Abstract
Cornelia de Lange syndrome (CdLS) is a genetic disorder associated with delayed growth, intellectual disability, limb reduction defects and characteristic facial features. Germline mosaicism has been a described mechanism for CdLS when there are several affected offspring of apparently unaffected parents. Presently, the recurrence risk for CdLS has been estimated to be as high as 1.5%; however, this figure may be an underrepresentation. We report on the molecularly defined germline mosaicism cases from a large CdLS database, representing the first large case series on germline mosaicism in CdLS. Of the 12 families, eight have been previously described; however, four have not. No one specific gene mutation, either in the NIPBL or the SMC1A gene, was associated with an increased risk for germline mosaicism. Suspected or confirmed cases of germline mosaicism in our database range from a conservative 3.4% up to 5.4% of our total cohort. In conclusion, the potential reproductive recurrence risk due to germline mosiacism should be addressed in prenatal counseling for all families who have had a previously affected pregnancy or child with CdLS.
Keywords: Cornelia de Lange syndrome, Germline Mosaicism, Germ Cell, Mosaicism, Genetic Counseling
INTRODUCTION
Germline mosaicism has been reported either as a probable or confirmed mechanism of inheritance in more than 60 genetic diseases [Helderman-van den Enden et al., 2009]. The conventional view of germline mosaicism involves a change in gametocytes during oogenesis or spermatogenesis in meiosis [Gardner and Sutherland, 2004]. If the abnormality arises in embryogenesis before the differentiation of the germline, termed somatic-germline mosaicism, other somatic tissues may be involved [Gardner and Sutherland, 2004]. To date, only a few conditions have documented estimated recurrence risks for a subsequent pregnancy.
Cornelia de Lange syndrome is a genetically heterogeneous disorder associated with delayed growth, intellectual disability, limb reduction defects and characteristic facial features. Fifty percent of patients have sporadic mutations in the NIPBL gene [Krantz et al., 2004; Gillis et al., 2004; Tonkin et al., 2004]. Approximately four percent of cases are due to mutations in the gene SMC1A located on the X chromosome [Musio et al., 2006]. Prior to gene testing, other reported cases of apparently unaffected parents who had two or more children with CdLS were hypothesized to be the result of germline mosaicism [Beratis et al., 1971; Lieber et al., 1973; Fryns et al., 1987; Naguib et al., 1987; Jackson et al., 1993; Krajewska-Walasek et al., 1995]. These reports estimated germline mosaicism recurrence rates to be around 1.5%.
Although germline mosaicism is an accepted mechanism for transmission in CdLS, there is limited literature on the molecular status of these families. The purpose of this family series is to describe the cases of suspected germline mosaicism that have been molecularly defined from our large CdLS database. This represents the first large case series report on germline mosaicism in CdLS. Some cases have been previously described; however many cases have not.
MATERIALS AND METHODS
Molecular studies
Genomic DNA was isolated from peripheral blood lymphocytes (Gentra Systems) in all families. The entire NIPBL coding region (exons 2–47) was screened for mutations via sequencing. Primer sequences, annealing temperatures, and sizes of PCR products are identical to those previously described [Gillis et al., 2004]. Primer pairs were designed to amplify exons, exon/intron boundaries, and short flanking intronic sequences. Larger exons were subdivided to allow for optimal product lengths. SMC1A analysis was performed as previously described [Deardorff et al., 2007].
RESULTS
Clinical Reports
Family I
The proband was born to unaffected parents, had classic features of CdLS and died at 17 months of age. A second child was unaffected. A third pregnancy was identified to have micrognathia and limb reduction abnormalities by fetal ultrasound. This pregnancy was terminated and facial features were consistent with CdLS upon post-mortem evaluation. A fourth pregnancy was closely followed due to suspected mosaicism in one of the parents. No signs of CdLS were identified by ultrasound; however CdLS was clinically diagnosed at birth. Subsequently, single nucleotide polymorphism (SNP) array identified a partial gene deletion of exons 1–8 in NIPBL. This was confirmed in the proband, terminated fetus, and the offspring from the fourth pregnancy. Parental peripheral blood studies were conducted and neither parent was found to have the deletion.
Family II
The proband was the second child born to unaffected parents. He had classic facial features of CdLS, as well as bilateral upper limb reduction abnormalities. He was found to carry a p.R1723X mutation in exon 26 of NIPBL. The couple’s third pregnancy was detected by fetal ultrasound to have the same upper limb abnormalities, and this pregnancy was terminated. The same NIPBL mutation was found in the products of conception from the third pregnancy. The first born son was unaffected, and the mother and father were not found to be carriers of this mutation in their blood.
Family III
Two children both with a severe CdLS phenotype including classic facial features and upper limb reduction defects were born to unaffected parents. A frame shift mutation, c.7151del5 in exon 42 of NIPBL was found in both siblings. There were no other affected children or pregnancies in the family. Both parents tested negative for the mutation in their blood.
Family IV
The proband was diagnosed with CdLS based on characteristic facial features, small hands and feet and developmental delay. A younger sister was born with similar features and diagnosed with a mild form of CdLS. Both girls were found to carry a p.R496H in exon 9 of SMC1A. The parents did not have this mutation in their blood.
Family V
The female proband had classic facial and physical features of CdLS. A second female child was born unaffected. A third child (male) was born with similar characteristics to his affected sister including small hands and feet. A c.7780delC in exon 45 of NIPBL mutation was identified in both affected siblings, but not in the unaffected sister or parents.
Family VI
The proband is a mildly affected female with characteristic facial features of CdLS and small hands. She is the only child born to her parents. With another partner, her father had four children. The first female child born in this partnership had facial features, overall small size, and developmental delay consistent with CdLS. A c.358+4G>C mutation in the intron downstream of exon 4 of NIPBL was identified in both half sisters and not found in blood lymphocytes from their unaffected father. The father’s three other children tested negative for the mutation as well.
Family VII
Two affected brothers were diagnosed with CdLS based on characteristic facial features and small hands. They were born to unaffected parents who had five unaffected children. A sample, available on one of the affected children, showed a p.R1856T mutation in exon 29 of NIPBL. The other affected brother died before a sample could be obtained. The mutation was not found in the blood of either parent.
Family VIII
The proband is a boy born to unaffected parents. The diagnosis of CdLS was made based on his characteristic facial features, growth parameters below the 5th centile including head circumference, and small hands and feet. His medical history included gastroesophageal reflux requiring fundoplication, hearing loss, and developmental delay. A sister was born after the proband was diagnosed, and CdLS was suspected at birth based on her physical features. Subsequently the parents had another unaffected girl. Two half siblings through the mother (by different fathers) were unaffected. Blood was obtained on all three full siblings and the parents. The two clinically affected children were found to carry a splice site mutation c.6763+5G>T in the intron downstream of exon 39 of NIPBL. The mutation was not found in the blood of the other unaffected sister, mother or father.
Family IX
Three siblings with moderate growth and cognitive delay, small hands without reduction defects, and typical facial features of CdLS were all shown to carry the same p.M1K, mutation in exon 2 of NIPBL. Each sibling had a different father. The mutation was not present in the blood of their mother, or in the two fathers from whom samples were available.
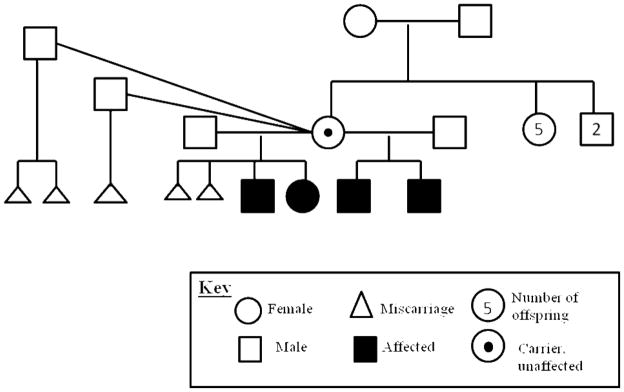
Family X
Four affected children with CdLS were born to an unaffected mother. Two children shared the same father; the remaining two children had different fathers (Fig 1). An identical splice site mutation, c.64+1 G>A in the intron downstream of exon 2 of NIPBL, was seen in all four affected children. The mutation was not present in their mother’s blood of.
Figure 1.
Pedigree of Family X. The mother (carrier symbol) has no physical features of Cornelia de Lange syndrome on exam. Her blood was tested and was not found to have the same 64+1 G>A in exon 2 of NIPBL mutation seen in her children. All miscarriages were spontaneous. The mother has no unaffected children. The miscarried products of conception were not molecularly evaluated for NIPBL mutations.
Family XI
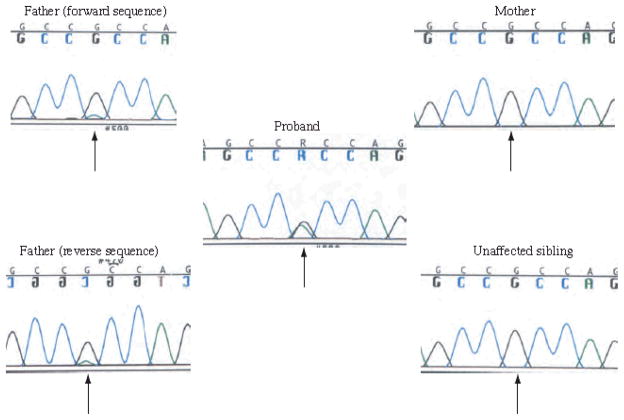
The proband is an affected girl with CdLS born to unaffected parents. She presented with facial features consistent with CdLS and small hands and feet. She had significant intellectual disability with no speech at age 23 years. Subsequent to this pregnancy, an unaffected boy was born. The last child was a girl who presented with similar physical features to the proband and was also diagnosed clinically with CdLS. She was reevaluated at 15 years of age and had significant intellectual disability with absent speech. Both children were found to carry a p.R496H mutation in exon 9 of SMC1A. The mother did not carry the mutation. However, chromatogram performed on the father’s DNA displayed a small peak underneath the normal base, seen in forward and reverse sequences (Fig 2), at the same position mutated in his children. While not quantitative, this is indicative of low-level tissue mosaicism. Photos of the father were reviewed and he had no physical features consistent with CdLS, and normal cognition.
Figure 2.
Family XI chromatograms. The proband’s chromatogram is in the center demonstrating heterozygosity for the p.R496H mutation. The unaffected mother and sibling are depicted on the right with normal sequences (homozygous for the “G”). The unaffected father’s forward and reverse sequence are depicted on the left, in both cases demonstrating a small sub “A” peak beneath the normal “G” peak at this base position, indicating a low level mosaicism for the p.R496H mutation. Arrows indicate the base in which the p.R496H mutation occurs.
Family XII
The proband was a boy born to unaffected parents who was diagnosed shortly after birth with CdLS due to classic facial features, microcephaly, proportionate growth retardation, and small hands and feet. He died at 89 days of age due to complications from bronchopulmonary dysplasia and a patent ductus arteriosis. The couple subsequently had a second pregnancy that was delivered at 37 weeks by cesarean after a prenatal ultrasound showed a slightly small left ventricle and a small aortic valve with increased peak systolic flow. Following delivery of this female child, CdLS was suspected due to facial features, growth retardation, and small hands and feet. A blood sample from this girl was obtained and a p.R308X mutation in exon 9 of NIPBL was identified. A banked DNA sample from the first born boy was analyzed and the same mutation was found. Both parents were screened for this mutation in their blood and were negative. The next pregnancy for this couple was initiated by in-vitro fertilization (IVF) with preimplantation genetic diagnosis (PGD). The IVF procedure resulted in 10 viable embryos; each was tested for the known mutation in the family. Five of the 10 embryos (50%) were positive for the p.R308X mutation. Two of the remaining five were inconclusive. Implantation of the unaffected embryos resulted in a viable singleton pregnancy, confirmed by amniocentesis to be negative for the familial NIPBL mutation.
DISCUSSION
Increased understanding of the inheritance and ability to molecularly define the gene defects in CdLS have unmasked multiple families with suspected germline mosaicism. We reported our experience of families with recurrence from our molecular database. A literature review of germline mosaicism in CdLS would be far from complete without molecular information, as in the past many older cases of potential germline mosaicism were only clinically described, with some likely representing the X-linked form of the disorder. Also, as the clinical phenotype of CdLS is variable and sometimes subtle, parental germline mosaicism may have been mistaken for autosomal dominant, X-linked inheritance, or low-level tissue mosaicism before the advent of molecular techniques, as shown by the father in Family XI who had low levels of mosaicism on his chromatogram (Fig 2). However, as tissues other than blood were not obtained, and CdLS features may be mild, low level somatic mosaicism cannot be completely ruled out in the other cases [Jackson et al., 1993].
Of the 12 families, four (Families I, VI, X, and XII) have not been previously reported (Table I). Two Families, IV and XI, were shown to have X-linked mutations in SMC1A with variable phenotypes. Also, all cases reported herein are due to exon deletions or sequence changes. Our data of cases with germline mosaicism, though limited, shows that no one specific gene mutation, either in the NIPBL or SMC1A, is associated with an increased risk for germline mosaicism. This observation is important when counseling for recurrence risk.
Table I. Molecularly defined families with suspected Cornelia de Lange syndrome germline mosaicism.
A synopsis of Cornelia de Lange syndrome families in our database with molecular support for germline mosiacism. If the families have been previously reported in the literature the citation is given. All cases, except two, include exonal mutations or deletions in NIPBL. Family XI was the only family that had proven low level tissue mosaicism in a phenotypically normal parent.
| Family | Mutation | Previously reported |
|---|---|---|
| I | exon 1–8 NIPBL deletion | No |
| II | p.R1723X in exon 26 of NIPBL | Gillis et al., 2004 |
| III | c.7151del5 in exon 42 of NIPBL | Gillis et al., 2004 |
| IV | p.R496H in exon 9 of SMC1A | Deardorff et al., 2007 |
| V | c.7780delC in exon 45 of NIPBL | Gillis et al., 2004 |
| VI | c.358+4G>C in the intron downstream of exon 4 of NIPBL | No |
| VII | p.R1856T in exon 29 of NIPBL | Gillis et al., 2004 |
| VIII | c.6763+5G>T in the intron downstream of exon 39 of NIPBL | Krantz et al., 2004 |
| IX | p.M1K in exon 2 of NIPBL | Krantz et al., 2004 |
| X | c.64+1 G>A in the intron downstream of exon 2 of NIPBL | No |
| XI | p.R496H in exon 9 of SMC1A | Deardorff et al., 2007 |
| XII | p.R308X in exon 9 of NIPBL | No |
Presently, the recurrence risk for CdLS has been estimated to be as high as 1.5%; however, this may be an underrepresentation. Although we are presently reporting 12 families in our cohort of 351 probands with CdLS, 19 database families had more than one affected individual with unaffected parents. Of these 19, 12 are presented here with confirmed proband mutations and unaffected parents. The additional seven families were presumed to be due to germline mosaicism as we have either met the parents in person or reviewed photos and feel comfortable they are not affected. However, no mutation has been found in the probands, even though they have all been screened for all genes known to cause CdLS and therefore there is a chance the proband has been misdiagnosed. Therefore, suspected or confirmed germline mosaicism (or low level somatic germline mosaicism with normal phenotype) in our database range from a conservative 3.4% (12 families presented herein of 351) up to 5.4% of our total cohort (19 families of 351). This represents a highly biased sample since familial cases were sought after to give power to the initial linkage studies to identify the causative gene. Thus, this data may be skewed as the true number of familial cases in the general population is unknown.
Germline mosaicism has been reported in cytogenetic and single-gene disorders. Up to 5% of trisomy 21 may be attributed to germline mosaicism [Delhanty, 2011]. Few estimates of the relative frequency of germline mosiacism in single-gene disorders are available. In Tuberous sclerosis, up to 2% of cases are reportedly due to germline mosaicism [Roach and Sparagana, 2004]. Other conditions have documented estimated recurrence risks for a subsequent pregnancy. Some examples include osteogenesis imperfecta (5 to 7%), Sotos syndrome (<1%) and achondroplasia (0.02%) [Byers et al., 1988; Baujat and Cormier-Daire, 2007; Mettler and Fraser, 2000]. Our estimate of around 4% seems to fall in the higher end of this range. In general, germline mosaicism should be considered whenever a dominant disorder recurs despite apparently unaffected parents. There is no clear cytogenetic or molecular explanation for the different frequencies of germline mosaicism in different disorders, but DNA structure may make certain areas of the genome more susceptible to de novo mutations [Strachan and Read, 1999].
In summary, our data supports that germline mosiacism recurrence risk should be addressed in prenatal counseling for all families who have had an affected pregnancy or child with CdLS. Additionally, parental testing for low level somatic moasicism for the deleterious mutation is recommended to better quantify recurrence risk, even if the parents appear unaffected.
Acknowledgments
We would like to acknowledge the patients and their families for their kind cooperation as well as all clinical and laboratory personnel involved in their care. This work was supported by the NICHD PO1 HD052860.
Footnotes
The authors declare that they have no disclosures or competing financial interests.
References
- Baujat G, Cormier-Daire V. Sotos Syndrome. Orphanet J Rare Dis. 2007;7:36. doi: 10.1186/1750-1172-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beratis NG, Hsu LY, Hirschhorn K. Familial de lange syndrome. report of three cases in a sibship. Clin Genet. 1971;2:170–176. [PubMed] [Google Scholar]
- Byers PH, Tsipouras P, Bonadio JF, Starman BJ, Schwartz RC. Perinatal lethal osteogenesis imperfecta (OI type II): A biochemically heterogeneous disorder usually due to new mutations in the genes for type I collagen. Am J Hum Genet. 1988;42:237–248. [PMC free article] [PubMed] [Google Scholar]
- Deardorff MA, Kaur M, Yaeger D, Rampuria A, Korolev S, Pie J, Gil-Rodriguez C, Arnedo M, Loeys B, Kline AD, Wilson M, Lillquist K, Siu V, Ramos FJ, Musio A, Jackson LS, Dorsett D, Krantz ID. Mutations in cohesin complex members SMC3 and SMC1A cause a mild variant of cornelia de lange syndrome with predominant mental retardation. Am J Hum Genet. 2007;80:485–494. doi: 10.1086/511888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhanty JD. Inherited aneuploidy: germline mosaicism. Cytogenet Genome Res. 2011;133:136–140. doi: 10.1159/000323606. [DOI] [PubMed] [Google Scholar]
- Fryns JP, Dereymaeker AM, Hoefnagels M, D'Hondt F, Mertens G, van den Berghe H. The brachmann-de lange syndrome in two siblings of normal parents. Clin Genet. 1987;31:413–415. doi: 10.1111/j.1399-0004.1987.tb02835.x. [DOI] [PubMed] [Google Scholar]
- Garder and Sutherland. Chromosome abnormalities and genetic counseling. New York: Oxford University Press; 2004. p. 44. [Google Scholar]
- Gillis LA, McCallum J, Kaur M, DeScipio C, Yaeger D, Mariani A, Kline AD, Li HH, Devoto M, Jackson LG, Krantz ID. NIPBL mutational analysis in 120 individuals with cornelia de lange syndrome and evaluation of genotype-phenotype correlations. Am J Hum Genet. 2004;75:610–623. doi: 10.1086/424698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helderman-van den Enden AT, de Jong R, den Dunnen JT, Houwing-Duistermaat JJ, Kneppers AL, Ginjaar HB, Breuning MH, Bakker E. Recurrence risk due to germ line mosaicism: Duchenne and becker muscular dystrophy. Clin Genet. 2009;75:465–472. doi: 10.1111/j.1399-0004.2009.01173.x. [DOI] [PubMed] [Google Scholar]
- Jackson L, Kline AD, Barr MA, Koch S. De lange syndrome: A clinical review of 310 individuals. Am J Med Genet. 1993;47:940–946. doi: 10.1002/ajmg.1320470703. [DOI] [PubMed] [Google Scholar]
- Krajewska-Walasek M, Chrzanowska K, Tylki-Szymanska A, Bialecka M. A further report of brachmann-de lange syndrome in two sibs with normal parents. Clin Genet. 1995;47:324–327. doi: 10.1111/j.1399-0004.1995.tb03974.x. [DOI] [PubMed] [Google Scholar]
- Krantz ID, McCallum J, DeScipio C, Kaur M, Gillis LA, Yaeger D, Jukofsky L, Wasserman N, Bottani A, Morris CA, Nowaczyk MJ, Toriello H, Bamshad MJ, Carey JC, Rappaport E, Kawauchi S, Lander AD, Calof AL, Li HH, Devoto M, Jackson LG. Cornelia de lange syndrome is caused by mutations in NIPBL, the human homolog of drosophila melanogaster nipped-B. Nat Genet. 2004;36:631–635. doi: 10.1038/ng1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber E, Glaser JH, Jhaveri R. Brachmann-de lange syndrome. report of two cases in a sibship. Am J Dis Child. 1973;125:717–718. doi: 10.1001/archpedi.1973.04160050065013. [DOI] [PubMed] [Google Scholar]
- Mettler G, Fraser FC. Recurrence risk for sibs of children with “sporadic” achondroplasia. Am J Med Genet. 2000;90:250–251. [PubMed] [Google Scholar]
- Musio A, Selicorni A, Focarelli ML, Gervasini C, Milani D, Russo S, Vezzoni P, Larizza L. X-linked cornelia de lange syndrome owing to SMC1L1 mutations. Nat Genet. 2006;38:528–530. doi: 10.1038/ng1779. [DOI] [PubMed] [Google Scholar]
- Naguib KK, Teebi AS, Al-Awadi SA, Marafie MJ. Brachmann-de lange syndrome in sibs. J Med Genet. 1987;24:627–629. doi: 10.1136/jmg.24.10.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach ES, Sparagana SP. Diagnosis of tuberous sclerosis complex. J Child Neurol. 2007;19:643–649. doi: 10.1177/08830738040190090301. [DOI] [PubMed] [Google Scholar]
- Strachan T, Read AP. Human Molecular Genetics. 2. New York: Wiley-Liss; 1999. [Last accessed 2/3/2012]. pp. 9.1–9.5.5. Available at http://www.ncbi.nlm.nih.gov/books/NBK7566/ [Google Scholar]
- Tonkin ET, Wang TJ, Lisgo S, Bamshad MJ, Strachan T. NIPBL, encoding a homolog of fungal Scc2-type sister chromatid cohesion proteins and fly nipped-B, is mutated in cornelia de lange syndrome. Nat Genet. 2004;36:636–641. doi: 10.1038/ng1363. [DOI] [PubMed] [Google Scholar]