Abstract
INTRODUCTION
The clinical manifestations of abdominal ‘cocoon’ are non-specific and hence its diagnosis is rarely made preoperatively and the management is often delayed. Surgery remains the main stay of treatment with satisfactory outcome and comprises excision of the fibrous membrane, meticulous adhesionolysis and release of the entrapped small bowel.
PRESENTATION OF CASE
A 45-year-old male patient presented with 6-month history of progressive subacute small bowel obstruction. After initial radiological investigations, he underwent diagnostic laparoscopy and was misdiagnosed as abdominal tuberculosis. He was started on anti-tuberculous therapy, but exploratory laparotomy was carried out after failure to respond to anti-tuberculous therapy. At laparotomy, the abdominal ‘cocoon’ which was encapsulating the entire small bowel was excised, and the adhesions were carefully lysed. The patient remained well and without recurrence at 1-year follow-up.
DISCUSSION
Abdominal ‘cocoon’ is a rare cause of subacute, acute and chronic small bowel obstruction. Its diagnosis is rarely made preoperatively.
CONCLUSION
Abdominal ‘cocoon’ should be thought of as a rare cause of small bowel obstruction. It may be mistaken with abdominal tuberculosis. Surgery remains the mainstay of curative treatment.
Keywords: Abdominal cocoon, Intestinal obstruction, Surgery, Adhesionlysis
1. Introduction
Abdominal ‘cocoon’ was first described in adolescent girls by Foo et al. in 19781 It is characterized by partial or total encasement of variable lengths of small intestine by a thick white fibrous cocoon-like membrane leading to acute, subacute or chronic intestinal obstruction. It is also called sclersoing encapsulating peritonitis and can be either primary (idiopathic) or secondary to chronic inflammatory systemic diseases.2,3
The clinical manifestations are non-specific and hence the diagnosis is rarely made preoperatively and management is often delayed. However, features on contrast computed tomography scan may be characteristic and therefore diagnostic. Surgery remains the main stay of treatment with satisfactory outcome. It comprises excision of the encasing fibrous membrane, meticulous division of adhesions and the release of the entrapped small bowel.
We report a case of abdominal ‘cocoon’ which presented with intermittent subacute intestinal obstruction. It was misdiagnosed initially as abdominal tuberculosis on diagnostic laparoscopy. Surgery was later carried out after failure of the patient to responds to anti-tuberculous treatment.
2. Case report
A 45-year-old male dentist presented with 6-month history of colicky central abdominal pain. The pain typically starts after eating and was associated with abdominal distension, and nausea, but relieved by vomiting. During the painful attacks, a right-sided abdominal mass commonly appears. He also reported a weight loss (18 kg over 3 months) with night sweats, but no other constitutional symptoms. Clinically he looked well but emaciated without pallor, jaundice or lymphadenopathy. The vital signs were normal and abdominal examination revealed a compressible soft but mildly tender abdominal mass (10 cm × 10 cm) in the central and extending to the right lumbar and iliac quadrants. Routine laboratory blood tests were all normal. Computerized tomography (CT) scan showed a conglomerate of multiple intestinal loops surrounded by a thick sac-like membrane. There was also a delayed passage of contrast from the jejunal loops to the mid small bowel loops which was suggestive of partial obstruction (Fig. 1). The patient underwent diagnostic laparoscopy that showed multiple adhesions in the peritoneal cavity between the small bowel and the parietal peritoneum. Small bowel loops were adherent and covered by a thick fibrinous layer appearing as a central mass. Free straw-colored ascitic fluid was present and the left hepatic lobe was plastered to the anterior abdominal wall. Some multiple subcentimetric nodules were noted over the visceral and parietal peritoneum. Abdominal tuberculosis was suspected and hence, multiple visceral and parietal peritoneal biopsies were taken. The biopsy came to be reactive mesothelial cells. Gram stain was negative, and Zeil–Neilson stain for acid-fast bacilli (AFB) was also negative. The patient was empirically put on anti-tuberculous medication while awaiting the PCR results. He reported symptomatic improvement, but there was no appreciable clinical improvement on follow-up. The culture for AFB and PCR for tuberculosis became later available and was negative. Repeat CT scan showed no interval improvement and the diagnosis of abdominal ‘cocoon’ was entertained. Therefore, he underwent exploratory laparotomy and the abdominal ‘cocoon’ which was encapsulating the entire small bowel and appendix was excised, the adhesions were carefully lysed and appendicectomy was performed (Fig. 2A–D). The postoperative period was uneventful and he was discharged home on the 6th post-operative day. The pathology of the resected encapsulating wall was consistent with sclerosing peritonitis. Follow-up CT scan after 6 months was normal and he remained well and without recurrence at one year follow-up.
Fig. 1.
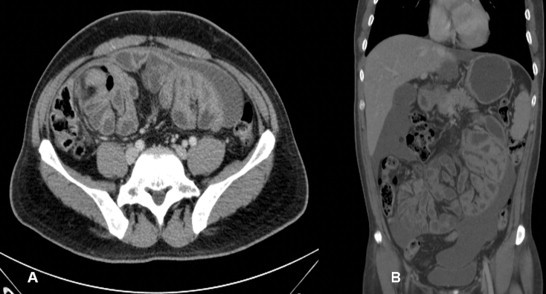
Computerized tomography scan showing a conglomerate of multiple intestinal loops surrounded by a thick sac-like membrane.
Fig. 2.
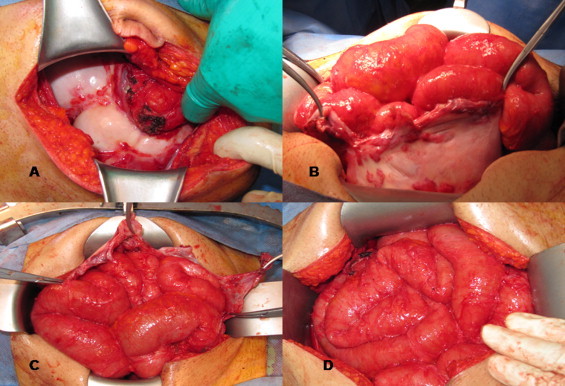
Operative views: (A) showing the ‘cocoon’ cover of the small bowel. (B) The ‘cocoon’ was opened to release the contained small bowel loops. (C) The adherent loops of small bowel were freed from the sac. (D) Complete excision of the sac and the release of entire small bowel.
3. Discussion
The exact pathogenesis of abdominal ‘cocoon’ remains unknown. However, the pathogenesis correlates to chronic asymptomatic peritonitis, some drugs such as practolol, peritoneal dialysis, endometriosis, abdominal tuberculosis and postoperatively especially after liver transplantatin.3–7
Some authors believe it may be a rare congenital condition due to abnormal return of mid-gut loops to the peritoneal cavity in the early stage of development.8 It is also postulated that during the embryological development the membranous layer of the greater omentum descends along the transverse colon and encase the small intestine within a fibrous membrane ‘cocoon’.2 Also, it may well be that in some cases, chronic peritonitis results in profuse exudation and malabsorption of fibrin in the peritoneal cavity with the formation of the thick fibrinous membrane that encapsulates small bowel.
The disease present with acute, subacute or chronic abdominal pain with or without abdominal mass. It is estimated that 87.5% of patients may present with partial or complete obstruction and 54% with abdominal mass. In this case both presentations were evident. Abdominal ‘cocoon’ may present acutely with acute small bowel obstruction which necessitates emergency surgery. CT scan may reveal features that are suggestive of abdominal ‘cocoon’. However, such striking features may – as in this case – be reported as internal herniation by inexperienced radiologists.
The diagnosis is rarely made preoperatively; in a retrospective analysis of 24 cases, only 4 patients were diagnosed before surgery by CT scan (n = 3) and barium examination (n = 1).2
In this case the diagnostic laparoscopy showed features that mimicked abdominal tuberculosis and it was felt that this cocoon was secondary to tuberculosis. It was therefore planned to start anti-TB therapy and reevaluate thereafter to decide about the need for surgery. The diagnosis of tuberculosis was only dismissed after the PCR results came back negative and the clinical improvement was negligible. Diagnostic laparoscopy is well-known for its pitfalls in visually diagnosing abdominal TB without the histological confirmation.9
Surgery is the main stay of treatment and it is often delayed due to delay in diagnosis especially in the absence of acute symptoms. During surgery, careful release of trapped small bowel loops from the ‘cocoon’ is mandatory together with release of adhesions between the bowel loops and the excision of the fibrous cover of the ‘cocoon’. It is also of importance to release the entire small bowel from the dudeno-jejunal junction to the ileocaecal valve as it was carried out in this case. Early postoperative complications such as intrabdominal sepsis, bowel perforation and enterocutaneous fistula may occur. Recurrence of the ‘cocoon’ is uncommon but future adhesive small bowel obstruction may occur with a morbidity of 12%.2 However, this can often be treated by non-operative means. In this case the author tried to minimize future adhesive small bowel obstruction by instilling some anti-adhesive agent in the peritoneal cavity at the conclusion of the operation prior to abdominal closure. It was reassuring that CT scan of the abdomen at 6 months revealed no evidence of ‘cocoon’ recurrence.
This case highlights the dilemma which is encountered by the surgeon on management of abdominal ‘cocoon’ presenting with non acute symptoms. It also further highlights the visual pitfall of diagnostic laparoscopic in erroneously diagnosing abdominal tuberculosis.9 Moreover, careful surgical intervention with the use of anti-adhesive agents may prove to be of a long-lasting favorable outcome.
Conflict of interest
None declared.
Funding
None.
Ethical approval
Patient's consent was obtained and is available for review upon editor's request.
Authors’ contribution
A.-W. Meshikhes initiated the manuscript and revised and wrote the final draft. S. Bojal wrote the first draft of the manuscript and searched the literature.
References
- 1.Foo K.T., Ng K.C., Rauff A., Foong W.C., Sinniah R. Unusual small intestinal obstruction in adolescent girls: the abdominal cocoon. Br J Surg. 1978;65:427–430. doi: 10.1002/bjs.1800650617. [DOI] [PubMed] [Google Scholar]
- 2.Wei B., Wei H.-B., Guo W.-P., Zheng Z.-H., Huang Y., Hu B.-G. Diagnosis and treatment of abdominal cocoon: a report of 24 cases. Am J Surg. 2009;198:348–353. doi: 10.1016/j.amjsurg.2008.07.054. [DOI] [PubMed] [Google Scholar]
- 3.Gadodia A., Sharma R., Jeyaseelan N. Tuberculous abdominal cocoon. Am J Trop Med Hyg. 2011;84(1):1–2. doi: 10.4269/ajtmh.2011.10-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahoo S.P., Gangopadhyay A.N., Gupta D.K., Gopal S.C., Sharma S.P., Dash R.N. Abdominal cocoon in children: a report of four cases. J Pediatr Surg. 1996;31:987–988. doi: 10.1016/s0022-3468(96)90431-5. [DOI] [PubMed] [Google Scholar]
- 5.Afthentopoulos I.E., Passadakis P., Oreopoulos D.G., Barqman J. Sclerosing peritonitis in continuous ambulatory peritoneal dialysis patients: one center's experience and review of the literature. Adv Ren Replace Ther. 1998;5:157–167. doi: 10.1016/s1073-4449(98)70028-7. [DOI] [PubMed] [Google Scholar]
- 6.Mann R.D. An instructive example of long-latency adverse drug reaction – sclerosing peritonitis due to practolol. Pharmacoepidemiol Drug Saf. 2007;16:1211–1216. doi: 10.1002/pds.1466. [DOI] [PubMed] [Google Scholar]
- 7.Maguire D., Srinivasan P., O’Grady J., Rela M., Heaton N.D. Sclerosing encapsulating peritonitis after orthotopic liver transplantation. Am J Surg. 2001;182(2):151–154. doi: 10.1016/s0002-9610(01)00685-7. [DOI] [PubMed] [Google Scholar]
- 8.Chew M.H., Sophian Hadi I., Chan G., Ong H.S., Wong W.K. A problem encapsulated: the rare peritoneal encapsulation syndrome. Singapore Med J. 2006;47(9):808–810. [PubMed] [Google Scholar]
- 9.Meshikhes A.-W. Pitfalls of diagnostic laparoscopy in abdominal tuberculosis. Surg Endosc. 2010;24(4):908–910. doi: 10.1007/s00464-009-0692-z. [DOI] [PubMed] [Google Scholar]


