Abstract
Pulmonary infection with Pseudomonas aeruginosa and neutrophilic lung inflammation significantly contribute to morbidity and mortality in cystic fibrosis (CF). High-mobility group box 1 protein (HMGB1), a ubiquitous DNA binding protein that promotes inflammatory tissue injury, is significantly elevated in CF sputum. However, its mechanistic and potential therapeutic implications in CF were previously unknown. We found that HMGB1 levels were significantly elevated in bronchoalveolar lavage fluids (BALs) of CF patients and cystic fibrosis transmembrane conductance regulator (CFTR )−/− mice. Neutralizing anti-HMGB1 monoclonal antibody (mAb) conferred significant protection against P. aeruginosa–induced neutrophil recruitment, lung injury and bacterial infection in both CFTR−/− and wild-type mice. Alveolar macrophages isolated from mice treated with anti-HMGB1 mAb had improved phagocytic activity, which was suppressed by direct exposure to HMGB1. In addition, BAL from CF patients significantly impaired macrophage phagocytotic function, and this impairment was attenuated by HMGB1-neutralizing antibodies. The HMGB1-mediated suppression of bacterial phagocytosis was attenuated in macrophages lacking toll-like receptor (TLR)-4, suggesting a critical role for TLR4 in signaling HMGB1-mediated macrophage dysfunction. These studies demonstrate that the elevated levels of HMGB1 in CF airways are critical for neutrophil recruitment and persistent presence of P. aeruginosa in the lung. Thus, HMGB1 may provide a therapeutic target for reducing bacterial infection and lung inflammation in CF.
INTRODUCTION
Cystic fibrosis (CF) is the most common fatal autosomal-recessive disease among Caucasian populations, with a frequency of 1 in 2,000–3,000 live births (1). This lethal disease is caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel, resulting in dysfunctional salt and water transport across epithelia (2). Despite the identification of the gene for this disease and advances in its management, life expectancy of CF patients remains in the late 30s (3). Although CF is a multisystem disorder, pulmonary disease remains the leading cause of morbidity and mortality (4). Characteristics of CF lung pathology are neutrophilic inflammation and chronic bacterial airway infection, notably with Pseudomonas aeruginosa (5–7). Despite intensive antibiotic regimens and therapies targeting lung damage caused by thick mucus and infection, P. aeruginosa continues to be one of the most prevalent bacterial pathogens affecting the majority of patients with CF (8,9). This persistent infection can lead to sustained inflammation and chronic neutrophil recruitment. In parallel, much neutrophil DNA accumulate in the lung, further obstructing the airway (4).
The mechanisms that allow P. aeruginosa infection to progress and persist in CF lungs remain poorly understood. With increasing difficulties in achieving satisfactory efficacy for current antibiotic regimen, efforts have focused on investigating host defense mechanisms against bacterial infections. It has been hypothesized that the deficiency in CFTR can result in intrinsic alterations to phagocyte functions, which may lead to defective bacterial clearance (10,11). Alveolar macrophages, one of the major components of innate immunity in the lung, are principally responsible for phagocytosis and killing of invading pathogens before the infiltration of neutrophils (12). Although increased numbers of alveolar macrophages were found in airways of fetuses and children with CF (13,14), few studies have addressed the role of alveolar macrophages in P. aeruginosa infection in CF. Interestingly, alveolar macrophages isolated from lungs of CF patients exhibited no significant differences in either morphological characteristics or the capability to phagocytose P. aeruginosa compared with those isolated from normal subjects (15,16). Here we sought to identify factors in CF airways that can impair the ability of alveolar macrophages to phagocytose P. aeruginosa.
High-mobility group box 1 protein (HMGB1), a ubiquitous nuclear DNA binding protein, can be released into the extracellular milieu from both activated immune cells and dying somatic cells. Once released, HMGB1 functions as a potent proinflammatory cytokine and causes epithelial leakage (17–19). Direct instillation of HMGB1 into the lung induces pulmonary neutrophil recruitment, tissue injury and inflammation that can be inhibited by coadministration of anti-HMGB1 antibodies (18,20). Overwhelming neutrophilic inflammation in the lung plays a critical role in the pathogenesis of CF. Elevated levels of HMGB1 were observed in sputum of CF patients (4), although it was not clear whether the elevated levels of HMGB1 were mainly from the many dead leukocytes in CF sputum. On the basis of the critical role that HMGB1 plays in the recruitment and efferocytosis of neutrophils (21,22), it was proposed that HMGB1 is an ideal target for reducing neutrophilic inflammation in CF (23). However, the clearance of invading pathogens in the lung depends on pulmonary inflammatory responses. Therefore, it is theoretically possible that inhibition of HMGB1 might hinder P. aeruginosa clearance in CF.
In this study, we found that administration of specific neutralizing anti-HMGB1 mAbs in CF mice infected with P. aeruginosa not only inhibited neutrophilic inflammation, but also significantly increased the clearance of P. aeruginosa in the lung and markedly reduced lung injury. Our results demonstrate that HMGB1 significantly contributes to CF pathogenesis by provoking inflammation and suppressing bacterial clearance in the lung.
MATERIALS AND METHODS
Special Reagents
Neutralizing anti-HMGB1 monoclonal and polyclonal antibodies were generated as described previously (17,24). Recombinant HMGB1 with a purity of <0.1 pg/μg endotoxin was generated using rat HMGB1 cDNA, which was cloned onto a pCAL-n vector and expressed in Escherichia coli BL21 (DE3) pLysS cells (17,25,26). Contaminating endotoxin was removed from HMGB1 preparation by Triton X-114 extraction (27). The extent of endotoxin contamination was assessed using the chromogenic Limulus amebocyte lysate assay (Endo-chrome; Charles River). Green fluorescent protein (GFP)-expressing PAO1, a nonmucoid strain of P. aeruginosa, was cultured as described previously (28,29).
Preparation of Bronchoalveolar Lavage Fluids
All human bronchoalveolar lavage fluid (BAL) samples analyzed were obtained from subjects with written informed consent through protocols approved by the Institutional Review Board of University of Vermont, Fein-stein Institute for Medical Research and St. John’s University. For this study, all CF BAL samples were collected from patients in the midst of a respiratory exacerbation requiring intravenous antibiotics and hospital admission as part of routine clinical care at the time of an acute respiratory exacerbation. Samples were deidentified as per collection protocol. These BAL samples were centrifuged, and the resultant supernatants were stored at −80°C. Mouse BAL was obtained as described previously (30,31). Briefly, mice were anesthetized by in-traperitoneal injection of sodium pentobarbital (70–90 mg/kg). After a 1- to 2-cm incision was made on the neck, the trachea was exposed and a 20-gauge × 1-inch intravenous catheter was inserted caudally into the lumen of the exposed trachea. The lungs were gently lavaged twice with 1 mL sterile nonpyrogenic phosphate-buffered saline (PBS) solution (Mediatech, Hendon, VA, USA). The samples were then centrifuged and assessed for cell numbers and differentials. The resultant supernatants were stored at −80°C until assayed for the levels of HMGB1 and the total protein content.
Animal Studies
The experimental use of animals presented in this study was approved by the Institutional Animal Care and Use Committees of St. John’s University, University of Vermont and Feinstein Institute for Medical Research and conformed to National Institutes of Health standards. Adult male (8- to 12-wk-old) Toll-like receptor (TLR)2−/− and TLR4−/− mice were obtained from Helena Erlandsson-Harris (Department of Medicine, Karolinska Institute, Stockholm, Sweden) (32); CFTR−/− mice [Cftrtm1Unc-Tg(FABPCFTR)1Jaw/J] (33,34) and C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and housed in a specific pathogen-free environment. Mice were inoculated with 5 × 108 colony-forming units (CFUs) of P. aeruginosa PAO1 (35) or 2.5 × 108 CFUs of P. aeruginosa PAO1 and 2.5 × 108 opsonized fluorescein isothiocyanate (FITC)-labeled latex beads (Polysciences, Warrington, PA, USA) via oropharyngeal aspiration after brief anesthesia with 2% isoflourane (28,29). Mice were randomized to receive either neutralizing anti-HMGB1 mAb (24) or an isotype control mAb, administrated by intraperitoneal injection 12 h before, and at the time of, bacterial inoculation. At 4, 18 or 24 h after bacterial inoculation, mice were anesthetized with intraperitoneal sodium pentobarbital (70–90 mg/kg) to obtain either lung tissues or BAL, as described above, and cannulated. After the pulmonary vascular trees were perfused with PBS, the lungs were either excised and immediately placed into 1 mL cold PBS and homogenized or inflated under 25 cm water pressure with 10% formalin. The lungs were then removed and fixed in 10% formalin and the tissue process and embedded in paraffin. The lung blocks were then sectioned and stained with hematoxylin and eosin for histological analysis.
Macrophage Cultures
Murine macrophage-like RAW 264.7 cells (American Type Culture Collection), primary mouse alveolar macrophages obtained from lung lavage fluids and primary peritoneal macrophages obtained from peritoneal fluids were cultured in RPMI 1640 medium (Gibco BRL, Grand Island, NY, USA) at 37°C in 5% CO2 and supplemented with 10% fetal bovine serum (FBS) (Gemini Biological Products, Calabasas, CA, USA), penicillin and streptomycin (Life Technologies, Grand Island, NY, USA) as described previously (30). Peritoneal exudate macrophages were obtained by lavaging the peritoneal cavity with 5 mL sterile 11.6% sucrose of either TLR2−/− or TLR4−/− or wild-type (WT) C57BL/6 mice after injection of 2 mL sterile 4% thioglycolate broth intraperitoneally to elicit peritoneal macrophages as described (32). Primary macrophages in either lung lavage fluids or peritoneal exudates were plated in RPMI 1640 medium on FBS-treated chamber slides as described (30,32). After removal of nonadherent cells, peritoneal macrophages were treated with BAL from either CF patients or normal healthy controls for 24 h, and alveolar macrophages/monocytes were fixed in 2% paraformaldehyde and stained with Texas Red X-phalloidin in 1% bovine serum albumin.
HMGB1 Measurement
Levels of HMGB1 in BAL were determined using immunoblotting analysis with the anti-HMGB1 antibody as described previously (17). In brief, samples were separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), proteins were electro-transferred onto a nitrocellulose membrane and then blocked with 5% nonfat dry milk in Tris-buffered saline with 0.1% Tween-20. The membrane was then incubated with anti-HMGB1 antibodies and then anti- rabbit horseradish peroxidase–coupled secondary antibodies (Bio-Rad, Hercules, CA, USA). After washing, antibody binding was detected by enhanced chemiluminescence plus Western blotting detection reagents (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Western blots were scanned with a silver image scanner (Silverscaner II; Lacie, Beaverton, OR, USA), and the band intensity was quantified using ImageJ software (NIH, Bethesda, MD, USA).
Phagocytosis Assay
Phagocytic activity of macrophages was determined as described previously with minor modifications (30). After different exposures, both RAW cells and primary peritoneal macrophages grown on chamber slides were incubated with GFP-expressing P. aeruginosa PAO1 or opsonized FITC-labeled latex beads (Polysciences, Warrington, PA, USA) at 37°C for 60 min. Macrophages were then treated with 0.04% trypan blue and washed with chilled (4ºC) PBS to quench the extracellular adherent particles. To visualize the uptake of P. aeruginosa, both RAW cells and primary macrophages were fixed with 2% paraformaldehyde, washed with PBS and stained with Texas Red X-phalloidin (Molecular Probes, Eugene, OR, USA) in 1% bovine serum albumin. The slides were analyzed using an epifluorescence microscope (Nikon, Melville, NY, USA). The internalization of P. aeruginosa was confirmed by a Bio-Rad MRC 600 confocal scanning microscope as described (30). Uptake of P. aeruginosa by macrophages was quantified by counting 50 macrophages/slide and no less than six slides from three independent experiments.
Quantitative Bacteriology
Viable bacterial counts in lungs were determined using CFU assay by plating serial dilutions of the lung homogenates onto BD Difco™ Pseudomonas Isolation Agar (BD, Franklin Lakes, NJ, USA) and cultured at 37°C as described (30).
Statistical Analysis
Results are presented as mean ± standard error of the mean (SEM) from at least three independent experiments. The data were analyzed for statistical significance using the Student t test or analysis of variance (ANOVA) (Microsoft, Seattle, WA, USA). A P value of ≤0.05 was considered statistically significant.
RESULTS
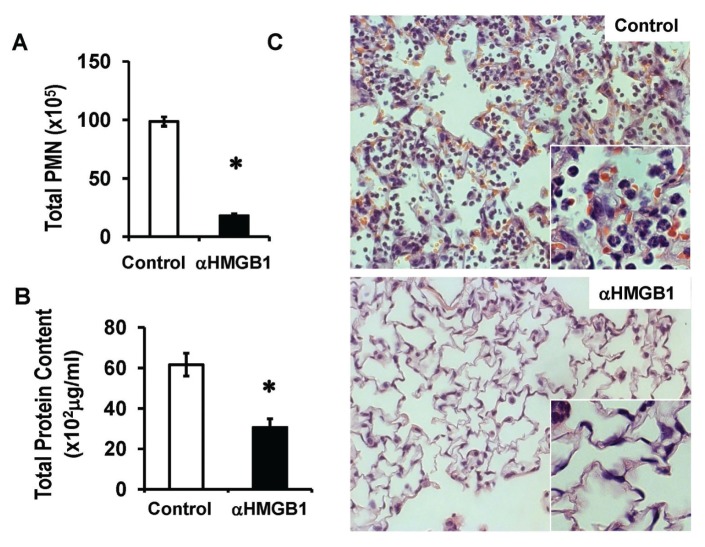
Anti-HMGB1 mAb reduces P. aeruginosa–induced pulmonary neutrophil recruitment and lung injury in CFTR−/− mice. Inoculation of C57BL/6 mice with P. aeruginosa via oropharyngeal aspiration leads to a severe pulmonary infection and substantial lung injury with marked neutrophil recruitment 24 h after the inoculation (28,29). To determine the effects of HMGB1 on infection-induced inflammatory lung injury and bacterial clearance in a CF model, we inoculated P. aeruginosa into CFTR−/− mice that are deficient in CFTR (36,37). Neutralizing anti-HMGB1 mAb was administered to CFTR−/− mice inoculated with PAO1, a nonmucoid strain of P. aeruginosa. Infection with nonmucoid strains of P. aeruginosa were observed in children with CF (38). Anti-HMGB1 mAb significantly reduced P. aeruginosa–induced lung neutrophil infiltration (98.7 ± 4 × 105 versus 18.1 ± 1.5 × 105 cells/mL; Figure 1A). The extent of lung injury, measured by total protein content in BALs and histological damage, was also significantly decreased in these mice (62 ± 5.6 × 102 versus 31 ± 4.2 × 102 μg/mL; Figures 1B, C). Most of the CFTR−/− mice that had received anti-HMGB1 mAb were observed to be active and healthy, whereas mice that had received isotype control mAb exhibited clinical signs of severe illness, including lethargy and huddling together in the corners of their cages. Together, these data indicate that anti-HMGB1 mAb confers significant protection against neutrophilic inflammation and lung injury in P. aeruginosa–infected CFTR−/− mice. Therefore, even in the presence of bacterial infection, inhibition of HMGB1 is still an effective approach to reduce neutrophilic inflammation and to mitigate the resulting lung injury in CF.
Figure 1.
Anti-HMGB1 mAb reduces P. aeruginosa–induced pulmonary neutrophil recruitment and lung injury in CFTR−/− mice. Male CFTR−/− mice were treated with 250 μg/mouse of either anti-HMGB1 mAb (αHMGB1) or isotype control antibody (control) 12 h before and at the time of inoculation with GFP-expressing P. aeruginosa PAO1. Eighteen hours later, mice were euthanized, and lungs and BALs were harvested. Neutrophilic inflammation was assessed by the PMN in the BAL (A). Total protein content in BAL (B) and histological analysis (C) were used to assess lung injury. Mean ± SEM, *P < 0.05 (n = 5–6 mice per group of three independent experiments). (C) Representative images of HE-staining lung sections.
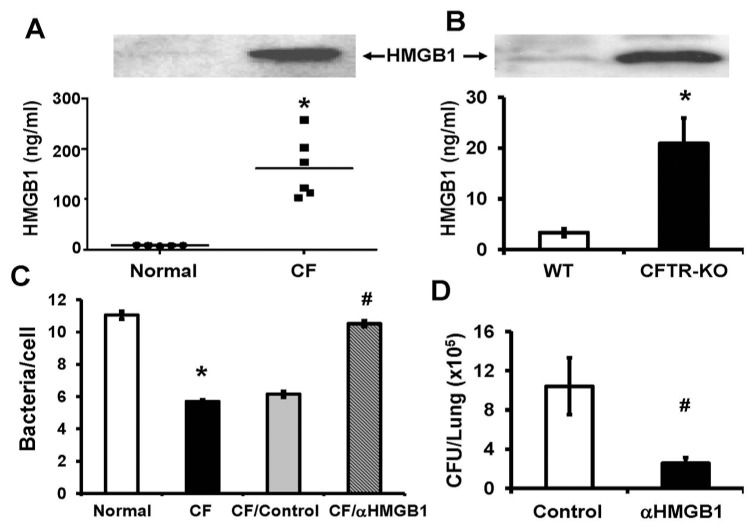
Elevated levels of HMGB1 in CF BAL inhibit bacterial clearance. To determine whether airway HMGB1 plays a critical role in the pathogenesis of CF lung disease, we measured the levels of HMGB1 in BAL obtained from patients with CF. HMGB1 levels were significantly higher in these samples compared with normal healthy volunteers (161.9 ± 24.8 versus 8.05 ± 0.39 ng/mL; Figure 2A). In addition, we found that the levels of airway HMGB1 in CFTR−/− mice (CFTR-KO) were significantly elevated, even in the absence of bacterial infection compared with that of sex- and age-matched WT mice (Figure 2B). This finding is consistent with previous observations in another murine model of CF (4). Together, these data indicate that extracellular HMGB1 is elevated in the airways of subjects with CF and high levels of HMGB1 can be accumulated in CF airways even in the absence of bacterial infection.
Figure 2.
Elevated levels of HMGB1 in BAL of CF patients inhibit bacterial clearance. The levels of HMGB1 in BAL of either CF patients or normal healthy volunteers (A) and of either CFTR−/− mice (CFTR-KO) or WT mice (B) were determined by immunoblotting analysis and quantified. Representative immunoblots are shown (A) and (B). (C) The phagocytic activity of RAW 264.7 macrophages was determined using heat-killed PAO1 in the presence of BAL from either CF patients (CF) or normal healthy volunteers (normal). Some CF BAL was pretreated with either anti-HMGB1 polyclonal antibodies (CF/αHMGB1) or control antibodies (CF/control). Macrophages were stained with phalloidin, and internalized PAO1 was counted and analyzed. (D). CFTR−/− mice were treated with either anti-HMGB1 mAbs (αHMGB1) or isotype control antibody as described in Figure 1. Viable bacteria in the lung were quantified by plating serial dilutions of homogenized lungs and expressed as CFUs/lung (n = 5–6 mice per group of three independent experiments). Mean ± SEM. * and #, P < 0.05 compared with either normal healthy volunteers (A and C) or WT mice (B) or subjects treated with isotype control antibodies (C and D), respectively.
Macrophages are the first line of defense in innate immunity and play critical roles in the clearance of invading pathogens (12). To determine whether HMGB1 in CF airways affects innate immunity in bacterial clearance, we examined whether BAL from CF patients can alter macrophage function of phagocytosis. Figure 2C shows that CF BAL significantly suppressed the ability of macrophage RAW 264.7 cells to phagocytose P. aeruginosa compared with BAL from healthy volunteers (CF versus normal; Figure 2C). Moreover, the addition of neutralizing anti-HMGB1 antibodies abolished the ability of CF BAL to sup-press macrophage phagocytic activity (CF/αHMGB1 versus CF/control; Figure 2C). These results indicate that HMGB1 in the airways of CF patients is necessary for the decreased phagocytosis of P. aeruginosa by macrophages. Intriguingly, bacterial counts in lungs of the CFTR−/− mice that had received anti-HMGB1 mAbs were significantly reduced even with the diminished neutrophil infiltration, as compared with mice treated with isotype control antibody (10.4 ± 2.91 × 105 versus 2.5 ± 0.57 × 105 CFUs/lung; Figure 2D). Thus, neutralizing anti-HMGB1 antibodies not only confer protection against inflammatory lung injury (Figure 1), but also enhance the host defense to clear bacteria from lungs of CFTR−/− mice in response to acute infection with P. aeruginosa.
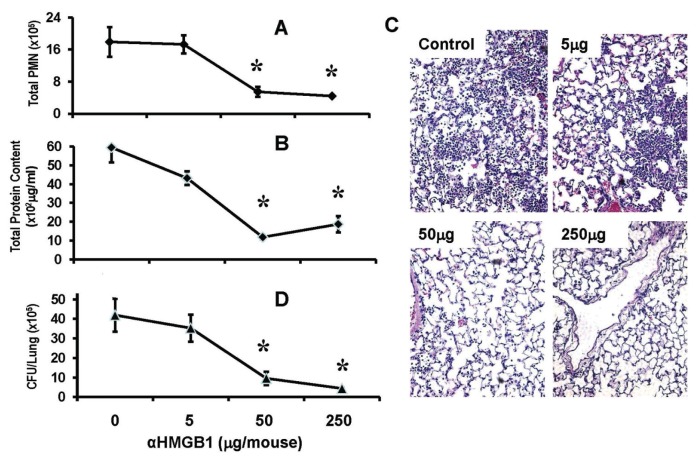
Anti-HMGB1 mAb confers significant protection against non-CF P. aeruginosa pneumonia. To address whether inhibition of HMGB1 also enhances bacterial clearance in non-CF bacterial pneumonia, WT C57BL/6 mice were administered with neutralizing anti-HMGB1 mAb before inoculation with P. aeruginosa PAO1. Similar to the observations made in the CF model (Figures 1, 2), treatment with anti-HMGB1 mAb significantly reduced P. aeruginosa–induced lung neutrophil recruitment (Figure 3A). In addition, anti-HMGB1 mAb significantly reduced lung injury (Figures 3B, C) and bacterial counts in the lung (Figure 3D). The effects of anti-HMGB1 mAb on neutrophilic inflammatory lung injury and bacterial clearance in these mice were dose dependent (Figure 3).
Figure 3.
Anti-HMGB1 mAb confers significant protection against P. aeruginosa pneumonia. C57BL/6 mice were treated with either anti-HMGB1 mAb (αHMGB1) or isotype control mAb (control) at doses of 5, 50 and 250 μg/mouse, 12 h before and at the time of inoculation with P. aeruginosa PAO1. Then, 24 h later, mice were euthanized and lungs and BAL harvested. (A) Neutrophil infiltration was assessed by the PMN in the BAL. Total protein content in BAL (B) and histological analysis (C) was assessed to determine the extent of lung injury. (D) Viable bacteria in the lung were quantified by plating serial dilutions of homogenized lungs and expressed as CFUs/lung. Data represent mean ± SEM. *P < 0.05 compared with the control group (n = 5–10 mice per group of three independent experiments).
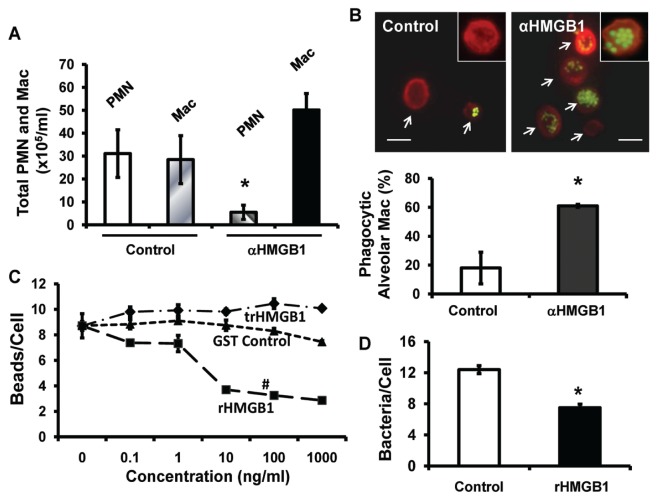
HMGB1 is sufficient to impair macrophage phagocytotic function. To investigate mechanisms underlying the enhanced ability to clear P. aeruginosa after inhibition of HMGB1, differential cell counts were evaluated in lung lavage fluids obtained from mice that had received anti-HMGB1 mAbs. Even though anti-HMGB1 mAbs significantly reduced neutrophil influx into the airways (poly-morphonuclear leukocytes [PMN]: αHMGB1 versus control; Figure 4A), the numbers of monocytes/macrophages in airways of mice treated with anti-HMGB1 mAbs were increased, but were not significantly compared with mice that had received isotype control mAbs (Mac: αHMGB1 versus control; Figure 4A). However, the majority of the monocytes/macrophages isolated from mice that had received anti-HMGB1 mAb exhibited active phagocytosis (61% ± 1%, αHMGB1; Figure 4B). In contrast, only 18% ± 11% of monocytes/macrophages from control mice were phagocytically active, and phagocytosed microorganisms were not observed in many of these monocytes/macrophages (control; Figure 4B). To determine whether HMGB1 is sufficient to suppress macrophage phagocytosis, RAW 264.7 cells were treated with recombinant HMGB1 (rHMGB1) (17). At concentrations ≥10 ng/mL, which are comparable to the levels of HMGB1 detected in CF airways (Figure 2), rHMGB1 significantly inhibited the ability of macrophages to phagocytose inert beads in a concentration-dependent manner (Figure 4C), as well as P. aeruginosa PAO1 (Figure 4D) compared with controls, including trypsinized rHMGB1 or GST peptide tag (GST control) (39). These data indicate that HMGB1 is sufficient in suppressing the efficiency of airway monocyte/macrophages in bacterial phagocytosis. This suppression can be attenuated by inhibiting airway HMGB1 with anti-HMGB1 antibodies.
Figure 4.
HMGB1 is sufficient to impair macrophage phagocytotic function. C57BL/6 mice (A, B) were treated with 50 μg/mouse of either anti-HMGB1 mAb (αHMGB1) or isotype control mAb (control), 12 h before the inoculation with GFP-expressing P. aeruginosa PAO1 and FITC-labeled latex beads. Four hours after inoculation, mice were sacrificed and BAL harvested. (A) PMN and monocytes/macrophages in BAL were quantified. (B) Alveolar macrophages/monocytes in BAL were plated on chamber slides and stained with Texas Red X-phalloidin. Phagocytic activity of macrophages was quantified by counting 50 cells/slide and no less than six slides from three independent experiments. Scale bar, 10 μm. (C, D) RAW 264.7 macrophages were treated for 24 h with either the indicated concentrations (C) or 10 ng/mL (D) of either recombinant HMGB1 (rHMGB1) or GST peptide tag control (control) or trypsinized rHMGB1 (trHMGB1) and then exposed for 1 h to either FITC-labeled latex beads (C) or heat-killed GFP-expressing PAO1 (D). Macrophages/monocytes in the fields were stained with phalloidin and internalized PAO1 or beads were counted and analyzed (B–D). Data represent mean ± SEM of at least three independent experiments. *P < 0.05 (t test) and #P < 0.001 (ANOVA), compared with the groups that were treated with either GST control or trypsinized rHMGB1. Mac, macrophages.
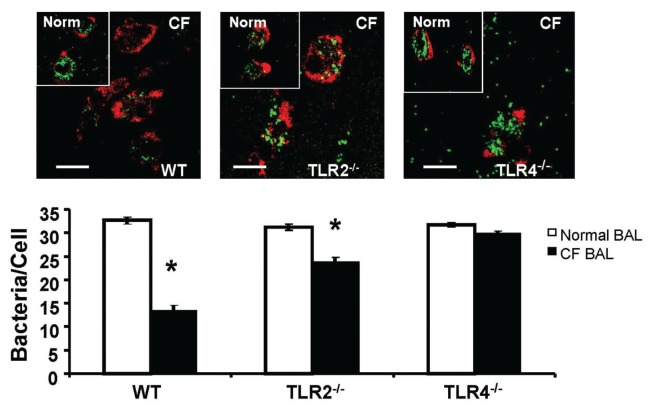
TLR4 mediates macrophage dysfunction in phagocytosis. Recent data indicate that TLR4 is the cognate receptor for HMGB1-mediated activation of macrophages (32). To investigate whether TLR4 on macrophages is required for signaling macrophage phagocytosis, peritoneal macrophages from either WT C57BL/6 mice or mice that were deficient in TLR2 or TLR4 were used. Similar to the observations made in cultured macrophages (Figure 2C), BAL of CF patients markedly suppressed the phagocytic activity of macrophages that were isolated from WT mice (Figure 5, WT). However, macrophages isolated from mice that were deficient in either TLR2 or TLR4 were less susceptible to the effects of CF BAL on macrophage phagocytic activity (Figure 5, TLR2−/− and TLR4−/−). Interestingly, no significant suppression in phagocytosing P. aeruginosa was observed in CF BAL-treated TLR4−/− macrophages (Figure 5, TLR4−/−). Although more susceptible than that of TLR4−/− mice, macrophages isolated from TLR2−/− mice were less susceptible to CF BAL than those from WT mice (Figure 5, TLR2−/−). These results suggest that functional TLR4 and TLR2 signaling pathways are critical for HMGB1-mediated suppression of P. aeruginosa phagocytosis in macrophages.
Figure 5.
TLR4 mediates macrophage dysfunction in phagocytosis. Peritoneal macrophages were harvested from either C57BL/6 WT or mice deficient in TLR2 (TLR2−/−) or TLR4 (TLR4−/−). Macrophages were allowed to grow for 48 h and then treated with either normal human BAL or CF patient BAL and then exposed to heat-killed PAO1 for 1 h. Actin cytoskeleton was visualized by staining macrophages with phalloidin, and internalized PAO1 were counted and analyzed. Insets are images of peritoneal macrophages treated with normal healthy volunteer BAL (Norm). Scale bar, 10 μm. Histogram graphs represent mean ± SEM (n = 4). *P < 0.05 compared to macrophages treated with BAL samples of control subjects.
DISCUSSION
Airway infection with P. aeruginosa and neutrophilic inflammation are characteristics of the lung pathology in CF (5–7). HMGB1 was implicated in mediating inflammatory lung disease in CF on the basis of its presence in sputum of CF patients (4). However, the impact of HMGB1 on infection-elicited inflammation, lung injury and the host defense against bacterial infection was not previously known. Using a mouse model of P. aeruginosa pneumonia, we found that not only did a neutralizing anti-HMGB1 mAb decrease P. aeruginosa–induced neutrophilic inflammation, it also reduced bacterial burden and injury in lungs of CF mice. Similar effects by anti-HMGB1 mAbs were observed in non-CF mice with bacterial pneumonia. Alveolar macrophages isolated from mice that received anti-HMGB1 mAbs had significantly higher phagocytic activity than macrophages isolated from mice receiving isotype control mAbs. In parallel with the findings in animal studies, levels of HMGB1 were significantly elevated in airways of CF patients. HMGB1 in human CF lung lavage fluids was necessary and sufficient in the impairment of macrophage ability to phagocytose P. aeruginosa. These findings reveal a novel role for HMGB1 in impairing bacterial clearance as well as neutrophilic inflammation in both CF and non-CF bacterial pneumonia.
Pulmonary inflammation is discordantly regulated in CF. For example, elevated levels of proinflammatory cytokines and pronounced neutrophil infiltration were detected in lung lavage fluids even in the absence of detectable bacteria (40–42). However, the mechanisms for this discordant inflammation are not fully elucidated, although several factors, including decreased levels of glutathione (43) and the antiinflammatory cytokine interleukin (IL)-10 (44,45) have been implicated. HMGB1 may play a prominent role in the enhanced inflammatory responses in CF lungs. Administration of HMGB1 markedly provoked an increase in both levels of proinflammatory cytokines, including IL-8, and pronounced neutrophil infiltration in mouse models (17,18,21). The significantly elevated levels of HMGB1 in lung lavage fluids of CF patients (Figure 2A) provide compelling evidence for the presence of HMGB1 in CF airways. Together with the detection of substantially elevated levels of HMGB1 in sputum of CF patients (4) and airways of CF mice, including CFTR−/−(Figure 2B) and Scnn1b-transgenic (Scnn1b-Tg) mice (4), these data strongly support the hypothesis that HMGB1 plays a critical role in CF pathogenesis. However, until now, potential mechanisms underlying the role of HMGB1 in CF lungs were not elucidated.
P. aeruginosa pneumonia is characterized by pronounced infiltration of neutrophils into the lung, especially in patients with CF (46). Although vigorous pulmonary inflammation contributes significantly to CF pathogenesis, there are few effective antiinflammatory strategies for CF patients with bacterial lung infection. In this study, we found that anti-HMGB1 mAbs can effectively diminish neutrophil recruitment into lungs of mice in the presence of P. aeruginosa infection (Figures 1, 4). Intriguingly, anti-HMGB1 mAb–attenuated pulmonary inflammation was associated with significantly reduced infection-elicited acute lung injury in both CF and non-CF mouse models of P. aeruginosa pneumonia (Figures 1, 3). These findings suggest that HMGB1 is critical in mediating inflammatory lung injury resulting from bacterial infection by augmenting recruitment of neutrophils to the lung. Therefore, inhibition of HMGB1 may provide a potential therapeutic approach to control pulmonary inflammation in CF patients. However, diminished neutrophil recruitment might impair bacterial clearance in P. aeruginosa pneumonia because infiltration of neutrophils into the lung is a critical response of the host defense to invading pathogens, including P. aeruginosa (46–48).
Besides neutrophils, other leukocytes, notably airway and alveolar macrophages, can play significant roles in bacterial clearance in normal lungs. Compromising macrophage functions in bacterial clearance may result in injury and disease. In patients with chronic obstructive pulmonary disease, impairment of the ability of alveolar macrophages in phagocytosis can comprise the host defense in clearing bacteria, including P. aeruginosa (49). In addition, hyperoxia-induced suppression of macrophage phagocytosis of P. aeruginosa causes increased susceptibility to bacterial infection, which can lead to increased mortality (30,50). Moreover, depletion of corneal macrophages in a mouse model of P. aeruginosa infection resulted in diminished bacterial clearance and keratitis (51). We found that airway and alveolar macrophages isolated from mice that were inoculated with P. aeruginosa had low phagocytic activity, which can be enhanced by inhibition of HMGB1 (Figure 4B). As shown in Figures 2 and 4, inhibition of HMGB1 in lung lavage fluids from either CF patients or CF mice significantly improved macrophage function and reduced bacterial loads in the lung. Serum from CF patients was found to have an inhibitory effect on alveolar macrophage ability to phagocytose P. aeruginosa, regardless of whether the macrophages were isolated from CF patients or normal human controls (15,16,52). Interestingly, Rowe et al. (4) demonstrated a significant elevation in the levels of HMGB1 in serum of CF patients. These studies support our conclusion on the role of HMGB1 in impairing macrophage phagocytotic function. Together, the significantly elevated levels of airway HMGB1 in CF subjects, even in the absence of bacterial infection (Figure 2 [4]), and the role of HMGB1 in macrophage dysfunction may contribute to the finding that the mice deficient in CFTR had dampened host defense to eliminate bacterial infection (53). Thus, the results presented in this study demonstrate a novel mechanism by which HMGB1 can impair innate immunity through inhibiting macrophage phagocytosis and interfere with the clearance of P. aeruginosa in CF airways.
At least three lines of evidence demonstrate that the levels of HMGB1 in airway fluids play a critical role in the impairment of macrophage phagocytotic function. Results shown in Figure 4C indicate that HMGB1 impairs macrophage function of phagocytosis in a dose-dependent manner. The phagocytic activity was reduced by >50% when macrophages were exposed to HMGB1 at concentrations of ≥100 ng/mL (Figure 4C). The levels of HMGB1 in BAL samples of CF patients were 162 ± 25 ng/mL, with a variation of <3-fold (Figure 2A) and thus sufficient to impair macrophage function. Indeed, results shown in Figure 2C confirmed this notion: BAL samples from CF patients significantly suppressed macrophage phagocytotic function, whereas neutralizing HMGB1 by specific anti-HMGB1 antibodies attenuated the effectiveness of this impairment (Figure 2C). Furthermore, the amount of anti-HMGB1 mAbs used in treating mice with P. aeruginosa infection was critical in modulating host defense to clear invading bacteria. Administration of 5 μg/mouse neutralizing anti-HMGB1 mAb did not significantly affect the host defense to clear bacterial infection compared with the isotype control mAb (Figure 3). In contrast, bacterial clearance, infection-elicited acute lung injury and macrophage phagocytic activity were significantly improved with higher amounts (50 and 250 μg/mouse) of anti-HMGB1 mAbs (Figures 3, 4). Together, these results indicate that the elevated levels of HMGB1 in CF airway fluids correlate with the impairment of macrophage phagocytotic function.
The role of HMGB1 in impairing macrophage phagocytotic function was observed only when it was present in the extracellular milieu. When little or no detectable HMGB1 was present in the extracellular milieu, macrophages exhibited normal phagocytic activity even in the presence of abundant HMGB1 in the nuclei (data not shown). On the other hand, addition of HMGB1 protein to the culture media compromised macrophage function in a dose-dependent manner (Figure 4C). HMGB1 is a ubiquitous nuclear protein and has been implicated in many vital cellular functions including gene expression, nucleosomal stabilization, mitochondrial respiration and ATP synthesis. HMGB1 knockout mice died of lethal hypoglycemia within 24 h after birth (54). However, the recent interest in targeting HMGB1 for treating inflammatory diseases is mainly attributed to its role as a potent inflammatory cytokine once released into the extracellular milieu (17). Results presented in this study reveal another pathogenic function of extracellular HMGB1 in impairing innate immunity to clear invading bacteria. Understanding these distinct intracellular and extracellular functions of HMGB1 is critical in designing effective therapeutic strategies to selectively inhibit the extracellular (pathogenic) function of HMGB1, while maintaining its intracellular (essential physiological) functions.
TLR4 has been shown to be important in mediating HMGB1-modulated proinflammatory responses (32,55–58). It is also involved in inflammatory responses to P. aeruginosa infection (59,60). Interestingly, we found that the effect of CF lung lavage fluids on impairing macrophage phagocytotic function was attenuated in the absence of functional TLR4 (Figure 5). These results suggest that TLR2 and TLR4 play critical roles in HMGB1- mediated macrophage dysfunction in phagocytosing bacteria. However, the effective clearance of invading bacteria and improvement of infection-induced lung injury involves processes more than just phagocytosis of bacteria by macro phages. While TLR4 is critical in HMGB1- mediated macrophage dysfunction, it may not be involved in all of the effects that HMGB1 has in bacterial clearance and lung injury. In addition, besides TLR4, other receptors might also mediate the effect of HMGB1 on macrophage functions. Indeed, results presented in Figure 5 indicate that HMGB1-compromised macrophage phagocytotic function was also diminished in the absence of functional TLR2, suggesting the involvement of TLR2. Comparably, although TLR4 is one of the most important receptors, other receptors, such as RAGE and TLR2, have been shown to be involved in mediating HMGB1-modulated proinflammatory responses (56). Moreover, HMGB1 can directly interact with other molecules, such as phosphatidylserine, resulting in downstream effects, such as compromised phagocytosis of apoptotic neutrophils by macrophages (22). Thus, while administration of neutralizing anti-HMGB1 mAbs improved lung injury and bacterial clearance in this study (Figures 1–3), it is not surprising that deficiency in TLR4 does not result in altered bacterial clearance in response to lung infection with P. aeruginosa as observed (59). This result is because HMGB1 can impair bacterial clearance and affect lung injury via molecules more than just TLR4, whereas TLR4 mediates bacterial clearance by interacting with molecules more than just HMGB1. Therefore, these results suggest that there is convergence as well as divergence between HMGB1-mediated and TLR4- mediated pathways in clearing invading P. aeruginosa in the lung.
CONCLUSION
In summary, we show that levels of HMGB1 are elevated in lung lavage fluids of CF patients and the HMGB1 in CF BAL is necessary and sufficient in impairing macrophage phagocytosis of P. aeruginosa. Neutralizing anti-HMGB1 antibodies can rescue CF BAL-induced macrophage dysfunction and reduce P. aeruginosa–induced neutrophilic inflammation, bacterial counts in the lung and alveolar injury in both CFTR−/− and WT animals. These findings reveal a novel role for HMGB1 in host defense by both mediating neutrophil infiltration and attenuating bacterial clearance in P. aeruginosa pneumonia. Therefore, inhibition of HMGB1 may provide a novel therapeutic approach to treat patients with CF and bacterial pneumonia.
ACKNOWLEDGMENTS
This work was supported by grants (to LL Mantell) from the National Heart, Lung, and Blood Institute (NHLBI) (HL093708), St. John’s University and The Feinstein Institute for Medical Research, North Shore-Long Island Jewish Health System. The authors would like to thank Jenna L Bement, Koichiro Taka-hashi, Ashwini Gore and Vivek Patel for insightful discussions and excellent assistance. M Entezari’s current affiliation is LaGuardia Community College, City University of New York, New York, NY, USA.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Ratjen F, Doring G. Cystic fibrosis. Lancet. 2003;361:681–9. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 2.Riordan JR, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–73. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 3.Whittaker LA. Preface. Clin Chest Med. 2007;28:xiii–xiv. [Google Scholar]
- 4.Rowe SM, et al. Potential role of high- mobility group box 1 in cystic fibrosis airway disease. Am J Respir Crit Care Med. 2008;178:822–31. doi: 10.1164/rccm.200712-1894OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Høiby N, Koch C. Cystic fibrosis. 1. Pseudomonas aeruginosa infection in cystic fibrosis and its management. Thorax. 1990;45:881–4. doi: 10.1136/thx.45.11.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson RL, Burns JL, Ramsey BW. Patho-physiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–51. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 7.Brennan AL, Geddes DM. Cystic fibrosis. Curr Opin Infect Dis. 2002;15:175–82. doi: 10.1097/00001432-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld M, et al. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr Pulmonol. 2001;32:356–66. doi: 10.1002/ppul.1144. [DOI] [PubMed] [Google Scholar]
- 9.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 10.Pier GB, et al. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science. 1996;271:64–7. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pier GB, Grout M, Zaidi TS. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc Natl Acad Sci U S A. 1997;94:12088–93. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg S, Grinstein S. Phagocytosis and innate immunity. Curr Opin Immunol. 2002;14:136–45. doi: 10.1016/s0952-7915(01)00309-0. [DOI] [PubMed] [Google Scholar]
- 13.Brennan S, et al. Alveolar macrophages and CC chemokines are increased in children with cystic fibrosis. Eur Respir J. 2009;34:655–61. doi: 10.1183/09031936.00178508. [DOI] [PubMed] [Google Scholar]
- 14.Hubeau C, Puchelle E, Gaillard D. Distinct pattern of immune cell population in the lung of human fetuses with cystic fibrosis. J Allergy Clin Immunol. 2001;108:524–9. doi: 10.1067/mai.2001.118516. [DOI] [PubMed] [Google Scholar]
- 15.Di A, et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol. 2006;8:933–44. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 16.Thomassen MJ, et al. Ultrastructure and function of alveolar macrophages from cystic fibrosis patients. Pediatr Res. 1980;14:715–21. doi: 10.1203/00006450-198005000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 18.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. Cutting edge: HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–4. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 19.Sappington PL, et al. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002;123:790–802. doi: 10.1053/gast.2002.35391. [DOI] [PubMed] [Google Scholar]
- 20.Lin X, et al. Alpha-chemokine receptor blockade reduces high mobility group box 1 protein-induced lung inflammation and injury and improves survival in sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;289:L583–90. doi: 10.1152/ajplung.00091.2005. [DOI] [PubMed] [Google Scholar]
- 21.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 22.Liu G, et al. High mobility group protein-1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine. J Immunol. 2008;181:4240–6. doi: 10.4049/jimmunol.181.6.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaggar A, Rowe SM, Hardison M, Blalock JE. Proline-glycine-proline (PGP) and high mobility group box protein-1 (HMGB1): potential mediators of cystic fibrosis airway inflammation. Open Respir Med J. 2010;4:32–8. doi: 10.2174/1874306401004010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin S, et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med. 2006;203:1637–42. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson U, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–70. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, et al. Recombinant HMGB1 with cytokine-stimulating activity. J Immunol Methods. 2004;289:211–23. doi: 10.1016/j.jim.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 27.Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods. 1990;132:191–5. doi: 10.1016/0022-1759(90)90029-u. [DOI] [PubMed] [Google Scholar]
- 28.Allard JB, et al. Aspergillus fumigatus generates an enhanced Th2-biased immune response in mice with defective cystic fibrosis transmembrane conductance regulator. J Immunol. 2006;177:5186–5194. doi: 10.4049/jimmunol.177.8.5186. [DOI] [PubMed] [Google Scholar]
- 29.Wargo MJ, Ho TC, Gross MJ, Whittaker LA, Hogan DA. GbdR regulates Pseudomonas aeruginosa plcH and pchP transcription in response to choline catabolites. Infect Immun. 2009;77:1103–11. doi: 10.1128/IAI.01008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrow DMP, et al. Antioxidants preserve macrophage phagocytosis of Pseudomonas aeruginosa during hyperoxia. 2007;42:1338–49. doi: 10.1016/j.freeradbiomed.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantell LL, et al. Unscheduled apoptosis during acute inflammatory lung injury. Cell Death Differ. 1997;4:600–7. doi: 10.1038/sj.cdd.4400278. [DOI] [PubMed] [Google Scholar]
- 32.Yang H, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010;107:11942–7. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou L, et al. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science. 1994;266:1705–8. doi: 10.1126/science.7527588. [DOI] [PubMed] [Google Scholar]
- 34.Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med. 2006;173:171–9. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stover CK, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–64. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 36.Snouwaert JN, et al. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–8. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 37.Clarke LL, et al. Defective epithelial chloride transport in a gene-targeted mouse model of cystic fibrosis. Science. 1992;257:1125–8. doi: 10.1126/science.257.5073.1125. [DOI] [PubMed] [Google Scholar]
- 38.Burns JL, et al. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J Infect Dis. 2001;183:444–52. doi: 10.1086/318075. [DOI] [PubMed] [Google Scholar]
- 39.Yang H, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan TZ, et al. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–82. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 41.Balough K, et al. The relationship between infection and inflammation in the early stages of lung disease from cystic fibrosis. Pediatr Pulmonol. 1995;20:63–70. doi: 10.1002/ppul.1950200203. [DOI] [PubMed] [Google Scholar]
- 42.Noah TL, Black HR, Cheng PW, Wood RE, Leigh MW. Nasal and bronchoalveolar lavage fluid cytokines in early cystic fibrosis. J Infect Dis. 1997;175:638–47. doi: 10.1093/infdis/175.3.638. [DOI] [PubMed] [Google Scholar]
- 43.Roum J, Buhl R, McElvaney N, Borok Z, Crystal R. Systemic deficiency of glutathione in cystic fibrosis. J Appl Physiol. 1993;75:2419–24. doi: 10.1152/jappl.1993.75.6.2419. [DOI] [PubMed] [Google Scholar]
- 44.Bonfield TL, Konstan MW, Berger M. Altered respiratory epithelial cell cytokine production in cystic fibrosis. J Allergy Clin Immunol. 1999;104:72–8. doi: 10.1016/s0091-6749(99)70116-8. [DOI] [PubMed] [Google Scholar]
- 45.Bonfield T, et al. Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am J Respir Cell Mol Biol. 1995;13:257–61. doi: 10.1165/ajrcmb.13.3.7544594. [DOI] [PubMed] [Google Scholar]
- 46.Davis PB, Drumm M, Konstan MW. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1229–56. doi: 10.1164/ajrccm.154.5.8912731. [DOI] [PubMed] [Google Scholar]
- 47.Knapp S, Schultz MJ, Poll T. Pneumonia models and innate immunity to respiratory bacterial pathogens. Shock. 2005;24(Suppl 1):12–8. doi: 10.1097/01.shk.0000191385.41689.f3. [DOI] [PubMed] [Google Scholar]
- 48.Zhang P, Summer WR, Bagby GJ, Nelson S. Innate immunity and pulmonary host defense. Immunol Rev. 2000;173:39–51. doi: 10.1034/j.1600-065x.2000.917306.x. [DOI] [PubMed] [Google Scholar]
- 49.Harvey CJ, et al. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci. Transl. Med. 2011;3:78ra32. doi: 10.1126/scitranslmed.3002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reddy NM, et al. Innate immunity against bacterial infection following hyperoxia exposure is impaired in NRF2-deficient mice. J Immunol. 2009;183:4601–8. doi: 10.4049/jimmunol.0901754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun Y, et al. TLR4 and TLR5 on corneal macrophages regulate Pseudomonas aeruginosa keratitis by signaling through MyD88-dependent and -independent pathways. J Immunol. 2010;185:4272–83. doi: 10.4049/jimmunol.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomassen MJ, et al. Inhibitory effect of cystic fibrosis serum on pseudomonas phagocytosis by rabbit and human alveolar macrophages. Pediatr Res. 1979;13:1085–8. doi: 10.1203/00006450-197909000-00030. [DOI] [PubMed] [Google Scholar]
- 53.Stoltz DA, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci. Transl. Med. 2010;2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calogero S, et al. The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat Genet. 1999;22:276–80. doi: 10.1038/10338. [DOI] [PubMed] [Google Scholar]
- 55.van Zoelen MAD, et al. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock. 2009;31:280–4. doi: 10.1097/SHK.0b013e318186262d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu M, et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–9. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 57.Park JS, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 58.Park JS, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–24. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 59.Skerrett SJ, Wilson CB, Liggitt HD, Hajjar AM. Redundant Toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol. 2007;292:L312–22. doi: 10.1152/ajplung.00250.2006. [DOI] [PubMed] [Google Scholar]
- 60.John G, Yildirim AO, Rubin BK, Gruenert DC, Henke MO. TLR-4-mediated innate immunity is reduced in cystic fibrosis airway cells. Am J Respir Cell Mol Biol. 2010;42:424–31. doi: 10.1165/rcmb.2008-0408OC. [DOI] [PMC free article] [PubMed] [Google Scholar]