Abstract
The epithelial-mesenchymal transition (EMT) is thought to be a critical step along the metastasis of carcinomas. In addition to gaining motility and invasiveness, tumor cells that undergo EMT also acquire increased resistance to many traditional cancer treatment modalities, including chemotherapy and radiation. As such, EMT has become an attractive, potentially targetable process for therapeutic interventions against tumor metastasis. The process of EMT is driven by a group of transcription factors designated as EMT transcription factors, such as Snail, Slug, Twist, and the recently identified T-box family member Brachyury. In an attempt to determine which of these drivers of EMT is more amenable to targeted therapies and, in particular, T-cell–mediated immunotherapeutic approaches, we have examined their relative expression levels in a range of human and murine normal tissues, cancer cell lines, and human tumor biopsies. Our results demonstrated that Brachyury is a molecule with a highly restricted human tumor expression pattern. We also demonstrated that Brachyury is immunogenic and that Brachyury-specific CD8+ T cells expanded in vitro are able to lyse Brachyury-positive tumor cells. We thus propose Brachyury as an attractive target for vaccination strategies designed to specifically target tumor cells undergoing EMT.
Introduction
The phenotypic switch of carcinomas designated as the epithelial-mesenchymal transition (EMT) has been recently proposed as a critical process during the progression of solid tumors.1,2 During an EMT, carcinoma cells lose characteristics associated with the epithelial phenotype, including cellular polarity, cell-to-cell contacts, and the expression of epithelial markers, such as E-cadherin and cytokeratins, and gain a fibroblast-like morphology and the expression of the mesenchymal-associated proteins Fibronectin and Vimentin, and various matrix metalloproteinases (MMPs). As a consequence of this phenotypic switch, tumor cells acquire the ability to move and to invade the surrounding tissues, properties that are essential for their dissemination and the formation of metastases at distant sites from the primary tumor mass.3–6 Additionally, tumor EMT has recently been associated with the acquisition of cancer stem cell-like features, or tumor stemness, which include the acquisition of resistance to the conventional therapeutics chemotherapy and radiation, and some small-molecule targeted therapies.7–9 With the identification and characterization of signaling pathways and transcriptional regulators that play a fundamental role in this phenotypic switch, it can be envisioned that novel strategies could be designed for the targeting of populations of tumor cells that exhibit metastatic propensity, self-renewing capacity, and therapeutic resistance.
Transcriptional Control of EMT
Multiple studies pertaining to the control of tumor EMT have been focused on the characterization of a group of transcriptional regulators, designated as EMT transcription factors, which are molecules normally involved in the control of epithelial-mesenchymal transitions in the early embryo. During the course of tumor EMT, the levels of these transcriptional regulators are enhanced and, as a consequence, the expression of genes regulated by them is either repressed (epithelial markers) or enhanced (mesenchymal markers). Examples of EMT transcription factors include the zinc finger proteins Snail10,11 and Slug,12,13 and the helix-loop-helix (HLH) transcription factor Twist.14,15 Recently, our group identified Brachyury, a T-box transcription factor, as a novel driver of EMT in human epithelial carcinomas. Brachyury was initially identified as a gene predominantly expressed in human tumors vs. normal tissues by utilizing a computer-based differential display tool for analysis of expressed sequence tags (ESTs) in the human Unigene database.16 In a subsequent study17 we demonstrated that overexpression of Brachyury in epithelial carcinoma cell lines induces changes characteristic of an EMT, including increased tumor cell motility and invasiveness. Conversely, we have also shown that inhibiting Brachyury expression in tumor cells having a more mesenchymal phenotype resulted in changes characteristic of a mesenchymal-to-epithelial transition (MET), with loss of expression of mesenchymal markers, gain of epithelial markers, and concurrent loss of tumor cell motility and invasiveness. The relevance of Brachyury expression in vivo has been demonstrated in a xenograft model with the H460 human lung cancer cell line that showed a diminished ability to form lung metastasis as a result of Brachyury-silencing.17
The important role of some EMT transcription factors has been acknowledged in human cancer progression. For example, in several studies, expression of Snail has been shown to correlate with tumor histological grade and lymph node status.11 Moreover, it has been demonstrated that increased Snail expression in breast and ovarian cancers correlates with a significant decrease in patient survival.18 Similarly, the expression of Slug has been shown to correlate with disease progression; in lung, colon, and ovarian cancer, for example, low Slug expression in the primary tumor has been correlated with decreased disease recurrence and increased overall survival.13,19 High levels of Twist expression have also been associated with highly invasive breast cancer,14 poor prognosis in cervical cancer,20 and high Gleason score prostate cancer.15 The T-box transcription Brachyury has been shown to be predominantly expressed among lung tumor tissue samples of higher stage (II–IV) as compared to stage I tumors or normal lung tissues,17 and recently it has been identified as a poor prognostic factor in colon cancer.21 Taken together, these results demonstrate the importance of these transcriptional regulators of EMT in the progression of human cancer and suggest they may offer unique opportunities as targets for future cancer treatment modalities.
Targeting of EMT transcription factors
Overall, the study of the phenomenon of tumor EMT is an extremely active area of cancer research and yet, little has been done so far concerning the exploitation of this process for therapeutic purposes. We might speculate on two major reasons accounting for the scarcity of studies aimed at targeting EMT: (a) initiation and maintenance of EMT in various tumor types may depend on the cooperation of multiple, redundant signaling pathways that might require simultaneous blockade to successfully revert the tumor phenotype into a fully epithelial one; and (b) targeting of EMT transcription factors appears largely difficult, as transcription factors are currently regarded as “non druggable” molecules with canonical small molecule inhibitor modalities. We thus propose the use of immunotherapeutic modalities to specifically target the EMT transcription factors as a means to eliminate mesenchymal-like tumor cell populations. Because of their intracellular localization, EMT transcription factors cannot be reached by targeted modalities that depend on the expression of the targeted molecule on the surface of tumor cells, such as monoclonal antibodies. Unlike antibodies, T cells recognize a target irrespective of its cellular localization in the form of short peptides presented in the context of the major histocompatibility complex (MHC). T-cell–based immunotherapeutic approaches thus appear to be a viable option to specifically target tumor cells that express EMT transcription factors.
Immunotherapeutic interventions against cancer are expected to elicit a long-term memory T-cell response against the targeted antigen; in order to avoid potential autoimmune reactions, the tissue distribution of the target is an important consideration. In the present work, we sought to investigate the tissue distribution of the EMT transcription factors Brachyury, Snail, Slug, and Twist in multiple human and murine normal tissues and tumor cell lines originated from various types of carcinomas. Our results demonstrate a diverse pattern of expression of EMT transcription factors across normal tissues, both human and murine, as well as a diverse pattern of expression in human versus murine carcinoma cell lines.
Expression of transcriptional regulators of EMT in human normal tissues and tumor cell lines
Multiple studies have demonstrated the ability of transcription factors such as Brachyury, Snail, Slug and Twist to modulate the epithelial/mesenchymal characteristics of human carcinoma cell lines. Most of these studies have been conducted either in vitro or using xenograft models in immune deficient mice.22,23 In addition to this newly proposed role in promoting tumor EMT, tumor invasiveness and metastasis, each of the EMT transcription factors Brachyury, Snail, Slug, and Twist plays anessential non-redundant ro le during embryogenesis. Although these genes primarily function in utero, it is well appreciated that their expression could also be maintained in some adult normal tissues. To our knowledge, no reports exist so far on a comprehensive analysis of the expression patterns of these genes in multiple human adult normal tissues. We believe such knowledge is critical to allow the development of therapeutics targeting these mediators of EMT and to appreciate the potential toxicities such treatments may generate.
We examined the relative mRNA expression levels of Brachyury, Snail, Slug, and Twist in panels of commercially available cDNAs generated from histologically normal human adult tissues, by real-time PCR as previously described.17 As shown in Figure 1A, Brachyury has a very restrictive pattern of expression among human normal tissues, being undetectable in most tissues examined. The highest level of Brachyury mRNA expression was detected in normal testis, followed by lower levels in spleen and small intestine (3- and 4-fold lower, respectively, than the testis). Unlike Brachyury, mRNA encoding for the transcription factors Snail, Slug, and Twist is detected in almost all of the human normal tissues evaluated here. The median expression for Snail, Slug, and Twist among human normal tissues was approximately 103- 104-fold higher than the median expression for Brachyury across the same tissue panels (Figure 1B–D). These data are in agreement with previous reports demonstrating detectable levels of mRNA encoding for Snail, Slug and Twist in many adult normal tissues.24–26 For example, the expression of human Snail was previously demonstrated in adult heart and lung and, at lower levels, in adult brain, liver and skeletal muscle.25 Similarly, the expression of Twist has been previously reported in mesodermally derived human adult normal tissues, including the heart and skeletal muscle.26 Altogether, these observations seem difficult to reconcile with studies showing positive correlations between Snail, Slug, and Twist expression and human tumor progression. In this regard, it is important to point out that the high expression of Snail, Slug, and Twist mRNA observed in normal tissues may be due to their selective expression in a particular subset of cells, and their pattern of expression may be best examined by further assessing protein expression by immunohistochemistry analysis. Moreover, the expression of mRNA could not necessarily correspond to that of protein expression, as multiple microRNAs are now being identified that could potentially inhibit protein translation.27,28
Figure 1. Relative mRNA expression of four EMT mediators in human normal tissues and carcinoma cell lines.
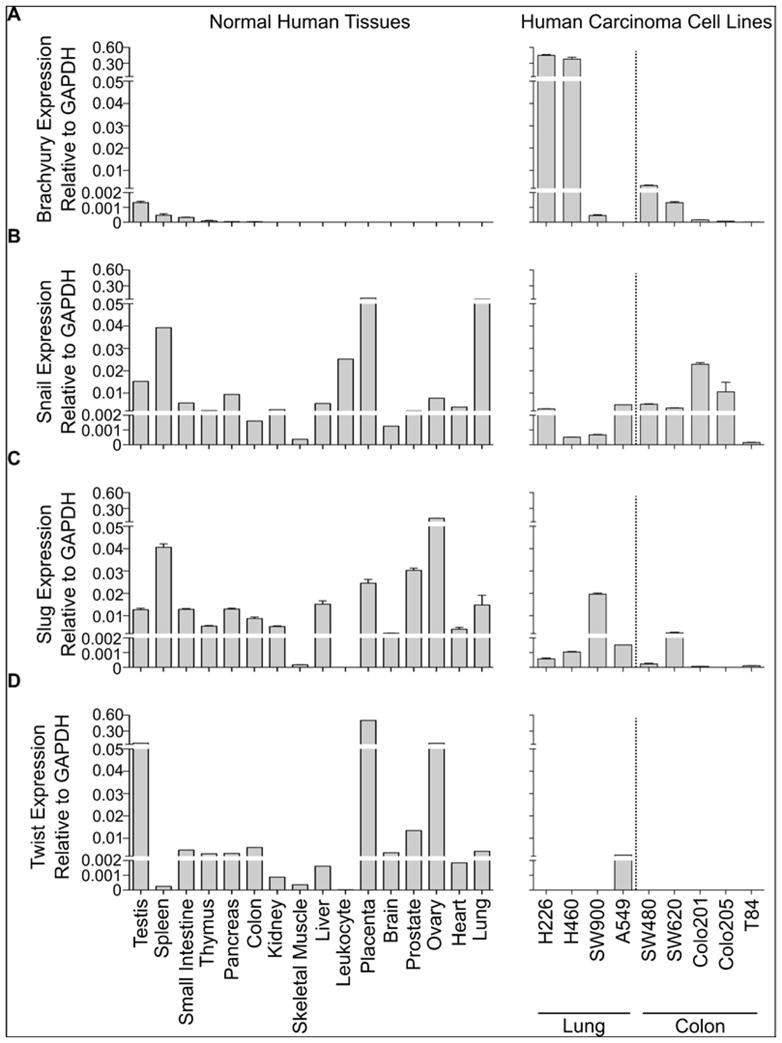
Real-time PCR was performed for Brachyury (A), Snail (B), Slug (C), and Twist (D) in a multi-tissue, commercially available cDNA panel library (Clontech), and several human lung and colon carcinoma cell lines as previously described.17 All values are expressed as a ratio to the endogenous control GAPDH.
In addition to evaluating the expression of Brachyury, Snail, Slug, and Twist mRNA across multiple human normal tissues, we also examined their level of expression in a number of human lung and colon cancer cell lines. We observed high Brachyury levels in the H226 and H460 lung cancer cell lines and relatively high levels in the SW480 and SW620 colon cancer cell lines, as compared to the expression observed in normal human tissues (Figure 1A). Although Snail and Slug mRNA were detected in almost all tumor cell lines analyzed, the levels of expression were not enhanced when compared to the median expression across normal tissues (Figures 1B–C). Most human cancer cell lines analyzed here resulted negative for Twist mRNA expression (Figure 1D). Hence, regarding the restricted expression in normal tissues, Brachyury appears to bean appealing target among the EMT transcription factors evaluated here.
Expression of transcriptional regulators of EMT in murine normal tissues and tumor cell lines
Although the majority of studies involving EMT have been performed with human cancer cell lines, EMT has also been shown to play an important role in tumor progression in syngeneic murine tumor models. The 4T1 breast cancer model for example has been used to demonstrate the importance of Twist in the ability of breast tumor cells to metastasize to the lungs.14 In the TRAMP model of spontaneous prostate cancer,29 Snail expression has been shown to correlate with loss of E-cadherin expression and increase in tumor stage.30 In a doxycycline-inducible HER2/neu model of breast cancer,31 it has been shown that Snail mediates an EMT and it is upregulated in recurrent tumorsas compared to primary tumors. 32
It is clear that additional murine models of EMT must be established to help in assessing the feasibility and effectiveness of targeting the EMT process as a strategy to reduce the metastatic spreading and resistant phenotype of tumor cells. In murine studies similar to our studies in human tissues and tumor cell lines, we examined the expression of Brachyury, Snail, and Twist in numerous normal murine tissues and tumor cell lines. As with human normal tissues, Brachyury mRNA was undetectable or expressed at very low levels in most normal murine tissues analyzed (Figure 2A). However, unlike with human carcinomas, the majority of mouse tumor cell lines were also negative for the expression of Brachyury. The exceptions were the embryonal teratocarcinoma cell lines NF-1 and P19, which showed significant expression of Brachyury, an observation consistent with Brachyury being a gene normally expressed along embryonic development.33 Similar to our results with human tissues and cell lines, Snail mRNA was detected at comparable levels across all murine normal tissues and tumor cell lines assayed. Twist mRNA was expressed at relatively low levels in many normal murine tissues, and we observed an increased expression in most murine tumor lines analyzed, up to 60-fold, compared to the median expression across all murine normal tissues. Twist expression was predominant in the murine TRAMP prostate cancer cell lines.29,34 The lack of endogenous Brachyury expression in the majority of murine cancer cell lines assayed here suggests that it may not be an appropriate target for pursuing murine preclinical studies. Instead, Twist is differentially expressed between murine tumor cell lines and normal tissues and may be employed as a model target antigen in preclinical murine studies aimed at evaluating the utility of targeted modalities against EMT.
Figure 2. Relative mRNA expression of three EMT mediators in murine normal tissues and tumor cell lines.
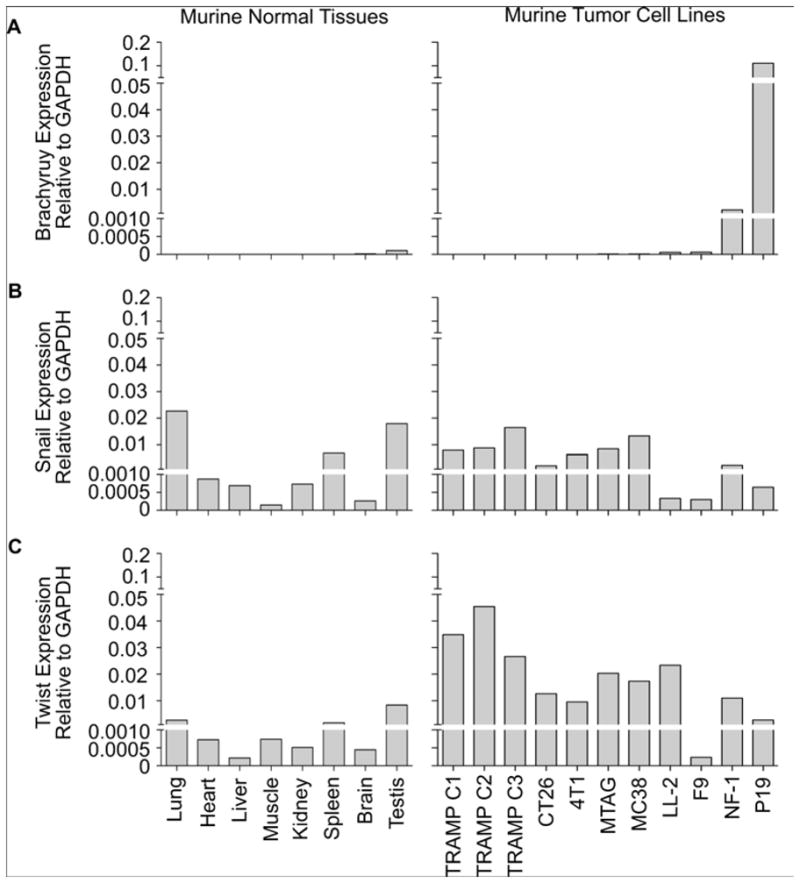
Real-time PCR was performed for Brachyury (A), Snail (B), and Twist (C) in a panel of normal murine tissue cDNAs (Clontech) and several murine tumor cell lines. The following TaqMan probes (AB Biosystems) were used: Brachyury (Mm01318249_m1), Snail (Mm00441533_g1), Twist (Mm00442036_m1). All values are expressed as a ratio to the endogenous control GAPDH as previously reported.17
Selective expression of Brachyury in human tumor vs. normal tissues
We have demonstrated increased Brachyury expression in human carcinoma cell lines as compared to normal tissues. To ascertain the clinical relevance of Brachyury as a potential target, we measured the Brachyury mRNA expression in tumor biopsies from 80 lung cancer patients and histologically normal tissue adjacent to tumors from 16 individuals. Forty-two of80 (52.5%) lung tumor tissues and only 2/16 (12.5%) normal adjacent lung tissues had detectable Brachyury mRNA levels (Figure 3). In a previous report, we demonstrated a positive correlation between Brachyury expression and lung tumor stage; Brachyury mRNA was expressed in more lung tumor tissue samples from stages II, III, and IV (30 of 48 [62.5%]) than samples from stage I disease (12 of 32 [37.5%]).17 These observations suggested that Brachyury potentially associates with lung tumor progression, making it an interesting target for the treatement of lung cancer, particularly in the more aggressive stages of disease. As can be seen in Figure 3, the expression of Brachyury mRNA in lung tumor tissues varied from undetectable to levels that approximately spanned a 4-log range. Interestingly, when comparing the levels of expression in the lung clinical samples and the various tumor cell lines of lung origin used for experimental preclinical studies, a comparable range of Brachyury expression can be observed (Palena, unpublished observations). These results strongly suggest that available human tumor cell line models reflect the heterogeneity seen in patients and may be useful in developing strategies attempting to target the EMT process.
Figure 3. Relative mRNA expression of Brachyury in lung tumor biopsies, normal adjacent tissues and several human lung and colon carcinoma cell lines.
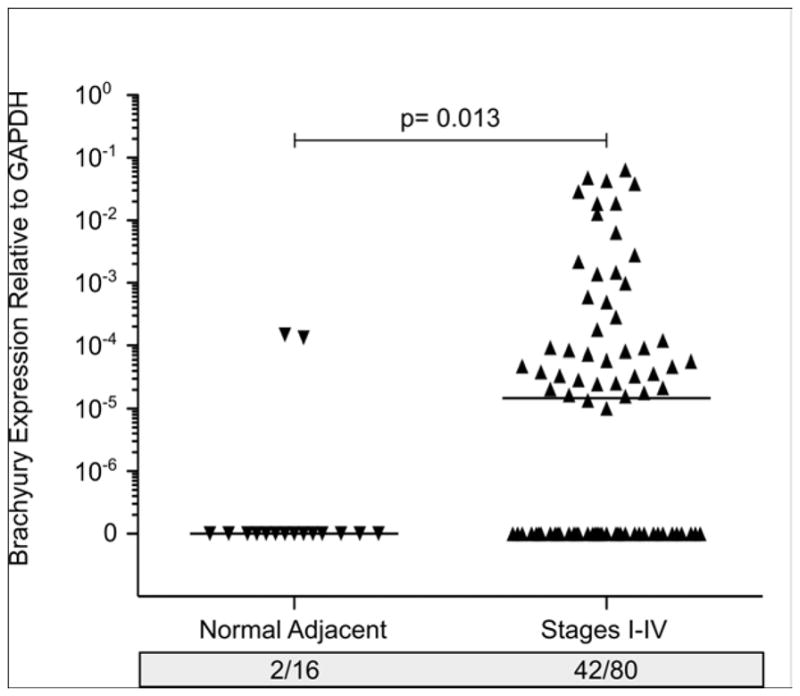
Relative Brachyury mRNA expression in a commercial panel of cDNAs (Origene TissueScan qPCR Arrays) generated from 80 lung tumors at various stages of disease (I–IV), along with 16 histologically normal lung tissues adjacent to the tumor. All values and the median for each group are expressed as a ratio to the endogenous control GAPDH.
EMT and cancer stem cells
In addition to promoting tumor spreading and metastasis, it has been proposed that EMT may also confer ontumor cells a cancer stem cell-like phenotype. 8 Cancer stem cells (CSCs) are a relatively new concept in tumor biology; theyare thought to represent a small fraction of tumor cells with the capacity for self-renewal, and whose progeny comprise the bulk of the tumor mass.35 In addition to their self-renewal capacity, CSCs are also more resistant to traditional cancer treatments and several studies have recently demonstrated that conventional treatments would select for tumor cells with a CSC phenotype.36–39 CSCs are commonly identified based upon their expression of a few molecules designated as “stem cell markers” (Table 1). In breast and prostate cancer, for example, cell populations expressing CD44high/CD24low appear to be enriched for CSCs.40 The surface glycoprotein CD133 in lung, pancreas, prostate, and colon cancer,41–44 or enhanced Aldehyde dehydrogenase 1 (ALDH1) activity in breast, lung, and colon cancer45–47 have also been used as markers for CSCs. In addition, the transcription factors Nanog, Oct4, and Sox2 have been used for identification of CSCs in prostate, breast, colon, pancreas, and lung cancer.48–50 Altogether, it can be said that currently available phenotypic markers of CSC are tumor type-dependent, and in many cases the CSC phenotype should be confirmed utilizing functional assays such as tumorosphere formation efficiency. Moreover, from the point of view of designing specific therapies to target CSCs in human cancer, the expression of these markers has also been demonstrated in normal adult tissues, normal stem and/or progenitor cells, 51–56 a fact that would preclude their use as potential therapeutic targets.
Table 1.
Stem Cell Markers Identified in Human Tumors and/or Tumor Cell Lines
As mentioned above, recent reports support the notion that cells that have undergone an EMT share many characteristics with CSCs, including an increased capacity for self-renewal, and increased resistance to chemotherapy.57–59 Although further studies are necessary, we hypothesize that, in the absence of definitive markers for CSCs, the identification of tumor cells that have undergone an EMT in human tumor tissue samples may be useful in selecting patients whose tumors may be enriched for populations with stem cell-like features. The restrictive pattern of Brachyury expression in normal tissues and its known association with tumor EMT may allow it to act not only as a surrogate marker of tumor cells with stem-cell like features but also, more importantly, to utilize it as an effective target for eliminating stem-cell–like cancer cells.
Brachyury as a target for cancer vaccine approaches
To function as a viable target for a cancer vaccine a tumor-associated antigen (TAA) must (a) have a very restricted expression profile in normal adult tissues, (b) have an increased expression in tumor cells, and (c) be able to induce an immune response. Unlike Snail, Slug, and Twist, we have demonstrated that Brachyury expression is very limited in normal human tissues as assessed by real-time PCR16,17 and confirmed by immunohistochemical detection of Brachyury protein (Palena, unpublished observations). We were also able to demonstrate a significant increase in the expression of Brachyury in human carcinoma cell lines and human tumor tissues (Figures 1, 3, and previous observations).16,17 Previously we have been able to demonstrate that Brachyury is immunogenic in humans, as one can expand Brachyury-specific CD8+ cytotoxic T cells capable of killing Brachyury-expressing tumor cells in an HLA-restricted manner (Figure 4)16. It is important to note that, in the H441 lung cancer cell line shown in Figure 4, Brachyury expression is slightly lower than the median expression seen among lung tumor tissue samples (Figure 3), suggesting that a T-cell response directed against Brachyury will be able to target tumor cells perhaps initiating the process of Brachyury-mediated EMT and therefore exhibiting low levels of expression of the target protein.
Figure 4. Lysis of Brachyury positive tumor cells by Brachyury-specific T cells.
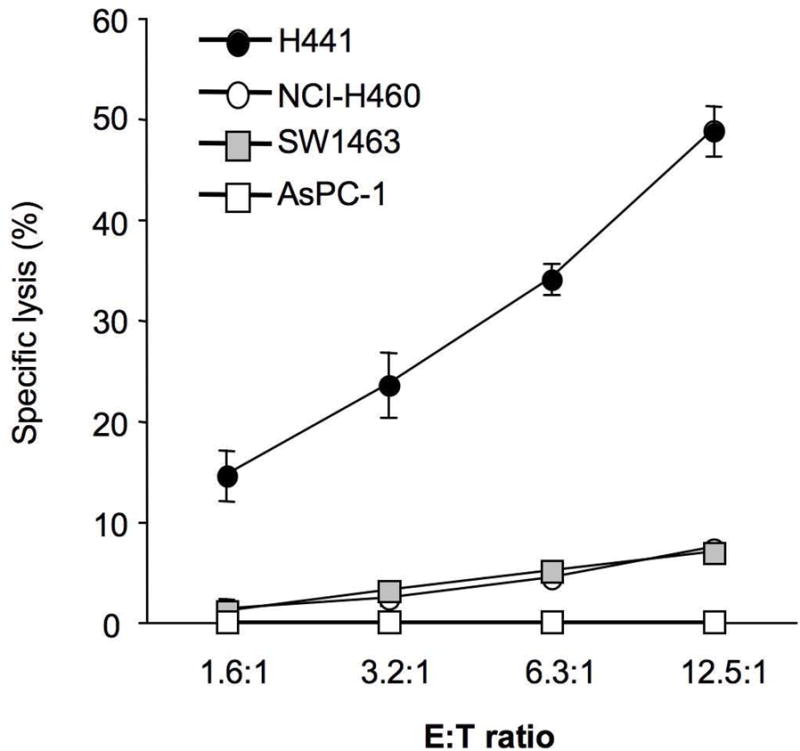
Brachyury-specific human T cells were expanded from the blood of normal donors and utilized in vitro for lysis of H441 (HLA-A2+/Brachyury+), SW1463 (HLA-A2+/Brachyury−), NCI-H460 (HLA-A2− Brachyury+) or AsPC-1 (HLA-A2−/Brachyury−) carcinoma cell lines. (Reprinted with permission from the American Association for Cancer Research; see reference 16)
Conclusions
The process by which epithelial carcinoma cells acquire a mesenchymal phenotype (EMT) is being characterized as a critical step in tumor metastasis, as a promoter of tumor dissemination and acquisition of resistance to chemotherapy, radiation, and some small molecule inhibitors. As such, targeting of EMT represents a potentially novel approach not only in treating metastatic disease, but also in alleviating therapeutic resistance. However, targeting of master drivers of the EMT process, i.e., transcription factors, is still seen as a very difficult approach. The use of vaccine-based strategies in whicha specific T-cell immune response can be elicited against a driver of EMT appears as one of a few viable options for the elimination of mesenchymal-like tumor cells. Promising results with the use of vaccines for the therapy of solid tumors, including the recently approved Sipuleucel-T60 as well as PSA-TRICOM (Prostvac)61 for the treatment of prostate cancer, support the development of an immunotherapeutic approach against EMT. As we have discussed here, the tumor-restricted pattern of expression of the transcription factor Brachyury and its proven immunogenicity make it an appealing protein for a cancer vaccine strategy targeting EMT. Such treatments will presumably have low toxicities and one can envision their future use for therapeutic purposes in the neoadjuvant or adjuvant settings and, in the long term, perhaps in the pre-neoplastic and/or preventive settings.
Acknowledgments
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH
The authors thank Dr. Jeffrey Schlom for helpful discussions on this manuscript, Margie Duberstein for technical assistance, and Debra Weingarten for editorial assistance.
Footnotes
Financial disclosures: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 2.Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23(10):912–23. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- 3.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 5.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68(10):3645–54. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 6.Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39(3):305–18. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- 7.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–73. doi: 10.1038/nrc2620. Epub 2009 Mar 5. [DOI] [PubMed] [Google Scholar]
- 9.Thomson S, Buck E, Petti F, Griffin G, Brown E, Ramnarine N, Iwata KK, Gibson N, Haley JD. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 2005;65(20):9455–62. doi: 10.1158/0008-5472.CAN-05-1058. [DOI] [PubMed] [Google Scholar]
- 10.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 11.Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, Nieto MA. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21(20):3241–6. doi: 10.1038/sj.onc.1205416. [DOI] [PubMed] [Google Scholar]
- 12.Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116(Pt 3):499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- 13.Shih JY, Tsai MF, Chang TH, Chang YL, Yuan A, Yu CJ, Lin SB, Liou GY, Lee ML, Chen JJ, et al. Transcription repressor slug promotes carcinoma invasion and predicts outcome of patients with lung adenocarcinoma. Clin Cancer Res. 2005;11(22):8070–8. doi: 10.1158/1078-0432.CCR-05-0687. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C, Zhang X, Chua CW, Chan KW, Chan FL, Glackin C, et al. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res. 2005;65(12):5153–62. doi: 10.1158/0008-5472.CAN-04-3785. [DOI] [PubMed] [Google Scholar]
- 16.Palena C, Polev DE, Tsang KY, Fernando RI, Litzinger M, Krukovskaya LL, Baranova AV, Kozlov AP, Schlom J. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin Cancer Res. 2007;13(8):2471–8. doi: 10.1158/1078-0432.CCR-06-2353. [DOI] [PubMed] [Google Scholar]
- 17.Fernando RI, Litzinger M, Trono P, Hamilton DH, Schlom J, Palena C. The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J Clin Invest. 2010;120(2):533–44. doi: 10.1172/JCI38379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elloul S, Elstrand MB, Nesland JM, Trope CG, Kvalheim G, Goldberg I, Reich R, Davidson B. Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer. 2005;103(8):1631–43. doi: 10.1002/cncr.20946. [DOI] [PubMed] [Google Scholar]
- 19.Shioiri M, Shida T, Koda K, Oda K, Seike K, Nishimura M, Takano S, Miyazaki M. Slug expression is an independent prognostic parameter for poor survival in colorectal carcinoma patients. Br J Cancer. 2006;94(12):1816–22. doi: 10.1038/sj.bjc.6603193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibata K, Kajiyama H, Ino K, Terauchi M, Yamamoto E, Nawa A, Nomura S, Kikkawa F. Twist expression in patients with cervical cancer is associated with poor disease outcome. Ann Oncol. 2008;19(1):81–5. doi: 10.1093/annonc/mdm344. [DOI] [PubMed] [Google Scholar]
- 21.Kilic N, Feldhaus S, Kilic E, Tennstedt P, Wicklein D, Wasielewski R, Viebahn C, Kreipe H, Schumacher U. Brachyury expression predicts poor prognosis at early stages of colorectal cancer. Eur J Cancer. 47(7):1080–5. doi: 10.1016/j.ejca.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Zhang A, Chen G, Meng L, Wang Q, Hu W, Xi L, Gao Q, Wang S, Zhou J, Xu G, et al. Antisense-Snail transfer inhibits tumor metastasis by inducing E-cadherin expression. Anticancer Res. 2008;28(2A):621–8. [PubMed] [Google Scholar]
- 23.Smit MA, Geiger TR, Song JY, Gitelman I, Peeper DS. A Twist-Snail axis critical for TrkB-induced epithelial-mesenchymal transition-like transformation, anoikis resistance, and metastasis. Mol Cell Biol. 2009;29(13):3722–37. doi: 10.1128/MCB.01164-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parent AE, Choi C, Caudy K, Gridley T, Kusewitt DF. The developmental transcription factor slug is widely expressed in tissues of adult mice. J Histochem Cytochem. 2004;52(7):959–65. doi: 10.1369/jhc.4A6277.2004. [DOI] [PubMed] [Google Scholar]
- 25.Paznekas WA, Okajima K, Schertzer M, Wood S, Jabs EW. Genomic organization, expression, and chromosome location of the human SNAIL gene (SNAI1) and a related processed pseudogene (SNAI1P) Genomics. 1999;62(1):42–9. doi: 10.1006/geno.1999.6010. [DOI] [PubMed] [Google Scholar]
- 26.Wang SM, Coljee VW, Pignolo RJ, Rotenberg MO, Cristofalo VJ, Sierra F. Cloning of the human twist gene: its expression is retained in adult mesodermally-derived tissues. Gene. 1997;187(1):83–92. [PubMed] [Google Scholar]
- 27.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–66. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 28.Pillai RS. MicroRNA function: multiple mechanisms for a tiny RNA? RNA. 2005;11(12):1753–61. doi: 10.1261/rna.2248605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MJ, Angelopoulou R, Rosen JM, Greenberg NM. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56(18):4096–102. [PubMed] [Google Scholar]
- 30.Raina K, Rajamanickam S, Singh RP, Deep G, Chittezhath M, Agarwal R. Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2008;68(16):6822–30. doi: 10.1158/0008-5472.CAN-08-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moody SE, Sarkisian CJ, Hahn KT, Gunther EJ, Pickup S, Dugan KD, Innocent N, Cardiff RD, Schnall MD, Chodosh LA. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell. 2002;2(6):451–61. doi: 10.1016/s1535-6108(02)00212-x. [DOI] [PubMed] [Google Scholar]
- 32.Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, Notorfrancesco KL, Cardiff RD, Chodosh LA. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8(3):197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Inman KE, Downs KM. Localization of Brachyury (T) in embryonic and extraembryonic tissues during mouse gastrulation. Gene Expr Patterns. 2006;6(8):783–93. doi: 10.1016/j.modgep.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57(16):3325–30. [PubMed] [Google Scholar]
- 35.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100(9):672–9. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 37.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 38.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5(4):275–84. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 39.Diehn M, Clarke MF. Cancer stem cells and radiotherapy: new insights into tumor radioresistance. J Natl Cancer Inst. 2006;98(24):1755–7. doi: 10.1093/jnci/djj505. [DOI] [PubMed] [Google Scholar]
- 40.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106(33):13820–5. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–51. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 42.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15(3):504–14. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 43.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 44.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 45.Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, Fields JZ, Wicha MS, Boman BM. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69(8):3382–9. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F, Dutcher J, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69(4):1302–13. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ezeh UI, Turek PJ, Reijo RA, Clark AT. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer. 2005;104(10):2255–65. doi: 10.1002/cncr.21432. [DOI] [PubMed] [Google Scholar]
- 49.Jeter CR, Badeaux M, Choy G, Chandra D, Patrawala L, Liu C, Calhoun-Davis T, Zaehres H, Daley GQ, Tang DG. Functional evidence that the self-renewal gene NANOG regulates human tumor development. Stem Cells. 2009;27(5):993–1005. doi: 10.1002/stem.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monk M, Holding C. Human embryonic genes re-expressed in cancer cells. Oncogene. 2001;20(56):8085–91. doi: 10.1038/sj.onc.1205088. [DOI] [PubMed] [Google Scholar]
- 51.Hess DA, Meyerrose TE, Wirthlin L, Craft TP, Herrbrich PE, Creer MH, Nolta JA. Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood. 2004;104(6):1648–55. doi: 10.1182/blood-2004-02-0448. [DOI] [PubMed] [Google Scholar]
- 52.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952–8. [PubMed] [Google Scholar]
- 53.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97(26):14720–5. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90(12):5002–12. [PubMed] [Google Scholar]
- 55.Bhat PV, Samaha H. Kinetic properties of the human liver cytosolic aldehyde dehydrogenase for retinal isomers. Biochem Pharmacol. 1999;57(2):195–7. doi: 10.1016/s0006-2952(98)00261-5. [DOI] [PubMed] [Google Scholar]
- 56.Kastan MB, Schlaffer E, Russo JE, Colvin OM, Civin CI, Hilton J. Direct demonstration of elevated aldehyde dehydrogenase in human hematopoietic progenitor cells. Blood. 1990;75(10):1947–50. [PubMed] [Google Scholar]
- 57.Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15(9):1010–2. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- 58.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15(6):740–6. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 59.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138(4):645–59. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 61.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(7):1099–105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10(2):R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1(4):389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]


