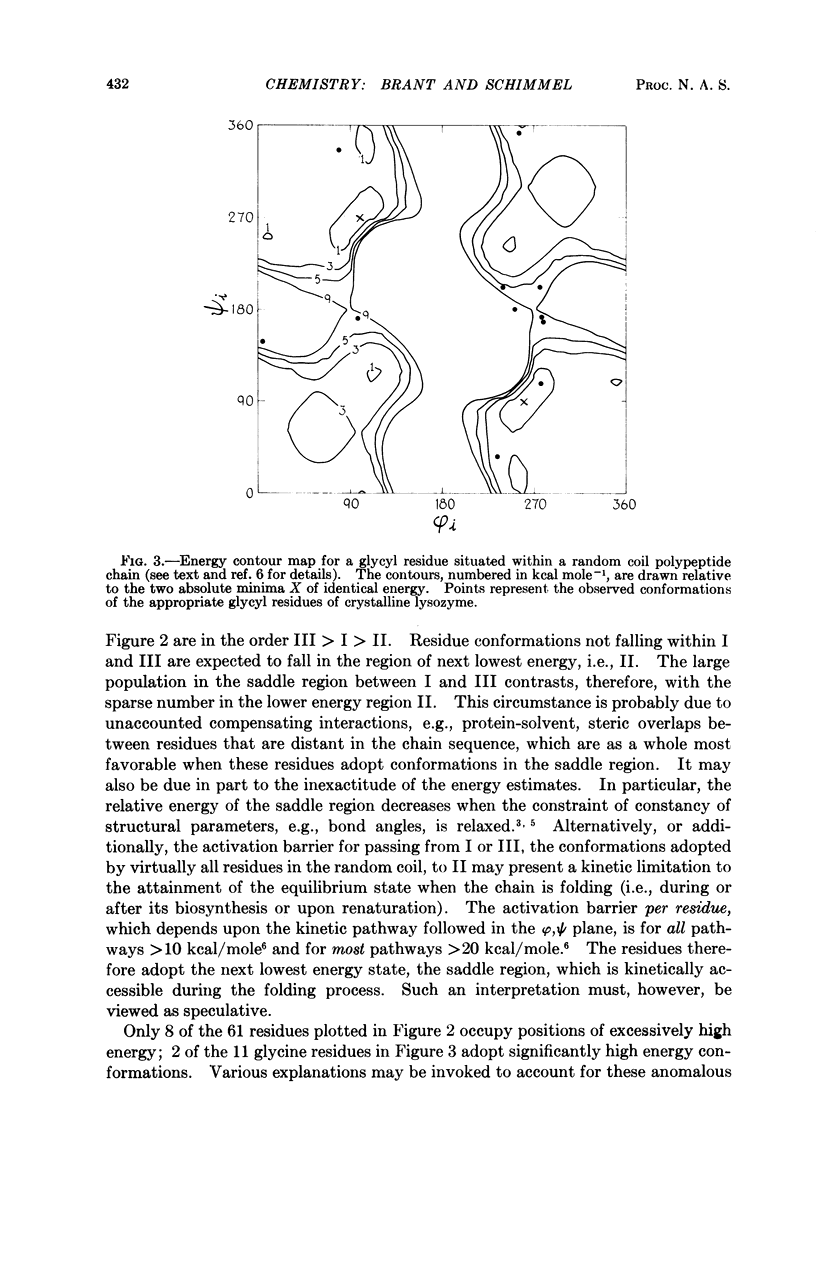
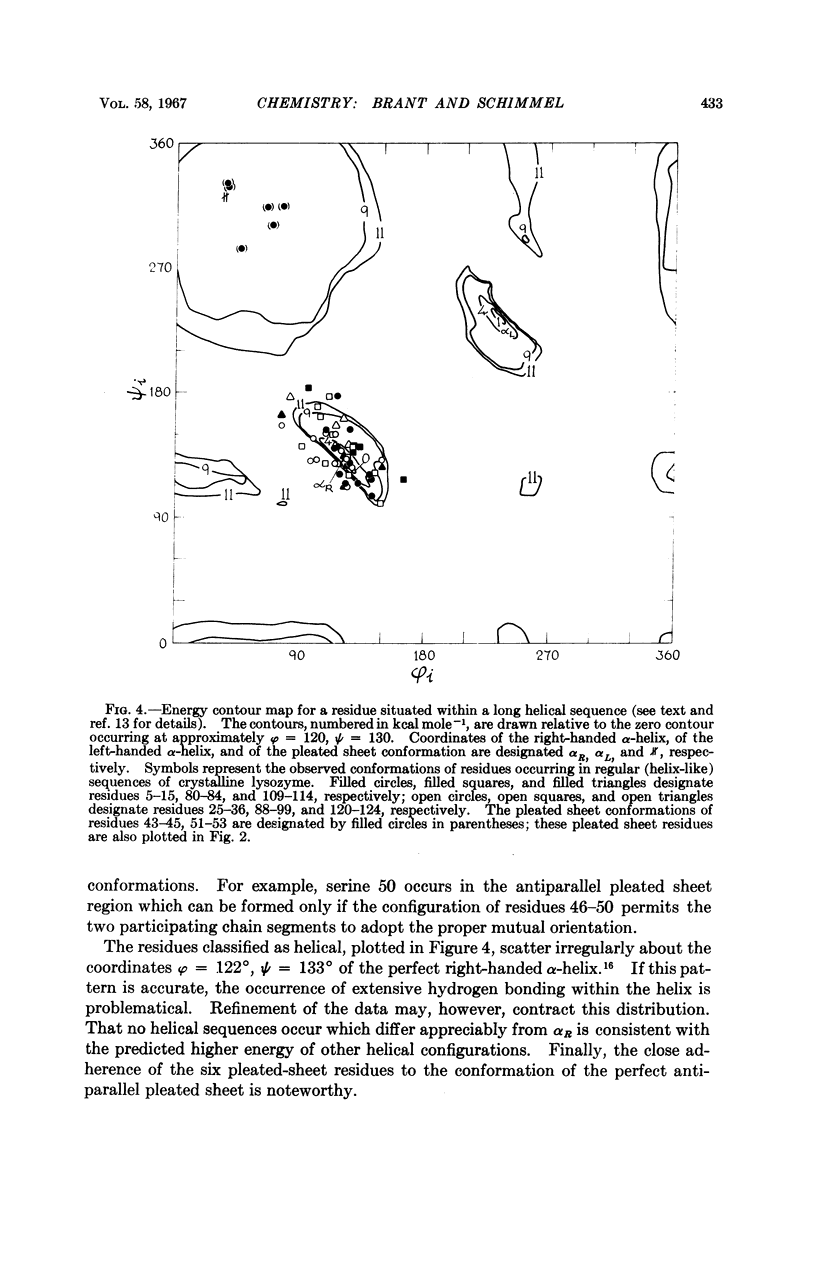
Full text
PDF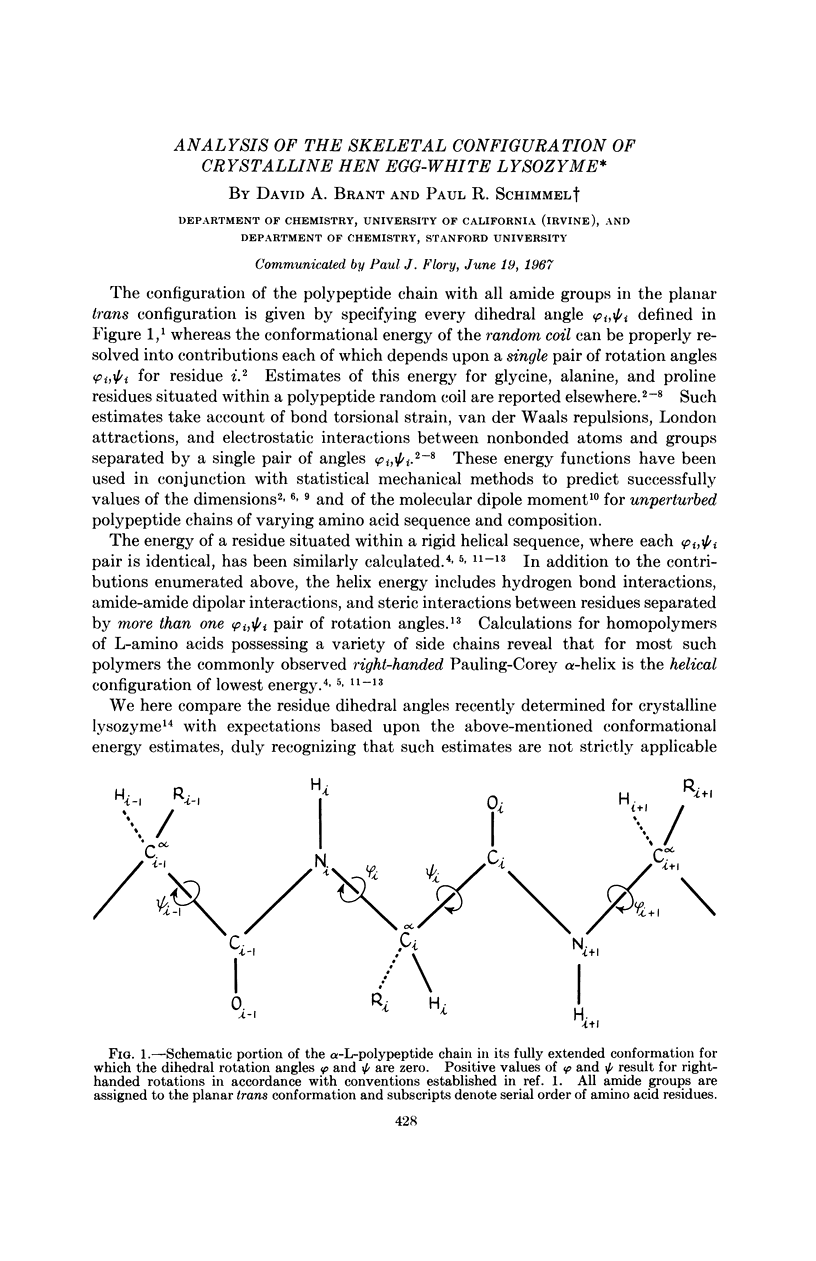





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CANFIELD R. E., LIU A. K. THE DISULFIDE BONDS OF EGG WHITE LYSOZYME (MURAMIDASE). J Biol Chem. 1965 May;240:1997–2002. [PubMed] [Google Scholar]
- De Santis P., Giglio E., Liquori A. M., Ripamonti A. Van der Waals interaction and the stability of helical polypeptide chains. Nature. 1965 May 1;206(983):456–458. doi: 10.1038/206456a0. [DOI] [PubMed] [Google Scholar]
- Gibson K. D., Scheraga H. A. Influence of flexibility on the energy contours of dipeptide maps. Biopolymers. 1966 Jul;4(6):709–712. doi: 10.1002/bip.1966.360040611. [DOI] [PubMed] [Google Scholar]
- JOLLES J., JAUREGUI ADELL J., BERNIER I., JOLLES P. LA STRUCTURE CHIMIQUE DU LYSOZYME DE BLANC D'OEUF DE POULE: 'ETUDE D'ETAILL'EE. Biochim Biophys Acta. 1963 Dec 13;78:668–689. doi: 10.1016/0006-3002(63)91033-3. [DOI] [PubMed] [Google Scholar]
- Leach S. J., Némethy G., Scheraga H. A. Computation of the sterically allowed conformations of peptides. Biopolymers. 1966 Apr-May;4(4):369–407. doi: 10.1002/bip.1966.360040402. [DOI] [PubMed] [Google Scholar]
- Ramachandran G. N., Venkatachalam C. M., Krimm S. Stereochemical criteria for polypeptide and protein chain conformations. 3. Helical and hydrogen-bonded polypeptide chains. Biophys J. 1966 Nov;6(6):849–872. doi: 10.1016/s0006-3495(66)86699-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan C., Ramachandran G. N. Stereochemical criteria for polypeptide and protein chain conformations. II. Allowed conformations for a pair of peptide units. Biophys J. 1965 Nov;5(6):909–933. doi: 10.1016/S0006-3495(65)86759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel P. R., Flory P. J. Conformational energy and configurational statistics of poly-L-proline. Proc Natl Acad Sci U S A. 1967 Jul;58(1):52–59. doi: 10.1073/pnas.58.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]



