Abstract
This Perspective describes the discovery and development of silyl glyoxylates, a new family of conjunctive reagents for use in multicomponent coupling reactions. The selection of the nucleophilic and electrophilic components determines whether the silyl glyoxylate reagent will function as a synthetic equivalent to the dipolar glycolic acid synthon, the glyoxylate anion synthon, or the α-keto ester homoenolate synthon. The ability to select for any of these reaction modes has translated to excellent structural diversity in the derived three- and four-component coupling adducts. Preliminary findings on the development of catalytic reactions using these reagents are detailed, as are the design and discovery of new reactions directed toward particular functional group arrays embedded within bioactive natural products.
Introduction
α-Hydroxy acids occupy a central role in organic chemistry. Simple members of this family such as glycolic acid, mandelic acid, and lactic acid are widely used naturally occurring substances. The latter two are valuable chiral building blocks for asymmetric synthesis.1 Methods for synthesizing α-hydroxy acids are abundant, but tactics for accessing α,α-disubstituted glycolic acids are more limited,2 and those that introduce both alkyl substituents in the same step are more unusual still.3, 4 The notion that one might “mix and match” complementary reactive components to achieve diverse access to α,α-disubstituted glycolic (hydroxyacetic) acids and related substances was attractive from the standpoint of modularity and creation of molecular complexity. In this Perspective we provide insight into the conception, discovery, and development of α-oxo-α-silyl esters (silyl glyoxylates) as linchpin reagents that enable the synthesis of a range of substituted glycolic acids.
Conception
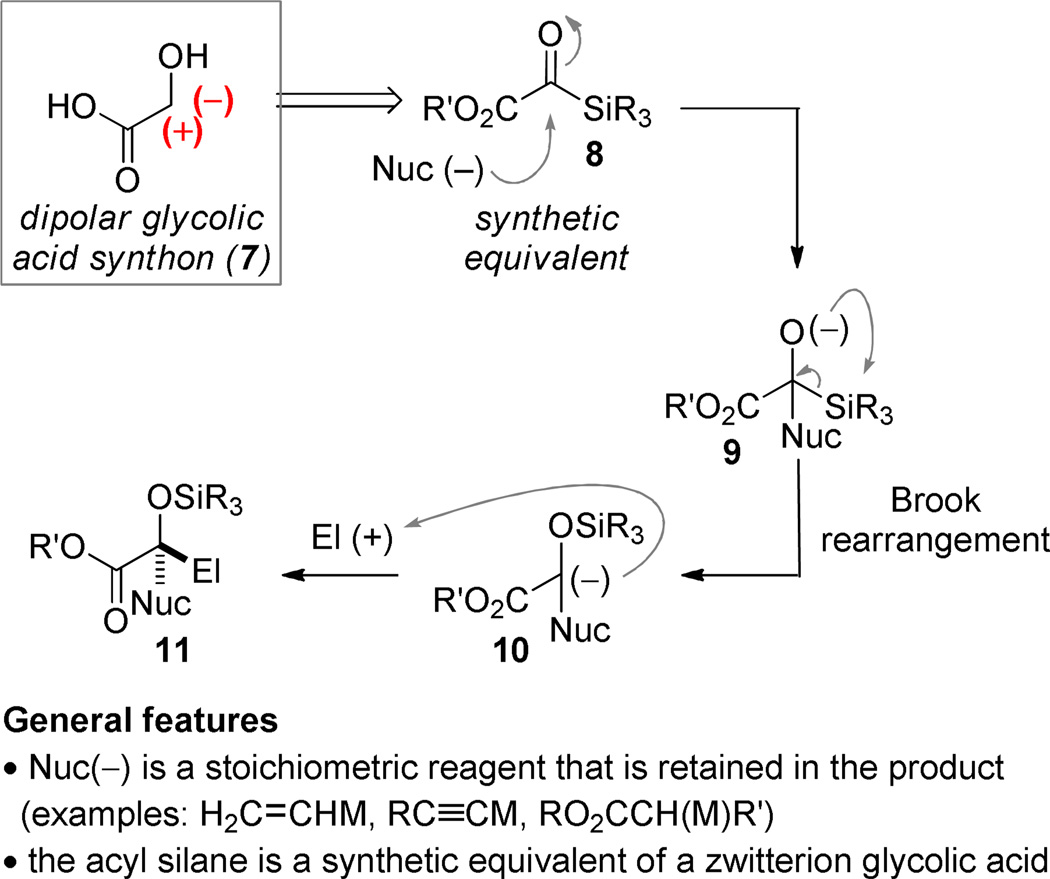
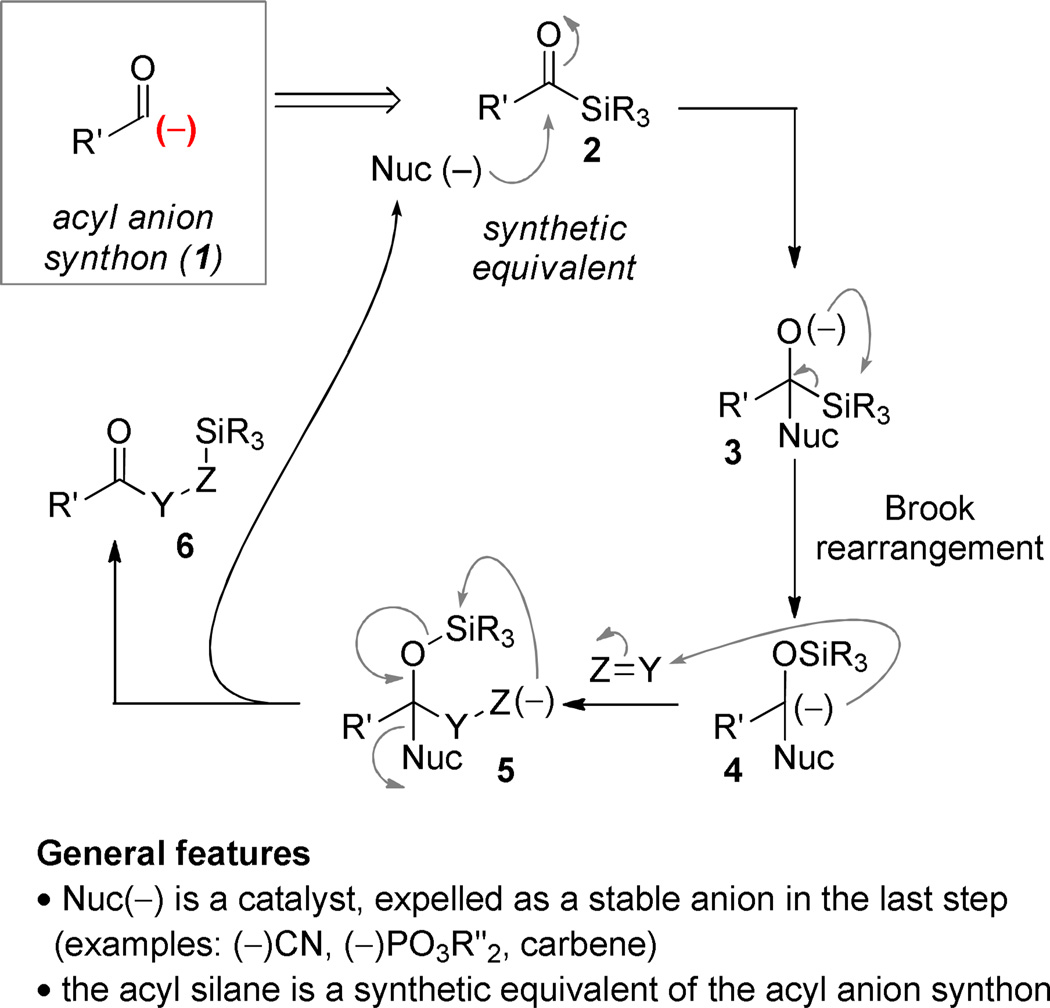
Acyl silanes (2) are reagents that are useful for umpolung (polarity reversal) applications by taking advantage of the [1,2]-anion transposition that is the defining feature of the Brook rearrangement.5–9 Nucleophilic addition generates a tetrahedral intermediate (3) that can give rise to C→O silyl migration driven by the strength of the O–Si bond.10 The facility of the migration is impacted by the identity of the acyl silane substituent and the nucleophile: since a carbanion is being generated, electron-withdrawing groups stabilize the intermediate. For applications in catalysis, the reaction of acyl silanes with metal cyanides,11–20 metallophosphites,12, 21–24, or carbenes25–27 provides a convenient method to generate intermediates of the general form 4 under mild conditions by stabilizing the carbanion generated. Nucleophilic addition to a π-electrophile, here generically represented as Y=Z, generates a new anionic intermediate 5. If O→Z silyl transfer induces breakdown of the tetrahedral intermediate by expulsion of the nucleophile, then the catalytic cycle is closed. The acyl silane has functioned as an acyl anion synthetic equivalent (1) and the nucleophile can be used substoichiometrically. It is an ongoing challenge of this subfield that the number of nucleophiles that can be used in such reactions is quite small because of the requirement that “Nuc” must be a strong anion stabilizing group.
The project that is the topic of this Perspective emerged from the hypothesis that the introduction of strong electron-withdrawing functionality on the acyl silane could broaden the scope of the nucleophilic component employed by easing the requirement that the nucleophile accept carbanion burden in the rearranged intermediate. It was important that the selected group deliver useful functionality; an ester was selected in our thought experiments (8). It moreover occurred to us that the implementation of this concept would necessarily entail a change in reaction manifold since more potent nucleophiles would be less likely to act as catalysts by being expelled as stable leaving groups at the end of the reaction (cf. 5→6). In the scenario outlined in Scheme 2, the acyl silane would function as template to conjoin complementary nucleophilic and electrophilic components at a glycolic acid junction. Rather than function as an acyl anion equivalent, the unusual dipolar glycolic acid synthon (7) would be achieved (Scheme 2).
Scheme 2.
Reagent Preparation
Silyl glyoxylates (8) were first prepared in the laboratory of Ando by dye-sensitized photooxidation of the corresponding α-silyl-α-diazo ester.28, 29 Ozonolysis of the latter was subsequently reported as another preparative method,30 although neither of these exceeded 40% yield for the diazo cleavage step and concurrent formation of the silyl ester by overoxidation was problematic. Bolm employed propylene oxide as the oxo transfer reagent in conjunction with a Rh(II) catalyst that presumably generates a transient Rh-carbenoid.31 This method is excellent for the preparation of a range of silyl glyoxylates. In our hands, the only time this approach breaks down is when the steric bulk is great in both the ester and the silyl groups. An alternative method that is applicable for a range of R/R′ combinations takes advantage of acetone/Oxone® to achieve the diazo→oxo conversion. The efficacy of this oxidant had been previously demonstrated in the synthesis of α-keto esters32, 33 and it proved to be the method of choice for preparing silyl glyoxylates on multigram scale, obviating the need for the Rh(II)-catalyst.34 The products are stable yellow liquids and can be stored in a freezer for several months.
Reactivity
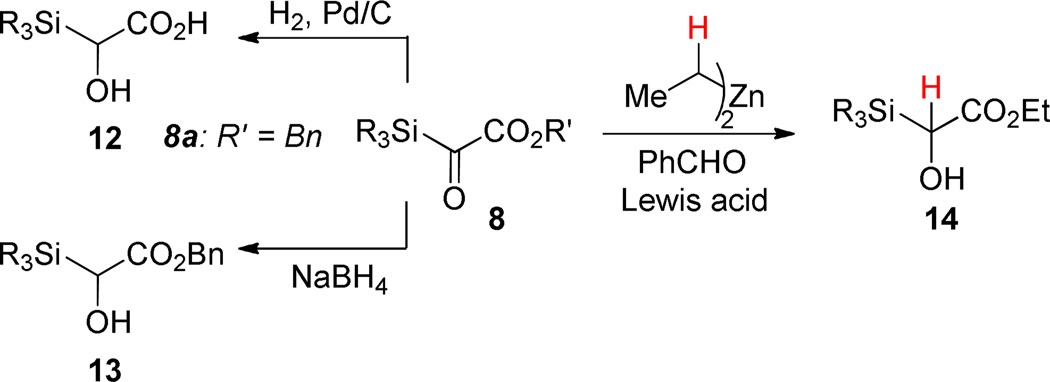
Bolm performed the first reactions with silyl glyoxylates.31 Exposure of a benzyl silyl glyoxylate 8a to H2 and Pd/C resulted in hydrogenation of the silyl ketone and hydrogenolysis of the benzyl ester to give α-silyl-α-hydroxyacid 12. Reduction of the silyl ketone alone was achieved with NaBH4. In neither of these transformations was silyl migration observed.
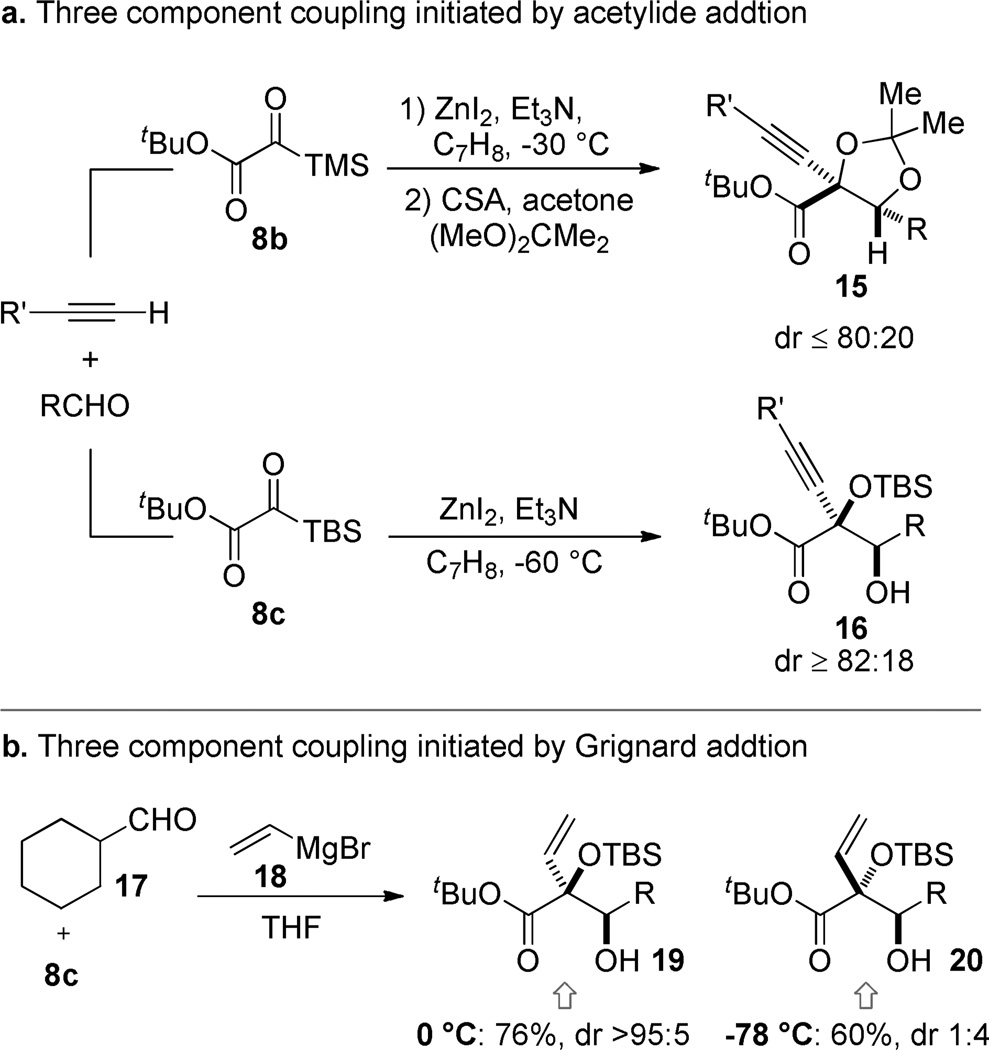
Reduction, whether desired or not, is a recurring theme in the chemistry of α-dicarbonyls.35, 36 Indeed, conversion to reduced compounds like 14 was the dominant observed pathway in the exploratory studies we conducted to assess the viability of the reaction pathway generalized by Scheme 2. In the presence of Lewis acids, organometals with β-hydrogens gave little or no alkylation, only reduction, and Brook rearrangement was seldom observed. To combat the undesired reduction pathway, we examined nucleophilic components that lacked β-hydrogens but could deliver useful functionality. Our first positive results came when we applied metal acetylides generated in situ from the terminal alkynes using Zn(II) and Et3N (Scheme 4).37 Using an aldehyde as the terminating electrophile, it was possible to obtain aldol products (15,16) in good yield. With a trimethylsilyl group a mixture of products was obtained where the silyl group was found on either the secondary or tertiary oxygen. For the purposes of isolation and characterization this mixture was converted to a single acetonide (15), but a better solution was the use of a tert-butyldimethylsilyl (TBS) group that had the dual effect of inhibiting silyl migration to the secondary alkoxide and enhancing the diastereoselectivity (16).38
Scheme 4.
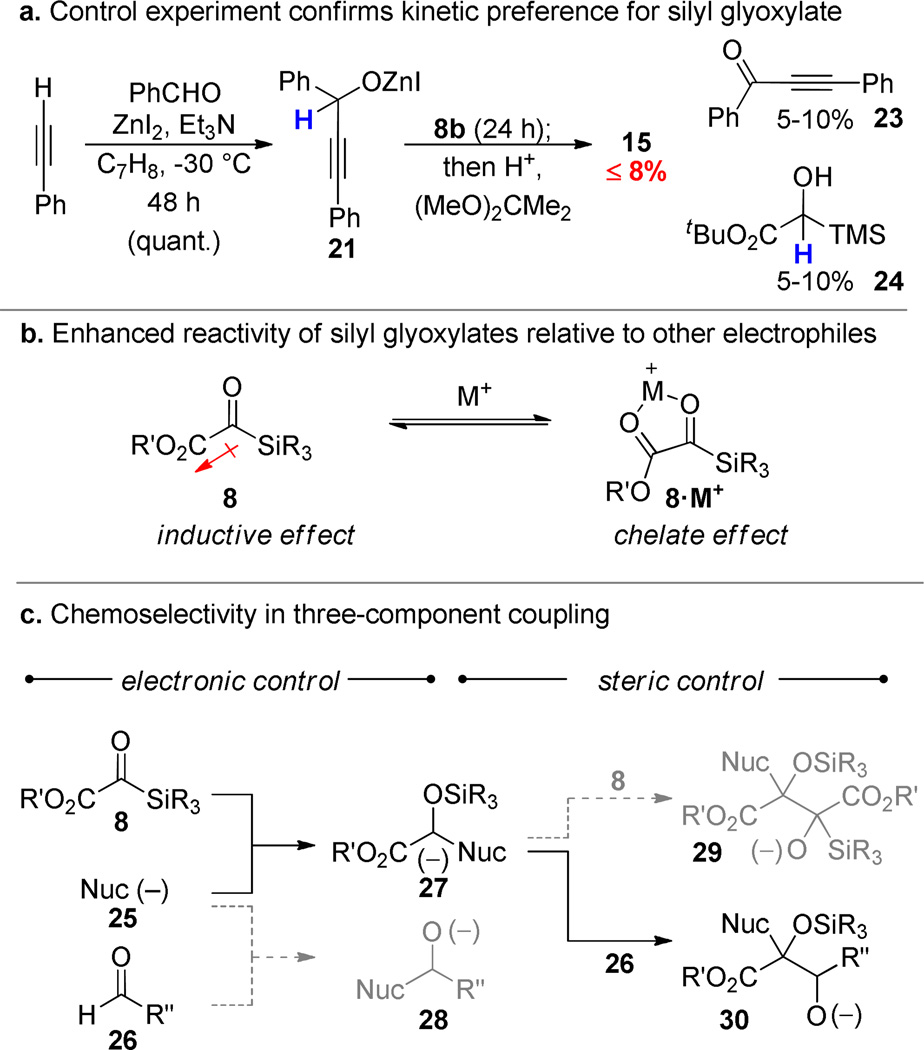
The three component alkyne/silyl glyoxylate/aldehyde coupling is noteworthy insofar as the direct addition of acetylides to aldehydes is a known process.39 A control experiment was performed to ascertain whether the silyl glyoxylate is kinetically a more competent electrophile than the aldehyde or whether the aldehyde reacts first, but reversibly with the acetylide. Omitting the silyl glyoxylate under otherwise identical conditions led to the quantitative formation of the secondary zinc alkoxide 21. This intermediate is apparently not on the productive reaction pathway since introduction of the silyl glyoxylate at this point does not provide appreciable amounts of the aldol 15. Foreshadowing future chemistries, the intermediate Zn-alkoxide 21 does react with the silyl glyoxylate in a Meerwein-Ponndorf-Verley/Oppenauer redox reaction to provide α,β-ynone 23 and hydroxysilane 24 in small amounts.
The preference exhibited by a range of nucleophiles for the silyl glyoxylate reagent over many different electrophiles has turned out to be a surprising, but fortuitous hallmark of this chemistry. We ascribe this chemoselectivity to both the inductive effect of the neighboring ester and the ability to form a highly activated 5-membered chelate intermediate 8·M+.40, 41 A more subtle, but perhaps more remarkable chemoselectivity issue becomes manifest when one considers the fate of the Brook rearrangement product 27. Given the preceding analysis regarding the reactivity of the silyl glyoxylate, it seems reasonable that the generic intermediate 27 would prefer to react with another molecule of silyl glyoxylate 8 rather than the secondary electrophile. In fact, this manifold is seldom observed and very good selectivity is observed for the secondary electrophile (e.g., 26). Our working hypothesis is that the steric interactions associated with bringing together the fully substituted glycolate enolate 28 and the large silyl glyoxylate tips the preference toward the more sterically accessible aldehyde electrophile. A striking example of this selectivity transpires upon addition of vinylmagnesium bromide (18) to a solution of silyl glyoxylate 8c and cyclohexanecarboxaldehyde (17): the three component coupling product is obtained in good yield. The syn isomer 19 is obtained at 0 °C while the anti product 20 predominated at −78 °C.42 These results are consistent with reversible aldolization.
α-Coupling
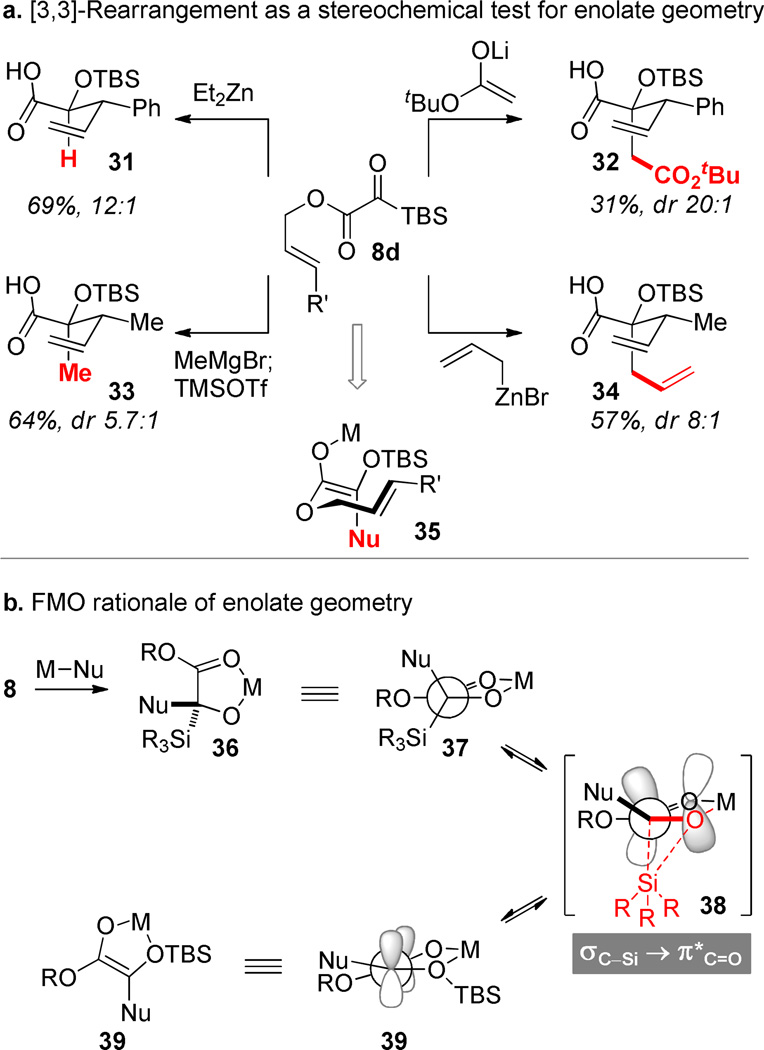
The Brook rearrangement that follows nucleophilic addition to a silyl glyoxylate delivers a silyloxy enolate, the geometry of which will impinge upon the stereochemistry of derived addition products. A stereochemical test was devised to indirectly probe the enolate geometry. The ester enolate Claisen rearrangement43 was selected because of its tendency to faithfully transmit enolate stereochemical information to the tetrahedral stereogenic centers of the product via a chair-like transition structure. O-Allyl silyl glyoxylates (8d) were prepared via acidic hydrolysis of the tert-butyl ester and nucleophilic esterification.44 A number of nucleophiles were tested in the nucleophilic addition. Yields were independently optimized on the basis of the countercation. Intermediates derived from CH3MgBr required the addition of TMSOTf in order to achieve [3,3]-rearrangement, while Li(I)- and Zn(II)-derivatives rearranged as the unsilylated metalloenolate. Reaction stereoselectivity was variable, but the stereoisomer derived from the (Z)-enolate 35 always predominated. A plausible explanation for the formation of the (Z)-isomer flows from the presumed intermediacy of the rigid chelated tetrahedral intermediate 36 that results from nucleophilic addition to the silyl glyoxylate. The organization of this structure appears to perfectly align the electron-rich σC–Si orbital with the vacant π*C=O orbital that will become occupied upon C→O migration. While C–Cα bond rotation (leading to the (E)-isomer) could conceivably accompany silyl migration, it would be necessary to break chelation in the transition state and this reorganization would deviate from a least motion path. Direct formation of the C-metallo tautomer of the enolate via stereospecific Brook rearrangement is also plausible,10 but seems unlikely given the presence of the ester.
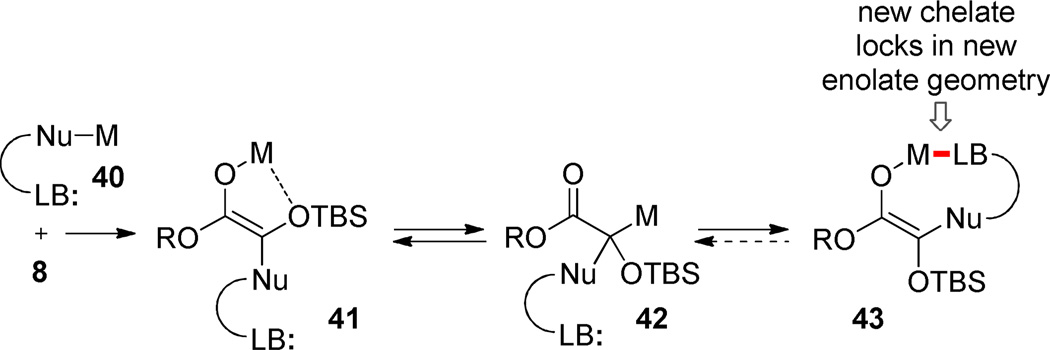
The stereoselectivity in these ester enolate Claisen rearrangements is not perfect. Stereochemical leakage via competing boat- or twist-boat-like transition structures is unlikely since unconstrained acyclic ester enolate Claisen rearrangements regularly proceed through chairlike transition states. It is more likely that imperfect stereocontrol arises from loss of enolate geometric homogeneity at some point during the reaction sequence. Intermediates derived from organozinc intermediates exhibit the greatest range of diastereoselectivities and this could arise from the well-established ability of zinc enolates to exist in the C-metallo tautomeric form,45 a circumstance that opens a pathway for enolate equilibrations (and thence stereoerosion in the [3,3]-rearrangement). While less appreciated, fluxional geometric behavior has also been observed with Mg-enolates; transient C-magnesiation was proposed as the mechanism for interconversion.46 The presence of a polar Lewis basic group (LB) within the nucleophile may lead to new stable chelates (e.g., 43) and drive the equilibration (vide infra).
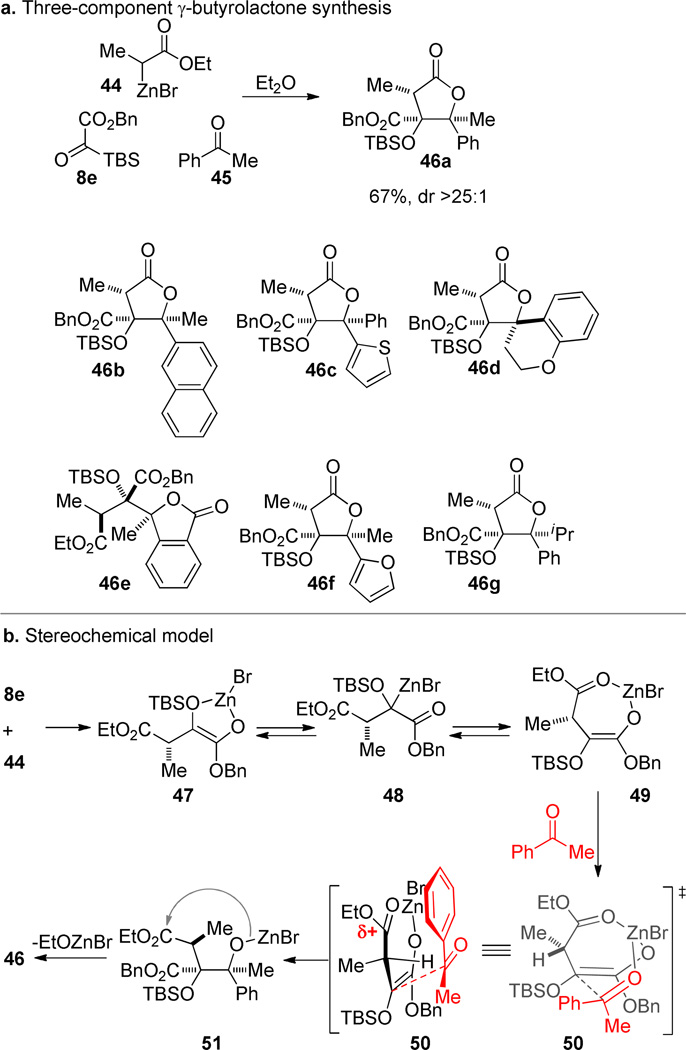
The stereochemical lability of the organozinc intermediates can be used advantageously. Reformatsky reagents 44 react with silyl glyoxylates (8e) and ketones (45) to yield pentasubstituted γ-butyrolactones (46).47 The assembly is modular and allows for highly congested structures to be assembled in short order. Remarkable levels of diastereocontrol are observed in the lactone products and the aryl group is consistently syn to the silyl ether. The reaction also works well with unsubstituted Zn-enolates and aldehydes, but diastereoselectivity is negligible in those cases. The combination of a substituted enolate and ketone terminating electrophile is uniquely effective for delivering products in high diastereoselectivity.
The ester of the Reformatsky reagent may be critical in regulating the stereochemical course of the reaction. The kinetically formed (Z)-enolate 47 has the stereocenter exo to the five-membered chelate. A1,3-strain could conceivably provide a rationale for the influence of the stereocenter, but a more likely scenario is that enolate O–C–O equilibration leads to a new chelate 49 that unambiguously differentiates the diastereotopic faces of the enolate. If one stipulates approach of the ketone over the methine hydrogen (rather than the methyl), the next question is what controls electrophile orientation. The illustrated coordination of the ethyl ester would generate a partial positive charge on the carbonyl that could be stabilized by the aromatic ring in the illustrated transition structure 50. Electronically differentiated diarylketones (e.g. thienyl vs. phenyl) prefer to place the electron-rich aromatic ring in the β-configuration, a finding that is consistent with the proposed electronic orienting effect.
The application of enolate-based bond constructions with silyl glyoxylates has been demonstrated by other research groups. Scheidt and coworkers published an example of Li-amide enolate addition to silyl glyoxylate 8 followed by alkylation of the rearranged intermediate with benzyl bromide.48 Lu and Yao showed the three component coupling of metalated sulfonylimidates, silyl glyoxylates, and N-tert-butanesulfinyl aldmines gave good yields of five-membered cyclic N-sulfonylamidines with essentially perfect control. These latter reactions constitute rare examples of successful chiral auxiliary-mediated silyl glyoxylate-based multicomponent couplings.49
Multicomponent γ-Coupling (Vinylogous Trapping)
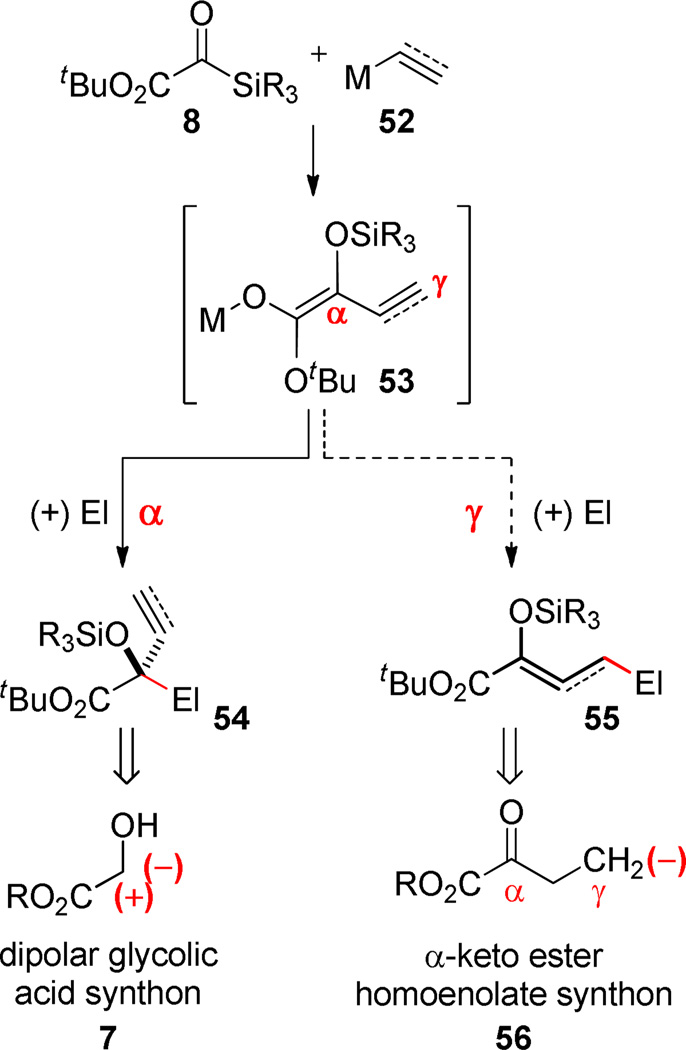
When unsaturated nucleophiles (vinylmetal, metal acetylide) are used with silyl glyoxylates, the rearranged intermediate can exhibit ambident behavior (Scheme 9). As noted in Scheme 4, aldehydes undergo trapping at the α-carbon of the extended π-system in preference to the γ-site. This reaction mode has the effect of rendering the silyl glyoxylate as the synthetic equivalent to the dipolar glycolic acid synthon 7. In contrast, the hypothetical γ-mode would regenerate the carbonyl (masked in the form of the enolsilane 55) and as such would represent a method to capture the unusual α-keto ester homoenolate synthon (56). Important precedent for this trapping mode for silyloxyallyl- and silyloxylpropargyl anionic systems lacking the ester group may be found in the seminal publications from the Kuwajima and Reich groups.50–56
Scheme 9.
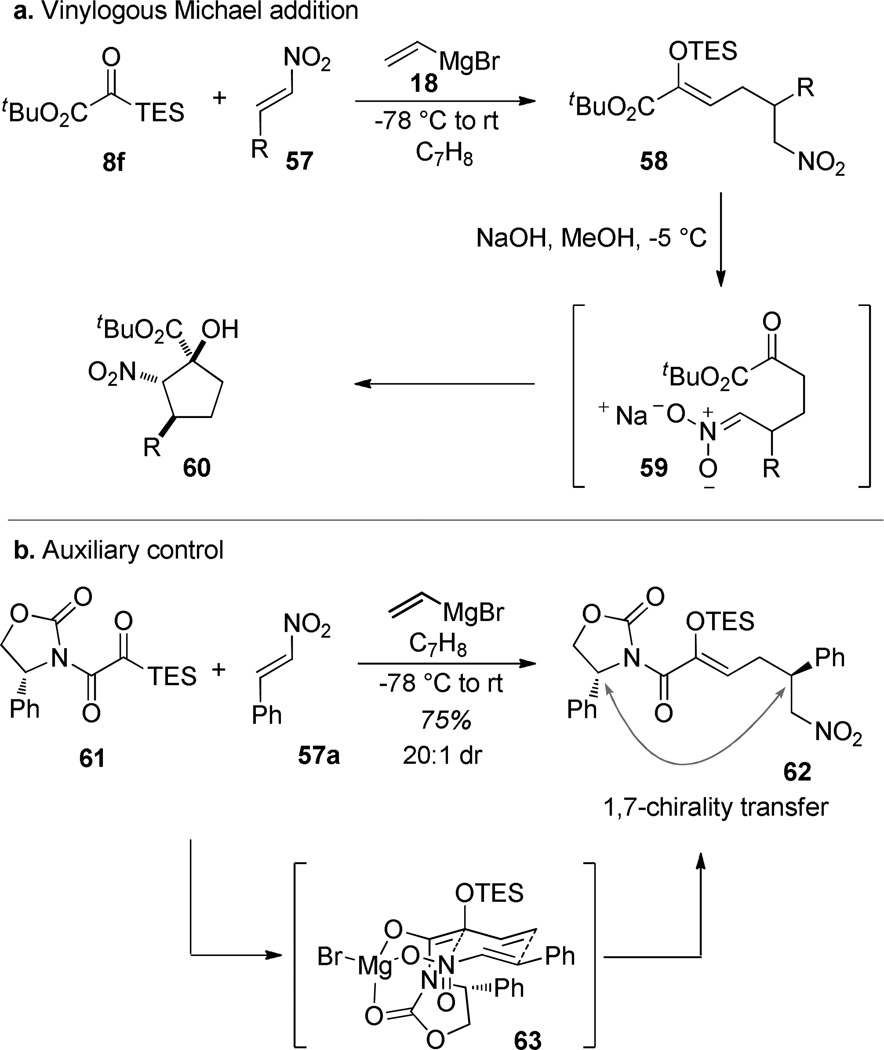
Electrophile identity determines the operative reaction mode. Nitroalkenes, silyl glyoxylates, and vinylmagnesium bromide react to give products of γ-trapping of the intermediate 53, leading to the formation of ε-nitro-α-silyloxy-α,β-unsaturated esters 58.57 In this case, a triethylsilyl glyoxylate and tert-butyldimethylsilyl glyoxylate functioned analogously, while the trimethylsilyl and triisopropylsilyl glyoxylates gave lower yields. The nitroalkene can be substituted with aryl and alkyl groups, with even a tert-butyl group giving reasonable amounts of product. Deprotection of the enolsilane under basic conditions simultaneously generates the nitronate anion 59 which can cyclize onto the nascent α-keto ester to generate the cyclopentanol 60.58 The acyl silane carbonyl functions as the electrophilic site twice during this sequence but still delivers the fully substituted glycolic ester in the end product. This reaction is one of the few instances where we have been successful in applying a chiral auxiliary for absolute stereocontrol. Chiral silyl glyoxamide 6159 reacts under analogous reaction conditions to provide the enolsilane 62 with excellent diastereocontrol. 1,7-Stereotransmission is operative in this case and the high diastereoselection could conceivably arise from a highly ordered trans-decalin transition structure such as 63.
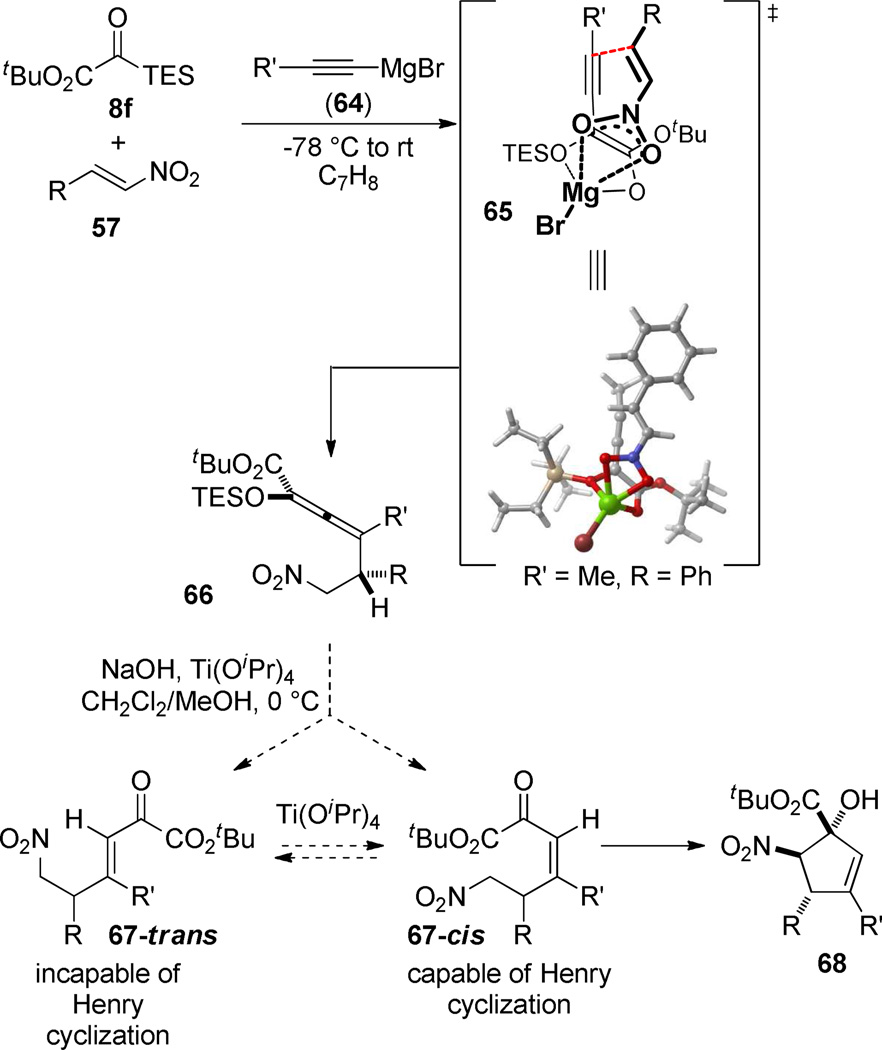
The use of acetylide nucleophiles in related three-component couplings retains the vinylogous addition mode, but introduces an additional stereochemical element, since a tetrasubstituted allene is produced in the product.60 The relationship between the stereogenic axis and the tetrahedral chiral center is controlled perfectly and >20:1 diastereocontrol is observed for a range of nucleophile/electrophile combinations. DFT calculations point to the chelated transition structure 65 as the one responsible for the observed diastereomer. As in the vinylmagnesium-initiated three-component coupling, the carbonyl functionality is preserved in the form of an enolsilane, with the added advantage of an additional degree of unsaturation that can be used for other transformations. The analogous deprotection/Henry cyclization conditions did not work for these substrates, but the addition of Ti(OiPr)4 aided in promoting successful, diastereoselective cyclizations. A subtle feature of this deprotection/cyclization is that the presence of the alkene means that only one geometry of the enone can cyclize. It is unknown at this point whether the deprotection is selective for the correct geometric isomer. Ti(OiPr)4 could conceivably assist with stereoselective deprotection to give the needed isomer 67-cis, or it could promote isomerization of the cis- and trans-isomers. The successful cyclization gives a highly functionalized cyclopentanol product 68. Many manipulations of the functionality can be envisioned. To date we have demonstrated that Ru-catalyzed ketohydroxylation61 can occur and proceeds diastereoselectively from the α-face.
Catalysis
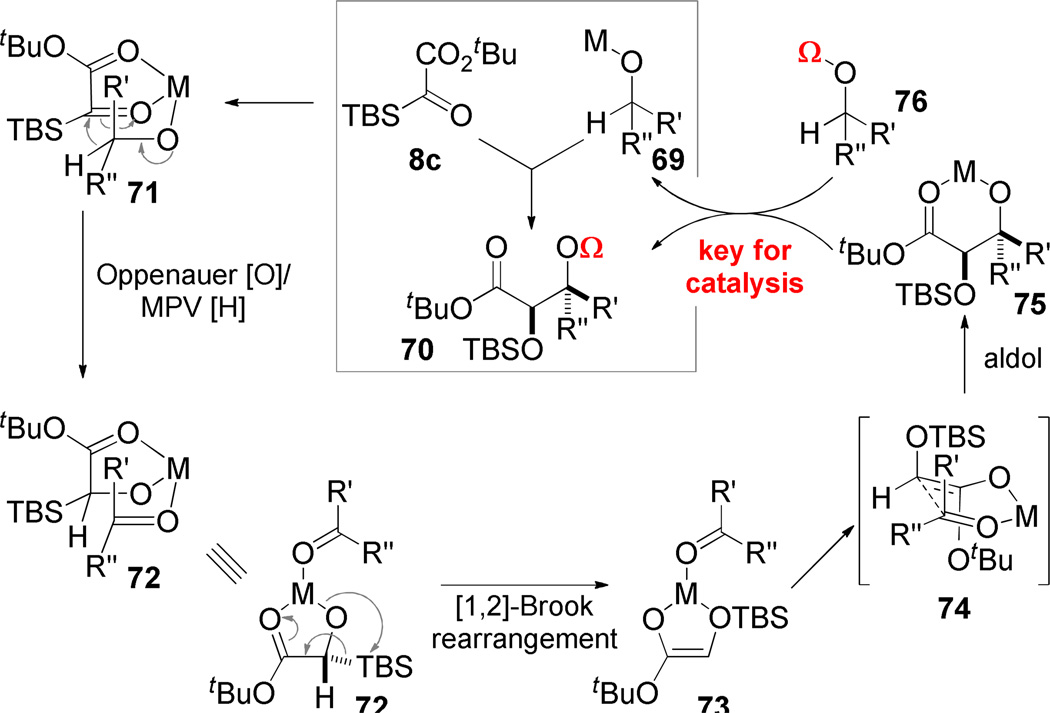
A comparison of Schemes 1 and 2 reveals that in order to achieve catalysis in multicomponent reactions involving silyl glyoxylates, it would be necessary for the nucleophile to be regenerated at the end of the reaction. Our point of departure for establishing proof of concept was the observation of by-products 23 and 24 during our early mechanistic studies. This reaction suggested the possibility of developing a new reaction where the MPV-Oppenauer redox process initiates a chain of events that mutually activates both reaction components for coupling. In particular, if the hydride addition into the silyl glyoxylate (71 → 72) triggered Brook rearrangement (→ 73), a glycolate enolate would result. Because the MPV-Oppenauer reaction simultaneously creates a ketone or aldehyde, aldolization (74) between the two symbiotically-generated intermediates might be expected. Indeed, the combination of silyl glyoxylates and magnesium alkoxides (generated from deprotonation of the alcohol or Grignard addition to an aldehyde) causes the proposed redox-initiated aldol cascade to occur in good yield.62 Nonetheless, this is still a process that is stoichiometric in the metal reagent: the product of the reaction is the magnesium aldolate 75 (which gives 70 (Ω = H) upon aqueous workup). To achieve catalysis, the aldolate must be quenched by transfer of some fragment “Ω” that concurrently releases the alkoxide 69.
Scheme 1.
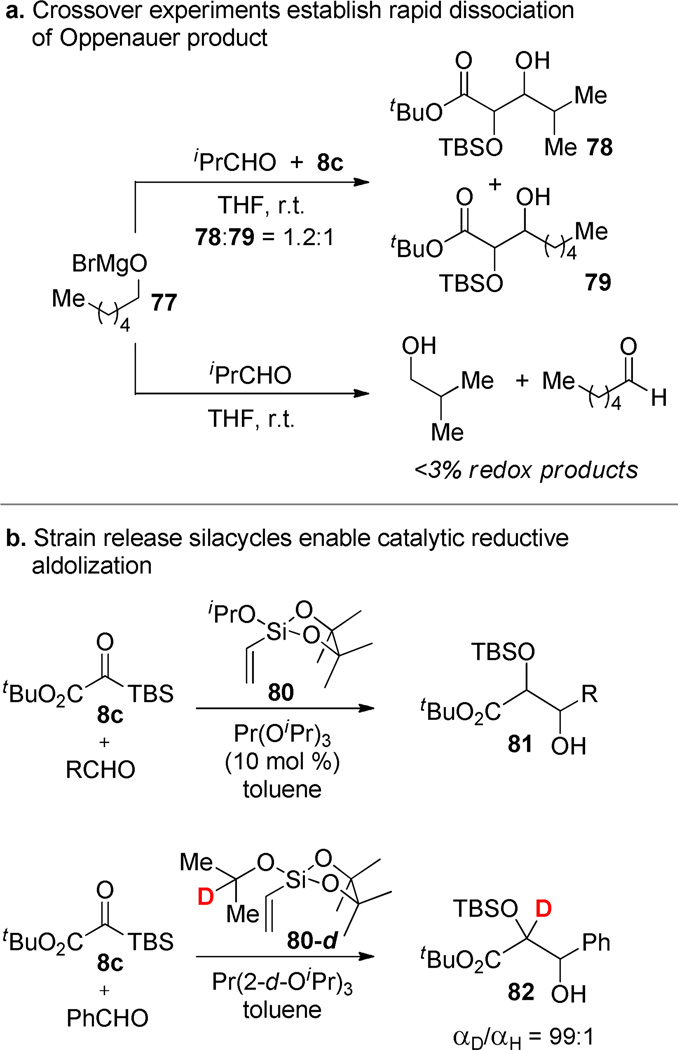
To obviate the need to prepare a unique Ω–OCHRR´ reagent (76) corresponding to each desired electrophile, we pursued the use of a single reagent that would release the reductant alkoxide and initiate reductive coupling of the silyl glyoxylate and an aldehyde. Crossover experiments had revealed that dissociation of the Oppenauer oxidation product is rapid and reversible (Scheme 13a); therefore, selection of an alkoxide that would give a relatively poor electrophile (ketone) was the projected method of choice. Many possibilities for Ω can be envisioned, but in practice the most effective was the silacycle 80 (Scheme 13b).63 Other candidates were inefficient at inducing alkoxide methathesis, presumably due to the fact that the alkoxide is a poor nucleofuge. The unique effectiveness of the silacycle undoubtedly stems from its high Lewis acidity64 that favors association with the aldolate. Mg-alkoxides were not effective for this catalytic variant. Lanthanum alkoxides were the most effective catalysts for this reaction and Pr(OiPr)3 distinguished itself for its reaction rate among those screened. Chiral variants provided some modest enantiocontrol, confirming involvement of the metal center in the stereoselectivity-determining transition state. While this strategy is not the final chapter in the development of catalytic reactions involving silyl glyoxylates, the associated mechanistic experiments are instructive and the studies should provide a useful template for future experiments.
Scheme 13.
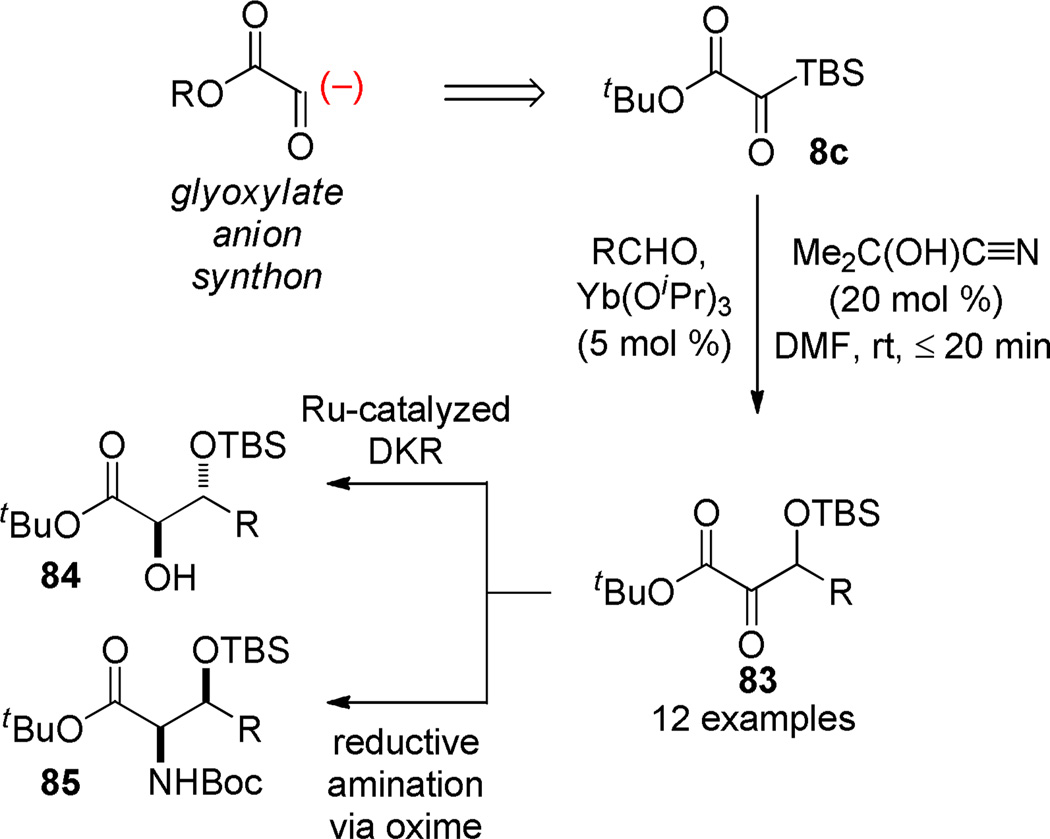
A second catalytic mode revisits our earlier work on the use of acyl silanes as acyl anion equivalents in the presence of umpolung catalysts. The addition of silyl glyoxylates to aldehydes in the presence of catalytic quantities of lanthanum cyanides (conveniently generated in situ from the lanthanum alkoxides and acetone cyanohydrin) provides a convenient method for the synthesis of β-silyloxy-α-keto esters via a silyl benzoin mechanism (Scheme 14).65 The products can be used in reductive amination for the synthesis of β-hydroxy-α-amino acid derivatives and promising preliminary results on their use in dynamic kinetic resolutions were obtained. In this application, the silyl glyoxylate functions as the synthetic equivalent to the glyoxylate anion synthon.
Scheme 14.
Natural Product Synthesis
Our philosophy in undertaking the synthesis of structurally interesting bioactive natural products flows from our interest in developing new complexity-building reactions based on mechanistic studies. We have avoided undertaking the preparation of any natural products where we already have the key reaction in hand. Instead, it strikes us as more interesting to use the structure of a given compound as an impetus to explore some undeveloped concept or reactivity pattern. Silyl glyoxylates are clearly well suited to the creation of new glycolic acid building blocks, and this is where our search for new chemistry began.
(a) Leustroducsin B.66, 67
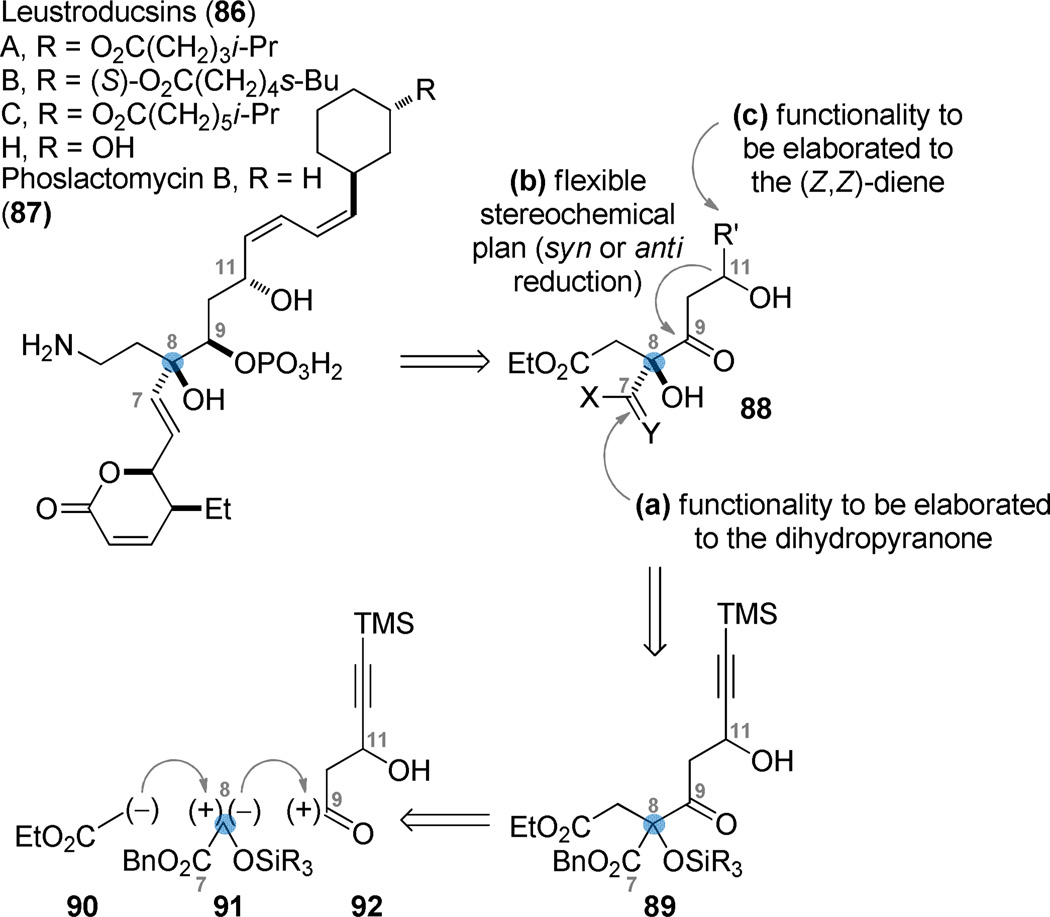
The leustroducsin (86)68, 69 and phoslactomycin (87)70, 71 families of natural products are isolates of the culture broth of Streptomyces platensis. In addition to exhibiting a range of compelling bioactivities, the natural products contain a number of structurally interesting features, such as an aminoethyl sidechain, an unsaturated δ-lactone, and a phosphonic acid vicinal to a tertiary alcohol. We envisioned that the tertiary alcohol at C8 (blue dot) might function as the central organizational point from which a convergent assembly of the leustroducsin backbone could be considered. Key to our thinking was the notion that the tertiary allylic alcohol might arise from functionality other than an alkene, and that elaboration of a C7 aldehyde via a Wittig (or related) olefination could provide a method for appending the dihydropyrone or its precursor (cf. 88(a)). A flexible synthetic strategy for the creation of the C9/C11 stereodiad was desired such that the configuration of the congested C9 phosphate could be reliably created from a C9 ketone under the directing influence of a C11 alcohol (cf. 88(b)). The functionality in R´ should be amenable to elaboration of the characteristic (Z,Z)-diene (cf. 88(c)). These design criteria led back to a structure 89 that collectively constitutes a retron for a (hypothetical) three-component enolate/silyl glyoxylate/acyl electrophile coupling (corresponding to synthons 90 + 91 + 92). In considering the pivotal intermediate 89 in the context of the final desired functionality, the synthetic strategy would for the first time employ silyl glyoxylates for the synthesis of something other than an embedded glycolate.
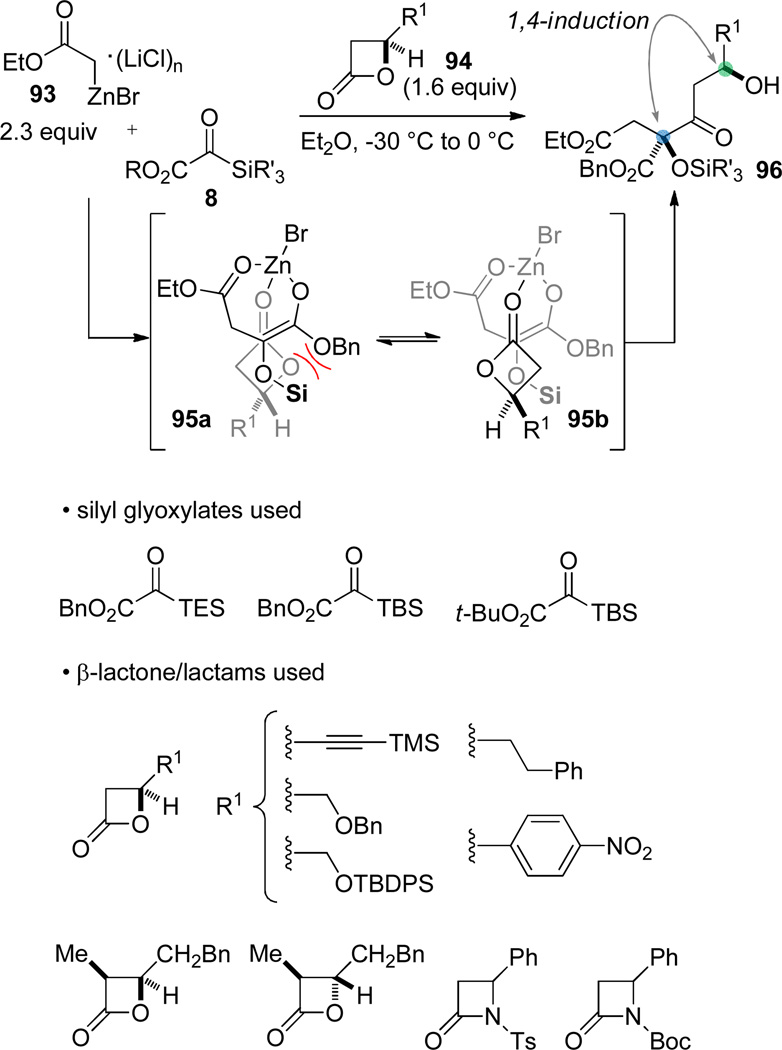
Since the targeted structure was a β-keto ester, the addition of 91 to 92 formally represented a Claisen condensation. The creation of fully substituted Claisen adducts (where deprotonation of the β-keto ester product is not feasible) is unusual, as are examples of stereoselective Claisen condensations.72, 73 We selected β-lactones as the acyl donor74 with the expectation that strain release would preclude the retro-Claisen75 pathway. An open question at the outset was the degree to which the stereogenic center in the β-lactone would impact the emerging fully substituted stereocenter. In the event, the three component coupling of acetate Reformatsky reagents 93, silyl glyoxylates 8, and β-lactones76 afforded the β-keto esters 96 with good stereocontrol. The superior electrophilicity of the silyl glyoxylate relative to the β-lactone allowed the synchronous addition of these two reagents to the nucleophile. Efficient coupling was observed and excellent 1,4-chirality transfer from the stereogenic center of the β-lactone to the incipient fully substituted center was observed. A stereochemical model where the Zn(II) center organizes the approach of the enolate intermediate to the β-lactone is proposed (95). Enolate approach opposite the alkyl substituent is presumed, leading to two limiting transition structures that might differentiate themselves on the basis of destabilizing electrostatic interactions in 95a.
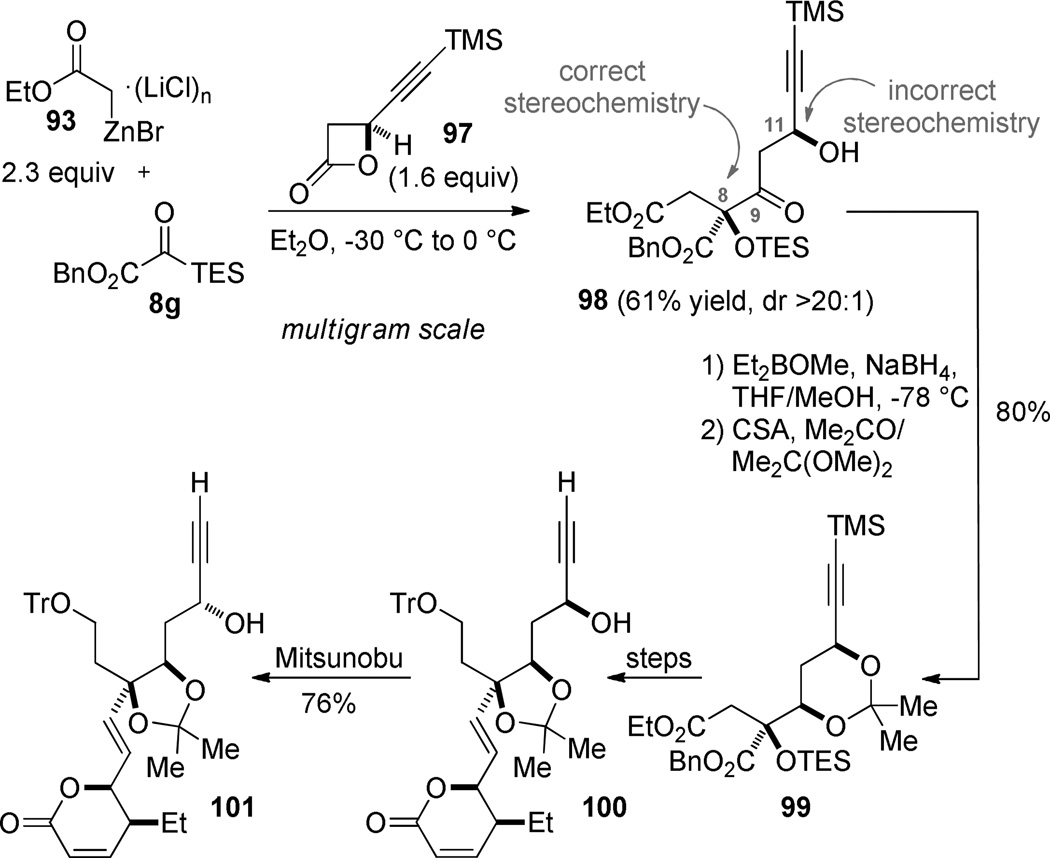
For the purposes of the leustroducsin synthesis, the incorrect relative stereochemical relationship is established during the enolate acylation. In our analysis, a stereochemical mistake at C11 would be easier to correct than at the C8 stereocenter; therefore, the “wrong” β-lactone 97 was used to create the C8 tertiary silyl ether with the correct absolute configuration (98). The correct C9 configuration was then established by using the C11 alcohol to mediate a syn reduction.77 The stereochemistry at C11 was carried as the incorrect configuration until late in the synthesis, when it was inverted using a Mitsunobu reaction,78 allowing us to intersect with an intermediate in Imanishi’s synthesis. While this synthetic endeavor ultimately involved more problems with protecting groups than we anticipated, a positive attribute of this approach to leustroducsin B was the rapid assembly of the carbon backbone and the application of reliable ensuing transformations to parlay the existing stereochemistry into those centers required to complete the synthesis.
(b) Trachyspic acid.79
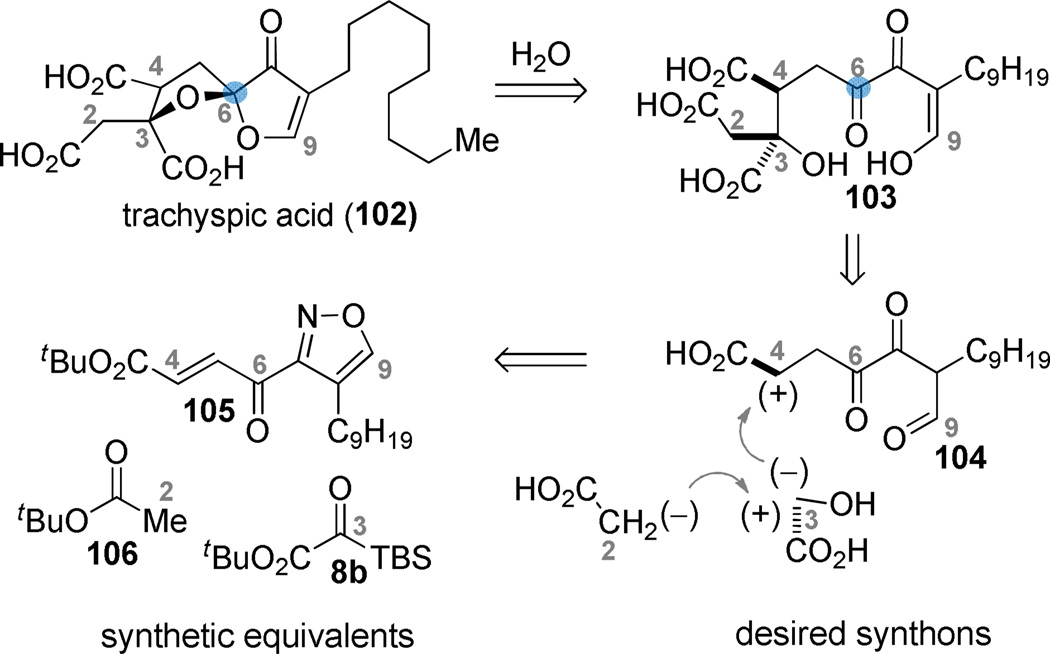
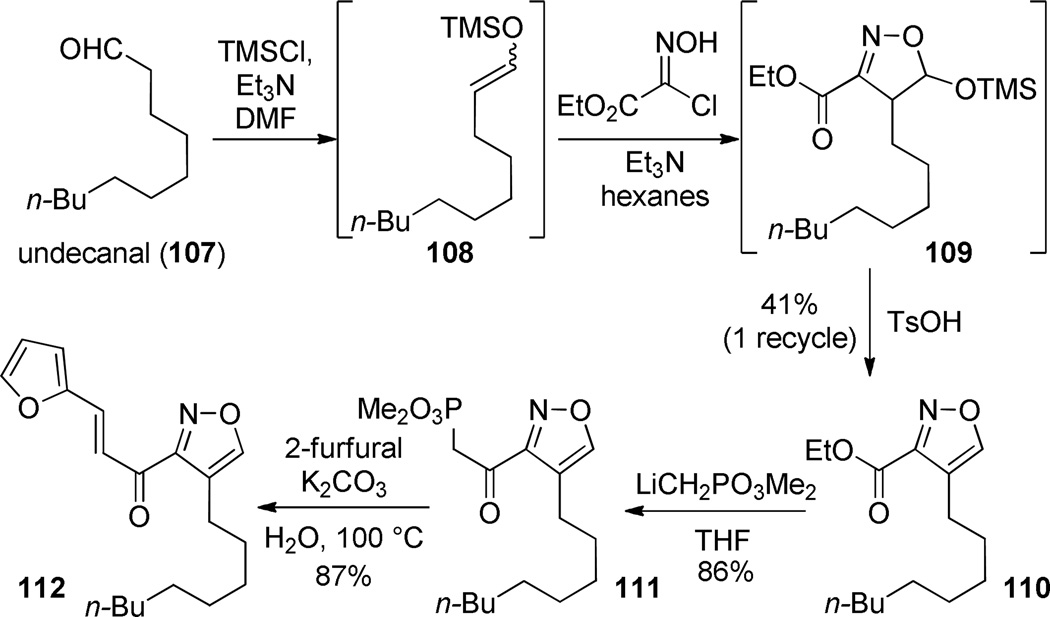
Trachyspic acid (102) is an alkyl citrate natural product that is an inhibitor of tumor heparanase.80, 81 Its interesting structure and biological activity attracted us to consider efficient approaches for its preparation. Our initial interest naturally resided with the C3 glycolate, disconnection of which suggested the opportunity for the development of a new enolate-initiated/Michael-terminated three-component coupling. Further inspection of the hypothetical acyclic hydrated form 103 revealed an unusual β,γ-diketoaldehyde from C6 to C9. We expected that a maximally efficient preparation of the target would entail the least manipulation of this reactive substructure. “Packaging” the C6–C9 functional array in a way that closely resembled 103, insulated the reactive functionality, and could be easily revealed at an opportune time late in the synthesis became an important goal. Masking the C7/C9-keto aldehyde as an isoxazole82 was viewed as an attractive possibility since the N–O bond could be cleaved to break the heteroaromatic ring late in the synthesis, but the C6 ketone could remain available to activate the C4–C5 alkene for conjugate addition.
The isoxazole 105 necessitated the development of a new nitrile oxide cycloaddition to prepare the 3,4-disubstitution pattern.83, 84 Nitrile oxide-alkyne dipolar cycloadditions are the method of choice for the preparation of isoxazoles, but typically lead to the 3,5-disubstituted product with terminal alkynes. To prepare the needed constitutional isomer, we used the enolsilane 108 as the alkyne equivalent. The silyloxy group presumably provides electronic direction during the [3+2] cycloaddition, and then facilitates the aromatization via acid catalyzed elimination (→ 110). The ester 110 is converted to the β-keto phosphonate 111 via Claisen condensation. The latter undergoes olefination with furfural in high yield (K2CO3, 100 °C). The furan group was selected as a convenient surrogate for the C4 carboxyl group.85
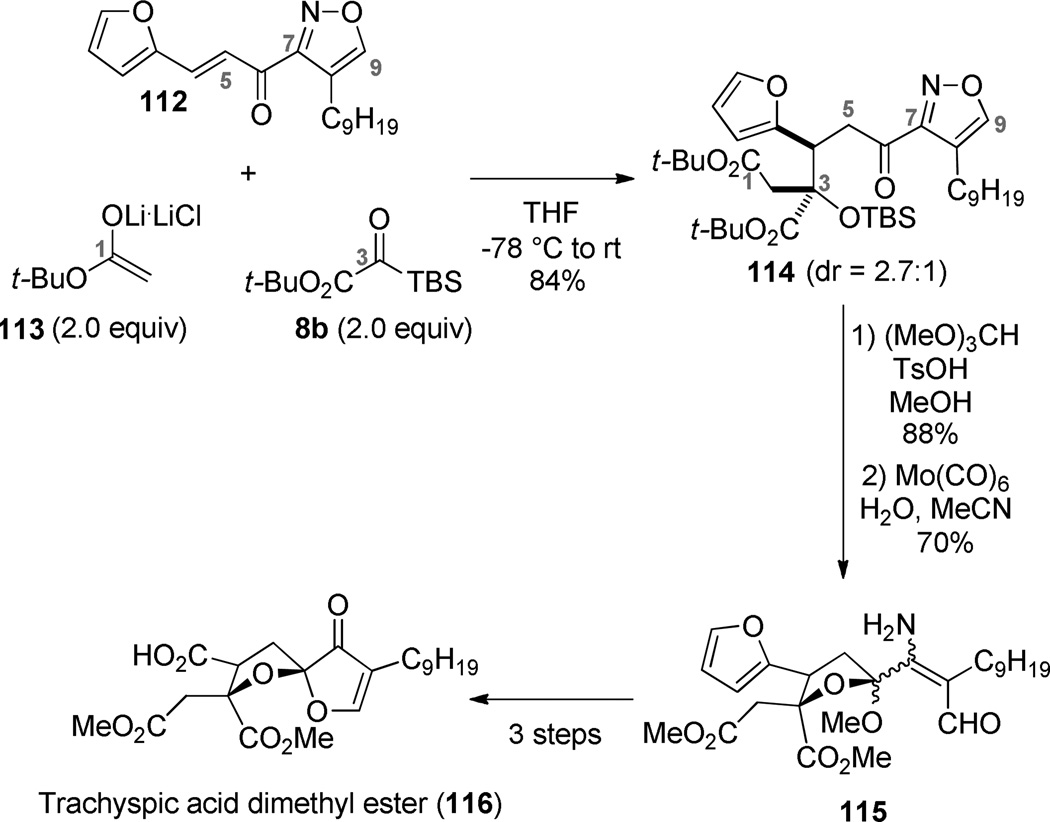
With the Michael acceptor 112 in hand, we were in a position to develop the requisite three-component coupling proposed in Scheme 18. The success of the reaction was found to be a function of the enolate counterion. Various zinc enolates gave mixtures of 1,2- and 1,4-adducts, while the lithium acetate enolate gave exclusively the desired Michael adduct 114. The formation of the desired diastereomer was favored when THF was employed as the solvent. This three-component coupling installed the complete carbon framework of trachyspic acid with a favorable disposition of functionality that was advanced according to our synthetic design. The furan and isoxazole masking groups performed their assigned roles, giving way to the C4 carboxylate and C7/C9 keto aldehyde respectively. Unfortunately, the extreme steric hindrance associated with the C3 ester in the penultimate intermediate 116 precluded the needed saponification. Our initial use of tert-butyl esters looked ideal from the vantage point of late-stage deprotection; however, the tert-butyl esters were unavoidably exchanged for methyl esters when the C6 ketone was engaged as its mixed methyl ketal. This latter step was obligatory since isoxazole cleavage was unsuccessful in the presence of the C6 ketone. Advancing intermediate 114 in a manner that retains the tert-butyl esters should preserve the excellent efficiency of this route, but create a more facile deprotection in the last step.
Scheme 18.
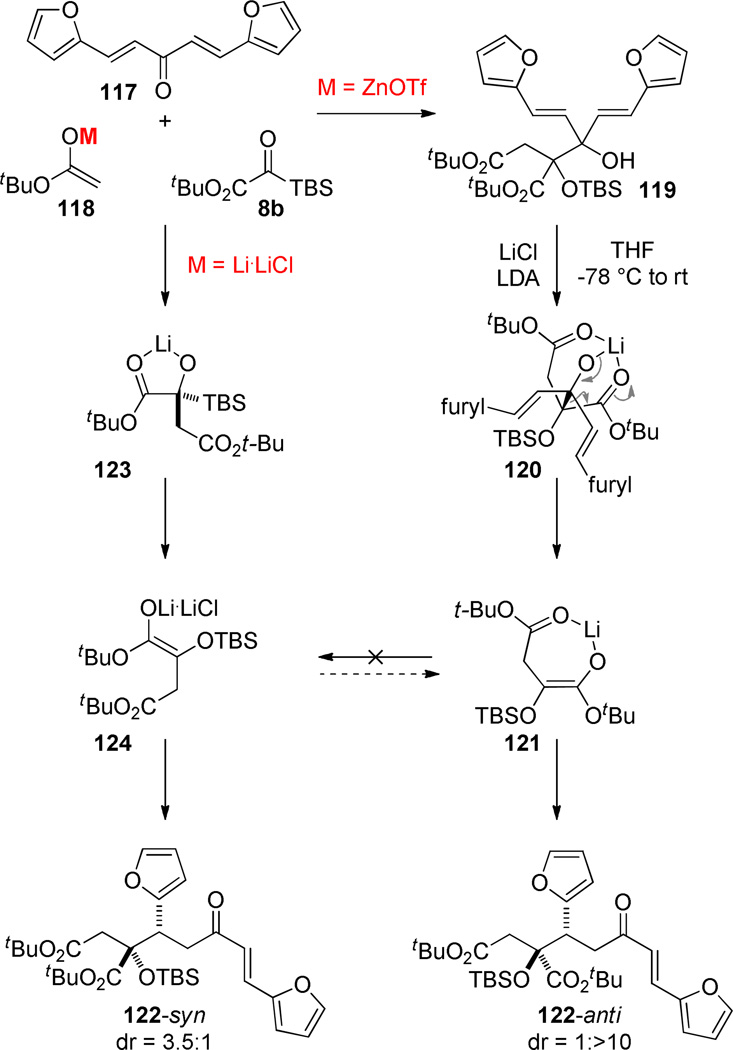
Experiments were performed to examine the subtleties of the cation-dependent 1,2-/1,4-selectivity in the three-component coupling. In the case of model dienone 117, the 1,2-adduct 119 is obtained exclusively with the Zn-enolate (118, M = ZnOTf). Deprotonating this tertiary alcohol 119 with LDA·LiCl did indeed convert the derived alkoxide 120 to the Michael adduct, but it delivered the epimeric product (122-anti) to the one obtained when the acetate lithium enolate was used directly (122-syn). A plausible explanation for this turnover in diastereoselectivity is that the retro-aldol reaction of 120 selectively forms the chelated (E)-enolate 121 via substrate organization around the Li-cation, while the reaction of the Li-enolate 118 (M = Li·LiCl) with the silyl glyoxylate affords the (Z)-geometry (124). The latter is already known from our Ireland-Claisen studies to flow from FMO considerations, and the chelated enolate 121 may be thermodynamically favored when there is a kinetically viable mechanism for its formation (cf. Scheme 8). These experiments suggest that the 1,4-adduct is the kinetic product when the lithium enolate is employed and a corollary finding is that the (E)-enolate 121 does not equilibrate with the (Z)-enolate 124 on the time scale competitive with electrophilic trapping. Whether the reverse reaction happens (124 → 121) is not clear (i.e. we do not know the inherent diastereoselectivity associated with (Z)-124), but to the extent that any of 121 is generated, the effect would be to erode the selectivity for the desired isomer 122-syn.
Scheme 8.
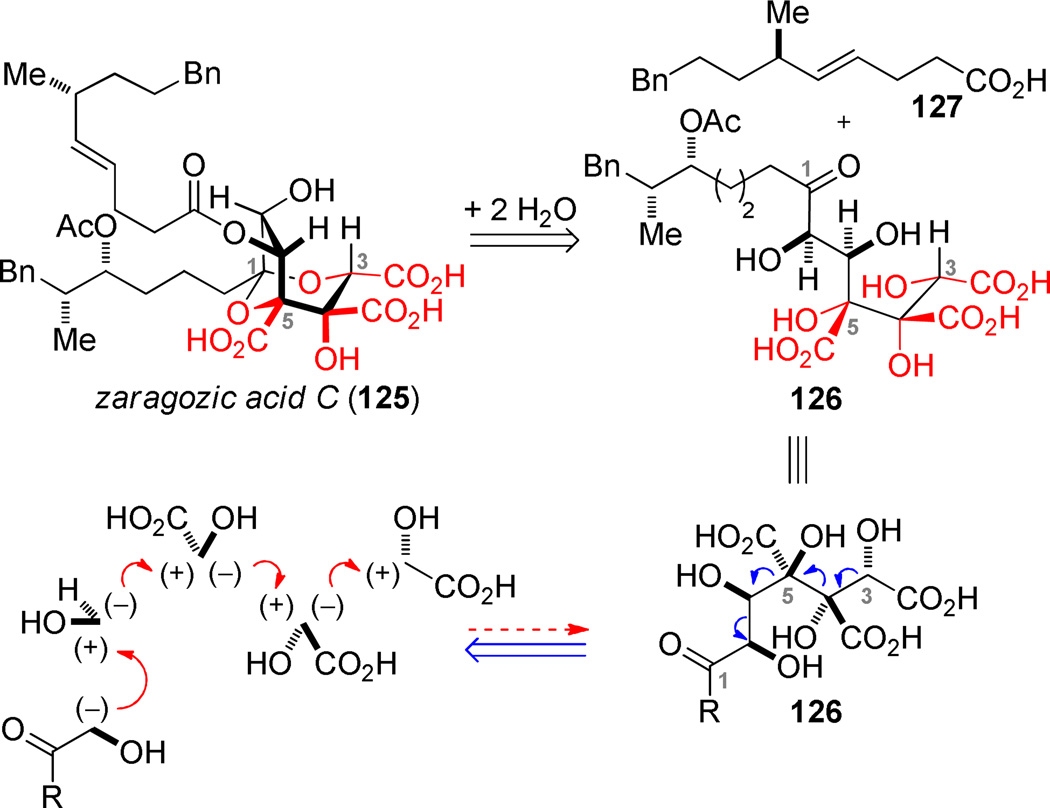
(c) Zaragozic Acid C.86
The zaragozic acids have been fascinating targets for synthetic chemists since the disclosure of their structures in the early 1990s.87–90 A defining feature of the highly oxygenated structures is the occurrence of three contiguous subunits of glycolic acid (highlighted in red) embedded within the dioxabicyclo[3.2.1]octane core. Zaragozic acid C (125) exists as internal ketal derived from the C1 ketone and the C3/C5 diol. Retrosynthetic hydration of the ketal (and concurrent hydration of the C6 ester) reveals the acyclic polyfunctional zaragozic acid core 126. A fascinating hypothesis that we wished to interrogate was whether the C3–C5 glycolic acid “trimer” could be efficiently assembled by employing multiple equivalents of the silyl glyoxylate reagent. This hypothetical cascade could in principle be uniquely effective in assembling the core structure in a way that directly delivers the functional groups desired in the natural product, without need for recourse to oxidation state changes and functional group manipulation.91–93 The new reaction to be developed simply entailed a change in reaction stoichiometry to use two equivalents of silyl glyoxylate relative to the initiating nucleophile: the Brook rearrangement product should only encounter only another molecule of silyl glyoxylate. Since our reaction would be terminated with a highly reactive glyoxylate ester, this electrophile would need to be added last to avoid premature termination of the cascade. We were cognizant that even successful formation of the desired bonds was insufficient in and of itself. Four diastereomeric products are possible and a stereorandom outcome was viewed as a distinct possibility.
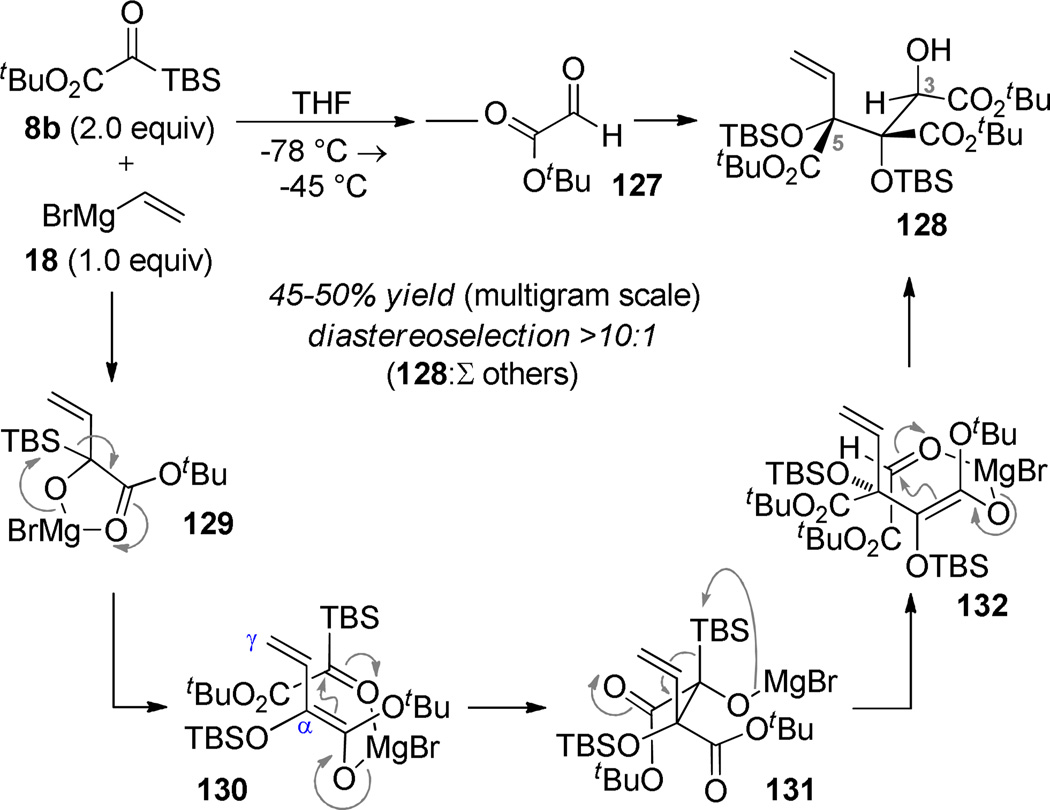
The addition of two equivalents of silyl glyoxylate 8b to vinylmagnesium bromide (18) in THF at −78 C, followed by addition of tert-butyl glyoxylate (127) gave the crystalline four-component coupling adduct 128 in 45–50% yield with >10:1 diasteroselection for the illustrated isomer. The relative stereochemistry of the product was established by an X-ray diffraction study.94 The major by-product arises from vinylogous (γ) trapping of the dienolate 130. Crucial to the ultimate success of the synthesis was the fact that the vicinal C4/C5 tertiary alcohols were established in the correct relative orientation. The incorporation of the C3 hydroxyl group in the incorrect relative configuration was initially disappointing, but ultimately critical for executing downstream reactions. Although we discovered that DBU-promoted epimerization provided the correct C3 configuration, several steps later in the synthesis were unworkable with this stereoarray. A risky decision was made to advance the synthesis using the wrong isomer with the expectation that the stereoerror could be corrected at some later stage of the synthesis.
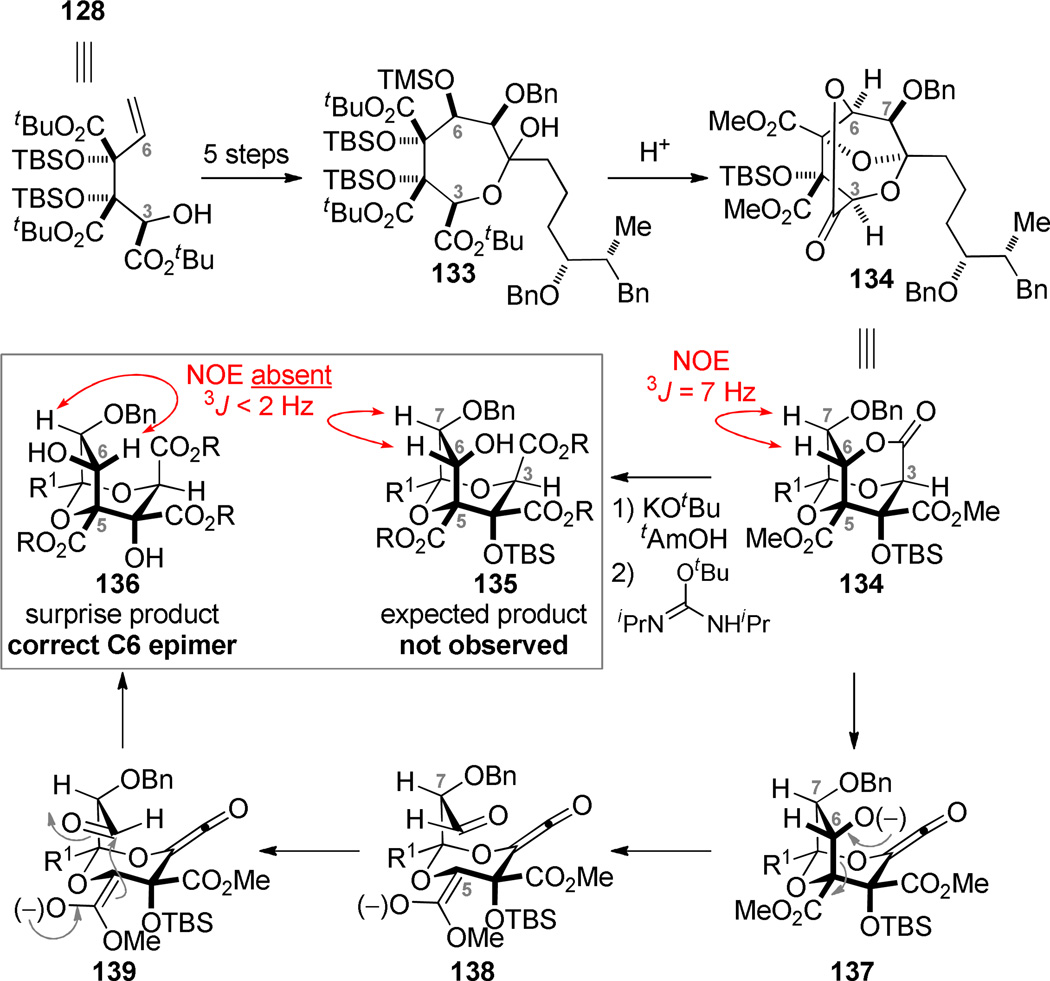
The hydroxy ester 128 was advanced to the lactol 133 in five steps. (The first is an oxidative kinetic resolution95 that oxidizes the undesired enantiomer to the α-keto ester and leaves behind enantioenriched 128). A second stereoerror (at C6) was introduced in this sequence, but in only six steps from silyl glyoxylate 8b, the entire carbon framework in the correct oxidation state was put in place. Treating lactol 133 with TsOH/MeOH engaged the C5 and C3 alcohols in the required internal ketal at C1. Because of the incorrect configurations at C3 and C6, the functionality at these carbons became engaged in the illustrated lactone 134.
Many efforts to invert the stereochemistry at C6 were met with failure. Attempts to achieve nucleophilic acyl substitution to open the lactone were largely unsuccessful with the exception of the somewhat unusual reaction conditions using KOtBu/tAmOH. Inspection of the NMR data for the ring-opened product exhibited puzzling characteristics. The starting lactone 134 displayed the expected NOE interaction between the C6/C7 methine hydrogens and a 3J value of 7 Hz. The reaction product displayed no NOE interaction between the C6/C7 methine hydrogens and a 3J value of <2 Hz was noted. We concluded that the C6 stereochemistry had inverted during the lactone opening (a surprising and fortuitous turn!) to give diol 136, not the expected 135. The most likely mechanism for the inversion is retro-aldol/aldol equilibration once the C6 alkoxide is revealed (137).96 The unique effectiveness of KOtBu may point to a ketene intermediate rather than a nucleophilic acyl substitution pathway.
We emphasize this epimerization in the context of designing the synthesis in such a way as to preserve the oxidation state of all the functional groups. Had the C5 carboxylate existed in some other (e.g. reduced) form, the retro-aldol reaction could not have occurred and the stereochemical correction would not have transpired. Thus, strategic decisions made early in the route to maximize efficiency were unexpectedly critical for the ultimate completion of the synthesis. The opening of the lactone allowed the C3 stereochemical correction to ensue (via basic epimerization), placing the ester in the more thermodynamically favorable equatorial position. Having the correct functionality and stereochemistry present in the core allowed the synthesis to be completed in short order.97
Conclusion
By applying many of the principles already developed during the long history of the [1,2]-Brook rearrangement in the context of the rationally modified reagent 8, exciting new reaction chemistry and product types were discovered. This phase of our investigation into the chemistry of silyl glyoxylates has revealed myriad reaction pathways that can lead to interesting, functionalized reaction products. To date, silyl glyoxylates have been deployed as synthetic equivalents to the geminal dipolar glycolic acid, α-keto ester homoenolate, and glyoxylate anion synthons.
The development of this program was driven by both mechanistic studies and the structures of complex natural products. Challenges that remain revolve around extending and improving the extant examples of absolute stereocontrol and catalysis. Endeavors in this regard will need to take advantage of the high inherent reactivity of this unusual α-dicarbonyl electrophile. Judging from the product types that have been prepared to date through the application of 8, it seems reasonable to think that new reactions relying on the concepts detailed herein might lead to the modular creation of even greater structural diversity.
Scheme 3.
Scheme 5.
Scheme 6.
Scheme 7.
Scheme 10.
Scheme 11.
Scheme 12.
Scheme 15.
Scheme 16.
Scheme 17.
Scheme 19.
Scheme 20.
Scheme 21.
Scheme 22.
Scheme 23.
Scheme 24.
Acknowledgments
Funding for this work was provided by the National Institutes of Health (National Institute of General Medical Sciences), Eli Lilly, Amgen, GlaxoSmithKline, and Novartis. D.A.N. and J.T.M. acknowledge ACS Division of Organic Chemistry Fellowships. K.M.S. acknowledges a BMS Minority Chemist Fellowship and an NIH NRSA Fellowship to Promote Diversity in Health-Related Research. J.S.J. acknowledges an Alfred P. Sloan Fellowship and a Camille-Dreyfus Teacher Scholarship. X-ray crystallography was performed by Dr. Peter White.
Biography

Jeffrey Johnson received a B.S. degree in chemistry from the University of Kansas in 1994, graduating with Highest Distinction and Honors in Chemistry. He was an NSF Graduate Fellow in the laboratory of David Evans at Harvard University, where he earned his Ph.D. in 1999. He undertook an NIH Postdoctoral Fellowship in the laboratory of Robert Bergman at the University of California at Berkeley from 1999–2001 and began as an Assistant Professor at the University of North Carolina in July, 2001. Since 2010, he has been a Professor of Chemistry.
References
- 1.Coppola GM, Schuster HF. Chiral α-Hydroxy Acids in Enantioselective Synthesis. Weinheim, Germany: Wiley-VCH; 1997. [Google Scholar]
- 2.Cristau H-J, Marat X, Vors J-P, Virieux D, Pirat JL. Curr. Org. Synth. 2005;2:113–119. [Google Scholar]
- 3.Bertrand MB, Wolfe JP. Org. Lett. 2006;8:4661–4663. doi: 10.1021/ol062016l. [DOI] [PubMed] [Google Scholar]
- 4.Giampietro NC, Kampf JW, Wolfe JP. J. Am. Chem. Soc. 2009;131:12556–12557. doi: 10.1021/ja905930s. [DOI] [PubMed] [Google Scholar]
- 5.Moser WH. Tetrahedron. 2001;57:2065–2084. [Google Scholar]
- 6.Page PCB, McKenzie MJ. Product Subclass 25: Acylsilanes. In: Fleming I, editor. Science of Synthesis. Vol. 4. Thieme: 2001. pp. 513–568. [Google Scholar]
- 7.Patrocinio AF, Moran PJS. J. Braz. Chem. Soc. 2001;12:7–31. [Google Scholar]
- 8.Cirillo PF, Panek JS. Org. Prep. Proced. Int. 1992;24:553–582. [Google Scholar]
- 9.Page PCB, Klair SS, Rosenthal S. Chem. Soc. Rev. 1990;19:147–195. [Google Scholar]
- 10.Brook AG. Acc. Chem. Res. 1974;7:77–84. [Google Scholar]
- 11.Degl'Innocenti A, Ricci A, Mordini A, Reginato G, Colotta V. Gazz. Chim. Ital. 1987;117:645–648. [Google Scholar]
- 12.Reich HJ, Holtan RC, Bolm C. J. Am. Chem. Soc. 1990;112:5609–5617. [Google Scholar]
- 13.Takeda K, Ohnishi Y. Tetrahedron Lett. 2000;41:4169–4172. [Google Scholar]
- 14.Linghu X, Nicewicz DA, Johnson JS. Org. Lett. 2002;4:2957–2960. doi: 10.1021/ol0263649. [DOI] [PubMed] [Google Scholar]
- 15.Linghu X, Johnson JS. Angew. Chem. Int. Ed. 2003;42:2534–2536. doi: 10.1002/anie.200250554. [DOI] [PubMed] [Google Scholar]
- 16.Nicewicz DA, Yates CM, Johnson JS. Angew. Chem. Int. Ed. 2004;43:2652–2655. doi: 10.1002/anie.200353354. [DOI] [PubMed] [Google Scholar]
- 17.Nicewicz DA, Yates CM, Johnson JS. J. Org. Chem. 2004;69:6548–6555. doi: 10.1021/jo049164e. [DOI] [PubMed] [Google Scholar]
- 18.Bausch CC, Johnson JS. J. Org. Chem. 2004;69:4283–4285. doi: 10.1021/jo0496143. [DOI] [PubMed] [Google Scholar]
- 19.Linghu X, Bausch CC, Johnson JS. J. Am. Chem. Soc. 2005;127:1833–1840. doi: 10.1021/ja044086y. [DOI] [PubMed] [Google Scholar]
- 20.Tarr JC, Johnson JS. J. Org. Chem. 75:3317–3325. doi: 10.1021/jo100312w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeda K, Tanaka T. Synlett. 1999:705–708. [Google Scholar]
- 22.Linghu X, Potnick JR, Johnson JS. J. Am. Chem. Soc. 2004;126:3070–3071. doi: 10.1021/ja0496468. [DOI] [PubMed] [Google Scholar]
- 23.Nahm MR, Linghu X, Potnick JR, Yates CM, White PS, Johnson JS. Angew. Chem. Int. Ed. 2005;44:2377–2379. doi: 10.1002/anie.200462795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrett MR, Tarr JC, Johnson JS. J. Am. Chem. Soc. 2007;129:12944–12945. doi: 10.1021/ja076095n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattson AE, Bharadwaj AR, Scheidt KA. J. Am. Chem. Soc. 2004;126:2314–2315. doi: 10.1021/ja0318380. [DOI] [PubMed] [Google Scholar]
- 26.Mattson AE, Scheidt KA. Org. Lett. 2004;6:4363–4366. doi: 10.1021/ol0481129. [DOI] [PubMed] [Google Scholar]
- 27.Mattson AE, Bharadwaj AR, Zuhl AM, Scheidt KA. J. Am. Chem. Soc. 2006;71:5715–5724. doi: 10.1021/jo060699c. [DOI] [PubMed] [Google Scholar]
- 28.Sekiguchi A, Kabe Y, Ando W. Tetrahedron Lett. 1979:871–872. [Google Scholar]
- 29.Sekiguchi A, Kabe Y, Ando W. J. Org. Chem. 1982;47:2900–2903. [Google Scholar]
- 30.Sekiguchi A, Ando W. J. Chem. Soc. Chem. Commun. 1979:575–576. [Google Scholar]
- 31.Bolm C, Kasyan A, Heider P, Saladin S, Drauz K, Guenther K, Wagner C. Org. Lett. 2002;4:2265–2267. doi: 10.1021/ol025911n. [DOI] [PubMed] [Google Scholar]
- 32.Yao W, Wang J. Org. Lett. 2003;5:1527–1530. doi: 10.1021/ol0343257. [DOI] [PubMed] [Google Scholar]
- 33.Darkins P, McCarthy N, Anthony M, McKervey, O'Donnell K, Ye T, Walker B. Tetrahedron: Asymmetry. 1994;5:195–198. [Google Scholar]
- 34.Nicewicz DA, Brétéché G, Johnson JS. Org. Synth. 2008;85:278. [Google Scholar]
- 35.Sugimura H, Watanabe T. Synlett. 1994;1994:175–177. [Google Scholar]
- 36.DiMauro EF, Kozlowski MC. J. Am. Chem. Soc. 2002;124:12668–12669. doi: 10.1021/ja026498h. [DOI] [PubMed] [Google Scholar]
- 37.Frantz DE, Faessler R, Carreira EM. J. Am. Chem. Soc. 1999;121:11245–11246. [Google Scholar]
- 38.Nicewicz DA, Johnson JS. J. Am. Chem. Soc. 2005;127:6170–6171. doi: 10.1021/ja043884l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anand NK, Carreira EM. J. Am. Chem. Soc. 2001;123:9687–9688. doi: 10.1021/ja016378u. [DOI] [PubMed] [Google Scholar]
- 40.Masuyama Y, Tsunoda T, Kurusu Y. Chem. Lett. 1989;18:1647–1650. [Google Scholar]
- 41.Evans DA, Burgey CS, Kozlowski MC, Tregay SW. J. Am. Chem. Soc. 1999;121:686–699. [Google Scholar]
- 42. Linghu X, Johnson JS. Unpublished results Our original publication showed −78 °C as the reaction temperature in the graphic but did not indicate that the reaction was warmed to 0 °C prior to the quench. (The experimental procedure was detailed in the Supporting Information accompanying that paper). Unbeknownst to us at the time, this method was very effective for giving the thermodynamic isomer. The kinetic aldolization was discovered subsequently.
- 43.McFarland CM, McIntosh MC. The Ireland–Claisen Rearrangement (1972–2004) In: Hiersemann M, Nubbemeyer U, editors. The Claisen Rearrangement: Methods and Applications. Weinheim: Wiley-VCH; 2007. pp. 117–210. [Google Scholar]
- 44.Schmitt DC, Johnson JS. Org. Lett. 2010;12:944–947. doi: 10.1021/ol9029353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dekker J, Budzelaar PHM, Boersma J, Van der Kerk GJM, Spek AJ. Organometallics. 1984;3:1403–1407. [Google Scholar]
- 46.Hurley ER, He X, Brown SN, Henderson KW. J. Am. Chem. Soc. 2009;131:6056–6057. doi: 10.1021/ja809716j. [DOI] [PubMed] [Google Scholar]
- 47.Greszler SN, Johnson JS. Angew. Chem. Int. Ed. 2009;48:3689–3691. doi: 10.1002/anie.200900215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lettan RB, Galliford CV, Woodward CC, Scheidt KA. J. Am. Chem. Soc. 2009;131:8805–8814. doi: 10.1021/ja808811u. [DOI] [PubMed] [Google Scholar]
- 49.Yao M, Lu CD. Org. Lett. 2011;13:2782–2785. doi: 10.1021/ol201029b. [DOI] [PubMed] [Google Scholar]
- 50.Kuwajima I, Kato M. J. Chem. Soc. Chem. Commun. 1979:708–709. [Google Scholar]
- 51.Reich HJ, Olson RE, Clark MC. J. Am. Chem. Soc. 1980;102:1423–1424. [Google Scholar]
- 52.Kuwajima I, Kato M. Tetrahedron Lett. 1980;21:623–626. [Google Scholar]
- 53.Enda J, Matsutani T, Kuwajima I. Tetrahedron Lett. 1984;25:5307–5310. [Google Scholar]
- 54.Kato M, Mori A, Oshino H, Enda J, Kobayashi K, Kuwajima I. J. Am. Chem. Soc. 1984;106:1773–1778. [Google Scholar]
- 55.Enda J, Kuwajima I. J. Am. Chem. Soc. 1985;107:5495–5501. [Google Scholar]
- 56.Matsuoka R, Horiguchi Y, Kuwajima I. Tetrahedron Lett. 1987;28:1299–1302. [Google Scholar]
- 57.Boyce GR, Johnson JS. Angew. Chem. Int. Ed. 2010;49:8930–8933. doi: 10.1002/anie.201003470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luzzio FA. Tetrahedron. 2001;57:915–945. [Google Scholar]
- 59.Al-Rashid ZF, Johnson WL, Hsung RP, Wei Y, Yao P-Y, Liu R, Zhao K. J. Org. Chem. 2008;73:8780–8784. doi: 10.1021/jo8015067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boyce GR, Liu S, Johnson JS. Org. Lett. 2012;14:652–655. doi: 10.1021/ol2033527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plietker B. J. Org. Chem. 2004;69:8287–8296. doi: 10.1021/jo048822s. [DOI] [PubMed] [Google Scholar]
- 62.Linghu X, Satterfield AD, Johnson JS. J. Am. Chem. Soc. 2006;128:9302–9303. doi: 10.1021/ja062637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greszler SN, Johnson JS. Org. Lett. 2009;11:827–830. doi: 10.1021/ol802828d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kinnaird JWA, Ng PY, Kubota K, Wang X, Leighton JL. J. Am. Chem. Soc. 2002;124:7920–7921. doi: 10.1021/ja0264908. [DOI] [PubMed] [Google Scholar]
- 65.Steward KM, Johnson JS. Org. Lett. 2010;12:2864–2867. doi: 10.1021/ol100996w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greszler SN, Malinowski JT, Johnson JS. J. Am. Chem. Soc. 2010;132:17393–17395. doi: 10.1021/ja108848d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greszler SN, Malinowski JT, Johnson JS. Org. Lett. 2011;13:3206–3209. doi: 10.1021/ol2011192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.(a) Kohama T, Enokita R, Okazaki T, Miyaoka H, Torikata A, Inukai M, Kaneko I, Kagasaki T, Sakaida Y, Satoh A, Shirashi A. J. Antibiot. 1993;46:1503. doi: 10.7164/antibiotics.46.1503. [DOI] [PubMed] [Google Scholar]; (b) Kohama T, Nakamura T, Kinoshita T, Kaneko I, Shiraishi A. J. Antibiot. 1993;46:1512. doi: 10.7164/antibiotics.46.1512. [DOI] [PubMed] [Google Scholar]; (c) Matsuhashi H, Shimada K. Tetrahedron. 2002;58:5619. [Google Scholar]
- 69.For leustroducsin syntheses, see: Shimada K, Kaburagi Y, Fukuyama T. J. Am. Chem. Soc. 2003;125:4048. doi: 10.1021/ja0340679. Miyashita K, Tsunemi T, Hosokawa T, Ikejiri M, Imanishi T. Tetrahedron Lett. 2007;48:3829. Miyashita K, Tsunemi T, Hosokawa T, Ikejiri M, Imanishi T. J. Org. Chem. 2008;73:5360. doi: 10.1021/jo8005599. Moise J, Sonowane RP, Corsi C, Wendeborn SV, Arseniyadis S, Cossy J. Synlett. 2008:2617.
- 70.(a) Fushimi S, Nishikawa S, Shimazo A, Seto H. J. Antibiot. 1989;42:1019. doi: 10.7164/antibiotics.42.1019. [DOI] [PubMed] [Google Scholar]; (b) Fushimi S, Furihata K, Seto H. J. Antibiot. 1989;42:1026. doi: 10.7164/antibiotics.42.1026. [DOI] [PubMed] [Google Scholar]; (c) Ozasa T, Suzuki K, Sasamata M, Tanaka K, Koburi M, Kadota S, Nagai K, Saito T, Watanabe S, Iwanami MJ. Antibiot. 1989;42:1331. doi: 10.7164/antibiotics.42.1331. [DOI] [PubMed] [Google Scholar]; (d) Ozasa T, Tanaka K, Sasamata M, Kaniwa H, Shimizu M, Matsumoto H, Iwanami M. J. Antibiot. 1989;42:1339. doi: 10.7164/antibiotics.42.1339. [DOI] [PubMed] [Google Scholar]; (e) Shibata T, Kurihara S, Yoda K, Haruyama H. Tetrahedron. 1995;51:11999. [Google Scholar]; (f) Sekiyama Y, Palaniappan N, Reynolds KA, Osada H. Tetrahedron. 2003;59:7465. doi: 10.1074/jbc.M305082200. [DOI] [PubMed] [Google Scholar]; (g) Choudhuri SD, Ayers S, Soine WH, Reynolds KA. J. Antibiot. 2005;58:573. doi: 10.1038/ja.2005.78. [DOI] [PubMed] [Google Scholar]; (h) Mizuhara N, Usuki Y, Ogita M, Fujita K, Kuroda M, Doe M, Lio H, Tanaka T. J. Antibiot. 2007;60:762. doi: 10.1038/ja.2007.101. [DOI] [PubMed] [Google Scholar]
- 71.For phoslactomycin syntheses, see: Wang Y-G, Takeyama T, Kobayashi Y. Angew. Chem. Int. Ed. 2006;45:3320. doi: 10.1002/anie.200600458. Nonaka H, Maeda N, Kobayashi Y. Tetrahedron Lett. 2007;48:5601. Shibahara S, Fujina M, Tashiro Y, Takahashi K, Ishihara J, Hatakeyama S. Org. Lett. 2008;10:2139. doi: 10.1021/ol8004672. Druais V, Hall MJ, Corsi C, Wendeborn SV, Meyer C, Cossy J. Org. Lett. 2009;11:935. doi: 10.1021/ol8029142. Konig CM, Gebhardt B, Schleth C, Dauber M, Koert U. Org. Lett. 2009;11:2728. doi: 10.1021/ol900757k. Shibahara S, Fujino M, Tashiro Y, Nanako O, Esumi T, Takahashi K, Ishihara J, Hatakeyama S. Synthesis. 2009:2935. Gebhardt B, König CM, Schleth C, Dauber M, Koert U. Chem. Eur. J. 2010;16:5934–5941. doi: 10.1002/chem.201000104.
- 72.Evans DA, Ennis MD, Le T, Mandel N, Mandel G. J. Am. Chem. Soc. 1984;106:1154. [Google Scholar]
- 73.(a) Tanabe Y, Hamasaki R, Funakoshi S. Chem. Commun. 2001:1674. [PubMed] [Google Scholar]; (b) Iida A, Nakazawa S, Okabayshi T, Horii A, Misaki T, Tanabe Y. Org. Lett. 2006;8:5215. doi: 10.1021/ol0619361. [DOI] [PubMed] [Google Scholar]; (c) Mermerian A, Fu GC. J. Am. Chem. Soc. 2005;127:5604. doi: 10.1021/ja043832w. [DOI] [PubMed] [Google Scholar]
- 74.For reactions of β-lactones with unsubstituted enolates, see: Shen X, Wasmuth AS, Zhao J, Zhu C, Nelson SG. J. Am Chem. Soc. 2006;128:7438. doi: 10.1021/ja061938g. Green ME, Rech JC, Floreancig PE. Angew. Chem. Int. Ed. 2008;47:7317. doi: 10.1002/anie.200802548. Shimizu M, Ishii K, Fujisawa T. Chem. Lett. 1997:765. (d) For a review of reactivity, see: Wang Y, Tennyson RL, Romo D. Heterocycles. 2004;64:605.
- 75.(a) Iida A, Takai K, Okabayashi T, Misaki T, Tanabe Y. Chem. Commun. 2005:3171. doi: 10.1039/b504750a. [DOI] [PubMed] [Google Scholar]; (b) Hauser CR, Renfrow WB. J. Am. Chem. Soc. 1937;59:1823. [Google Scholar]; (c) Yatluk YG, Chernyak SV, Suvorov AL, Khrustaleva EA, Abramova VI. Russ. J. Gen. Chem. 2001;71:965. [Google Scholar]
- 76.Nelson SG, Peelen TJ, Wan Z. J. Am. Chem. Soc. 1999;121:9742–9743. [Google Scholar]
- 77.Chen K-M, Gunderson KG, Hardtmann GE, Prasad K, Repic O, Shapiro MJ. Chem. Lett. 1987:1923–1926. [Google Scholar]
- 78.Swamy KCK, Kumar NNB, Balaraman E, Kumar KVPP. Chem. Rev. 2009;109:2551–2651. doi: 10.1021/cr800278z. [DOI] [PubMed] [Google Scholar]
- 79.Schmitt DC, Lam L, Johnson JS. Org. Lett. 2011;13:5136–5139. doi: 10.1021/ol202002r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shiozawa H, Takahashi M, Takatsu T, Kinoshita T, Tanzawa K, Hosoya T, Furuya K, Takahashi S, Furihata K, Seto H. J. Antibiot. 1995;48:357–362. doi: 10.7164/antibiotics.48.357. [DOI] [PubMed] [Google Scholar]
- 81.Synthetic studies: Morokuma K, Taira Y, Uehara Y, Shibahara S, Takahashi K, Ishihara J, Hatakeyama S. Tetrahedron Lett. 2008;49:6043–6045. Calo F, Richardson J, White AJP, Barrett AGM. Tetrahedron Lett. 2009;50:1566–1567. Zammit SC, White JM, Rizzacasa MA. Org. Biomol. Chem. 2005;3:2073–2207. doi: 10.1039/b504258e. Zammit SC, Ferro V, Hammond E, Rizzacasa MA. Org. Biomol. Chem. 2007;5:2826–2834. doi: 10.1039/b708594j. Hirai K, Ooi H, Esumi T, Iwabuchi Y, Hatakeyama S. Org. Lett. 2003;5:857–859. doi: 10.1021/ol0275264.
- 82.Evans DA, Ripin DHB, Halstead DP, Campos KR. J. Am. Chem. Soc. 1999;121:6816–6826. [Google Scholar]
- 83.Hosomi A, Shoji H, Sakurai H. Chem. Lett. 1985;14:1049–1052. [Google Scholar]
- 84.Burkhard JA, Tchitchanov BH, Carreira EM. Angew. Chem. Int. Ed. 2011;50:5379–5382. doi: 10.1002/anie.201100260. [DOI] [PubMed] [Google Scholar]
- 85.Noji M, Sunahara H, Tsuchiya Ki, Mukai T, Komasaka A, Ishii K. Synthesis. 2008:3835–3845. [Google Scholar]
- 86.Nicewicz DA, Satterfield AD, Schmitt DC, Johnson JS. J. Am. Chem. Soc. 2008;130:17281–17283. doi: 10.1021/ja808347q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dawson MJ, Farthing JE, Marshall PS, Middleton RF, Oneill MJ, Shuttleworth A, Stylli C, Tait RM, Taylor PM, Wildman HG, Buss AD, Langley D, Hayes MV. J. Antibiot. 1992;45:639–647. doi: 10.7164/antibiotics.45.639. [DOI] [PubMed] [Google Scholar]
- 88.Bergstrom JD, Kurtz MM, Rew DJ, Amend AM, Karkas JD, Bostedor RG, Bansal VS, Dufresne C, VanMiddlesworth FL, Hensens OD. Proc. Nat. Acad. Sci. 1993;90:80–84. doi: 10.1073/pnas.90.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.(a) Carreira EM, Du Bois J. J. Am. Chem. Soc. 1994;116:10825–6. [Google Scholar]; (b) Carreira EM, Du Bois J. J. Am. Chem. Soc. 1995;117:8106–25. [Google Scholar]; (c) Nicolaou KC, Yue EW, Naniwa Y, De Riccardis F, Nadin A, Leresche JE, La Greca S, Yang Z. Angew. Chem. Int. Ed. Engl. 1994;33:2184–2187. [Google Scholar]; (d) Nicolaou KC, Nadin A, Leresche JE, La Greca S, Tsuri T, Yue EW, Yang Z. Angew. Chem. Int. Ed. Engl. 1994;33:2187–2190. [Google Scholar]; (e) Nicolaou KC, Nadin A, Leresche JE, Yue EW, La Greca S. Angew. Chem. Int. Ed. Engl. 1994;33:2190–2191. [Google Scholar]; (f) Nicolaou KC, Yue EW, La Greca S, Nadin A, Yang Z, Leresche JE, Tsuri T, Naniwa Y, De Riccardis F. Chem. Eur. J. 1995;1:467–494. [Google Scholar]; (g) Evans DA, Barrow JC, Leighton JL, Robichaud AJ, Sefkow M. J. Am. Chem. Soc. 1994;116:12111–12112. [Google Scholar]; (h) Stoermer D, Caron S, Heathcock CH. J. Org. Chem. 1996;61:9115–9125. [Google Scholar]; (i) Caron S, Stoermer D, Mapp AK, Heathcock CH. J. Org. Chem. 1996;61:9126–9134. [Google Scholar]; (j) Sato H, Nakamura S, Watanabe N, Hashimoto S. Synlett. 1997:451–454. [Google Scholar]; (k) Nakamura S, Sato H, Hirata Y, Watanabe N, Hashimoto S. Tetrahedron. 2005;61:11078–11106. [Google Scholar]; (l) Armstrong A, Jones LH, Barsanti PA. Tetrahedron Lett. 1998;39:3337–3340. [Google Scholar]; (m) Armstrong A, Barsanti PA, Jones LH, Ahmed G. J. Org. Chem. 2000;65:7020–7032. doi: 10.1021/jo000700m. [DOI] [PubMed] [Google Scholar]; (n) Tomooka K, Kikuchi M, Igawa K, Suzuki M, Keong PH, Nakai T. Angew. Chem. Int. Ed. 2000;39:4502–4505. doi: 10.1002/1521-3773(20001215)39:24<4502::aid-anie4502>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]; (o) Freeman-Cook KD, Halcomb RL. J. Org. Chem. 2000;65:6153–6159. doi: 10.1021/jo000665j. [DOI] [PubMed] [Google Scholar]; (p) Nakamura S, Hirata Y, Kurosaki T, Anada M, Kataoka O, Kitagaki S, Hashimoto S. Angew. Chem. Int. Ed. 2003;42:5351–5355. doi: 10.1002/anie.200352629. [DOI] [PubMed] [Google Scholar]; (q) Hirata Y, Nakamura S, Watanabe N, Kataoka O, Kurosaki T, Anada M, Kitagaki S, Shiro M, Hashimoto S. Chem. Eur. J. 2006;12:8898–8925. doi: 10.1002/chem.200601212. [DOI] [PubMed] [Google Scholar]; (r) Bunte JO, Cuzzupe AN, Daly AM, Rizzacasa MA. Angew. Chem. Int. Ed. 2006;45:6376–6380. doi: 10.1002/anie.200602507. [DOI] [PubMed] [Google Scholar]; (s) Wang YZ, Metz P. Chem. Eur. J. 2011;17:3335–3337. doi: 10.1002/chem.201003399. [DOI] [PubMed] [Google Scholar]
- 90.Reviews: Nadin A, Nicolaou KC. Angew. Chem. Int. Ed. Engl. 1996;35:1622–1656. Jotterand N, Vogel P. Curr. Org. Chem. 2001;5:637–661. Armstrong A, Blench TJ. Tetrahedron. 2002;58:9321–9349.
- 91.Hendrickson JB. Top. Curr. Chem. 1976;62:49–172. doi: 10.1007/BFb0046047. [DOI] [PubMed] [Google Scholar]
- 92.Hendrickson JB. J. Am. Chem. Soc. 1977;99:5439–5450. [Google Scholar]
- 93.Burns N, Baran P, Hoffmann R. Angew. Chem. Int. Ed. 2009;48:2854–2867. doi: 10.1002/anie.200806086. [DOI] [PubMed] [Google Scholar]
- 94.This rather small compound contains three tert-butyl esters and two TBS groups; it is remarkable that it is crystalline. We could not have easily established the relative stereochemistry in the absence of an X-ray structure.
- 95.Radosevich AT, Musich C, Toste FD. J. Am. Chem. Soc. 2005;127:1090–1091. doi: 10.1021/ja0433424. [DOI] [PubMed] [Google Scholar]
- 96.White JD, Cutshall NS, Kim TS, Shin H. J. Am. Chem. Soc. 1995;117:9780–9781. [Google Scholar]
- 97.Short order ≈1.5 years. Compound 136 differentiates the C6 and C7 alcohols in a way that is perfectly set up to complete the synthesis insofar as zaragozic acid C has an acyl group on the C6 alcohol. The fact that C7 was protected looked very promising for direct conversion to the natural product. Unfortunately the C6 acyl side chain contains an alkene in the side chain and all efforts to deprotect the C7 benzyl ether while retaining the C=C p bond were unsuccessful. We were fortunate that Carreira and Du Bois had worked out the differentiation of a C6/C7 diol this was ultimately the method that allowed us to complete the synthesis. An even further streamlined synthesis might be feasible with a somewhat different C7 protecting group but this strategy was not extensively pursued.