Abstract
Introduction
Efficacy of daptomycin has been recorded in adult Gram-positive bone and joint infections OAI (1) and daptomycin has been used as secondary or tertiary agent when primary agents have failed (1, 2) in the treatment of osteoarticular infections caused by Staphylococcus aureus.
Presentation of case
We report a 16-year-old schoolboy with Panton-Valentine Leucocidin (PVL) positive methicillin susceptible S. aureus osteomyelitis, who was refractory to 9 days of recognised antimicrobial chemotherapy with progressive multifocal haematogenous spread. Subsequent addition of daptomycin promptly cleared the bacteraemia and arrested the disease process within 9 days.
Discussion
Although cases have been reported of daptomycin usage in children with invasive staphylococcus bacteraemia, endocarditis and OAI (2), we believe this to be the first case report describing the use of daptomycin in paediatric osteomyelitis caused by PVL positive S. aureus.
Conclusion
Repercussions of osteomyelitis, in particular those caused by PVL S. aureus, and evolving resistance patterns internationally, highlight the need for further evaluation of daptomycin in the paediatric arena. The response seen with the addition of Daptomycin in this case suggests possible reduction in hospital stay and number of surgical procedures when compared to other published series using conventional antibiotic regimens.
Abbreviations: PVL, Panton-Valentine Leucocidin; MSSA, methicillin-sensitive Staphylococcus aureus; OAI, Osteoarticular infection
Keywords: Osteomyelitis, Daptomycin, Panton-Valentine Leucocidin, Off label drug use, Paediatric
1. Introduction
PVL is an exotoxin that destroys leukocytes by creating pores in cell membranes. Genes encoding PVL are present in less than 5% of community-acquired methicillin-susceptible Staphylococcus aureus (CA-MSSA) in Europe,3–5 though significant geographic variations exist.
PVL positive S. aureus infection has a broad presentation profile and has been reported in a number of young people with a history of contact sports.6 The condition may start with relatively non-specific symptoms and include skin lesions3 and deep abscesses7 but can include necrotizing pneumonia3 and complicated musculoskeletal infection.8,9 Sepsis related multi-organ failure and/or necrotising pneumonia account for the high fatality rates observed3 and the requirement of intensive care facilities. Greater severity of infection is seen when compared with a PVL negative infection.6,10
Daptomycin binds to bacterial membranes in a calcium-dependent manner, causing subsequent potassium efflux. This leads to arrest of several intracellular processes, including Deoxyribonucleic acid and Ribonucleic acid production and protein synthesis, thereby hampering PVL production. This cascade eventually leads to bacterial death.11 Daptomycin's efficacy has been demonstrated in animal models12,13 with eradication of blood born and osteoarthritic infections with antibiotic-resistant and antibiotic-sensitive Gram-positive bacteria.1 Efficacy has been recorded in adult Gram-positive bone infections14 and it has been used in adults as a secondary or tertiary agent when primary agents have failed.1
Health Protection Agency guidelines15 for treatment of PVL-induced skin and soft tissue infections in children recommend flucloxacillin, clindamycin, linezolid. In adults with osteomyelitis or other deep seated infections, daptomycin is recommended as a second-line agent.15 Daptomycin, can be considered in therapy-refractory PVL-positive staphylococcal infections in children and we present such a case.
2. Presentation of case
A previously well 16-year-old boy presented with a 3-day history of rigors, vomiting, diarrhoea and 2-day history of lethargy, loss of appetite and worsening pain of his right knee. He had played football 2 days prior to onset of symptoms, but had no history of trauma.
On examination he was pyrexial, tachycardic and tachypnoeic. There was a mild effusion of the right knee. He was able to perform a straight-leg raise, but had pain on flexion and weight-bearing. The right hip and contralateral lower limb were unremarkable. Initial investigations included normal chest radiograph, white blood count (WBC) of 6.24 × 109/mm3, platelets 115 × 109/L, erythrocyte sedimentation rate of 74, C-reactive protein (CRP) of 302.4 mg/L and normal chemistry. The right knee aspirate demonstrated no organisms or crystals. Clinical impression was of a viral illness, possibly gastroenteritis, with reactive arthritis and he was treated with anti-inflammatories.
On Day 2 he remained pyrexial with increasing right knee pain. Repeat examination showed venous prominence on the medial aspect and effusion with tenderness around the right knee, but specifically in the popliteal fossa. Joint aspirate from admission remained culture negative and therefore the knee was re-aspirated. Initial blood cultures from admission grew methicillin-susceptible S. aureus and, following discussion with local microbiology, he was commenced on intravenous flucloxacillin (2 g four times daily) and oral clindamycin (300 mg four times daily). An urgent arthroscopic washout did not show evidence of synovitis.
On day 4 he developed coughing and an oxygen requirement. He was tachycardic, tachypnoeic and hypotensive. WBC rose to 12.61 × 109/mm3, platelets of 148 × 109/mm3 and CRP 185.2 mg/L.
On Day 8 with increasing right knee pain and associated oedema, magnetic resonance imaging (MRI) (Fig. 1) confirmed right proximal tibial osteomyelitis. He underwent incision and drainage of the right proximal tibia. Subperiosteal pus was drained and cortex windowed to release intramedullary pus.
Fig. 1.
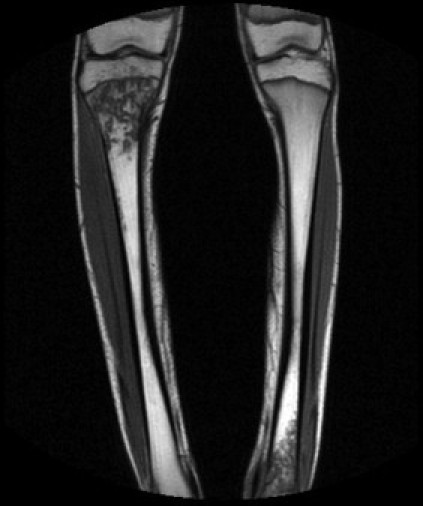
Magnetic resonance imaging demonstrating osteomyelitc change in the right proximal tibia and left distal tibia.
Other symptoms included right-sided chest pain, worsening dry cough and vomiting. Bilateral chest crepitations on examination and chest radiograph with right upper lobe shadowing prompted computed tomography that showed cavitating lung lesions in keeping with a diagnosis of multi-lobar pneumonia. Transthoracic echocardiogram revealed pericardial effusion, but without evidence of intravascular infection. Given the adequate views attained, the Cardiology consultant did not think a trans-oesophageal echo would be additionally instructive.
On day 9 his clinical condition deteriorated and he was intubated, ventilated and transferred to Paediatric Intensive Care. Samples were sent to the reference laboratory because of suspicion of PVL septicaemia, and contact isolation of the patient and screening of close family members was commenced. Intravenous flucloxacillin was discontinued and intravenous linezolid (600 mg twice daily) was commenced.
Second washout and debridement of right proximal tibia and primary drainage of left distal tibia were performed. All 8 specimens taken and repeat blood cultures were culture positive for S. aureus.
Despite further surgical debridement and double antibiotic therapy, his CRP continued to rise (227 mg/L), with no clinical improvement and so on day 11 intravenous daptomycin at 8 mg/kg was added to linezolid and clindamycin. The isolate was confirmed as PVL-positive on day 13.
His clinical condition improved, his chest cleared and by day 20 a repeat echocardiograph showed the pericardial effusion had resolved and CRP was 14.8 mg/L. Continued left ankle symptoms triggered a further MRI of distal tibia and it's subsequent debridement. All specimens including repeat blood cultures were culture negative. A radioisotope bone scan excluded silent multifocal disease (Fig. 2).
Fig. 2.
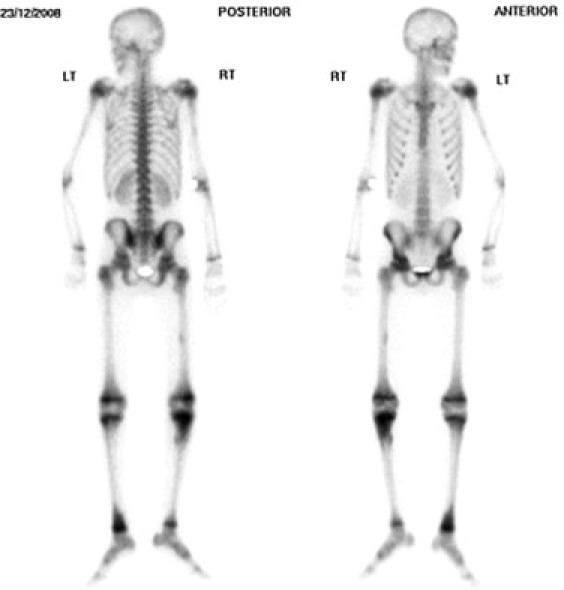
Isotope bone scan demonstrating hot spots at right proximal tibia and left ankle.
With subjective and objective improvement he was discharged home on day 30 with oral clindamycin and by day 44 his left ankle wound had completely healed, while his right tibial wound had decreased in size and CRP was 1.8 mg/L. At 1 year the left ankle was asymptomatic with full range of movement, right tibia was pain-free but the proximal growth plate had fused leaving 1 cm leg length discrepancy at skeletal maturity. No further episodes of infection have taken place.
3. Discussion
Data on effectiveness of antibiotic therapy and optimal duration in the management of osteomyelitis is limited. A 2001 meta-analysis concluded that little high-quality evidence-based therapy for osteomyelitis and septic arthritis exists.16 An open-label randomized control trial of standard treatment with vancomycin versus daptomycin in adults with osteoarticular infections secondary to staphylococcal infection, suggested daptomycin may be considered an alternative to standard therapy.14
Published use of daptomycin in paediatric infections are also limited and focus mainly on invasive staphylococcal disease of soft tissues. Addition of daptomycin has been reported in both adults and children and resulted in bacteriologic cure following a previously persistent bacteraemia in adults17 and children2 secondary to infection with Gram-positive-cocci. However, none of these reports look specifically at PVL positive S. aureus infection.
As in the case described, PVL-positive infections may be non-specific on presentation, but in the presence of joint pain with a negative aspirate one must consider osteomyelitis and perform MRI early. An earlier MRI is likely to have been helpful in this child's case. If the clinical picture is progressive despite treatment, follow-up blood cultures at 48 h should be taken. A persistent bacteraemia represented biochemically by sharp secondary rise in inflammatory markers, was our trigger for alternative and/or additional therapy. In the light of the severely deteriorating clinical picture, and in spite of the relative contraindication of pneumonia,18 we had to consider other pharmaceutical options regardless of Level 1 evidence.
Our institution attempts to apply a protocol to trigger early laboratory referral for PVL. In positive cases managed in our hospital the inflammatory cascade is so marked and prolonged that despite a resolving bacteraemia indicated by falling inflammatory markers, one frequently witnesses sporadic pyrexia. Isolated pyrexial manifestations should not be used as the sole reason to change anti-microbial therapy, neither should they be thought of as secondary to drug side effects, without supplementary evidence.
Table 1 shows a comparison of our case of PVL positive paediatric osteomyelitis treated with dapatomycin, another published case report and two series of children with PVL S. aureus osteoarticular infections managed with standard regimens.
Table 1.
Comparison of our case to another paediatric published case report and two series of children with PVL S. aureus osteoarticular infections managed with standard regimens.
4. Conclusion
Rapid bactericidal activity against growing and stationary-phase bacteria, a once-daily dosing regimen, and no requirement for drug monitoring contribute to daptomycin's potential therapeutic utility.2 Although licensed only for treatment of complicated skin and soft-tissue infections in adults, with activity against a range of aerobic and anaerobic Gram-positive bacteria including methicillin-resistant S. aureus and MSSA, glycopeptide-intermediate S. aureus, methicillin-resistant coagulase-negative Staphylococcus spp, and vancomycin-resistant enterococci, its success, albeit as an adjucnt to clindamycin and linezolid in this case, shows that further research is necessary to assess its true role in the management of paediatric osteomyelitis.
Conflict of interest
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Funding
None.
Authors’ contribution
Gurhan Erturan collected the data. All authors contributed to paper design, writing and editing.
References
- 1.Rice D.A., Mendez-Vigo L. Daptomycin in bone and joint infections: a review of the literature. Arch Orthop Trauma Surg. 2009;129(11):1495–1504. doi: 10.1007/s00402-008-0772-x. Epub 2008/11/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ardura M.I., Mejias A., Katz K.S., Revell P., McCracken G.H., Jr., Sanchez P.J. Daptomycin therapy for invasive Gram-positive bacterial infections in children. Pediatr Infect Dis J. 2007;26(12):1128–1132. doi: 10.1097/INF.0b013e31814523f8. Epub 2007/11/29. [DOI] [PubMed] [Google Scholar]
- 3.Gillet Y., Issartel B., Vanhems P., Fournet J.C., Lina G., Bes M. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359(9308):753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 4.Moumile K., Cadilhac C., Lina G., Berche P., Glorion C., Ferroni A. Severe osteoarticular infection associated with Panton-Valentine leukocidin-producing Staphylococcus aureus. Diagn Microbiol Infect Dis. 2006;56(1):95–97. doi: 10.1016/j.diagmicrobio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Holmes A., Ganner M., McGuane S., Pitt T.L., Cookson B.D., Kearns A.M. Staphylococcus aureus isolates carrying Panton-Valentine leucocidin genes in England and Wales: frequency, characterization, and association with clinical disease. J Clin Microbiol. 2005;43(5):2384–2390. doi: 10.1128/JCM.43.5.2384-2390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunnington A., Brick T., Cooper M., Danin J., Hunt D., Jeanes A. Severe invasive Panton-Valentine Leucocidin positive Staphylococcus aureus infections in children in London, UK. J Infect. 2009;59(1):28–36. doi: 10.1016/j.jinf.2009.05.003. Epub 2009/06/30. [DOI] [PubMed] [Google Scholar]
- 7.Reichert B., Birrell G., Bignardi G. Severe non-pneumonic necrotising infections in children caused by Panton-Valentine leukocidin producing Staphylococcus aureus strains. J Infect. 2005;50(5):438–442. doi: 10.1016/j.jinf.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Bocchini C.E. Panton-Valentine Leukocidin genes are associated with enhanced inflammatory response and local disease in acute hematogenous Staphylococcus aureus osteomyelitis in children. Pediatrics. 2006;117(2):433–440. doi: 10.1542/peds.2005-0566. [DOI] [PubMed] [Google Scholar]
- 9.Dohin B., Gillet Y., Kohler R., Lina G., Vandenesch F., Vanhems P. Pediatric bone and joint infections caused by Panton-Valentine leukocidin-positive Staphylococcus aureus. Pediatr Infect Dis J. 2007;26(11):1042–1048. doi: 10.1097/INF.0b013e318133a85e. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Aguilar G., Avalos-Mishaan A., Hulten K., Hammerman W., Mason E.O., Kaplan S.L. Community-acquired, methicillin-resistant and methicillin-susceptible Staphylococcus aureus musculoskeletal infections in children. Pediatr Infect Dis J. 2004;23(8):701–706. doi: 10.1097/01.inf.0000133044.79130.2a. [DOI] [PubMed] [Google Scholar]
- 11.Hancock R.E. Mechanisms of action of newer antibiotics for Gram-positive pathogens. Lancet Infect Dis. 2005;5(4):209–218. doi: 10.1016/S1473-3099(05)70051-7. [DOI] [PubMed] [Google Scholar]
- 12.Yin L.Y., Lazzarini L., Li F., Stevens C.M., Calhoun J.H. Comparative evaluation of tigecycline and vancomycin, with and without rifampicin, in the treatment of methicillin-resistant Staphylococcus aureus experimental osteomyelitis in a rabbit model. J Antimicrob Chemother. 2005;55(6):995–1002. doi: 10.1093/jac/dki109. [DOI] [PubMed] [Google Scholar]
- 13.Mader J.T., Adams K. Comparative evaluation of daptomycin (LY146032) and vancomycin in the treatment of experimental methicillin-resistant Staphylococcus aureus osteomyelitis in rabbits. Antimicrob Agents Chemother. 1989;33(5):689–692. doi: 10.1128/aac.33.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lalani T., Boucher H.W., Cosgrove S.E., Fowler V.G., Kanafani Z.A., Vigliani G.A. Outcomes with daptomycin versus standard therapy for osteoarticular infections associated with Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2008;61(1):177–182. doi: 10.1093/jac/dkm437. Epub 2007/11/15. [DOI] [PubMed] [Google Scholar]
- 15.Agency H.P. Diagnosis and management of PVL-SA infections in England and Wales: an update. Health Protect Rep. 2008:33. [Google Scholar]
- 16.Stengel D., Bauwens K., Sehouli J., Ekkernkamp A., Porzsolt F. Systematic review and meta-analysis of antibiotic therapy for bone and joint infections. Lancet Infect Dis. 2001;1(3):175–188. doi: 10.1016/S1473-3099(01)00094-9. Epub 2002/03/02. [DOI] [PubMed] [Google Scholar]
- 17.Falagas M.E., Siempos I.I., Papagelopoulos P.J., Vardakas K.Z. Linezolid for the treatment of adults with bone and joint infections. Int J Antimicrob Agents. 2007;29(3):233–239. doi: 10.1016/j.ijantimicag.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 18.Silverman J.A., Mortin L.I., Vanpraagh A.D., Li T., Alder J. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191(12):2149–2152. doi: 10.1086/430352. [DOI] [PubMed] [Google Scholar]
- 19.Swaminathan A., Massasso D., Gotis-Graham I., Gosbell I. Fulminant methicillin-sensitive Staphylococcus aureus infection in a healthy adolescent, highlighting Panton?.Valentine leucocidin syndrome. Intern Med J. 2006;36(11):744–747. doi: 10.1111/j.1445-5994.2006.01220.x. [DOI] [PubMed] [Google Scholar]


