Abstract
This article is intended to raise awareness of the adaptive value of endurance exercise (particularly running) in the evolutionary history of humans, and the implications of the genetic disposition to exercise for the aging populations of modern technology-driven societies. The genome of Homo sapiens has evolved to support the svelte phenotype of an endurance runner, setting him/her apart from all other primates. The cellular and molecular mechanisms underlying the competitive advantages conferred by exercise capacity in youth can also provide a survival benefit beyond the reproductive period. These mechanisms include up-regulation of genes encoding proteins involved in protecting cells against oxidative stress, disposing of damaged proteins and organelles, and enhancing bioenergetics. Particularly fascinating are the signaling mechanisms by which endurance running changes the structure and functional capabilities of the brain and, conversely, the mechanisms by which the brain integrates metabolic, cardiovascular and behavioral responses to exercise. As an emerging example, I highlight the roles of brain-derived neurotrophic factor (BDNF) as a mediator of the effects of exercise on the brain, and BDNF s critical role in regulating metabolic and cardiovascular responses to endurance running. A better understanding of such healthspan-extending actions of endurance exercise may lead to new approaches for improving quality of life as we advance in the coming decades and centuries.
Introduction
The take home message of this article can be appreciated from the following few sentences in which I comment on daily life in modern societies. Ah, this is the life – no need to run, nary even to walk. We curse the ‘inconvenience’ of a broken elevator and the ‘annoying’ bicycle rider who slows our drive home. We work with our fingertips and relax by watching television, while consuming large amounts of omnipresent tasty morsels. As a consequence, our body and brain experience a chronic positive energy balance. Unless we are motivated to rectify this dangerous lifestyle, obesity and/or insulin resistance occur, hastening the development of age-related diseases including diabetes, cardiovascular disease, cancers and neurodegenerative disorders. Because this scenario has become hauntingly common, our society must endure the increasing burden on the health care system and the workforce as the time window of productivity of persons in positive energy balance (PPEBs) shrinks.
Recently, several investigators have placed the ongoing epidemic of obesity and associated diseases in the context of the evolutionary history of Homo sapiens (Booth and Lees, 2007; O’ Keefe et al., 2010). The idea is that a sedentary lifestyle betrays the evolutionary history encoded in our genes, a genetic code for periodic endurance running and intermittent feeding. Here I focus on the notion that for optimal fitness our genetic code demands challenges to the cells within which it resides, challenges that stimulate the expression of ‘survival genes’ that encode for proteins that enhance the ability of the cells to withstand oxidative and metabolic stress. The latter ‘use it or lose it’ hypothesis falls within the broader concept of hormesis which we have defined previously as “an evolutionarily conserved process in which a low dose of a stressful stimulus activates an adaptive response that increases the resistance of the cell or organism to a moderate to severe level of stress” (Calabrese et al., 2007). Though adaptive stress responses occur in cells throughout the body, I will comment on the adaptive responses of muscle and nerve cells to the stress of physical exercise.
Homo sapiens are unique among primates in their capability for endurance running
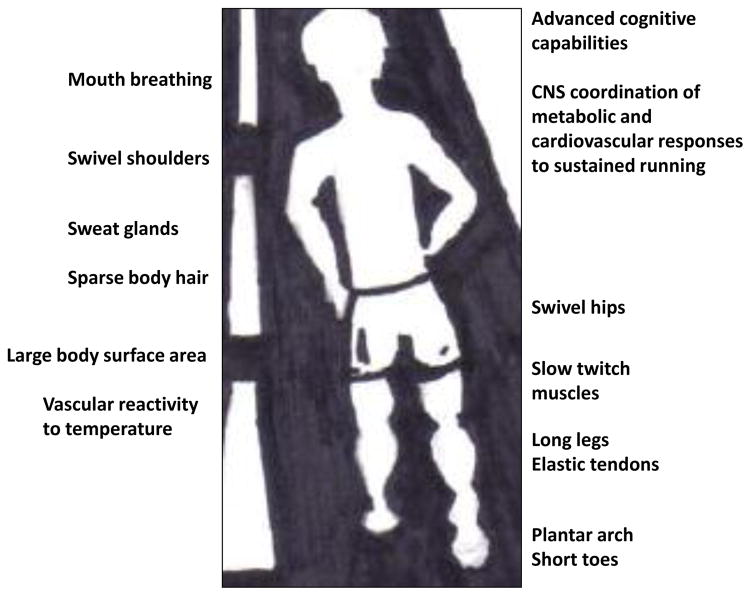
Before delving into an exploration of the molecular events occurring within muscle and brain cells during and after exercise that may protect against aging, I will briefly review some of the emerging information concerning the evolution of the remarkable capacity of humans for endurance exercise. An analysis of the structural and physiological underpinnings of endurance running ability among mammals revealed several features of the genus Homo that evolved within the past 2 million years that endow humans with superior sustained long distance running capability (Bramble and Lieberman, 2004). Humans are the only primates capable of sustained endurance running. One structural feature of humans that is believed to facilitate energetic efficiency during endurance running is legs with long spring-like tendons (e.g., the Achilles tendon) that attach short muscle fibers to leg and foot bones (Figure 1). The plantar arch of the foot may also be an adaptation for endurance running in humans which functions as a spring that returns up to 20% of the energy generated during the weight-loading phase of the running stride. Additional adaptations for endurance running in humans (compared to lower primates and quadriped mammals) include: long legs and stride length; relatively small feet with short toes (compared to other nonhuman primates) (Rolian et al., 2009); slow twitch muscle cells; large gluteus maximus muscle; structural modifications of the hips and shoulders that generate counter-balancing forces to enable smooth transitions between strides; sweat glands, reduced body hair and an elongated body form for heat dissipation (Marino, 2008); and mouth breathing.
Figure 1.
Adaptions of humans for endurance running.
A range of genetic and environmental factors may determine endurance running capabilities among individuals. A recent example is a study reporting that a polymorphism in the gene encoding α-actinin 3 was present in significantly higher frequencies in both elite endurance athletes and centenarians compared to elite power athletes and non-centenarians (Fiuza-Luces et al., 2011). Another example is the level of testosterone experienced by the developing fetus. A low ratio of the length of the second digit (index finger) to the fourth digit (ring finger), which has long been known to be associated with relatively high levels of fetal testosterone, was recently reported to be associated with superior endurance running (Manning et al., 2007).
A brain – endurance connection?
Interestingly, the increased size of the human brain relative to other primates, and its resultant cognitive capabilities, may have played an important role in the endurance running phenotype. Consistent with the latter possibility, a study in which brain size and maximum metabolic rate (MMR; a proxy for exercise capacity) were measured in a range of mammals revealed a positive correlation between brain size and MMR across a wide range of species (Raichlen and Gordon, 2011). Why might this be so? One reason is that distance running in our human ancestors was purposeful and required complex cognitive processes. The retention and recall of the details (topography, potential food sources, water sources, etc.) of large areas of land was likely required to maximize the acquisition of resources that were ‘spread thin’ in a timely manner. Individuals who possessed superior cognitive processing ability and endurance running capacity would be expected to have an advantage over those with lesser mental and endurance capabilities. The nervous system controls all aspects of body movements over a large time scale from milliseconds to hours, days, months and years. It is the planning of locomotion/behaviors that is mediated by nerve cell circuits housed in the evolutionarily expanded regions of the cerebral cortex in humans.
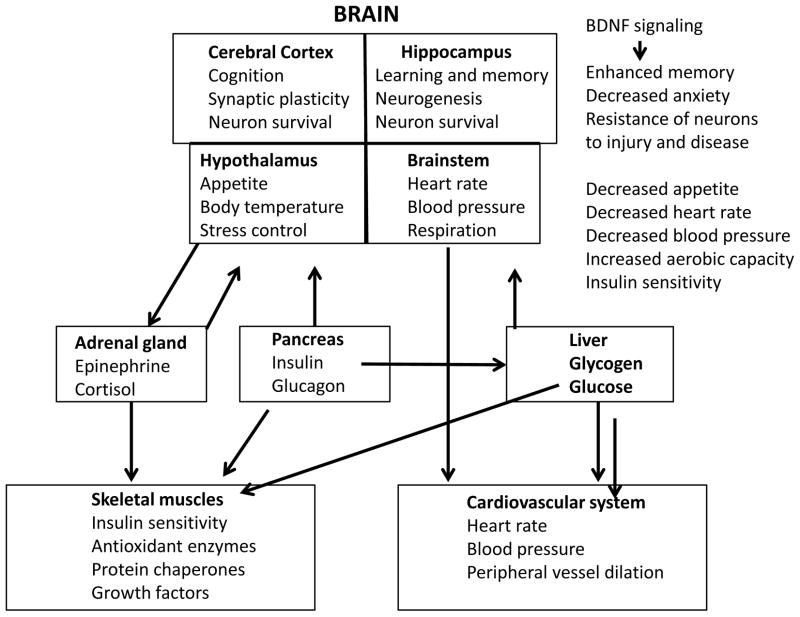
Not only does exercise strengthen muscle cells, it also strengthens brain cells (Figure 2). Indeed, it is now well-established that endurance exercise can stimulate the growth of brain cells and can improve cognitive function (see articles by Stranahan et al. and van Praag et al. in this issue). Running stimulates the production of neurotrophic factors, most notably brain-derived neurotrophic factor (BDNF), which promotes the growth of dendrites, the strengthening of synapses and even the production of new nerve cells from stem cells in some brain regions (van Praag et al., 1999; Duman, 2004; Stranahan et al., 2009; Kobilo et al., 2011a, 2011b). The latter scenario makes sense from the standpoint of optimizing the ability of the animal or human to acquire, store and protect resources. For example, during a 1 hour run many different potential sources of food or materials that could be used for shelter could be encountered, and up-regulation of the expression of BDNF at synapses activated during the encounter with the resource would promote strengthening of those synapses and long-term retention of the memory of the resource and its location. The cellular and molecular mechanisms by which BDNF expression is increased in response to exercise, and the signal transduction pathways activated by BDNF and some of the genes induced by BDNF that strengthen neurons are described below and have been reviewed in more detail recently (Lu et al., 2008; Greenberg et al., 2009; Cohen-Coray et al., 2010). Interestingly, emerging evidence indicates that BDNF plays prominent roles in the regulation of energy metabolism via actions at multiple sites in the nervous system (Noble et al., 2011). For example,
Figure 2.
Brain-derived neurotrophic factor (BDNF) is an integrator of adaptive responses of the brain and body to endurance running.
Exercise retards age-related decrements in muscle strength and endurance via hormesis
While it is well-known that muscles atrophy if not used, and that regular exercise will increase muscle strength and endurance, the underlying molecular mechanisms have only recently emerged. As reviewed by Jackson and McArdle (2011), in young animals exercise induces superoxide and nitric oxide in the muscle cells, and the latter reactive oxygen species (ROS) stimulate several transcription factors (AP-1, HSF-1 and NF-κB) which, in turn induce the expression of antioxidant enzymes and protein chaperones. The increased levels of the latter cytoprotective proteins extend well beyond the exercise period such that the stress during the exercise results in a long-lasting resilience of the muscle cells. The importance of ROS-mediated signaling in the beneficial effects of exercise on muscle cells is supported by data showing that treatment of human subjects with antioxidants (vitamins E and C) abolish the ability of exercise to activate PGC-1a and enhance insulin sensitivity in muscle cells (Ristow et al., 2009). The oxidative stress that occurs in muscle cells during exercise can also trigger mitochondrial biogenesis, a process mediated by PGC-1α, a master regulator of the growth and division of mitochondria (Wright et al., 2007). Endurance exercise engages cytoprotective responses in all of the major subcellular organelles. For example, the endoplasmic reticulum (ER) responds to exercise by engaging a sequence of events called the unfolded protein response (UPR) involving the up-regulation and/or activation of multiple ER proteins (e.g., glucose-regulated protein 78 and calreticulin) which ensure that newly synthesized proteins are properly folded. The latter adaptive response to exercise is mediated by PGC-1α and coactivation of ATF6α (Wu et al., 2011). In addition to these cell-autonomous signaling events that occur in muscle cells in response to exercise, those cells also produce factors that are released and promote the growth and resilience of adjacent muscle cells; such factors include insulin-like growth factor 1 (Harridge, 2003) and vascular endothelial cell growth factor (Olfert et al., 2009).
The ability of muscle cells to respond adaptively to exercise is compromised during the aging process. Thus, it was found that levels of protein chaperones and antioxidant enzymes were not increased in skeletal muscle in response to exercise in very old (28 month-old) rats (Vasilaki et al., 2002, 2006). Interestingly, muscle cells in very old animals exhibit elevated constitutive activation of the redox-sensitive transcription factor NF-κB, and some antioxidant enzymes and pro-inflammatory cytokines (Jackson and McArdle, 2011). Exercise also fails to induce mitochondrial biogenesis in muscles from very old animals. The combination of increased basal oxidative stress/damage and an inflammatory state may render the old muscle cells incapable of responding adaptively to exercise. Because regular exercise during midlife and continuing into old age can delay the onset of age-related muscle atrophy (Rogers and Evans, 1993; Peterson et al., 2010), it is likely that the ROS-mediated signaling pathways that mediate the beneficial effects of exercise on muscles are preserved as a result of the midlife exercise. However, numerous studies of human subjects have shown that endurance exercise benefits muscles and the cardiovascular system even when initiated in elderly subjects who were relatively sedentary in midlife (Hollmann et al., 2007; Williams and Stewart, 2009).
Neurotrophic factors mediate anti-aging effects of endurance exercise on the brain
It has become clear that there is a widespread activation of signaling pathways involved in adaptive stress responses in cells of many different organ systems that occurs during and after endurance exercise (Radak et al., 2008). Nerve cells in the brain are remarkably responsive to exercise. Studies of animal models and of human subjects support the hypothesis that exercise induces the expression of neurotrophic factors which, in turn, promote structural and functional plasticity of neurons and their resistance to injury and disease (Gomez-Pinilla, 2008; Mattson and Wan, 2008; Zoladz and Pilc, 2010). BDNF has been the most intensively studied exercise-induced neurotrophic factor. Recent studies of human subjects have shown that age-related decrements in cognitive performance are associated with reduced circulating BDNF levels (Erickson et al, 2010) and that aerobic exercise training can elevate BDNF levels, increase the size of the hippocampus and improve memory in elderly subjects (Erickson et al., 2011). Two mechanisms by which BDNF expression is induced by running are synaptic activity-mediated activation of the transcription factor CREB (cyclic AMP response element-binding protein (CREB; Conti et al., 2002; Chen and Russo-Neustadt, 2009) and energetic stress-mediated activation of the transcription factor NF-κB (Marini et al., 2004; Kairisalo et al., 2009). A major signaling pathway upstream of CREB involves activation of synaptic glutamate receptors which results in calcium influx and activation of a calcium/calmodulin-dependent protein kinase (Hu et al., 1999). CREB can be considered a stress sensor because it not only induces BDNF expression, but also expression of multiple genes that encode proteins that protect neurons against oxidative stress, including the DNA repair enzyme APE1 (Yang et al., 2010) and Bcl-2 (Meller et al., 2005).
Multiple intercellular signaling pathways have evolved that coordinate the adaptive responses of the brain to exercise. For example, in addition to BDNF, exercise also induces the expression of fibroblast growth factor 2 (FGF2) and VEGF in the brain (Gomez-Pinilla and Kesslak, 1998; Fabel et al., 2003). FGF2 can protect neurons against oxidative, metabolic and excitotoxic injury (Cheng and Mattson, 1991; Mattson et al., 1995) and also promotes the growth of astrocytes, a type of glial cell that provides metabolic support to neurons (Petroski et al., 1991). FGF2 also promotes the proliferation of neural progenitor cells and may, together with BDNF, play a key role in exercise-induced neurogenesis (Bull and Bartlett, 2005). In addition, it has been shown that running increases the expression of VEGF and enhances angiogenesis in the hippocampus of rodents (Fabel et al., 2003; Kerr et al., 2010), which would be expected to increase the supply of nutrients to the brain cells. During endurance exercise, energy utilization by skeletal and cardiac muscle are greatly increased, but it is critical that the energy supply to the brain be maintained because nerve cells require a constant supply of glucose and will quickly become dysfunctional if glucose levels are reduced as occurs in hypoglycemia and ischemic conditions. Endurance training can, therefore, enhance the availability of glucose to neurons.
In order to begin to understand the scope and integration of adaptive changes that occur in brain cells in response to endurance exercise in the context of aging, we performed a large-scale gene array analysis of brain tissue samples from old mice that were life-long runners compared to age-matched non-runner control mice (Stranahan et al., 2010). Prior to euthanizing the mice, they were trained in a water maze to stimulate activity in nerve cell circuits involved in learning and memory processes. Compared to the more sedentary mice, cells in the runners brains exhibited greater activation of genes involved in mitochondrial function and synaptic plasticity, and lower levels of activation of genes involved in oxidative stress and lipid metabolism. One interesting pathway modified by running was the pathway activated by the Wnt protein (Stranahan et al., 2010). It was recently reported that mice lacking the Wnt receptor frizzled-related protein exhibit reduced running exercise performance (Lories et al., 2009), although future studies will be required to establish whether Wnt signaling in the brain (or other tissues) is critical for endurance running.
BDNF has emerged as a pivotal regulator of energy metabolism and a mediator of many different adaptive responses of the brain and body to endurance exercise (see Pedersen et al., 2009; Noble et al., 2011 for review). Mice with reduced levels of mice exhibit hyperphagia, and develop obesity and diabetes (Kernie et al., 2000; Duan et al., 2003). Increased expression of BDNF may mediate, in part, the enhanced insulin sensitivity that occurs in response to endurance exercise. The latter possibility is consistent with studies showing that infusion of BDNF into the brain can reduce plasma glucose levels and ameliorate diabetes in mice (Tonra et al., 1999; Nakagawa et al., 2000). A recent study provided evidence that hypothalamic BDNF signaling can induce the generation of brown fat cells within white adipose deposits by a mechanism involving modulation of the autonomic innervation of the white fat (Cao et al., 2011). Cardiovascular adaptations to endurance running may also involve BDNF signaling in the nervous system. We found that intermittent food deprivation (which is known to extend lifespan in rodents) results in reductions in resting heart rate and blood pressure, and improved cardiovascular adaptation to stress in rats (Wan et al., 2003). These effects of intermittent food deprivation are similar to those that occur in response to endurance running, and involve increased parasympathetic tone and increased heart rate variability (Mager et al., 2006). More recently we provided evidence that BDNF signaling in the brainstem enhances parasympathetic tone and reduces heart rate (Griffioen et al., Society for Neuroscience Abstract 859.14/M5; 2010), suggesting a role for BDNF as a mediator of the effects of exercise on the heart. Thus, the emerging picture of the brain – body interface and endurance includes a prominent role for BDNF as an integrator of many of the major systems that regulate neural, endocrine and cardiovascular adaptations to and for endurance exercise.
Conclusions
In the not too distant past pizzas were not delivered, there were no drive-through fast-food restaurants, and no automobiles. Quite the contrary, during our evolutionary history, there was selection for genes that ‘posed the questions’ : Why sit when you can walk and why walk when you can run? From the perspective of optimal health, we have over-engineered our lives, not only from the perspective of technologies that reduce the necessity of exercise, but also in more subtle ways. One example is the running shoe industry, which has promulgated the myth that cushion and toe compartment stability reduce injuries. In fact, it has recently been directly determined that barefoot runners experience less strain on their legs than do shod runners because the barefoot runners are ‘toe strikers’ and the shod runners are ‘heel strikers’ (Lieberman et al., 2010). Humans exhibit numerous adaptations that enable endurance running that include features of musculoskeletal anatomy and physiology, cardiovascular regulation, and metabolic efficiency. A fascinating aspect of the evolution of endurance running capability is the apparent co-evolution of signaling pathways that regulate neuroplasticity and peripheral adaptations to exercise. I have highlighted BDNF signaling as one such pathway, and it will be of interest to delve deeply into the evolutionary origins of BDNF and its receptor trkB to determine if and how it influenced selection for endurance running phenotypes. Other signaling mechanisms that integrate the response of multiple organ systems to endurance running will undoubtedly be discovered. Modern methods for genome-wide DNA sequencing, and analysis of epigenetic modifications (e.g., methylation and acetylation), will enable discovery of the inherited and acquired molecular factors involved in endurance capacity. In the more distant future, knowledge of the various pathways that promote endurance phenotypes may lead to novel interventions that promote optimal health.
HIGHLIGHTS.
Humans are anatomically and metabolically suited for running long distances
Endurance running improves health by enabling cells to cope with stress
The human brain evolved to process complex information related to resource acquisition
Brain-derived neurotrophic factor mediates multiple effects of exercise on the brain
Acknowledgments
This research was supported entirely by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345–352. doi: 10.1038/nature03052. [DOI] [PubMed] [Google Scholar]
- Bull ND, Bartlett PF. The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J Neurosci. 2005;25:10815–10821. doi: 10.1523/JNEUROSCI.3249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, Cedergreen N, Cherian MG, Chiueh CC, Clarkson TW, Cook RR, Diamond DM, Doolittle DJ, Dorato MA, Duke SO, Feinendegen L, Gardner DE, Hart RW, Hastings KL, Hayes AW, Hoffmann GR, Ives JA, Jaworowski Z, Johnson TE, Jonas WB, Kaminski NE, Keller JG, Klaunig JE, Knudsen TB, Kozumbo WJ, Lettieri T, Liu SZ, Maisseu A, Maynard KI, Masoro EJ, McClellan RO, Mehendale HM, Mothersill C, Newlin DB, Nigg HN, Oehme FW, Phalen RF, Philbert MA, Rattan SI, Riviere JE, Rodricks J, Sapolsky RM, Scott BR, Seymour C, Sinclair DA, Smith-Sonneborn J, Snow ET, Spear L, Stevenson DE, Thomas Y, Tubiana M, Williams GM, Mattson MP. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, During MJ. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14:324–338. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Russo-Neustadt AA. Running exercise-induced up-regulation of hippocampal brain-derived neurotrophic factor is CREB-dependent. Hippocampus. 2009;19:962–972. doi: 10.1002/hipo.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B, Mattson MP. NGF and bFGF protect rat hippocampal and human cortical neurons against hypoglycemic damage by stabilizing calcium homeostasis. Neuron. 1991;7:1031–1041. doi: 10.1016/0896-6273(91)90347-3. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol. 2010;70:271–288. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti AC, Cryan JF, Dalvi A, Lucki I, Blendy JA. cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J Neurosci. 2002;22:3262–3268. doi: 10.1523/JNEUROSCI.22-08-03262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Mattson MP. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology. 2003;144:2446–2453. doi: 10.1210/en.2002-0113. [DOI] [PubMed] [Google Scholar]
- Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, Pence BD, Martin SA, Vieira VJ, Woods JA, McAuley E, Kramer AF. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30:5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Fiuza-Luces C, Ruiz JR, Rodríguez-Romo G, Santiago C, Gómez-Gallego F, Yvert T, Cano-Nieto A, Garatachea N, Morán M, Lucia A. Are 'endurance' alleles 'survival' alleles?. Insights from the ACTN3 R577X polymorphism. PLoS One. 2011 Mar 3;6(3):e17558. doi: 10.1371/journal.pone.0017558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Pinilla F, So V, Kesslak JP. Spatial learning and physical activity contribute to the induction of fibroblast growth factor: neural substrates for increased cognition associated with exercise. Neuroscience. 1998;85:53–61. doi: 10.1016/s0306-4522(97)00576-9. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F. The influences of diet and exercise on mental health through hormesis. Ageing Res Rev. 2008;7:49–62. doi: 10.1016/j.arr.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci. 2009;29:12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harridge SD. Ageing and local growth factors in muscle. Scand J Med Sci Sports. 2003;13:34–39. doi: 10.1034/j.1600-0838.2003.20235.x. [DOI] [PubMed] [Google Scholar]
- Hollmann W, Strüder HK, Tagarakis CV, King G. Physical activity and the elderly. Eur J Cardiovasc Prev Rehabil. 2007;14:730–739. doi: 10.1097/HJR.0b013e32828622f9. [DOI] [PubMed] [Google Scholar]
- Hu SC, Chrivia J, Ghosh A. Regulation of CBP-mediated transcription by neuronal calcium signaling. Neuron. 1999;22:799–808. doi: 10.1016/s0896-6273(00)80738-2. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, McArdle A. Age-related changes in skeletal muscle reactive oxygen species generation and adaptive responses to reactive oxygen species. J Physiol. 2011;589:2139–2145. doi: 10.1113/jphysiol.2011.206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kairisalo M, Korhonen L, Sepp M, Pruunsild P, Kukkonen JP, Kivinen J, Timmusk T, Blomgren K, Lindholm D. NF-kappaB-dependent regulation of brain-derived neurotrophic factor in hippocampal neurons by X-linked inhibitor of apoptosis protein. Eur J Neurosci. 2009;30:958–966. doi: 10.1111/j.1460-9568.2009.06898.x. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr AL, Steuer EL, Pochtarev V, Swain RA. Angiogenesis but not neurogenesis is critical for normal learning and memory acquisition. Neuroscience. 2010;171:214–226. doi: 10.1016/j.neuroscience.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011a;18:605–609. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Yuan C, van Praag H. Endurance factors improve hippocampal neurogenesis and spatial memory in mice. Learn Mem. 2011b;18:103–107. doi: 10.1101/lm.2001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman DE, Venkadesan M, Werbel WA, Daoud AI, D'Andrea S, Davis IS, Mang'eni RO, Pitsiladis Y. Foot strike patterns and collision forces in habitually barefoot versus shod runners. Nature. 2010;463:531–535. doi: 10.1038/nature08723. [DOI] [PubMed] [Google Scholar]
- Lories RJ, Peeters J, Szlufcik K, Hespel P, Luyten FP. Deletion of frizzled-related protein reduces voluntary running exercise performance in mice. Osteoarthritis Cartilage. 2009;17:390–396. doi: 10.1016/j.joca.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89:312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager DE, Wan R, Brown M, Cheng A, Wareski P, Abernethy DR, Mattson MP. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 2006;20:631–637. doi: 10.1096/fj.05-5263com. [DOI] [PubMed] [Google Scholar]
- Manning JT, Morris L, Caswell N. Endurance running and digit ratio (2D:4D): implications for fetal testosterone effects on running speed and vascular health. Am J Hum Biol. 2007;19:416–421. doi: 10.1002/ajhb.20603. [DOI] [PubMed] [Google Scholar]
- Marini AM, Jiang X, Wu X, Tian F, Zhu D, Okagaki P, Lipsky RH. Role of brain-derived neurotrophic factor and NF-kappaB in neuronal plasticity and survival: From genes to phenotype. Restor Neurol Neurosci. 2004;22:121–130. [PubMed] [Google Scholar]
- Marino FE. The evolutionary basis of thermoregulation and exercise performance. Med Sport Sci. 2008;53:1–13. doi: 10.1159/000151545. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Lovell MA, Furukawa K, Markesbery WR. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J Neurochem. 1995;65:1740–1751. doi: 10.1046/j.1471-4159.1995.65041740.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Wan R. Neurotrophic factors in autonomic nervous system plasticity and dysfunction. Neuromolecular Med. 2008;10:157–168. doi: 10.1007/s12017-007-8021-y. [DOI] [PubMed] [Google Scholar]
- Meller R, Minami M, Cameron JA, Impey S, Chen D, Lan JQ, Henshall DC, Simon RP. CREB-mediated Bcl-2 protein expression after ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:234–246. doi: 10.1038/sj.jcbfm.9600024. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Tsuchida A, Itakura Y, Nonomura T, Ono M, Hirota F, Inoue T, Nakayama C, Taiji M, Noguchi H. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes. 2000;49:436–444. doi: 10.2337/diabetes.49.3.436. [DOI] [PubMed] [Google Scholar]
- Noble EE, Billington CJ, Kotz CM, Wang C. The lighter side of BDNF. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1053–1069. doi: 10.1152/ajpregu.00776.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe JH, Vogel R, Lavie CJ, Cordain L. Organic fitness: physical activity consistent with our hunter-gatherer heritage. Phys Sportsmed. 2010;38:11–18. doi: 10.3810/psm.2010.12.1820. [DOI] [PubMed] [Google Scholar]
- Olfert IM, Howlett RA, Tang K, Dalton ND, Gu Y, Peterson KL, Wagner PD, Breen EC. Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol. 2009;587:1755–1767. doi: 10.1113/jphysiol.2008.164384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Pedersen M, Krabbe KS, Bruunsgaard H, Matthews VB, Febbraio MA. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp Physiol. 2009;94:1153–1160. doi: 10.1113/expphysiol.2009.048561. [DOI] [PubMed] [Google Scholar]
- Peterson MD, Rhea MR, Sen A, Gordon PM. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev. 2010;9:226–237. doi: 10.1016/j.arr.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroski RE, Grierson JP, Choi-Kwon S, Geller HM. Basic fibroblast growth factor regulates the ability of astrocytes to support hypothalamic neuronal survival in vitro. Dev Biol. 1991;147:1–13. doi: 10.1016/s0012-1606(05)80002-8. [DOI] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med. 2008;44:153–159. doi: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Raichlen DA, Gordon AD. Relationship between exercise capacity and brain size in mammals. PLoS One. 2011;6(6):e20601. doi: 10.1371/journal.pone.0020601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MA, Evans WJ. Changes in skeletal muscle with aging: effects of exercise training. Exerc Sport Sci Rev. 1993;21:65–102. [PubMed] [Google Scholar]
- Rolian C, Lieberman DE, Hamill J, Scott JW, Werbel W. Walking, running and the evolution of short toes in humans. J Exp Biol. 2009;212:713–721. doi: 10.1242/jeb.019885. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19:951–961. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Becker KG, Zhang Y, Maudsley S, Martin B, Cutler RG, Mattson MP. Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiol Aging. 2010;31:1937–1949. doi: 10.1016/j.neurobiolaging.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonra JR, Ono M, Liu X, Garcia K, Jackson C, Yancopoulos GD, Wiegand SJ, Wong V. Brain-derived neurotrophic factor improves blood glucose control and alleviates fasting hyperglycemia in C57BLKS-Lepr(db)/lepr(db) mice. Diabetes. 1999;48:588–594. doi: 10.2337/diabetes.48.3.588. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilaki A, Jackson MJ, McArdle A. Attenuated HSP70 response in skeletal muscle of aged rats following contractile activity. Muscle Nerve. 2002;25:902–905. doi: 10.1002/mus.10094. [DOI] [PubMed] [Google Scholar]
- Vasilaki A, McArdle F, Iwanejko LM, McArdle A. Adaptive responses of mouse skeletal muscle to contractile activity: The effect of age. Mech Ageing Dev. 2006;127:830–839. doi: 10.1016/j.mad.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Wan R, Camandola S, Mattson MP. Intermittent food deprivation improves cardiovascular and neuroendocrine responses to stress in rats. J Nutr. 2003;133:1921–1929. doi: 10.1093/jn/133.6.1921. [DOI] [PubMed] [Google Scholar]
- Williams MA, Stewart KJ. Impact of strength and resistance training on cardiovascular disease risk factors and outcomes in older adults. Clin Geriatr Med. 2009;25:703–714. doi: 10.1016/j.cger.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J Biol Chem. 2007;282:194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- Wu J, Ruas JL, Estall JL, Rasbach KA, Choi JH, Ye L, Boström P, Tyra HM, Crawford RW, Campbell KP, Rutkowski DT, Kaufman RJ, Spiegelman BM. The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1α/ATF6α complex. Cell Metab. 2011;13:160–169. doi: 10.1016/j.cmet.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Tadokoro T, Keijzers G, Mattson MP, Bohr VA. Neurons efficiently repair glutamate-induced oxidative DNA damage by a process involving CREB-mediated up-regulation of apurinic endonuclease 1. J Biol Chem. 2010;285:28191–28199. doi: 10.1074/jbc.M109.082883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoladz JA, Pilc A. The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. J Physiol Pharmacol. 2010;61:533–541. [PubMed] [Google Scholar]