Abstract
The aim was to expand recently published information regarding the significance of the interleukin (IL)-8/p-STAT-3 (signal transducer and activator of transcription) pathway in astrocytomas, focusing on the IL-8 receptor, chemokine (C-X-C motif) receptor 2 (CXCR2), and the STAT-3 inhibitor SOCS-3 (suppressors of cytokine signaling). A total of 91 paraffin-embedded human astrocytoma tissues (grades II–IV) were investigated for the association of SOCS-3 and CXCR2 expression with clinicopathologic and morphometric microvascular characteristics, vascular endothelial growth factor (VEGF), IL-8 and p-STAT-3 expression and patient survival. Peripheral IL-8 secretion levels were assessed by enzyme-linked immunosorbent spot (ELISPOT). SOCS-3, p-STAT-3 and CXCR2 protein levels were also quantified by Western immunoblotting in six cases, and the protein levels of SOCS-3 and CXCR2 were correlated with the immunohistochemical expression of the respective proteins. All CXCR2-positive cases by Western immunoblotting displayed increased peripheral IL-8 secretion levels. Treatment of primary glioblastoma cell cultures with exogenous IL-8 enhanced proliferation, and this effect was inhibited by treatment with a neutralizing anti-CXCR2 antibody. SOCS-3 and CXCR2 were expressed by neoplastic astrocytes in 92.4% and 48.78% of cases, respectively, with their levels increasing with histological grade and extent of necrosis. VEGF expression and microvessel density, CXCR2 and IL-8 levels were interrelated. SOCS-3 and p-STAT-3 were co-expressed in 85.7% of cases, although they were not interrelated. In univariate survival analysis, increased SOCS-3 expression and the presence of CXCR2 adversely affected survival, whereas in multivariate analysis, only CXCR2 remained significant. The prognostic significance of CXCR2 was validated in an independent set of 63 patients. Our data implicate IL-8/CXCR2 signaling pathway in the progression and regulation of angiogenesis in astrocytomas and provide a rationale for CXCR2 therapeutic exploitation in these tumors.
INTRODUCTION
Glioblastoma, the most common neoplasm among diffuse infiltrating astrocytomas, is notorious for its ability to evade immunosurveillance as well as for its invasive and angiogenic properties (1). Despite current multidisciplinary approaches, the overall prognosis remains dismal because of tumor refractoriness to therapy. Deciphering the well-orchestrated signaling pathways coordinating the complex biology of glioma cells is crucial to optimizing treatment strategies.
Direct and indirect evidence links the chemoattractant and proinflammatory CXC chemokine interleukin (IL)-8 to the invasive potential and rapid growth of glioblastoma cells. Most importantly, IL-8, like all ELR + CXC chemokines, is known to harbor potent angiogenic properties related to the Glu-Leu-Arg (ELR) motif immediately preceding its first N-terminal cysteine residue (2). The biologic effects of IL-8 are mediated by two highly related G protein–coupled receptors: chemokine (C-X-C motif) receptor 1 (CXCR1; IL-8 R1) and CXCR2 (IL-8 R2). CXCR2 is promiscuous in nature, since it can also bind other CXC chemokines (3). IL-8/CXCR1 autocrine signaling is presumably partly responsible for the glioblastoma-invasive phenotype, since downregulation of IL-8 or CXCR1 or silencing of the IL-8 gene by siRNA results in decreased invasive potential of glioblastoma cell lines (4). While CXCR1 and CXCR2 share many functions, they are also capable of activating distinct downstream pathways and can therefore exhibit different physiologic roles (5).
Signal transducer and activator of transcription (STAT)-3 is a transcription factor strategically positioned at the convergence point of several signaling pathways that becomes phosphorylated in response to cytokines, growth factors and extracellular signals (6). The conflicting data regarding the significance of aberrant phosphorylation of STAT-3 in malignant astrocytomas (rev. in 7) reflect the dual role of STAT-3, which harbors tumor-suppressive properties in the context of phosphatase and tensin homolog (PTEN) deficiency and oncogenic properties after expression of epidermal growth factor receptor variant III (EGFRvIII) (8).
The disastrous biologic consequences of uncontrolled cytokine signaling have generated a surge of interest into the identification of upstream regulators of the cytokine-driven STAT activation pathway (9). To date, the only known inducible inhibitors in this regard are the suppressors of cytokine signaling (SOCS) proteins, comprising SOCS1–SOCS7 and the cytokine-inducible SH2 domain-containing proteins. SOCS proteins can recognize cytokine receptors of the associated Janus-associated kinases (JAKs) phosphorylating STAT-3, thereby attenuating signal transduction. STAT-3 in particular induces SOCS-3, which feeds back to negatively regulate JAK/STAT signaling (10). Although SOCS proteins serve as robust tools to ensure tight cytokine signaling control, recent data argue in favor of their action as multi-functional molecular “switches,” facilitating or suppressing neoplastic transformation depending on cellular context (11).
Most recently, we have established the IL-8/STAT-3 signaling pathway as a novel regulatory mechanism promoting the acquisition of an aggressive and angiogenic phenotype in astroglial tumors (7). We herein pursue our investigation to two components interacting upstream and downstream with this pathway, namely IL-8 receptor CXCR2 and STAT-3 inhibitor SOCS-3, since existing information in this context is limited and controversial. We chose to focus on CXCR2 only because the role of CXCR1 in glioblastomas is well known (as opposed to that of CXCR2) (4) and because recent data in other tumors favor CXCR2 as a more likely mediator of IL-8 angiogenic properties (12,13). First, we analyzed the relationships of CXCR2 and SOCS-3 immunohistochemical expression with clinicopathologic data, IL-8 and p-STAT-3. Immunohistochemistry was validated by Western blot in a subset of cases. Second, we tested the implication of the IL-8/CXCR2 axis in the proliferation of primary glioblastoma cells. Third, we examined the relationships of these molecules with microvascular characteristics and vascular endothelial growth factor (VEGF), aiming to gain insight into their potential implication in the angiogenic process. Fourth, we tested the prognostic impact of SOCS-3 and CXCR2 by univariate and multivariate analysis and validated the results in an independent set of patients.
MATERIALS AND METHODS
Patient Description
This is a study of 91 adult patients with supratentorial diffuse infiltrating astrocytomas (grades II–IV) for whom archival primary tumor material at diagnosis, before radiotherapy, was available. A total of 28 patients and 23 healthy controls without evidence of any other type of cancer were also investigated for IL-8 secretion levels in peripheral blood. Patients had been diagnosed and treated in the First Department of Pathology, “Laiko” Hospital, University of Athens Medical School, and follow-up occurred in the “Evangelismos,” “Asklepeeion” and “Red Cross” Hospitals between 1999 and 2006. In all cases, the diagnoses and grading were peer-reviewed according to the principles laid down in the latest World Health Organization classification (14). Distinction between primary and secondary glioblastomas was on the basis of World Health Organization criteria (14). Informed consent was obtained from all patients, and the study was approved by the University of Athens Medical School Ethics Committee. The presence (62 cases) and extent of necrosis (focal, 14 cases; extensive, 48 cases) were assessed on the basis of imaging data and compared with that in tissue specimens. The demographic data of our patients are summarized in Table 1.
Table 1.
Demographic data of patient cohort and validation cohort.
| Patient cohort | Validation cohort | |
|---|---|---|
| Age [median (range)] | 59 (19–82) | 61 (9–84) |
| Sex | ||
| Male | 55 | 37 |
| Female | 36 | 26 |
| Location | ||
| Frontal | 18 | 20 |
| Temporal | 19 | 18 |
| Parietal | 23 | 13 |
| Occipital | 1 | 3 |
| >1 lobe | 30 | 9 |
| Grade | ||
| II | 18 | 11 |
| III | 8 | 7 |
| IV | 65 | 45 |
| Events | ||
| Death | 65 (follow-up: 6–104 months, median 17 months) | 41 (follow-up: 2–39 months, median 12 months) |
| Censored | 26 (follow-up: 8–48 months, median 13 months) | 22 (follow-up: 16–52 months, median 16.5 months) |
| Surgery | ||
| Partial | 55 | 24 |
| Complete | 36 | 39 |
| Radiotherapy | ||
| Yes | 72a | 57a |
| No | 19 | 6 |
| Chemotherapy | ||
| Yes | 23b | 43 |
| No | 35 | 6 |
| Information not available | 33 | 14 |
Data are n unless otherwise stated.
Postoperative radiotherapy (a total dose of 60 Gy in 30–33 fractions).
Gliadel in 4 cases, Temodal in 14 cases and Gliadel plus Temodal in 5 cases.
Peripheral blood was obtained before therapeutic administration of corticosteroids or any other treatment that consisted of surgery (partial resection [36 patients] or almost complete resection [55 patients]) and postoperative radiation—a total dose of 60 Gy in 30–33 fractions (72 patients).
Western Immunoblotting
Western blot assays were performed on six frozen astroglial tumor samples that were also analyzed immunohistochemically for IL-8, CXCR2 and SOCS-3. After homogenization and fractionation of fresh frozen tumor tissue, 100 μg protein was separated on a 10% polyacrylamide gel and blotted onto nitrocellulose membranes, probed with primary antibody overnight, followed by incubation with horseradish peroxidase–conjugated goat-anti-rabbit IgG secondary antibody (AP132P; Chemicon, Millipore, Temecula, CA, USA). Western immunoblotting was performed with the same antibodies used for immunohistochemistry as described below. Antibody dilutions were 1:2,000 for anti–p-STAT-3, 1:200 for anti–SOCS-3 and 1:200 for anti-CXCR2. Anti-actin antibody (MAB1501; Chemicon) was used as the loading control. Bands were visualized using ECL chemiluminescence detection reagents (Perkin Elmer, Athens, Greece).
Enzyme-Linked Immunosorbent Spot Assay
Peripheral blood mononuclear cells (PBMCs) isolated from fresh acid citrate dextrose peripheral blood samples from 28 patients by gradient density centrifugation were used for IL-8 enzyme-linked immunosorbent spot (ELISPOT) assay as previously described (15,16). Samples were also obtained from 23 healthy controls.
Primary Glioma Cell Cultures
Three fresh glioblastoma samples were obtained in collection medium (Dulbecco’s modified Eagle’s medium [DMEM]/Ham’s F12, penicillin and streptomycin, amphotericin B), and primary glioma cell cultures were established as previously described (15,16).
Proliferation Assay
To investigate the effect of IL-8/CXCR2 signaling on glioma cell proliferation, we performed a proliferation assay using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Briefly, primary glioma cells were seeded at a density of 5 × 103 cells/100 μL into 96-well flat-bottomed plates in triplicate and allowed to adhere overnight. These cultures were re-fed with fresh growth medium (DMEM/Ham’s F12, penicillin and streptomycin, amphotericin B, 10% fetal calf serum) with or without 10 ng/mL recombinant IL-8 (R&D Systems, Abingdon, U.K.). After 48 h incubation, the medium was replaced with MTT (Sigma-Aldrich, Athens, Greece) dissolved at a final concentration of 1 mg/mL in serum-free, phenol red (PR)-free medium, for a further 3 h at 37°C incubation. Then, the MTT-formazan product was solubilized thoroughly in isopropanol, and the absorbance was then measured with a test wavelength of 550 nm and a reference wavelength of 690 nm. To assess the effect of anti-CXCR2 antibody on the enhanced glioma proliferation, glioma cells were pretreated with 15 μg/mL anti-CXCR2 antibody (MAB 331; R&D Systems, Minneapolis, MN, USA) or an isotype-matched antibody (Sigma-Aldrich, Athens, Greece) for 1 h before stimulation with IL-8, and cell proliferation was determined as described above after 48 h incubation.
Immunohistochemical Staining
The 4-μm serial sections cut from formalin-fixed paraffin-embedded tumor tissue were immunostained using the two-step peroxidase technique with a peroxidase-conjugated polymer (DAKO Envision kit; DAKO, Carpentaria, CA). The following antibodies were used: anti–SOCS-3 (SC 9023, Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1:100 and anti-CXCR2 antibody (MAB 331) diluted 1:50. Both antibodies were incubated overnight at 4°C. CXCR2 staining data were available in 82 cases. Positive reaction was visualized using DAB as chromogen. Before primary antibody application, sections were treated with 10 mmol/L citrate buffer, pH 6.0, in a microwave oven at 750 W for 30 min. All cases had also been analyzed previously for IL-8, p-STAT-3 and VEGF expression (7).
SOCS-3 and CXCR2 evaluation was performed by light microscopy by two experienced pathologists (PK, GL) without knowledge of the clinical information. If a discrepancy occurred between the assessments of the two observers, the slides were reassessed in a combined session without information of the previous scores. In each case, the percentage of any neoplastic cells with cytoplasmic SOCS-3 and CXCR2 immunoreactivity was calculated in at least 500 tumor cells counted in several randomly chosen high-power fields. To ensure that positive cells were indeed neoplastic, parallel sections in cases in which there was any doubt about the nature of positive cells were also stained for the macrophage marker PGM1 and for leukocyte common antigen (LCA). Cholangiocarcinoma and normal tonsillar tissue were used as positive controls for SOCS-3 and CXCR2, respectively. Also, endothelial cells were used as internal positive controls for SOCS-3 to ensure optimal staining. Negative controls included sections where primary antibody had been substituted by nonimmune serum. Micro vessel counting was performed as detailed in our previous study (17) on CD34 immunostained slides (85 cases: 15 grade II, 8 grade III and 62 grade IV). Several morphometric parameters relating to microvessel cross-section (major and minor axis length, perimeter, area, Feret diameter) and shape (shape factor) as well as to the extent of microvascular network represented by microvessel density [MVD] and the total area occupied by microvessels (total vascular area) were estimated by using an image analysis software (Sigma Scan Pvo 5.0 Software; Jandel Scientific, Erkrath, Germany).
Statistical Analysis
Statistical analysis was performed by a biostatistician (G Levidou, MSc). In the basic statistical analysis, IL-8, SOCS-3, CXCR2, VEGF and p-STAT-3 expression and morphometric microvascular characteristics were treated as continuous variables to avoid any “data-driven” categorization. Proliferation assay results were tested with the t test. Associations of SOCS-3 and CXCR2 expression with clinicopathologic parameters and microvascular characteristics were tested using nonparametric tests with correction for multiple comparisons (Kruskal-Wallis analysis of variance [ANOVA], Mann-Whitney U test, Fisher exact test and Spearman rank correlation coefficient, as appropriate). The associations of IL-8, VEGF and p-STAT-3 expression with grade and necrosis in astrocytic tumors had been examined previously by our group (7) and have not been included in the results of the present investigation.
Survival analysis was performed using death by disease as an end point. The effect of various clinicopathologic parameters (age, sex, chemotherapy, radiotherapy, extent of surgical resection and histological grade) as well as SOCS-3, p-STAT-3, IL-8 and CXCR2 immunoreactivity on clinical outcome was assessed by plotting survival curves according to the Kaplan-Meier method and comparing groups using the log-rank test, as appropriate. Numerical variables were categorized on the basis of cutoff values provided by receiver operating characteristic curves. Multivariate analysis was performed using Cox proportional hazard estimation model to evaluate the predictive power of each parameter independently of others. To improve the stability and power of the adjusted model, Cox stepwise backward selection strategy was used. SOCS-3 and CXCR2 expression was categorized using the same cut points as in univariate analysis. The administration of chemotherapy was not included in multivariate analysis because of the significant number of cases with missing information. Also, VEGF and microvascular characteristics were not included in survival analysis because their prognostic significance has been the subject of previous investigations (17,18). Statistical calculations were performed using the Statistical package STATA 9.0 for Windows. All results with a two-sided P value ≤0.05 were considered statistically significant. The adequacy of our cohort size prognostic analysis was tested with the Statistical Package Pass 2005 for Windows according to the respective literature for power and sample size calculation for survival analysis (19).
Validation Cohort
An independent set of patients with astrocytomas was used to validate our results of univariate and multivariate survival analysis and test the validity of the chosen cutoff values for the expression of CXCR2. The validation group we used consisted of 63 patients, diagnosed and treated at “Evangelismos” Hospital after the selection of the patient cohort, between 2006 and 2010, and subject to a similar therapy regime. The demographic data are shown in Table 1. The follow-up period ranged from 2 to 52 months (median 12 months). The results of univariate survival analysis for CXCR2 expression in the population group were used to calculate the required number of patients in the validation group for an adequately powered analysis (90%) (20). A total of 48 patients would be needed to detect a difference of 0.3193 between 0.3662 and 0.0469 (the probability of surviving in CXCR2 expressor and CXCR2 non-expressor groups after 86 months, as calculated in the population group) using a two-sided log-rank test and to achieve 90% power at a 0.05 significance level.
RESULTS
Western Blot Analysis
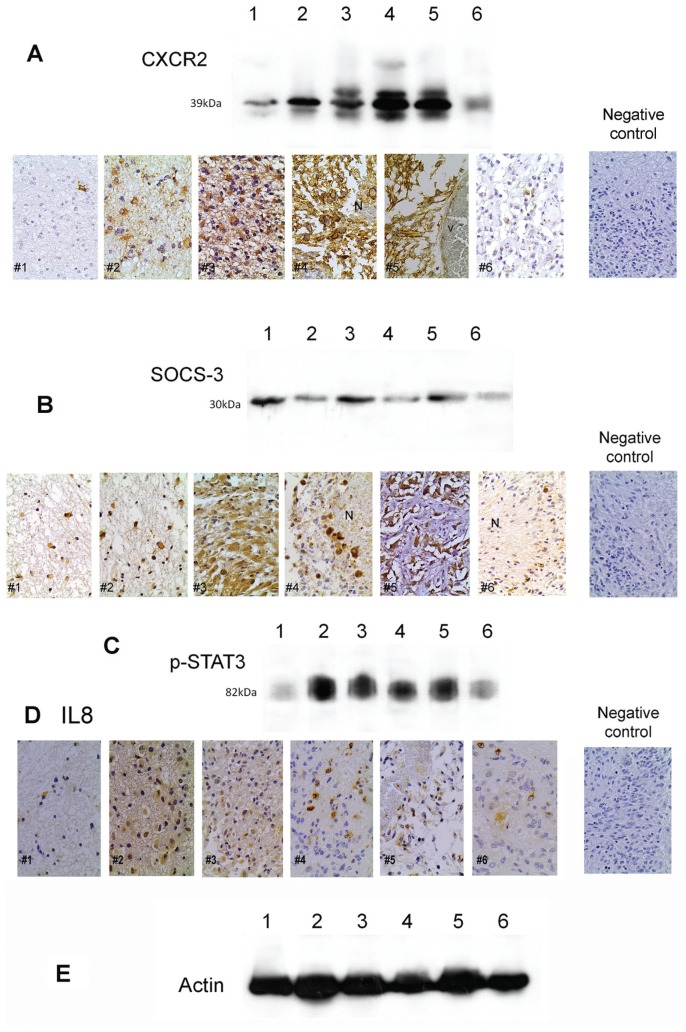
The CXCR2 and SOCS-3 expression levels by Western blot in the examined six cases were found to correlate with the immunohistochemical expression of the respective proteins in the same cases (Figures 1A, B).
Figure 1.
CXCR2, SOCS-3 and STAT-3 protein levels in fresh-frozen tissue specimens by Western blot and immunohistochemical expression of CXCR2, SOCS-3 and IL-8 in formalin-fixed tissue from the same six specimens. (A) Detection of CXCR2 (Western blot and immunohistochemistry) in the six cases. Tumors 4 and 5 showed the highest CXCR2 levels in frozen and paraffin-embedded samples. (B) Detection of SOCS-3 (Western blot and immunohistochemistry) in the six cases. Tumors 1, 3 and 5 showed the highest SOCS-3 levels in frozen and paraffin-embedded samples. (C) Detection of p-STAT-3 by Western blot in the six cases. (D) Immunohistochemical detection of IL-8 in the six cases. (E) Actin used as an internal control for Western blot analysis in the six cases. Negative controls indicate cases in which CXCR2, SOCS-3 and IL-8 have been substituted by nonimmune serum. N, area at necrosis; V, blood vessel.
IL-8 Secretion from PBMCs in Patients and Controls
The number of IL-8–secreting cells was markedly increased in patients with astrocytomas (median value 147) compared with healthy controls (median value 93; Wilcoxon matched pairs signed-rank test, P < 0.0001) as previously described (15). Within the patient group, all CXCR2-positive cases as determined by Western blot also exhibited increased peripheral IL-8 secretion levels by ELISPOT and IL-8–positive neoplastic cells by immunohistochemistry (Figure 1D). However, the levels of circulating IL-8 were not measured.
Effect of IL-8/CXCR2 Signaling on Glioblastoma Cell Proliferation
Treatment of primary glioblastoma cells with recombinant IL-8 for 48 h followed by MTT assay significantly enhanced their proliferation. Moreover, treatment with anti-CXCR2 antibody inhibited glioma proliferation enhanced by recombinant IL-8 (Figure 2). An isotype-matched control antibody had no effect on cell proliferation.
Figure 2.
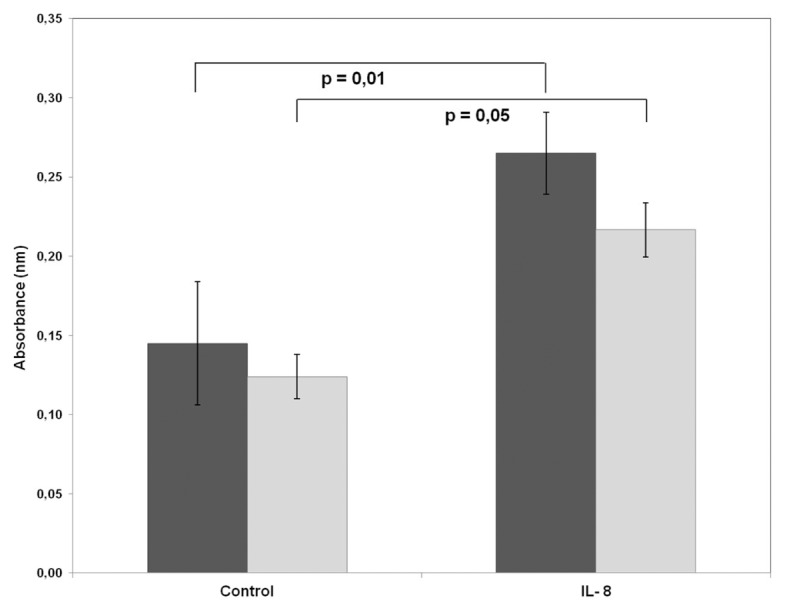
Effect of IL-8/CXCR2 signaling on glioma cell proliferation. Primary glioblastoma cells were seeded in 96-well plates and cultured for 48 h with or without IL-8 (Control). Furthermore, to examine the effects of anti-CXCR2 antibody (Ab) on IL-8–mediated glioma proliferation, cells were pretreated with anti-CXCR2 Ab for 1 h before stimulation with IL-8. After 48 h incubation, glioblastoma proliferation was assessed by MTT assay (P values refer to the t test). ■, Without CXCR2 Ab; □, with CXCR2 Ab.
Immunohistochemical Assessment of CXCR2 Expression in Astrocytomas
CXCR2 expression was recorded in 34 of 82 (48.78%) cases. Positive cells represented 0.5% to 70% (median value 3%) of the neoplastic population (Table 2). Cases with a small number of positive cells were also stained for glial fibrillary acidic protein (GFAP) to ensure that positive cells were astrocytes. Immunoreactivity was observed in the cytoplasm of neoplastic cells (Figure 1A) and was mainly localized in perivascular regions, especially in glioblastomas (Figure 1A, #5), as well as in endothelial and inflammatory cells. Scattered positive neoplastic cells were also encountered throughout the tumor parenchyma and around necrosis (Figure 1A, #4).
Table 2.
Distribution of SOCS-3 and CXCR2 expression per histological grade in patients with astrocytomas.
| Grade II | Grade III | Grade IV | Total | |
|---|---|---|---|---|
| SOCS-3 (%) | 3.5 (0–70) | 1.75 (0–70) | 30 (0–95) | 20 (0–95) |
| CXCR2 (%) | 0 (0–5) | 0.25 (0–5) | 0.5 (0–70) | 0 (0–70) |
Data are medians (range).
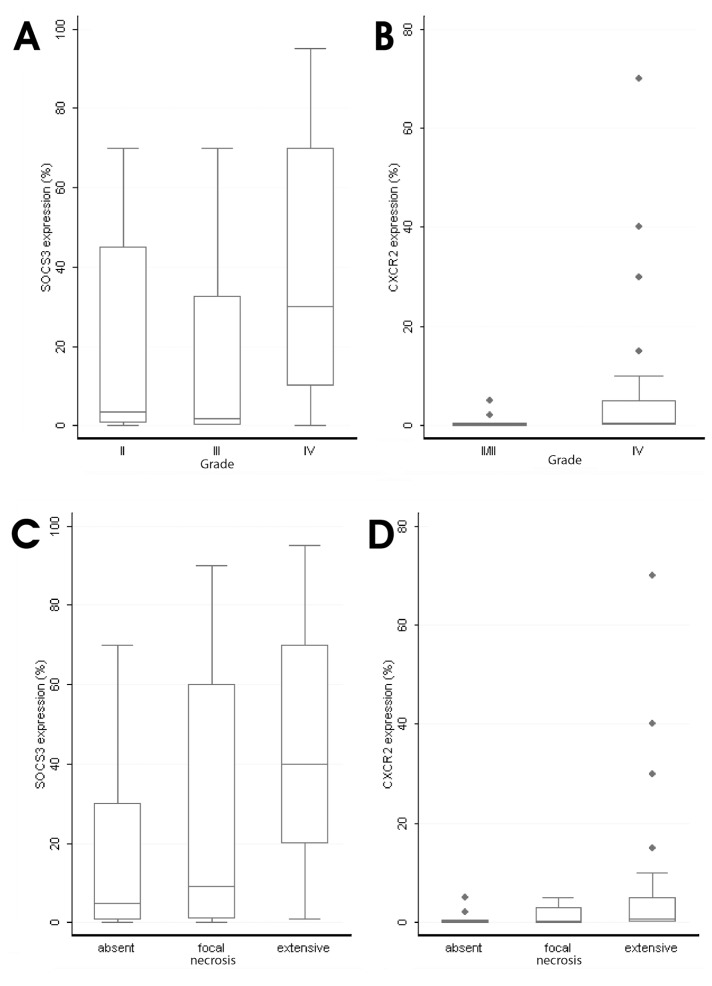
CXCR2 expression increased with tumor histological grade (Mann Whitney U test, II/III versus IV, P = 0.0139, Figure 3B) and with the extent of necrosis (Kruskal-Wallis P = 0.020, Figure 3D). All but three cases with CXCR2 expression by neoplastic astrocytes also showed IL-8–positive neoplastic cells. Moreover, CXCR2 expression was positively correlated with IL-8 levels (Spearman’s rank correlation coefficient, P < 0.0001, R = 0.4708).
Figure 3.
Box-plots representing the association of SOCS-3 (A, C) and CXCR2 (B, D) expression levels with grade (A, B) and extent of necrosis (C, D).
Immunohistochemical Assessment of SOCS-3 Expression in Astrocytomas
SOCS-3 expression was detected in 84 of 91 (92.4%) cases with the percentage of SOCS-3–positive neoplastic cells ranging from 0.5% to 95% (median 30% in positive cases, see Table 2). Immunoreactivity was localized in the cytoplasm and the cell membrane of neoplastic cells (Figure 1B) with a propensity for gemistocytes (Figure 1B, #3) and pseudopalisating cells around necrosis in glioblastoma (Figure 1B, #4 and #6). Endothelial cells also expressed SOCS-3. Scattered positive cells were also encountered throughout the tumor parenchyma away from necrotic areas. No staining was seen in normal brain tissue adjacent to the tumor.
SOCS-3 expression levels were higher in glioblastomas than in diffuse and anaplastic astrocytomas (Kruskal-Wallis ANOVA, II versus III versus IV, P < 0.0001, Figure 3A) and were positively associated with the extent of necrosis (Kruskal-Wallis P = 0.0003, Figure 3C) and IL-8 immunoreactivity levels (R = 0.4802, P < 0.0001). SOCS-3 and p-STAT-3 were coexpressed in 78/91 (85.7%) cases but were not interrelated (P = 0.0816).
Relationship of SOCS-3 and CXCR2 Expression with VEGF and Microvascular Characteristics
VEGF staining data were available in 91 cases and morphometric microvascular characteristics in 85 cases. Both SOCS-3 and CXCR2 immunoreactivity levels increased with increasing VEGF expression (R = 0.4366, P < 0.0001, and R = 0.3478, P = 0.0014, respectively) and MVD (R = 0.3200, P = 0.0028, and R = 0.3549, P = 0.0017, respectively).
Survival Analysis
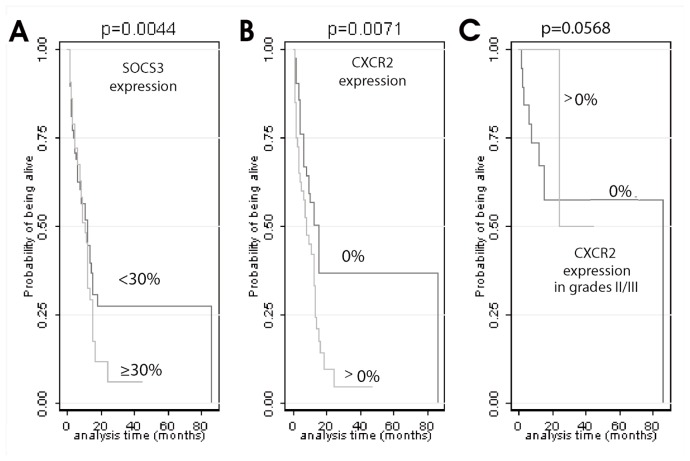
Univariate survival analysis was carried out in the entire cohort as well as in grades II/III and grade IV, separately (Table 3). Increased (≥5%) IL-8 expression (P = 0.0005), increased SOCS-3 expression (≥30%, P = 0.0044, Figure 4A) and the presence of CXCR2 expression (P = 0.0071, Figure 4B) adversely affected survival in the entire cohort (see Table 3). The median survival time for the SOCS-3 high expressor group was 8 months compared with 14 months for the SOCS-3 low expressor group. The corresponding value for those patients who expressed CXCR2 was 8 months compared with 15 months for patients who were negative for CXCR2.
Table 3.
Univariate analysis in the entire cohort as well as in grades II/III and grade IV separately.
| Parameter | All grades | Grades II/III | Grade IV |
|---|---|---|---|
| Histological grade (II versus III versus IV) | <0.0001 | 0.0327 | — |
| Age (>35 versus ≤35 years) | 0.0253 | 0.6588 | 0.1082 |
| Sex (1: male, 2: female) | 0.7155 | 0.5600 | 0.7978 |
| Surgical excision (0: partial, 1: almost complete) | 0.1191 | 0.0660 | 0.0741 |
| Radiotherapy (0: absence, 1: presence) | 0.3824 | 0.4535 | 0.0243 |
| Chemotherapy (0: absence, 1: presence) | 0.5346 | a | 0.1288 |
| SOCS-3 expression (30% versus ≥30%) | 0.0044 | 0.8808 | 0.0833 |
| CXCR2 expression (negative versus positive) | 0.0071 | 0.0568 | 0.3617 |
| IL-8 expression (<5% versus ≥5%) | 0.0005 | 0.2985 | 0.1470 |
| p-STAT-3 expression (<8% versus ≥8%) | 0.9407 | 0.5477 | 0.0430 |
Analysis was performed using the log-rank test (P values). The numbers in bold are P values ≤0.05 and are considered statistically significant.
Cannot be calculated.
Figure 4.
Kaplan-Meier curves for SOCS-3 (A) and CXCR2 (B, C) in the entire cohort (A, B) and grades II/III (C).
The presence of CXCR2 expression also adversely affected survival of patients with grade II/III astrocytomas (P = 0.0568, Figure 4C), although the relationship was of marginal significance. Decreased expression of p-STAT-3 (<8%, P = 0.0430) and SOCS-3 (P = 0.0833) adversely affected survival in grade IV astrocytomas, although the latter relationship was of marginal significance.
Multivariate survival analysis results including all parameters for the 82 patients, for whom SOCS-3, CXCR2, IL-8 and p-STAT-3 staining results were available, are presented in Table 4. The presence of CXCR2 expression remained significant along with histological grade, patient age and treatment.
Table 4.
Cox proportional hazard estimation in 82 patients for whom staining results for CXCR2, SOCS-3, IL-8 and p-STAT-3 were available using a stepwise backward selection strategy.
| Parameter | HR | Pa | 95% Confidence interval of HR | |
|---|---|---|---|---|
| CXCR2 expression | 1.861 | 0.035 | 1.045 | 3.314 |
| Surgery (partial versus almost complete) | 0.529 | 0.019 | 0.310 | 0.901 |
| Radiotherapy (absence versus presence) | 0.402 | 0.028 | 0.178 | 0.904 |
| Histological grade | 6.379 | <0.001 | 2.566 | 15.856 |
Significant at P < 0.05.
Both log-rank test and multivariate Cox regression analysis for CXCR2 expression in our cohort had an adequate power (>0.80) at a significant level of 5%. For power calculation, we used the results of our analysis (the ratio of the survival functions in each arm of CXCR2 expression for the log-rank test as well as the log hazard ratio of CXCR2 expression adjusted for all the investigated parameters, the standard deviation of CXCR2 expression and the existing sample size for the multivariate Cox regression analysis). In the latter calculations, the sample size was adjusted, since a multiple regression of CXCR2 expression on the other covariates in the Cox regression was expected to have an R2 value of 0.3600 and an anticipated event rate of 70.73% (58 deaths of disease in the 82- patient cohort, for whom staining results for CXCR2 were available).
Survival Analysis: Validation Group
The overall survival was significantly lower in the CXCR2 expressor compared with the CXCR2 non-expressor group (log-rank test, P = 0.0036). Furthermore, the significant correlations of CXCR2 expression with survival from Cox modeling in the initial cohort were reproduced within the validation cohort (hazard ratio [HR] 1.009, P = 0.042), thus corroborating the effect of CXCR2 upon survival established in the patient cohort.
DISCUSSION
Despite accumulating evidence highlighting IL-8 as a critical factor modulating cell proliferation, invasion and angiogenesis in astroglial tumors (9), the presence of IL-8 receptors in neoplastic astrocytes has been disputed (4). Initially, the perivascular distribution of CXCR1 and CXCR2 mRNA, detected by means of in situ hybridization, was assigned to the infiltrating leucocytes (21), leading to the hypothesis that IL-8 derived from neoplastic glial cells (and macrophages) exerts its biologic effects on blood vessels and inflammatory cells. RNase protection assay subsequently revealed both IL-8 receptor mRNA in 5 of 16 glioma cell lines tested, albeit at low levels (22). Most recently, flow cytometric analysis of CXCR protein expression indicated the presence of CXCR1, but not CXCR2, in all glioblastoma cell lines tested (4). We herein provide for the first time immunohistochemical evidence for the presence of CXCR2 on neoplastic astrocytes, validated by Western blot analysis. The distribution of positive neoplastic cells was mainly perivascular, conforming to that originally reported by Desbaillets et al. (21) and differing from the perinecrotic pattern of IL-8 mRNA and protein distribution observed by us and other investigators (21,23). Taken together, these findings advocate that IL-8 could act as an autocrine growth factor secreted by neoplastic astrocytes to promote their own growth.
The fact that occasional pseudopalisading cells expressed CXCR2 and that CXCR2 expression correlated with the extent of necrosis indicates that hypoxia is one of the mechanisms upregulating CXCR2. Indeed, experimental data support this notion indicating the presence of hypoxia-response elements in the CXCR2 promoter (24). CXCR2 expression was recorded in approximately half of our cases, usually at low levels, as reported by Zhou et al. (22), and was higher in glioblastomas compared with astrocytomas and anaplastic astrocytomas. No immunoreactivity in adjacent normal brain tissue was seen. IL-8 and CXCR2 protein levels were strongly interrelated, and all CXCR2-positive cases by Western blot exhibited increased peripheral IL-8 secretion. This result attests to the intimate functional link between this chemokine and its receptor. Peripheral IL-8 secretion was reported to correlate with its levels in astrocytic tumor tissues (25) and likely stimulates central IL-8 expression through the penetration of leukocytes via the blood-brain barrier, as hypothesized for IL-6 (19). Our proliferation assay showed enhanced proliferation of primary glioblastoma cells after stimulation with exogenous IL-8 that was, however, inhibited by preincubation of cells with anti-CXCR2 antibody. What emerges from the aforementioned findings is that IL-8/CXCR2 biologic axis underpins both the development and progression of diffuse astrocytic tumors. A similar autocrine signaling between IL-1 and CXCR2 was suggested to operate in early-stage colorectal cancer (26). Upregulation of CXCR2 mediates IL-8– induced invasive and migratory phenotype of melanoma cells (24) and is associated with a more aggressive phenotype in hepatocellular (27), gastric (28) and colorectal (29) carcinoma. The mechanisms by which CXCR2 facilitates transformation and migratory responses evoked during oncogenesis are, at least partially, due to manipulation of a comprehensive signaling network comprising phosphatidylinositol 3-kinase/protein kinase B (AKT), nuclear factor κB, Ras, mitogen-activated protein kinase (MAPK) and JAK/STAT-3 (28,30–33).
The indisputable role of IL-8/CXCR2 axis in astrocytic tumors goes far beyond the autocrine loop signaling and clearly involves the induction of angiogenesis (4). Our finding that CXCR2 correlated with VEGF levels and MVD fits well with the proposed model favoring CXCR2 as a more likely candidate than CXCR1 mediating the proangiogenic effects of IL-8 (12,13). In harmony with this argument, CXCR2 was shown to upregulate VEGF and downregulate thrompospondin-1 (an inhibitor of angiogenesis) in ovarian carcinomas (30), whereas neutralization of CXCR2 reduced microvascular endothelial cell migration (34) and MVD (25).
One of the most important findings emerging from the present investigation is the adverse prognostic significance of CXCR2 expression by neoplastic astrocytes established by both univariate and multivariate analysis and validated in an independent set of patients. When grades II/III and grade IV were examined separately, the prognostic significance of CXCR2 was established in the lower-grade group. Several lines of experimental evidence attest to the implication of CXCR2 in the biologic aggressiveness of various tumors. A significant inhibition of tumor growth and experimental lung metastasis formation was seen in CXCR2−/− mice compared with wild-type nude mice (33), whereas blockade or silencing of the CXCR2 gene attenuated human pancreatic cancer growth (34) and arrested ovarian carcinoma cells at G0/G1 and G2/M (30). Interestingly, CXCR2 has been shown to suppress the expression of proapoptotic factors while enhancing the expression of antiapoptotic proteins (30), thereby providing a mechanism by which astrocytoma cells escape apoptotic deletion caused by radiotherapy. It is worthy to note that CXCR2 retained its prognostic significance in the presence of IL-8, lending credence to the idea that targeting CXCR2, which binds all ELR + CXC chemokines, may be a more effective and biologically rational therapeutic strategy than targeting individual ELR + CXC chemokines or CXCR1 (12). The adverse prognostic significance of CXCR2 was recently documented in gastric (28) and high-grade serous ovarian cancer (30) patients.
A hallmark of glioblastomas is, among others, the presence of activated STAT-3 (6). Because SOCS-3 is a negative regulator of STAT-3 activation, it was assumed initially that SOCS-3 might function as a tumor suppressor and, hence, its expression might be repressed in glioblastoma tissues. Indeed, defective expression of SOCS-3 due to hypermethylation of its promoter, with consequent loss of feedback inhibition of STAT-3 activation, has been described in glioblastoma by Martini et al. (36). However, we observed increasing expression of SOCS-3 in astrocytic tumors culminating in glioblastomas, the vast majority of which were SOCS-3 positive, in agreement with two previous reports (6,11), raising the idea that SOCS-3 overexpression might endow glioblastoma cells with survival advantage. SOCS-3 was also shown to increase during development and progression of prostate cancer, being important for the survival machinery of neoplastic cells (37), whereas in breast cancer, higher expression of SOCS-3 was associated with earlier tumor stage (38). What emerges from such discrepant findings is that SOCS-3 may act as a tumor suppressor or a tumor protector, depending on cell type (39).
In our glioblastoma specimens, SOCS-3 exhibited a perinecrotic and perivascular distribution, also described by Zhou et al. (11). The significance of this finding is unclear, but it may indicate SOCS-3 regulation by intracellular oxygen concentration, also suggested by its association with the degree of necrosis in our series. A similar perinecrotic distribution of IL-8 was seen in the same cases, resulting from hypoxic upregulation by virtue of the presence of the oxygen-sensitive transcriptional factor AP-1 in the IL-8 promoter (2). This spatial relationship implicates hypoxia as the common denominator of IL-8, SOCS-3 and VEGF overexpression, accounting for the intriguing associations of SOCS-3 with IL-8 or VEGF emerging from our study.
Our failure to substantiate the expected negative correlation between SOCS-3 and p-STAT-3 levels is not without precedence, having also been observed in urothelial carcinomas (40). This result may suggest that other pathways inhibit STAT-3 activation in glioblastomas, namely PIAS (protein inhibitors of activated STAT-3) (6), or that neoplastic cells have developed strategies to overcome negative correlation by SOCS-3 (40). Furthermore, the parallel elevation of SOCS-3 and STAT-3 levels may be attributed to the fact that SOCS-3 is also a transcriptional target of STAT-3, according to in vitro experiments, where introduction of a phosphorylation-deficient dominant-negative (DN) STAT-3 mutant into the U87 cell line strongly repressed SOCS-3 mRNA and protein expression (11).
Univariate analysis indicated that enhanced SOCS-3 expression was predictive of worse patient survival. These findings seem to contradict those of Martini et al. (36) that downregulation of SOCS-3 is an indicator of worse patient survival because of the loss of feedback inhibition of STAT-3 activation contributing to tumor progression. On the contrary, Zhou et al. (11) demonstrated in vitro that SOCS-3 enhanced glioblastoma cell survival and upregulated ERK/MAPK signaling, converting the anti-apoptotic function of STAT-3 into proapoptotic (41) and promoting radio-resistance. This result explains why the adverse prognostic effect of SOCS-3 was marginally maintained in univariate analysis of our glioblastoma cases. Obviously, these conflicting reports underline the need for more comprehensive studies to elucidate the function of SOCS-3 in glioblastoma. It is worthy to note, though, that the prognostic significance of SOCS-3 established in univariate analysis did not hold true in multivariate analysis, proving inferior to histological grade, CXCR2 and type of treatment.
CONCLUSION
In summary, this is the first study to propose the IL-8/CXCR2 axis as a signaling pathway facilitating progression, contributing to proliferation and regulating angiogenesis in astroglial tumors. More importantly, our data bring forward CXCR2 expression by neoplastic astrocytes as a promising marker of prognostic value. SOCS-3, on the other hand, is overexpressed with p-STAT-3 in glioblastomas but appears less influential than CXCR2 as a prognostic marker. Validation of these findings in prospective clinical studies is warranted, before CXCR2 is exploited for therapeutic intervention in the management of these devastating tumors.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Maher EA, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–33. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 2.Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7:122–33. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253:1280–3. doi: 10.1126/science.1891716. [DOI] [PubMed] [Google Scholar]
- 4.Raychaudhuri B, Vogelbaum MA. IL-8 is a mediator of NFκB induced invasion by gliomas. J Neurooncol. 2011;101:227–35. doi: 10.1007/s11060-010-0261-2. [DOI] [PubMed] [Google Scholar]
- 5.Richardson RM, Marjoram RJ, Barak LS, Snyderman R. Role of the cytoplasmic tails of CXCR1 and CXCR2 in mediating leukocyte migration, activation, and regulation. J Immunol. 2003;170:2904–11. doi: 10.4049/jimmunol.170.6.2904. [DOI] [PubMed] [Google Scholar]
- 6.Brantley EC, et al. Loss of protein inhibitors of activated STAT-3 expression in glioblastoma multiforme tumors: implications for STAT-3 activation and gene expression. Clin Cancer Res. 2008;14:4694–704. doi: 10.1158/1078-0432.CCR-08-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piperi C, et al. Prognostic significance of IL-8-STAT-3 pathway in astrocytomas: correlation with IL-6, VEGF and microvessel morphometry. Cytokine. 2011;55:387–95. doi: 10.1016/j.cyto.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 8.de la Iglesia N, et al. Deregulation of a STAT3-interleukin 8 signaling pathway promotes human glioblastoma cell proliferation and invasiveness. J Neurosci. 2008;28:5870–8. doi: 10.1523/JNEUROSCI.5385-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wormald S, Hilton DJ. Inhibitors of cytokine signal transduction. J Biol Chem. 2004;279:821–4. doi: 10.1074/jbc.R300030200. [DOI] [PubMed] [Google Scholar]
- 10.Baker BJ, Akhtar LN, Benveniste EN. SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol. 2009;30:392–400. doi: 10.1016/j.it.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou H, et al. Reciprocal regulation of SOCS 1 and SOCS3 enhances resistance to ionizing radiation in glioblastoma multiforme. Clin Cancer Res. 2007;13:2344–53. doi: 10.1158/1078-0432.CCR-06-2303. [DOI] [PubMed] [Google Scholar]
- 12.Matsuo Y, et al. CXC-chemokine/CXCR2 biological axis promotes angiogenesis in vitro and in vivo in pancreatic cancer. Int J Cancer. 2009;125:1027–37. doi: 10.1002/ijc.24383. [DOI] [PubMed] [Google Scholar]
- 13.Li A, et al. Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis. 2005;8:63–71. doi: 10.1007/s10456-005-5208-4. [DOI] [PubMed] [Google Scholar]
- 14.Louis DN, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samaras V, et al. Application of the ELISPOT method for comparative analysis of interleukin (IL)-6 and IL-10 secretion in peripheral blood of patients with astroglial tumours. Mol Cell Biochem. 2007;304:343–51. doi: 10.1007/s11010-007-9517-3. [DOI] [PubMed] [Google Scholar]
- 16.Zisakis A, et al. Comparative analysis of peripheral and localised cytokine secretion in glioblastoma patients. Cytokine. 2007;39:99–105. doi: 10.1016/j.cyto.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Korkolopoulou P, et al. Prognostic implications of microvessel morphometry in diffuse astrocytic neoplasms. Neuropathol Appl Neurobiol. 2002;28:57–66. doi: 10.1046/j.1365-2990.2002.00367.x. [DOI] [PubMed] [Google Scholar]
- 18.Korkolopoulou P, et al. Expression of hypoxia-related tissue factors in astrocytic gliomas: a multivariate survival study with emphasis upon carbonic anhydrase IX. Hum Pathol. 2007;38:629–38. doi: 10.1016/j.humpath.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh FY, Lavori PW. Sample-size calculations for the Cox proportional hazards regression model with nonbinary covariates. Control Clin Trials. 2000;21:552–60. doi: 10.1016/s0197-2456(00)00104-5. [DOI] [PubMed] [Google Scholar]
- 20.Lachin JM, Foulkes MA. Evaluation of sample size and power analysis of survival with allowance for nonuniform patient entry, losses to follow-up noncompliance and stratification. Biometrics. 1986;42:507–19. [PubMed] [Google Scholar]
- 21.Desbaillets I, Diserens AC, Tribolet N, Hamou MF, Van Meir EG. Upregulation of interleukin 8 by oxygen-deprived cells in glioblastoma suggests a role in leukocyte activation, chemotaxis, and angiogenesis. J Exp Med. 1997;186:1201–12. doi: 10.1084/jem.186.8.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, Larsen PH, Hao C, Yong VW. CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J Biol Chem. 2002;277:49481–7. doi: 10.1074/jbc.M206222200. [DOI] [PubMed] [Google Scholar]
- 23.Van Meir E, et al. Interleukin-8 is produced in neoplastic and infectious diseases of the human central nervous system. Cancer Res. 1992;52:4297–305. [PubMed] [Google Scholar]
- 24.Gabellini C, et al. Functional activity of CXCL8 receptors, CXCR1 and CXCR2, on human malignant melanoma progression. Eur J Cancer. 2009;45:2618–27. doi: 10.1016/j.ejca.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Samaras V, et al. Analysis of interleukin (IL)-8 expression in human astrocytomas: associations with IL-6, cyclooxygenase-2, vascular endothelial growth factor, and microvessel morphometry. Human Immunol. 2009;70:391–7. doi: 10.1016/j.humimm.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Oladipo O, et al. The expression and prognostic impact of CXC-chemokines in stage II and III colorectal cancer epithelial and stromal tissue. Br J Cancer. 2011;104:480–7. doi: 10.1038/sj.bjc.6606055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Z, Yang L, Xu J, Zhang X, Wang B. Enhanced expression and clinical significance of chemokine receptor CXCR2 in hepatocellular carcinoma. J Surg Res. 2011;166:241–6. doi: 10.1016/j.jss.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Cheng WL, et al. Overexpression of CXCL1 and its receptor CXCR2 promote tumor invasion in gastric cancer. Ann Oncol. 2011;22:2267–76. doi: 10.1093/annonc/mdq739. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto M, et al. TSU68 prevents liver metastasis of colon cancer xenografts by modulating the premetastatic niche. Cancer Res. 2008;68:9754–62. doi: 10.1158/0008-5472.CAN-08-1748. [DOI] [PubMed] [Google Scholar]
- 30.Yang G, et al. CXCR2 promotes ovarian cancer growth through dysregulated cell cycle, diminished apoptosis, and enhanced angiogenesis. Clin Cancer Res. 2010;16:3875–86. doi: 10.1158/1078-0432.CCR-10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D, et al. MGSA/GRO-mediated melanocyte transformation involves induction of Ras expression. Oncogene. 2000;19:4647–59. doi: 10.1038/sj.onc.1203820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burger M, Hartmann T, Burger JA, Schraufstatter I. KSHV-GPCR and CXCR2 transforming capacity and angiogenic responses are mediated through a JAK2-STAT3-dependent pathway. Oncogene. 2005;24:2067–75. doi: 10.1038/sj.onc.1208442. [DOI] [PubMed] [Google Scholar]
- 33.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 34.Singh S, Varney M, Singh RK. Host CXCR2-dependent regulation of melanoma growth, angiogenesis, and experimental lung metastasis. Cancer Res. 2009;69:411–5. doi: 10.1158/0008-5472.CAN-08-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li A, et al. Overexpression of CXCL5 is associated with poor survival in patients with pancreatic cancer. Am J Pathol. 2011;178:1340–9. doi: 10.1016/j.ajpath.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martini M, et al. Prognostic relevance of SOCS3 hypermethylation in patients with glioblastoma multiforme. Int J Cancer. 2008;123:2955–60. doi: 10.1002/ijc.23805. [DOI] [PubMed] [Google Scholar]
- 37.Puhr M, et al. Down-regulation of suppressor of cytokine signaling-3 causes prostate cancer cell death through activation of the extrinsic and intrinsic apoptosis pathways. Cancer Res. 2009;69:7375–84. doi: 10.1158/0008-5472.CAN-09-0806. [DOI] [PubMed] [Google Scholar]
- 38.Sasi W, Jiang WG, Sharma A, Mokbel K. Higher expression levels of SOCS 1,3,4,7 are associated with earlier tumour stage and better clinical outcome in human breast cancer. BMC Cancer. 2010;10:178. doi: 10.1186/1471-2407-10-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brender C, et al. STAT3-mediated constitutive expression of SOCS-3 in cutaneous T-cell lymphoma. Blood. 2001;97:1056. doi: 10.1182/blood.v97.4.1056. [DOI] [PubMed] [Google Scholar]
- 40.Huang WT, et al. Expression of signal transducer and activator of transcription 3 and suppressor of cytokine signaling 3 in urothelial carcinoma. Kaohsiung J Med Sci. 2009;25:640–6. doi: 10.1016/S1607-551X(09)70569-8. [DOI] [PubMed] [Google Scholar]
- 41.Lu Y, et al. Loss of SOCS3 gene expression converts STAT3 function from anti-apoptotic to pro-apoptotic. J Biol Chem. 2006;281:36683–90. doi: 10.1074/jbc.M607374200. [DOI] [PubMed] [Google Scholar]