Abstract
Cancers so much resemble self that they prove difficult for the immune system to eliminate, and those that have already escaped natural immunosurveillance have gotten past the natural immune barriers to malignancy. A successful therapeutic cancer vaccine must overcome these escape mechanisms. Our laboratory has focused on a multistep “push-pull” approach in which we combine strategies to overcome each of the mechanisms of escape. If tumor epitopes are insufficiently immunogenic, we increase their immunogenicity by epitope enhancement, improving their binding affinity to major histocompatibility complex (MHC) molecules. If the anti-tumor response is too weak or of the wrong phenotype, we use cytokines, costimulatory molecules, Toll-like receptor ligands, and other molecular adjuvants to increase not only the quantity of the response, but also its quality, to push the response in the right direction. Finally, the tumor invokes multiple immunosuppressive mechanisms to defend itself, so we need to overcome those as well, including blocking or depleting regulatory cells or inhibiting regulatory molecules, to pull the response by removing the brakes. Some of these strategies individually have now been translated into human clinical trials in cancer patients. Combinations of these in a push-pull approach are promising for the successful immunotherapy of cancer.
Introduction
Cancers that escape natural immunosurveillance and become clinical tumors, like viruses that cause chronic infections, need something more than just the tumor cell or the virus to induce an immune response sufficient to eradicate the disease. Vaccines other than live attenuated organisms often use adjuvants to improve the immune response, what Janeway called immunology’s “dirty little secret” 1. Recent improvements in our understanding of immunology have allowed the design of vaccines using immune modulators as defined molecular adjuvants, to more rationally improve the immune response. In this review, we will focus on our work on vaccines to induce T cells, especially CD8+ T cells, but antibodies can play an important role in cancer as well, as witnessed by the clinical success of a number of monoclonal antibodies. Indeed, we recently described an adenoviral vector vaccine expressing the extracellular and transmembrane domains of HER-2 that cured mice of large (2 cm) established tumors and lung metastases, by a mechanism that involved only antibodies, and not effector T cells at all 2–5. That preclinical study is currently being translated into a vaccine to treat HER-2 positive human tumors such as breast cancer.
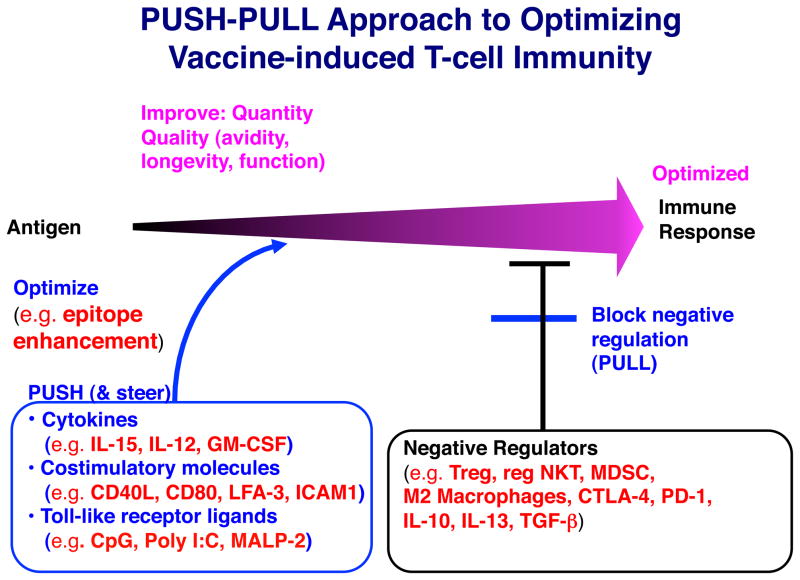
Immune modulators used in vaccines are of two broad types, ones that amplify the response or change its quality, and ones that remove negative regulatory mechanisms or check point inhibitors, so as to allow the response to reach its full potential. We call the former the “push” and the latter the “pull” of our “push-pull” strategy (See Fig. 1) 6–8. The push involves not only increasing the magnitude of the response, but also improving the quality of the response, and we have found that the quality often is more important for protection and efficacy than the quantity. In quality, we include the avidity and the longevity of the response, as well as functional activities such as the cytokine profile. We will discuss the use of cytokines, costimulatory molecules, and Toll-like receptor (TLR) ligands to achieve this. The pull is intended to release the parking brake so the vehicle can move. We remove or inhibit suppressive cells such as Foxp3+ T regulatory cells (Treg), regulatory type II NKT cells, or myeloid-derived suppressor cells (MDSC) or block inhibitory molecules such as PD-1 or CTLA-4 receptors on T cells or inhibitory cytokines such as TGF-beta or IL-138–13. The review will thus be divided into sections based on these complementary strategies, but first we may also improve the structure of the antigen itself, by a process we call “epitope enhancement,” which will be discussed first.
Figure 1.
Push-pull approach to optimizing vaccine efficacy for induction of T cell immunity. The basic skeleton of a vaccine is an antigen used to induce an immune response. We can improve on this at each of several steps as described in the text. First, we can improve the immunogenicity of the T-cell epitopes by substituting amino acids that interact with the MHC molecule to increase affinity, without altering the 3-dimensional configuration seen by the TCR. We call this epitope enhancement. Next, we can push the response with cytokines, costimulatory molecules and TLR ligands as molecular adjuvants, not only to increase the magnitude of the response, but also to improve the quality of the response. Even this is not sufficient because of the negative regulatory cells, surface molecules and cytokines that are often present and inhibit the immune response, so we need to overcome those to finally optimize the response (the “pull” of the approach). The examples shown are ones described in the text, but are not intended to be an exhaustive list. We call the overall strategy a “push-pull” approach. Modified from 96 with permission.
Epitope Enhancement
Tumor antigens, like viral antigens, did not evolve to be good vaccines, so we could in principle do better. In addition to strategies such as using long peptides 14, we have focused on increasing the affinity of the peptide epitopes for Major Histocompatibility Complex (MHC) molecules that present the peptides to T cells, a process we call epitope enhancement. We have shown that increasing the affinity for MHC molecules increases the immunogenicity, in accord with the finding of others that immunogenicity of natural epitopes correlates with affinity for MHC molecules 15, 16. The concept is to alter amino acids that bind to the MHC molecule without altering the face of the peptide that is exposed out of the MHC groove to interact with the T cell receptor (TCR). Thereby, we hope to increase immunogenicity while eliciting T cells with receptors that still recognize the natural cancer or viral antigen. We have carried out successful epitope enhancement of both CD4+ and CD8+ T cell epitopes from tumor antigens as well as HIV and hepatitis C virus (HCV) antigens 7, 17–24. For one of these, T-cell alternate reading frame protein (TARP) 22, a breast and prostate cancer antigen, we currently have an ongoing clinical trial using an epitope-enhanced peptide, as will be discussed in the section on translation.
Pushing the T cell response with cytokines, costimulatory molecules, and TLR ligands to improve the quantity and quality of the immune response
Over more than a quarter century, our lab has studied immunomodulatory cytokines and costimulatory molecules and more recently TLR ligands as molecular adjuvants 9, 25. We first found that incorporation of IL-2 into an emulsion adjuvant with the antigen could induce responses in low responder strains of mice that were as high as those in high responder strains, overcoming genetic low responsiveness 26. Incorporation in the emulsion created a depot for slow release of the cytokine with the antigen to the same draining lymph nodes, while concurrently avoiding systemic side effects of systemic administration of cytokines. We then compared multiple cytokines as adjuvants and found that some increased most types of immune responses (e.g. GM-CSF), whereas others more selectively amplified some responses and not others (e.g. IL-12) 27. We found that GM-CSF and IL-12 synergized for increasing the CD8+ T cell response, whereas TNF-α and IL-12 synergized to markedly increase the interferon-gamma (IFN-γ) response 27. Mechanistically, we found that GM-CSF increases antigen presenting cells and activity in the draining lymph node, whereas TNF-α and IL-12 synergized to increase expression of the IL-12 receptor, and thus increase sensitivity to IL-12 to induce IFN-γ 28, 29. We also found synergy between GM-CSF and CD40L, on the basis that GM-CSF increased the antigen-presenting dendritic cells (DCs) and then CD40L activated or matured them through ligation of CD40 6.
One key qualitative feature of CD8+ T cells is their functional avidity for antigen, which we measure by titrating peptide on antigen-presenting cells (APCs) 30. Early on, using adoptive transfer into SCID mice that lacked their own T and B cells, we first discovered that high avidity CD8+ T cells were much more effective at clearing a virus infection than low avidity T cells specific for the same peptide-MHC complex 30. The functional avidity depended not only on the intrinsic affinity of the T-cell receptor (TCR), but also the TCR density, the density of the CD8 coreceptor31, 32, and probably other factors involved in signal transduction. This finding was confirmed in other viral studies 33 and extended to killing of tumor cells 34–36. Thus, induction of high avidity CD8+ T cells became a key goal of our vaccine strategy.
However, unlike the situation in vitro, in which one could select for higher avidity T cells by culturing them with very low concentrations of antigen 30, in vivo lowering the dose of antigen was not sufficient, as one reached a threshold below which a response could not be induced. We reasoned that increasing costimulation (signal 2) might allow induction of T cells with less antigen (signal 1), and tested this hypothesis in collaboration with Hodge, Schlom and coworkers who had developed poxviral vectors expressing a triad of costimulatory molecules, B7-1, ICAM-1 and LFA-3 37. We found that immunizing with the vector expressing the costimulatory molecules along with antigen induced higher avidity CD8+ T cells than did immunizing with vector expressing antigen alone 38. This finding was extended to show dramatic increases in avidity and protection against cancer that could be achieved with such vectors 39.
We also explored the use of cytokines as adjuvants to augment T cell avidity. In a collaborative study with Waldmann and Perera, using a vaccinia vector expressing both antigen and IL-15, we found that expression of IL-15 by the vector, presumably in the same APCs presenting antigen expressed by that vector, led to greater avidity maturation of CD8+ T cells after several months 40. This correlated with apparent selection for T cells expressing their own IL-15Rα, the receptor chain on dendritic cells that presents IL-15 in trans to T cells and NK cells 41, 42, possibly because they could respond to IL-15 independently of other cells. IL-15 is known to promote the homeostatic proliferation of CD8+ T cells 43, 44. We found that the CD8+ T cells induced by the vector expressing IL-15 had higher levels of their own IL-15Rα and underwent more homeostatic proliferation when adoptively transferred into naïve animals without any antigen exposure 40. Over time, the high avidity T cells thus persisted, whereas the low avidity response fell off, so the average avidity of the population increased, accounting for avidity maturation 40. Avidity maturation of T cells had been an enigma, because their receptor, unlike antibodies, does not undergo somatic mutation 45. In addition, we found that IL-15 increased expression of the CD8 coreceptor, which also contributes to functional avidity 40. These two mechanisms complement each other to account for the higher avidity induced by IL-15.
We also found that vectors expressing IL-15 with antigen increased the longevity of CD8+ T cells for as long as 14 months, more than half a mouse lifetime, compared to similar vectors with antigen alone or with IL-2 expressed 46. Since CD4+ helper T cells also promote T cell longevity 47–49, and can induce the DC presenting antigen to make IL-15, like cells infected with a vector expressing both antigen and IL-15, we hypothesized that IL-15 might be a natural mediator of help. A corollary was that in the absence of CD4+ T cell help, it would be hard to induce such long-lived CD8+ T cells, but that IL-15 might substitute. To test this, we depleted mice of CD4+ T cells and compared immunization with vectors expressing antigen with or without IL-15. When CD4-depleted animals were immunized with the vector expressing only antigen, they had no long-term memory from 2 months to a year. However, when such depleted animals were immunized with the vector expressing IL-15 with antigen, their long-term memory was almost as high as that of non-depleted immunized animals 50. Such T cells induced without help had been shown to be more susceptible to TRAIL-mediated antigen-induced death when re-exposed to antigen 51. We found that IL-15 could substitute for CD4+ T cell help to prevent this TRAIL-mediated death 50. Also, if the DCs presenting antigen were incapable of making IL-15, they did not mediate normal help, so we concluded that IL-15 was not only sufficient to replace help, but also necessary for help, and thus was an important natural mediator of help 50.
Another approach to accomplish the same goal might be to use Toll-like receptor (TLR) ligands, which can also activate DCs to express costimulatory molecules and cytokines 52. These ligands are natural microbial products that probably occur in combinations in nature, so we reasoned that selected combinations might manifest synergy. Indeed, we and others found synergistic combinations of TLR ligands 52–54, and we demonstrated that they function synergistically as vaccine adjuvants to induce greater CD8+ T cell responses 54. We found that the synergy depended in part on use of different adaptor molecules, MyD88 for TLR2/6 or 9, and TRIF for TLR3, and that there was unidirectional cross-talk between these pathways so that the signal through TRIF amplified the induction of IL-12 through MyD88 54. Thus, TLR3 synergized with TLR2/6 and with TLR9, but the triple combination did not induce more specific T cells than the effective double combinations. Therefore, we were surprised when the triple combination induced better protection against virus challenge in mice 55. If not explicable by increased T cell quantity, we asked whether this was due to increased quality, and found that indeed the CD8+ T cell functional avidity induced by the triple combination of TLR ligands was substantially greater than that induced by the double combination 55. This could be explained by increased induction of IL-15. Thus, again, the quality of T cell response was more important than the quantity of the response in providing protection.
We translated these findings into a macaque study of an intrarectal mucosal lentiviral SIV vaccine, using a vaccine consisting of a group of antigen peptides boosted by recombinant poxviral vectors. Comparing groups given IL-15, the triple combination of TLR ligands, both or neither, along with the vaccines, we found that only the combination of both induced protection against high dose intrarectal challenge with pathogenic SIVmac251 56. We found both innate and adaptive correlates of protection. The molecular adjuvant combination upregulated expression of the virus restriction molecule APOBEC3G, a cytidine deaminase, especially in mucosal dendritic cells, lasting almost 2 months after the last vaccination. The combination also most effectively induced polyfunctional CD8+ T cells that correlated with protection 56. Thus, combinations of defined molecular adjuvants such as cytokines, costimulatory molecules, and TLR ligands can be used to improve both the magnitude and more importantly the quality of the T-cell immune response to vaccines.
Overcoming negative regulation
As mentioned above, now it is apparent that patients’ immune systems are dampened by negative regulatory cells and factors that serve as roadblocks for the induction of optimal immune responses. Thus, removing these roadblocks is a key step for the success of cancer immunotherapy. One example supporting this idea is the recent success of the anti-CTLA-4 monoclonal antibody (Ipilimumab) in the treatment of metastatic melanoma patients.
TGF-β is one of the most potent immunosuppressive cytokines. This cytokine affects the immune system in multiple ways. TGF-β induces immunosuppressive Foxp3+ Tregs from non-Treg CD4 T cells 57. It has been reported that TGF-β deficiency causes defects in the number as well as functions of Treg cells. TGF-β also can directly suppress activation/function of Th1 and CD8 T cells 58 and has been shown to recruit CD11b+Gr1+ myeloid derived suppressor cells (MDSC) into the tumor microenvironment 59. TGF-β also supports cancer growth through multiple mechanisms such as enhancing angiogenesis, invasion and metastasis. In fact, it has been shown that TGF-β levels in the peripheral blood are inversely correlated with the prognosis of many types of cancer 60. In terms of the sources of TGF-β, it was originally considered that cancer cells are the major producer. However, accumulated literature suggests that immune regulatory cells are also important sources of TGF-β involved in immune suppression. Foxp3+ Treg cells are reported to have TGF-β on the cell surface, which contributes to the cell-cell contact dependent suppression 61. It is also reported that regulatory NKT cells (type II NKT cells), through IL-13 and TNF-α, induce TGF-β production by CD11b+Gr1+ myeloid cells that suppresses tumor specific CD8 T cells 62, 63.
Since TGF-β is involved in multiple mechanisms that can support tumor growth, it is a very attractive target for cancer therapy. Consequently it is not surprising that TGF-β blockade has been shown to suppress tumor growth 62, 64, 65 in some mouse models. However, monotherapy with a TGF-β antagonist is not always sufficient to induce clinical response. The combination of TGF-β antagonists and a vaccine has been reported to enhance vaccine efficacy in multiple mouse models. In a s.c. tumor model with the TC-1 tumor cell line, which is a lung epithelial cell line transfected with HPV 16 E6 and E7 oncogenes, monotherapy with 1D11, a monoclonal antibody that neutralizes all three isoforms of TGF-β, does not show any impact on tumor growth. However, when combined with a peptide vaccine comprising a minimal CTL epitope derived from the E7 antigen, this anti-TGF-β antibody significantly improved vaccine efficacy to reduce tumor size 66. The clinical effect was accompanied by improved frequency, IFN-γ production and lytic activity of tumor antigen-specific CD8+ T cells. In the eradication of tumors by CD8+ T cells, the T cells with higher functional avidity, which can recognize targets with a lower number of antigen-MHC complex as described above, are more effective. With combination treatment, as the magnitude of the anti-tumor response was enhanced, the number of tumor-specific CD8+ T cells with higher functional avidity was concomitantly increased, suggesting a contribution toward better tumor suppression. Surprisingly, there was no effect of anti-TGF-β treatment on the number Treg cells in tumor draining lymph nodes or in tumors. This result was consistent with the observation that Treg depletion (by anti-CD25) did not affect the vaccine efficacy.
A similar effect of 1D11 was observed with an irradiated whole tumor cell vaccine, in a s.c. CT26 colon carcinoma model 67. The combined treatment significantly improved survival mediated by CD8+ T cells. However, there was no effect on the number of Treg cells. The combination of 1D11 and peptide vaccines was also shown to improve the clinical response against the mouse glioma GL261, with enhanced CD8+ T cell response including higher frequency of tumor specific T cells and higher level of pro-inflammatory cytokine production 68. In this model, reduction of tumor infiltrating Treg cells was observed in the mice with the combination therapy. Another anti-TGF-β monoclonal antibody, 2G7, has been shown to improve the efficacy of a recombinant Listeria vaccine expressing HPV16 E7 in the TC1 tumor model when given 7 and 14 days after tumor challenge 69.
Another way to inhibit TGF-β signaling is to use a small molecule inhibitor of the receptor kinase. When an adenoviral vector expressing HPV16 E7 is given with SM16, an inhibitor of TGF-β receptor kinase, the vaccine efficacy is significantly facilitated, inducing significant regression of tumors in a TC1 tumor model 70. These reports strongly suggest that blockade of TGF-β or its signaling has a great potential to enhance the efficacy of vaccines with different platforms including peptide, viral vector, and bacterial vector. As discussed below, currently clinical trials of an anti-TGF-β monoclonal antibody and antagonists are ongoing and a combination study with a therapeutic vaccine is a high priority.
In the immunoregulatory pathway mentioned above, in which type II NKT cells and CD11b+Gr-1+ myeloid cells are involved, IL-13 together with TNF-α is shown to induce the production of TGF-β by myeloid cells 11, 62, 63, 71–73. Thus, the effect of IL-13 blockade by soluble IL-13Rα2-Fc was examined on a CTL-inducing peptide vaccine derived from the HIV envelope protein gp120 6. The blockade significantly enhanced induction of lytic acivity of antigen-specific CTL compared to the vaccine without IL-13Rα2-Fc. Moreover, the combination of vaccine and IL-13Rα2-Fc induced significantly better protection against a vaccinia viral infection expressing HIV gp120. Similarly the vaccine alone without IL-13Rα2-Fc was more effective in NKT cell-deficient CD1d KO mice compared with in wild-type mice. This result is consistent with the previous observation that type II NKT cells are necessary for IL-13 induced immune suppression. IL-13 also induces other immune suppressive mechanisms in MDSC and M2 macrophages that have been shown to play a critical role in immune suppression in cancer patients 74,75. Hence, inhibiting IL-13 and its signaling can block multiple pathways of immune suppression.
Although we do not have enough space to discuss other immune inhibitory molecules such as CTLA-4 and PD-1 that can be good targets to enhance vaccine efficacy, it is worth noting that recent reports suggest synergistic effects of anti-CTLA-4 and anti-PD-1 agents when given with a cancer vaccine 76. Thus, simultaneous inhibition of multiple pathways may be necessary for optimal impact of therapeutic cancer vaccines on clinical outcomes.
Clinical translation of therapeutic cancer vaccine strategies
The basic bench discoveries outlined above investigating molecular enhancement of antigens, optimization of T cell responses with cytokines and TLR ligands and overcoming negative regulation of immune responses, provide the foundation for clinical translation and first-in-human studies of promising therapeutic cancer vaccines, checkpoint inhibitors and cytokines. Challenges that accompany clinical translation include identifying the appropriate study populations and clinical endpoints, the mode of vaccine delivery and frequency, monitoring the response to vaccination, and identifying the immunologic responses that correlate with clinical outcomes. Indeed, the immunomodulatory landscape is littered with therapeutic cancer vaccines and other agents that failed to successfully address these challenges, but along the way have allowed us to gain insight into approaches that are more likely to lead to clinical success.
A critical understanding gained is that immune-based therapies have a better chance of demonstrating preliminary activity and clinical efficacy in patients with less advanced disease, thereby avoiding the immune dysregulation of high tumor burden 77 and marrow depletion from repeated chemotherapies characteristic of very advanced stage disease. In keeping with this observation is the clinical indication for PROVENGE® (sipuleucel-T), the first therapeutic cancer vaccine approved in April 2010 for use in humans: its efficacy and improvement in overall survival is limited to men with minimal disease burden i.e. those with asymptomatic or minimally symptomatic metastatic castrate resistant (hormone refractory) prostate cancer 78. Among clinical endpoints, improvement in overall survival (OS) is the platinum standard and the endpoint that matters most to patients battling cancer. However, again using PROVENGE® as an example, the immunologic correlates predictive of its associated median 4.1 month improvement in OS remain poorly understood 79. Importantly, this improvement in OS was not associated with changes in time to disease progression and the OS effect was not observed until after 6 months of therapy, consistent with observations that the clinical impact of immune-based therapies may be delayed relative to chemotherapeutic and other molecularly targeted agents 80.
As a consequence of challenges identified with the development of other cancer immunotherapies, we elected to conduct our initial studies of a T-cell alternative reading frame protein (TARP) peptide vaccine in men with Stage D0 prostate cancer that have PSA biochemical recurrence without radiologic evidence of metastatic disease, a patient population with presumably normal (or near normal) immune function. TARP is expressed by both normal and malignant prostate tissue and is overexpressed in 95% of prostate cancer specimens 81–83 making it a good target antigen for therapeutic vaccination. The current vaccine platform builds on our approach of epitope enhancement. It consists of two HLA-A*0201 TARP peptide epitopes documented in our preclinical laboratory studies in mice and human cell lines to generate cytolytic CD8 T-cell responses: TARP27–35 and epitope-enhanced (EE) TARP29-37-9V 22. As the optimal method for therapeutic vaccination with peptide vaccines is unclear, our prospective study is examining in a 1:1 randomization, delivery of TARP peptides as a patient-specific, autologous intradermal dendritic cell vaccine or a non-cellular, generic emulsion of TARP peptides in combination with Montanide® ISA51 VG adjuvant plus Sagramostim (GM-CSF) administered by deep subcutaneous injection. A total of 1.1 mg of each peptide is delivered per vaccine given every three weeks for an initial course of 5 vaccinations with subsequent options for a 6th dose of vaccine at 36 weeks based on immune response or PSA doubling time (PSADT) criteria; all patients subsequently undergo booster vaccination at Weeks 48 and 96. Primary study endpoints include safety and immunogenicity with secondary evaluation of the impact of TARP vaccination on PSADT which has been validated to be a reliable and important predictor of clinical recurrence, progression and survival, especially in men with PSA failure only i.e. Stage D0 disease 84,85. To date TARP vaccination has been safe and well tolerated with adverse events limited to local injection site reactions. A preliminary interim analysis among pooled patients enrolled to date in the two arms documents a significant decline in the slope log (PSA) (and subsequent lengthening of the PSADT) from 3–24 weeks and 3–48 weeks post vaccination when compared to baseline, with no statistically significant difference found between the arms. Analysis of immunogenicity to TARP WT27–35 and EE29-37-9V vaccine epitopes as well as cross-reactivity to native WT29–37 as assessed by IFN-γ ELISPOT and TARP tetramer assays is ongoing.
In addition to inducing anti-tumor immune responses using optimized antigens in therapeutic cancer vaccines and molecular adjuvants to “push” the immune system, we have also explored blocking the negative regulation and checkpoints that interfere with generation of effective anti-tumor responses. Since blockade of TGF-β has been shown by us and others to have anti-tumor and anti-metastatic effects in multiple pre-clinical animal models utilizing a variety of cancers 62, 64, 65, neutralization and blockade of this highly prevalent and pleotropic cytokine represents a truly novel therapeutic target in cancer treatment. Multiple approaches targeting TGF-β are planned or currently under investigation in clinical trials, including a monoclonal antibody (GC1008), 86 an antisense oligodeoxynucleotide specific for human TGF-β2 (AP12009, trabedersen), 87 and a type I receptor TGF-β kinase antagonist (LY2157299) 88.
The NCI participated in a multi-institution phase I study of GC1008, a human IgG4 monoclonal antibody capable of neutralizing all three isoforms of TGF-β. Interim data were reported at ASCO.86 Patients with advanced malignant melanoma (MM) (n=21) or renal cell carcinoma (RCC) (n=1) who had progressed on at least one prior therapy, were treated with GC1008 at 0.1, 0.3, 1, 3, 10 or 15 mg/kg dose levels in a traditional 3+3 cohort design. Lack of dose limiting toxicity (DLT) was confirmed in the first 28 days following initial GC1008 dosing, with patients subsequently receiving 3 additional doses every 2 weeks for a cycle of therapy followed by restaging. Patients achieving stable disease (SD) of at least 8 weeks duration, partial response (PR), mixed response (MR) or complete response (CR) by RECIST criteria, 89, 90 were offered extended treatment with GC1008 every 2 weeks for a total of 4 doses per cycle and a maximum of two extended treatment cycles. There were no DLTs at the highest dose level of 15 mg/kg and evidence of clinical benefit (SD or better) was seen in 5 of 22 (23%) patients. Interestingly, this activity was observed in the lower dose cohorts: 5 of 5 objective responses in cohorts with initial GC1008 dosing of 0.1 to 1 mg/kg compared to 0 responses at initial doses greater than or equal to 3mg/kg. An additional 7 patients (all with MM) were enrolled in an expansion cohort at 15mg/kg to further explore activity at this dose level and final analyses are pending. Notably, a similar atypical pattern of response i.e. a trend towards greater frequency of responses and survival also at lower rather than higher doses of drug has also been reported with another agent using an anti-sense approach to target TGF-β 87, 91, highlighting the unique characteristics of this molecular target. The relationship of GC1008 dose and response is unclear at this time due to small patient numbers and selection bias that may occur during the course of a Phase I study. Based on our preliminary evidence of efficacy, a phase II study in patients with MM examining different dose levels is important to further explore the dose relationship of GC1008.
Administration of GC1008 was associated with the development of eruptive keratoacanthomas (KAs) 92 in 3 patients, 2 of whom had received at least 1 cycle of extended treatment. KAs are unique epidermal tumors that classically occur in sun-exposed areas and are characterized by rapid, abundant growth and spontaneous resolution. Diagnosing KAs is a challenge within the dermatopathology community due to lack of sensitive or specific histologic features that distinguish it from well-differentiated squamous cell carcinoma (SCC). 92 Consistent with these observations, the KAs documented in this study patients were non-life threatening and resolved following discontinuation of GC1008. In addition, a single case of well-differentiated SCC was diagnosed in a patient with a history of this cancer. Of note, well-differentiated SCC has been reported in association with loss of TGF-β epithelial homeostasis 93. In addition, emerging data indicates there is a clinical disorder characterized by multiple KAs in which TGF-β signaling is disrupted.94 Ferguson-Smith disease is an autosomal-dominant skin cancer condition characterized by multiple squamous carcinoma-like locally invasive skin tumors (KAs) that grow rapidly for a few weeks before spontaneously regressing. Genomic analyses indicate a genotype-phenotype correlation between loss-of-function of TGF-βR1 mutations and KAs, a biologic scenario which might be mimicked through significant neutralization or blockade of TGF-β.
The current enthusiasm within the field of cancer immunotherapy associated with the recent approvals of Provenge® in 2010 and Ipilimumab in 2011 has fueled significant interest in investigating combinatorial approaches in the hopes of achieving even better clinical outcomes. Pre-clinical animal models provide a scientific rationale for doing so and suggest these approaches have the potential for mechanistic synergy. A tantalizing (and potentially infinite) spectrum of multiple different combinations of vaccine and adoptive cell transfer platforms, cytokines, chemotherapeutic agents and checkpoint inhibitors of negative regulation is already possible. Indeed, the improved vaccine efficacy demonstrated with an anti-TGF-β monoclonal antibody combined with an HPV E7 peptide vaccine 66 or with a whole irradiated tumor cell vaccine 67 makes clinical translation of this combination type using our TARP peptide vaccine and GC1008 a scientific priority. However, it also highlights the need for combination approaches to be driven by well-designed pre-clinical studies able to characterize and further elucidate our basic understanding of immunology. By doing so, we can seek to maximize the promise and minimize the pitfalls of combination therapy, the cornerstone and continued hallmark of future cancer treatment.
Conclusions
Overall, we believe that this multistep push-pull approach has promise for successful cancer vaccine immunotherapy. In preclinical studies, proof of principle for each step has been shown in both cancer and viral vaccine models. Individually, epitope enhancement, cytokine and TLR ligand molecular adjuvants, and blockade of negative regulators like TGF-β or IL-13 can enhance vaccine efficacy. Some of these have individually been translated into clinical trials in human cancer, such as the epitope enhanced peptides in the TARP prostate cancer vaccine, which also incorporates a cytokine adjuvant, or the trial of anti-TGF-β as a blocker of negative regulation in melanoma and renal cell cancer patients. Blockers of other checkpoints, such as anti-CTLA-4 have been tested by others 95 and are now licensed drugs. The goal now will be to put these components together in a multipronged combination immunotherapy approach that hopefully will prove synergistic in reliably curing human cancers.
Acknowledgments
Supported by funds of the Intramural Research Program, Center for Cancer Research, National Cancer Institute, NIH, Bethesda, Maryland 20892
Footnotes
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Sakai Y, Morrison BJ, Burke JD, Park JM, Terabe M, Janik JE, et al. Vaccination by Genetically Modified Dendritic Cells Expressing a Truncated neu Oncogene Prevents Development of Breast Cancer in Transgenic Mice. Cancer Research. 2004;64:8022–8028. doi: 10.1158/0008-5472.CAN-03-3442. [DOI] [PubMed] [Google Scholar]
- 3.Park JM, Terabe M, Sakai Y, Munasinghe J, Forni G, Morris JC, et al. Early Role of CD4+ Th1 cells and antibodies in HER-2 adenovirus-vaccine protection against autochthonous mammary carcinomas. J Immunol. 2005;174:4228–4236. doi: 10.4049/jimmunol.174.7.4228. [DOI] [PubMed] [Google Scholar]
- 4.Park JM, Terabe M, Steel JC, Forni G, Sakai Y, Morris JC, et al. Therapy of advanced established murine breast cancer with a recombinant adenoviral ErbB-2/neu vaccine. Cancer Res. 2008;68:1979–1987. doi: 10.1158/0008-5472.CAN-07-5688. [DOI] [PubMed] [Google Scholar]
- 5.Steel JC, Ramlogan CA, Yu P, Sakai Y, Forni G, Waldmann TA, et al. Interleukin-15 and its receptor augment dendritic cell vaccination against the neu oncogene through the induction of antibodies partially independent of CD4 help. Cancer Res. 2010;70:1072–1081. doi: 10.1158/0008-5472.CAN-09-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahlers JD, Belyakov IM, Terabe M, Koka R, Donaldson DD, Thomas E, et al. A push-pull approach to maximize vaccine efficacy: abrogating suppression with an IL-13 inhibitor while augmenting help with GM-CSF and CD40L. Proc Natl Acad Sci U S A. 2002;99:13020–13025. doi: 10.1073/pnas.192251199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berzofsky JA, Ahlers JD, Belyakov IM. Strategies for designing and optimizing new generation vaccines. Nature Reviews Immunology. 2001;1:209–219. doi: 10.1038/35105075. [DOI] [PubMed] [Google Scholar]
- 8.Berzofsky JA, Ahlers J, Janik J, Morris J, Oh S, Terabe M, et al. Progress on new vaccine strategies against chronic viral infections. J Clin Invest. 2004;114:450–462. doi: 10.1172/JCI22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berzofsky JA, Terabe M, Oh S, Belyakov IM, Ahlers JD, Janik JE, et al. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Invest. 2004;113:1515–1525. doi: 10.1172/JCI21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 11.Terabe M, Berzofsky JA. Immunoregulatory T cells in tumor immunity. Current Opinion in Immunology. 2004;16:157–162. doi: 10.1016/j.coi.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Sinha P, Clements VK, Ostrand-Rosenberg S. Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res. 2005;65:11743–11751. doi: 10.1158/0008-5472.CAN-05-0045. [DOI] [PubMed] [Google Scholar]
- 13.Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55:237–245. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res. 2008;14:169–177. doi: 10.1158/1078-0432.CCR-07-1881. [DOI] [PubMed] [Google Scholar]
- 15.Alexander J, Snoke K, Ruppert J, Sidney J, Wall M, Southwood S, et al. Functional consequences of engagement of the T cell receptor by low affinity ligands. JImmunol. 1993;150:1–7. [PubMed] [Google Scholar]
- 16.Alexander J, Sidney J, Southwood S, Ruppert J, Oseroff C, Maewal A, et al. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1994;1:751–761. doi: 10.1016/s1074-7613(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 17.Berzofsky JA. Epitope selection and design of synthetic vaccines: molecular approaches to enhancing immunogenicity and crossreactivity of engineered vaccines. AnnNYAcadSci. 1993;690:256–264. doi: 10.1111/j.1749-6632.1993.tb44014.x. [DOI] [PubMed] [Google Scholar]
- 18.Berzofsky JA. Designing peptide vaccines to broaden recognition and enhance potency. AnnNYAcadSci. 1995;754:161–168. doi: 10.1111/j.1749-6632.1995.tb44449.x. [DOI] [PubMed] [Google Scholar]
- 19.Ahlers JD, Takeshita T, Pendleton CD, Berzofsky JA. Enhanced immunogenicity of HIV-1 vaccine construct by modification of the native peptide sequence. ProcNatlAcadSciUSA. 1997;94:10856–10861. doi: 10.1073/pnas.94.20.10856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarobe P, Pendleton CD, Akatsuka T, Lau D, Engelhard VH, Feinstone SM, et al. Enhanced in vitro potency and in vivo immunogenicity of a CTL epitope from hepatitis C virus core protein following amino acid replacement at secondary HLA-A2.1 binding positions. JClinInvest. 1998;102:1239–1248. doi: 10.1172/JCI3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berzofsky JA, Ahlers JD, Derby MA, Pendleton CD, Arichi T, Belyakov IM. Approaches to improve engineered vaccines for human immunodeficiency virus (HIV) and other viruses that cause chronic infections. ImmunolRev. 1999;170:151–172. doi: 10.1111/j.1600-065x.1999.tb01336.x. [DOI] [PubMed] [Google Scholar]
- 22.Oh S, Terabe M, Pendleton CD, Bhattacharyy A, Bera TK, Epel M, et al. Human CTL to wild type and enhanced epitopes of a novel prostate and breast tumor-associated protein, TARP, lyse human breast cancer cells. Cancer Research. 2004;64:2610–2618. doi: 10.1158/0008-5472.can-03-2183. [DOI] [PubMed] [Google Scholar]
- 23.Okazaki T, Pendleton DC, Lemonnier F, Berzofsky JA. Epitope-enhanced conserved HIV-1 peptide protects HLA-A2-transgenic mice against virus expressing HIV-1 antigen. J Immunol. 2003;171:2548–2555. doi: 10.4049/jimmunol.171.5.2548. [DOI] [PubMed] [Google Scholar]
- 24.Okazaki T, Pendleton CD, Sarobe P, Thomas EK, Harro C, Schwartz D, et al. Epitope-enhancement of a CD4 HIV epitope toward the development of the next generation HIV vaccine. Journal of Immunol. 2006;176:3753–3759. doi: 10.4049/jimmunol.176.6.3753. [DOI] [PubMed] [Google Scholar]
- 25.Ahlers JD, Belyakov IM, Berzofsky JA. Cytokine, chemokine and costimulatory molecule modulation to enhance efficacy of HIV vaccines. Current Molecular Medicine. 2003;3:285–301. doi: 10.2174/1566524033479843. [DOI] [PubMed] [Google Scholar]
- 26.Kawamura H, Rosenberg SA, Berzofsky JA. Immunization with antigen and interleukin-2 in vivo overcomes Ir genetic low responsiveness. JExpMed. 1985;162:381–386. doi: 10.1084/jem.162.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahlers JD, Dunlop N, Alling DW, Nara PL, Berzofsky JA. Cytokine-in-adjuvant steering of the immune response phenotype to HIV-1 vaccine constructs: GM-CSF and TNFa synergize with IL-12 to enhance induction of CTL. JImmunol. 1997;158:3947–3958. [PubMed] [Google Scholar]
- 28.Ahlers JD, Belyakov IM, Matsui S, Berzofsky JA. Mechanisms of cytokine synergy essential for vaccine protection against viral challenge. Int Immunol. 2001;13:897–908. doi: 10.1093/intimm/13.7.897. [DOI] [PubMed] [Google Scholar]
- 29.Ahlers JD, Belyakov IM, Matsui S, Berzofsky JA. Signals delivered through TCR instruct IL-12R expression: IL-12 and TNFα Synergize for IL-12R expression at low antigen dose. Int Immunol. 2001;13:1433–1442. doi: 10.1093/intimm/13.11.1433. [DOI] [PubMed] [Google Scholar]
- 30.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high or low avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. ProcNatlAcadSciUSA. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cawthon AG, Lu H, Alexander-Miller MA. Peptide requirement for CTL activation reflects the sensitivity to CD3 engagement: correlation with CD8alphabeta versus CD8alphaalpha expression. J Immunol. 2001;167:2577–2584. doi: 10.4049/jimmunol.167.5.2577. [DOI] [PubMed] [Google Scholar]
- 32.Cawthon AG, Alexander-Miller MA. Optimal colocalization of TCR and CD8 as a novel mechanism for the control of functional avidity. J Immunol. 2002;169:3492–3498. doi: 10.4049/jimmunol.169.7.3492. [DOI] [PubMed] [Google Scholar]
- 33.Gallimore A, Dumrese T, Hengartner H, Zinkernagel RM, Rammensee HG. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J Exp Med. 1998;187:1647–1657. doi: 10.1084/jem.187.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yee C, Savage PA, Lee PP, Davis MM, Greenberg PD. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. JImmunol. 1999;162:2227–2234. [PubMed] [Google Scholar]
- 35.Zeh HJ, III, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. JImmunol. 1999;162:989–994. [PubMed] [Google Scholar]
- 36.Derby MA, Alexander-Miller MA, Tse R, Berzofsky JA. High avidity CTL exploit two complementary mechanisms to provide better protection against viral infection than low avidity CTL. J Immunol. 2001;166:1690–1697. doi: 10.4049/jimmunol.166.3.1690. [DOI] [PubMed] [Google Scholar]
- 37.Hodge JW, Sabzevari H, Yafal AG, Gritz L, Lorenz MGO, Schlom J. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59:5800–5807. [PubMed] [Google Scholar]
- 38.Oh S, Hodge JW, Ahlers JD, Burke DS, Schlom J, Berzofsky JA. Selective induction of high avidity CTL by altering the balance of signals from antigen presenting cells. J Immunol. 2003;170:2523–2530. doi: 10.4049/jimmunol.170.5.2523. [DOI] [PubMed] [Google Scholar]
- 39.Hodge JW, Chakraborty M, Kudo-Saito C, Garnett CT, Schlom J. Multiple costimulatory modalities enhance CTL avidity. J Immunol. 2005;174:5994–6004. doi: 10.4049/jimmunol.174.10.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15R alpha-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:15154–15159. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 42.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 43.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 Controls both Proliferation and Survival of a Subset of Memory-Phenotype CD8(+) T Cells. J Exp Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis MM. Molecular genetics of the T cell-receptor beta chain. AnnuRevImmunol. 1985;3:537–560. doi: 10.1146/annurev.iy.03.040185.002541. [DOI] [PubMed] [Google Scholar]
- 46.Oh S, Berzofsky JA, Burke DS, Waldmann TA, Perera LP. Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc Natl Acad Sci U S A. 2003;100:3392–3397. doi: 10.1073/pnas.0630592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaech SM, Ahmed R. IMMUNOLOGY: CD8 T Cells Remember with a Little Help. Science. 2003;300:263–265. doi: 10.1126/science.1084511. [DOI] [PubMed] [Google Scholar]
- 48.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 49.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8(+) T cells during homeostatic proliferation requires CD4(+) T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 50.Oh S, Perera LP, Terabe M, Ni L, Waldmann TA, Berzofsky JA. IL-15 as a mediator of CD4+ help for CD8+ T cell longevity and avoidance of TRAIL-mediated apoptosis. Proc Natl Acad Sci U S A. 2008;105:5201–5206. doi: 10.1073/pnas.0801003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 52.Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol Rev. 2004;199:227–250. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 53.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu Q, Egelston C, Vivekanandhan A, Uematsu S, Akira S, Klinman DM, et al. Toll-like receptor ligands synergize through distinct dendritic cell pathways to induce T cell responses: Implications for vaccines. PNAS. 2008;105:16260–16265. doi: 10.1073/pnas.0805325105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu Q, Egelston C, Gagnon S, Sui Y, Belyakov IM, Klinman DM, et al. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. J Clin Invest. 2010;120:607–616. doi: 10.1172/JCI39293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sui Y, Zhu Q, Gagnon S, Dzutsev A, Terabe M, Vaccari M, et al. Innate and adaptive immune correlates of vaccine and adjuvant-induced control of mucosal transmission of SIV in macaques. Proc Natl Acad Sci U S A. 2010;107:9843–9848. doi: 10.1073/pnas.0911932107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teicher BA. Transforming growth factor-beta and the immune response to malignant disease. Clin Cancer Res. 2007;13:6247–6251. doi: 10.1158/1078-0432.CCR-07-1654. [DOI] [PubMed] [Google Scholar]
- 61.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Terabe M, Matsui S, Park J-M, Mamura M, Noben-Trauth N, Donaldson DD, et al. Transforming Growth Factor-β production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block Cytotoxic T Lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fichtner-Feigl S, Terabe M, Kitani A, Young CA, Fuss I, Geissler EK, et al. Restoration of tumor immunosurveillance via targeting of interleukin-13 receptor-alpha 2. Cancer Res. 2008;68:3467–3475. doi: 10.1158/0008-5472.CAN-07-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nam JS, Terabe M, Kang MJ, Chae H, Voong N, Yang YA, et al. Transforming Growth Factor {beta} Subverts the Immune System into Directly Promoting Tumor Growth through Interleukin-17. Cancer Res. 2008;68:3915–3923. doi: 10.1158/0008-5472.CAN-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nam JS, Terabe M, Mamura M, Kang MJ, Chae H, Stuelten C, et al. An Anti-Transforming Growth Factor {beta} Antibody Suppresses Metastasis via Cooperative Effects on Multiple Cell Compartments. Cancer Res. 2008;68:3835–3843. doi: 10.1158/0008-5472.CAN-08-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Terabe M, Ambrosino E, Takaku S, O’Konek JJ, Venzon D, Lonning S, et al. Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-beta monoclonal antibody. Clin Cancer Res. 2009;15:6560–6569. doi: 10.1158/1078-0432.CCR-09-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takaku S, Terabe M, Ambrosino E, Peng J, Lonning S, McPherson JM, et al. Blockade of TGF-beta enhances tumor vaccine efficacy mediated by CD8(+) T cells. Int J Cancer 2009. 2010;126:1666–1674. doi: 10.1002/ijc.24961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ueda R, Fujita M, Zhu X, Sasaki K, Kastenhuber ER, Kohanbash G, et al. Systemic inhibition of transforming growth factor-beta in glioma-bearing mice improves the therapeutic efficacy of glioma-associated antigen peptide vaccines. Clin Cancer Res. 2009;15:6551–6559. doi: 10.1158/1078-0432.CCR-09-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167:6471–6479. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 70.Kim S, Buchlis G, Fridlender ZG, Sun J, Kapoor V, Cheng G, et al. Systemic blockade of transforming growth factor-beta signaling augments the efficacy of immunogene therapy. Cancer Res. 2008;68:10247–10256. doi: 10.1158/0008-5472.CAN-08-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matsui S, Ahlers JD, Vortmeyer AO, Terabe M, Tsukui T, Carbone DP, et al. A model for CD8+ CTL tumor immunosurveillance and regulation of tumor escape by CD4 T cells through an effect on quality of CTL. JImmunol. 1999;163:184–193. [PubMed] [Google Scholar]
- 72.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nature Immunology. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 73.Park JM, Terabe M, van den Broeke LT, Donaldson DD, Berzofsky JA. Unmasking immunosurveillance against a syngeneic colon cancer by elimination of CD4+ NKT regulatory cells and IL-13. International J of Cancer. 2004;114:80–87. doi: 10.1002/ijc.20669. [DOI] [PubMed] [Google Scholar]
- 74.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 75.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 76.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 78.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 79.Higano CS, Small EJ, Schellhammer P, Yasothan U, Gubernick S, Kirkpatrick P, et al. Sipuleucel-T. Nat Rev Drug Discov. 2010;9:513–514. doi: 10.1038/nrd3220. [DOI] [PubMed] [Google Scholar]
- 80.Hoos A, Parmiani G, Hege K, Sznol M, Loibner H, Eggermont A, et al. A clinical development paradigm for cancer vaccines and related biologics. J Immunother. 2007;30:1–15. doi: 10.1097/01.cji.0000211341.88835.ae. [DOI] [PubMed] [Google Scholar]
- 81.Essand M, Vasmatzis G, Brinkmann U, Duray P, Lee B, Pastan I. High expression of a specific T-cell receptor gamma transcript in epithelial cells of the prostate. Proc Natl Acad Sci U S A. 1999;96:9287–9292. doi: 10.1073/pnas.96.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wolfgang CD, Essand M, Vincent JJ, Lee B, Pastan I. TARP: a nuclear protein expressed in prostate and breast cancer cells derived from an alternate reading frame of the T cell receptor gamma chain locus. Proc Natl Acad Sci U S A. 2000;97:9437–9442. doi: 10.1073/pnas.160270597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolfgang CD, Essand M, Lee B, Pastan I. T-cell receptor gamma chain alternate reading frame protein (TARP) expression in prostate cancer cells leads to an increased growth rate and induction of caveolins and amphiregulin. Cancer Res. 2001;61:8122–8126. [PubMed] [Google Scholar]
- 84.Arlen PM, Bianco F, Dahut WL, D’Amico A, Figg WD, Freedland SJ, et al. Prostate Specific Antigen Working Group guidelines on prostate specific antigen doubling time. J Urol. 2008;179:2181–2185. doi: 10.1016/j.juro.2008.01.099. discussion 2185–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 86.Morris JC, Shapiro GI, Tan AR, Lawrence DP, Olencki TE, Dezube BJ, et al. Phase I/II Study of GC1008: A human anti-transforming growth factor-beta (TGFb) monoclonal antibody (MAb) in patients with advanced malignant melanoma (MM) or renal cell carcinoma (RCC) J Clin Oncol. 2008;26(Suppl):9028. doi: 10.1371/journal.pone.0090353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hau P, Bogdahn U, Mahapatra AK, et al. Results of a phase IIb active-controlled study with AP120009 for patients with recurrent or refractory anaplastic astrocytoma. J Clin Oncol. 2007;25(Suppl):12521. [Google Scholar]
- 88.Bueno L, de Alwis DP, Pitou C, Yingling J, Lahn M, Glatt S, et al. Semi-mechanistic modelling of the tumour growth inhibitory effects of LY2157299, a new type I receptor TGF-beta kinase antagonist, in mice. Eur J Cancer. 2008;44:142–150. doi: 10.1016/j.ejca.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 89.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 90.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 91.Bogdahn U, Hau P, Stockhammer G, Venkataramana NK, Mahapatra AK, Suri A, et al. Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol. 2011;13:132–142. doi: 10.1093/neuonc/noq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Karaa A, Khachemoune A. Keratoacanthoma: a tumor in search of a classification. Int J Dermatol. 2007;46:671–678. doi: 10.1111/j.1365-4632.2007.03260.x. [DOI] [PubMed] [Google Scholar]
- 93.Guasch G, Schober M, Pasolli HA, Conn EB, Polak L, Fuchs E. Loss of TGFbeta signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell. 2007;12:313–327. doi: 10.1016/j.ccr.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goudie DR, D’Alessandro M, Merriman B, Lee H, Szeverenyi I, Avery S, et al. Multiple self-healing squamous epithelioma is caused by a disease-specific spectrum of mutations in TGFBR1. Nat Genet. 2011;43:365–369. doi: 10.1038/ng.780. [DOI] [PubMed] [Google Scholar]
- 95.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sui Y, Berzofsky JA. Vaccine strategies to prevent mucosal transmission of HIV-1. In: Hefferon KL, editor. Novel Approaches to Vaccine Research. Research Signpost; 2011. pp. 137–180. [Google Scholar]