Abstract
Background
Motivational interviewing (MI) is widely used for adolescent smoking cessation but empirical support for this approach is mixed.
Methods
Adolescent cigarette smokers 14–18 years old (N = 162) were recruited from medical, school, and community settings and randomly assigned to enhanced MI or brief advice (BA) for smoking cessation. MI comprised an in-person individual session, a telephone booster session one week later, and a brief telephone-based parent intervention. BA consisted of standardized brief advice to quit smoking. Assessments occurred at baseline, post-treatment and at 1-, 3-, and 6-month follow ups.
Results
Biochemically-confirmed 7-day point prevalence abstinence rates were low (e.g., 4.5% for MI; 1.4% for BA at 1 month) and did not differ significantly by group at any follow up. Only those in MI reported significant decreases in cigarettes smoked per day (CPD) from baseline to 1 month. At 3 and 6 months, smokers in both groups reported significantly reduced CPD with no differences between groups. MI reduced perceived norms regarding peer and adult smoking rates, while BA had no effect on normative perceptions. No group differences emerged for self-reported motivation or self-efficacy to quit smoking.
Conclusions
Findings support the efficacy of MI for addressing normative misperceptions regarding peer and adult smoking and for modestly reducing CPD in the short-term; however, these effects did not translate to greater smoking abstinence. MI may have more promise as a prelude to more intensive smoking intervention with adolescents than as a stand-alone intervention.
Keywords: Tobacco, Cessation, Adolescents, Motivational Interviewing, Intervention, Parent
1. Introduction
Tobacco use is the leading preventable cause of death globally (Beaglehole et al., 2011). Each day, between 82,000 to 99,000 youth worldwide begin smoking (Mackay et al., 2006), and in the US this results in approximately 1.5 million new smokers under the age of 18 every year (Substance Abuse and Mental Health Services Administration, 2009). According to the Monitoring the Future national surveillance study, 7% of eighth graders, 13% of tenth graders and 20% of twelfth graders report current smoking (Johnston et al., 2010). Smoking during adolescence is particularly concerning because early smoking onset is associated with higher adult smoking rates and lower odds of successfully quitting smoking (Breslau and Peterson, 1996; Coambs et al., 1992). Following declines in adolescent smoking in the US, smoking prevalence among adolescents has stalled at these unacceptably high rates.
The adolescent smoking cessation literature based on randomized clinical trials provides some support for motivational, cognitive-behavioral, and/or social influence approaches for adolescent smoking cessation (Gervais A. et al., 2007; Grimshaw and Stanton, 2006; Heckman et al., 2010; Hettema and Hendricks, 2010; Sussman and Sun, 2009) with limited evidence to support the use of pharmacological interventions for adolescent smoking cessation (Colby & Gwaltney, 2007; Curry et al., 2009).
Motivational enhancement interventions are among the most widely used approaches for adolescent smoking cessation (Curry, et al., 2009). Motivational interviewing (MI) is a brief, client-centered approach focused on resolving ambivalence regarding quitting and increasing self-efficacy for change. MI uses a nonjudgmental, directive, and supportive therapeutic style that emphasizes personal responsibility for making decisions about change (Miller & Rollnick, 2002) and often incorporates personalized feedback, designed to correct normative misperceptions and heighten awareness of personally-relevant consequences of smoking. An individual change plan can be developed collaboratively from a menu of options (Colby et al., 1998; Colby et al., 2005).
Although several studies have failed to demonstrate significant effects of MI for promoting confirmed abstinence in adolescents (Brown et al., 2003; Colby, et al., 2005; Horn et al., 2007), two recent multi-study analyses found significantly greater abstinence among adolescent smokers following MI versus a comparison intervention (Heckman, et al., 2010; Hettema & Hendricks, 2010). In one study, pooled data from eight adolescent trials indicated that MI roughly doubled the odds of smoking abstinence at follow up, from 6% in comparison conditions to 11.5% in MI (Heckman, et al., 2010). In a meta-analysis of 23 studies (including 6 adolescent trials), the overall effect of MI versus comparison intervention was significant at long-term follow ups (≥ 6 months) but not shorter-term follow ups. Subgroup analysis of the adolescent studies found significant combined effects at short- and long-term follow ups (Hettema & Hendricks, 2010). All effect sizes fell below Cohen’s criterion for a small effect (Cohen, 1988), thus the large samples required to detect treatment effects may explain the discrepancy between non-significant findings in individual trials versus significant effects in pooled analyses and meta-analyses.
In the current trial, we attempted to bolster the effect size of MI by adding two new components, a one-week telephone booster session and a brief parent intervention, also administered via telephone. The basis for incorporating parent intervention was the widely documented influence of parent smoking behaviors and attitudes on adolescent smoking (Chassin et al., 2008; den Exter Blokland et al., 2004; Flay et al., 1998; Gilman et al., 2009; Thomas et al., 2009; Tyas & Pederson, 1998; White et al., 2002; Withers et al., 2000. Adolescents whose parents smoke are more likely to smoke themselves, and the odds of smoking initiation and progression are greater for those who have two parents who smoke relative to having one parent who smokes (Gilman, et al., 2009; Peterson et al., 2006). In contrast, parental disapproval of smoking, stronger anti-smoking beliefs, and household smoking restrictions decrease the odds of adolescent smoking (Andersen et al., 2004; Huver et al., 2006; Kodl and Mermelstein, 2004) and adolescent experimental smokers who perceive strong parental disapproval are less likely to progress to regular smoking, even if their parents are smokers (Sargent & Dalton, 2001).
This study compared enhanced MI to Brief Advice (BA) for adolescent smoking cessation at 1, 3, and 6 months follow up. We hypothesized that MI would result in significantly greater smoking abstinence and smoking reductions compared with BA. A secondary aim was to examine the proximal effects of MI on motivation to change, quitting self-efficacy, and normative perceptions about smoking.
2. Method
2.1. Recruitment
Participants were primarily recruited from various sites where MI, if determined efficacious, could potentially be diffused, including an emergency department (ED), a hospital-based adolescent outpatient clinic, a pediatrician’s office, and five high schools. In medical settings, flyers advertising the study were posted and research staff proactively screened and recruited patients who were waiting for appointments/treatment. In high schools, classroom presentations were made and table displays in school cafeterias provided study information during lunch. For students who expressed interest, research staff explained the project and assessed eligibility. Adolescents in the general community who heard about the study through flyers, radio ads, and word of mouth called the research office and were screened for eligibility.
2.2. Eligibility Criteria
Participants were required to be ages 14 to 18, speak English, and smoke at least once per week for the past month. Patients with suicidal ideation or recent traumatic injury (in medical settings) were excluded. Interest in reducing/quitting smoking was not required for participation. Signed informed assent (if < 18 years) and consent (adult participants and parents of minors) were obtained prior to enrollment. In order to minimize barriers to adolescent participation in the trial, parent participation was encouraged but not required.
2.3. Participants
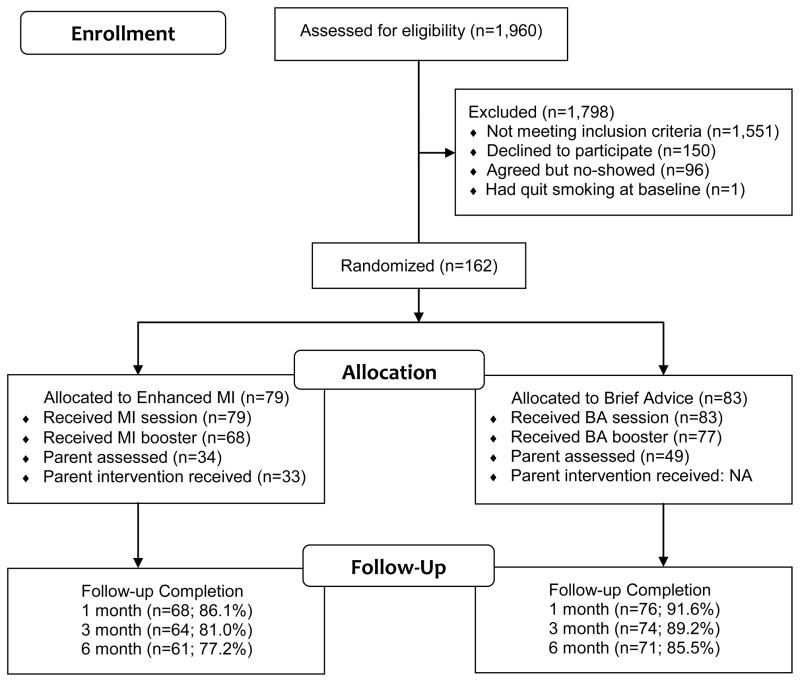
Of 1,960 screened adolescents, 409 (20.9%) were eligible; most ineligibility was due to nonsmoking status. Of the 150 who declined, 149 did so prior to enrollment, and 1 withdrew prior to randomization. Reasons for refusal included: disinterest (44%), time commitment (17%), and not wanting to disclose smoking to parents (11%). The randomized sample (N=162) consisted of 85 males and 77 females, and were 72% non-Hispanic White, 7% Black/African American, 6% Hispanic/Latino, and 15% other race or more than one race.
Most participants were from school (44%) or medical (36%) settings; 20% were from the community. Participants from these three sources were compared in terms of demographics, smoking history, patterns, dependence, biomarkers, smoking status of parents and friends, and depression symptoms. They were found to differ on three variables. Participants from medical settings were more ethnically and racially diverse, χ2(2) = 9.35, p = .009, with 40.7% minorities compared with 16.7% in schools and 29.0% in the community; 2) settings differed in terms of baseline CO level, F(2,161)=5.67, p=.004; participants recruited from schools had lower CO levels (M=7.21; SD=5.26) than those in medical settings (M=11.15; SD=9.17, p=.008) and those in community settings (M=11.16; SD=7.98), which may have been a consequence of the after-school CO assessment following prolonged smoking restriction while at school. Settings also differed on CES-D depression scores, F(2,161)=4.07, p=.019, with those in medical settings scoring significantly higher (M=16.90; SD=10.61) than those in schools (M=12.08; SD=9.37, p=.014). Importantly, the two intervention conditions were equivalent on the distribution of participants from the three recruitment sources, χ2(2)=0.57, p=.753.
2.4. Procedure
Procedures were approved by university and hospital Institutional Review Boards. Study sessions were confidential and conducted individually in a private setting. Adolescents completed in-person assessments at baseline and 1-, 3-, and 6-month follow up, for which they received a $30 shopping mall gift certificate and cash payments of $15, $20, and $25 respectively, plus a $20 bonus if all appointments were completed on time. Parents were assessed by telephone at baseline (within one week of adolescent baseline) and at 1-, 3-, and 6-month follow ups; they received $5 cash for the 1- and 3-month follow ups, and $10 at the 6-month follow up. Baseline assessments were conducted by interventionists, follow-up assessments were conducted by research assistants (RAs); all interviewers were blind to condition assignment during assessments. At follow up, self-reported smoking data were collected retrospectively if the prior follow up had not been completed.
2.4.1. Randomization
A computer-generated random number sequence allocated participants to treatment groups prior to enrollment; assignments were sealed in envelopes which were filed in a series of sequentially numbered folders. Interventionists used folders in order and completed baseline assessment before opening the envelope.
2.4.2. Measures
2.4.2.1. Adolescent assessments
Interviewers read questions aloud and entered responses into a laptop computer. A demographic questionnaire assessed age, gender, years of education completed, ethnicity/race, and living arrangements. Motivation to Quit (how much the participant “would like to quit smoking”) and Quitting Self-efficacy (SE) (confidence in being able to “quit smoking for good” were rated on 5-point scales (1=not at all, 5 = very) at baseline and follow up. The Timeline Follow Back (TLFB), a calendar-assisted structured interview that uses memory cues to increase recall accuracy, was used to record daily smoking behavior (Lewis-Esquerre et al., 2005) for the past 30 days at baseline and for the past 30, 60, and 90 days at each follow up respectively; these data were used to calculate average number of cigarettes per day (CPD). The Stanford Dependence Inventory (SDI; Rojas et al., 1998) provided a validated index of baseline nicotine dependence (O’Loughlin et al., 2002). Scores range from 5–25; higher scores indicate greater dependence. The Smoking History and Patterns Questionnaire (Colby, et al., 2005) assessed age at progression through smoking milestones (first cigarette, weekly smoking, and daily smoking) and quit attempts. The Interpersonal Influences Questionnaire (IIQ; Colby, et al., 2005) assessed parent smoking status and disapproval of smoking, and smoking status of five closest friends. The 20-item Center for Epidemiological Studies-Depression Scale (CES-D; Radloff, 1991) assessed past-week depressive symptoms. A drug use assessment queried the number of days (out of the past 30) the participant used alcohol and other drugs. At baseline and 1-month follow up, normative perceptions were assessed by asking participants to estimate smoking rates for same-age same-gender adolescents and for adults.
Two biochemical markers were used to validate self-reported abstinence at follow up: (1) expired carbon monoxide (CO), obtained via a Bedfont Micro Smokerlyzer®; and (2) saliva cotinine, analyzed via gas chromatography by an independent laboratory. CO levels < 9 ppm and cotinine < 14 ng/ml confirmed abstinence.
2.4.2.2. Parent assessments
At baseline, parents provided demographics and smoking status for both parents and smoking pattern data for the reporting parent. At each assessment, disapproval and perceived harm of adolescent smoking were rated on 5-point Likert-type scales (1=strongly disapprove to 5=strongly approve; 1=strongly disagree to 5=strongly agree, respectively). Frequency of discussing smoking with the adolescent, warning of health consequences, and scolding were assessed with a 5-point scale at baseline (1=never, 5=daily) and by asking the number of times at each follow up interval. Smoking restrictions were classified as 1=adolescent not allowed to smoke at all; 2=adolescent not allowed to smoke inside home; 3=adolescent allowed to smoke/no smoking restrictions.
2.4.3. Interventions
Adolescent MI and BA sessions were manualized and have been described in detail elsewhere (Colby, et al., 2005) so are briefly summarized here.
2.4.3.1. BA
BA emphasized strong directive advice to quit smoking as soon as possible. Participants were advised that “quitting smoking is the most important thing you can do to protect your current and future health.” Participants were provided with a pamphlet on quitting smoking and a list of local smoking treatment referrals. BA was delivered in about 5 minutes.
2.4.3.2. MI
Interventionists’ therapeutic style followed MI principles (Miller & Rollnick, 2002). The MI manual included the following sections: 1) establishing rapport; 2) exploring pros and cons of smoking and quitting; 3) delivery of computer-generated personalized assessment feedback; 4) imagining the future with and without smoking; 5) reviewing a menu of change options and developing a change plan; and 6) enhancing self-efficacy for change. MI participants were provided with the same handouts as in BA. They also received their assessment feedback sheet and change plan. The length of the baseline session was 45 minutes.
2.4.3.3. Telephone boosters
Interventionists contacted participants one week after baseline. In MI, this 15–20 minute discussion was designed to reinforce progress toward goals. The interventionist assisted in problem-solving, discussed coping skills, promoted self-efficacy for change, and updated change plans if appropriate. Revised change plans were mailed to participants afterwards. In BA, the 5-minute discussion reiterated strong directive advice to quit smoking and maintain abstinence. BA participants who reported quit attempts were praised; those who reported continued smoking were strongly encouraged to try to quit as soon as possible.
2.4.3.4. Parent intervention
Following the baseline parent assessment by telephone, parents of MI participants were asked to participate in a 15–20 minute discussion. This intervention was designed to be consistent with MI principles, emphasized the adolescent’s responsibility for making decisions/changes related to smoking, and focused on increasing parent support for the adolescent’s goals for changing smoking, increasing clear communication, and establishing home smoking rules. Interventionists used open-ended questions to elicit information about the parent’s attitudes and behavior relevant to these topics and, based on parent interest, introduced strategies for enhancing communication, enforcing household smoking restrictions, and reinforcing adolescent efforts toward change goals. Parents in the BA condition completed the same assessments but did not participate in a discussion with the interventionist. Parents in both conditions were mailed informational materials on helping adolescents quit smoking.
2.4.4. Training, supervision, and adherence
Fifteen interventionists, each with a Bachelors or Masters degree and at least one year of clinical research experience, delivered both interventions. Training involved reading Motivational Interviewing (Miller & Rollnick, 2002) and related articles, viewing training videotapes, and 40 hours of interactive training sessions, which involved practicing MI techniques and style, and role-playing sessions with supervisor feedback. Interventionists participated in weekly group supervision.
Post-MI and BA, interventionists and adolescent participants completed session ratings. To limit demand characteristics, participants sealed responses in an envelope that was delivered to RAs. Interventionist style (e.g., rapport, empathy, self-efficacy enhancement), support of autonomy (“supported my choices and decisions about smoking and quitting”), and providing expert advice (“told me what to do about smoking and quitting”) were rated from 1 (strongly disagree) to 4 (strongly agree). Participants rated their satisfaction from 1 (not at all) to 5 (very satisfied). In MI, participants and interventionists also rated whether each of 16 prescribed session elements had been discussed. Following the parent MI, interventionists rated whether each of 12 prescribed session elements had been discussed.
2.5. Data Analysis
Chi-square analyses and independent t-tests were used to analyze group differences on key baseline characteristics, rates of treatment and follow-up completion, and intervention session ratings. Analyses included all randomized participants with data regardless of whether they completed all intervention components. Chi-square analyses were used to test group differences on point-prevalence abstinence at each follow up. CPD was analyzed using 2 × 2 (group × time) ANOVA from baseline to each follow up. Intervention effects on adolescent-reported proximal outcomes were tested using repeated measures 2 × 2 (group × time) ANOVA from baseline to 1-month follow up. For parent-reported proximal outcomes, analysis of covariance (ANCOVA) tested for group differences at 1-month follow up while covarying the corresponding variable at baseline.
3. Results
3.1. Preliminary Analyses
3.1.1. Participation and attrition
Participant flow through the study is presented in Figure 1. There were no significant group differences on booster or follow-up completion rates. However, parents in BA were more likely to participate than parents in MI, χ2(1) = 4.15, p = .042. Adolescents who lived with their parent(s) were more likely to have parent participation (85/130; 65%) than those not living with parent(s) (14/32; 44%), χ2(1) = 5.06, p< .05. Participants who missed one or more follow ups (22%) did not differ from those who completed all three (78%) on any variable tested, including demographics, parent participation, treatment assignment, motivation, smoking rate, or tobacco dependence.
Figure 1.
Participant Flow Diagram
3.1.2. Baseline characteristics by treatment assignment
There were no significant differences between intervention groups on any baseline variables with the exception of CO level, which was greater in MI than BA participants (see Table 1). Participants smoked about a half-pack of cigarettes daily on average and reported high motivation to quit smoking but relatively low quitting self-efficacy. The majority of participants used alcohol and marijuana and had a parent who currently smoked cigarettes.
Table 1.
Baseline Demographic and Smoking Characteristics by Group
| Variable | MI (n = 79) | BA (n = 83) | t or χ2 | p |
|---|---|---|---|---|
| Demographic Characteristics | ||||
| Male, n (%) | 41 (51.9) | 44 (53.0) | 0.02 | .89 |
| Age, M (SD) | 16.2 (1.3) | 16.2 (1.2) | 0.06 | .96 |
| Non-Hispanic White, n (%) | 57 (72.2) | 60 (72.3) | 0.00 | .98 |
| Total years education, M (SD) | 9.5 (1.3) | 9.6 (1.3) | −0.83 | .41 |
| Smoking Characteristics | ||||
| # Cigarettes/day in past 30 days, M (SD) | 11.3 (8.5) | 9.2 (7.0) | 1.69 | .09 |
| # Days smoked in past 30 days, M (SD) | 28.0 (4.7) | 27.2 (4.9) | 1.00 | .32 |
| Made a quit attempt in past year, n (%) | 69 (90.8) | 71 (91.0) | 0.00 | .96 |
| Stanford Dependence Index, M (SD) | 14.1 (4.0) | 13.5 (4.0) | 1.07 | .29 |
| CO level (ppm), M (SD) | 11.1 (8.7) | 7.8 (6.0) | 2.72 | .007 |
| Saliva cotinine (ng/ml), M (SD) | 252.3 (201.7) | 212.4 (174.1) | 1.34 | .18 |
| Parent smokes cigarettes, n (%) | 56 (70.9) | 57 (68.7) | 0.09 | .76 |
| # of 5 closest friends who smoke, M (SD) | 3.9 (1.1) | 3.9 (1.3) | 0.36 | .72 |
| 1 to 5 Rating Scales | ||||
| Motivation to quit smoking, M (SD) | 4.1 (1.0) | 4.2 (1.0) | −0.20 | .84 |
| Quitting self-efficacy, M (SD) | 2.5 (1.4) | 2.6 (1.5) | −0.47 | .64 |
| Past 7-Day Depressive Symptoms | ||||
| CES-D total score, M (SD) | 14.6 (9.5) | 13.8 (10.2) | 0.49 | .63 |
| Past 30-Day Substance Use | ||||
| Drank alcohol, n (%) | 60 (75.9) | 63 (76.8) | 0.02 | .90 |
| Used marijuana, n (%) | 57 (72.2) | 50 (61.0) | 2.26 | .13 |
| # Days drank alcohol, M (SD) | 5.9 (5.0) | 5.9 (5.8) | 0.04 | .97 |
| # Days used marijuana, M (SD) | 15.1 (10.7) | 14.0 (11.2) | 0.53 | .60 |
Notes. MI=Motivational Interviewing; BA=Brief Advice; CES-D = Center for Epidemiologic Studies Depression Scale.
3.1.3. Intervention acceptability and fidelity
Satisfaction ratings were equally high in both conditions. MI participants tended to rate the interventionist as more empathic and more helpful in increasing quitting self-efficacy compared with those in BA (Table 2). Other session ratings did not differ by group. Interventionists and adolescents both indicated that nearly all 16 MI session components were delivered (M = 15.6, SD = .80 and M = 15.4, SD = 1.39 respectively). Interventionists indicated that they had provided 10.7 of 12 (SD = 1.78) parent MI components.
Table 2.
Intervention Session Ratings by Group
| Variable | MI (n = 79) | BA (n = 83) | t | p |
|---|---|---|---|---|
| Participant ratings of interventionista | ||||
| Rapport, M (SD) | 3.84 (0.47) | 3.88 (0.46) | −0.59 | .56 |
| Empathy, M (SD) | 3.51 (0.66) | 3.30 (0.68) | 1.91 | .06 |
| Enhance self-efficacy, M (SD) | 3.66 (0.58) | 3.48 (0.65) | 1.88 | .06 |
| Support autonomy, M (SD) | 3.73 (0.57) | 3.59 (0.67) | 1.52 | .13 |
| Expert advice, M (SD) | 3.37 (1.01) | 3.54 (0.85) | −1.12 | .26 |
| Participant ratings of sessionb | ||||
| Satisfaction, M (SD) | 4.37 (0.81) | 4.26 (0.75) | 0.91 | .37 |
Notes. MI=Motivational Interviewing; BA=Brief Advice.
1 = strongly disagree; 4 = strongly agree;
1 = not at all satisfied; 5 = very satisfied.
3.2. Intervention Effects on Smoking Outcomes
3.2.1. Point prevalence abstinence
Intervention effects on biochemically-confirmed 7-day abstinence were not significant. Verified abstinence rates for MI and BA were 3/66 (4.5%) and 1/72 (1.4%) at 1-month follow up, χ2(1) = 1.21, p = .27; 2/61 (3.3%) and 5/73 (6.8%) at 3-month follow up, χ2(1) = 0.86, p = .36; and 3/61 (4.9%) and 2/71 (2.8%) at 6-month follow up, χ2(1) = 0.40, p = .53, respectively. Analyses that recoded all missing data as “smoking” yielded lower abstinence rates and were also not significant.
3.2.2. CPD
A significant group × time interaction effect was found at 1-month follow up, F(1, 145) = 5.65, p = .019. Simple effects tests showed that MI participants reported significantly reduced CPD from baseline (M = 11.27, SD = 8.71) to follow up (M = 8.52, SD = 6.39), F(1,145) = 25.09, p < .001; those in BA showed a non-significant trend toward reduction (M = 9.41, SD = 7.11 and M = 8.45, SD = 6.59) respectively, F(1,145) = 3.44, p = .065. At 3- and 6-month follow ups, both groups reported significant reductions from baseline in CPD with no significant group effects.
3.3. Intervention Effects on Proximal Outcomes at 1-Month Follow Up
3.3.1. Adolescents
Intervention effects on motivation to quit smoking and quitting self-efficacy were not significant, nor did motivation or self-efficacy change significantly from baseline to 1-month follow up (see Table 3). There was a significant group by time effect on perceived adolescent smoking norms, F(1,136) = 34.25, p < .001. Simple effects tests showed that perceived adolescent smoking norms reduced in MI from baseline to 1-month (F(1,136) = 90.56, p < .001) but did not change among adolescents in BA (F(1,136) = 2.18, p = .142). The group by time effect on perceived adult smoking norms was similar, F(1,136) = 22.16, p < .001; perceived adult smoking norms reduced in MI from baseline to 1-month follow up (F(1,136) = 51.96, p < .001) but did not change in BA (F(1,136) = 0.52, p = .472).
Table 3.
Effects of MI and BA on Adolescent Proximal Outcomes
| Baseline | 1M FU | |||
|---|---|---|---|---|
| M | SD | M | SD | |
| Motivation to Quita | ||||
| MI | 4.12 | 1.03 | 4.12 | 1.11 |
| BA | 4.07 | 1.06 | 4.15 | 1.12 |
| Quitting Self-Efficacya | ||||
| MI | 2.31 | 1.30 | 2.67 | 1.38 |
| BA | 2.50 | 1.44 | 2.53 | 1.20 |
| Perceived Adolescent Smoking Rateb | ||||
| MI | 58.68 | 21.45 | 32.15 | 19.55 |
| BA | 57.74 | 18.51 | 53.79 | 19.13 |
| Perceived Adult Smoking Rateb | ||||
| MI | 60.74 | 20.94 | 42.92 | 21.32 |
| BA | 59.24 | 19.13 | 57.53 | 20.17 |
Notes. All measures based on adolescent participant self-report. MI=Enhanced MI; BA=Brief Advice.
No significant effects of group, time or group × time.
Significant group × time effects (p < .001). MI decreased from baseline to 1M FU (1-month follow up) while BA did not change from baseline to follow up.
3.3.2. Parents
At 1-month follow up, analyses of intervention effects on parent attitudes and behaviors related to adolescent smoking revealed that compared to parents in BA, parents in MI reported somewhat weaker disapproval of adolescent smoking and less frequent warnings about the health consequences of smoking (see Table 4). Groups did not differ on smoking rules at 1 or 3 months, but at 6 months, MI parents were more likely to report having no restrictions on adolescent smoking (45.0%) compared to BA parents (17.4%), χ2(2) = 11.07, p = .004.
Table 4.
Intervention Effects on Parent Proximal Outcomes: 1-Month Follow Up Variables Adjusted for Baseline Levels
| Variable, Adj. M (SD) | MI (N=31) | BA (N=38) | F(1,66) | p |
|---|---|---|---|---|
| Parent Attitudes and Perceptions | ||||
| Approval of adolescent’s smoking | 1.43 (0.62) | 1.26 (0.54) | 3.81 | .055 |
| Perceived harm of adolescent’s smoking | 4.25 (0.85) | 4.48 (0.72) | 0.54 | .467 |
| Parent Behaviors (# times) | ||||
| Discussed smoking with adolescent | 14.16 (10.49) | 15.06 (12.13) | 0.13 | .720 |
| Warned about health consequences | 9.73 (9.01) | 13.88 (11.55) | 3.86 | .054 |
| Scolded about smoking | 6.08 (11.37) | 7.03 (10.31) | 0.16 | .686 |
Notes. All measures based on parent self-report. MI=Enhanced MI; BA=Brief Advice.
4. Discussion
Following both enhanced MI, which included a telephone booster session and an optional parent session, or BA for adolescent smokers, participants reported declines in smoking rate (cigarettes per day) with greater declines for MI than BA at 1-month follow up. Rates of biochemically-confirmed abstinence were low at each follow up, did not differ between groups at any follow up, and were not consistently greater in the MI condition (i.e., making it unlikely that lack of significance was exclusively attributable to statistical power limitations). Results are largely consistent with our previous work (Colby, et al., 1998; Colby, et al., 2005) and other MI trials (Audrain-McGovern et al., 2011; Brown, et al., 2003; Horn, et al., 2007) that demonstrate that MI has a modest impact on self-reported smoking rates among adolescent smokers, but little to no impact on biochemically-confirmed cessation.
Proximal outcomes thought to mediate changes in smoking behavior, including motivation and self-efficacy to quit smoking, as well as perceptions regarding peer and adult smoking norms were also examined at 1 month. Contrary to our expectations, MI did not lead to increased motivation or quitting self-efficacy; however, both groups demonstrated relatively high motivation to quit at baseline, so lack of change may reflect ceiling effects. Adolescents in MI versus BA reported significantly greater decreases in their perceptions of the percentage of peers and adults who smoke, decreasing by about half in MI, with no significant change in BA. This finding is important because the perception that smoking is a normative behavior is associated with greater rates of smoking among adolescents (Botvin et al., 1992; Castrucci et al., 2002; Simons-Morton, 2002).
A pattern of results emerged from the parent data that was unexpected: MI parents endorsed less restrictive family smoking rules compared to BA parents at the final follow up. There were also trends for MI parents to report less disapproval of adolescent smoking and less frequent discussion of smoking’s health consequences than BA parents at 1-month follow up. It is possible that MI’s emphasis on supporting the adolescent’s personal choices and responsibility for behavior change related to smoking may have undermined empirically-supported parental strategies for reducing smoking, such as expressing clear disapproval of use, as well as parental monitoring and enforcing rules regarding smoking (Andersen, et al., 2004; Sargent & Dalton, 2001). Our low-intensity parent intervention was designed to be feasible and easily disseminable; however, evidence from other substance use intervention trials suggests that a one-session, face-to-face parent intervention with both adolescent and parent present, may have more promise (Spirito et al., 2011; Stanton et al., 2004). Further research on the effects and optimal attributes of parent interventions on adolescent smoking outcomes seems warranted.
Following substantial declines in adolescent smoking prevalence in the U.S., youth who continue to smoke tend to be heavier or more frequent smokers (Chassin et al., 2007; Curry, et al., 2009) who may require more intensive interventions. Sussman and Sun’s (2008) review recommends a minimum of 5 intervention sessions to achieve substantial effects on adolescent smoking abstinence. Brief interventions may not be sufficient to produce substantial rates of smoking cessation among adolescents.
Our findings should be interpreted within the context of some limitations. First, this trial was not designed to test the incremental effects of the parent intervention; effects of the parent component of MI cannot be disentangled from the other MI intervention components. Second, parent involvement was optional and incentives for parent participation were minimal. The resulting low rates of parent participation led to an underpowered and less informative test of the full intervention. Third, due to low rates of smoking abstinence and lack of group differences at follow up, we were unable to conduct formal mediation analyses. We instead tested intervention effects on proximal outcomes, which yielded information about which variables are affected by MI and may be useful for further intervention development.
4.1. Conclusions
There is a need for efficacious interventions for adolescent smokers. In this trial, MI modestly reduced smoking rates (average cigarettes per day) in the short-term compared to BA. Compared to BA, MI also led to substantial reductions in perceived smoking norms for adolescents and for adults. These findings suggest that MI may provide an efficacious first step toward smoking cessation. An important direction for future intervention development research is how best to capitalize on these proximal effects to lead to longer-term abstinence for adolescent smokers.
Highlights.
We compared two brief smoking cessation interventions for adolescents.
Motivational interviewing (MI) reduced smoking more than brief advice (BA).
MI also reduced perceptions of adolescent and adult smoking norms.
Rates of smoking cessation were low and did not differ by group.
We conclude that MI has positive proximal effects but does not lead to cessation.
Acknowledgments
Role of Funding Sources
This research and manuscript preparation was funded by NIDA grant#1R01 DA11204. Preparation of the manuscript was also supported by NIAAA grant #1R01 AA016000 and NIDA grant # 1T32 DA016184. NIDA and NIAAA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
The authors express appreciation to Cheryl A. Eaton for her assistance with data analysis.
Footnotes
Contributors
Authors Colby, O’Leary Tevyaw, Barnett, Rohsenow, and Monti designed the study and wrote the protocol. Authors Colby, Nargiso, O’Leary Tevyaw, and Metric managed the literature review and summaries of previous related work, undertook the statistical analysis, and wrote the first draft of the manuscript. Authors Lewander and Woolard provided guidance for implementation of the protocol in the medical settings and participated in study design and interpretation of results. All authors contributed to and have approved the final manuscript.
Conflict of Interest
All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen MR, Leroux BG, Bricker JB, Rajan KB, Peterson AV., Jr Antismoking Parenting Practices Are Associated With Reduced Rates of Adolescent Smoking. Arch Pediatr Adolesc Med. 2004;158(4):348–352. doi: 10.1001/archpedi.158.4.348. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Stevens S, Murray PJ, Kinsman S, Zuckoff A, Pletcher J, et al. The efficacy of motivational interviewing versus brief advice for adolescent smoking behavior change. Pediatrics. 2011;128(1):e101–111. doi: 10.1542/peds.2010-2174. [DOI] [PubMed] [Google Scholar]
- Beaglehole R, Bonita R, Horton R, Adams C, Alleyne G, Asaria P, et al. Priority actions for the non-communicable disease crisis. The Lancet. 2011;377(9775):1438–1447. doi: 10.1016/S0140-6736(11)60393-0. [DOI] [PubMed] [Google Scholar]
- Botvin GJ, Botvin EM, Baker E, Dusenbury L, Goldberg CJ. The false consensus effect: predicting adolescents’ tobacco use from normative expectations. Psychol Rep. 1992;70(1):171–178. doi: 10.2466/pr0.1992.70.1.171. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL. Smoking cessation in young adults: Age at initiation of cigarette smoking and other suspected influences. American Journal of Public Health. 1996;86(2):214–220. doi: 10.2105/ajph.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Ramsey SE, Strong DR, Myers MG, Kahler CW, Lejuez CW, et al. Effects of motivational interviewing on smoking cessation in adolescents with psychiatric disorders. Tob Control. 2003;12(Suppl 4):IV3–10. doi: 10.1136/tc.12.suppl_4.iv3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrucci BC, Gerlach KK, Kaufman NJ, Orleans CT. The association among adolescents’ tobacco use, their beliefs and attitudes, and friends’ and parents’ opinions of smoking. Matern Child Health J. 2002;6(3):159–167. doi: 10.1023/a:1019774028526. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson C, Morgan-Lopez A, Sherman SJ. “Deviance proneness” and adolescent smoking 1980 versus 2001: Has there been a “hardening” of adolescent smoking? Journal of Applied Developmental Psychology. 2007;28(3):264–276. doi: 10.1016/j.appdev.2007.02.005. [DOI] [Google Scholar]
- Chassin L, Presson C, Seo DC, Sherman SJ, Macy J, Wirth RJ, et al. Multiple trajectories of cigarette smoking and the intergenerational transmission of smoking: a multigenerational, longitudinal study of a Midwestern community sample. Health Psychol. 2008;27(6):819–828. doi: 10.1037/0278-6133.27.6.819. [DOI] [PubMed] [Google Scholar]
- Coambs RB, Li S, Kozlowski LT. Age Interacts with Heaviness of Smoking in Predicting Success in Cessation of Smoking. American Journal of Epidemiology. 1992;135(3):240–246. doi: 10.1093/oxfordjournals.aje.a116277. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. New Jersey: Lawrence Erlbaum; 1988. [Google Scholar]
- Colby SM, Gwaltney CJ. Pharmacotherapy for adolescent smoking cessation. JAMA. 2007;298(18):2182–2184. doi: 10.1001/jama.298.18.2182. [DOI] [PubMed] [Google Scholar]
- Colby SM, Monti PM, Barnett NP, Rohsenow DJ, Weissman K, Spirito A, et al. Brief motivational interviewing in a hospital setting for adolescent smoking: a preliminary study. J Consult Clin Psychol. 1998;66(3):574–578. doi: 10.1037//0022-006x.66.3.574. [DOI] [PubMed] [Google Scholar]
- Colby SM, Monti PM, O’Leary Tevyaw T, Barnett NP, Spirito A, Rohsenow DJ, et al. Brief motivational intervention for adolescent smokers in medical settings. Addict Behav. 2005;30(5):865–874. doi: 10.1016/j.addbeh.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Curry SJ, Mermelstein RJ, Sporer AK. Therapy for Specific Problems: Youth Tobacco Cessation. Annual Review of Psychology. 2009;60:229–255. doi: 10.1146/annurev.psych.60.110707.163659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Exter Blokland EA, Engels RC, Hale WW, 3rd, Meeus W, Willemsen MC. Lifetime parental smoking history and cessation and early adolescent smoking behavior. Prev Med. 2004;38(3):359–368. doi: 10.1016/j.ypmed.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Flay BR, Hu FB, Richardson J. Psychosocial predictors of different stages of cigarette smoking among high school students. Prev Med. 1998;27(5 Pt 3):A9–18. doi: 10.1006/pmed.1998.0380. [DOI] [PubMed] [Google Scholar]
- Gervais A, O’Loughlin J, Dugas E, Eisenberg MJ, Wellman RJ, JRD A systematic review of randomized controlled trials of youth smoking cessation interventions. Drogués Santé et société. 2007;6(1 Suppl 2):ii1–ii26. [Google Scholar]
- Gilman SE, Rende R, Boergers J, Abrams DB, Buka SL, Clark MA, et al. Parental smoking and adolescent smoking initiation: an intergenerational perspective on tobacco control. Pediatrics. 2009;123(2):e274–281. doi: 10.1542/peds.2008-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimshaw GM, Stanton A. Tobacco cessation interventions for young people. Cochrane Database Syst Rev. 2006;(4):CD003289. doi: 10.1002/14651858.CD003289.pub4. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Egleston BL, Hofmann MT. Efficacy of motivational interviewing for smoking cessation: a systematic review and meta-analysis. Tob Control. 2010;19(5):410–416. doi: 10.1136/tc.2009.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JE, Hendricks PS. Motivational interviewing for smoking cessation: a meta-analytic review. J Consult Clin Psychol. 2010;78(6):868–884. doi: 10.1037/a0021498. [DOI] [PubMed] [Google Scholar]
- Horn K, Dino G, Hamilton C, Noerachmanto N. Efficacy of an emergency department-based motivational teenage smoking intervention. Prev Chronic Dis. 2007;4(1) [PMC free article] [PubMed] [Google Scholar]
- Huver RME, Engels RCME, de Vries H. Are anti-smoking parenting practices related to adolescent smoking cognitions and behavior? Health Education Research. 2006;21:66–77. doi: 10.1093/her/cyh045. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2009. Bethesda, MD: National Institute on Drug Abuse; 2010. [Google Scholar]
- Kodl MM, Mermelstein R. Beyond modeling: Parenting practices, parental smoking history, and adolescent cigarette smoking. Addictive Behaviors. 2004;29(1):17–32. doi: 10.1016/s0306-4603(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Lewis-Esquerre JM, Colby SM, Tevyaw TO, Eaton CA, Kahler CW, Monti PM. Validation of the timeline follow-back in the assessment of adolescent smoking. Drug Alcohol Depend. 2005;79(1):33–43. doi: 10.1016/j.drugalcdep.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Mackay J, Eriksen M, Shafey O. The tobacco atlas. Atlanta, GA: American Cancer Society; 2006. [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. New York: Guilford Press; 2002. [Google Scholar]
- O’Loughlin J, Tarasuk J, Difranza J, Paradis G. Reliability of selected measures of nicotine dependence among adolescents. Ann Epidemiol. 2002;12(5):353–362. doi: 10.1016/s1047-2797(01)00312-x. [DOI] [PubMed] [Google Scholar]
- Peterson AV, Jr, Leroux BG, Bricker J, Kealey KA, Marek PM, Sarason IG, et al. Nine-year prediction of adolescent smoking by number of smoking parents. Addict Behav. 2006;31(5):788–801. doi: 10.1016/j.addbeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The Use of the Center for Epidemiologic Studies Depression Scale in Adolescents and Young-Adults. Journal of Youth and Adolescence. 1991;20(2):149–166. doi: 10.1007/BF01537606. [DOI] [PubMed] [Google Scholar]
- Rojas NL, Killen JD, Haydel KF, Robinson TN. Nicotine dependence among adolescent smokers. Arch Pediatr Adolesc Med. 1998;152(2):151–156. doi: 10.1001/archpedi.152.2.151. [DOI] [PubMed] [Google Scholar]
- Sargent JD, Dalton M. Does Parental Disapproval of Smoking Prevent Adolescents From Becoming Established Smokers? Pediatrics. 2001;108(6):1256–1262. doi: 10.1542/peds.108.6.1256. [DOI] [PubMed] [Google Scholar]
- Simons-Morton BG. Prospective analysis of peer and parent influences on smoking initiation among early adolescents. Prev Sci. 2002;3(4):275–283. doi: 10.1023/a:1020876625045. [DOI] [PubMed] [Google Scholar]
- Spirito A, Sindelar-Manning H, Colby SM, Barnett NP, Lewander W, Rohsenow DJ, et al. Individual and Family Motivational Interventions for Alcohol-Positive Adolescents Treated in an Emergency Department Results of a Randomized Clinical Trial. Archives of Pediatrics & Adolescent Medicine. 2011;165(3):269–274. doi: 10.1001/archpediatrics.2010.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton B, Cole M, Galbraith J, Li X, Pendleton S, Cottrel L, Marshall S, Wu Y, Kaljee L. Randomized trial of a parent intervention: parents can make a difference in long-term adolescent risk behaviors, perceptions, and knowledge. Arch Pediatr Adolesc Med. 2004;158(10):947–955. doi: 10.1001/archpedi.158.10.947. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. NSDUH Series H-36, HHS Publication No SMA 09-4434. Office of Applied Studies; Rockville, MD: 2009. Results from the 2008 National Survey on Drug Use and Health: National Findings. [Google Scholar]
- Sussman S, Sun P. Youth tobacco use cessation: 2008 update. Tob Induc Dis. 2009;5:3. doi: 10.1186/1617-9625-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RE, Baker PR, Lorenzetti D. Cochrane review: Family-based programmes for preventing smoking by children and adolescents. Evidence-Based Child Health: A Cochrane Review Journal. 2009;4(2):826–882. doi: 10.1002/14651858.CD004493.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyas SL, Pederson LL. Psychosocial factors related to adolescent smoking: A critical review of the literature. Tobacco Control. 1998;7:409–420. doi: 10.1136/tc.7.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HR, Pandina RJ, Chen PH. Developmental trajectories of cigarette use from early adolescence into young adulthood. Drug Alcohol Depend. 2002;65(2):167–178. doi: 10.1016/s0376-8716(01)00159-4. [DOI] [PubMed] [Google Scholar]
- Withers NJ, Low JL, Holgate ST, Clough JB. Smoking habits in a cohort of U.K. adolescents. Respir Med. 2000;94(4):391–396. doi: 10.1053/rmed.1999.0746. [DOI] [PubMed] [Google Scholar]