Abstract
TNF-α has been reported to be a key component of the functional priming, of both myeloid and non-myeloid cells, that is thought to contribute to the lung’s increased susceptibility to injury following shock. Not surprisingly, we found that mice deficient in TNF-α exhibited reduced acute lung injury (ALI) resultant from the combined insults of hemorrhagic shock and sepsis. However, we found that when we adoptively transferred neutrophils from mice expressing TNF-α to neutrophil depleted mice that lacked TNF-α, they were not able to serve as priming stimulus for the development of ALI. Based on these findings we proposed that resident lung tissue cells mediate TNF-α priming. To begin to unravel the complex signaling pathway of various resident lung tissue cells in TNF-α induced priming, we compared the effect of local [intratracheal (i.t.)] verses systemic [intravenous (i.v.)] delivery of TNF-α small interference (si)RNA. We hypothesized that alternately suppressing expression of TNF-α in lung endothelial (i.v.) or epithelial (i.t.) cells would produce a differential effect in shock induced ALI. We found that when in vivo siRNA i.t. or i.v. against TNF-α was administered to C57/BL6 mice at 2 hours post hemorrhage, 24 hours prior to septic challenge, that systemic/i.v., but not i.t., delivery of TNF-α siRNA following hemorrhage priming significantly reduces expression of indices of ALI compared to controls. These findings suggest that an absence of local lung tissue TNF-α significantly reduces lung tissue injury following hemorrhage priming for ALI and that pulmonary endothelial and/or other possible vascular resident cells, not epithelial cells, play a greater role in mediating the TNF-α priming response in a mouse model of hemorrhage/sepsis induced ALI.
Keywords: tumor necrosis factor-alpha, acute lung injury, neutrophil, short interference(si)-RNA, endothelial cells
Introduction
For the traumatically injured, there appears to be a window of time in which patients exhibit an increased susceptibility to the development of morbid events, such as organ failure and/or life threatening septic/infectious episodes. Indirect acute lung injury (ALI) is a common manifestation of this susceptibility. ALI is a progressive disease, which if left unresolved, has the potential to advance in severity to acute respiratory distress syndrome (ARDS) or more critically, multiple organ failure (MOF) and death. Despite improvements in therapeutic strategies, mortality in trauma patients remains high, 20–40% [4,28]. While the cause of this trauma-induced susceptibility is not yet understood, studies suggest that the immune response to secondary inflammatory/infectious challenge in these individuals is dysfunctional due to an initial priming of resident lung tissue cells and/or immune cells, i.e., neutrophils, in the peripheral vasculature [11,13,20,34,42]. In this context, the recruitment of neutrophils from the peripheral blood and their retention in the lungs of these patients is not only characteristic of the pathology associated with ALI, but also thought to be a contributing element. Importantly, our laboratory along with others, has shown that traumatic shock serves not only to potentiate the chemotactic response of the blood neutrophils, but also primes these cells. This “priming” event serves to augment their oxidative burst/proteolytic response to subsequent innocuous stimuli in such a fashion as to precipitate marked ALI and death following the combined insults of shock and sepsis [5,6,20,22,38]. We have, however, recently shown that many of the aspects of ALI (neutrophil influx, tissue damage and the rise of pro-inflammatory cytokines in the lungs of shocked mice) can be mitigated by systemic antibody blockade of murine neutrophil chemokines, keratinocyte-derived chemokine (KC) and macrophage inflammatory chemokine (MIP-2) and/or local pulmonary knock-down of KC and MIP-2 with short interference (si)RNA [20,22,24]. Nonetheless, while these studies demonstrate the importance of these chemokines in mediating the trafficking of neutrophils to the lungs in ALI, the early signaling events and various cellular interactions within the lung, specific to hemorrhage induced priming, have not been clearly identified. With respect to the latter, several studies, including our own [21,25,37,39], have shown that resident cells in the lungs, such as pulmonary epithelial cells, endothelial cells and alveolar macrophages appear to be making important proximal responses to shock induced stimulation. These may serve to shape the pulmonary response to subsequent septic challenge that is typified by neutrophil influx to the lung. However, the nature of local-tissue cell priming/activation and/or their respective contribution(s) to the development of indirect pulmonary ALI is poorly understood.
TNF-α has been reported to be one of the earliest mediators produced in response to a wide variety of inflammatory stimuli [30,33]. TNF-α levels are significantly elevated in humans with ALI and in our murine shock/sepsis model for the development of ALI [7,22,38]. It has been shown to increase the expression of intracellular adhesion molecules on endothelial cells, maximizing the targeting of immune cells from the vasculature [16,33,35] as well as stimulating local cytokine production [19,36]. TNF-α has been reported to be a key component in the functional priming, of both myeloid and non-myeloid cells, that is thought to contribute to the lung’s increased susceptibility to injury following shock [1,15,39]. However, while TNF-α expression/release is clearly central to the initiation of the processes culminating in indirect ALI, much debate exists at the cellular level as to which early TNF-α interactions are most critical.
In as much, we set out to test the hypothesis that neutrophil mediated release of TNF-α is critical to the induction of indirect ALI resultant from the dual insult of shock followed by septic challenge.
Materials and Methods
Mice
Male mice 8–10 weeks were used for all in vivo/in vitro experiments. B6 129S6-Tnftm1Gkl/J (TNF−/−) and B6 129SF2/J (TNF+/+) strains were used for TNF-α knockout, neutrophil depletion/adoptive transfer experiments, C57BL/6 were used for siRNA experiments and the transgenic mice, B6.Cg-Tg(TIE2GFP)287Sato/1J, were used for Tie2 experiments and C57BL/6-TgN(ACTbEGFP)1Osb, for GFP siRNA controls (Jackson Laboratory, Bar Harbor, ME ). Experiments were performed in accordance with National Institutes of Health guidelines and with approval from the Animal Use Committee of Rhode Island Hospital.
Reagents
KC and MIP-2 antibodies for ELISA assays were purchased from R&D Systems, Minneapolis, MN. Mouse IL-6, IL-10 and TNF-α ELISA kits were purchased from BD Bioscience, San Diego, CA. Custom designed siRNA duplex for TNF-α (5′-GACAACCAACUAGUGGUGCUU-3′)[23,44], GFP (GGCUACGUCCAGGAGCGCACCUU-3′)[37] and chromophore labeled RISC-free control siRNA (siGLO) were synthesized by Dharmacon Research, Inc., Lafayette, CO. Fluorescently-labeled (Alexa647) siRNA targeted against mouse endothelial growth factor, Angiopoietin-2, was synthesized by Qiagen, Valencia, CA. Fluorescently labeled antibodies for flowcytometry were purchased from Abcam, Cambridge, MA (FITC mouse Cytokeratin 18) and eBioscience, San Diego, CA (eFluor 450 anti-mouse CD31/PECAM-1). All other chemicals were analytical reagent grade and purchased from Sigma Chemical, St Louis, MO.
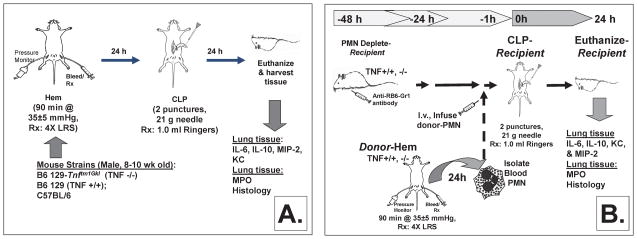
Mouse Hemorrhage/Sepsis Model for ALI (Figure 1A)
Figure 1.
Murine model of hemorrhage-induced priming for the development of ALI (A). Model for the study of adoptive transfer of hemorrhage-induced priming for the development of ALI (B).
Hemorrhage
The non-lethal fix-pressure hemorrhage model used for these experiments has been previously described [20]. In brief, mice were anesthetized with isoflurane, restrained in supine position and catheters were inserted into both femoral arteries. Anesthesia was discontinued and blood pressure was continuously monitored through one catheter attached to a blood pressure analyzer (BPA, MicroMed, Louisville, KY). When fully awake, as determined by a mean blood pressure of ~95mmHg, the mice were bled over a 5–10 minute period to a mean blood pressure of 35mmHg (± 5mmHg) and kept stable for 90 minutes. Immediately following hemorrhage mice were resuscitated intravenously (i.v.) with Ringers lactate at 4 times drawn blood volume. Following resuscitation, arteries were ligated, catheters removed and catheter sites bathed with lidocaine and sutured closed. Sham hemorrhage was performed as a control, these mice were anesthetized, restrained and their femoral arteries ligated, but no blood was drawn.
Polymicrobial Sepsis/CLP
24 hours post hemorrhage (or sham hemorrhage), sepsis was induced as a secondary challenge via cecal ligation and puncture (CLP) as previously described [20]. To summarize, mice were anesthetized with isoflurane and restrained in supine position. A 1cm midline incision was made; the cecum was ligated with 5-0 silk thread and punctured twice with a 21-gauge needle. The cecum was then replaced and the incision. Mice were resuscitated with 1ml Ringers lactate s.c. and returned to their cages.
Neutrophil Adoptive Transfer Protocol/Donor- Post Hemorrhage (Figure 1B)
Neutrophil isolation
Neutrophil isolation from individual donor mouse whole blood was performed as previously described [20]. In summary, whole blood was collected from hemorrhaged (Hem) or sham hemorrhaged (SHem) mice via cardiac puncture into a heparin rinsed syringe. An equal volume of 3% Dextran in PBS was added to the heparinized blood in a 15ml conical tube; the mixture was shaken vigorously and let stand at room temperature for 45 minutes. The top leukocyte rich layer was removed, transferred to a new 15ml tube, centrifuged 10 minutes at 300×s(25°C) and washed once with PBS containing 0.1% BSA. Cells were re-suspended in 2ml PBS with 0.1% BSA and layered over a discontinuous density Percoll gradient (1.097 density 2ml bottom layer carefully overlaid with 3ml of 1.077 density) and centrifuged 1 hour at 500× g and 25°C. PMN were removed from lower interface, transferred to a 15ml tube with PBS, centrifuged 15 minutes at 500× g and 4°C. Cells were washed with 15ml PBS once. The number of viable cells per sample was determined using Trypan blue exclusion.
Neutrophil Adoptive Transfer Protocol/Recipient Septic Challenge (Figure 1B)
Neutrophil depletion
Mice which were to be recipients of donor animal cells were depleted of resident PMNs via tail vein injection of 500μg rat anti-mouse neutrophil antibody (clone RB6-8C5, rat IgG2b) per mouse 48 hours prior to CLP [20]. Efficacy of antibody treatment was determined to be greater than 95% based on the reduction of the number of neutrophils in peripheral blood smears assessed 48 hours post treatment.
Transfer of donor neutrophils to antibody depleted mice
Un-pooled purified donor mouse neutrophils (1×106 cells in 100μl PBS) from TNF+/+ or TNF−/− hemorrhaged (Hem) or sham hemorrhaged (SHem) mice were transfer via tail vein injection to neutropenic recipient mice. One hour later all recipient mice were made septic via CLP.
Local lung vs. systemic in vivo siRNA delivery
For validation experiments mice were given Alexa 647 (Invitrogen, Carlsbad, CA) labeled Angiopoietin-2 siRNA (10 nM/mouse) either naked through intratracheal delivery or by intravenous tail vein injection in a liposome encapsulated preparation (DOTAP, Roche Applied Science, Indianapolis, IN). Mice were euthanized 20 hours later and their lungs were enzymatically digested. Single cell suspensions (2×106) were stained for endothelial cell marker; CD31 using Pacific Blue or cytokeratin-18, an epithelial cell marker using FITC. Samples were analyzed [17,50] using a FACS Diva flowcytometer (BD Biosciences, San Jose, CA) and FCS Express (De Novo Software, Los Angeles, CA)
For TNF-α experiments, 50μg of TNF-α or RISC-free siRNA (unlabeled or fluorescently labeled) in 100μl saline was administered 2 hours post hemorrhage, either intratracheally (naked) or via tail vein injection (liposomal encapsulated) into isoflurane anesthetized mice. The dosage of siRNA used for these experiments was determined based on published studies done with other siRNA constructs and as reported by our laboratory [24,37,53]. As we have previously observed, following administration of control or GFP siRNA, levels of IL-6, TNF-α, and IFN-α were not elevated in lung tissue or plasma following either intravenous tail vein (i.v.) or intratracheal (i.t.) delivery [37,53].
Sample collection
24hours post CLP mice were euthanized with an overdose of Isoflurane (Pitman-Moore, Mundelein, IL).
Blood was collected via cardiac puncture into heparinized syringes. Blood samples were centrifuge, plasma collected and stored at −70°C for later cytokine analysis.
Lung tissue was harvested for assessment of TNF-α mRNA by RT-PCR, cytokine levels, myeloperoxidase activity (MPO), neutrophil influx (esterase+ cells), flowcytometry and tissue architecture. Samples for ELISAs, and MPO were collected in potassium buffer or lysis buffer for processing. For histological assessment, the trachea was cannulated and lungs were gently inflated with formalin. Lungs were excised into formalin for later processing of frozen sections.
Methods of Assessment
Cytokine and chemokine ELISAs for IL-6, IL-10, KC, MIP-2 and TNF-α were performed as per manufacturer’s protocols (R&D systems & BD Biosciences) on lung tissue homogenates and plasma samples collected from experimental mice.
Real-Time Quantitative Polymerase Chain Reaction
To quantify TNF-α gene expression in lung tissue homogenates, total RNA was isolated and purified using TriPure Isolation Reagent (Boehringer, Mannheim, Germany) from frozen, homogenized mouse lung tissue as previously described [24,50,52]. Complementary DNA was synthesized using iScript cDNA as previously described [24,50,52]. Primer sequences for mouse TNF-α (5′ to 3′)-forward: AGGCTCATCCTTGCCTTTGTCTCT and reverse: TCAGCAGCTACCCACACTTCACTT [55].
Enzymatic lung digest was performed to obtain a single cell suspension for sample assessment by flowcytometry. Lung tissue cells were isolated from PBS perfused whole mouse lungs described [17,50]. In summary, following euthanasia, the pleural and peritoneal cavities were opened via a midline incision. Clamps were placed on the superior vena cava and inferior vena cava and lungs were flushed with cold Hank’s Balanced Salt Solution (HBSS) infused through the right ventricle, and draining through an incision made in the left ventricle. The whole lung was placed in a P100 plastic culture dish in 2ml enzyme solution [2.4 U/ml Dispase II, 0.1% Collagenase A (Roche Applied Science, Indianapolis, IN), 2.5mM CaCl2 (Sigma-Aldrich, St. Louis, MO) in HBSS (GIBCO, Carlsbad, CA)]. Lungs were then minced using Metzenbaum scissors, transferred into a 50 ml conical tube and incubated in a 37° C water bath for 45 minutes. 1 ml of cold HBSS was added to lung digest and the digest solution was pipetted using a P100 pipettor to break up remaining tissue. Lung digest solution was then passed through a 40μm filter into a clean 50 ml conical tube, the volume was increased to 20 ml with HBSS and the digest was centrifuged for 10 minutes, 500 × g, at 4° C. The cellular digest was washed with HBSS, re-suspended in 1 ml of HBSS and total viable cell number was determined using Trypan exclusion and a hemocytometer.
Lung myeloperoxidase (MPO) activity as an assessment of neutrophil influx was measured according to established protocols [20]. Briefly, lung tissue was homogenized in 0.5ml of 50mM potassium phosphate buffer pH 7.4 and centrifuged at 40,000×g at 4°C for 30 minutes. The supernatant was reserved for cytokine analysis. The remaining pellet was re-suspended in 0.5 ml of 50mM potassium buffer pH 6.0 with 0.5% hexadecyltrimethylammonium bromide, sonicated on ice and then centrifuged at 12,000×g at 4°C for 10 minutes. Supernatants were then assayed at a 1:20 dilution in reaction buffer (530nmol/L o-dianisidine, 150 nmol/L H2O2 in 50mM potassium phosphate buffer) and read at 490nm.
Immunohistochemical staining for assessment of neutrophil influx and tissue architecture
Staining for leukocyte specific esterase, Naphthol AS-D chloroacetate esterase (Sigma Diagnostics, St. Louis, MO) was performed on frozen tissue sections fixed in citrate-acetone-formaldehyde [20,24]. Slides were incubated in a solution of sodium nitrate, Fast Red Violet BL base solution, TRIZMAL 6.3 buffer and Naphthol AS-D chloroacetate solution in de-ionized water for 15 minutes at 37°C. Following rinsing, slides were counterstained with Gills Hemotoxylin solution and cover-slipped. Stained lung sections were examined microscopically for morphology and positively stained cells. To establish the total number (%) of cells (per field) that were neutrophils (esterase +) present in the sample, tissue sections were randomly screened (7–8 fields/slide) at 400x (25μm2/field).
Statistical Analysis
Data is expressed as mean ± SEM of mice examined in each group. Statistical significance was determined using One Way ANOVA and a post hoc multiple comparisons TUKEY’s test was done when so indicated. Calculations were performed using SigmaStat for Windows version 2.03. P values<0.05 were considered significant.
Results
TNF-α plays a role in the development of ALI resulting from the dual insults of shock followed by sepsis
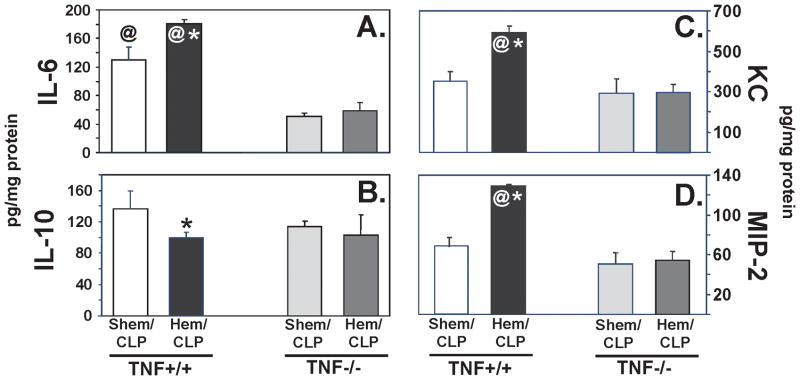
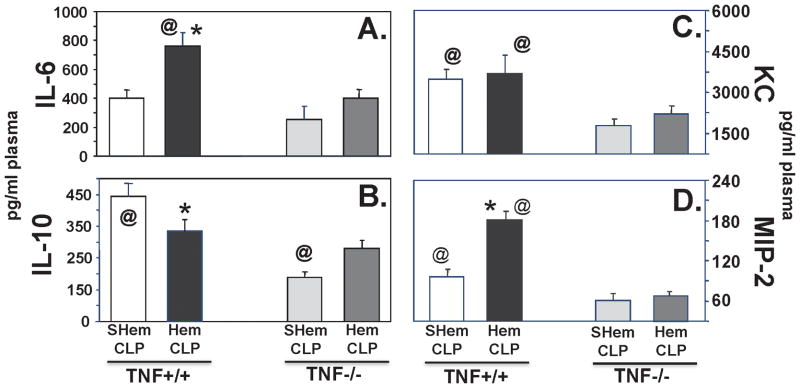
Since TNF-α is purported to be a central upstream mediator of the pathological processes, such as priming and/or activation, contributing to the development of ALI, we initially sought to determine if TNF-α gene expression contributed to the general (non-cell selective) development of indirect-pulmonary ALI resultant from the dual insults of non-lethal shock (hemorrhage) followed by septic challenge. Using TNF −/− mice, we found that following Hem/CLP, cytokine, IL-6 (Fig. 2A) and neutrophil chemotactic proteins, KC (Fig. 2C) and MIP-2 (Fig. 2D), were reduced to, or below levels measured in lung tissue homogenates from background-control, sham hemorrhaged (abbreviated SHem), septic mice (CLP) (SHem/CLP, TNF +/+). Levels of IL-10 (Fig. 2B), however, were not significantly affected in either the SHem or Hem, TNF −/− mice when compared to the TNF +/+ background controls. Similar decreases in IL-6 (Fig. 3A) and MIP-2 (Fig. 3D) were observed in plasma from Hem/CLP TNF −/− mice, however, plasma KC (Fig. 3C) was not significantly decreased when compared to Hem/CLP TNF +/+ control.
Figure 2.
Lung tissue homogenate levels of pro-inflammatory cytokine, IL-6 (A), and neutrophil chemotactic proteins, MIP-2 (D), were significantly decreased in lungs from Hem/CLP TNF−/− mice when compared with Hem/CLP background, TNF+/+ mice. Neutrophil chemotactic protein, KC, was significantly decreased in lung tissue (C) and IL-10 (F) showed significant decreased in plasma. (n=7–8. *p<0.05 vs. equivalent Sham, @<p0.05vs TNF−/− Hem/CLP).
Figure 3.
Plasma levels of IL-6 (A), and neutrophil chemotactic proteins, KC (C) and MIP-2 (D), in blood were significantly decreased following Hem/CLP in TNF−/− mice as compared to background, TNF+/+ mice. This was not observe in plasma IL-10 levels (B), where only mice in the Sham Hem (SHem)/CLP groups showed significant difference.
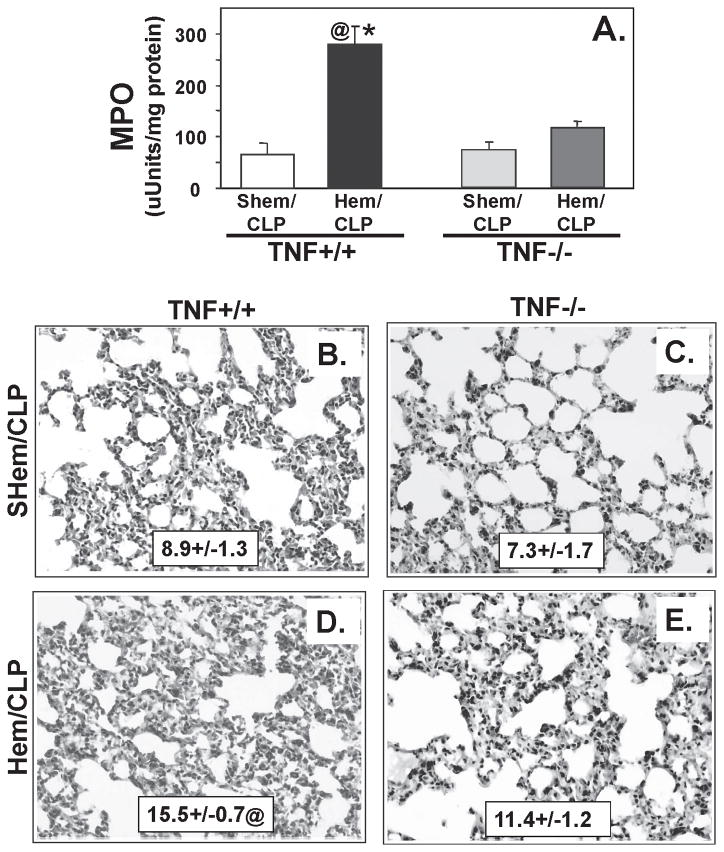
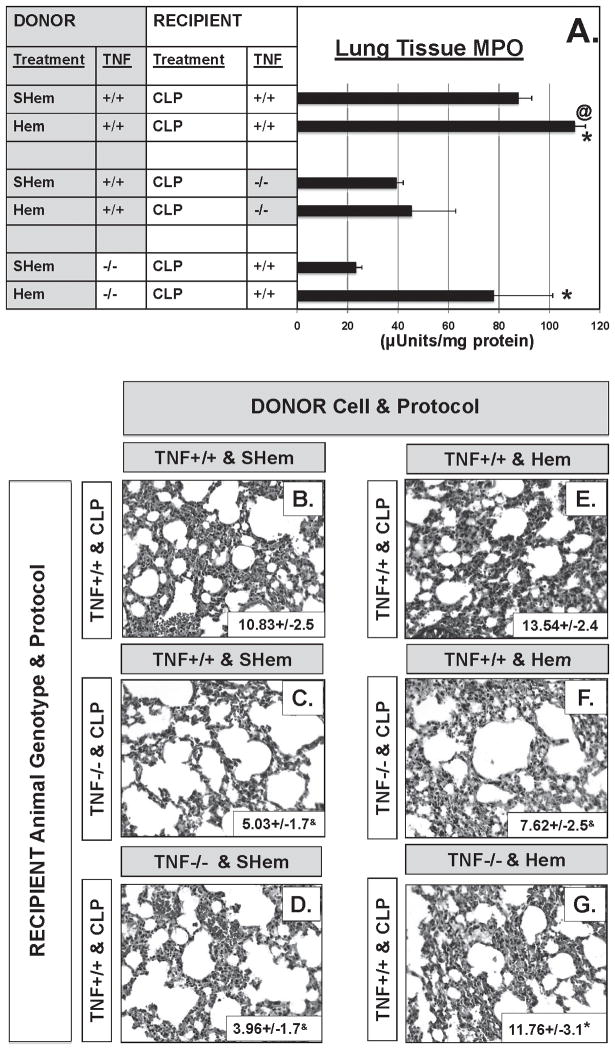
In addition, a significant decrease in neutrophil influx (MPO activity) (Fig. 4A) was evident in the lungs of the TNF −/− mice following Hem/CLP when compared to TNF +/+ mice. Lung tissue sections stained for a neutrophil specific esterase confirmed these findings (Fig. 4B–E). Lung tissue sections from Hem/CLP TNF −/− (Fig. 4E) showed a significant decrease in esterase + (neutrophils) cells as well as a decrease in alveolar thickening and cellular infiltrate compared to Hem/CLP, TNF +/+ mice (Fig. 4D).
Figure 4.
Lung tissue myeloperoxidase activity (A), a measure of neutrophil influx to lung, was reduced greater than 50% in lungs from Hem/CLP, TNF−/− mice relative to Hem/CLP TNF+/+ mice. This is consistent with histological findings of significantly reduced lung tissue septal thickening and cellular infiltrate (E) and esterase+ (neutrophil specific) cells (E/insert box) compared to lung tissue from Hem/CLP TNF+/+ mice (D/insert box). (n=7–8. *p<0.05 vs. equivalent Sham, @<p0.05vs TNF−/− Hem/CLP)
What is the cellular basis for TNF-α priming in our hemorrhage/sepsis model for ALI?
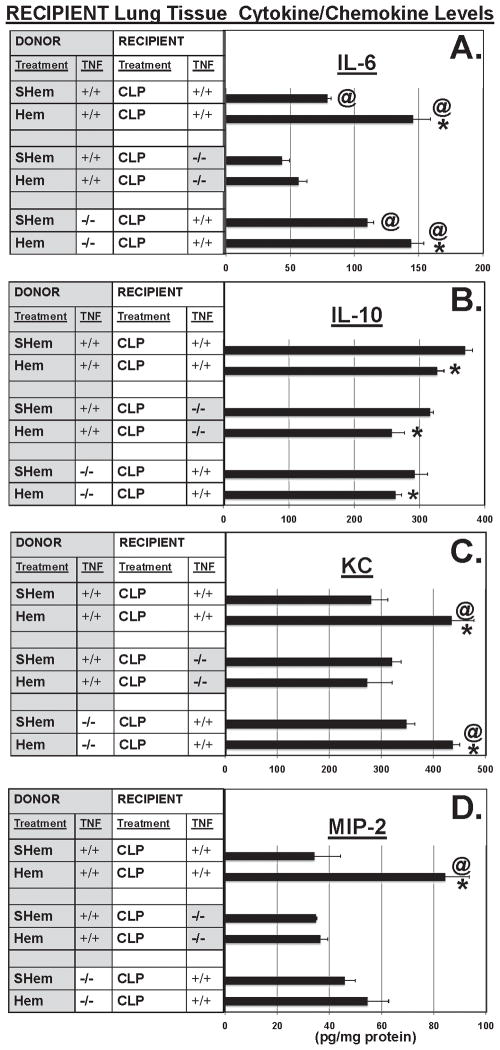
In previously published experiments, we have shown that non-lethal hemorrhage serves to transiently prime the capacity of neutrophils to induce lung injury in response to a subsequent or otherwise occult septic challenge [20,21] and that this capacity can be adoptively transferred to naïve, neutropenic recipients. In light of the observations made from the above-described TNF knockout mice, we attempted to address a more cell-selective hypothesis, which proposed that the autocrine/paracrine release of TNF-α by neutrophils was the critical component driving the development of ALI in response to the combined insults of shock and sepsis. To address this hypothesis, neutrophil adoptive transfer was used to assess the impact of the absence of TNF-α signaling/stimulation during shock, in local tissue or migrating cells (neutrophils), in recipient lung tissue (Fig. 5). These conditions, TNF-α competent (TNF +/+) vs. TNF-α deficient (TNF −/−), in local environment or migrating neutrophils, were evaluated by assessing the ability of donor neutrophils to induce aspects of ALI when transferred to a neutropenic recipient mouse that was subjected to septic insult. To address the counter hypothesis that the absence of TNF-α signal/stimulation involved in the priming and/or activation of local pulmonary cells, and not neutrophils, is critical to the development of indirect-ALI resulting from the dual insults of shock followed by septic insult; we compared the impact of neutrophil adoptive transfer to neutropenic recipients which were either TNF-α competent (TNF +/+) or TNF-α deficient (TNF −/−). Recipient lung tissue levels of pro-inflammatory cytokine, IL-6, was reduced by over 50% in TNF−/− mice that received neutrophils from hemorrhaged TNF +/+ mice when compared to the TNF +/+ recipients that received neutrophils from either the Hem TNF −/− or TNF +/+ donors (Fig. 5A). In contrast, levels of IL-10 were similar within each treatment group (Hem or SHem) for all recipient lung tissue/donor neutrophil combinations (Fig. 5B).
Figure 5.
TNF−/− recipients that received neutrophils from Hem TNF+/+ donors showed significantly reduced levels of IL-6 (A) in lung tissue homogenates in contrast to TNF+/+ competent Donor/Recipient and TNF+/+ Recipients that received TNF−/− neutrophils from Hem Donors. However, IL-10 (B), while reduced between Donor treatments (SHem vs. Hem) was not different between neutrophil Donor/lung tissue Recipient combinations. Levels of KC (C) and MIP-2 (D) in TNF−/− Recipients were significantly reduced in contrast to TNF+/+ Donor/Recipient combination, however, TNF−/− Donor neutrophils also reduced MIP-2 in TNF+/+ recipients (D). (n=7–8. *p<0.05 vs. equivalent Sham, @<p0.05 vs. Recipient TNF−/− CLP)
Levels of the neutrophil chemotactic proteins, KC (Fig. 5C) and MIP-2 (Fig. 5D), were also reduced in the TNF −/− recipients of TNF +/+ donor neutrophils, however, donor neutrophils from TNF −/− mice also reduced levels of MIP-2 in TNF +/+ recipient mice.
Neutrophil influx, as assessed by MPO activity, was reduced in lung tissue from the TNF −/− recipient and TNF +/+ recipient mice that received neutrophils from TNF −/− donors (Fig. 6A). This was also observed in lung tissue histology; the number of neutrophil specific esterase+ (neutrophils) cells was significantly reduced in the TNF −/− mice that received neutrophils from TNF +/+ Hem donors (Fig. 6F insert box). Disruption of lung tissue architecture, cellularity and septal thickening was also reduced in lung tissue sections from these mice (Fig. 6F).
Figure 6.
Lung tissue myeloperoxidase activity (A), was significantly reduced in TNF−/− recipient lung tissue compared to either TNF+/+ Recipient combination (n=7–8. *p<0.05 vs. equivalent Sham, @<p0.05vs TNF−/− Hem/CLP). This is consistent with histological findings of reduced lung tissue septal thickening and cellular infiltrate (F) and esterase+ (neutrophil specific) cells (F insert box) compared to TNF+/+ Donor/Recipient combination (E/insert box). Representative lung tissue sections (n=7–8) *p<0.05 vs. equivalent SHem, &p<0.05 vs. TNF+/+ Donor with TNF+/+ Recipient.
Selective targeting of resident lung tissue cells established by route of siRNA delivery
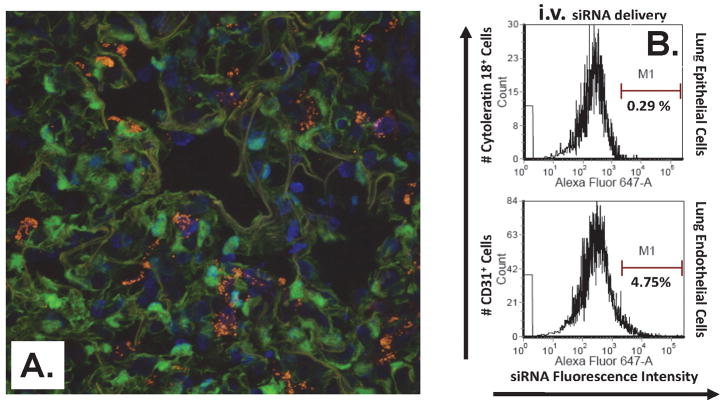
Since data from our adoptive transfer experiments strongly suggested that it is the local tissue (cell) environment, not immigrating cells, that appear to drive the development of ALI in our hemorrhage-priming model, and, given that neutrophil expression of TNF-α did not appear to be critical to the development of indirect pulmonary ALI seen here; we set out to further test the alternative hypothesis that a local pulmonary cellular source of TNF-α is critical to the development of shock/sepsis induced lung injury. To address this hypothesis we chose to take advantage of our recent finding that local intra-tracheal delivery of naked siRNA constructs appear to be sequestered/taken up primarily by lung epithelial cells [46] (but not macrophages or endothelial cells) [37], as opposed to intra-venous delivery of liposomal encapsulated siRNA, which is reported to be restricted primarily to cells in direct contact with the vasculature [3,27,29,32,41,47]. To the extent that i.v. administration of liposomal siRNA was actually targeting, being taken up by, vascular endothelial cells and not lung epithelial cells, we assessed the co-localization of chromophore-labeled siGLO RISC-free siRNA in Tie-2 GFP expressing transgenic mice. Uptake of chromophore -labeled siRNA (punctate fluorescence/white arrows) was confirmed in primarily lung Tie2+ endothelial cells and a small percentage of resident macrophages (Fig. 7A). This observation is in agreement with the findings of Santel et al. [41]. Additional assessment of route-of-delivery specific uptake was performed using flowcytometric detection of siRNA against Angiopoietin (Ang)-2, an endothelial cell growth factor synthesized primarily by endothelial cells [12]. Uptake of Alexa 647 labeled Ang-2 siRNA via i.v. delivery was observed in CD31+ (endothelial cells), while i.t. delivered siRNA was observed in cytokeratin 18+ (epithelial cells) and a small percent of macrophages (as assessed by forward/side scatter analysis) (Fig. 7B).
Figure 7.
Confocal microscopy of i.v. delivered, fluorochrome-labeled (SiGlo RISC-free) siRNA uptake in lung endothelial (Tie2+) cells (A). Uptake of Alexa 647 labeled endothelial cell growth factor, Angiopoietin-2, siRNA via i.v. delivery was observed in CD31+ (endothelial cells), while i.t. delivered siRNA was observed in cytokeratin 18+ (epithelial cells) and a small percent of macrophages (as assessed by forward/side scatter analysis) (B).
Suppression of TNF-α-induced lung tissue injury with systemic (i.v.), but not local pulmonary (i.t.) delivery of TNF-α siRNA post hemorrhage
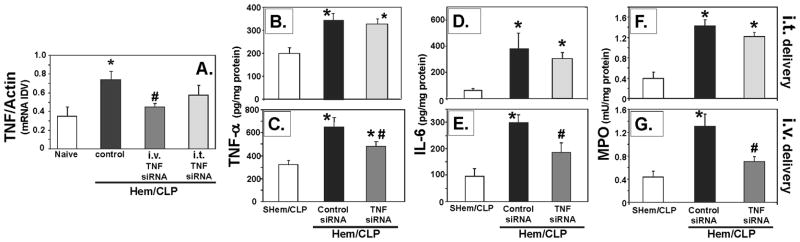
Suppression of inflammatory lung injury associated with TNF-α priming was observed in mice that received systemically (i.v.) administered liposomal siRNA against TNF-α as opposed to animals receiving i.t. delivery of naked siRNA against TNF-α siRNA following hemorrhage, subsequent to the CLP (Fig. 8B–G) I.V. delivery significantly reduced levels of lung tissue TNF-α (Fig. 8B–C), IL-6 (Fig. 8D–E) and MPO (Fig. 8F–G) when compared to Control-siRNA and SHem/CLP. Alternatively, i.t. delivery of TNF-α siRNA did not serve to significantly reduce these levels when compared to control-siRNA treated mice. Representative lung tissue histology show a decreased in inflammatory lung injury, septal thickening and cellular infiltrate, in lungs from i.v. treated mice, further supportingn these findings (Fig. 9D). Additionally, TNF-α gene expression in lung tissue homogenates, as measured by total RNA (Rt-PCR), while not statistically significant, showed a consistently lower gene expression following i.v. delivery that i.t (8A).
Figure 8.
i.v. delivery of TNF-α siRNA 2 hours post hemorrhage priming significantly reduced levels of TNF-α (C), IL-6 (E) as well as MPO activity (G) in mouse lungs following CLP when compared with siRNA control, this was not observed in the naked i.t. delivered TNF-α treated mice. (n=6–7. * p< 0.05 vs. SHem/CLP control, #p<0.05 vs. Hem/CLP Control-siRNA). TNF-α gene expression in lung tissue homogenates, as measured by total RNA (Rt-PCR) (n=4), was consistently lower following i.v. as opposed to i.t delivery of TNF-α siRNA (8A).
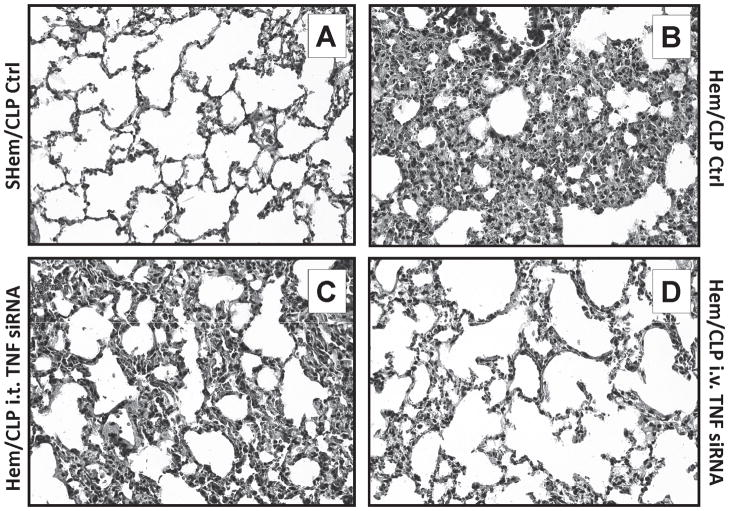
Figure 9.
Comparison of representative hemotoxylin and eosin stained lung tissue sections show a discernible decrease in lung inflammation, cellular infiltrate and septal thickening, in the i.v. TNF-α siRNA (D), but not i.t. (C) TNF-α siRNA route of delivery when compared to Hem/CLP control mice (B). (n=4/group)
Discussion
TNF-α is accepted by most investigators in the field to be one of the most central/proximal mediators to the initiation and maintenance of the inflammatory response to a host of inflammatory/infectious stimuli [8–10,14,18,33,40,43,49,51]. Its role in lung injury both direct and indirect is well documented and the contributions TNF-α makes at a cellular level have been well described based largely on ex vivo and in vitro studies. However, translating the contribution of any mediator, including TNF-α, in the setting of in vivo pathogenesis of a complex condition such as organ dysfunction resulting from the indirect stimuli like shock/infection, is difficult even in the experimental rodent, let alone the critically injured patient. In this study we have therefore tried to clarify whether the hypothesis that TNF-α serves as an autocrine/paracrine neutrophil priming agent and that such TNF-α priming contributes to shock/sepsis induced lung injury, actually occurs in mice. Not surprisingly, we found that mice deficient in the TNF-α gene/protein exhibited reduced indices of inflammation and lung tissue injury when subjected to the combined insults of hemorrhagic shock and sepsis, thus confirming the critical role of upstream/proximal TNF-α signaling in the pathogenesis of ALI.
We have previously shown, in a murine model of adoptive transfer, that neutrophils from shocked mice have the capacity to induce lung inflammation/injury in neutrophil depleted mice that are subsequently subjected to septic challenge. These neutrophils are thus phenotypically, as well as functionally distinct from neutrophils derived from naïve and/or Sham-treated animals [20]. Having shown here that generalized/whole animal TNF-α depletion alters the development of shock/sepsis induced ALI, including neutrophil influx to the lung; we next asked whether neutrophils derived from mice that could not produce TNF-α when exposed to shock, retained the capacity to induce ALI when transferred to TNF (−/−) deficient recipient mice verses a wild-type/background control (TNF+/+). Our data showed that when we adoptively transferred neutrophils from Hem donor mice expressing TNF-α to neutrophil depleted recipient mice that lacked the ability to make TNF-α, they were not able to stimulate the development of ALI. This data shows that the lack of TNF-α production in the recipient’s tissue environment, not the effect of TNF-α released in either in a paracrine or autocrine fashion by the donor neutrophils during Hem, is central to hemorrhage induced priming for ALI in our model.
These findings raise an interesting question, relative to lung, as to which tissue cells might contribute to local TNF-α mediated priming (effect)? To address this question we sought to suppress TNF-α production in different cell populations in the lung that might be contributing to TNF-α priming/ALI. In this regard, our laboratory has previously used i.t. delivery of siRNA to knock down the expression of proteins, MIP-2, KC, Fas, FasL, Caspase-3, in the lungs of shocked septic mice [24,37,46,54]. Silencing RNA target validation experiments showed that i.t. delivery of fluorescently labeled siRNA was taken up by primarily cytokeratin-18 positive lung tissue/epithelial cells as opposed to local tissue macrophage and/or endothelial cells [37].
From the systemic/vascular side, we sought to target delivery into vascular endothelial cells using i.v. delivery. While we appreciate this approach targets a number of vascular beds, the sensitivity of the lung to systemic/vascular inflammation makes them a valuable organ for assessing the source (tissue or vascular) of TNF-α priming using route of delivery specific siRNA. In this respect, numerous studies using liposomal carriers for either plasmid DNA [27,47] or siRNA [3,29,41] indicate that intravenous administration primarily leads to vascular endothelial cell uptake, with only modest interaction with leukocytes and virtually no epithelial cell targeting [3,27,29,41,47]. In light of this we felt the differential cell targeting of i.t. vs. i.v. approach would allow us to determine potentially which cell sub-populations within the lungs might be involved in TNF-α priming. As proof of principle, we delivered chromophore-labeled siGLO RISC-free siRNA, i.v. and observed co-localization (punctate fluorescence) mainly in Tie-2 GFP expressing endothelial cells and a small number of resident tissue macrophage in the lung. In addition, to further confirm specific targeting of i.v. tail vein delivery, liposomal-encapsulated chromophore-labeled Ang-2 siRNA was observed in CD31+ (endothelial cells) and not in cytokeratin-18+ (epithelial cells) using flowcytometery. As endothelial cells are the primary source of Ang-2 [12], this experiment enabled us to not only visualize its uptake, but also assess resultant endothelial cell-specific inhibition of Ang-2 in lung tissue and plasma (data not shown).
Importantly, the observation that i.v. delivery of siRNA against TNF-α, as opposed to i.t. delivery of naked anti-TNF-α siRNA, altered the ability to produce ALI implies that it is the capacity of the vascular cells, such as endothelial cells, and to a lesser extent macrophages, to express TNF-α, that is critical to processes involved in activation/priming of the lung for the increased susceptibility to inflammatory injury following shock. Alternatively, pulmonary epithelial cells do not appear to be as critical in mediating this aspect of TNF-α regulation/contribution to the development of ALI in our model. This is intriguing in light of the recent observation made by several laboratories [26], including our own [37], indicating that pulmonary epithelial cell response to/role in the development of direct and/or indirect lung injury appeared to be more proximal than the activation and recruitment of neutrophils to the lung. The inability of i.t. delivered anti-TNF-α siRNA to reduce ALI in our model also suggests a scenario in which shock, in the form of hemorrhage, as described here, initially acts on vascular endothelial and/or local tissue macrophage, and as such serves as stimulus to release other local mediators. In as much; while TNF-α may not be the neutrophil released autocrine/paracrine-priming agent in shock, we and others have shown that agents like MIP-2α [20,22,24], platelet activating factor (PAF) [2,48] and/or complement [31,45] might be playing this role. These participants, lipid mediators, chemokines and complement, may in turn act on the epithelial cells to up-regulate the expression of pro-inflammatory mediators, such as chemokines, cytokines, etc., and/or pro-apoptotic proteins like Fas/FasL and caspase-3 [37,38,46]. Such changes then serve to predispose (prime) the local tissue cell environment to capture and sequester neutrophils and/or macrophages that are activated by subsequent inflammatory and/or septic/infectious stimuli. To the extent this is true, we have recently shown, using this same, i.t. vs. i.v. siRNA approach, that epithelial, but not endothelial cell expression of FasL is involved in mediating/inducing ALI in this model [46].
In conclusion, we believe our study not only serves to clarify our understanding of the contributions of TNF-α expression to the pathological process of shock/sepsis as it relates to the development of lung injury, but also begins to identify the role of specific cellular components that express TNF-α in the development of shock-induced priming for ALI in mice. Finally, these finding also suggest the feasibility of therapeutically targeting protein expression using siRNA silencing in specific cell populations, via mediators, like TNF-α, that have been associated with priming and de-regulated immune/inflammatory responsiveness.
Footnotes
Authors have no existing conflicts of interest related to the subject matter of this manuscript.
Reference List
- 1.Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1137–L1145. doi: 10.1152/ajplung.2000.279.6.L1137. [DOI] [PubMed] [Google Scholar]
- 2.Alarcon P, Conejeros I, Carretta MD, Concha C, Jara E, Tadich N, Hidalgo MA, Burgos RA. D-Lactic acid interferes with the effects of platelet activating factor on bovine neutrophils. Veterinary Immun and Immunopath. 2011;144:68–78. doi: 10.1016/j.vetimm.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Aleku M, Fisch G, Mopert K, Keil O, Woflgang A, Kaufmann J, Santel A. Intracellular localization of lipoplexed siRNA in vascular endothelial cells of different mouse tissues. Microvasc Res. 2008;76:31–41. doi: 10.1016/j.mvr.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Ayala A, Chung CS, Lomas J, Grutkoski PS, Doughty LA, Simms HH. A mouse model of priming for acute lung injury following shock. Shock. 2001;15:83S. [Google Scholar]
- 6.Ayala A, Chung CS, Lomas JL, Song GY, Doughty LA, Gregory SH, Cioffi WG, LeBlanc BW, Reichner J, Simms HH, Grutkoski PS. Shock induced neutrophil mediated priming for acute lung injury in mice: divergent effects of TLR-4 and TLR-4/FasL deficiency. Amer J Pathol. 2002;161:2283–2294. doi: 10.1016/S0002-9440(10)64504-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayala A, Wang P, Ba ZF, Perrin MM, Ertel W, Chaudry IH. Differential alterations in plasma IL-6 and TNF levels following trauma and hemorrhage. Am J Physiol. 1991;260:R167–R171. doi: 10.1152/ajpregu.1991.260.1.R167. [DOI] [PubMed] [Google Scholar]
- 8.Beutler B, Poltorak A. Sepsis and evolution of the innate immune response. Crit Care Med. 2001;29:S2–S6. doi: 10.1097/00003246-200107001-00002. [DOI] [PubMed] [Google Scholar]
- 9.Czermak BJ, Breckwoldt M, Ravage ZB, Huber-Lang M, Schmal H, Bless NM, Friedl HP, Ward PA. Mechanisms of enhanced lung injury during sepsis. Amer J Pathol. 1999;154:1057–1065. doi: 10.1016/S0002-9440(10)65358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunican AL, Leuenroth SJ, Grutkoski P, Ayala A, Simms HH. TNF-α induced suppression of PMN apoptosis is mediated through IL-8 production. Shock. 2000;14:284–289. doi: 10.1097/00024382-200014030-00007. [DOI] [PubMed] [Google Scholar]
- 11.Fan J, Marshall JC, Jimenez M, Shek PN, Zagorski J, Rotstein OD. Hemorrhagic shock primes for increased expression of cytokine-induced neutrophil chemoattractant in the lung: role in pulmonary inflammation following lipopolysaccaride. J Immunol. 1998;161:440–447. [PubMed] [Google Scholar]
- 12.Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG. The tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103:4150–4156. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- 13.Friese RS, Rehring TF, Wollmering M, Moore EE, Ketch LL, Banerjee A, Harken AH. Trauma primes cells: editorial review. Shock. 1994;1:388–394. doi: 10.1097/00024382-199405000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Garnacho-Montero J, Aldabo-Pallas T, Garnacho-Montero C, Cayuela A, Jimenez R, Barroso S, Ortiz-Leyba C. Timing of adequate antibiotic therapy is a greater determinant of outcome than are TNF and IL-10 polymorphisms in patients with sepsis. Critical Care. 2006;10 doi: 10.1186/cc4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgieva GS, Kurata S, Ikeda S, Eishi Y, Mitaka C, Imai T. Nonischemic lung injury by mediators from unilateral ischemic reperfused lung:ameliorating effect of tumor necrosis factor-alpha-coverting enzyme inhibitor. Shock. 2007;27(1):84. doi: 10.1097/01.shk.0000235131.89986.45. [DOI] [PubMed] [Google Scholar]
- 16.Henninger DD, Gerritsen ME, Granger DN. Low-density lipoprotein receptor knockout mice exhibit exaggerated microvascular responses to inflammatory stimuli. Circ Res. 1997;81(2):274. doi: 10.1161/01.res.81.2.274. [DOI] [PubMed] [Google Scholar]
- 17.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci USA. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keane MP, Strieter RM. Chemokine signaling in inflammation. Crit Care Med. 2000;28:N13–N26. doi: 10.1097/00003246-200004001-00003. [DOI] [PubMed] [Google Scholar]
- 19.Lee WL, Downey GP. Neutrophil activation and acute lung injury. Curr Opin Crit Care. 2001;7:1–7. doi: 10.1097/00075198-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Lomas JL, Chung CS, Grutkoski PS, LeBlanc BW, Lavigne L, Reichner J, Gregory SH, Doughty LA, Cioffi WG, Ayala A. Differential effects of macrophage inflammatory protein-2 and keratinocyte-derived chemokine on hemorrhage-induced neutrophil priming for lung inflammation: assessment by adoptive cell transfer in mice. Shock. 2003;19:358–365. doi: 10.1097/00024382-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Lomas-Neira J, Chung CS, Perl M, Gregory S, Biffl W, Ayala A. Role of alveolar macrophage and migrating neutrophils in hemorrhage-induced priming for ALI subsequent to septic challenge. Amer J Physiol. 2006;290:L51–L58. doi: 10.1152/ajplung.00028.2005. [DOI] [PubMed] [Google Scholar]
- 22.Lomas-Neira J, Chung CS, Grutkoski P, Dunican AL, Simms HH, Cioffi WG, Ayala A. Divergent roles of murine neutrophil chemokines in hemorrhage induced priming for acute lung injury. Cytokine. 2005;31:169–179. doi: 10.1016/j.cyto.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Lomas-Neira J, Perl M, Soldato D, Venet F, Chung CS, Ayala A. TNF-α priming for the development of shock induced acute lung injury (ALI) is mediated by local tissue not circulating cells. J Leukoc Biol. 2007;82(abst supplt):30. [Google Scholar]
- 24.Lomas-Neira JL, Chung CS, Wesche DE, Perl M, Ayala A. In vivo gene silencing (with siRNA) of pulmonary expression of MIP-2 versus KC results in divergent effects on hemorrhage-induced, neutrophil-mediated septic acute lung injury. J Leukocyte Biol. 2005;77:1–8. doi: 10.1189/jlb.1004617. [DOI] [PubMed] [Google Scholar]
- 25.Matute-Bello G, Liles WC, Radella IF, Steinberg KP, Ruzinski JT, Jonas M, Chi EY, Hudson LD, Martin TR. Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;156:1969–1977. doi: 10.1164/ajrccm.156.6.96-12081. [DOI] [PubMed] [Google Scholar]
- 26.Matute-Bello G, Martin TR. Science Review: Apoptosis in acute lung injury. Critical Care. 2003;7:355–358. doi: 10.1186/cc1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLean JW, Fox EA, Baluk P, Bolton PB, Haskell A, Pearlman R, Thurston G, Umemoto EY, McDonald DM. Organ-specific endothelial cell uptake of cationic liposome-DNA complexes in mice. Am J Physiol. 1997;273:H387–H404. doi: 10.1152/ajpheart.1997.273.1.H387. [DOI] [PubMed] [Google Scholar]
- 28.Minino A, Heron M, Smith B. Preliminary data for 2004. National vital statistics reports. CDC; 2004. [PubMed] [Google Scholar]
- 29.Miyawaki-Shimizu K, Predescu D, Shimizu J, Broman M, Predescu S, Malik Ab. siRNA-induced caveolin-1 knockdown in mice increases lung vascular permeability via the junctional pathway. Am J Physiol Lung Cell Mol Physiol. 2005;290:L405–L413. doi: 10.1152/ajplung.00292.2005. [DOI] [PubMed] [Google Scholar]
- 30.Mizgerd JP, Peschon JJ, Doerschuk CM. Roles of Tumor Necrosis Factor receptor signaling during murine Escherichia coli pneumonia. Am J Respir Cell Mol Biol. 2000;22:85. doi: 10.1165/ajrcmb.22.1.3733. [DOI] [PubMed] [Google Scholar]
- 31.Morris AC, Brittan M, Wilkinson TS, McAuley DF, Antonelli J, McCulloch C, Barr LC, McDonald NA, Dhaliwal K, Jones RO, Mackellar A, Haslett C, Hay AW, Swann DG, Anderson N, Laurenson IF, Davidson DJ, Rossi AG, Walsh TS, Simpson AJ. C5a-mediated neutrophil dysfunction is RhoA-dependent and predicts infection in critically ill patients. Blood. 2011;117:5178–5188. doi: 10.1182/blood-2010-08-304667. [DOI] [PubMed] [Google Scholar]
- 32.Moschos SA, Williams AE, Lindsay MA. Cell-penetrating-peptide-mediated siRNA lung delivery. Biochemical Society Transactions. 2007;35:807–810. doi: 10.1042/BST0350807. [DOI] [PubMed] [Google Scholar]
- 33.Mulligan MS, Vaporciyan AA, Miyasaka M, Tamatani T, Ward PA. Tumor necrosis factor alpha regulates in vivo intrapulmonary expression of ICAM-1. Am J Pathol. 1993;142:1739–1749. [PMC free article] [PubMed] [Google Scholar]
- 34.Ogura H, Tanaka H, Koh T, Hashiguchi N, Kuwagata Y, Hosotsubo H, Shimazu T, Sugimoto H. Priming, second-hit priming, and apoptosis in leukocytes from trauma patients. J Trauma. 1999;46:774–781. doi: 10.1097/00005373-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Panes J, Perry MA, Anderson DC, Manning A, Leone B, Cepinskas G, Rosenbloom C, Miyasaka M, Kveitys PR, Granger DN. Regional differences in constitutive and induced ICAM-1 expression in vivo. Am J Physiol (Heart Circ Physiol 38) 1995;269:H1955–H1964. doi: 10.1152/ajpheart.1995.269.6.H1955. [DOI] [PubMed] [Google Scholar]
- 36.Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, Wheeler AP and NHLBI Acute Respiratory Distress Syndrome Clinical Trials Network. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Critical Care Medicine. 2005;33(1):1. 1–2. doi: 10.1097/01.ccm.0000149854.61192.dc. [DOI] [PubMed] [Google Scholar]
- 37.Perl M, Chung CS, Lomas-Neira J, Rachel TM, Biffl WL, Cioffi WG, Ayala A. Silencing of Fas- but not caspase-8 in lung epithelial cells ameliorates pulmonary apoptosis, inflammation, and neutrophil influx after hemorrhagic shock and sepsis. Am J Pathol. 2005;167:1545–1559. doi: 10.1016/S0002-9440(10)61240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perl M, Chung CS, Perl U, Lomas-Neira JL, De Paepe ME, Cioffi WG, Ayala A. Fas induced pulmonary apoptosis and inflammation during extrapulmonary acute lung injury. Am J Respir Crit Care Med. 2007;176:591–601. doi: 10.1164/rccm.200611-1743OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pittet JF, Griffiths MJD, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LS, Gotwals PJ, Koteliansky VE, Matthay MA, Sheppard D. TGF-[beta] is a critical mediator of acute lung injury. J Clin Invest. 2001;107:1537–1544. doi: 10.1172/JCI11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Remick DG, Strieter RM, Eskandari MK, Nguyen DT, Genord MA, Raiford CL, Kunkel SL. Role of tumor necrosis factor-alpha in lipopolysaccharide-induced pathologic alterations. Am J Pathol. 1990;136:49–60. [PMC free article] [PubMed] [Google Scholar]
- 41.Santel A, Aleku M, Keil O, Endruschat J, Esche V, Fisch G, Dames S, Loffler K, Fechtner M, Arnold W, Giese K, Klippel A, Kaufmann J. A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene Therapy. 2006;13:1222–1234. doi: 10.1038/sj.gt.3302777. [DOI] [PubMed] [Google Scholar]
- 42.Sarin EL, Moore EE, Moore JB, Masuno T, Moore JL, Banerjee A, Silliman CC. Systemic neutrophil priming by lipid mediators in post-shock mesenteric lymph exits across species. J Trauma. 11-1-2004;57(5):950–954. doi: 10.1097/01.ta.0000149493.95859.6c. [DOI] [PubMed] [Google Scholar]
- 43.Smith MR, Munger WE, Kung HF, Takacs L, Durum SK. Direct evidence for an intracellular role for tumor necrosis factor-α. Microinjection of tumor necrosis factor kills target cells. J Immunol. 1990;144:162–169. [PubMed] [Google Scholar]
- 44.Sorenson DR, Leirdal M, Sioud M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J Mol Biol. 2003;327:761–766. doi: 10.1016/s0022-2836(03)00181-5. [DOI] [PubMed] [Google Scholar]
- 45.Stahl A, Sartz L, Karpman D. Complement activation on platelet-leukocyte complexes and microparticles in enterohemorrhagic Escherichia coli-induced hemolytic uremic syndrom. Blood. 2011;117:5503–5513. doi: 10.1182/blood-2010-09-309161. [DOI] [PubMed] [Google Scholar]
- 46.Thakkar RK, Chung CS, Chen Y, Monaghan SF, Lomas-Neira J, Cioffi WG, Ayala A. Local tissue expression of the cell death ligand, FasL, plays a central role in the development of extra-pulmonary acute lung injury. Shock. 2011;36:138–143. doi: 10.1097/SHK.0b013e31821c236d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thurston G, McLean JW, Rizen M, Baluk P, Haskell A, Murphy TJ, Hannahan D, McDonald DM. Cationic liposomes target angiogenic endothelial cells in tumors and chronic inflammation in mice. J Clin Invest. 1998;101:1401–1413. doi: 10.1172/JCI965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tintinger GR, Theron AJ, Steel HC, Cockeran R, Pretorius L, Anderson R. Protein kinase C promotes restoration of calcium homeostasis to platelet activating factor-stimulated human neutrophils by inhibition of phospholipase C. Journal of Inflammation(Lond) 2009;6 doi: 10.1186/1476-9255-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tracey KJ, Beutler B, Lowry SF, Merryweather J, Wolpe S, Milsark IW, Hariri RJ, Fahey TJ, III, Zentella A, Albert JD, Shires GT, Cerami A. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 50.Venet F, Chung CS, Huang X, Lomas-Neira J, Chen Y, Ayala A. Lymphocytes in the development of lung inflammation: a role for regulatory CD4+ T cells in indirect pulmonary lung injury. J Immunol. 2009;183:3472–3480. doi: 10.4049/jimmunol.0804119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang P, Ba ZF, Chaudry IH. The pivotal role of tumor necrosis factor (TNF) in producing endothelial cell dysfunction in sepsis. Surg Forum. 1993;44:77–79. [Google Scholar]
- 52.Wesche-Soldato DE, Chung CS, Lomas-Neira J, Gregory SH, Ayala A. In vivo delivery of caspase 8 siRNA improves the survival of septic mice. Shock. 2004;21(Suppl 2):23. [Google Scholar]
- 53.Wesche-Soldato DE, Chung CS, Lomas-Neira JL, Doughty LA, Gregory SH, Ayala A. In vivo delivery of caspase 8 or Fas siRNA improves the survival of septic mice. Blood. 2005;106:2295–2301. doi: 10.1182/blood-2004-10-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wesche-Soldato DE, Lomas-Neira JL, Perl M, Chung CS, Ayala A. Hydrodynamic Delivery of siRNA in a Mouse Model of Sepsis. In: Barik S, editor. Methods in Molecular Biology: siRNA, shRNA and miRNA. Vol. 442. Totowa, NJ: Humana Press Inc; 2008. pp. 67–73. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X, Shan P, Jiang G, Zhang SSM, Otterbein LE, Fu XY, Lee PJ. Endothelial STAT3 is essential for the protective effects of HO-1 in oxidant-induced lung injury. FASEB J. 2006;20:E1528–E1538. doi: 10.1096/fj.06-5668fje. [DOI] [PubMed] [Google Scholar]