Abstract
Insect survival depends on contact chemosensation to sense and avoid consuming plant-derived insecticides, such as l-canavanine. Members of a family of ∼60 gustatory receptors (GRs) comprise the main peripheral receptors responsible for taste sensation in Drosophila. However, the roles of most Drosophila GRs are unknown. In addition to GRs, a G protein-coupled receptor, DmXR, has been reported to be required for detecting l-canavanine. Here, we showed that GRs are essential for responding to l-canavanine and that flies missing DmXR displayed normal l-canavanine avoidance and l-canavanine-evoked action potentials. Mutations disrupting either Gr8a or Gr66a resulted in an inability to detect l-canavanine. We found that l-canavanine stimulated action potentials in S-type sensilla, which were where Gr8a and Gr66a were both expressed, but not in Gr66a-expressing sensilla that did not express Gr8a. l-canavanine-induced action potentials were also abolished in the Gr8a and Gr66a mutant animals. Gr8a was narrowly required for responding to l-canavanine, in contrast to Gr66a, which was broadly required for responding to other noxious tastants. Our data suggest that GR8a and GR66a are subunits of an l-canavanine receptor and that GR8a contributes to the specificity for l-canavanine.
Introduction
Contact chemosensation is crucial for animals to discriminate safe, nutritious foods from toxic substances in the environment. In insects such as the fruit fly, Drosophila melanogaster, this ability is essential for survival since many plants produce compounds with insecticidal properties. One such botanically derived insecticide is l-canavanine, which is an analog of the amino acid l-arginine (Dahlman and Rosenthal, 1975; Rosenthal and Dahlman, 1986; Rosenthal, 2001). Consumption of l-canavanine causes lethality to fruit flies and many other insects, since this amino acid derivative is incorporated into proteins in place of l-arginine, thereby hindering protein function.
In fruit flies, tastants are detected in gustatory receptor neurons (GRNs), which are housed in gustatory hairs (sensilla) (Vosshall and Stocker, 2007; Montell, 2009). The main class of taste receptors consists of ∼60 gustatory receptors (GRs), and these multipass transmembrane proteins are unrelated to G protein-coupled receptors (GPCRs) (Clyne et al., 2000; Robertson et al., 2003; Montell, 2009). Currently, only four GRs (GR32a, GR33a, GR66a, and GR93a) are known to be required for sensing aversive tastants, ranging from caffeine to quinine, strychnine, and the synthetic repellent DEET (Moon et al., 2006, 2009; Lee et al., 2009, 2010). In contrast to the fructose receptor (GR43a) (Sato et al., 2011) and possibly the glycerol receptor (GR64e) (Wisotsky et al., 2011), the subunit composition of GRs that respond to sugars (Dahanukar et al., 2007; Jiao et al., 2007, 2008) and to aversive chemicals is complex and appears to consist of at least three subunits (Lee et al., 2009; Moon et al., 2009). The TRPA1 channel as well as GRs are expressed in GRNs and respond to noxious compounds (Kang et al., 2010; Kim et al., 2010). In addition to GRs and TRP channels, a GPCR, DmXR, encoded by the mangetout (mtt) gene, has been reported to be required for the behavioral avoidance to l-canavanine (Mitri et al., 2009).
Here, we found that both the behavioral and electrophysiological responses to l-canavanine were indistinguishable between mtt mutant and control flies. Rather, Gr8a and Gr66a were indispensible for sensing l-canavanine. While Gr66a was required for detecting multiple aversive tastants (Moon et al., 2006; Lee et al., 2010), Gr8a mutant flies responded normally to all other repellent compounds tested. We propose that GRs that function in the sensation of aversive tastants are comprised of assemblies consisting of a narrowly tuned subunit, as well as at least one broadly required coreceptor.
Materials and Methods
Generation of the Gr8a1 mutant and transgenic flies.
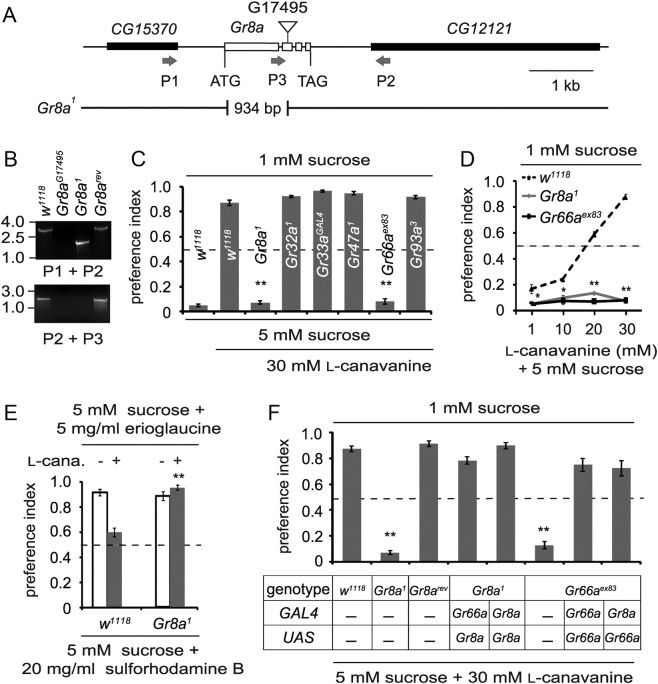
The P-element line G17495, which was inserted in the Gr8a coding region, was donated by the Drosophila Library Facility Biomedical Research Center (Korea Advanced Institute of Science and Technology, Daejeon, South Korea). To generate the Gr8a1 mutant, the P-element was mobilized by genetically introducing transposase using the P[ry(Δ2–3)] line. The Δ2-3 transgene results in a high level of transposase expression as a result of deleting an intron that is normally spliced out inefficiently (Laski et al., 1986). To identify a deletion that removed Gr8a, we conducted a PCR-based screen using the following primers: P1 (TCCGAAACATACAGTGGCGTCGAT) and P2 (TGTTCTTCGTGCTGACTGGCTACA) (Fig. 2A,B). We recovered one P-element excision line out of 200 lines screened. The deletion was confirmed by genomic PCR using the following primers: P2 and P3 (GCTCAAATGTTTCCAGGGTGCCTT) (Fig. 2A,B). To determine the ends of the deletion, we amplified the genomic DNA and obtained the DNA sequence. The deletion was 934 base pairs and removed the sequences encoding the amino acid residues 15–305.
Figure 2.
GRs required for l-canavanine avoidance. A, Physical map of the Gr8a genomic region. The P-element (G17495) that inserted into the Gr8a coding region is indicated by the inverted triangle. The deletion and precise excision were confirmed using the indicated PCR primers (arrows) to amplify genomic DNA (P1, P2, and P3), followed by DNA sequencing. B, PCR analyses of genomic DNA. The PCR products were generated using the indicated fly stocks and PCR primer pairs and fractionated on an agarose gel. C, Screen for Grs required for l-canavanine avoidance. The two-way choice tests were performed using the indicated chemicals and fly stocks; n = 5–15. D, Dose-avoidance relationship determined using the two-way choice behavioral assay. Flies of the indicated genotypes were tested using l-canavanine over a range of concentrations (1–30 mm); n = 4–11. E, Two-way choice assays using 5 mm sucrose plus 5 mg/ml erioglaucine (in the presence or absence of 30 mm l-canavanine) versus 5 mm sucrose plus 20 mg/ml sulforhodamine B. F, Rescue of l-canavanine avoidance defects by expressing wild-type Gr transgenes in GRNs using the GAL4/UAS system; n = 6–13. The error bars indicate SEMs; *p < 0.05, **p < 0.01.
To obtain P[UAS-Gr8a] and P[UAS-Gr66a] transgenic flies, we prepared RNA from the labellum of wild-type flies and amplified the Gr8a and Gr66a cDNA by RT-PCR. We subcloned the cDNA into the pUAST vector (Brand and Perrimon, 1993). The transformation vector was injected into w1118 embryos.
Fly stocks.
We reported the following mutants previously and deposited them with the Bloomington Stock Center: Gr33aGAL4 (Moon et al., 2009), Gr66ex83 (Moon et al., 2006), and Gr93a3 (Lee et al., 2009). mttf06268 and Df(2R)Exel7096 were obtained from Y. Grau (University of Montpellier, Montpellier, France; Mitri et al., 2009). H. Amrein (Texas A&M Health Science Center, College Station, TX) provided the Gr32a1 (Miyamoto and Amrein, 2008) and the P[Gr66a-GAL4] flies (Thorne et al., 2004). The P[Gr8a-GAL4] flies and P[Gr66a-I-GFP] were from J. Carlson (Yale University, New Haven, CT; Weiss et al., 2011) and K. Scott (University of California, Berkeley, CA; Wang et al., 2004), respectively. The fly stocks were outcrossed for at least five generations into a w1118 background, and w1118 was used as the “wild-type” control.
Immunohistochemistry.
The labella of GR8a-GAL4/+; UAS-DsRed/GR66a-I-GFP flies were dissected and fixed using 4% paraformaldehyde with 0.2% Triton X-100 for 15 min at room temperature. The labella were washed three times with PBST (1× PBS and 0.2% Triton X-100), cut in half with a razor blade, and blocked with 0.5% goat serum in PBST for 30 min at room temperature. The labella were transferred to new blocking buffer containing the primary antibodies and incubated overnight at 4°C: mouse anti-GFP (Molecular Probe, 1:1000) or rabbit anti-DsRed (Clontech, 1:1000). The labella were washed three times with PBST at room temperature and incubated with secondary antibodies (goat anti-mouse Alexa Fluor 488 and goat anti-rabbit Alexa Fluor 568) for 1 h at room temperature. The tissues were washed three times with PBST and mounted in VECTASHELD (Vector Laboratories). The samples were viewed using a Carl Zeiss LSM510 confocal microscope. The sensilla expressing Gr8a were identified by examining the dendrites of the GRNs in the fluorescent image and the bristles by differential interference contrast imaging.
Chemicals.
Sucrose, caffeine, l-canavanine, DEET, denatonium, lobeline, papaverine, quinine, strychnine, l-arginine and N-methyl-l-arginine (NMA), sulforhodamine B, erioglaucine, and KCl were purchased from Sigma-Aldrich. Berberine sulfate trihydrate and Brilliant Blue FCF were obtained from Wako Pure Chemical Industries.
Two-way choice behavioral assays.
With the exception of the data shown in Figure 1G and Figure 2E, the two-way choice assays were conducted as described previously (Meunier et al., 2003; Moon et al., 2006). Briefly, we starved 50 flies (3–6 d old) for 24 h and then introduced the animals into 72-well microtiter dishes. Alternating wells were filled with 1% agarose combined with one of two types of test mixtures: 1 mm sucrose or 5 mm sucrose plus an avoidance chemical. To monitor food intake, one test mixture contained a blue dye (Brilliant Blue FCF, 0.125 mg/ml) while the other contained a red dye (sulforhodamine B, 0.2 mg/ml). After allowing the flies to feed for 90 min at room temperature in the dark, the animals were frozen at −20°C. The numbers of flies that were blue (NB), red (NR), or purple (NMIX) were determined in a blind fashion based on the colors of the abdomen, and the preference index (P.I.) values were calculated according to one of the two following equations: (NB + 0.5Nmix)/(NR + NB + Nmix) or (NR + 0.5Nmix)/(NR + NB + Nmix), depending on the dye/tastant combinations. P.I.s equal to 1.0 and 0 indicated complete preferences for either the 1 or 5 mm sucrose, respectively. A P.I. equal to 0.5 indicated no preference between the two food alternatives.
Figure 1.
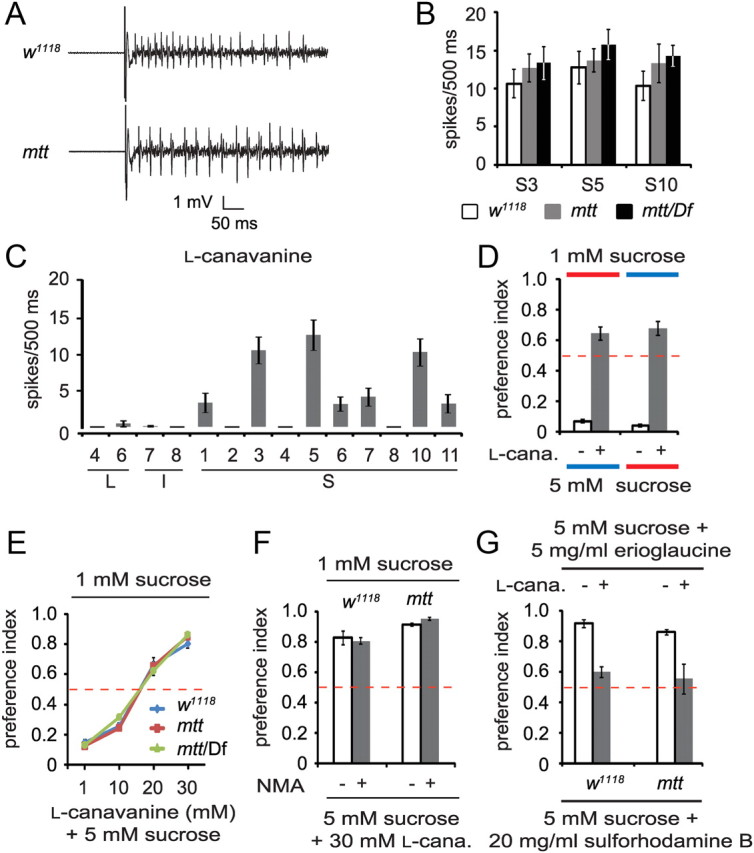
mtt was not required for l-canavanine-induced action potentials or for behavioral avoidance. A, Representative traces of l-canavanine triggered nerve firings from w1118 and mttf06268 flies. The tip recordings were performed in S3 sensilla using 25 mm l-canavanine. B, Mean number of action potentials in response to 25 mm l-canavanine recorded from S3, S5, and S10 sensilla. The genotypes are indicated. Df stands for Df(2R)Exel7096; n = 10–12. C, Tip recordings surveying nerve firings in GRNs from w1118 labellar sensilla in response to 25 mm l-canavanine. Shown are the mean action potentials; n = 10–18. D, Effects of red and blue food dyes on two-way choice assays. The flies were given a choice between 1 mm sucrose and 5 mm sucrose (plus or minus 30 mm l-canavanine). The red and blue bars indicate the color of the food dye added to the 1 or 5 mm sucrose. The dashed lines indicate P.I. = 0.5, which results if there is no preference; n = 5. Unless indicated otherwise, the two-way choice tests were performed with the 0.125 mg/ml Brilliant Blue FCF and 0.2 mg/ml sulforhodamine B. E, Effects of different concentrations of l-canavanine on the selection of 5 mm versus 1 mm sucrose; n = 4–10 for each concentration. Shown are means ± SEMs. F, Two-way choice tests in the presence or absence of 30 mm NMA. G, Two-way choice tests using 5 mm sucrose plus 5 mg/ml erioglaucine (with or without 30 mm l-canavanine) versus 5 mm sucrose plus 20 mg/ml sulforhodamine B.
To test further a possible role for mtt in l-canavanine avoidance, we also performed two-way choice behavioral assays as described previously (Mitri et al., 2009). In these assays (Fig. 1G and 2E), we added 5 mm sucrose to all wells in the microtiter dishes. Alternating wells also contained either a different blue dye than that described above (5 mg/ml erioglaucine), or 100-fold more of the red dye than that used in our standard assays (20 mg/ml sulforhodamine B). Flies were allowed to feed in the dark for 2 h. As reported, the high concentration of red dye was repulsive, causing the flies to prefer the 5 mm sucrose plus erioglaucine (Mitri et al., 2009). To test for aversion to l-canavanine, we added 30 mm l-canavanine to the 5 mm sucrose that contained the 5 mg/ml erioglaucine.
Electrophysiology.
Tip recordings (Hodgson et al., 1955; Wieczorek and Wolff, 1989) were performed as described previously (Moon et al., 2006) using either 25 mm l-canavanine or 25 mm l-arginine. Briefly, newly eclosed flies were immobilized by inserting a glass capillary filled with Ringer's solution into the abdomen all the way to the head. This electrode also served as the indifferent electrode. The labellar hairs were stimulated with tastants dissolved in the buffer solution of the recording pipette (10–20 μm tip diameter). KCl at 1 mm was used as the electrolyte for all recordings. The recording electrode was connected to a preamplifier (TastePROBE, Syntech), and the signals were collected and amplified (10×) using a signal connection interface box (Syntech) in conjunction with a 100–3000 Hz bandpass filter. Recordings of action potentials were acquired using a 12 kHz sampling rate and analyzed using Autospike 3.1 software (Syntech).
Data analyses.
All error bars represent SEMs. Unpaired Student's t tests were used to compare two sets of data. ANOVA with the Tukey post hoc tests were used to compare multiple sets of data. Asterisks indicate statistical significance (*p < 0.05, **p < 0.01).
Results
mtt was dispensable for l-canavanine-induced action potentials and avoidance
Mutation of the mtt locus, which disrupts a GPCR (DmXR), has been reported to be necessary for the behavioral avoidance to l-canavanine (Mitri et al., 2009). In this prior study, the electrophysiological responses of gustatory receptor neurons were not tested. Therefore, we performed tip recordings to assess l-canavanine-induced action potentials. GRNs are housed in gustatory hairs (sensilla) situated on several body parts, including the main taste organ, the labellum, and are broadly grouped into three size classes [small (S); intermediate (I); large, (L)] (Vosshall and Stocker, 2007; Montell, 2009). We recorded from S3 sensilla, which respond to most aversive tastants (Weiss et al., 2011). In control flies, addition of l-canavanine stimulated action potentials (10.6 ± 1.9) immediately upon contact with the recording pipette (Fig. 1A,B). Surprisingly, we found that mttf06268 mutant and wild-type flies displayed similar frequencies of l-canavanine-induced action potentials (Fig. 1A,B). Because mttf06268 produced normal l-canavanine action potentials, we confirmed the reported transposon insertion in mttf06268 by genomic PCR and analyzed mttf06268 in trans with a deletion that completely removed mtt, Df(2R)Exel7096. These latter flies also showed a wild-type electrophysiological response to l-canavanine (Fig. 1B).
Since the mttf06268 S3 sensilla responded normally to l-canavanine, we wondered whether other mutant sensilla might be impaired in l-canavanine-induced action potentials. To conduct this analysis, we first surveyed wild-type sensilla. Most I- and S-type sensilla, but none of the L-type sensilla, respond to aversive compounds (Weiss et al., 2011). Therefore, we recorded l-canavanine-induced action potentials from the 10 S-type sensilla (out of 12 total) that were most accessible for recordings. In addition, we sampled two I-type and two L-type sensilla, the latter of which we did not expect to be stimulated by l-canavanine. The S3, S5, and S10 sensilla were most responsive to l-canavanine (>10 spikes/500 ms), while the S1, S6, S7, and S11 sensilla displayed a low-level activity (Fig. 1C). The remaining three S-type sensilla tested, S2, S4, and S8, were insensitive to l-canavanine (Fig. 1C). None of the L- or I-type sensilla examined were stimulated by l-canavanine (Fig. 1C). Given the high responsiveness of wild-type S5 and S10, we recorded from these latter sensilla from mttf06268 and mttf06268/Df flies and found that the responses were also indistinguishable from those of wild-type flies (Fig. 1B).
Due to the preceding results, we tested mttf06268 and mttf06268/Df for behavioral avoidance to l-canavanine using a two-way choice behavioral assay (Meunier et al., 2003; Moon et al., 2006). We starved young flies for 24 h and allowed them to feed for 1.5 h in the dark in a microtiter dish that contained alternating wells consisting of 1 mm sucrose or 5 mm sucrose plus 30 mm l-canavanine. Each of the two tastants was mixed with red or blue food coloring. We then counted the number of flies with red, blue, or purple abdomens. Complete preferences for the 1 or 5 mm sucrose yield P.I.s of 1.0 and 0 respectively, while a P.I. of 0.5 results if there were a lack of bias for the two concentrations of sucrose. In the absence of l-canavanine, the control flies (w1118) selected the higher concentration of sucrose, and this preference was unaffected by the red or blue food coloring (P.I. = 0.07 or 0.04; Fig. 1D). When given a choice between 1 mm sucrose and 5 mm sucrose plus 30 mm l-canavanine, the w1118 flies decreased their preference for 5 mm sucrose (Fig. 1D). We assayed the behavioral responses to a range of l-canavanine concentrations. In control flies, 1 mm l-canavanine did not affect the preference for 5 mm sucrose over 1 mm sucrose, while 20 and 30 mm l-canavanine induced large avoidance responses (Fig. 1E). Consistent with the electrophysiological results, the mttf06268 mutant displayed normal behavioral avoidance to l-canavanine. Wild-type and mttf06268 flies also showed indistinguishable l-canavanine avoidance using a modified two-way choice test employed previously to characterize mttf06268 (Fig. 1G; see Materials and Methods for detailed description). Based on the combination of electrophysiological and behavioral assays, we conclude that DmXR, which is encoded by mtt, was not required for detecting l-canavanine.
Requirement for GR8a and GR66a for avoiding l-canavanine
To address whether a Drosophila GR was required for avoiding l-canavanine, we tested the available mutants that disrupt GRs that function in sensing aversive chemicals. These include Gr32a, Gr33a, Gr66a, Gr93a (Moon et al., 2006, 2009; Lee et al., 2009, 2010), and Gr47a (Y. Lee, S. J. Moon, and C. Montell, unpublished observations). In addition, we generated a null mutation in Gr8a by mobilizing a P-element that inserted in the second exon, thereby deleting most of the coding region (Fig. 2A,B). The Gr8a1 flies were homozygous viable and fertile.
We found that mutations disrupting Gr8a1 and Gr66aex83 severely impaired l-canavanine avoidance (Fig. 2C). These mutant flies displayed the same preference for 5 mm sucrose, even at the highest concentration of l-canavanine (30 mm; Fig. 2D). Loss of the remaining four GRs that are known to affect the responses to aversive compounds had no impact on the repulsion to l-canavanine (Fig. 2C). These include Gr33aGAL4, which is required for sensing all other noxious tastants tested (Moon et al., 2009), and Gr32a1 (Lee et al., 2010), which affected the responses to all avoidance tastants examined, except caffeine. We tested Gr8a1 using the previously described two-way choice test (Mitri et al., 2009) and found that the mutant was also impaired in l-canavanine repulsion using this alternative assay (Fig. 2E).
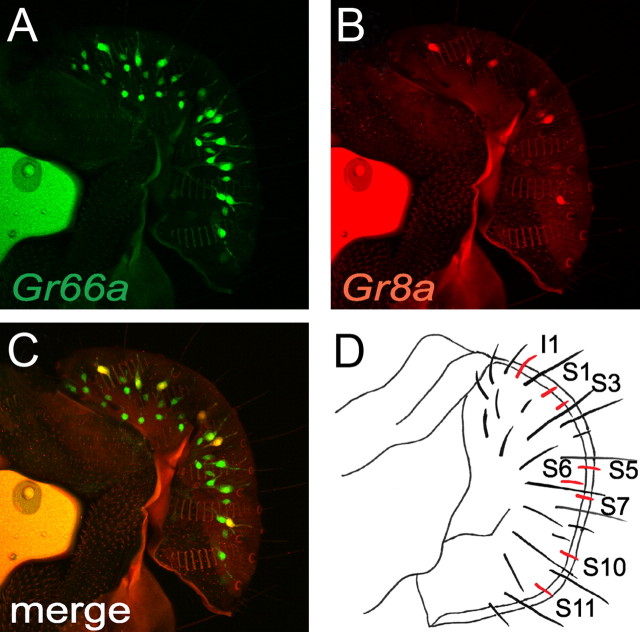
To confirm that the deficits in l-canavanine avoidance were due to the mutations affecting Gr8a and Gr66a, we tested for rescue of the mutant phenotypes with wild-type transgenes. Expression of UAS-Gr8a under control of either the Gr66a promoter (Gr66a-GAL4) or the Gr8a promoter (Gr8a-GAL4) rescued the Gr8a1 phenotype (Fig. 2F). Similarly, we rescued the Gr66aex83 phenotype using UAS-Gr66a and either the Gr66a-GAL4 or the Gr8a-GAL4 (Fig. 2F). We detected expression of the Gr8a-GAL4 reporter in S-type sensilla (S1, S3, S5, S6, S7, S10, and S11) and one of I-type (I1) sensillum, but not other I- or L-type sensilla (Fig. 3), similar to that reported previously (Weiss et al., 2011).
Figure 3.
Expression of the Gr8a reporter in subsets of Gr66a-positive GRNs. A–C, Confocal images. A, Gr66a-I-GFP labeled all bitter responsive GRNs (anti-GFP, green). B, UAS-DsRed was expressed under control of the Gr8a-GAL4 (anti-DsRed, red). Most but not all Gr8a-postive cells were detected in single confocal images C, Merged image of A and B. D, Schematic illustration of a fly labellum showing gustatory sensilla that expressed the Gr8a reporter (red). The nomenclature for the sensilla is as described previously (Hiroi et al., 2002). This differs somewhat from another nomenclature (Weiss et al., 2011).
Elimination of l-canavanine-induced action potentials
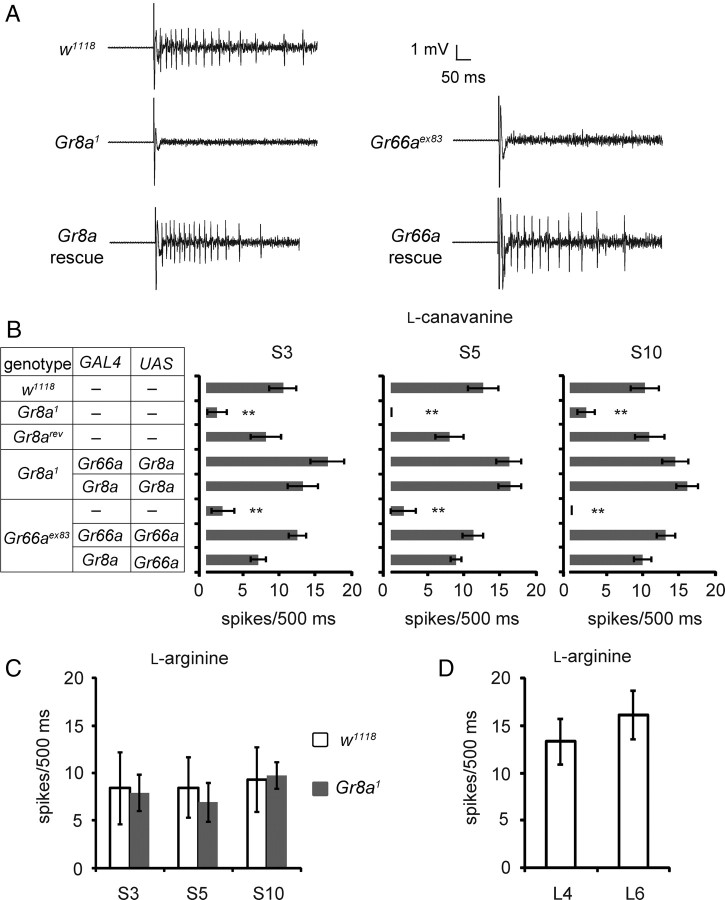
To test whether GR8a and GR66a were required for the electrophysiological responses to l-canavanine, we recorded from S3, S5, and S10 sensilla, which in control flies elicited the largest responses to l-canavanine. We found that l-canavanine-induced action potentials were eliminated in either the Gr8a1 or Gr66aex83 mutants and were restored upon expression of the wild-type transgenes that were driven under control of the Gr8a-GAL4 or Gr66a-GAL4 (Fig. 4A,B). The S3, S5, and S10 sensilla also responded to l-arginine, which is a structural analog of l-canavanine (Fig. 4C). l-Arginine evoked similar frequencies of action potentials in Gr8a1 as in wild type (Fig. 4C). L-type sensilla, which did not express the Gr8a reporter (Weiss et al., 2011), also responded to l-arginine (Fig. 4D).
Figure 4.
GR8a and GR66a are indispensible for l-canavanine-induced nerve firings. A, Representative traces of l-canavanine evoked action potentials from control flies (w1118), Gr8a1, Gr66aex83, and mutants with rescue transgenes (Gr8a1;Gr8a-GAL4/UAS-Gr8a and Gr8a-Gal4/UAS-Gr66a;Gr66aex83). The recordings were performed from S5 sensilla. B, Tip recordings showing that GR8a and GR66a are required for l-canavanine-induced nerve firings. The assays were performed from S3, S5, and S10 sensilla using animals of the indicted genotypes; n = 10–12. The error bars represent SEMs; **p < 0.01. C, Tip recordings showing l-arginine-induced action potentials in S3, S5, and S10 sensilla; n = 10–11. The error bars represent SEMs. D, l-Arginine-induced action potentials in L4 and L6 sensilla; n = 10–11. Shown are means ± SEMs.
Distinct requirements for GR8a and GR66a
Loss of GR66a diminishes the repulsion to caffeine, lobeline, and papaverine and the synthetic insect repellent DEET (Moon et al., 2006; Lee et al., 2010). To investigate whether Gr8a1 displayed impaired avoidance to other noxious chemicals or was narrowly required for l-canavanine, we performed additional behavioral assays. Gr8a1 displayed normal responses to all other repulsive tastants tested: caffeine, quinine, berberine, denatonium, lobeline, papaverine, strychnine, and DEET (Fig. 5A). The frequencies of action potentials induced by application of these bitter chemicals were also similar between the mutant and the control flies (Fig. 5B). Thus, GR8a was more narrowly tuned to l-canavanine than GR66a.
Figure 5.
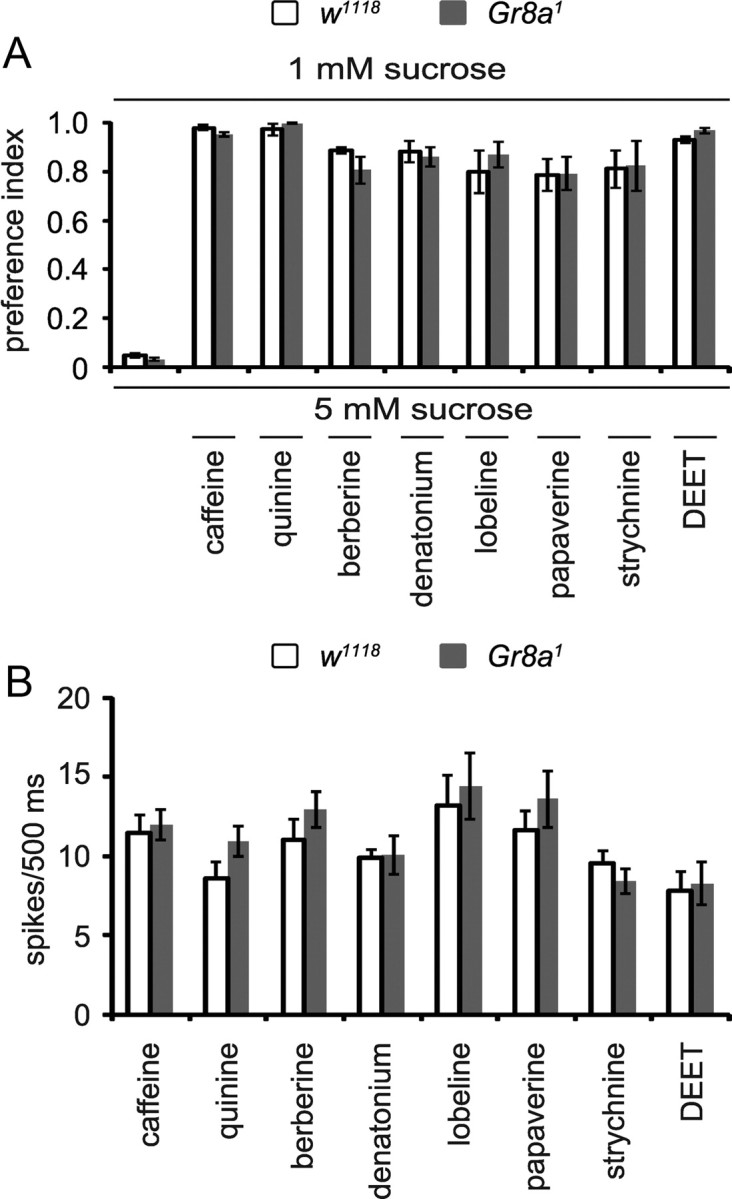
Behavioral and electrophysiological responses of Gr8a1 to multiple noxious tastants. A, Behavioral responses of w1118 and Gr8a1 using two-way choice assays and one of the following concentrations of chemicals: 6 mm caffeine, 1 mm quinine, 0.05 mm berberine, 0.1 mm denatonium, 0.1 mm lobeline, 0.5 mm papaverine, 0.5 mm strychnine, or 0.2% DEET; n = 5–12. The error bars indicate SEMs. B, Mean nerve firing responses from w1118 and Gr8a1 by performing tip recordings from S6 sensilla using the indicated concentrations of chemicals: 10 mm caffeine, 1 mm quinine, 0.1 mm berberine, 1 mm denatonium, 1 mm lobeline, 1 mm papaverine, 1 mm strychnine, 0.2% DEET; n = 11–18. Shown are means ± SEMs.
Discussion
GRs but not DmXR are required for avoiding l-canavanine
We conclude that GRs rather than the GPCR (DmXR) were essential for detecting the plant-derived toxin l-canavanine. Gr8a and Gr66a were required since mutations disrupting each of these genes eliminated the behavioral repulsion to the insecticide. These deficits were more dramatic than those associated with mutations disrupting the sensation of other aversive tastants, since avoidance to l-canavanine was abolished even at the highest concentrations examined. Both the Gr8a and Gr66a mutants displayed the same strong preference for 5 mm sucrose over 1 mm sucrose, even when the 5 mm sucrose was laced with 30 mm l-canavanine. Thus, in contrast to other aversive tastants that inhibit the sucrose response, thereby causing a reduced preference for 5 mm sucrose (Meunier et al., 2003), l-canavanine did not appear to inhibit the sugar response. In further support of the findings that Gr8a and Gr66a were required for sensing l-canavanine, we found that the electrophysiological responses to l-canavanine were greatly reduced in the mutant flies (≥7–10 fold). Confirmation that Gr8a and Gr66a were essential for l-canavanine repulsion was that we rescued the mutant phenotypes by introduction of wild-type transgenes.
In contrast to the requirements for Gr8a and Gr66a, the GPCR (DmXR) encoded by mtt was dispensable for the repellent action of l-canavanine, since the behavior and electrophysiological responses of the mtt mutant were indistinguishable from those of control flies. This finding was not due to contamination of the mttf06268 stock with wild-type flies, since we confirmed the presence of the mtt mutation by PCR. The lack of requirement for DmXR described here was not due to differences in behavioral assays, since DmXR was also dispensable when we employed the two-way choice test used previously to assay mttf06268 mutant flies. Furthermore, we found that l-canavanine avoidance was not blocked with NMA, which has been reported to be a DmXR antagonist. Finally, we performed tip recordings and found that l-canavanine-induced action potentials depended on GR8a and GR66a and not on DmXR. Thus, while DmXR appears to be activated in vitro by l-canavanine, we conclude that GRs are the critical receptors for avoiding this plant-derived insecticide in vivo.
Gr8a and Gr66a are corequired in S-type sensilla
While Gr8a and Gr66a were both essential for avoiding l-canavanine, these two Grs displayed different expression patterns. Gr66a is widely expressed in most sensilla that respond to aversive compounds, which include the I-type sensilla, such as I7 and I8, which do not respond to l-canavanine (Hiroi et al., 2002; Thorne et al., 2004; Wang et al., 2004; Marella et al., 2006; Moon et al., 2006; Weiss et al., 2011). However, there was a strong correlation between sensilla that expressed Gr8a and displayed l-canavanine-induced activity. Sensilla with undetectable expression of the Gr8a reporter were unresponsive to l-canavanine (L-type, I-type, S2, S4, and S8), while the S-type sensilla with relatively high expression displayed l-canavanine-evoked action potentials. We were not able to record from the one I-type sensillum that expressed Gr8a (I1) due to poor accessibility of this sensillum. Gr66a and Gr8a were necessary for sensing l-canavanine in Gr8a-expressing cells, since the loss of the l-canavanine response in the mutant flies was reversed by expression of the wild-type transgenes under control of the Gr8a promoter.
The requirements for GR8a and GR66a were also distinct. GR8a functioned narrowly in sensing l-canavanine. In contrast and consistent with the wider expression pattern of the Gr66a reporter, this latter GR is necessary for the repulsion to most (six of nine) noxious tastants examined (Moon et al., 2006; Lee et al., 2010). The other available Gr mutations did not affect l-canavanine repulsion. We suggest that GR8a and GR66a may form a multisubunit receptor that functions in a subset of S-type sensilla for l-canavanine repulsion. GR8a may contribute to the specificity for sensing l-canavanine, and GR66a may be a coreceptor for multiple GRs. However, GR8a and GR66a were not sufficient for responding to l-canavanine, since coexpression of the two GRs in tissue culture cells, ectopic coexpression of Gr8a and Gr66a in sugar-responsive GRNs, or Gr8a in Gr66a-expressing GRNs that did not express Gr8a (e.g., I7 or I8) did not confer l-canavanine sensitivity (data not shown).
Aversive GRs comprised of subunits with narrow and broad specificities
The widely differing specificities for the two GRs that act in sensing l-canavanine is reminiscent of our previous findings concerning the repertoire of GRs that function in caffeine repulsion. GR93a is required for avoidance of caffeine only (Lee et al., 2009), while mutations disrupting two other GRs that impair caffeine aversion (GR33a and GR66a) disrupt the repulsion to most deterrent tastants (Moon et al., 2006, 2009; Lee et al., 2009, 2010). Thus, while a single GR appears to be sufficient for the response to fructose (Sato et al., 2011), we propose that the GRs that participate in sensing noxious compounds are complexes comprised of at least one subunit with a narrow specificity and others that are broadly required coreceptors.
Footnotes
This work was supported by a grant to C.M. from the NIDCD (DC007864) and by grants to S.J.M. from the Converging Research Center Program funded by the Ministry of Education, Science, and Technology (2011K000678) and from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (2010-0016576). We thank T. Sokabe for examining the function of GRs in vitro and J. Kim, Y. Grau, K. Scott, and J. Carlson for fly stocks.
References
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Clyne PJ, Warr CG, Carlson JR. Candidate taste receptors in Drosophila. Science. 2000;287:1830–1834. doi: 10.1126/science.287.5459.1830. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlman DL, Rosenthal GA. Non-protein amino acid-insect interactions–I. Growth effects and symptomology of l-canavanine consumption by tobacco hornworm, Manduca sexta (L.) Comp Biochem Physiol A Comp Physiol. 1975;51:33–36. doi: 10.1016/0300-9629(75)90409-0. [DOI] [PubMed] [Google Scholar]
- Hiroi M, Marion-Poll F, Tanimura T. Differentiated response to sugars among labellar chemosensilla in Drosophila. Zoolog Sci. 2002;19:1009–1018. doi: 10.2108/zsj.19.1009. [DOI] [PubMed] [Google Scholar]
- Hodgson ES, Lettvin JY, Roeder KD. Physiology of a primary chemoreceptor unit. Science. 1955;122:417–418. doi: 10.1126/science.122.3166.417-a. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Moon SJ, Montell C. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc Natl Acad Sci U S A. 2007;104:14110–14115. doi: 10.1073/pnas.0702421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Moon SJ, Wang X, Ren Q, Montell C. Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Curr Biol. 2008;18:1797–1801. doi: 10.1016/j.cub.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, Garrity PA. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464:597–600. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Lee Y, Akitake B, Woodward OM, Guggino WB, Montell C. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc Natl Acad Sci U S A. 2010;107:8440–8445. doi: 10.1073/pnas.1001425107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laski FA, Rio DC, Rubin GM. Tissue specificity of Drosophila P element transposition is regulated at the level of mRNA splicing. Cell. 1986;44:7–19. doi: 10.1016/0092-8674(86)90480-0. [DOI] [PubMed] [Google Scholar]
- Lee Y, Moon SJ, Montell C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc Natl Acad Sci U S A. 2009;106:4495–4500. doi: 10.1073/pnas.0811744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kim SH, Montell C. Avoiding DEET through insect gustatory receptors. Neuron. 2010;67:555–561. doi: 10.1016/j.neuron.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Meunier N, Marion-Poll F, Rospars JP, Tanimura T. Peripheral coding of bitter taste in Drosophila. J Neurobiol. 2003;56:139–152. doi: 10.1002/neu.10235. [DOI] [PubMed] [Google Scholar]
- Mitri C, Soustelle L, Framery B, Bockaert J, Parmentier ML, Grau Y. Plant insecticide l-canavanine repels Drosophila via the insect orphan GPCR DmX. PLoS Biol. 2009;7:e1000147. doi: 10.1371/journal.pbio.1000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Amrein H. Suppression of male courtship by a Drosophila pheromone receptor. Nat Neurosci. 2008;11:874–876. doi: 10.1038/nn.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. A taste of the Drosophila gustatory receptors. Curr Opin Neurobiol. 2009;19:345–353. doi: 10.1016/j.conb.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SJ, Köttgen M, Jiao Y, Xu H, Montell C. A taste receptor required for the caffeine response in vivo. Curr Biol. 2006;16:1812–1817. doi: 10.1016/j.cub.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Moon SJ, Lee Y, Jiao Y, Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol. 2009;19:1623–1627. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson HM, Warr CG, Carlson JR. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2003;100(Suppl 2):14537–14542. doi: 10.1073/pnas.2335847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal GA. l-Canavanine: a higher plant insecticidal allelochemical. Amino Acids. 2001;21:319–330. doi: 10.1007/s007260170017. [DOI] [PubMed] [Google Scholar]
- Rosenthal GA, Dahlman DL. l-Canavanine and protein synthesis in the tobacco hornworm Manduca sexta. Proc Natl Acad Sci U S A. 1986;83:14–18. doi: 10.1073/pnas.83.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Tanaka K, Touhara K. Sugar-regulated cation channel formed by an insect gustatory receptor. Proc Natl Acad Sci U S A. 2011;108:11680–11685. doi: 10.1073/pnas.1019622108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 2011;69:258–272. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek H, Wolff G. The labellar sugar receptor of Drosophila. J Comp Physiol A. 1989;164:825–834. [Google Scholar]
- Wisotsky Z, Medina A, Freeman E, Dahanukar A. Evolutionary differences in food preference rely on Gr64e, a receptor for glycerol. Nat Neurosci. 2011;14:1534–1541. doi: 10.1038/nn.2944. [DOI] [PubMed] [Google Scholar]