demonstrate here that neutrophil apoptosis in sepsis is rather additionally regulated by factors modulating the extrinsic pathway, as shown by diminished caspase-8 activity and reduced Bid cleavage.
Our results showed many pro-and antiapoptotic genes to be upregulated after major trauma, supporting the regulation of gene activity by inflammatory mediators (38,39). Interestingly, Weber and colleagues have recently described a massive upregulation of proapoptotic factors in circulating blood cells from patients suffering from severe sepsis (40). They further concluded that the proapoptotic pattern of gene expression must be generated by lymphocytes, displaying increased apoptosis in sepsis, rather than neutrophils with prolonged life span (40). However, this work demonstrates that the gene expression of most pro apoptotic genes such as Bax, Bad, and Bid is also upregulated in neutrophils after major trauma showing prolonged life span.
Previous studies have suggested that neutrophil spontaneous apoptosis highly depends on the integrity of the mitochondrial membrane and is regulated by Bcl-2 family members (20,22,29). Several lines of evidence indicate that under inflammatory conditions, such as for example, after major trauma, the neutrophil life span becomes modified owing to the action of proinflammatory cytokines present in the circulation. In this context, it has been already shown by our group and others that GM-CSF– and also interleukin (IL)-18–mediated suppression of neutrophil apoptosis is associated with an increase in antiapoptotic Mcl-1 protein (10,41). So far, it remains speculative whether additional serum factors beside GM-CSF and IL-18 are involved in the regulation of neutrophil apoptotic factors after severe trauma.
The antiapoptotic Bcl-2 members Mcl-1 and A1 are known to impair the intrinsic pathway by maintaining the mitochondrial transmembrane potential (10,42). Both proteins were found to be markedly up-regulated in all patients after trauma while Bax protein levels were slightly downregulated when compared with healthy controls. Whereas Mcl-1 protein expression significantly declined at d 10 after trauma in the sepsis group, there was a strong decrease in A1 protein levels from d 5, suggesting a downregulation of antiapoptotic factors in sepsis.
A1 has already been reported to interact with human Bax (43). However, in contrast to the work of Werner and coworkers (42), reporting that A1 blocks the mitochondrial apoptotic pathway, here the reduction in A1 protein alone in the sepsis group at d 5 after trauma did not seem to abrogate intrinsic neutrophil apoptosis resistance as shown by staurosporine treatment and mitochondrial staining. However, it is likely that a simultaneously downregulation of other antiapoptotic Bcl-2 proteins is required to sensitize neutrophils to intrinsic apoptosis inducers.
In a previous study, we have demonstrated that intrinsic apoptosis resistance in neutrophils is accompanied by an increase in Mcl-1 protein levels. Further, incubation of control neutrophils with sera collected from severely injured patients was sufficient to reproduce this effect and to maintain mitochondrial membrane integrity after apoptosis induction with staurosporine (10). Consistent with these previous observations, the reduced Mcl-1 as well as A1 levels found in septic patients at d 10 after trauma were associated with an increased permeabilization of the mitochondrial membrane. In addition, our knockdown experiments using neutrophils expressing elevated levels of Mcl-1 (d 1 after trauma) further confirmed the regulation of the intrinsic apoptotic pathway by this factor. Interestingly, we found here that despite diminished levels of antiapoptotic Bcl-2 members, for example, Mcl-1, the apoptosis of neutrophils from patients with sepsis development after major trauma was still delayed. Based on these findings, we further suggested that additional upstream regulatory mechanisms are involved in the inhibition of neutrophil cell death in sepsis. In spontaneous neutrophil apoptosis Bid is cleaved to tBid which has been reported to activate Bax, thus promoting its translocation from the cytosol to the mitochondria (29,35). In the current study, the production of tBid was inhibited in neutrophils isolated from patients with sepsis development at d 5 and d 10 after trauma. This reduced Bid truncation was further associated with significantly reduced caspase-8 activity at d 10. These findings are in line with recently published results demonstrating that prolonged neutrophil life span in septic patients was associated with elevated serum levels of soluble Fas (sFas). sFas may functionally antagonize the Fas/FasL pathway and thus extrinsic caspase-8–mediated apoptosis. Indeed, patient’s SOFA and MOD scores showed a positive correlation with serum sFas levels (25) as well as an inverse correlation with neutrophil apoptosis at d 10. Hence, sFas in the circulation of trauma patients could represent another factor responsible for the dysregulation of apoptosis in severely injured patients.
In addition, in this article, we also show for the first time a strong upregulation of Dad1 protein in septic patients at d 10, but its mode of action is not yet understood. Dad1 has largely been implicated in N-linked glycosylation. The inhibition of N-linked protein glycosylation in cells that undergo apoptosis after loss of Dad1 function suggests that loss of N-linked glycoproteins is associated with onset of apoptotic cell death (44). In addition, Dad1 has been also shown to bind to antiapoptotic Mcl-1 protein and his expression in animal cells was reportedly down-regulated prior to the induction of programmed cell death (45). However, it remains unclear whether Dad1 might diminish neutrophil apoptosis by the modulation of Mcl-1 activity or by maintaining accurate N-glycosylation of proteins thus preventing endoplasmic reticulum stress. In fact, diminished neutrophil apoptosis in sepsis seems to be related to both, increased Dad1 levels and impaired extrinsic pathway, respectively.
Overall, most of the effects have been observed at d 10 after trauma. At the earlier time point (d 5), five of twelve patients in our cohort already displayed clinical signs of sepsis. In contrast, at d 10 all patients included in the sepsis group had clinical manifested septic disease.
One important limitation of this study is that we only investigated apoptotic signalling pathways in circulating neutrophils. Because these cells are known to infiltrate tissues and also to retain to the circulation after contact with the inflamed tissue, their function and apoptotic status might change. Being aware that posttraumatic tissue destruction is rather mediated by infiltrated than circulating neutrophils, it was not yet possible to investigate apoptosis of tissue neutrophils in patients, which however is a common limitation in patient studies. Further, we did not differentiate between different subpopulations of neutrophils, for example, newly released cells from the bone marrow, and therefore further discrepancies regarding apoptotic signalling in these cells cannot be ruled out.
CONCLUSION
In summary, our study demonstrates that sepsis development after major trauma is associated with changes in the expression of neutrophil antiapoptotic factors. In the early phase after trauma, neutrophil apoptosis seems to be mainly regulated by antiapoptotic Bcl-2 members which inhibit the intrinsic mitochondria-dependent pathway. Importantly, our data indicate that neutrophil apoptosis do not always correlate with Mcl-1 protein levels. Neutrophils from patients with clinically diagnosed sepsis at d 10 after trauma displayed reduced neutrophil apoptosis despite diminished levels of antiapoptotic Mcl-1 and A1 protein. In these patients, a predominant inhibition of the extrinsic apoptotic pathway could be found. The correlation between reduced neutrophil apoptosis and the severity of illness further supports the importance of neutrophil activity in the pathophysiology of sepsis. On the basis of these findings, drugs designed to target extrinsic rather than intrinsic apoptotic signalling in neutrophils during sepsis may help to modulate neutrophil life span and to prevent host tissue damage under these conditions.
ACKNOWLEDGMENTS
This study was, in part, supported by a grant from the Forschungskomission of the Heinrich-Heine University Düsseldorf. We are grateful to S Seghrouchni and J Schneider for excellent technical assistance.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Angus DC, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Papathanassoglou ED, Moynihan JA, McDermott MP, Ackerman MH. Expression of Fas (CD95) and Fas ligand on peripheral blood mononuclear cells in critical illness and association with multiorgan dysfunction severity and survival. Crit Care Med. 2001;29:709–18. doi: 10.1097/00003246-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Power C, Fanning N, Redmond HP. Cellular apoptosis and organ injury in sepsis: a review. Shock. 2002;18:197–211. doi: 10.1097/00024382-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Vaki I, et al. An early circulating factor in severe sepsis modulates apoptosis of monocytes and lymphocytes. J Leukoc Biol. 2011;89:343–9. doi: 10.1189/jlb.0410232. [DOI] [PubMed] [Google Scholar]
- 5.Hotchkiss RS, Tinsley KW, Karl IE. Role of apoptotic cell death in sepsis. Scand J Infect Dis. 2003;35:585–92. doi: 10.1080/00365540310015692. [DOI] [PubMed] [Google Scholar]
- 6.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–82. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 7.Tsukamoto T, Chanthaphavong RS, Pape HC. Current theories on the pathophysiology of multiple organ failure after trauma. Injury. 2010;41:21–6. doi: 10.1016/j.injury.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195–9. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 9.Savill J. Apoptosis in resolution of inflammation. J Leukoc Biol. 1997;61:375–80. doi: 10.1002/jlb.61.4.375. [DOI] [PubMed] [Google Scholar]
- 10.Paunel-Görgülü A, et al. Mcl-1-mediated impairment of the intrinsic apoptosis pathway in circulating neutrophils from critically ill patients can be overcome by Fas stimulation. J Immunol. 2009;183:6198–206. doi: 10.4049/jimmunol.0901264. [DOI] [PubMed] [Google Scholar]
- 11.Härter L, Mica L, Stocker R, Trentz O, Keel M. Mcl-1 correlates with reduced apoptosis in neutrophils from patients with sepsis. J Am Coll Surg. 2003;197:964–73. doi: 10.1016/j.jamcollsurg.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Taneja R, et al. Delayed neutrophil apoptosis in sepsis is associated with maintenance of mitochondrial transmembrane potential and reduced caspase-9 activity. Crit Care Med. 2004;32:1460–9. doi: 10.1097/01.ccm.0000129975.26905.77. [DOI] [PubMed] [Google Scholar]
- 13.Wesche DE, Lomas-Neira JL, Perl M, Chung CS, Ayala A. Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol. 2005;78:325–37. doi: 10.1189/jlb.0105017. [DOI] [PubMed] [Google Scholar]
- 14.Ertel W, et al. Circulating mediators in serum of injured patients with septic complications inhibit neutrophil apoptosis through up-regulation of protein-tyrosine phosphorylation. J. Trauma. 1998;44:767–75. doi: 10.1097/00005373-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Chitnis D, Dickerson C, Munster AM, Winchurch RA. Inhibition of apoptosis in polymorphonuclear neutrophils from burn patients. J Leukoc Biol. 1996;59:835–9. doi: 10.1002/jlb.59.6.835. [DOI] [PubMed] [Google Scholar]
- 16.Lee WL, Downey GP. Neutrophil activation and acute lung injury. Curr. Opin. Crit. Care. 2001;7:1–7. doi: 10.1097/00075198-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Matute-Bello G, et al. Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome. Crit Care Med. 2000;28:1–7. doi: 10.1097/00003246-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Daigle I, Simon HU. Critical role for caspases 3 and 8 in neutrophil but not eosinophil apoptosis. Int Arch Allergy Immunol. 2001;126:147–56. doi: 10.1159/000049506. [DOI] [PubMed] [Google Scholar]
- 19.Murphy BM, O’Neill AJ, Adrain C, Watson RW, Martin SJ. The apoptosome pathway to caspase activation in primary human neutrophils exhibits dramatically reduced requirements for cytochrome C. J Exp Med. 2003;197:625–32. doi: 10.1084/jem.20021862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moulding DA, Akgul C, Derouet M, White MR, Edwards SW. BCL-2 family expression in human neutrophils during delayed and accelerated apoptosis. J Leukoc Biol. 2001;70:783–92. [PubMed] [Google Scholar]
- 21.Dzhagalov I, St John A, He YW. The anti-apoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2007;109:1620–6. doi: 10.1182/blood-2006-03-013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moulding DA, Quayle JA, Hart CA, Edwards SW. Mcl-1 expression in human neutrophils: regulation by cytokines and correlation with cell survival. Blood. 1998;92:2495–502. [PubMed] [Google Scholar]
- 23.Akgul C, Moulding DA, Edwards SW. Molecular control of neutrophil apoptosis. FEBS Lett. 2001;487:318–22. doi: 10.1016/s0014-5793(00)02324-3. [DOI] [PubMed] [Google Scholar]
- 24.Akgul C, Edwards SW. Regulation of neutrophil apoptosis via death receptors. Cell Mol Life Sci. 2003;60:2402–8. doi: 10.1007/s00018-003-3110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paunel-Görgülü A, Flohé S, Scholz M, Windolf J, Lögters T. Increased serum soluble Fas after major trauma is associated with delayed neutrophil apoptosis and development of sepsis. Crit. Care. 2011;15:R20. doi: 10.1186/cc9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenspan L, McLellan BA, Greig H. Abbreviated injury scale and injury severity score: a scoring chart. J. Trauma. 1985;25:60–4. doi: 10.1097/00005373-198501000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Calandra T, Cohen J. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. 2005;33:1538–48. doi: 10.1097/01.ccm.0000168253.91200.83. [DOI] [PubMed] [Google Scholar]
- 28.Marshall JC, et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–52. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Maianski NA, Mul FP, van Buul JD, Roos D, Kuijpers TW. Granulocyte colony-stimulating factor inhibits the mitochondria-dependent activation of caspase-3 in neutrophils. Blood. 2002;99:672–9. doi: 10.1182/blood.v99.2.672. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Akilesh S, Shaffer DJ, Roopenian D. Customized molecular phenotyping by quantitative gene expression and pattern recognition analysis. Genome Res. 2003;13:1719–27. doi: 10.1101/gr.533003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derouet M, Thomas L, Cross A, Moots RJ, Edwards SW. Granulocyte macrophage colony-stimulating factor signaling and protea-some inhibition delay neutrophil apoptosis by increasing the stability of Mcl-1. J Biol Chem. 2004;279:26915–21. doi: 10.1074/jbc.M313875200. [DOI] [PubMed] [Google Scholar]
- 33.Makishima T, Yoshimi M, Komiyama S, Hara N, Nishimoto T. A subunit of the mammalian oligosaccharyltransferase, DAD1, interacts with Mcl-1, one of the bcl-2 protein family. J Biochem. 2000;128:399–405. doi: 10.1093/oxfordjournals.jbchem.a022767. [DOI] [PubMed] [Google Scholar]
- 34.Simmons MJ, et al. Bfl-1/A1 functions, similar to Mcl-1, as a selective tBid and Bak antagonist. Oncogene. 2008;27:1421–8. doi: 10.1038/sj.onc.1210771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheel-Toellner D, et al. Reactive oxygen species limit neutrophil life span by activating death receptor signaling. Blood. 2004;104:2557–64. doi: 10.1182/blood-2004-01-0191. [DOI] [PubMed] [Google Scholar]
- 36.Grinberg M, et al. tBID Homooligomerizes in the mitochondrial membrane to induce apoptosis. J Biol Chem. 2002;277:12237–45. doi: 10.1074/jbc.M104893200. [DOI] [PubMed] [Google Scholar]
- 37.Maianski NA, Roos D, Kuijpers TW. Bid truncation, bid/bax targeting to the mitochondria, and caspase activation associated with neutrophil apoptosis are inhibited by granulocyte colony-stimulating factor. J Immunol. 2004;172:7024–30. doi: 10.4049/jimmunol.172.11.7024. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, et al. Gene expression in mature neutrophils: early responses to inflammatory stimuli. J Leukoc Biol. 2004;75:358–72. doi: 10.1189/jlb.0903412. [DOI] [PubMed] [Google Scholar]
- 39.Malcolm KC, Arndt PG, Manos EJ, Jones DA, Worthen GS. Microarray analysis of lipopolysaccharide-treated human neutrophils. Am J Physiol Lung Cell Mol Physiol. 2003;284:L663–70. doi: 10.1152/ajplung.00094.2002. [DOI] [PubMed] [Google Scholar]
- 40.Weber SU, et al. Induction of Bim and Bid gene expression during accelerated apoptosis in severe sepsis. Crit. Care. 2008;12:R128. doi: 10.1186/cc7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akhtar S, Li X, Kovacs EJ, Gamelli RL, Choudhry MA. Interleukin-18 delays neutrophil apoptosis following alcohol intoxication and burn injury. Mol Med. 2011;17:88–94. doi: 10.2119/molmed.2010.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Werner AB, de Vries E, Tait SW, Bontjer I, Borst J. Bcl-2 family member Bfl-1/A1 sequesters truncated bid to inhibit is collaboration with pro-apoptotic Bak or Bax. J Biol Chem. 2002;277:22781–8. doi: 10.1074/jbc.M201469200. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H, et al. Structural basis of BFL-1 for its interaction with BAX and its anti-apoptotic action in mammalian and yeast cells. J Biol Chem. 2000;14:11092–9. doi: 10.1074/jbc.275.15.11092. [DOI] [PubMed] [Google Scholar]
- 44.Hauptmann P, et al. Defects in N-glycosylation induce apoptosis in yeast. Mol Microbiol. 2006;59:765–78. doi: 10.1111/j.1365-2958.2005.04981.x. [DOI] [PubMed] [Google Scholar]
- 45.Makishima T, Yoshimi M, Komiyama S, Hara N, Nishimoto T. A subunit of the mammalian oligosaccharyltransferase, DAD1, interacts with Mcl-1, one of the bcl-2 protein family. J Biochem. 2000;128:399–405. doi: 10.1093/oxfordjournals.jbchem.a022767. [DOI] [PubMed] [Google Scholar]