Abstract
Advancing age and adiposity contribute to musculoskeletal degenerative diseases and the development of sarcopenic obesity. The etiology of muscle loss is multifactorial, and includes inflammation, oxidative stress and hormonal changes, and is worsened by activity avoidance due to fear of pain. The risk for mobility disability and functional impairment rises with severity of obesity in the older adult. Performance measures of walking distance, walking speed, chair rise, stair climb, body transfers and ability to navigate obstacles on a course are adversely affected in this population, and this reflects decline in daily physical functioning. Exercise training is an ideal intervention to counteract the effects of aging and obesity. The 18 randomized controlled trials of exercise studies with or without diet components reviewed here indicate that 3–18 month programs that included aerobic and strengthening exercise (2–3 days per week) with caloric restriction (typically 750 kcal deficit/day), induced the greatest change in functional performance measures compared with exercise or diet alone. Importantly, resistance exercise attenuates muscle mass loss with the interventions. These interventions can also combat factors that invoke sarcopenia, including inflammation, oxidative stress and insulin resistance. Therefore, regular multimodal exercise coupled with diet appears to be very effective for counteracting sarocpenic obesity and improving mobility and function in the older, obese adult.
Keywords: Obesity, Aging, Physical function, Mobility, Strength
1. Introduction
The confluence of aging and obesity may create an ideal environment for skeletal muscle catabolism and decline in physical function. Advancing age and obesity contribute to the development of sarcopenic obesity. Sarcopenic obesity currently affects 16% of women and 17.5% of men over the age of 80 years (Bales and Ritchie, 2002). This condition is defined by appendicular skeletal muscle mass index of >2 standard deviations compared to a young referent group 20–30 years of age, or a body fat percentage >60th percentile for the same gender and age (Cederholm et al., 2011). A recent statement from the International Working Groups on Sarcopenia indicates that populations who are targets include those who have difficulty rising from a chair unassisted and have gait speeds <1.0 m/s (Fielding et al., 2011).
Numerous changes have been made in the health care of obese individuals over the last four decades, enabling the individual to live longer; however, longevity comes at a price, as the individual copes with longer exposure to carrying excessive weight (Alley and Chang, 2007). As a consequence, the relationship between obesity and musculoskeletal-related functional disability is strengthened (Alley and Chang, 2007). Projections indicate that as younger obese individuals age, the prevalence of physical disability will escalate beginning in the year 2012 (Manton, 2007). Sarcopenic obesity is characterized by inadequate muscle strength, mobility disability and difficulty with weight-bearing activities (Broadwin et al., 2001). Routine activities of daily living such as walking, climbing steps, traveling or maneuvering within public spaces are physically difficult or impossible for the severely obese individual; these limitations also restrict or prevent participation in recreational activities or exercise programs (Pain and Wiles, 2006).
The seriousness of this issue lies in the fact that obesity-related physical limitations are even evident in young obese children, where lower body transfers, jumps and chair rises are already compromised (Riddiford-Harland et al., 2006). From the global perspective, strategies that target the musculoskeletal system need to be developed to prevent or reduce the magnitude of physical impairment in the growing older demographic with excessive weight (Kelly et al., 2008). In our opinion, exercise may be an ideal intervention to counteract some of the aging effects in skeletal muscle and the unfavorable shift in body composition in the older obese adult. This review presents the available high-quality evidence of various exercise modalities on functional ability and mobility. A brief summary of the potential mechanisms underlying the onset of sarcopenia in the older, obese adult is presented along with future directions for further research.
2. Adiposity, muscle strength, phenotype, and aging
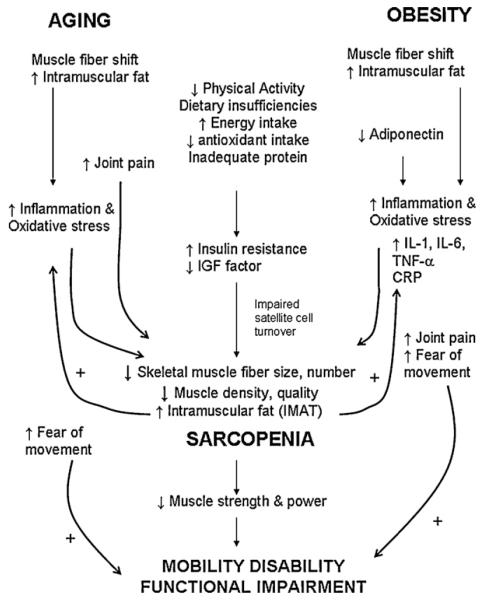
A hypothetical model of the major influential factors on muscle deficiency and sarcopenia in the older, obese adult and potential pathways that lead to functional impairment and mobility disability are shown in Fig. 1. Given that both obesity and aging can independently contribute to muscle inadequacy and deterioration of mobility, the combination of the two likely exacerbates negative physiological changes in the musculoskeletal system and shapes an environment ideal for sarcopenia and loss of independence. The main physiological mechanisms that underlie skeletal muscle decline in aging and obesity include changes in body composition, muscle fiber type shifting, hormonal level decline, inflammation, psychosocial aspects, development of joint pain and sedentary behavior.
Fig. 1.
A proposed model of the contributing major factors to muscle mass insufficiency and sarcopenia in obese, older adults (AOX, antioxidant; IGF, insulin like growth factor; IMAT, intramuscular adipose tissue; IL-1, interleukin 1; IL-6, interleukin 6; TNF-α, tumor necrosis factor α; CRP, C reactive protein). + denotes a positive effect on the downstream variable.
There is a relative decline in muscle mass and strength (Baumgartner et al., 2004) concurrent with elevations in fat mass (Schultz et al., 2002). By the age of 80 years, the older adult has lost 40% of their muscle mass compared to when they were 20 years of age, while fat mass increases differentially depending on the characteristics of the individual (Baumgartner et al., 1995; Gallagher et al., 1997). In addition, the number and size of skeletal muscle fibers (with preferential loss to the type II fibers) decreases with aging. In the obese, older adult, a lower proportion of type II fibers has been documented. Skeletal muscle protein synthesis decreases concurrent with muscle mitochondrial function (Marcell, 2003). Hence, aging is related with an overall reduction in muscle quality. Obesity may even exacerbate muscle fiber loss over the lifespan by reducing the muscle satellite cell DNA content and cell turnover (Peterson et al., 2008), potentially reducing the muscle’s ability to increase in size and keep a proportional tissue volume in light of increasing overall body volume. The importance of the fiber type alterations observed in aging and obesity is that muscle power and strength decline with this shift. A loss in muscle strength and power can directly lead to functional limitations and mobility disability (Puthoff and Nielsen, 2007).
Excessive adiposity is also disruptive to muscle architecture. Fat infiltrates muscle tissue to create a “marbling” effect (Zamboni et al., 2007). Histochemical data show elevations in triglyceride content in obese individuals, particularly in slower twitch fibers, compared to non-obese counterparts (Malenfant et al., 2001). Fat accumulation within muscle is also higher in elderly than young persons at any given BMI value (Beaufrere and Morio, 2000). Additionally, fat deposition is redistributed during aging, with fat accrual occurring more readily in the intra-abdominal (visceral) area compared to subcutaneous depot. Importantly, this redistribution of fat mass and relative decline in muscle mass can occur in even relatively weight-stable individuals (Newman et al., 2005).
The low proportion of muscle mass relative to the high proportion of fat mass can create frailty in the obese adult (Jarosz and Bellar, 2009). Frailty was originally characterized by Fried et al. (2001) as a condition that consists of at least three of five phenotypic criteria: unintentional weight loss, exhaustion, low physical activity, slowness, and weakness. Frailty has also been proposed more recently as a medical syndrome (Bandeen-Roche et al., 2006). Because sarcopenic obesity sufficiently fulfills the criteria of frailty, sarcopenic obesity is now being recognized as a syndrome of obese frailty (Roubenoff, 2004). A recent data analysis of 3055 community-dwelling men and women aged >65 years who participated in the English Longitudinal Study of Ageing (Hubbard et al., 2009) has shown that frailty or frailty index was related to BMI in a U-shaped manner (i.e., higher frailty in people with extremes of low or high BMI). However, in people with large WC (≥88 cm in women and ≥102 cm in men), frailty exists in all BMI categories.
3. Hormonal influences on muscle
In general, men 65 years and older and women over 55 years of age, there is a withdrawal of growth hormone production and anabolic sex hormones from the circulation (“andropause” in men and menopause in women) (Seidman, 2007). Importantly, obesity exacerbates the drop in anabolic hormone production (Isidori et al., 2000), and testosterone levels are even lower in sarcopenic obese adults compared to their older obese counterparts. The growth hormone-insulin like growth factor (IGF-1) axis declines with aging and with obesity (Waters et al., 2008). This is problematic for skeletal muscle, as both GH and IGF-1 preserve muscle protein synthesis rates and prevent muscle degradation. Lower levels of anabolic hormones are directly related to low muscle strength (Stenholm et al., 2008).
Insulin resistance is a hallmark of obesity and can develop with advancing age (Zeyda and Stulnig, 2009). In normal healthy muscle, insulin is a potent stimulator of muscle anabolism; in aging and obesity, insulin levels significantly increase (Zeyda and Stulnig, 2009). Adipose infiltrate in skeletal muscle impairs the efficacy of insulin and glucagon while pancreatic adipose deposition causes decreased insulin secretion. Accumulation of visceral adipose tissue is directly toxic to the surrounding cells in organ tissues such as skeletal muscle and pancreas (Jarosz and Bellar, 2009). Also, the accumulation of fatty acids within muscle cells in obesity and aging interferes with the phosphorylation pathway of the insulin receptor and subsequently inhibits the GLUT-4 translocation (Evans et al., 2010). Excessive insulin levels in the resistant state impair several cellular pathways relating to signaling of muscle anabolism, mitochondrial function and protein synthesis (Guillet and Boirie, 2005). The muscle fiber quality and contractile efficiency decreases (Evans et al., 2010). In addition, insulin resistance is related to many clinical features such as skeletal muscle weakness and lower extremity mobility disability (Abbatecola and Paolisso, 2008).
4. Inflammation, oxidative stress and muscle strength
Sedentary behavior that occurs with obesity and older age leads to skeletal muscle mitochondrial dysfunction and fat accumulation between tissues and within muscle itself (Malenfant et al., 2001; Stenholm et al., 2008). High adipose tissue volumes in the viscera and the intramuscular and perimuscular spaces are insidious in nature as they produce high levels of inflammatory proteins and cytokines such as C-reactive protein, tumor necrosis factor-α (TNF-α) interleukin-6 and interleukin 1β among others (Cesari et al., 2005). Adiposity creates a chronic, smoldering inflammatory environment that relates with muscle atrophy and the progression of sarcopenia (Schaap et al., 2006). TNF-α has a direct catabolic effect on muscle by impairing protein synthesis (Lambert et al., 2008), and rodent studies show that TNF-α induces loss of myonuclei and satellite cells due to mitochondrial-mediated apoptosis or activation of the death receptor pathway (Thornell, 2011).
Also, high circulating IL-6 levels and low IGF-1 levels act synergistically to impair mobility (Cappola et al., 2003). These cytokines have autocrine and paracrine effects that induce a feed-forward process of muscle catabolism. IL-6 acts directly on skeletal muscle and inhibits the anabolic activities of IGF-1, thereby attenuating protein synthesis and promoting muscle degradation. IL-6 and IGF-1 are also epidemiologically important because they are associated with low muscle strength in obese, older adults (Schrager et al., 2007).
Adipose tissue itself generates a myriad of hormones and other bioactive proteins, including leptin (in normal concentrations induces satiety, regulates body composition) and adiponectin (anti-inflammatory, antiatherogenic) (Wozniak et al., 2008). Obesity induces a state of leptin insensitivity and reduces adiponectin levels. Additional hypotheses suggest that excessive leptin and tumor necrosis factor-α contribute indirectly to sarcopenia by causing insulin resistance and attenuation of growth hormone levels. Attenuation of adiponectin perpetuates chronic inflammation in muscle tissue. Of recent interest is the association of inflammatory biomarkers and the presence of intermuscular adipose tissue (IMAT). Both aging and obesity are physiological states in which infiltration of adipose tissue within skeletal muscle occurs. IMAT has been defined as the adipose tissue embedded under the fascia and between muscle groups; IL-6 levels are positively related with IMAT, and expression of inflammatory cytokines might locally control accrual of IMAT in specific muscles such as the erector spinae (Zoico et al., 2010). This is clinically significant as fat infiltration into muscle tissue is related with mobility limitations (Visser et al., 2002).
Evidence is mounting to suggest that a common mechanism that might link old age and obesity-related insulin resistance, inflammation and sarcopenia is oxidative stress (Vincent et al., 2007). Oxidative stress is the imbalance between the endogenous antioxidant defenses within tissues (enzymes, dietary vitamins and minerals, non-enzymatic antioxidants) and the overproduction of reactive oxygen species and free radicals. The net result of these processes is damage to contractile and mitochondrial enzymatic muscle proteins, lipids and DNA. Accumulation of these damaged mitochondrial and nuclear DNA molecules is believed to activate pro-apoptotic factors, fiber atrophy and myocyte loss (Meng and Yu, 2010). Advancing age (Combaret et al., 2009) and obesity (Vincent et al., 2007; Vincent and Taylor, 2006) have both independently been shown to create oxidative stress, but, together, exacerbate this unfavorable condition. Free radical activity has been implicated in the contractile dysfunction within skeletal muscle through impairment of the actomyosin interaction, thereby reducing muscle force (Cooke, 2007). Also, elevation of inflammatory cytokines such as TNFα can act at the skeletal muscle to increase cytosolic oxidant activity; these oxidants impair myofibrillar activity and reduce specific force values of the muscle (Reid and Moylan, 2011). Recently, it has been postulated that oxidative stress biomarkers might be useful predictors of mobility impairment in the elderly (Cesari et al., 2006).
5. Psychosocial aspects in functional decline
Physical restrictions may be due in part to psychosocial factors. For example, we recently found that obese individuals demonstrated higher levels of kinesiophobia (fear of movement due to pain) compared with non-obese individuals (Vincent et al., 2010a,b). Interestingly, kinesiophobia corresponds with significantly higher perceptions of disability with regard to performing activities such as jumping, climbing stairs, running, rising from a chair and other tasks of mobility. However, fear of movement was not related with direct functional tests of knee or back flexibility or strength in obese persons. Other studies indicate that obese and morbidly obese patients with knee osteoarthritis (Somers et al., 2008, 2009), pain catastrophizing levels are proportional to the severity of pain. This is particularly true when somatization and catastrophic thinking patterns are present. A focus on the pain and negative aspects of pain likely contribute to hypervigilance to pain sensations and physical inactivity (Roelofs et al., 2004). In some obese individuals, the anticipation of pain heightens anxiety and somatic arousal with exercise and other physical activities (Smits et al., 2010); this may foster preferential activity avoidance due to anticipation of pain. Therefore, physical activity plans should encompass components that positively address the fear of movement, negative anticipation of exercise and reducing somatic focus to ensure long-term success with exercise adherence.
6. Pain during exercise
A major contributing factor to obesity-related disability is musculoskeletal pain (Lamb et al., 2000). The most probable cause for pain in obese adults is development of, or flaring of, osteoarthritis-induced inflammation (Grotle et al., 2008). Pain is a psychosocial factor that may contribute to avoidance of physical activity and the development of muscle deficiency in the obese, older adult. Pain, especially in the low back and in the lower extremity contributes to mobility disability in obese individuals (Lamb et al., 2000; Weaver et al., 2009). Worse yet, multiple comorbidities that commonly exist with either obesity or old age may generate exercise-induced pain and discomfort, such as chronic obstructive pulmonary disease, vascular disease, fibromyalgia (Okifuji et al., 2010), gout and anxiety/depression. With regard to musculoskeletal issues, excessive weight and age are major, independent risk factors for joint degeneration and osteoarthritis. The underlying mechanisms of joint pain in obesity may be due to adiposity-related joint loading and aberrant biomechanics. Pain with physical activity initiates a downward spiral of avoidance of activity and muscle disuse; over time, sedentary behavior worsens muscle weakness and contributes to muscle wasting. Joint pain may be due to the mechanical stress of loading on weight-bearing joints, systemic or localized inflammation, and cartilaginous degradation. Pain triggers activity avoidance behaviors and fear of movement, and consequential sedentary behaviors lead to progressive weight gain. While evidence in the obese, older demographic is sparse, our laboratory showed that obese individuals have higher fear of movement than non-obese counterparts, and this was related with a higher self-reported disability level (Vincent et al., 2010a). Pain during activity can severely limit mobility and serves as a reliable indicator of physical function in elderly adults (Heuts et al., 2004).
Lower extremity pain, particularly in the knee and ankle, contributes to mobility disability in obese individuals (Lamb et al., 2000; Weaver et al., 2009). Obesity and lower body joint pain may accelerate the severity of mobility disability and worsen gait (Marks, 2007). The subclassifications of kinesiophobia, activity avoidance and the somatic focus of pain, both may perpetuate a vicious cycle in which avoidance of movement facilitates weight gain and worsening of joint pain. The alternative point-of-view is that weight loss reduces joint pain severity, and that pain attenuation is directly related to gains in physical function (Messier et al., 2004). The necessity of managing joint pain through weight loss has powerful implications for the maintenance of independence and functional mobility with aging.
7. Sedentary behavior
A sedentary life-style is a major risk for weight gain (LaMonte and Blair, 2006). Obese persons demonstrate patterns of less physical activity and this may perpetuate the decline in muscle strength (Duvigneaud et al., 2008). Muscle atrophy attenuates resting and exercise metabolic rates which can work in concert with sedentary behavior to promote weight gain (Stenholm et al., 2008). While sedentary behavior and lower levels of activity may coexist in obese adults (Martínez-González et al., 1999), the relationship between these factors in older obese adults is less studied. Existing cross-sectional data show that high body weight is related with low physical activity patterns (Chen and Guo, 2008; Kyle et al., 2004; Lebrun et al., 2006) and less participation in ambulatory activity such as walking (Swartz et al., 2007). One cross-sectional study found that among older men, low participation in leisure time physical activity and walking was associated with high rates of disability and obesity (Di Francesco et al., 2005). In this group, appendicular skeletal muscle mass was also directly related with participation in high-intensity exercise (e.g., brisk walking, gardening). The highest self-reported disability was found in sedentary participants who had BMI values exceeding 25 kg/m2 (Di Francesco et al., 2005). These findings were similar to others reporting that the more time engaged in leisure time physical activity rather than being sedentary was related to appendicular muscle mass (Raguso et al., 2006), and that physical activity was a key determinant of muscle strength in elderly obese women (Rolland et al., 2004).
Reductions in skeletal muscle cross-sectional area that occur with disuse can primarily be attributed to a decrease in protein synthesis; lower protein synthesis rates occur with decreased loading, and mRNA translation (an essential step in protein synthesis) is slowed (Urso, 2009). In clinical populations, the relationships between muscle disuse, sarcopenia and functional impairment are complicated by the use of comorbid medications for conditions such as hypertension. For example, recent evidence shows that losartan (antihypertensive) can activate the transforming growth factor-β signaling cascade and improve muscle remodeling processes and protect against disuse atrophy (Burks et al., 2011). An alternative view is that muscle disuse and sedentary behavior are also the endpoints secondary to pain symptoms and fear avoidance in the older, obese adult.
8. Obesity and exercise impairment in the older adult
Skeletal muscle loss and fat accrual, together, relate to reductions in physical function (Broadwin et al., 2001). Recent data demonstrate that muscle fat infiltration is associated with reduced strength (Visser et al., 2002) and higher incidence of disability (Visser et al., 2005). There is a strong likelihood that the additive effects of skeletal muscle decline and fat tissue accumulation (Zamboni et al., 2007) facilitate the cycle of weight gain, systemic inflammation and loss of skeletal muscle mass. With increases in body weight, the compressive forces increase within the load-bearing joints, contributing to incapacitating pain. The collective influence of pain and worsening inflammation increase the risk for physical weakness and functional impairment in the obese, older adult. There is higher mobility disability in women than men with advancing age. Presently, the mechanisms to explain this finding are not known, but could be related to sarcopenia, especially in severe obesity (Davison et al., 2002), higher perceived levels of functional disability in women than men (Merrill et al., 1997), and lower levels of physical activity across the lifespan in women than men (U.S. Department, 1996). Others have proposed that this finding may also be due in part to a “survivor effect” (Davison et al., 2002); typically, women live longer, and the aging male populations who were participants in these studies could have been self-selected as those with better health compared to the general U.S. population. When separated from other characteristics such as gender, ethnicity and race, obesity confers major adverse effects on the musculoskeletal system in the older adult. Among several treatment options for the obese older adult with functional limitations, exercise is a foundational treatment. Given that decreased muscle mass and/or excessive fat mass are related with diminished strength and exercise capacity (Thomas, 2007) and functional disability (Lebrun et al., 2006; Ramsay et al., 2006; Sternfeld et al., 2002; Visser et al., 1998a,b), exercise is ideal is address these issues.
9. Exercise interventions in the obese, older adult
Obesity interventions for the older adult have been controversial in the past due to the apparent medical risks that weight loss can pose with aging. In 2005, the American Society for Nutrition and the NAASO, The Obesity Society stated their position on the issue, recommending that obese older persons engage in weight-loss regimens that will minimize the loss of lean body mass if they are afflicted by functional limitations or medical complications as a result of their obesity (Villareal et al., 2005). While there are a significant number of exercise-related studies in the literature on the obese aging population, this review will focus on the randomized controlled trials (RCT) of exercise interventions in this group. All studies included some assessment of objective functional outcomes that may have reflected a positive influence on sarcopenia and/or frailty and improvement in functional independence. The sample studied had to include participants ≥60 years, and inclusion of outcomes relating to changes in body composition and physical function, particularly with maximal strength and mobility activities such as walking endurance, stair climb and chair rise, among others. Available studies have been grouped into those that examined (1) aerobic exercise and resistance exercise programs compared with control groups, (2) multimodal exercise programs compared with control groups, and (3) multimodal exercise with or without dietary change or caloric restriction.
9.1. Aerobic and resistance exercise programs vs. control groups
Several RCTs were identified that included resistance exercise (RX) and/or aerobic exercise (AX) (Table 1). RX encompasses resistance exercise machines, strengthening exercise with body weight or weights added to the body and home-based strengthening exercise. AX involves rhythmic, large muscle activity such as brisk walking, stationary cycling, stair stepping or aquatic exercise. Two studies were completed in the U.S. (Avila et al., 2010; Vincent et al., 2006), three in Canada (Bouchard et al., 2009, 2010; Davison et al., 2002), and one in Korea (Lim et al., 2010). Intervention periods ranged from 2 to 6 months in duration and were comprised of sample sizes of 27–75 participants.
Table 1.
Aerobic and resistance exercise interventions to increase physical function in the obese, older adult.
| Author | Population | N | Exercise program | Outcomes |
|---|---|---|---|---|
| Avila et al. (2010) | 67 ± 4 years 60% women; American |
27 | 2.5 months; two group design 1. RX Exercise (Cybex machines four sets of 8–12 reps on each machine 2. RX + Exercise + Diet group followed the DASH diet guidelines |
The RX + DASH diet group lost more weight (4 kg vs. 1.5 kg), increased mid thigh cross-sectional area more than the DASH diet group (p < 0.05); Muscle quality was higher, 400-m walk time was faster (240 vs. 260 s) in the RX + DASH diet, but both groups improved time to perform 5 chair stands (p < 0.05) |
| Bouchard et al. (2009) | 55–75 years women Canadian |
48 | 3 months; four group design 1. Diet (nutritionist directed program to reduce body weight 0.5–1 kg/week 2. RX Exercise (3× week, nine exercises, 3 sets of 8 repetitions each machine; supervised by kinesiologist) + Diet 3. Diet alone 4. Control (no intervention group) |
All intervention groups ↑ one legged squat strength by 56–105%, the 30 s chair stand time ↑ in the RX exercise group from 12.4 to 16.0 s, and time ↓ with walking backward (6.1–5.4 s; both p < 0.05). BMI ↓ in all intervention groups, and fat mass ↓ in the Diet and Exercise + Diet groups. Lean mass was maintained in the Exercise + Diet and Exercise groups |
| Bouchard et al. (2010) | 55–75 years women Canadian |
36 | 3 months; three group design 1. Diet (nutritionist directed program to reduce body weight 0.5–1 kg/week 2. RX Exercise (3× week, nine exercises, 3 sets of 8 repetitions each machine; supervised by kinesiologist) + Diet 3. Control (no intervention group) |
Global physical activitya improved across all activities except for lifting a 10 lb object and fastest walking speed, independent of intervention approach; the self-reported assessment of functional improvement did not correspond to the actual objective measures of physical improvement at month 3 |
| Davidson et al. (2009) | 60–80 years 58% women Canadian |
6 months; four group design 1. AX (5× week; 30 min walking at 60–75% VO2) 2. RX (20 min; 1 set of 9 exercises 3× week) 3. AX + RX (3× week; 50 min) 4. Control (no intervention) |
The AX and Combined exercise groups ↑ walking time to exhaustion at month 6; the chair stands and arm curls performed in 30 s, step test (steps completed in 2 min) and timed-up-and-go and 8 foot up and go test times improved in all three intervention groups, with the Combined exercise group showing greatest change (p < 0.05); total fat loss was highest in the AX and Combined exercise groups, and the skeletal muscle mass was ↑ in the RX and Combined exercise groups (p < 0.05) |
|
| Lim et al. (2010) | 65.9 7.9 yrs 87% women with knee OA; Korean |
75 | 2 months; three group design Aquatic exercise (40 min/3× week; 65% max HR) Conditioning exercise (40 min/3× week; 60% 1 RM for extensors Control (home-based exercise for legs) |
BMI changed minimally in both exercise groups body fat ↓ 1.1% in the aquatic group (p < 0.05); peak knee torque ↑ in all groups by 1.5–10.3%; WOMAC scores ↓ by 13.8 and 9.9 points in the aquatic and conditioning groups compared to 2.7 in the control (p < 0.05). |
| Vincent et al. (2006) | 60–72 years American; Stratified by BMI (< or ≥30 kg/m2) |
49 | 6 months; four group design Obese and non-obese RX vs. control; MedX machines (RX) 3 days/week, 12 machines, 1 set of 8–13 reps at 50–80% maximal lift; Control groups did not exercise |
The obese RX group lost 2% excess fat walk time to exhaustion increased in RX trained obese participants than obese CON group (1.7 increase vs. 1.5 min decrease); overall body strength increased by 15% in the obese RX and non-obese RX groups (p < 0.05) |
Ten different physical activities including the 6-min walk, climbing one set of stairs, 30 s-chair stands, lifting a 10 lb object, walking speed at normal and fastest pace, one-legged stand, putting on a jacket, picking up a penny from the floor, backwards walking.
Four studies examined the effects of RX protocols. Vincent et al. (2006) tested whether 6 months of RX training using weight machines three times a week at moderate to high intensity work-loads (16–18 on the Borg Rating of Perceived Exertion Scale [RPE]) would differentially improve body strength and walking time to exhaustion compared to a non-exercise control period in obese and non-obese adults. The obese RX training group lost fat mass and demonstrated a 15% increase in walking time and an 18% increase in overall strength compared to no significant changes in the control group. Bouchard et al. (2009, 2010) published two studies from a population of Canadian women. In the first study, participants were randomized to one of four groups for a 3-month intervention: diet, diet and RX, RX alone or a control group. Those in the RX groups performed supervised sessions of leg press, chest press, leg extension, shoulder press, sit-ups, seated rows, triceps extensions, arm curls, calf press (3 sets of 8 repetitions each). The dietary component included nutritionist-supervised food selection to match the American Heart Association recommendations for healthy diets. Objective assessment of physical function consisted of a comprehensive Global Physical Activity battery of ten different tasks (see Table 1 legend for the tasks) at baseline and month 3. Consistent improvements were made in number of chair rises made in 30 s, velocity of walking backward, and one-legged squat strength by the end of the study (Bouchard et al., 2009). In the second study by Bouchard et al. (2010), another analysis was performed on the groups that completed Diet and RX + Diet interventions and control. This correlation analysis demonstrated that the magnitude of the objective changes were consistently higher than those reported by participants with subjective assessment of physical function (Short Form 36 Physical Function subscore; r = 0.32, p = 0.07). A possibility is that these women were either already relatively high functioning before the study, or had adapted to the environment based on obesity limitations making the perceived improvements less sensitive to RX and RX + Diet intervention. Avila et al. (2010) compared the effects of 10 weeks of moderate RX + Diet on muscle quality, thigh tissue composition and various strength, power and physical function assessments. The dietary component consisted of 30-min dietician-led dietary education sessions on the Dietary Approaches to Stop Hypertension (DASH) on a weekly basis. The RX + Diet group demonstrated increases in leg press muscle strength compared with baseline (453 ± 36 to 513 39 N) and in muscle quality (1.97 ± 0.09 to 2.19 ± 0.13 N/cm2). Both the RX + Diet and Diet groups demonstrated improvements in the time to complete five chair stands (16% and 11.4%, respectively) and time to complete a 400-m walk (12% and 15% improvements, respectively). Compared with the Diet group, the RX + Diet group had significant reductions in body fat mass, thigh adipose tissue, intramuscular adipose tissue, subcutaneous adipose tissue and low density muscle areas, and increases in overall lean mass, total muscle area and normal density muscle (all p < 0.05).
A 6-month investigation was conducted to determine the independent and combined effects of RX and AX on functional limitations in abdominally obese older men and women (Davidson et al., 2009). Participants were randomized to AX (150 min weekly accumulated time), RX (60 min weekly accumulated time), AX + RX (150 min weekly accumulated time) or a control group. Compared with baseline, the number of chair stands completed in 30 s increased in all intervention groups, with greater changes in the RX and AX + RX groups than AX (changes of 4.3 and 5.89 vs. 4.0 rises). More steps were achieved during the 2-min step test in the AX + RX compared with the RX and AX groups (changes of 26.88 vs. 19.58 and 17.00 steps). Improvements were made in the 8-ft up-and-go test in all groups relative to the control group (−0.45 to −0.60 s vs. −0.05 s; p < 0.05). The greatest loss in fat mass occurred in the AX + RX group, and gain in skeletal muscle occurred in the RX and AX + RX groups at month 6 (p < 0.05). Hence, functional improvements are enhanced with the addition of RX to exercise programs.
Aquatic exercise (AQE) was used as an intervention to promote weight loss and increase physical function in women with knee osteoarthritis. The efficacy of 2 months of conditioning exercise (supervised; 40 min sessions of joint mobilization, strengthening exercise, range of motion, isometric leg press and leg extensions, bicycling) was compared to 2 months of AQE (40 min sessions at 65% maximal heart rate for underwater cycling, walking, running with weight cuffs) on knee strength, torque and subjective knee function scores (WOMAC scores). The control group performed home-based exercises for the legs. The WOMAC score decreased from 35.1 ± 11.3 points to 20.9 ± 9.9 points and from 33.6 ± 12.6 to 23.6 ± 12.8 points in the AQE and conditioning exercise at month 2 (p < 0.05). A 33% reduction in pain interference occurred in the AQE group compared to the remaining two groups (p < 0.05). These positive changes in function and pain were accompanied by 1.1% and 1.6% reductions in body fat and body weight, respectively in the AQE group (p < 0.05).
These data were supported by a non-randomized, small, Australian study (Henwood and Taaffe, 2005) in which adults 60–80 years of age were placed into an RX group (three sets of 8 repetitions at 65% of maximal strength on Maxim® machines: bench press, seated row, shoulder press, leg press, leg extension, leg curl, and seated calf press) or a control, non-exercise group for 8 weeks. Physical function assessments were collected at baseline and week 8. After RX, muscle strength on all machines increased. Also, the scores for the floor rise to standing, 6-m walking speed and chair rise time improved by 10.4%, 6.6% and 10%, respectively, with no change in the control group (p < 0.05).
9.2. Multimodal exercise vs. control groups
Some studies have incorporated a variety of exercise stimuli that provide a combination of aerobic, resistive and flexibility components to the obese older population (Table 2). Available RCTs from the U.K. and U.S. have provided multimodal activity programs ranging from 3 to 12 months in duration, with sample sizes ranging from 26 to 424 participants (Grant et al., 2004; Manini et al., 2010; Schlenk et al., 2011). Dietary advice was provided to all participants by nurses in one study, which consisted of adapting cooking methods with the goals of decreasing fat intake and increasing intake of fruits and vegetables (Grant et al., 2004).
Table 2.
Multimodal exercise compared with control groups.
| Author | Population | N | Exercise program | Outcomes |
|---|---|---|---|---|
| Grant et al. (2004) | 63 ± 4 years women; English |
26 | 3 months; 2× week (40 min) 1. Exercise (aerobic dance, flexibility and targeted strengthening muscle groups; dietary advice was provided by nurse 2. Control group had no intervention |
Compared with controls, the exercisers lost 1.7 kg (p < 0.05), but no fat mass at month 3; Chair rise, timed-up-and-go and 20 m walk times ↑ by 10%, 16%, and 10% after training; stair ascent and descent times by 9% and 16%, respectively (trend not significant) |
| Manini et al. (2010) | 70–89 years 69% women American LIFE-P subset |
424 | 12 months; transition from center based to home-based program; 3× week 40–60 min to 5× week for 150 min; included walking exercise and leg exercises (chair squats, toe stands, leg curl, leg ext, side leg raises with ankle weights) The Control group attended weekly, then monthly sessions on health education topics |
Total SPPB scores ↑ in the obese participants at month 6 and year 1 compared with obese persons in the control group; 400 m gait speed declined however in both groups at months 6 and 12 |
| Schlenk et al. (2011) | 63.2 ± 9.8 years 96% women American Knee OA |
26 | 6 months; two group design 1. Exercise (walking, lower limb targeted exercises) 2. Control group |
Total SPPB and 6-min walk scores improved in the Exercise group compared with the Control group at month 6 (p < 0.05) |
SPPB: Short Physical Performance Battery score consisting of 4-m walk, standing balance tests and repeated chair stands; LIFE-P: Lifestyle Interventions and Independence for Elders Pilot study.
Grant et al. (2004) randomized British women to either an exercise program or a control group for a 3-month study. The exercise group participated in 40-min classes twice a week; the sessions were comprised of 10 min of gentle, large body movements to warm up, 20 min of low impact aerobic dance activity to music, and 5 min of functional strengthening exercise (squats, side leg raises, inner thigh lifts, wall press ups, triceps extensions and abdominal exercise), followed by a 5 min flexibility-based cool-down period. At month 3, the exercise group improved chair rise time from 32.4 ± 7.4 to 29.1 ± 5.5 s, up-and-go test time from 9.4 2.7 to 7.9 ± 1.6 s and 20-m walk time from 17.4 ± 3.5 to 15.7 ± 1.7 s (all p < 0.05), whereas the control group did not ± demonstrate changes in these variables. A 1.7 kg weight loss occurred in the exercise group by month 3, but fat loss was not detected this was likely due to the use of less sensitive skin fold assessment.
A secondary analysis was performed from the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) study (Manini et al., 2010). Obese and non-obese participants were randomized to either a multimodal physical activity (PA) group or a “successful aging” control group (SA). The PA group participated in the program for 1 year; during months 1 and 2, participants performed supervised exercise sessions at moderate intensity three times a week (40–60 min per session, walking exercise, strength exercises [standing chair squats, toe stands, leg curl, knee extensions, side hip raises with ankle weights] and balance [dual and single legged standing movements]). From month 2 to 6, exercise volume was progressively increased and participants performed more home exercise sessions and fewer supervised sessions. From month 6 to 12, participants were encouraged to complete five sessions a week of exercise at home, with monthly visits to the lab. The SA group attended weekly group presentations on topics related to health and aging, with upper body stretching. The obese PA and SA groups made mean improvements in Short Physical Performance battery (BPPB) scores at month 6 (0.98 and 0.66 points, respectively; p < 0.05); at month 12, the obese group demonstrated a slow rate of decline in SPPB scores from month 6. The 400-m walk speed actually declined in both the PA and SA groups (mean difference in walk speed at months 6 and 12 were 0.009 m/s and 0.014 m/s). These data suggest that multimodal exercise with significant weight loss may increase overall physical function, but does not necessarily transfer to longer mobility tasks such as the 400-m walk.
Similar findings were reported by Schlenk et al. (2011) who studied obese persons with knee OA. A total of 26 randomized participants received either a 6-month lower extremity and fitness walking intervention or a non-exercise control. At month 6, the SPPB scores and 6-min walking performance increased. The WOMAC physical function subscale was a surrogate for self-efficacy, and those individuals randomized to the exercise group showed a tendency for self-efficacy by month 6 compared with the control group.
9.3. Multimodal exercise and dietary interventions vs. control groups
Several RCTS were identified that incorporated multimodal exercise with dietary modification. All studies were performed in the U.S., ranging in sample size from 24 to 316 participants (Table 3). Five studies focused on persons with knee OA (Focht et al., 2005; Messier et al., 2000, 2004; Miller et al., 2008; van Gool et al., 2005). Four studies used a two group, prospective study design in which the multimodal exercise program was compared with a control group (Anton et al., 2011; Frimel et al., 2008; Miller et al., 2008; Villareal et al., 2006).
Table 3.
Multimodal exercise interventions coupled with diet to combat physical decline in the obese, older adult.
| Author | Population | N | Interventions | Outcomes |
|---|---|---|---|---|
| Anton et al. (2011) | 55–79 years American |
34 | 6 months; 2 group design 1. Aerobic (walking, cycling) resistance (knee extension, leg raises, toe raise, leg curl, wide leg squat) using ankle weights-10 reps each; flexibility; dietician supervised 750 kcal daily deficit with self-selected foods 2. Control group (wait listed) |
400 m walking speed ↑ in t Exercise–Diet group compared to control (0.16 vs. 0.2 m/s); SPPB scores ↑ in both the Exercise–Diet and the Control groups (1.82 0.36 6 months vs. 0.8 0.29 points; p < 0.05) isokinetic leg strength did not change in either group by month 6 to healthy living 6 months |
| Focht et al. (2005) | ≥60 years American Knee OA |
ADAPT; 18 months four group design 1. Exercise (3 days/wk RX 2 sets, 12 reps of leg exercises, 15 min AX at 50–75% heart rate reserve) 2. Diet (5% loss of body weight using group sessions) 3. Exercise + Diet 4. Control group |
Exercise + Diet induced the highest improvements in pain, stair climb time and 6-min walk distance compared with the other three groups (p < 0.05); self-efficacy with physical tasks increased the most in this group |
|
| Frimel et al. (2008) | 70 ± 5 years 60% women American |
6 months; two group design Exercise vs. Exercise + Diet 1. Exercise (3 days/wk, 90 min sessions 30 min AX, 30 min RX, 30 min balance and flexibility; supervised by therapist 2. Diet + Exercise: prescribed balanced diet with 750 kcal daily deficit, weight loss goal was 10% body weight loss by month 6; Exercise was added to the diet in this group |
Strength ↑ in all muscle groups, and training volume (muscle endurance) ↑ in all muscle groups by 54–100% by month 6 in the Exercise + Diet group. Compared with the Diet only group, fat free mass decreased less, in both upper and lower limbs in the Exercise + Diet group (p < 0.05) |
|
| Messier et al. (2000) | ≥60 years 70% women American Knee OA |
24 | 6 months; two group design Exercise vs. Diet + Exercise 1. Exercise (3× week, 20 min walking, 20–30 min RX; 10–12 reps of 7 exercises) 2. Exercise + Diet (group sessions met 1 h/wk t help achieve at least 15 kg wt loss) |
6-min walk distance ↑ by 22% and 29% in the Exercise and Exercise + Diet groups; Stair climb time ↓ by 12% and 25% in the Exercise and Exercise + Diet groups (both p < 0.05) by month 6; knee pain symptom severity and frequency ↓ during walking, and body transfer activities |
| Messier et al. (2004) | ≥ 60 years 68–74% women; American Knee OA |
316 | ADAPT; 18 months 4 group design 1. Exercise (3 days/weeks RX 2 sets, 12 reps of leg exercises, 15 min AX at 50–75% Heart rate reserve) 2. Diet (5% loss of body weight using group sessions) 3. Exercise + Diet 4. Control group |
6-min walk distance increased in the Exercise + Diet group compared with the remaining groups by month 6 (482.4 m vs. 428.6–465.0 m; p < 0.05). Stair climb time decreased most in the Exercise + Diet group than the remaining three groups by month 6 (−2.54 s vs. −0.22 to −1.63 s; p < 0.05). WOMAC pain scores decreased the most in the Exercise + Diet group compared to all remaining groups (−2.2 points vs. −0.40 to −1.23 s; p < 0.05) |
| Miller et al. (2008) | 69.7 ± 0.6 yrs 57–65% women American; Knee OA; PAIBCT trial |
87 | 6 months, 3× week (45 min) Aerobic (walking, cycling; 50–85% HHR) Strength (leg extension, leg curl, heel raise ands step ups; 2 sets × 12 reps); meal replacements, structured menus, educational component; controls received lectures on health topics |
Exercisers lost 0.8% body weight compared with controls; 6 month changes in stair climb time ↓ from 9.5 to 9.2 s, and 6-min walk distance ↑ from 445.3 to 493.4 m (both p < 0.05); no significant change in IL-6 TNF-α, or C-reactive protein by month 6 |
| van Gool et al. (2005) | ≥60 years American Knee OA |
134 | ADAPT subsample at 18 months stratification of participants by adherence to the program at month 18; low adherence ≤40% of sessions Intermediate adherence 41–70% sessions High adherence ≥71% |
Tertile analyses showed a dose response association between adherence and improvement in 6-min walk distance at months 6 and 18 (p < 0.05) and with adherence and self-reported disability at these same time points (p < 0.05) |
| Villareal et al. (2006) | ≥65 years 66% women; American |
27 | 6.5 months; two group design 1. Exercise (90 min of flexibility, strength, endurance behavioral therapy, 3× week) + Diet (750 daily kcal deficit prescribed by dietician 2. Control group (no treatment) |
Exercisers lost 8.4% body weight and 15.6% body fat mass by week 12 (p < 0.05), fat free mass was maintained. PPT scores ↑ from 29.4 to 31.9 points compared with controls (no score change), and treadmill endurance ↑ 10.3% by week 26 (p < 0.05) |
| Villareal et al. (2011) | ≥65 years 62% women; American |
107 | 12 months; four group study 1. Exercise (90 min sessions of AX cycling, treadmill, stairs; RX 1–2 sets of upper and lower body machines at 65% of maximal lift, 8–12 reps, flexibility) 2. Diet (weekly group sessions with a Dietician to obtain prescriptions for a 750 kcal daily Deficit) 3. Exercise + Diet 4. Control group (no intervention) |
The Exercise + Diet group demonstrated a 21% change in PPT scores compared with exercise and diet groups by month 12 (12–15%); muscle strength ↑ 34–35% in the Exercise and Exercise + Diet group compared to the diet group (3%); obstacle course time to completion improved 10–13% in all intervention groups compared to control; single leg balance time also increased in these same three groups by week 52 (all p < 0.05) |
SPPB, Short Physical Performance Battery score consisting of 4-m walk, standing balance tests and repeated chair stands; ADAPT, Arthritis, Diet, and Activity Promotion Trial; PAIBCT, Physical activity, Inflammation and Body Composition Trial; PPT, Modified Physical Performance test consisting of timed trials of walking a 50 ft distance, putting on and taking off a lab coat, picking up a penny from the floor, standing up five times from a chair, lifting a 7-lb book to a shelf, climbing one flight of stairs, tandem and semi-tandem standing; AX, aerobic exercise; RX, resistance exercise.
Villareal et al. (2006) randomized frail, older, obese women to a 6.5-month intervention involving 90 min group sessions of flexibility, endurance, strength and balance exercises and a daily caloric deficit of 750 kcal/day, or a control group that was instructed to maintain normal diet and exercise patterns during the study period. The treatment group was targeted to lose 10% of body weight by the end of the study. The modified Physical Performance test (PPT; 7 tasks of physical ability, listed in Table 3 footnote) and body composition was measured at baseline and at the end of the study. The treatment group lost an average of 8.2 ± 5.7 kg (8.4% body weight) by the study’s end. Knee extension and flexion improved 12.9–25.5%, walking speed increased 7.6%, one legged balance stand increased 79.5% and the time to complete an obstacle course was reduced by 11.3% in the intervention group. The PPT score improved by 9.2% and decreased by 0.4% in the treatment and control groups, respectively (p < 0.05). At the end of the study, the intervention group decreased fat mass by an average of 6.6 ± 3.4%, vs. a 1.7 kg increase in the control group. In both groups, fat free mass was reduced by 1.2 ± 2.1 in the treatment group and by 1.0 ± 3.5 kg in the control group (p < 0.05). After controlling for changes in knee strength, changes in body weight correlated with changes in PPT scores (−0.51, p = 0.01).
In the Physical activity, Inflammation and Body Composition Trial, persons with knee OA were randomized to either a treatment group or a control group (Miller et al., 2008). The goal of the study was to lose 10% of body weight by month 6. The treatment consisted of a diet component: partial meal replacement (shakes, bars), dietician-led nutrition education sessions, lifestyle behavioral modifications in group sessions (3× week) and individually (1× month); and an exercise component: structured, supervised training (3× week) performing 20 min of strengthening (four stations of machines, weighted vests and cuffs: leg extension, leg curl, heel raise, step-ups), 20 min of AX (predominantly walking, occasionally stationary cycling at 50–85% of age-predicted heart rate reserve). The control group met bimonthly as a group to hear presentations on health topics, exercise and OA. Performance measures of 6-min walk distance and stair climb time were assessed at baseline and month 6. Pooled data analysis of the two groups revealed mean improvements in the 6-min walk distance (from 445.3 ± 11.2 m to 493.4 ± 12.4 m; p < 0.05), and in the stair climb time (from 9.5 ± 0.5 s to 9.2 ± 0.7 s; p < 0.05). While the treatment group lost 8.7 ± 0.8% body weight, the control group did not lose weight (0.0%).
Given the potential for diet-induced weight loss therapy to exacerbate sarcopenia, Frimel et al. (2008) tested whether adding multimodal exercise to a diet program could attenuate muscle mass loss and physical function decline in frail, obese, older adults. Participants were randomized to a Diet group alone (prescribed a daily 750 kcal deficit for a targeted total weight loss of 10%), or Exercise + Diet group (exercise consisted of three 90 min sessions per week, a total of 72 sessions; 15 min of flexibility, 30 min of low impact AX, 30 min of progressive, high intensity RX [8–12 repetitions at 65–85% of maximal strength on squats, leg press, knee extension, knee flexion, seated row, upright row, seated chest press, biceps curl and triceps extension using a squat rack and Hoist machine]). At month 6, there were significant improvements in strength for the Exercise + Diet group compared with the control group (p < 0.05) for all exercises except for biceps curl. While both groups lost body mass and fat mass, the loss of fat-free mass was attenuated in the Exercise + Diet group compared to the control (1.8 ± 1.5 kg vs. 3.5 ± 2.1 kg; p < 0.05). In addition, the reduction in the lean mass in both the upper and lower extremities were less in the Exercise + Diet group than the control group (both p < 0.05).
Sedentary African-American and Caucasian women with mild to moderate functional limitations were randomized to either a 6-month weight loss plus exercise group (WL + E) or a control group (Anton et al., 2011). Women in the WL + E group performed supervised sessions three times a week, for progressively longer durations. Once a daily exercise time of 150 min was achieved this was maintained for the study duration. Exercise consisted of walking exercise at an intensity corresponding to a score of ‘13’ on the Borg Rating of Perceived Exertion (RPE) scale, and resistive exercise (two sets of 10 repetitions of wide leg squat, standing leg curl, knee extension, side hip raise and toe stand) at and RPE of ‘15’. Compared with controls, the WL + E demonstrated an increase in 400-m walking speed by month 6 (0.16 0.03 vs. 0.02 0.03 m/s; p < 0.05) with no difference between ethnic groups (mean change by month 6 0.13–0.19 m/s). The SPPB scores improved in both study groups, but the WL + E group exhibited a greater improvement (mean change was 1.8 ± 0.4 points vs. 0.8 ± 0.3 points). Knee extension strength did not change in either group during the study.
Messier and colleagues have performed several rigorous research studies in obese older adults with knee OA and physical function. In 2000, a preliminary study examined the differential efficacy of exercise and Exercise + Diet on knee pain symptoms and physical function over a 6-month period (Messier et al., 2000). Exercise consisted of 1 h sessions of combined AX and RX 3 days a week. The RX program included seven upper and lower body exercises using dumbbells or cuff weights, and the AX was walking at 50–75% of heart rate reserve. Dietary therapy consisted of 1 h group sessions per week, during which participants would receive instruction, perform problem solving, sample healthful foods and food record keeping. Compared to baseline, 6 min walk distance increased 1408 to 1718 ± 41 feet and from 1408 to 1821 ± 36 in the Exercise and Exercise + Diet groups by month 6. Similarly, stair climb time improved from 9.81 s to 8.67 and 7.39 s in the Exercise and Exercise + Diet groups, respectively (all changes p < 0.05). These functional changes corresponded with 4.0 1.8 kg (Exercise group) and 18.8 ± 8.5 kg of body weight loss (Exercise Diet group) at month 6, with differences between groups (p < 0.05). Also, 17–20% improvements in self-reported Fitness Arthritis and Seniors Trial Functional Performance Inventory summary scores were reported for the two study groups. Importantly, OA pain symptoms severity and frequency decreased depending on the task performed (ambulation vs. transfers) and the group.
The Arthritis, Diet and Physical Promotion Trial (ADAPT) generated three papers relevant to this review. ADAPT was a single-blinded, controlled study in obese older adults (Messier et al., 2004) that was designed to compared the effects of four interventions over an 18-month period: (1) exercise only (15 min AX at 50–75% heart rate reserve, 15 min RX with two sets of 12 repetitions of leg extension, leg curl, heel raise and step-ups using cuff weights and vests, and cool down 15 min); (2) diet (three phase diet intervention with the goal of losing and maintaining 5% of body weight); (3) exercise and diet, and (4) a control group (“healthy lifestyle,” no intervention). An additive effect of exercise and diet was found for 6 min walk test distance and improvements in stair climb time. For example, the Diet, Exercise and Exercise + Diet groups increased walk distance by 9.7, 48.6 and 61.6 m, whereas the Control group decreased distance by 4.7 m at month 18 (p < 0.05). Similarly, the Diet, Exercise and Exercise + Diet groups decreased stair climb time by 1.3, 1.6 and 2.5 s, but the Control group demonstrated a 0.2 s decrease in time (p < 0.05). Eighteen-month reductions in knee pain were greater in the Exercise + Diet group compared to controls (30.3% vs. 16%; p < 0.05). Weight loss was greatest in the Exercise + Diet and Diet groups (5.7% and 4.9% of body weight) compared with the Exercise and Control groups (3.7% and 1.2% body weight). Focht et al. (2005) performed an analysis of a subgroup of obese participants from the ADAPT trial to determine whether self-efficacy and pain mediated improvements in physical function. Participants were asked to rate their level of certainty from 0 to 10 of being able to complete laps during walking or to climb sets of stairs. At month 18, the largest changes in self-efficacy occurred in the Exercise + Diet group for both activities (13.41 and 18.51 points), and the lowest changes in self-efficacy occurred in the control group (2.04 and 5.01 points; p < 0.05). Pain and self-efficacy were found to be independent predictors for physical function, and the participants who had the greatest reduction in pain and highest change in self-efficacy exhibited the greatest improvement in stair climbing ability and walking distance. The third paper presented a secondary analysis of the ADAPT trial data with 156 participants to determine whether adherence rate was related to changes in physical function at months 6 and 18 (van Gool et al., 2005). After stratification of participants on adherence level during training, tertile analysis revealed a dose response association between session completion rate and improvement in walking distance by month 18 (β = 0.39, p < 0.01). The 18-month change in BMI was also correlated with the change in 6-month walking distance (r = −0.24; p < 0.05).
Most recently, a four group RCT was performed by Villareal et al. (2011) to determine the comparative effects of multimodal exercise on PPT scores after a 12-month intervention. Secondary outcomes included the time to complete an obstacle course, fastest gait speed during a 25-foot walk and static balance. Participants were assigned to a Control group, a Diet group, an Exercise group or a Diet + Exercise group. The diet intervention consisted of a prescription of a 500–750 kcal daily deficit, with 1 g/kg body mass of high quality protein per day. The target weight loss was 10% of body weight. The exercise component included 90 min sessions of AX (treadmill walking, stationary cycling, stair climbing at 65% up to 75% peak heart rate), RX (1–2 sets at 12 repetitions on nine machines at 65% of their 1-repetition–maximal strength). All participants received 1500 mg calcium and 1000 IU vitamin D per day. The Control group received only monthly meetings with the staff to discuss healthy living. The average PPT scores increased more in the Diet + Exercise group than the Diet or Exercise groups at month 12 (5.4 ± 2.4 points vs. 3.1 ± 1.4 and 4.0 ± 2.5 points; p < 0.05). Similarly, the change in peak fitness values was greater in the Diet + Exercise group than the Diet or Exercise groups (16.1% vs. 8.0% and 9.6%; p < 0.05). Obstacle course completion times improved by 16% and 14% in the Diet + Exercise and Exercise groups, and walking speed increased by 23% and 11% in these same two groups by month 12 (p < 0.05). Muscle strength increased by 30% and 33% in the Diet + Exercise and Exercise groups (p < 0.05). Body weight decreased 8.6–9.3% and fat mass 15–17% in the Diet and Diet + Exercise groups, with minimal change in the other two groups after the intervention. Lean mass decreased the least in the Exercise group (2.2%), and the Diet group lost the most muscle mass (5.2%; p < 0.05). Interestingly, the changes in subjective functional status questionnaire scores were improved most in the groups who performed exercise, with persons in the Diet + Exercise group reporting the greatest change at month 12. While weight loss alone helped to attenuate frailty, the combination of exercise withdiet was most effective among the treatments for muscle strength and performance.
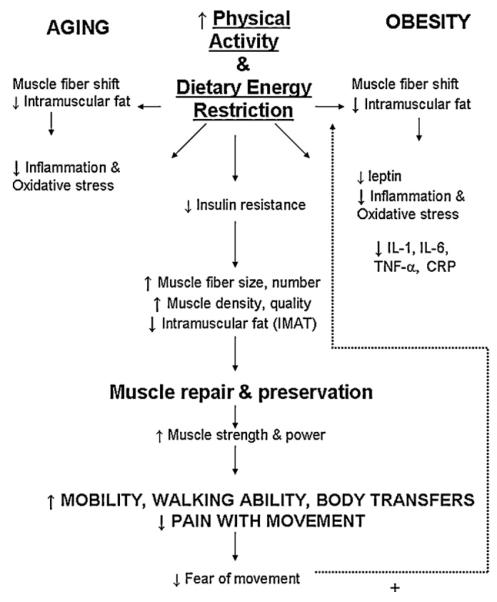
10. Additional benefits of exercise in obesity
Several physiological pathways that contribute to sarcopenia in the obese older adult may be favorably altered by exercise, such as inflammation and oxidative stress as shown in Fig. 2. Smaller studies that propose possible mechanisms linking body composition change, functional improvement and preservation of muscle mass and quality with exercise. For example, 12 weeks of low glycemic index diets coupled with supervised AX (60 min per session, 5 days/week at 80–85% heart rate) reduces BMI by approximately 7%, and decreases the cytokine production by monocytes (IL-6, TNF-α) in obese, older adults (Kelly et al., 2011). Dutheil et al. (2010) studied 14 participants who completed 3 weeks of an intensive supervised intervention followed by 5 months of autonomous lifestyle change. Exercise consisted of 3 h walking, pool and fitness at 40–60% heart rate reserve for 6 days/week; a nutritionist implemented a 500 kcal deficit diet for the group. At month 6, BMI decreased by 6%, body fat percentage decreased by 6.5% and lean mass decreased by 2.5% (p < 0.0001). Body composition changed corresponded with reductions in circulating TNF-α levels (from 11.1 to 0.8 pg/ml) and in CRP levels (from 3.92 to 2.45 mg/l) (Dutheil et al., 2010).
Fig. 2.
A proposed model of the effects of regular physical activity or exercise training can foster positive physiological and psychological changes to reduce sarcopenia and increase functional ability in the obese older adult (IMAT, intramuscular adipose tissue; IL-1, interleukin 1; IL-6, interleukin 6; TNF-α, tumor necrosis factor α; CRP, C reactive protein). + denotes a positive effect on physical activity and exercise.
Tai Chi exercise, when practiced for 12 weeks three times per week, compared with time-matched aerobic dance activity, can significantly reduce triglyceride levels, CRP levels and oxidative stress biomarkers such as malondialdehyde in obese older adults (Chen et al., 2010). Importantly, reductions in joint pain can occur with regular exercise training, as shown by reductions in knee pain up to 30% from baseline (Messier et al., 2004). With less pain, selfefficacy levels rise, fear of movement is reduced and this promotes increased participation in other physical activities. These collective improvements can positively mediate changes in functional capacity (Focht et al., 2005).
11. Caloric restriction strategies and complementing exercise benefits in older, obese adult
Evidence is emerging regarding different dietary restriction patterns on body composition and other physiological markers. In our opinion, dietary strategies that preserve muscle mass while reducing fat mass would be ideal to augment physical effects of regular exercise in the older, obese population. Long term caloric restriction can delay the age related reductions in upper leg muscle mass, preserve muscle cross-sectional area and type II fibers (McKiernan et al., 2011). Energy restriction can combat the age-related functional impairment of muscle mitochondria, reducing the volume of muscle fiber mitochondrial DNA mutations, electron transport abnormalities and fiber morphology aberrations (Marzetti et al., 2009). The amount of TNF-α expressed in myocytes and myocyte death triggered by TNF-α can also be reduced by caloric restriction (Phillips and Leeuwenburgh, 2005). When long term caloric restriction is combined with life-long exercise in rodents, the level of age-related oxidative stress and rate of skeletal muscle cell death is attenuated (Wohlgemuth et al., 2010). These beneficial protections afforded by caloric restriction show promise as an excellent adjuvant to regular exercise for preserving skeletal muscle mass and muscle function with aging.
Recent studies have compared whether daily caloric restriction or intermittent fasting (partial or complete) is more effective in favorably changing body composition and body weight (Harvie et al., 2011; Heilbronn et al., 2005; Johnson et al., 2007). Intermittent fasting involves alternating days of ad libitum feeding and restricted or partially restricted feeding (e.g., canned meal replacement shakes or 25% of baseline energy needs). The findings revealed that 22 days of intermittent fasting reduced body and fat mass by 2.5–4% while the rate of fat oxidation increased 57% while the rate of carbohydrate oxidation decreased by 53% (Heilbronn et al., 2005). Related studies documented significant reductions in insulin, TNF-α, oxidative stress biomarker levels following 8 weeks of alternate day calorie restriction (Johnson et al., 2007). Weight loss can be successfully achieved in both the continuous and the intermittent approaches (Harvie et al., 2011). These collective dietary-induced improvements can positively contribute to muscle preservation and reduction of body fat and would likely complement the exercise benefits reviewed in this paper. While most of these studies involved younger populations, similar methods may be applied to older, obese adults. Advantages of alternate day dietary restriction or fasting include a wider appeal to the obese population who may not be able to achieve the stringent behaviors necessary with a continuous diet of caloric restriction.
11.1. Unanswered questions
The reviewed studies provide exciting evidence that exercise can help combat sarcopenia and functional decline in the obese older adult; however, there are some limitations to the existing literature that deserve comment. Most of the interventions presented ranged from 2 to 18 months; additional studies are needed to prospectively follow the adherence patterns of this population to prescribed exercise and dietary recommendations after the study duration and what percentage of the participants relapse to unhealthy habits. This issue is critical, as health care utilization may be higher for those who relapse compared to those who maintain adopted exercise and diet patterns. While the preponderance of the RCTs used supervised exercise training programs, it is unclear whether home-based, community or partner-based programs are also effective in increasing muscle mass and performance. At the present time, we have little evidence evaluating the translational efficacy of the exercise program into other settings such as home, in community facilities or small groups. A major goal should include developing methods to help bring exercise programming and dietary modification to other sites than the laboratory to help reach as much of the aging obese demographic as possible. Interesting data in obese women aged 50–75 years from rural communities who were prescribed a 6-month exercise and diet intervention through the Cooperative Extension Services and followed by extended care (phone counseling or individual counseling sessions) reduced body weight and maintained most of that weight loss by month 18 (Perri et al., 2008). Application of extended care may be particularly useful in the aging obese population.
Additional evidence of the efficacy of exercise training for the severely obese demographic is also lacking. Because the morbidly obese population is on the rise, the prevalence of disability due to obesity will be increasing. It is not yet known whether there is an optimal exercise type, frequency or duration that might be most effective for the attenuation of sarcopenia in this group. Examination of the dosage effect of exercise on functional performance outcomes and mobility would provide practitioners, therapists and physiologists a framework from which to structure long term plans for the obese patients. The RCTs reviewed in this study primarily focused on comparing one type of treatment to another, rather than the dosage or exposure to a given exercise stimulus. Only one of the studies discussed the potential differences in responsiveness between racial groups, and most of the single sex studies were in women. Additional data examining different racial, ethnic and gender differences in functional improvement or sarcopenia would be highly valuable from the clinical standpoint. Finally, most studies reported that fat loss or body weight occurred with Exercise or Exercise + Diet interventions. In the populations who experience chronic joint pain, it is not yet clear how reductions in body fat, body weight, inflammation, oxidative stress relative and pain mediate changes in function.
11.2. Future directions
The trend for obesity-related disability in older adults is worsening. NHANES III data show that functional impairment for tasks related to lower body function are becoming more prevalent within the obese (≥30 kg/m2) segment of the population from the years 1994–2004. Among the obese segment of the population, 36.8% reported disability in 1994, whereas 42.2% reported disability and functional impairment in 2004 (Alley and Chang, 2007). Given that obesity and muscle loss has a multifaceted etiology, it is likely that obesity related disability will need to be examined from several aspects to fully understand how to counteract it. Areas to address in the future in obese, older adults include longitudinal examination of skeletal muscle phenotypic and contractile changes in relation to adiposity over time, and comparison of systemic inflammatory and biochemical parameters that can influence strength and body composition across the BMI spectrum or based on different assessments of body composition. Factors that can contribute to obesity disability in later life that require examination include personality traits, behavioral traits or genetic phenotypes associated with development of severe obesity.
Finally, an area ripe for research is the incorporation of technology and the use of extended care options to encourage wide spread use of exercise programs among the obese older population. If personalized counseling is not an option, or is cost prohibitive, interactive technology may helpful in providing exercise programs and dietary guidance for weight loss and improved function. Mobile phone-based applications or Internet-based websites could be very useful and easy to manage and could provide immediate feedback on patterns of daily exercise. Customized exercise plans (with or without diet) could also be developed for the individual to target specific functional or mobility goals. These options may be interactive, engaging, and non-threatening for the older, obese adult.
12. Conclusion
Exercise training is an effective intervention to combat the collective effects of aging and obesity on functional decline, sarcopenia and frailty. Exercise studies with and without diet components reviewed here show that comprehensive programs 3–18 months in duration that included AX and RX (2–3 days per week) with caloric restriction (approximately 750 kcal deficit/day) incurred the greatest improvement in functional performance compared with exercise or diet conditions alone. RX can minimize muscle mass loss with the interventions that include diet components. Exercise can also counteract factors that contribute to sarcopenia, including inflammation, oxidative stress and insulin resistance. Regular multimodal exercise coupled with diet appears to be effective for counteracting sarcopenic obesity and functional mobility disability in the older, obese adult.
Acknowledgments
This publication was made possible by Grant Number AR057552-01A1 from NIAMS/NIH (H Vincent). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAMS or NIH.
References
- Abbatecola AM, Paolisso G. Is there a relationship between insulin resistance and frailty syndrome? Curr. Pharm. Des. 2008;14:405–410. doi: 10.2174/138161208783497750. [DOI] [PubMed] [Google Scholar]
- Alley DE, Chang VW. The changing relationship of obesity and disability, 1988–2004. JAMA. 2007;298:2020–2027. doi: 10.1001/jama.298.17.2020. [DOI] [PubMed] [Google Scholar]
- Anton SD, Manini TM, Milsom VA, Dubyak P, Cesari M, Cheng J, et al. Effects of a weight loss plus exercise program on physical function in overweight, older women: a randomized controlled trial. Clin. Inter. Aging. 2011;6:141–149. doi: 10.2147/CIA.S17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila JJ, Gutierres JA, Sheehy ME, Lofgren IE, Delmonico MJ. Effect of moderate intensity resistance training during weight loss on body composition and physical performance in overweight older adults. Eur. J. Appl. Physiol. 2010;109:517–525. doi: 10.1007/s00421-010-1387-9. [DOI] [PubMed] [Google Scholar]
- Bales CW, Ritchie CS. Sarcopenia, weight loss and nutritional frailty in the elderly. Ann. Rev. Nutr. 2002;22:209–223. doi: 10.1146/annurev.nutr.22.010402.102715. [DOI] [PubMed] [Google Scholar]
- Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J. Geront. A. Biol. Sci. Med. Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Stauber PM, McHugh D, Koehler KM, Garry PJ. Cross-sectional age differences in body composition in persons 60+ years of age. J. Geront. A: Biol. Sci. Med. Sci. 1995;50:M307–M316. doi: 10.1093/gerona/50a.6.m307. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes. Res. 2004;12:1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- Beaufrere B, Morio B. Fat and protein redistribution with aging: metabolic considerations. Eur. J. Clin. Nutr. 2000;54(Supplement):S48–S53. doi: 10.1038/sj.ejcn.1601025. [DOI] [PubMed] [Google Scholar]
- Bouchard DR, Soucy L, Sénéchal M, Dionne IJ, Brochu M. Impact of resistance training with or without caloric restriction on physical capacity in obese older women. Meno. 2009;16:66–72. doi: 10.1097/gme.0b013e31817dacf7. [DOI] [PubMed] [Google Scholar]
- Bouchard DR, Soucy L, Sénéchal M, Dionne IJ, Brochu M. Changes in objective and self-reported measures of physical capacity after an intervention in obese older women. J. Wom. Aging. 2010;22:34–36. doi: 10.1080/08952840903489011. [DOI] [PubMed] [Google Scholar]
- Broadwin J, Goodman-Gruen D, Slymen D. Ability of fat and fat-free mass percentages to predict functional disability in older men and women. J. Am. Geriat. Soc. 2001;49:1641–1645. doi: 10.1046/j.1532-5415.2001.t01-1-49273.x. [DOI] [PubMed] [Google Scholar]
- Burks TN, Andres-Mateos E, Marx R, Mejias R, Van Erp C, Simmers JL, et al. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci. Trans. Med. 2011;3:82ra37. doi: 10.1126/scitranslmed.3002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J. Clin. Endocrin. Metab. 2003;88:2019–2025. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- Cederholm TE, Bauer JM, Boirie Y, Schneider SM, Sieber CC, Rolland Y. Toward a definition of sarcopenia. Clin. Geriat. Med. 2011;27:341–353. doi: 10.1016/j.cger.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BW, Lenchik L, et al. Sarcopenia, obesity, and inflammation: results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors Study. Am. J. Clin. Nutr. 2005;82:428–434. doi: 10.1093/ajcn.82.2.428. [DOI] [PubMed] [Google Scholar]
- Cesari M, Kritchevsky SB, Leeuwenburgh C, Pahor M. Oxidative damage and platelet activation as new predictors of mobility disability and mortality in elders. Antiox. Redox Sig. 2006;8:609–619. doi: 10.1089/ars.2006.8.609. [DOI] [PubMed] [Google Scholar]
- Chen H, Guo X. Obesity and functional disability in elderly Americans. J. Am. Geriat. Soc. 2008;56:689–694. doi: 10.1111/j.1532-5415.2007.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SC, Ueng KC, Lee SH, Sun KT, Lee MC. Effect of t’ai chi exercise on biochemical profiles and oxidative stress indicators in obese patients with type 2 diabetes. J. Altern. Comp. Med. 2010;16:1153–1159. doi: 10.1089/acm.2009.0560. [DOI] [PubMed] [Google Scholar]
- Combaret L, Dardevet D, Béchet D, Taillandier D, Mosoni L, Attaix D. Skeletal muscle proteolysis in aging. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:37–41. doi: 10.1097/MCO.0b013e32831b9c31. [DOI] [PubMed] [Google Scholar]
- Cooke R. Modulation of the actomyosin interaction during fatigue of skeletal muscle. Muscle. 2007;36:756–777. doi: 10.1002/mus.20891. [DOI] [PubMed] [Google Scholar]
- Davidson LE, Hudson R, Kilpatrick K, Kuk JL, McMillan K, Janiszewski PM, et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch. Int. Med. 2009;169:122–131. doi: 10.1001/archinternmed.2008.558. [DOI] [PubMed] [Google Scholar]
- Davison KK, Ford ES, Cogswell ME, Dietz WH. Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. J. Am. Geriat. Soc. 2002;50:1802–1809. doi: 10.1046/j.1532-5415.2002.50508.x. [DOI] [PubMed] [Google Scholar]
- Di Francesco V, Zamboni M, Zoico E, Bortolani A, Maggi S, Bissoli L, et al. Relationships between leisure-time physical activity, obesity and disability in elderly men. Aging Clin. Exper. Res. 2005;17:201–206. doi: 10.1007/BF03324597. [DOI] [PubMed] [Google Scholar]
- Dutheil F, Lesourd B, Courteix D, Chapier R, Doré E, Lac G. Blood lipids and adipokines concentrations during a 6-month nutritional and physical activity intervention for metabolic syndrome treatment. Lipids Health Dis. 2010;9:148. doi: 10.1186/1476-511X-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvigneaud N, Matton L, Wijndaele K, Deriemaeker P, Lefevre J, Philippaerts R, et al. Relationship of obesity with physical activity, aerobic fitness and muscle strength in Flemish adults. J. Sports Med. Phys. Fit. 2008;48:201–210. [PubMed] [Google Scholar]
- Evans WJ, Paolisso G, Abbatecola AM, Corsonello A, Bustacchini S, Strollo F, et al. Frailty and muscle metabolism dysregulation in the elderly. Biogerontology. 2010;11:527–536. doi: 10.1007/s10522-010-9297-0. [DOI] [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focht BC, Rejeski WJ, Ambrosius WT, Katula JA, Messier SP. Exercise, self-efficacy, and mobility performance in overweight and obese older adults with knee osteoarthritis. Arthr. Rheum. 2005;53:659–665. doi: 10.1002/art.21466. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J. Geront. A Biol. Sci. Med. Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Frimel TN, Sinacore DR, Villareal DT. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med. Sci. Sports Exerc. 2008;40:1213–1219. doi: 10.1249/MSS.0b013e31816a85ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher D, Visser M, De Meersman RE, Sepúlveda D, Baumgartner RN, Pierson RN, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J. Appl. Phys. 1997;83:229–239. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- Grant S, Todd K, Aitchison TC, Kelly P, Stoddart D. The effects of a 12-week group exercise programme on physiological and psychological variables and function in overweight women. Pub. Health. 2004;118:31–42. doi: 10.1016/S0033-3506(03)00131-8. [DOI] [PubMed] [Google Scholar]
- Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Muscul. Disord. 2008;2:132. doi: 10.1186/1471-2474-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet C, Boirie Y. Insulin resistance: a contributing factor to age-related muscle mass loss? Diab. Metab. 2005;31:5S20–25S26. doi: 10.1016/s1262-3636(05)73648-x. [DOI] [PubMed] [Google Scholar]
- Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int. J. Obesity. 2011;35:714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am. J. Clin. Nutr. 2005;81:69–73. doi: 10.1093/ajcn/81.1.69. [DOI] [PubMed] [Google Scholar]
- Henwood TR, Taaffe DR. Improved physical performance in older adults undertaking a short-term programme of high-velocity resistance training. Gerontology. 2005;51:108–115. doi: 10.1159/000082195. [DOI] [PubMed] [Google Scholar]
- Heuts PH, Vlaeyen JW, Roelofs J, de Bie RA, Aretz K, van Weel C, et al. Pain-related fear and daily functioning in patients with osteoarthritis. Pain. 2004;110:228–235. doi: 10.1016/j.pain.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Hubbard RE, Lang IA, Llewellyn DJ, Rockwood K. Frailty, body mass index, and abdominal obesity in older people. J. Gerontol. A Biol. Sci. Med. Sci. 2009;65:377–381. doi: 10.1093/gerona/glp186. [DOI] [PubMed] [Google Scholar]
- Isidori AM, Strollo F, Morè M, Caprio M, Aversa A, Moretti C, et al. Leptin and aging: correlation with endocrine changes in male and female healthy adult populations of different body weights. J. Clin. Endo. Metab. 2000;85:1954–1962. doi: 10.1210/jcem.85.5.6572. [DOI] [PubMed] [Google Scholar]
- Jarosz PA, Bellar A. Sarcopenic obesity. An emerging cause of frailty in older adults. Nat. Geronot. Assoc. 2009;30:65–70. doi: 10.1016/j.gerinurse.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Rad. Biol. Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KR, Haus JM, Solomon TP, Patrick-Melin AJ, Cook M, Rocco M, et al. A low-glycemic index diet and exercise intervention reduces TNF(alpha) in isolated mononuclear cells of older, obese adults. J. Nutr. 2011;141:1089–1094. doi: 10.3945/jn.111.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. (Lond.) 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- Kyle UG, Morabia A, Schutz Y, Pichard C. Sedentarism affects body fat mass index and fat-free mass index in adults aged 18 to 98 years. Nutrition. 2004;20:255–260. doi: 10.1016/j.nut.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Lamb SE, Guralnik JM, Buchner DM, Ferrucci LM, Hochberg MC, Simonsick EM, et al. Factors that modify the association between knee pain and mobility limitation in older women: the Women’s Health and Aging Study. Ann. Rheum. Dis. 2000;59:331–337. doi: 10.1136/ard.59.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert CP, Wright NR, Finck BN, Villareal DT. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J. Appl. Physiol. 2008;105:473–478. doi: 10.1152/japplphysiol.00006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMonte MJ, Blair SN. Physical activity, cardiorespiratory fitness, and adiposity: contributions to disease risk. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9:540–546. doi: 10.1097/01.mco.0000241662.92642.08. [DOI] [PubMed] [Google Scholar]
- Lebrun CE, van der Schouw YT, de Jong FH, Grobbee DE, Lamberts SW. Fat mass rather than muscle strength is the major determinant of physical function and disability in postmenopausal women younger than 75 years of age. Menopause. 2006;13:474–481. doi: 10.1097/01.gme.0000222331.23478.ec. [DOI] [PubMed] [Google Scholar]
- Lim JY, Tchai E, Jang SN. Effectiveness of aquatic exercise for obese patients with knee osteoarthritis: a randomized controlled trial. PMR. 2010;2:723–731. doi: 10.1016/j.pmrj.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Malenfant P, Joanisse DR, Thériault R, Goodpaster BH, Kelley DE, Simoneau JA. Fat content in individual muscle fibers of lean and obese subjects. Int. J. Obes. Relat. Metab. Dis. 2001;25:1316–1321. doi: 10.1038/sj.ijo.0801733. [DOI] [PubMed] [Google Scholar]
- Manini TM, Newman AB, Fielding R, Blair SN, Perri MG, Anton SD, et al. Effects of exercise on mobility in obese and nonobese older adults. Obesity (Silv. Spr.) 2010;18:1168–1175. doi: 10.1038/oby.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manton KG. Recent declines in chronic disability in the elderly U.S. population: risk factors and future dynamics. Ann. Rev. Pub. Health. 2007;29 doi: 10.1146/annurev.publhealth.29.020907.090812. doi:10.1146/annurev.publhealth.29.020907.090812. [DOI] [PubMed] [Google Scholar]
- Marcell TJ. Sarcopenia: causes, consequences, and preventions. J. Geront: A. Biol. Sci. Med. Sci. 2003;58:M911–M916. doi: 10.1093/gerona/58.10.m911. [DOI] [PubMed] [Google Scholar]
- Marks R. Obesity profiles with knee osteoarthritis: correlation with pain, disability, disease progression. Obesity (Silv. Spr.) 2007;15:1867–1874. doi: 10.1038/oby.2007.221. [DOI] [PubMed] [Google Scholar]
- Martínez-González MA, Martínez JA, Hu FB, Gibney MJ, Kearney J. Physical inactivity, sedentary lifestyle and obesity in the European Union. Int. J. Obes. Relat. Metab. Disord. 1999;23:1192–1201. doi: 10.1038/sj.ijo.0801049. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Lees HA, Wohlgemuth SE, Leeuwenburgh C. Sarcopenia of aging: underlying cellular mechanisms and protection by calorie restriction. Biofactors. 2009;35:28–35. doi: 10.1002/biof.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan SH, Colman RJ, Lopez M, Beasley TM, Aiken JM, Anderson RM, et al. Caloric restriction delays aging-induced cellular phenotypes in rhesus monkey skeletal muscle. Exp. Geront. 2011;46:23–29. doi: 10.1016/j.exger.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010;11:1509–1529. doi: 10.3390/ijms11041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill SS, Seeman TE, Kasl SV, Berkman LF. Gender differences in the comparison of self-reported disability and performance measures. J. Geront. A. Biol. Sci. Med. Sci. 1997;52:M19–M26. doi: 10.1093/gerona/52a.1.m19. [DOI] [PubMed] [Google Scholar]
- Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthr. Rheum. 2004;50:1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- Messier SP, Loeser RF, Mitchell MN, Valle G, Morgan TP, Rejeski WJ, et al. Exercise and weight loss in obese older adults with knee osteoarthritis: a preliminary study. J. Am. Geriat. Soc. 2000;48:1062–1072. doi: 10.1111/j.1532-5415.2000.tb04781.x. [DOI] [PubMed] [Google Scholar]
- Miller GD, Nicklas BJ, Davis C, Loeser RF, Lenchik L, Messier SP. Intensive weight loss program improves physical function in older obese adults with knee osteoarthritis. Obesity (Silv. Spr.) 2008;14:1219–1230. doi: 10.1038/oby.2006.139. [DOI] [PubMed] [Google Scholar]
- Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB, Tylavsky FA, et al. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am. J. Clin. Nutr. 2005;82:872–878. doi: 10.1093/ajcn/82.4.872. [DOI] [PubMed] [Google Scholar]
- Okifuji A, Donaldson GW, Barck L, Fine PG. Relationship between fibromyalgia and obesity in pain, function, mood, and sleep. J. Pain. 2010;11:1329–1337. doi: 10.1016/j.jpain.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain H, Wiles R. The experience of being disabled and obese. Disabil. Rehab. 2006;28:1211–1120. doi: 10.1080/09638280600554561. [DOI] [PubMed] [Google Scholar]
- Perri MG, Limacher MC, Durning PE, Janicke DM, Lutes LD, Bobroff LB, et al. Extended-care programs for weight management in rural communities: the treatment of obesity in underserved rural settings (TOURS) randomized trial. Arch. Int. Med. 2008;168:2347–2354. doi: 10.1001/archinte.168.21.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JM, Bryner RW, Alway SE. Satellite cells proliferation is reduced in muscles of obese Zucker rats but restored with loading. Am. J. Cell Physiol. 2008;295:C521–C528. doi: 10.1152/ajpcell.00073.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005;19:668–670. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- Puthoff ML, Nielsen DH. Relationships among impairments in lower-extremity strength and power, functional limitations, and disability in older adults. Phys. Ther. 2007;87:1334–1347. doi: 10.2522/ptj.20060176. [DOI] [PubMed] [Google Scholar]
- Raguso CA, Kyle U, Kossovsky MP, Roynette C, Paoloni-Giacobino A, Hans D, et al. A 3-year longitudinal study on body composition changes in the elderly: role of physical exercise. Clin. Nutr. 2006;25:573–580. doi: 10.1016/j.clnu.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Ramsay SE, Whincup PH, Shaper AG, Wannamethee SG. The relations of body composition and adiposity measures to ill health and physical disability in elderly men. Am. J. Epidemiol. 2006;164:459–469. doi: 10.1093/aje/kwj217. [DOI] [PubMed] [Google Scholar]
- Reid MB, Moylan JS. Beyond atrophy: redox mechanisms of muscle dysfunction in chronic inflammatory disease. J. Physiol. 2011;589:2171–2179. doi: 10.1113/jphysiol.2010.203356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddiford-Harland DL, Steele JR, Baur LA. Upper and lower limb functionality: are these compromised in obese children? Int. J. Pediat. Obes. 2006;1:42–49. doi: 10.1080/17477160600586606. [DOI] [PubMed] [Google Scholar]
- Roelofs J, Goubert L, Peters ML, Vlaeyen JW, Crombez G. The Tampa Scale for Kinesiophobia: further examination of psychometric properties in patients with chronic low back pain and fibromyalgia. Eur. J. Pain. 2004;8:495–502. doi: 10.1016/j.ejpain.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Rolland Y, Lauwers-Cances V, Pahor M, Fillaux J, Grandjean H, Vellas B. Muscle strength in obese elderly women: effect of recreational physical activity in a cross-sectional study. Am. J. Clin. Nutr. 2004;79:552–557. doi: 10.1093/ajcn/79.4.552. [DOI] [PubMed] [Google Scholar]
- Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes. Res. 2004;12:887–888. doi: 10.1038/oby.2004.107. [DOI] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006;119(52):e529–517. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Schlenk EA, Lias JL, Sereika SM, Dunbar-Jacob J, Kwoh CK. Improving physical activity and function in overweight and obese older adults with osteoarthritis of the knee: a feasibility study. Rehab. Nurs. 2011;36:32–42. doi: 10.1002/j.2048-7940.2011.tb00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J. Appl. Physiol. 2007;102:919–925. doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz Y, Kyle UU, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int. J. Obes. 2002;26:953–960. doi: 10.1038/sj.ijo.0802037. [DOI] [PubMed] [Google Scholar]
- Seidman SN. Androgens and the aging male. Psychopharm. Bull. 2007;40:205–218. [PubMed] [Google Scholar]
- Smits JA, Tart CD, Presnell K, Rosenfield D, Otto MW. Identifying potential barriers to physical activity adherence: anxiety sensitivity and body mass as predictors of fear during exercise. Cogn. Behav. Ther. 2010;39:28–36. doi: 10.1080/16506070902915261. [DOI] [PubMed] [Google Scholar]
- Somers TJ, Keefe FJ, Carson JW, Pells JJ, Lacaille L. Pain catastrophizing in borderline morbidly obese and morbidly obese individuals with osteoarthritic knee pain. Pain Res. Manag. 2008;13:401–406. doi: 10.1155/2008/652453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers TJ, Keefe FJ, Pells JJ, Dixon KE, Waters SJ, Riordan PA, et al. Pain catastrophizing and pain-related fear in osteoarthritis patients: relationships to pain and disability. J. Pain Symptom. Manag. 2009;37:863–872. doi: 10.1016/j.jpainsymman.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternfeld B, Ngo L, Satariano WA, Tager IB. Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. Am. J. Epidemiol. 2002;156:110–121. doi: 10.1093/aje/kwf023. [DOI] [PubMed] [Google Scholar]
- Swartz A, Strath S, Parker S, Miller N, Cieslik L. Ambulatory activity and body mass index in white and non-white older adults. J. Phys. Act. Health. 2007;4:294–304. doi: 10.1123/jpah.4.3.294. [DOI] [PubMed] [Google Scholar]
- Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin. Nutr. 2007;26:389–399. doi: 10.1016/j.clnu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Thornell LE. Sarcopenic obesity: satellite cells in the aging muscle. Clin. Opin. Clin. Nutr. Metab. Care. 2011;14:22–27. doi: 10.1097/MCO.0b013e3283412260. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . Physical Activity and Health: A Report on the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; Atlanta, GA: 1996. pp. 175–206. [Google Scholar]
- Urso ML. Disuse atrophy of human skeletal muscle: cell signaling and potential interventions. Med. Sci. Sports Exerc. 2009;41:1860–1868. doi: 10.1249/MSS.0b013e3181a6458a. [DOI] [PubMed] [Google Scholar]
- van Gool CH, Penninx BW, Kempen GI, Rejeski WJ, Miller GD, van Eijk JT, et al. Effects of exercise adherence on physical function among overweight older adults with knee osteoarthritis. Arthr. Rheum. 2005;53:24–32. doi: 10.1002/art.20902. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Apovian CM, Kushner RF, Klein S, American Society for Nutrition, NAASO, TOS Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes. Res. 2005;13:1849–1863. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch. Int. Med. 2006;17:172–173. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. New Engl. J. Med. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent HK, Bourguignon C, Vincent KR. Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity (Silv. Spr.) 2006;14:1921–1930. doi: 10.1038/oby.2006.224. [DOI] [PubMed] [Google Scholar]
- Vincent HK, Innes KE, Vincent KR. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diab. Obes. Metab. 2007;9:813–839. doi: 10.1111/j.1463-1326.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- Vincent HK, Lamb KM, Day TI, Tillman SM, Vincent KR, George SZ. Morbid obesity is associated with fear of movement and lower quality of life in patients with knee pain-related diagnoses. PM&R. 2010a;2:713–722. doi: 10.1016/j.pmrj.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Vincent HK, Omli MR, Day TI, Hodges M, Vincent KR, George S. Fear of movement, quality of life and self-reported disability in obese patients with chronic lumbar pain. Pain Med. 2010b;12:154–164. doi: 10.1111/j.1526-4637.2010.01011.x. [DOI] [PubMed] [Google Scholar]
- Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int. J. Obes. 2006;30:400–418. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Geront. A Biol. Sci. Med. Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- Visser M, Harris TB, Langlois J, Hannan MT, Roubenoff R, Felson DT, et al. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J. Gerontol. A Biol. Sci. Med. Sci. 1998a;53:M214–M221. doi: 10.1093/gerona/53a.3.m214. [DOI] [PubMed] [Google Scholar]
- Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J. Am. Geriatr. Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- Visser M, Langlois J, Guralnik JM, Cauley JA, Kronmal RA, Robbins J, et al. High body fatness, but not low fat-free mass, predicts disability in older men and women: the Cardiovascular Health Study. Am. J. Clin. Nutr. 1998b;68:584–590. doi: 10.1093/ajcn/68.3.584. [DOI] [PubMed] [Google Scholar]
- Waters DL, Qualls CR, Dorin RI, Veldhuis JD, Baumgartner RN. Altered growth hormone, cortisol, and leptin secretion in healthy elderly persons with sarcopenia and mixed body composition phenotypes. J. Geront. A Biol. Sci. Med. Sci. 2008;63:536–541. doi: 10.1093/gerona/63.5.536. [DOI] [PubMed] [Google Scholar]
- Weaver GD, Kuo YF, Raji MA, Al Snih S, Ray L, Torres E, et al. Pain and disability in older Mexican-American adults. J. Am. Geriatr. Soc. 2009;57:992–999. doi: 10.1111/j.1532-5415.2009.02263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp. Geront. 2010;45:138–148. doi: 10.1016/j.exger.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose tissue: the new endocrine organ? A review article. Digest. Dis. Sci. 2008 doi: 10.1007/s10620-008-0585-3. doi:10.1007/s10620-008-0585-3. [DOI] [PubMed] [Google Scholar]
- Zamboni M, Mazzali G, Fantin F, Rossi A, DiFrancesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr. Metab. Cardiovasc. Dis. 2007;18:388–395. doi: 10.1016/j.numecd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance: a mini-review. Gerontology. 2009;55:379–386. doi: 10.1159/000212758. [DOI] [PubMed] [Google Scholar]
- Zoico E, Rossi A, Di Francesco V, Sepe A, Olioso D, Pizzini F, et al. Adipose tissue infiltration in skeletal muscle of healthy elderly men: relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J. Geront. A Biol. Sci. Med. Sci. 2010;65:295. doi: 10.1093/gerona/glp155. [DOI] [PMC free article] [PubMed] [Google Scholar]