Abstract
Calcineurin inhibitors (CNIs) may promote post-transplantation cancer through altered expression of cytokines and chemokines in tumor cells. We found that there is a potential cross-talk among CNI-induced signaling molecules and mTOR. Here, we utilized a murine model of post-transplantation cancer to examine the effect of a combination therapy (CNI + mTOR-inhibitor rapamycin) on allograft survival and renal cancer progression. The therapy prolonged allograft survival; and significantly attenuated CNI-induced post-transplantation cancer progression, with down-regulation of mTOR and S6-kinase phosphorylation. Also, rapamycin inhibited CNI-induced over-expression of the angiogenic cytokine VEGF, and the chemokine receptor CXCR3 and its ligands in post-transplantation tumor tissues.
Keywords: Renal cancer, Angiogenesis, VEGF, Chemokine, Transplantation
1. INTRODUCTION
The development as well as rapid progression of post-transplantation cancer is a major limitation to the success of solid organ transplantation; it may occur de novo, as a recurrence of a pre-existing malignancy, or from transmission of malignancy from the donor [1, 2, 3]. Some forms of cancer, e.g. cancers of kidney and skin, lymphoma, and Kaposi's sarcoma are very common after organ transplantation [1, 4]. Although the immunosuppressive therapy has significantly increased the survival time of transplant patients in recent years, it can also promote tumor growth [5, 6, 7]. The association between immunosuppressive therapy and cancer is mediated through several pathogenic factors [8]. Indirectly, immunosuppressive drugs markedly increase the risk of post-transplantation cancer by impairing immune surveillance and facilitating the action of oncogenic viruses [1, 2]. However, the immunosuppressive agents may also have a direct pro- and anti-tumorigenic function [9, 10, 11, 12].
Recent studies have demonstrated that calcineurin inhibitors (CNIs) may play a major role in the development of post-transplantation cancer; and this effect of CNIs may not depend on their immunoregulatory function [7, 8, 9, 12, 13]. The pro-tumorigenic effect(s) of CNIs can be mediated through several effector molecules, including cytokines and chemokines. Chemokines are small cytokine-like secreted proteins that have chemoattractant properties [14]. It has been shown that the CNI cyclosporine (CsA) can promote tumor growth through the production of transforming growth factor-β [9]. We and others have demonstrated that CNIs can induce tumor growth through overexpression of the angiogenic cytokine vascular endothelial (VEGF) [10, 11, 15]. It is now established that apart from their functions in the immune system, different chemokines and their receptors can play significant role(s) in cancer development [16, 17, 18, 19]. We have found that CNIs can facilitate tumor progression through regulation of the chemokine receptor CXCR3 [20]. In addition, we have also discovered some novel tumorigenic pathway(s) in which CNIs can activate the proto-oncogenic ras and specific isoforms (ζ and δ) of protein kinase C (PKC) in human renal cancer cells to promote tumor growth [11, 15, 21].
The mammalian target of rapamycin (mTOR) inhibitor rapamycin (RAPA) is also used as an immunosuppressive agent to prevent allograft rejection in transplant patients [5, 22]. Some positive and clinically relevant effects of rapamycin include its capacity to interfere with fibrotic processes that often accompany transplant rejection, and to influence the preferential development of immunological tolerance [23]. Interestingly, in contrast to CNIs, RAPA can mediate an anti-angiogenic and anti-tumorigenic function [5, 10, 15, 24]. The mTOR pathway plays a major role in regulating the growth of angiogenic tumors (like, renal tumors) [25, 26]; and thus, the treatment with RAPA may play an important role for the prevention of tumor growth in transplant patients.
In our recent study, we have demonstrated that CNI-induced VEGF overexpression in renal cancer cells was significantly inhibited by the treatment with RAPA [27]. This suggests that mTOR pathway may have a potential cross-talk with the CNI-induced signaling molecules, and may play a critical role in CNI-induced VEGF overexpression. In support of this observation, we found that CNI-induced and Ras-PKC-mediated signals can indeed activate mTOR through the regulation of a proline-rich Akt substrate of 40 kDa (PRAS40) [27, 28]. Thus, a combination therapy using CNI and RAPA may have an important beneficial effect in preventing both allograft rejection and tumor growth in transplant patients.
In the present study, we utilized an in vivo murine model of post-transplantation cancer; and we examined the effect of a combination therapy using CNI and RAPA on the progression of renal cancer in mice undergoing cardiac transplantation. We show that the combination therapy can significantly attenuate the rapid progression of post-transplantation renal cancer through regulation of the angiogenic cytokine VEGF, and also the chemokine receptor CXCR3 and its ligands.
2. MATERIALS AND METHODS
2.1. Reagents and Antibodies
CsA (Novartis) was purchased from Children's Hospital Boston pharmacy, and RAPA was purchased from LC laboratories. The Western blot antibodies for mTOR, phospho-mTOR (Ser-2448), p70S6K and phosho-p70S6K (Thr-389) were obtained from Cell Signaling.
2.2. Cell Lines
The murine renal cancer cell line (RENCA) was obtained from the American Type Culture Collection. Cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum (GIBCO).
2.3. Renal Tissue Samples from Transplant Patients
Pathological tissue samples of human kidney were obtained from surgical specimens of two transplant patients at the Department of Nephrology, Transplant Center, Institute of Clinical and Experimental Medicine (Prague, Czech Republic). Patients underwent kidney transplantation and was under either CsA or tacrolimus therapy (calcineurin inhibitors); afterwards, the native kidneys had to be removed either due to the development of renal cell carcinoma or due to suspicion of tumor. Normal renal tissues were obtained from normal parts of the surgical specimens, and the normalcy of these tissues was confirmed by histology. The protocol to obtain tissue samples was approved by the review board of the Institute.
2.4. In Vivo Tumor Development
To evaluate the growth of tumors in allograft recipients, murine renal cancer cells (RENCA) were injected s.c. into BALB/c mice 6 days before heart transplantation. Tumor volume was measured using a digital caliper at regular intervals. The volume was estimated by following standard method [24], using the formula V = π/6 × a2 × b, wherein a is the short axis and b is the long tumor axis. Mice were sacrificed at designated times after injection or if complications occurred, which included signs of inactivity, cachexia, or decreased responsiveness. All animal works were approved by the animal care and use committee at Children's Hospital Boston and in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals.
2.5. Heart Transplantation
BALB/c mice were used as recipients of fully MHC mismatched C57BL/6 donor hearts. Vascularized intra-abdominal heterotopic heart transplantation was performed as described before [11, 24]. Mice were treated with different combinations of CsA (10 mg/kg/day) and RAPA (1.5 mg/kg/day). Donor hearts were monitored daily (by measuring palpation) for the development of rejection.
2.6. RNA Isolation and Real-time PCR
Total RNA was prepared using the RNeasy isolation kit (Qiagen), and cDNA was synthesized using cloned AMV first-strand synthesis kit (Invitrogen, Carlsbad, CA). To analyze the expression of VEGF, CXCL10, CXCL11, and CXCR3/CXCR3-A/CXCR3-B, real time PCR was performed using the Assays-on-Demand Gene Expression product (TaqMan, Mammalian Gene Collection probes) according to the manufacturer's instructions (Applied Biosystems, Foster City, CA). As an internal control, GAPDH mRNA was amplified. Gene-specific primers were obtained from Applied Biosystems. Ct value (the cycle number at which emitted fluorescence exceeded an automatically determined threshold) for gene of interest was corrected by the Ct value for GAPDH and expressed as ΔCt. Data were reported as fold change in mRNA amount, which was calculated as follows: (fold change) = 2X (where X = ΔCt for control group - ΔCt for experimental group).
2.7. Western Blot Analysis
Protein samples were run on SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (NEN Life Sciences Product, Inc.). The membranes were first incubated with primary antibody, and subsequently incubated with peroxidase-linked secondary antibody (Santa Cruz Biotechnology). All primary antibodies were diluted at 0.5 μg/ml; secondary antibodies were diluted at 0.2 μg/ml. The reactive bands were detected by using chemiluminescent substrate (Pierce).
2.8. Immunohistochemistry
For pathological tissue samples of human kidney, the tissue sections were incubated first with anti-CXCR3 / anti-CXCL10 / anti-CD31 (LS Bioscience), and second with a species-specific horseradish peroxidase-conjugated secondary antibody. For murine tissues, the sections were incubated first with anti-CD31 (BD PharMingen), and then with a species-specific horseradish peroxidase-conjugated secondary antibody. All the tissue sections were washed thoroughly in between incubations. Human tissue sections were developed in 3,3’-diaminobenzidine (BioGenex), and murine tissue sections were developed in 3-aminoethylcarbazole. Both the sections were then counterstained with hematoxylin using standard techniques. Vessel densities were quantified by grid-counting method at X400 magnification.
2.9. Statistical Analyses
Graft survival was compared using the Kaplan-Meier product-limit method with the log-rank test to determine whether time to rejection differed between treatment groups. Changes in mean tumor volume were compared by two-way repeated-measures analysis of variance (ANOVA) to test for differences in slopes between the treatment groups over time using the group-by-time interaction F-test. Fit to the data in handling the multiple tumor volume measurements from the same animal at the serial time points was accounted for using a compound symmetry covariance structure in the mixed-model longitudinal approach. Data are presented as mean and standard error of the mean (SEM). Statistical analysis was performed using SPSS version 19.0 (SPSS Inc./IBM, Chicago, IL). All two-tailed values of p < 0.05 were considered statistically significant.
For in vitro experiments, statistical evaluation for data analysis was determined by Student's t test. Differences with P < 0.05 were considered statistically significant.
3. RESULTS
3.1. Increased Expression of VEGF, CXCR3, CXCL10, and CXCL11 in Nephrectomized Kidney Tissues from Patients with Organ Transplantation
Renal epithelial cells (including renal cancer) have been shown to express the angiogenic cytokine VEGF, and the chemokine receptor CXCR3 and its ligands [11, 20]. In our previous studies [11, 15], we have demonstrated that the treatment with calcineurin inhibitors (CNIs) promotes a rapid progression of post-transplantation cancer through increased expression of VEGF. We have also observed that CNI treatment can mediate renal cancer progression through altered expression of CXCR3 splice variants [20]. Here, we examined the expression of VEGF, CXCR3 splice variants and CXCR3-binding ligands (CXCL10 and CXCL11) in nephrectomized kidney tissues of patients, who underwent kidney transplantation and received CNI therapy (either CsA or tacrolimus) as described in methods section. Gene expression in renal pathological tissues was compared with normal renal tissue. Patient-1 received CsA and developed renal cell carcinoma in the native kidney; while patient-2 received tacrolimus and had nephrectomy due to suspected tumor in the native kidney. As shown in Figure-1 (A-D), the mRNA expression of VEGF, CXCR3-A, CXCL10, and CXCL11 was significantly increased in the renal cancer tissue of patient-1 receiving CsA treatment. There was no significant change (data not shown) in the expression of CXCL9, another CXCR3-binding ligand. Based on our previous finding [20], we also expected that CsA treatment might down-regulate CXCR3-B expression. However, there was no significant change (data not shown) in the expression of CXCR3-B as well as the CXCR3-B-binding ligand CXCL4. In the nephrectomized kidney tissue of patient-2 receiving tacrolimus, there were also increases in the expression of CXCR3-A and CXCL10; although the increase in expression of these genes were much lower compared with patient-1 receiving CsA (Figure-1).
Figure-1. CNI-induced post-transplantation renal pathogenesis is associated with the overexpression of VEGF, CXCR3-A, CXCL10, and CXCL11.
Total RNA was isolated from nephrectomized renal tissues of patient-1 (CsA-treated), and patient-2 (tacrolimus-treated), and also from normal renal tissues, and was reverse transcribed. Fold changes in the mRNA expression of VEGF (A), CXCR3-A (B), CXCL10 (C), and CXCL11 (D) were measured by real-time PCR using gene-specific primers. Columns are average of triplicate readings of the samples; error bars are +/- SD. *, p < 0.01 compared with normal renal tissue.
We also performed immunohistochemistry to confirm the expression of some of these genes in nephrectomized renal tissues of the transplant patients. As shown in Figure-2, the expression of CXCR3 (top panel) and CXCL10 (middle panel) was markedly increased in pathological renal tissues (obtained from nephrectomized kidneys of patients receiving CNI therapy) compared with normal renal tissues. We also checked the blood vessel density in these tissues as observed by CD31 staining. We found that CD31 was primarily expressed in the glomeruli of normal kidney tissues; whereas, its expression was distributed throughout the nephrectomized kidney tissues of patients receiving CNI (Figure-2, bottom panel). Together, these observations suggest that CNI-induced post-transplantation renal cancer/pathogenesis can be associated with induced expression of angiogenic VEGF and increased renal vessel density; there may also be induced expression of growth-promoting CXCR3 (CXCR3-A), which has been shown to mediate tumor cell proliferation/migration through increased binding of its ligands in an autocrine manner [20].
Figure-2. CNI treatment is associated with increased expression of CXCR3, CXCL10, and CD31 in nephrectomized kidney tissues of transplant patients.
Representative photomicrographs show the expression of CXCR3, CXCL10, and CD31 in nephrectomized pathological kidney tissues (of transplant patients) and normal kidney tissues detected by immunohistochemistry. Dark brown color, expression of CXCR3, CXCL10, and CD31. Representative of two different tissues. Scale bar, 100 μm.
3.2. Inhibition of mTOR Prolongs Allograft Survival, and Down-Regulates CNI-Induced Rapid Progression of Post-Transplantation Renal Cancer
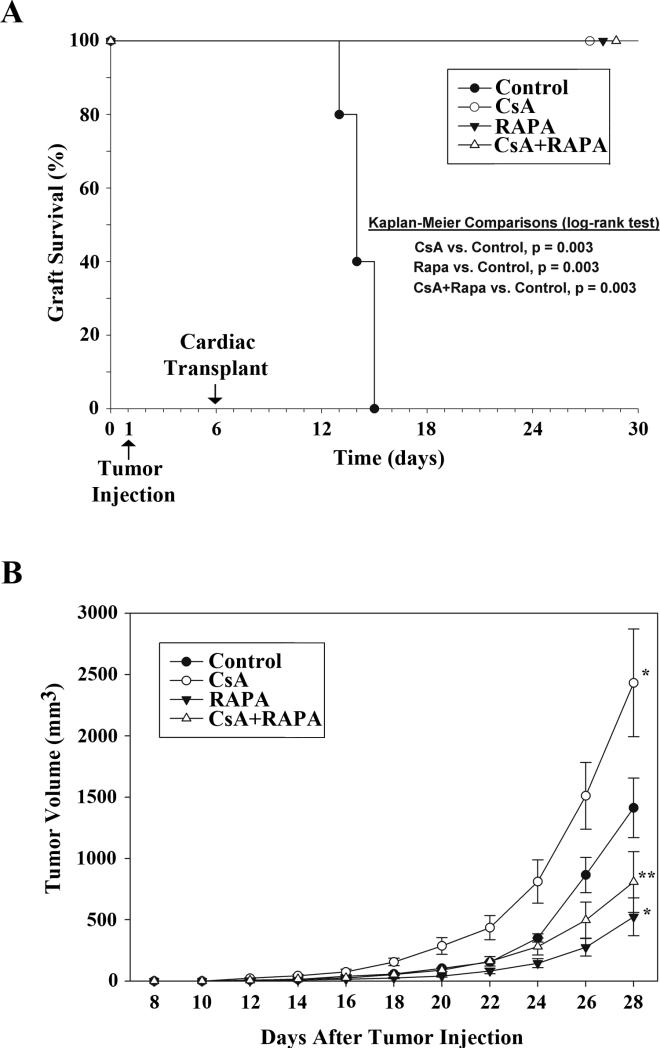
Our earlier experiments (as described above) suggest that CNI treatment in transplant patients can promote post-transplantation renal pathogenesis, including renal cancer. We have recently reported a novel mechanism by which the CNI treatment using CsA can induce the activation of mTOR through the regulation of PRAS40 [27]. Here, we examined the effect of a combination therapy using CNI and the mTOR inhibitor RAPA on the length of allograft survival as well as post-transplantation renal tumor growth in a murine model. We made use of a syngenic renal cancer cell line (RENCA) that can form angiogenic tumors. Tumor cells were injected (s.c.) into BALB/c mice (day-1), and fully MHC mismatched cardiac transplants (C57BL/6) were performed in these mice 5 days later (day-6) as described before [11, 24]. This model may mimic a clinical situation in which few preexisting tumor cells are present in patients undergoing transplantation. After cardiac transplantation, the mice (n = 5 in each group) were treated with different combinations of CsA (10 mg/kg/day) and RAPA (1.5 mg/kg/day). The mice in the control group were treated with vehicle alone. Treatments were continued for 22 days (i.e., up to 28 days after tumor injection). As shown in Figure-3A, vehicle-treated mice rejected allografts within 7-9 days of transplantation (i.e., days 13-15 post tumor injection), whereas there was significant prolongation of allograft survival (100% graft survival) in CsA and RAPA-treated groups up to 22 days following transplantation (i.e., day 28 post tumor injection).
Figure-3. A combination therapy using CsA+RAPA prolongs allograft survival, and inhibits CsA-induced post-transplantation renal cancer progression.
0.5 × 106 syngenic tumor cells (RENCA) were injected s.c. in BALB/c mice (day-1), and fully MHC mismatched cardiac transplants were performed in these animals 5 days later (day-6). After cardiac transplantation, the mice (n = 5 in each group) were treated with different combinations of CsA (10 mg/kg/day) and RAPA (1.5 mg/kg/day); the mice in the control group were treated with vehicle alone. Treatments were continued for 22 days (i.e., up to 28 days after tumor injection). A, Kaplan-Meier curves depicting cardiac allograft survival after tumor injection for the four treatment groups (n = 5 in each group). Grafts in the control group were rejected in 7-9 days (i.e., days 13-15 post tumor injection), whereas all the grafts in other treatment groups survived (100%), each showing significantly better graft survival than control (p = 0.003). B, Tumor volumes were monitored on alternate days for the four treatment groups (n = 5 in each group) using digital caliper. Tumors were harvested after 28 days of tumor injection. *, denotes significant differences compared to control, with a faster rate of tumor growth for CsA alone and a slower rate of tumor growth for RAPA alone (both p < 0.001, ANOVA). Tumor growth was significantly reduced with CsA+RAPA treatment compared to CsA alone (**, p < 0.001). Error bars are standard error of the mean (SEM).
Tumor volumes in different groups were monitored on alternate days. As shown in Figure-3B, ANOVA indicated slope differences in tumor volume over time with significantly more rapid tumor growth in CsA-treated group (F = 4.77, p < 0.001) and less rapid for RAPA-treated group (F = 11.83, p < 0.001) compared with vehicle-treated control. Interestingly, in the group treated with a combination of CsA + RAPA, the change in tumor volume was significantly reduced as depicted by a less steep slope in volume change compared with the group treated with CsA alone (F = 12.00, p < 0.001). Longitudinal analysis using a repeated-measures mixed model depicts the effect of treatment differences on tumor growth over the time course after cardiac transplantation (Figure 3B). Together, these observations suggest that a combination therapy using a CNI (CsA) and a mTOR inhibitor (RAPA) may play an important role in prolonging allograft survival, and also preventing the rapid progression of post-transplantation cancer.
3.3. Combination Treatment with CsA and RAPA Down-Regulates CNI-Induced mTOR Activation
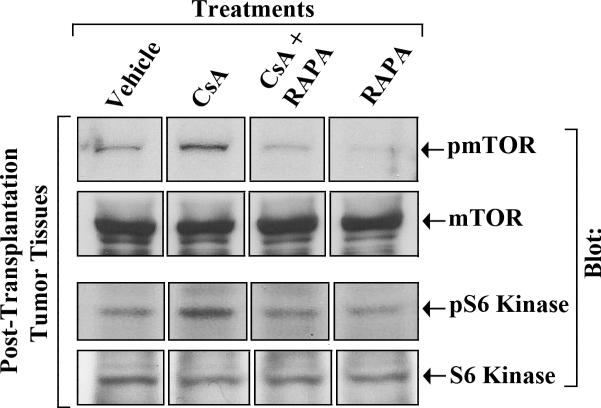
Our earlier experiment using a murine model of post-transplantation renal cancer (Figure-3) suggested a critical role of the mTOR inhibitor RAPA in preventing CNI-induced tumor progression. Here, tumors were harvested on day 28 following tumor injection, and we checked the status of the effector molecules in post-transplantation tumor tissues following immunosuppressive therapy. As shown in Figure-4, CsA treatment increased the phosphorylation of mTOR (top panel) as well as its down-stream target S6 kinase (third panel) in renal tumor tissues; and the combination therapy using CsA + RAPA markedly down-regulated CsA-induced phosphorylation of both mTOR and S6 kinase. There was no significant change in the expression of total mTOR (second panel) and total S6 kinase (bottom panel) following immunosuppressive therapy. These results suggest that the treatment with RAPA plays an important role in preventing CNI-induced mTOR activation and renal cancer progression.
Figure-4. A combination therapy using CsA+RAPA inhibits CsA-induced phosphorylation of mTOR and S6 kinase in post-transplantation renal cancer tissues.
Post-transplantation renal cancer tissues from four different groups of mice (vehicle-, CsA-, RAPA-, and CsA+RAPA-treated) were harvested at day 28 following tumor injection as described in Figure-2. Amounts of phospho-mTOR, total mTOR, phosho-S6 kinase, and total S6 kinase in the harvested tissue lysates were measured by Western blot analysis using anti-phosho-mTOR (anti-pmTOR), anti-mTOR, anti-phospho-S6 Kinase (anti-pS6 Kinase), and anti-S6 Kinase respectively. The lanes were grouped from different parts of the same gel and same blot. Representative of three different tissue samples from all the groups.
3.4. Inhibition of mTOR Prevents CNI-Induced Expression of VEGF, CXCR3, CXCL10, and CXCL11 in Post-Transplantation Renal Tumors
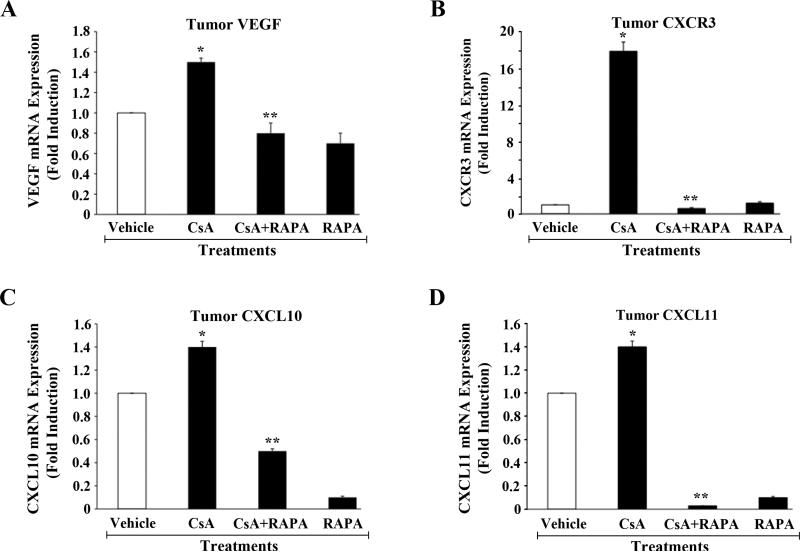
As discussed earlier, the overexpression of VEGF, CXCR3, CXCL10 and CXCL11 in renal tumor cells may play critical roles in CNI-induced renal cancer progression. Here, we examined the expression status of these genes in harvested post-transplantation renal tumor tissues following immunosuppressive therapy. As shown in Figure-5 (A-D), the mRNA expression of VEGF, CXCR3, CXCL10, and CXCL11 was increased following CsA treatment; there was no significant change (data not shown) in the expression of CXCL9. However, a combination therapy using CsA + RAPA significantly down-regulated CNI-induced overexpression of these genes. Thus, the inhibition of mTOR through RAPA treatment may prevent the rapid progression of CNI-induced post-transplantation renal cancer by down-regulating the expression of VEGF, CXCR3, CXCL10, and CXCXL11 in tumor tissues.
Figure-5. The combination therapy using CsA+RAPA inhibits CsA-induced overexpression of VEGF, CXCR3, CXCL10, and CXCL11 in post-transplantation renal cancer tissues.
Post-transplantation renal cancer tissues from four different groups of mice (vehicle-, CsA-, RAPA-, and CsA+RAPA-treated) were harvested at day 28 following tumor injection as described in Figure-2. Total RNA was isolated from harvested tumor tissues and reverse transcribed. Fold changes in the mRNA expression of VEGF (A), CXCR3 (B), CXCL10 (C), and CXCL11 (D) were measured by real-time PCR using gene-specific primers. Columns are average of duplicate readings of two different samples; error bars are +/- SD. *, p < 0.05 compared with vehicle-treated controls; **, p < 0.05 compared with CsA-treated group.
3.5. RAPA Treatment Inhibits CNI-Induced Vessel Density in Post-Transplantation Renal Tumors
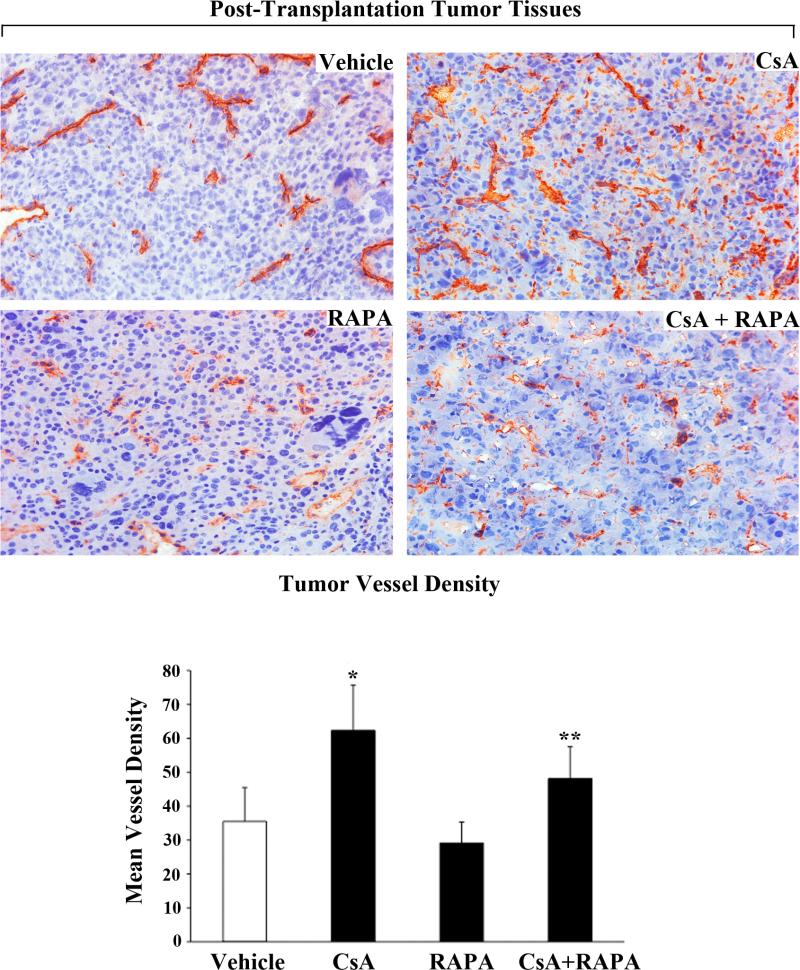
It is known that renal tumors are highly angiogenic [29], and we have previously demonstrated that CNI treatment can promote a rapid progression of renal cancer through the induction of angiogenesis [11]. Here, we sought to determine if the inhibition of mTOR could down-regulate CNI-induced blood vessel density in post-transplantation renal tumor tissues as observed by CD31 staining. We found that CsA treatment markedly increased tumor vessel density compared with vehicle-treated controls (Figure-6, top right panel). In contrast, a combination therapy using CsA + RAPA significantly down-regulated CNI-induced tumor vessel density (Figure-6, bottom right panel). Together, these observations suggest that the RAPA treatment should have important beneficial effects in prolonging allograft survival, and also restricting CNI-induced post-transplantation renal cancer progression partly by modulating tumor angiogenesis.
Figure-6. The combination therapy using CsA+RAPA inhibits CsA-induced tumor vessel density in post-transplantation renal cancer tissues.
Post-transplantation renal cancer tissues from four different groups of mice (vehicle-, CsA-, RAPA-, and CsA+RAPA-treated) were harvested at day 28 following tumor injection as described in Figure-2. The figure illustrates representative photomicrographs showing the immunohistochemical expression of CD31 in harvested tumor tissues (day 28; magnification, x400). Representative of three different tissues from all the groups. Bottom panel, mean vessel density calculated by standard grid counting of CD31-positive vessels at x400 magnification. The data reflect three different tissues from each group, in which three to four non-overlapping fields of each specimen were analyzed in a blinded manner. Columns, average value of vessel counts; bars, SD. *, p < 0.05 compared with vehicle-treated controls; **, p < 0.05 compared with CsA-treated group.
4. DISCUSSION
The development of cancer is an increasing and major problem in organ transplant recipients; and it is a challenge for the clinicians to fix a safe but effective immunosuppressive therapy to prevent allograft rejection as well as cancer progression in transplant patients. In this study, we show that a combination therapy using the CNI (CsA) and the mTOR inhibitor RAPA can prevent the rapid progression of post-transplantation renal cancer through down-regulation of the angiogenic cytokine VEGF, and the chemokine receptor CXCR3 and its ligands.
As discussed earlier, although CNIs are very good immunosuppressive agents to prevent allograft rejection, they play a significant role in cancer recurrence/progression in transplant patients [7, 8, 12]. In addition to compromising immune surveillance mechanism(s) for tumor cells, CNIs can directly activate some pro-tumorigenic pathways [8, 9]. We have recently demonstrated that the treatment with CNIs can activate the proto-oncogenic ras, and specific isoforms (ζ and δ) of PKC in human renal cancer cells [11, 15, 21]. It is established that renal tumors are highly vascular, and depend on the process of angiogenesis for their growth and progression [29]; and different cytokines and chemokines may play major roles in regulating these events. Our earlier studies have shown that CNI-induced signaling events can promote overexpression of the angiogenic cytokine VEGF for the growth of renal tumors [11, 15]; CNIs can also modulate the expression of the chemokine receptor CXCR3 splice variants to facilitate renal cancer progression [20].
CNI-induced pro-tumorigenic signals may have some complex cross-talks with other signaling pathways known to promote tumor growth. It has been shown that a novel molecule called carabin may act as an endogenous inhibitor for both calcineurin and Ras [30]; and we found that CNI may down-regulate the expression of carabin [21]. We suggest that CNI-mediated down-regulation of carabin may act as one of the possible mechanisms for Ras activation in renal cells. In our most recent study, we have demonstrated a novel pathway in which CNI-induced and Ras-PKC-mediated signals can involve mTOR complex1 (mTORC1) through the regulation of its inhibitory molecule PRAS40, and promote VEGF overexpression [27]. In support of our observations, Carriere et al. [31] have recently reported that mitogenic and oncogenic activation of the Ras pathway can induce mTORC1; and it has been shown the Akt-mTOR pathway is important for CNI-induced tumor growth [32]. Also, both PKC-ζ and PKC-δ can promote induction of the Akt-mTOR pathway [33, 34]; and the PI-3K/Akt/mTOR-mediated signals can be channeled through HIF and Sp1 [35, 36], two major transcription factors for VEGF expression [37]. All these observations strongly support the presence of novel cross-talks among CNI-induced signaling pathways and mTOR; thus, the mTOR inhibitors may have beneficial effect(s) in restricting CNI-induced cancers.
In this study, we show that VEGF, CXCR3 and CXCR3-binding ligands (CXCL10 and CXCL11) are overexpressed in post-transplantation renal cancer tissues following CNI (CsA) treatment. We suggest that these genes are expressed mostly by tumor cells, as they constitute bulk of the tumor mass; and it has been demonstrated that VEGF, CXCR3 and CXCR3-binding ligands are expressed in renal cancer epithelial cells [11, 19, 20]. Overexpressed VEGF in tumor cells can interact in a paracrine manner with the VEGF receptors expressed on endothelial cells to promote tumor angiogenesis. On the other hand, overexpressed CXCL10 and CXCL11 can interact in an autocrine manner with CXCR3 expressed on tumor cells to promote cell proliferation as shown by us and others [38, 39]. However, we cannot rule out the expression of CXCR3 and its ligands on other cell types, including tumor infiltrating immune cells. In addition, VEGF may also have pro-inflammatory function [40]; and the over-expressed VEGF following CNI treatment can also promote the infiltration of CXCR3-expressing immune cells within tumor tissues. Interestingly, in our animal model (with cardiac transplantation), a combination therapy using CNI and RAPA significantly inhibited the rapid progression of post-transplantation renal cancer; and the expression of VEGF, CXCR3, CXCL10, and CXCL11 in the tumor tissues were down-regulated following therapeutic treatment. It is expected that CNI-induced activation of the Ras-PKC-mTOR pathway lead to the overexpression of these cytokines and chemokines/chemokine receptor in tumor cells (RENCA); we and others have shown that Ras/PKC/mTOR are indeed involved in the regulation of these genes [11, 15, 37, 39, 41]. Our present observations clearly confirm the critical and positive role of mTOR in CNI-induced pro-tumorigenic pathways.
It is known that the tumor suppressor gene von Hippel-Lindau (VHL) is often lost/mutated in renal cell carcinoma, and plays an important role in pathogenesis of the disease [42]. The RENCA cells used in our study have a wild-type VHL [43]; and thus, CNI-induced renal cancer progression may not be directly associated with loss of VHL. However, it will be interesting to compare the growth-promoting effects of CNI in VHL-deficient versus VHL-wild type renal tumor cells in terms of post-transplantation cancer. Also, the effect of a combination therapy (CNI + RAPA) in preventing post-transplantation cancer progression can be compared in these cells with different VHL status.
In summary, the mTOR pathway may act as an important therapeutic target for the prevention of CNI-induced post-transplantation cancer. We suggest that a combination therapy using CNI in presence of an mTOR inhibitor (like RAPA) can prolong allograft survival, and can also prevent cancer progression in transplant patients through the down-regulation of some major tumor-promoting pathways. Together, our study should have potential clinical significance in terms of fixing effective immunosuppressive therapy to prevent the progression of post-transplantation cancer.
ACKNOWLEDGMENTS
We wish to thank Dr. Mohamed H. Sayegh for helpful suggestion about this work. This work was supported by National Institutes of Health Grant R01 CA131145 (to S. P.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT:
None.
REFERENCES
- 1.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am. J. Transplant. 2004;4:905–913. doi: 10.1111/j.1600-6143.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 2.Bustami RT, Ojo AO, Wolfe RA, Merion RM, Bennett WM, McDiarmid SV, Leichtman AB, Held PJ, Port FK. Immunosuppression and the risk of post-transplant malignancy among cadaveric first kidney transplant recipients. Am. J. Transplant. 2004;4:87–93. doi: 10.1046/j.1600-6135.2003.00274.x. [DOI] [PubMed] [Google Scholar]
- 3.Lutz J, Heemann U. Tumours after kidney transplantation. Curr. Opin. Urol. 2003;13:105–109. doi: 10.1097/00042307-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Wimmer CD, Rentsch M, Crispin A, Illner WD, Arbogast H, Graeb C, Jauch KW, Guba M. The janus face of immunosuppression - de novo malignancy after renal transplantation: the experience of the Transplantation Center Munich. Kidney Int. 2007;71:1271–1278. doi: 10.1038/sj.ki.5002154. [DOI] [PubMed] [Google Scholar]
- 5.Dantal J, Soulillou JP. Immunosuppressive drugs and the risk of cancer after organ transplantation. N. Engl. J. Med. 2005;352:1371–1373. doi: 10.1056/NEJMe058018. [DOI] [PubMed] [Google Scholar]
- 6.Campistol JM. Minimizing the risk of cancer in transplant patients. G. Ital. Nefrol. 2010;27(Suppl 50):S81–85. [PubMed] [Google Scholar]
- 7.Webster AC, Craig JC, Simpson JM, Jones MP, Chapman JR. Identifying high risk groups and quantifying absolute risk of cancer after kidney transplantation: a cohort study of 15,183 recipients. Am. J. Transplant. 2007;7:2140–2151. doi: 10.1111/j.1600-6143.2007.01908.x. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez-Dalmau A, Campistol JM. Immunosuppressive therapy and malignancy in organ transplant recipients: a systematic review. Drugs. 2007;67:1167–1198. doi: 10.2165/00003495-200767080-00006. [DOI] [PubMed] [Google Scholar]
- 9.Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, Shimbo T, Suthanthiran M. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530–534. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]
- 10.Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat. Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 11.Basu A, Contreras AG, Datta D, Flynn E, Zeng L, Cohen HT, Briscoe DM, Pal S. Overexpression of vascular endothelial growth factor and the development of post-transplantation cancer. Cancer Res. 2008;68:5689–5698. doi: 10.1158/0008-5472.CAN-07-6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guba M, Graeb C, Jauch KW, Geissler EK. Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation. 2004;77:1777–1782. doi: 10.1097/01.tp.0000120181.89206.54. [DOI] [PubMed] [Google Scholar]
- 13.Maluccio M, Sharma V, Lagman M, Vyas S, Yang H, Li B, Suthanthiran M. Tacrolimus enhances transforming growth factor-beta1 expression and promotes tumor progression. Transplantation. 2003;76:597–602. doi: 10.1097/01.TP.0000081399.75231.3B. [DOI] [PubMed] [Google Scholar]
- 14.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 15.Basu A, Datta D, Zurakowski D, Pal S. Altered VEGF mRNA stability following treatments with immunosuppressive agents: implications for cancer development. J. Biol. Chem. 2010;285:25196–25202. doi: 10.1074/jbc.M110.119446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zlotnik A. Chemokines in neoplastic progression. Semin. Cancer Biol. 2004;14:181–185. doi: 10.1016/j.semcancer.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Zipin-Roitman A, Meshel T, Sagi-Assif O, Shalmon B, Avivi C, Pfeffer RM, Witz IP, Ben-Baruch A. CXCL10 promotes invasion-related properties in human colorectal carcinoma cells. Cancer Res. 2007;67:3396–3405. doi: 10.1158/0008-5472.CAN-06-3087. [DOI] [PubMed] [Google Scholar]
- 18.Walser TC, Rifat S, Ma X, Kundu N, Ward C, Goloubeva O, Johnson MG, Medina JC, Collins TL, Fulton AM. Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer. Cancer Res. 2006;66:7701–7707. doi: 10.1158/0008-5472.CAN-06-0709. [DOI] [PubMed] [Google Scholar]
- 19.Suyama T, Furuya M, Nishiyama M, Kasuya Y, Kimura S, Ichikawa T, Ueda T, Nikaido T, Ito H, Ishikura H. Up-regulation of the interferon gamma (IFN-gamma)-inducible chemokines IFN-inducible T-cell alpha chemoattractant and monokine induced by IFN-gamma and of their receptor CXC receptor 3 in human renal cell carcinoma. Cancer. 2005;103:258–267. doi: 10.1002/cncr.20747. [DOI] [PubMed] [Google Scholar]
- 20.Datta D, Contreras AG, Grimm M, Waaga-Gasser AM, Briscoe DM, Pal S. Calcineurin inhibitors modulate CXCR3 splice variant expression and mediate renal cancer progression. J. Am. Soc. Nephrol. 2008;19:2437–2446. doi: 10.1681/ASN.2008040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Datta D, Contreras AG, Basu A, Dormond O, Flynn E, Briscoe DM, Pal S. Calcineurin inhibitors activate the proto-oncogene Ras and promote protumorigenic signals in renal cancer cells. Cancer Res. 2009;69:8902–8909. doi: 10.1158/0008-5472.CAN-09-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monaco AP. The role of mTOR inhibitors in the management of posttransplant malignancy. Transplantation. 2009;87:157–163. doi: 10.1097/TP.0b013e318193886e. [DOI] [PubMed] [Google Scholar]
- 23.Geissler EK, Schlitt HJ. The potential benefits of rapamycin on renal function, tolerance, fibrosis, and malignancy following transplantation. Kidney Int. 2010;78:1075–1079. doi: 10.1038/ki.2010.324. [DOI] [PubMed] [Google Scholar]
- 24.Koehl GE, Andrassy J, Guba M, Richter S, Kroemer A, Scherer MN, Steinbauer M, Graeb C, Schlitt HJ, Jauch KW, Geissler EK. Rapamycin protects allografts from rejection while simultaneously attacking tumors in immunosuppressed mice. Transplantation. 2004;77:1319–1326. doi: 10.1097/00007890-200405150-00002. [DOI] [PubMed] [Google Scholar]
- 25.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat. Rev. Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 26.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O'Toole T, Lustgarten S, Moore L, Motzer RJ. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N. Engl. J. Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 27.Basu A, Banerjee P, Contreras AG, Flynn E, Pal S. Calcineurin inhibitor-induced and Ras-mediated overexpression of VEGF in renal cancer cells involves mTOR through the regulation of PRAS40. PLoS One. 2011;6:e23919. doi: 10.1371/journal.pone.0023919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat. Cell. Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 29.Choueiri TK, Bukowski RM, Rini BI. The current role of angiogenesis inhibitors in the treatment of renal cell carcinoma. Semin. Oncol. 2006;33:596–606. doi: 10.1053/j.seminoncol.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Pan F, Sun L, Kardian DB, Whartenby KA, Pardoll DM, Liu JO. Feedback inhibition of calcineurin and Ras by a dual inhibitory protein Carabin. Nature. 2007;445:433–436. doi: 10.1038/nature05476. [DOI] [PubMed] [Google Scholar]
- 31.Carriere A, Romeo Y, Acosta-Jaquez HA, Moreau J, Bonneil E, Thibault P, Fingar DC, Roux PP. ERK1/2 phosphorylate Raptor to promote Ras-dependent activation of mTOR complex 1 (mTORC1) J. Biol. Chem. 2011;286:567–577. doi: 10.1074/jbc.M110.159046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han W, Ming M, He TC, He YY. Immunosuppressive cyclosporin A activates AKT in keratinocytes through PTEN suppression: implications in skin carcinogenesis. J. Biol. Chem. 2010;285:11369–11377. doi: 10.1074/jbc.M109.028142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leseux L, Laurent G, Laurent C, Rigo M, Blanc A, Olive D, Bezombes C. PKC zeta mTOR pathway: a new target for rituximab therapy in follicular lymphoma. Blood. 2008;111:285–291. doi: 10.1182/blood-2007-04-085092. [DOI] [PubMed] [Google Scholar]
- 34.Minhajuddin M, Bijli KM, Fazal F, Sassano A, Nakayama KI, Hay N, Platanias LC, Rahman A. Protein kinase C-delta and phosphatidylinositol 3-kinase/Akt activate mammalian target of rapamycin to modulate NF-kappaB activation and intercellular adhesion molecule-1 (ICAM-1) expression in endothelial cells. J. Biol. Chem. 2009;284:4052–4061. doi: 10.1074/jbc.M805032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mireuta M, Darnel A, Pollak M. IGFBP-2 expression in MCF-7 cells is regulated by the PI3K/AKT/mTOR pathway through Sp1-induced increase in transcription. Growth Factors. 2010;28:243–255. doi: 10.3109/08977191003745472. [DOI] [PubMed] [Google Scholar]
- 36.Harada H, Itasaka S, Kizaka-Kondoh S, Shibuya K, Morinibu A, Shinomiya K, Hiraoka M. The Akt/mTOR pathway assures the synthesis of HIF-1alpha protein in a glucose- and reoxygenation-dependent manner in irradiated tumors. J. Biol. Chem. 2009;284:5332–5342. doi: 10.1074/jbc.M806653200. [DOI] [PubMed] [Google Scholar]
- 37.Pal S, Datta K, Khosravi-Far R, Mukhopadhyay D. Role of protein kinase Czeta in Ras-mediated transcriptional activation of vascular permeability factor/vascular endothelial growth factor expression. J. Biol. Chem. 2001;276:2395–2403. doi: 10.1074/jbc.M007818200. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg-Bittman L, Neumark E, Sagi-Assif O, Azenshtein E, Meshel T, Witz IP, Ben-Baruch A. The expression of the chemokine receptor CXCR3 and its ligand, CXCL10, in human breast adenocarcinoma cell lines. Immunol. Lett. 2004;92:171–178. doi: 10.1016/j.imlet.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 39.Datta D, Flaxenburg JA, Laxmanan S, Geehan C, Grimm M, Waaga-Gasser AM, Briscoe DM, Pal S. Ras-induced modulation of CXCL10 and its receptor splice variant CXCR3-B in MDA-MB-435 and MCF-7 cells: relevance for the development of human breast cancer. Cancer Res. 2006;66:9509–9518. doi: 10.1158/0008-5472.CAN-05-4345. [DOI] [PubMed] [Google Scholar]
- 40.Reinders ME, Sho M, Izawa A, Wang P, Mukhopadhyay D, Koss KE, Geehan CS, Luster AD, Sayegh MH, Briscoe DM. Proinflammatory functions of vascular endothelial growth factor in alloimmunity. J. Clin. Invest. 2003;112:1655–1665. doi: 10.1172/JCI17712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melillo RM, Castellone MD, Guarino V, De Falco V, Cirafici AM, Salvatore G, Caiazzo F, Basolo F, Giannini R, Kruhoffer M, Orntoft T, Fusco A, Santoro M. The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J. Clin. Invest. 2005;115:1068–1081. doi: 10.1172/JCI22758. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Whaley JM, Naglich J, Gelbert L, Hsia YE, Lamiell JM, Green JS, Collins D, Neumann HP, Laidlaw J, Li FP, et al. Germ-line mutations in the von Hippel-Lindau tumor-suppressor gene are similar to somatic von Hippel-Lindau aberrations in sporadic renal cell carcinoma. Am. J. Hum. Genet. 1994;55:1092–1102. [PMC free article] [PubMed] [Google Scholar]
- 43.Chang YS, Adnane J, Trail PA, Levy J, Henderson A, Xue D, Bortolon E, Ichetovkin M, Chen C, McNabola A, Wilkie D, Carter CA, Taylor IC, Lynch M, Wilhelm S. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother. Pharmacol. 2007;59:561–574. doi: 10.1007/s00280-006-0393-4. [DOI] [PubMed] [Google Scholar]