Abstract
Voriconazole is the treatment of choice for invasive aspergillosis and its use is increasing in pediatrics. Minimal pharmacokinetic data exist in young children. We report voriconazole concentrations for 10 children less than <3 years of age and pharmacokinetic parameters for one infant who had therapeutic drug monitoring performed. Trough concentrations were unpredictable based on dose, highlighting the need to follow values during therapy.
Keywords: invasive aspergillosis, pediatric, voriconazole, pharmacokinetic, therapeutic drug monitoring
INTRODUCTION
Invasive aspergillosis (IA) is a major cause of morbidity and mortality in children with compromised immune systems. The treatment of choice for IA in adults is voriconazole.1 The pharmacokinetics (PK) of voriconazole in children 2–11 years old exhibit dose proportionality over the dosage range of 3–4 mg/kg every 12 hours and significantly lower drug exposure when compared with adults based on area-under-the-curve values.2 Minimal data exist in children aged younger than 2 years. Multiple factors affect drug clearance, including age, cytochrome P4502C19 polymorphisms, hepatic function, and drug interactions.
Studies in adults and older children suggest that voriconazole therapeutic drug monitoring (TDM) may improve antifungal efficacy and safety. Voriconazole trough concentrations ≥1 mcg/mL have been associated with improved response to therapy and survival.3,4 Increased adverse events have been associated with trough concentrations >5–6 mcg/mL.4–6 We report a case of successful treatment of disseminated aspergillosis in a premature infant using voriconazole and micafungin. We also summarize our experience with voriconazole TDM in this infant with IA and 9 other children less <3 years of age.
METHODS
We identified 10 children aged <3 years with proven or probable invasive fungal disease1 hospitalized at Duke University Medical Center (Durham, NC) or Primary Children’s Medical Center (Salt Lake City, UT) from 2008–2010 who received voriconazole and had voriconazole concentrations measured for TDM per standard of care. Voriconazole concentrations were measured with high-performance liquid chromatography with ultraviolet detection performed at FOCUS Diagnostics, Inc. (Cypress, CA) for the infant described in the case report, with a lower limit of quantification (LLOQ) of 0.1 mcg/mL. For the remainder of the patients, voriconazole concentrations were measured with solid phase extraction followed by analysis with high-performance liquid chromatography with ultraviolet detection performed at Duke University Medical Center, with an LLOQ of 0.2 mcg/mL. Voriconazole concentrations that were below the LLOQ were listed in the table as half of the LLOQ.7
The institutional review board of Duke University Medical Center approved the study for patients enrolled at that site. Written informed consent allowing use of identifiable information for publication was obtained for the patient enrolled at Primary Children’s Medical Center.
CASE REPORT
A male infant was born at 24 2/7 weeks’ gestation. Birth weight was 580 grams. He required high-frequency oscillatory ventilation for the first 6 days of life (DOL) and was treated with hydrocortisone for presumed adrenal insufficiency of prematurity and with ampicillin and gentamicin for 7 days for presumed sepsis. On the seventh DOL, he developed necrotizing enterocolitis with perforation of the small bowel and marked clinical deterioration. Broad-spectrum antibiotics, including vancomycin, cefotaxime, and piperacillin/tazobactam were started, and he underwent a laparotomy with resection of necrotic ileum and creation of an end ileostomy.
On DOL 12, marked skin breakdown and exudative drainage was noted at the site of a right antecubital, peripherally inserted central catheter. Upon removal of the catheter dressing, there was a large soft tissue defect, measuring 2 x 2.5 cm, with muscle damage. In addition, there were 3 small black lesions on the right palm. A black eschar was present on the infant’s abdomen at the laparotomy incision site. Antimicrobial therapy was changed to fluconazole, meropenem, and vancomycin.
On DOL 16, cultures from both the arm and abdominal wound sites grew Aspergillus fumigatus. Initial antifungal therapy included liposomal amphotericin B 6 mg/kg every 24 hours IV and caspofungin 5 mg/kg every 24 hours IV. Due to continued clinical deterioration, antifungal therapy was changed 48 hours later to voriconazole 9 mg/kg/dose IV every 12 hours and micafungin 10 mg/kg/dose IV every 24 hours. An abdominal ultrasound on DOL 17 revealed an echogenic mass measuring 8 x 9 x 8 mm within the left lobe of the liver and small hyperechoic foci in both kidneys consistent with fungal disease.
Within 2 days of initiating voriconazole therapy, the arm and abdominal lesions improved significantly, and the infant’s clinical status stabilized. A. fumigatus was isolated again from abdominal wound cultures obtained on day 8 of voriconazole therapy, but cultures were negative by day 28. After 6 weeks of antifungal therapy, the hepatic lesion measured 5 x 6 x 6 mm, and the kidney lesions improved. The arm lesion healed with minimal scarring and complete re-epithelialization after 8 days of voriconazole. The infant completed a 12-week course of combination antifungal therapy with voriconazole and micafungin. During voriconazole treatment, TDM was carried out 4 times. One dosing increase was made to achieve a trough concentration >1 mcg/mL.
Liver function tests were monitored throughout the course of antifungal therapy. Transaminases were unremarkable until 2 days prior to cessation of voriconazole. At that time, both transaminases (range 100–400 U/L) and alkaline phosphatase (range 476–50 U/L) became elevated and continued to be elevated for 6 weeks post treatment without a clear etiology. The conjugated bilirubin was mildly elevated (0.7 mg/dL) approximately 3 weeks after initiation of voriconazole and steadily increased throughout the duration of therapy (max 6.0 mg/dL). The gamma-glutamyl transpeptidase became elevated 19 days after beginning voriconazole therapy. The maximum value was 721 U/L after 5 weeks of therapy and gradually decreased after this. Three months after discontinuation of voriconazole, the transaminases, bilirubin, and alkaline phosphatase almost normalized.
The infant was discharged from the hospital at 6 months of age, without recurrence of fungal disease.
RESULTS
TDM data for the infant described above were combined with data from 9 additional children receiving voriconazole. When treatment was started, patients ranged in age from 2 weeks to 35 months, with a median age of 17.5 months (see table, Supplemental Digital Content 1). Four patients had proven fungal disease, and 6 had probable fungal disease. Three patients died, although no deaths were directly related to fungal disease. One patient received a concomitant medication (phenobarbital) that could have decreased voriconazole concentrations via hepatic CYP450 enzyme induction.8
Dosing regimens ranged from 3.4–14.7 mg/kg/dose every 12 hours. Thirteen voriconazole concentrations (in 8 patients) were measured while patients were receiving IV dosing, and 5 (in 4 patients) were obtained during oral dosing. Two patients (patients 8 and 9) had levels measured both during IV and oral therapy. The majority of patients received both formulations during their course of treatment. However, insufficient data exist in this cohort to compare trough concentrations obtained on IV and oral therapy.
Eighteen plasma voriconazole concentrations were measured, of which 17 were troughs. Most voriconazole concentrations (89%) were obtained after ≥3 voriconazole doses, and 12 (67%) concentrations were obtained after ≥10 doses (5 days) of therapy at the same dose as recommended by the manufacturer.9 Although the half-life of voriconazole in patients <3 years of age is unknown, based on prior pediatric PK studies,2 it is probable that our subjects were at steady state after 3 voriconazole doses, although this should be interpreted with caution as voriconazole half-life is dose-dependent and demonstrates non-linear elimination in children over higher dosing ranges.10
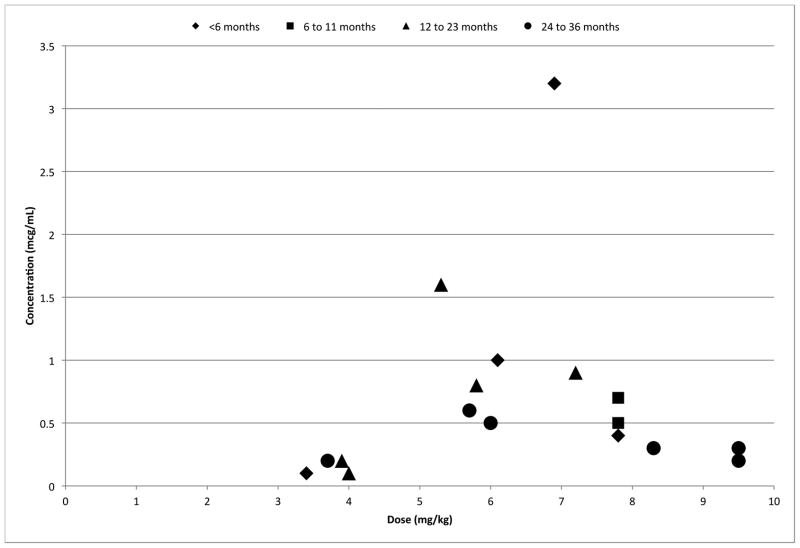
Trough concentrations ranged from 0.1–3.2 mcg/mL, with only 3 (18%) trough values ≥1 mcg/mL. These were associated with body weight-normalized voriconazole doses of 5.3, 6.1, and 6.9 mg/kg per dose after ≥10 doses. Figure 1 shows the relationship between observed voriconazole trough concentrations (mcg/mL) and weight-normalized (mg/kg) voriconazole dose. There was no correlation between dosage and trough concentrations; likewise, there was no correlation between age and trough concentrations.
FIGURE 1.
Relationship between voriconazole dosage and trough blood concentration by subject age (represented by data points; in months).
Eight patients had their voriconazole dose increased (total of 10 dosing increases made). One patient had his/her medication changed from voriconazole to fluconazole. All of the voriconazole trough concentrations temporally related to dosing changes were ≤1 mcg/mL, except for one level of 1.6 mcg/mL. Most patients did not have follow-up trough concentrations measured following dose changes.
The neonate described in the case report was the only patient with >3 plasma concentrations obtained. Using nonlinear regression, a 1-compartment model was fit to the data of this subject (WinNonLin v.6, Pharsight Inc., Cary, NC). The PK parameters for this infant were: volume of distribution 1045 mL/kg (coefficient of variation 56%), clearance 240 mL/h/kg (coefficient of variation 41%), and half-life 3.0 hours (coefficient of variation 42%). Clearance, half-life, and volume of distribution indices were obtained from the 1-compartment model fitted to the observed data. Because plasma values were obtained during different dosing intervals, the pharmacokinetic parameters represent an average over the total treatment period.
Two patients (including the neonate in the case report) had substantial elevation of liver enzymes during voriconazole treatment (as measured by aspartate aminotransferase, alanine aminotransferase, and bilirubin). Of note, the infant described in the case report received total parenteral nutrition, up to 2.3 g/kg/day of intralipids, for 67 of 78 days of voriconazole therapy. In the other patient, liver dysfunction was associated with graft-versus-host disease and ultimately death. Five patients had mild elevation in their transaminases, none exceeding twice the upper limit of normal for age. No patient had voriconazole stopped due to hepatic or other toxicity.
DISCUSSION
IA is a significant cause of morbidity and mortality in hospitalized children with impaired immunity. Voriconazole is more effective than other therapies for IA in adults.1 Data are lacking, however, regarding appropriate dosing strategies for young children; they are therefore at risk for treatment failure or toxicity. We present limited data from 10 patients aged <3 years treated with voriconazole who underwent TDM. This is the largest series to date describing voriconazole TDM in this young population. Although limited, these data strongly suggest that TDM should be used in children <3 years of age who receive voriconazole until additional data are available.
Based on several randomized trials, the 2008 guidelines of the Infectious Diseases Society of America recommend voriconazole for IA in adults.1 Voriconazole use in children is increasing, based on extrapolation from efficacy data in adults. In a recent large multicenter retrospective review of pediatric IA, voriconazole was prescribed for more than half of patients.11 However, published pharmacokinetic data for voriconazole in children 3 years of age and younger is limited to 24 patients.3,12–20 Doses and dosing intervals varied, ranging from 3.4 mg/kg/day to 23 mg/kg/day. Target voriconazole trough levels >1 mcg/mL were difficult to achieve, and weight-adjusted doses that did achieve target concentrations varied widely. These data are consistent with our experience that voriconazole concentrations are highly variable and are difficult to predict based on weight-based dosing.
The factors contributing to the difficulty in achieving target voriconazole concentrations in young children are poorly understood. One possible explanation is phenotypic differences in CYP2C19 enzymatic activity because this is the major route of voriconazole elimination.2 In young infants, this finding could be exacerbated by additional developmental changes associated with CYP2C19 maturation that have not been fully described.21 Genetic polymorphisms in a recently discovered voriconazole elimination pathway22 could also account for some of the observed inter-individual variability in drug concentrations; however, to our knowledge this has not been described. Another potential contributing factor is differences in voriconazole protein-binding capacity between young children and adults and among children as they age. However, because voriconazole is not highly protein-bound, substantial changes in this binding capacity or in the protein – drug-binding dynamics (nonlinear protein binding) would have to occur to observe an effect in the dose-exposure relationship. Lastly, underlying disease has been identified as a factor influencing voriconazole disposition in children.23 The mechanism for this association has not been described — it may be related to drug interactions or disease acuity in children with different underlying conditions.
Common side effects of voriconazole include visual disturbances, rash, and mild, reversible hepatitis.24 The visual disturbances caused by voriconazole are associated with reversible electroretinogram tracing alterations,24 and the safety of voriconazole in very young patients with developing retinas is unknown. Liver dysfunction in patients receiving voriconazole may be multifactorial and related to comorbid conditions and concomitant medications with liver toxicity.24 However, concerns exist that increased voriconazole values may be related to elevated transaminases.24 One study showed an increased association between neurotoxicity (reversible encephalopathy) and voriconazole trough concentrations ≥5.5 mcg/mL, but no correlation between severe hepatic toxicity and drug levels.4 Due to the retrospective nature of this study, laboratory data for many of our patients are limited, often obtained only on the day of the voriconazole concentration. Therefore, we are unable to comment upon a potential relationship between voriconazole concentration and serum transaminase values in our patients. Moreover, none of the trough concentrations obtained were in the toxic range (and all but 2 were ≤1 mcg/mL).
Limitations to our study include the retrospective study design, small number of patients, few patients with measurement of multiple drug levels, trough values not always obtained at steady-state, and a heterogeneous patient population with complex comorbid conditions. For subjects 1 through 9, voriconazole administration times were obtained from nursing documentation on the medical record.
Despite these limitations, these data support several conclusions regarding voriconazole dosing in children <3 years of age: 1) weight-based dosing did not reliably predict plasma trough concentrations, emphasizing the need for additional population PK studies in very young children and infants; 2) higher doses are needed in young children to achieve serum trough concentrations ≥1 mcg/mL; and 3) this study underscores the importance of TDM in young children receiving voriconazole and the need to follow levels during therapy. Given the difficulty in achieving target serum concentrations and the significant morbidity and mortality of IA in these very young patients, therapy with 2 active antifungal agents could be considered until target voriconazole trough concentrations are achieved.
Acknowledgments
This study was reviewed by the institutional review board of Duke University School of Medicine. This work was supported by the H.A. and Edna Benning Foundation.
Sources of funding: Dr. Doby receives support from the H.A. and Edna Benning Foundation. Dr. Blaschke receives support from NIH/NIAID 1K23AI079401 and 1U01AI082184-01, and CDC 1U18IP000303-01. Dr. Ward received support from NCRR 3UL1RR025764-02S3, NICHD HD060559-01, and NICHD 5 R01 HD060559-02. Dr. Pavia receives support from NIAID 1R01AI089489-01, CDC 1U181P000303, and NIAID U01-AI74419. Dr. Cohen-Wolkowiez receives support from NICHD 1K23HD064814-01, the non-profit organization Thrasher Research Foundation for his work in neonatal pharmacology, and from Pfizer for neonatal and pediatric drug development. Dr. Moran was supported in part by NIH CTSA grant 1UL 1RR024128-01. This work was also supported by the United States government (1R01HD057956-02, 1R01FD003519-01, 1U10-HD45962-06, 1K24HD058735-01, and Government Contract HHSN267200700051C to Dr. Benjamin).
Footnotes
Conflicts of interest: Dr. Benjamin is a consultant for Pfizer, Biosynexus, and Johnson & Johnson and a principal investigator for Astellas Pharma and AstraZeneca. Dr. Cohen-Wolkowiez is a consultant for Pfizer. Dr. Moran is a consultant for Pfizer and a principal investigator for Astellas. Dr. Pavia has consulted for Pfizer. The remaining authors have no potential conflicts to report.
References
- 1.Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 2.Walsh TJ, Karlsson MO, Driscoll T, et al. Pharmacokinetics and safety of intravenous voriconazole in children after single- or multiple-dose administration. Antimicrob Agents Chemother. 2004;48:2166–2172. doi: 10.1128/AAC.48.6.2166-2172.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neely M, Rushing T, Kovacs A, Jelliffe R, Hoffman J. Voriconazole pharmacokinetics and pharmacodynamics in children. Clin Infect Dis. 2010;50:27–36. doi: 10.1086/648679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46:201–211. doi: 10.1086/524669. [DOI] [PubMed] [Google Scholar]
- 5.Ueda K, Nannya Y, Kumano K, et al. Monitoring trough concentration of voriconazole is important to ensure successful antifungal therapy and to avoid hepatic damage in patients with hematological disorders. Int J Hematol. 2009;89:592–599. doi: 10.1007/s12185-009-0296-3. [DOI] [PubMed] [Google Scholar]
- 6.Zonios DI, Gea-Banacloche J, Childs R, Bennett JE. Hallucinations during voriconazole therapy. Clin Infect Dis. 2008;47:e7–e10. doi: 10.1086/588844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28:481–504. doi: 10.1023/a:1012299115260. [DOI] [PubMed] [Google Scholar]
- 8.Theuretzbacher U, Ihle F, Derendorf H. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet. 2006;45:649–663. doi: 10.2165/00003088-200645070-00002. [DOI] [PubMed] [Google Scholar]
- 9.Pfizer Inc. Vfend US physician prescribing information. New York, NY: Pfizer Inc; Jun, 2011. revised. [Google Scholar]
- 10.Walsh TJ, Driscoll T, Milligan PA, et al. Pharmacokinetics, safety, and tolerability of voriconazole in immunocompromised children. Antimicrob Agents Chemother. 2010;54:4116–4123. doi: 10.1128/AAC.00896-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgos A, Zaoutis TE, Dvorak CC, et al. Pediatric invasive aspergillosis: a multicenter retrospective analysis of 139 contemporary cases. Pediatrics. 2008;121:e1286–e1294. doi: 10.1542/peds.2007-2117. [DOI] [PubMed] [Google Scholar]
- 12.Spriet I, Cosaert K, Renard M, et al. Voriconazole plasma levels in children are highly variable. Eur J Clin Microbiol Infect Dis. 2011;30:283–287. doi: 10.1007/s10096-010-1079-8. [DOI] [PubMed] [Google Scholar]
- 13.Shima H, Miharu M, Osumi T, Takahashi T, Shimada H. Differences in voriconazole trough plasma concentrations per oral dosages between children younger and older than 3 years of age. Pediatr Blood Cancer. 2010;54:1050–1052. doi: 10.1002/pbc.22451. [DOI] [PubMed] [Google Scholar]
- 14.Gerin M, Mahlaoui N, Elie C, et al. Therapeutic drug monitoring of voriconazole after intravenous administration in infants and children with primary immunodeficiency. Ther Drug Monit. 2011;33:464–466. doi: 10.1097/FTD.0b013e3182241b2b. [DOI] [PubMed] [Google Scholar]
- 15.Bruggemann RJ, van der Linden JW, Verweij PE, Burger DM, Warris A. Impact of therapeutic drug monitoring of voriconazole in a pediatric population. Pediatr Infect Dis J. 2011;30:533–534. doi: 10.1097/INF.0b013e318204d227. [DOI] [PubMed] [Google Scholar]
- 16.Frankenbusch K, Eifinger F, Kribs A, Rengelshauseu J, Roth B. Severe primary cutaneous aspergillosis refractory to amphotericin B and the successful treatment with systemic voriconazole in two premature infants with extremely low birth weight. J Perinatol. 2006;26:511–514. doi: 10.1038/sj.jp.7211532. [DOI] [PubMed] [Google Scholar]
- 17.Maples HD, Stowe CD, Saccente SL, Jacobs RF. Voriconazole serum concentrations in an infant treated for Trichosporon beigelii infection. Pediatr Infect Dis J. 2003;22:1022–1024. doi: 10.1097/01.inf.0000095167.38306.76. [DOI] [PubMed] [Google Scholar]
- 18.Muldrew KM, Maples HD, Stowe CD, Jacobs RF. Intravenous voriconazole therapy in a preterm infant. Pharmacotherapy. 2005;25:893–898. doi: 10.1592/phco.2005.25.6.893. [DOI] [PubMed] [Google Scholar]
- 19.Santos RP, Sanchez PJ, Mejias A, et al. Successful medical treatment of cutaneous aspergillosis in a premature infant using liposomal amphotericin B, voriconazole and micafungin. Pediatr Infect Dis J. 2007;26:364–366. doi: 10.1097/01.inf.0000258698.98370.89. [DOI] [PubMed] [Google Scholar]
- 20.Walsh TJ, Lutsar I, Driscoll T, et al. Voriconazole in the treatment of aspergillosis, scedosporiosis, and other invasive fungal infections in children. Pediatr Infect Dis J. 2002;21:240–248. doi: 10.1097/00006454-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Ward RM, Tammara B, Sullivan SE, et al. Single-dose, multiple-dose, and population pharmacokinetics of pantoprazole in neonates and preterm infants with a clinical diagnosis of gastroesophageal reflux disease (GERD) Eur J Clin Pharmacol. 2010;66:555–561. doi: 10.1007/s00228-010-0811-8. [DOI] [PubMed] [Google Scholar]
- 22.Yanni SB, Annaert PP, Augustijns P, Ibrahim JG, Benjamin DK, Jr, Thakker DR. In vitro hepatic metabolism explains higher clearance of voriconazole in children versus adults: role of CYP2C19 and flavin-containing monooxygenase 3. Drug Metab Dispos. 2010;38:25–31. doi: 10.1124/dmd.109.029769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kassir N, Théorêt Y, Litalien C, et al. Population pharmacokinetics of intravenous and oral voriconazole in children [Abstract A1-586/40]. 49th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco, CA. September 12–14, 2009; Washington, DC: American Society for Microbiology; 2009. [Google Scholar]
- 24.Johnson LB, Kauffman CA. Voriconazole: a new triazole antifungal agent. Clin Infect Dis. 2003;36:630–637. doi: 10.1086/367933. [DOI] [PubMed] [Google Scholar]