Abstract
Although it was discovered more than two decades ago, new information concerning the biological activities of IL-9 has been provided in recent years, after the isolation of cells that selectively produce this cytokine, designated “Th9.” Th9 cells are generated in vitro by polarization, mainly by TGF-β and IL-4, during activation with the specific antigen, or with anti-CD3/CD28 antibodies. This review deals mainly with Th9 generated by the former, “physiological” mode of activation. Of particular interest is the unique production kinetics of IL-9: the cytokine is produced very rapidly, but after reaching its peak (day 3 in our studies), it declines sharply to trace levels. In addition to IL-9, Th9 cells also produce similar amounts of another cytokine, IL-10, but the production kinetics of these two cytokines are strikingly different. Antigen-activated Th9 in our studies also developed pathogenic capacity, but only during the short time period of peak IL-9 production. Interestingly, no IL-9-producing cells were detected in sites of inflammation induced by Th9, in contrast to Th1 and Th17. The unique features of Th9 cells and their products are discussed with regards to the known and assumed functions of the cytokine.
Keywords: IL-9, Th9, inflammation, eye, lymphocyte subsets
I. INTRODUCTION
IL-9 was discovered more than two decades ago by Van Snick and his colleagues,1,2 but more recent studies have shed new light on the unique features of this cytokine, its multiple biological activities, and the lymphoid cells that produce it. Excellent reviews on various aspects of IL-9 have been published in recent years3–7 and the present paper summarizes the outstanding pieces of information detailed in these reviews. The present review focuses, however, on certain recent observations concerning the unusual features of IL-9 and of the cells that produce it. Cells that selectively produce IL-9 (“Th9”) have been recently generated and their analysis revealed several unique features of the Th9 cells, which are detailed in this review.
II. IMMUNOOGICAL FUNCTIONS OF IL-9
IL-9 belongs to the family of common γ-chain cytokines and uses the γ-chain receptor along with its specific receptor, IL-9Rα, for delivering its signals into target cells. Analysis of the immunological functions of IL-9 has been carried out mainly by observations made in mice in which the IL-9 or its receptor genes was deleted8,9 or animals transgenically over-producing this cytokine.9–12 Accumulating data indicate that IL-9 plays a major role in the immune response against helminthes13,14 and it is assumed this is the cytokine's “physiological” function. Similar to other molecules of the same function (e.g., IgE), however, IL-9 also participates in the pathogenic process of allergy, in particular asthma.15–18 It is of note, however, that deficiency in IL-9 does not affect the development of allergic reactions in the respiratory system.19 These activities of IL-9 are mainly exerted by promoting proliferation and accumulation of mast cells, as well as other leukocytes, at the affected tissue, particularly the respiratory tract and the gut.7,20,21 In addition, IL-9 has been found to participate in other immunological activities, mostly related to pathogenic processes. In the majority of these systems, IL-9 was shown to promote, or even mediate, the pathogenic processes of conditions such as experimental autoimmune encephalomyelitis (EAE).22,23 On the other hand, IL-9 was reported to regulate the pathology induced by syncitial virus,24 to inhibit EAE by enhancing the function of FoxP3 nTreg cells,25 or to dampen the Th17-induced autoimmune gastritis.26
III. IL-9 PRODUCING CELLS
Whereas data of early studies suggested that IL-9 is produced by Th2 cells,27,28 two concurrent publications in 2008, by the groups of Stockinger14 and Kuchroo,29 showed that IL-9 is also produced exclusively by a population of Th cells that was designated “Th9.” Lineages of Th9 cells have since been established by several other groups30–34 and several of their unique features are discussed in detail below. In addition, IL-9 was found to be produced by Th17 cells,23,26 Treg cells,35,36 as well as by NKT cells.37,38 Production of IL-9 was mainly demonstrated in studies in vitro, but IL-9 and cells expressing it in vivo have been reported as well.23 In addition, a recent study by Stockinger group17 showed that IL-9 is also produced by innate lymphoid cells in response to papain sensitization in vivo. The data of this study also suggest that these cells are the main source of IL-9 in the lungs of allergen-sensitized mice.17
Generation of Th9 lineages has been based on the seminal observation made by Schmitt et al.39 that CD4 cells incubated with IL-4 and TGF-β produce IL-9. This pair of cytokines provides the polarizing environment for CD4 cells when concurrently activated by other agents to produce IL-9. The production of IL-9 was shown to be enhanced in cultures of CD4 in which additional cytokines were added to the polarizing cocktail, along with IL-4 and TGF-β: Angkasekwinai et al.40 found that intracellular IL-9 was remarkably elevated by the addition of IL-25, whereas a similar effect of IL-1 was reported in mouse41,42 and human cultures.43 Interestingly, Uyttenhove et al. reported recently that IL-9-producing T-cells are also generated in cultures in which IL-4 is replaced by IL-1.33
The existence of lineages of Th9 cells was further established by the findings of two transcription factors that are crucial for Th9 generation, i.e., PU.1 and interferon-regulatory factor 4 (IRF4). The critical role of PU.1 was shown by Kaplan and his colleagues,30 whereas the role of IRF4 was demonstrated by the group of Bopp.32 Deficiency in either one of these factors affected the generation of Th9 cells and reduced the pathological changes of asthma. Further, the combinational expression of both PU.1 and IRF4 is assumed to synergistically regulate IL-9 production in Th9 cells.
IV. ANTIGEN-SPECIFIC TH9 CELL LINES
Th lineages are generated in culture by activation of naïve CD4 cells during the polarization process induced by the cytokine cocktail. In the majority of published studies, the concurrent CD4 activation was mediated by anti-CD3 antibodies, usually in combination with anti-CD28 antibodies.14,29,30 The natural mode of CD4 cell activation, however, is by the specific antigen presented by APC, and recent studies by Van Snick and colleagues33 and our group34 have yielded data concerning Th9 lineages generated by this natural mode of activation. It is of note that a comparison between lines generated by activation with either the antibodies or the antigen/APC revealed remarkable differences between their phenotypes (Tan et al., unpublished data).
The study by Van Snick group33 analyzed activities of Th9 lines generated mainly with CD4 cells from pre-sensitized mice, following activation in vitro with the immunizing antigen. In our study, on the other hand, Th9 cell lines were generated by activating naïve CD4 cells with their target antigen. The CD4 cells in our study transgenically express a TCR specific against hen egg lysozyme (HEL) and were strongly activated in culture upon exposure to HEL presented by APC.34 As described below, analysis of antigen-activated Th9 cells, by both in vivo and in vitro methods, revealed several unique features that differentiate this lineage from all other known Th lineages.
V. UNIQUE KINETICS OF IL-9 PRODUCTION
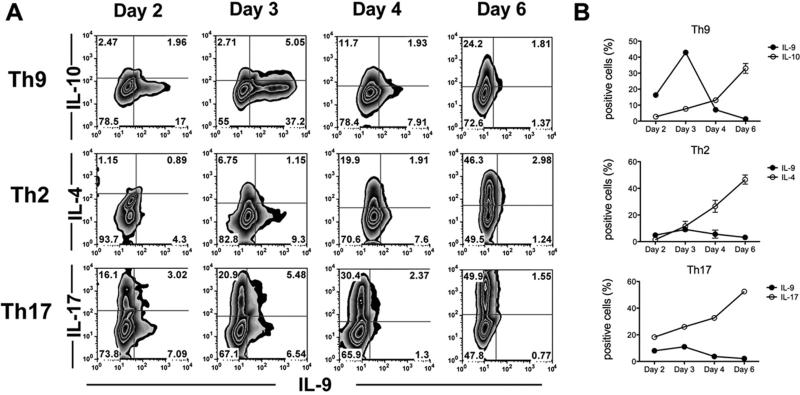
The kinetics of IL-9 production were analyzed in vitro by determining, at different time points of incubation, the levels of the mRNA transcript, the secreted amounts of the cytokine, and the proportions of cells that express it (Ref. 33 and Fig. 1A). The analysis was best achieved by the third parameter, in which IL-9 intracellular production was determined by flow cytometry. In addition to IL-9, all Th9 lines express IL-1014,29,34 and the uniqueness of IL-9 production was manifested when compared to that of IL-10 (Fig. 1). A remarkable proportion of Th9 cells (~15%) expressed IL-9 as soon as day 2 of incubation, reaching the peak (~45%) on day 3 and declining sharply, to just marginal levels, on day 4. In contrast, intracellular IL-10 in these cells increased gradually, reaching the highest level on day 6 in culture (Fig. 1). Moreover, the striking uniqueness of IL-9 production was further demonstrated in studies in which the kinetics of intracellular IL-9 expression was compared with that of IL-4 or IL-17 by cells of Th2 and Th17 lineages, respectively. Similar to IL-10 production by Th9 cells, levels of IL-4 and IL-17 increased gradually and reached their highest values on day 6 in culture (Fig. 1A). It is noteworthy that low levels of IL-9 are also made by Th2 and Th17 cell lineages, and the kinetics of IL-9 production by these cells resembled that by Th9 cells and strikingly differed from those of the corresponding cytokines, IL-4 and IL-17, respectively (Fig. 1B).
FIGURE 1.
Production kinetics of IL-9 differs from that of other cytokines. Naïve CD4 cells of 3A9 mice were activated with HEL and APC in the presence of polarizing cytokines specific for Th9, Th2 or Th17. Cells were collected at different time points, as indicated, and intracellular expression of IL-9, IL-10, IL-4, and IL-17 was measured by flow cytometry. (A) Data of a representative experiment. (B) Mean percent of cells expressing intracellularly the indicated cytokines. This figure is modified from Ref. 33, with permission from the Journal of Immunology.
The in vitro system also made it possible to analyze the “secondary” immune response of Th9 cells, by testing the cytokine production by Th9 cells in cultures re-stimulated with the specific antigen and the polarizing cytokines, following a period of resting.34 The cells responded with vigorous production of both IL-9 and IL-10, with ~4-fold higher levels than those following the first stimulation. Importantly, the intracellular production of IL-9 reached the peak just one day after re-stimulation, followed by a sharp decline on day 2; in contrast, IL-10 levels remained high throughout the 4 days of culture following re-stimulation.34
VI. PRODUCTION OF NON-IL-9 CYTOKINES BY TH9 CELLS
Antigen-activated Th9 cells produce marginal or low levels of other cytokines, namely, interferon-γ and IL-17,34 but as mentioned above, lines of antigen-specific Th9 cells produce high levels of IL-10, similar to the levels of IL-9.34 Similar observations on production of cytokines other than IL-9 were made in studies using anti-CD3/CD28 antibodies for generation of Th9.14,29 As shown above, the production kinetics of IL-10 are entirely different from that of IL-9, and we have also shown that the two cytokines are mainly produced by two different subpopulations within the Th9 lineages.34 The function of IL-10 producing “Th9” cells is not known, but it is possible they are involved in suppressive activities attributed to the Th9 population, as mentioned above.
VII. PLASTICITY OF TH9 CELLS
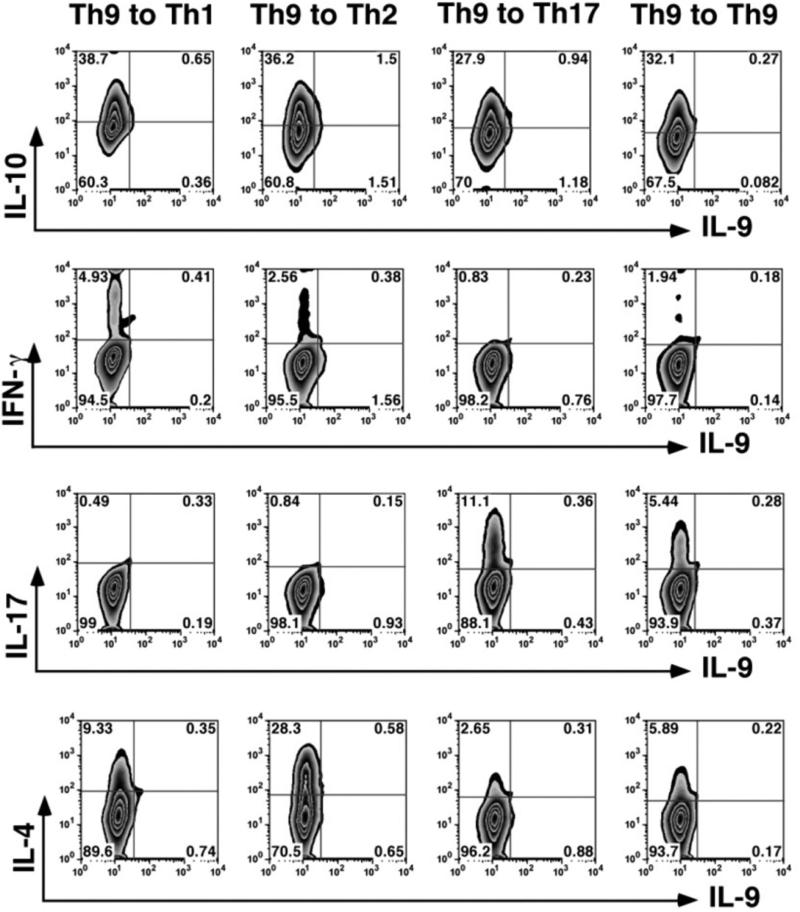
Studies of recent years revealed that Th cells exhibit various degrees of plasticity, namely, the capacity to change their phenotype when exposed to new cytokine environments.43–46 When cultured in media with polarization cytokine cocktails specific for Th1 or Th17, Th9 cells showed low levels of phenotype switching, but a substantial portion of Th9 acquired the Th2 phenotype (Fig. 2).34 The selective readiness to acquire the Th2 phenotype is in line with the close relationship between Th2 and Th9; Veldhoen et al.14 reported that Th9 lineages are generated by culturing Th2 cells in medium supplemented with TGF-β, without IL-4. It is noteworthy that, unlike the observations in vitro, a large portion of Th9 cells acquire the phenotype of Th1 and produce IFN-γ in the target organ, the eye in our study.34
FIGURE 2.
Assessment of Th9 cell plasticity. Th9 cells collected following 3 days of activation/polarization were expanded with IL-2 for 4 days, washed, and reactivated for 3 days with HEL and APC in the presence of polarizing cytokines specific for Th1, Th2, Th17, and Th9. Intracellular expression of IL-10, IFN-γ, IL-17, IL-4, and IL-9. A representative experiment; similar data were obtained in another experiment. This figure is modified from Ref. 33, with permission from the Journal of Immunology.
VIII. PATHOGENICITY OF TH9 CELLS
IL-9 plays a major role in the protection against and expulsion of helminthes, but similar to other molecules of the same function (e.g., IgE), IL-9 participates in the pathogenic processes of allergy, in particular asthma.15–17 In addition, Th9 cells were found to promote pathogenic processes,29 and data showing a direct pathogenic effect of Th9 were provided by Jager et al.,22 who induced severe EAE by using Th9 lines generated by activation with the target antigen, followed by re-activation with plate-bound anti-CD3/CD28 antibodies.
In our study,34 antigen-stimulated Th9 cells were examined for their pathogenicity using a system in which Th9 cells specific to HEL are injected into recipients transgenically expressing HEL in their eyes. Moderate inflammation developed in these recipients when the injected Th9 cells were harvested after 3 days in culture, at a time point when the expression of IL-9 reached its peak. In contrast, no disease was induced by Th9 harvested just one day later, on day 4 of incubation, when the IL-9 level was dramatically lower. The possible relationship between IL-9 and the expression of IL-9 by antigen-stimulated Th9 cells was further indicated by the finding that the inflammation severity was elevated when the injected Th9 cells produced higher levels of IL-9 by adding IL-1 and IL-25 to the polarizing cytokine cocktail (unpublished data).
A unique feature of Th9-induced inflammation is the actual absence of IL-9-producing cells at the affected site:34 flow cytometric analysis of inflammatory cells collected from eyes with Th9-induced inflammation failed to detect cells expressing IL-9. Similarly, only marginal proportions of cells expressing IL-9 were detected by Jager et al. in inflamed central nervous system of mice with EAE induced by antibody-activated Th9 cells.22 These observations are in contrast to the findings of large portions of cells producing IFN-γ or IL-17 in sites of inflammation induced by Th1 or Th17, respectively.44–47 As discussed in the Comments section below, we attribute this absence of cells expressing IL-9 to the re-exposure of the transferred Th9 cells to their target antigen, HEL, in the recipient's eye. In support of this assumption, we found that, unlike in the eye, a considerable portion of the transferred cells in the recipient's circulation retained their IL-9 expression.34
IX. COMMENTS
IL-9 is critical for defense against invasion by helminthes and it is conceivable that certain unique features that characterize this cytokine and the cells that produce it are related to this primordial function. The same unique features are also apparently related to the involvement of IL-9 in the allergic response. Both processes require quick responses, and IL-9 production by naïve CD4 cells was found in our study to reach its peak production as soon as day 3 of incubation, remarkably sooner than other tested cytokines (Fig. 1). Moreover, restimulation of pre-activated (“memory”) Th9 cells triggered an essentially immediate response.34 It may be speculated that memory Th9 cells exist and provide immediate high levels of IL-9 upon receiving the re-stimulation. It is also of interest that He et al.48 observed an abundance of Th9 cells in the jejunum of mice developing allergic response in that tissue 48 hours following the initiation of the response. These authors suggested, therefore, that Th9 cells serve as a link between immediate and late-phase allergic response.
Of particular interest is the sharp decline in IL-9 production that followed the rapid production of this cytokine. In our experimental system the intracellular level of IL-9 declined from its peak on day 3 to a level approximately 5-fold lower on day 4.34 The uniqueness of the steep decline of IL-9 level is particularly emphasized in view of the gradual increase in production of IL-10 by cells in the same Th9 cultures (Fig. 1). Furthermore, a gradual increase in production was also observed with other cytokines, IL-4 and IL-17.34 The sharp decline in IL-9 production in cultures stimulated with the target antigen, HEL in our study,34 provides an explanation for the observation that IL-9-expressing cells could not be detected at sites of inflammation mediated by Th9. The role and fate of Th9 cells following secretion of the cytokine are not known. A portion of these cells is assumed, however, to become memory cells.
There is still much unknown about IL-9 and the cells that produce it, but the data collected so far underscore the uniqueness of the IL-9 system. It can be expected that future studies will shed additional new light on the relationship between these unique features and the functions of Th9 and their products.
ACKNOWLEDGMENT
The research cited in this paper was supported by the Intramural Research Program of the National Eye Institute, NIH.
ABBREVIATIONS
- APC
antigen presenting cells
- CD
the cluster of differentiation
- EAE
experimental autoimmune encephalomyelitis
- HEL
hen egg lysozyme
- IFN-γ
interferon-gamma
- Ig
immunoglobulin
- IL
interleukin
- IRF4
interferon-regulatory factor 4
- NKT
natural killer T cells
- TCR
T cell receptor
- Th
T helper
- TGF-β
transforming growth factor beta
- Treg
regulatory T cells
REFERENCES
- 1.Uyttenhove C, Simpson RJ, Van Snick J. Functional and structural characterization of P40, a mouse glycoprotein with T-cell growth factor activity. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6934–8. doi: 10.1073/pnas.85.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Snick J, Goethals A, Renauld JC, Van Roost E, Uyttenhove C, Rubira MR, Moritz RL, Simpson RJ. Cloning and characterization of a cDNA for a new mouse T cell growth factor (P40). J Exp Med. 1989 Jan 1;169(1):363–8. doi: 10.1084/jem.169.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noelle RJ, Nowak EC. Cellular sources and immune functions of interleukin-9. Nat Rev Immunol. 2010 Oct;10(10):683–7. doi: 10.1038/nri2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goswami R, Kaplan MH. A brief history of IL-9. J Immunol. 2011 Mar 15;186(6):3283–8. doi: 10.4049/jimmunol.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh CK, Raible D, Geba GP, Molfino NA. Biology of the interleukin-9 pathway and its therapeutic potential for the treatment of asthma. Inflamm Allergy Drug Targets. 2011 Jun;10(3):180–6. doi: 10.2174/187152811795564073. [DOI] [PubMed] [Google Scholar]
- 6.Perumal NB, Kaplan MH. Regulating Il9 transcription in T helper cells. Trends Immunol. 2011 Apr;32(4):146–50. doi: 10.1016/j.it.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soroosh P, Doherty TA. Th9 and allergic disease. Immunology. 2009 Aug;127(4):450–8. doi: 10.1111/j.1365-2567.2009.03114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poulin LF, Richard M, Le Moine A, Kiss R, McKenzie AN, Goldman M, Renauld JC, Van Snick J, Braun MY. Interleukin-9 promotes eosinophilic rejection of mouse heart allografts. Transplantation. 2003 Aug 15;76(3):572–7. doi: 10.1097/01.TP.0000071201.32424.D2. [DOI] [PubMed] [Google Scholar]
- 9.Knoops L, Louahed J, Van Snick J, Renauld JC. IL-9 promotes but is not necessary for systemic anaphylaxis. J Immunol. 2005 Jul 1;175(1):335–41. doi: 10.4049/jimmunol.175.1.335. [DOI] [PubMed] [Google Scholar]
- 10.Arras M, Louahed J, Heilier JF, Delos M, Brombacher F, Renauld JC, Lison D, Huaux F. IL-9 protects against bleomycin-induced lung injury: involvement of prostaglandins. Am J Pathol. 2005 Jan;166(1):107–15. doi: 10.1016/S0002-9440(10)62236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Temann UA, Geba GP, Rankin JA, Flavell RA. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J Exp Med. 1998 Oct 5;188(7):1307–20. doi: 10.1084/jem.188.7.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Temann UA, Ray P, Flavell RA. Pulmonary overexpression of IL-9 induces Th2 cytokine expression, leading to immune pathology. J Clin Invest. 2002 Jan;109(1):29–39. doi: 10.1172/JCI13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan WI, Richard M, Akiho H, Blennerhasset PA, Humphreys NE, Grencis RK, Van Snick J, Collins SM. Modulation of intestinal muscle contraction by interleukin-9 (IL-9) or IL-9 neutralization: correlation with worm expulsion in murine nematode infections. Infect Immun. 2003 May;71(5):2430–8. doi: 10.1128/IAI.71.5.2430-2438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008 Dec;9(12):1341–6. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 15.Cheng G, Arima M, Honda K, Hirata H, Eda F, Yoshida N, Fukushima F, Ishii Y, Fukuda T. Anti-interleukin-9 antibody treatment inhibits airway inflammation and hyperreactivity in mouse asthma model. Am J Respir Crit Care Med. 2002 Aug 1;166(3):409–16. doi: 10.1164/rccm.2105079. [DOI] [PubMed] [Google Scholar]
- 16.Nicolaides NC, Holroyd KJ, Ewart SL, Eleff SM, Kiser MB, Dragwa CR, Sullivan CD, Grasso L, Zhang LY, Messler CJ, Zhou T, Kleeberger SR, Buetow KH, Levitt RC. Interleukin 9: a candidate gene for asthma. Proc Natl Acad Sci U S A. 1997 Nov 25;94(24):13175–80. doi: 10.1073/pnas.94.24.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, Sparwasser T, Helmby H, Stockinger B. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12(11):1071–7. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ying S, Meng Q, Kay AB, Robinson DS. Elevated expression of interleukin-9 mRNA in the bronchial mucosa of atopic asthmatics and allergen-induced cutaneous late-phase reaction: relationships to eosinophils, mast cells and T lymphocytes. Clin Exp Allergy. 2002 Jun;32(6):866–71. doi: 10.1046/j.1365-2222.2002.01376.x. [DOI] [PubMed] [Google Scholar]
- 19.McMillan SJ, Bishop B, Townsend MJ, McKenzie AN, Lloyd CM. The absence of interleukin 9 does not affect the development of allergen-induced pulmonary inflammation nor airway hyperreactivity. J Exp Med. 2002 Jan 7;195(1):51–7. doi: 10.1084/jem.20011732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryce PJ. Revolution 9: the backwards and forwards evidence surrounding interleukin-9. Am J Respir Crit Care Med. 2011 Apr 1;183(7):834–5. doi: 10.1164/rccm.201009-1464ED. [DOI] [PubMed] [Google Scholar]
- 21.Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, Ahrens R, Seidu L, McKenzie A, Strait R, Finkelman FD, Foster PS, Matthaei KI, Rothenberg ME, Hogan SP. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008 Apr 14;205(4):897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009 Dec 1;183(11):7169–77. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nowak EC, Weaver CT, Turner H, Begum-Haque S, Becher B, Schreiner B, Coyle AJ, Kasper LH, Noelle RJ. IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med. 2009 Aug 3;206(8):1653–60. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dodd JS, Lum E, Goulding J, Muir R, Van Snick J, Openshaw PJ. IL-9 regulates pathology during primary and memory responses to respiratory syncytial virus infection. J Immunol. 2009 Dec 1;183(11):7006–13. doi: 10.4049/jimmunol.0900085. [DOI] [PubMed] [Google Scholar]
- 25.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, Bettelli E, Oukka M, van Snick J, Renauld JC, Kuchroo VK, Khoury SJ. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009 Aug 4;106(31):12885–90. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephens GL, Swerdlow B, Benjamin E, Coyle AJ, Humbles A, Kolbeck R, Fung M. IL-9 is a Th17-derived cytokine that limits pathogenic activity in organ-specific autoimmune disease. Eur J Immunol. 2011 Apr;41(4):952–62. doi: 10.1002/eji.201040879. [DOI] [PubMed] [Google Scholar]
- 27.Gessner A, Blum H, Rollinghoff M. Differential regulation of IL-9-expression after infection with Leishmania major in susceptible and resistant mice. Immunobiology. 1993 Dec;189(5):419–35. doi: 10.1016/S0171-2985(11)80414-6. [DOI] [PubMed] [Google Scholar]
- 28.Dugas B, Renauld JC, Pene J, Bonnefoy JY, Peti-Frere C, Braquet P, Bousquet J, Van Snick J, Mencia-Huerta JM. Interleukin-9 potentiates the interleukin-4-induced immunoglobulin (IgG, IgM and IgE) production by normal human B lymphocytes. Eur J Immunol. 1993 Jul;23(7):1687–92. doi: 10.1002/eji.1830230743. [DOI] [PubMed] [Google Scholar]
- 29.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, Khoury S, Oukka M, Kuchroo VK. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol. 2008 Dec;9(12):1347–55. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, Jabeen R, McKinley C, Ahyi AN, Han L, Nguyen ET, Robertson MJ, Perumal NB, Tepper RS, Nutt SL, Kaplan MH. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010 May 2;11(6):527–34. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Putheti P, Awasthi A, Popoola J, Gao W, Strom TB. Human CD4 memory T cells can become CD4+IL-9+ T cells. PLoS One. 2010;5(1):e8706. doi: 10.1371/journal.pone.0008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, Gerlitzki B, Hoffmann M, Ulges A, Taube C, Dehzad N, Becker M, Stassen M, Steinborn A, Lohoff M, Schild H, Schmitt E, Bopp T. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010 Aug 27;33(2):192–202. doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 33.Uyttenhove C, Brombacher F, Van Snick J. TGF-beta interactions with IL-1 family members trigger IL-4-independent IL-9 production by mouse CD4(+) T cells. Eur J Immunol. 2010 Aug;40(8):2230–5. doi: 10.1002/eji.200940281. [DOI] [PubMed] [Google Scholar]
- 34.Tan C, Aziz MK, Lovaas JD, Vistica BP, Shi G, Wawrousek EF, Gery I. Antigen-specific Th9 cells exhibit uniqueness in their kinetics of cytokine production and short retention at the inflammatory site. J Immunol. 2010 Dec 1;185(11):6795–801. doi: 10.4049/jimmunol.1001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eller K, Wolf D, Huber JM, Metz M, Mayer G, McKenzie AN, Maurer M, Rosenkranz AR, Wolf AM. IL-9 production by regulatory T cells recruits mast cells that are essential for regulatory T cell-induced immune suppression. J Immunol. 2011 Jan 1;186(1):83–91. doi: 10.4049/jimmunol.1001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, Strom TB, Zheng XX, Noelle RJ. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006 Aug 31;442(7106):997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 37.Jones TG, Hallgren J, Humbles A, Burwell T, Finkelman FD, Alcaide P, Austen KF, Gurish MF. Antigen-induced increases in pulmonary mast cell progenitor numbers depend on IL-9 and CD1d-restricted NKT cells. J Immunol. 2009 Oct 15;183(8):5251–60. doi: 10.4049/jimmunol.0901471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lauwerys BR, Garot N, Renauld JC, Houssiau FA. Cytokine production and killer activity of NK/T-NK cells derived with IL-2, IL-15, or the combination of IL-12 and IL-18. J Immunol. 2000 Aug 15;165(4):1847–53. doi: 10.4049/jimmunol.165.4.1847. [DOI] [PubMed] [Google Scholar]
- 39.Schmitt E, Germann T, Goedert S, Hoehn P, Huels C, Koelsch S, Kuhn R, Muller W, Palm N, Rude E. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J Immunol. 1994 Nov 1;153(9):3989–96. [PubMed] [Google Scholar]
- 40.Angkasekwinai P, Chang SH, Thapa M, Watarai H, Dong C. Regulation of IL-9 expression by IL-25 signaling. Nat Immunol. 2010 Mar;11(3):250–6. doi: 10.1038/ni.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmitt E, Beuscher HU, Huels C, Monteyne P, van Brandwijk R, van Snick J, Ruede E. IL-1 serves as a secondary signal for IL-9 expression. J Immunol. 1991 Dec 1;147(11):3848–54. [PubMed] [Google Scholar]
- 42.Helmby H, Grencis RK. Interleukin 1 plays a major role in the development of Th2-mediated immunity. Eur J Immunol. 2004 Dec;34(12):3674–81. doi: 10.1002/eji.200425452. [DOI] [PubMed] [Google Scholar]
- 43.O'Shea JJ, Hunter CA, Germain RN. T cell heterogeneity: firmly fixed, predominantly plastic or merely malleable? Nat Immunol. 2008 May;9(5):450–3. doi: 10.1038/ni0508-450. [DOI] [PubMed] [Google Scholar]
- 44.Shi G, Cox CA, Vistica BP, Tan C, Wawrousek EF, Gery I. Phenotype switching by inflammation-inducing polarized Th17 cells, but not by Th1 cells. J Immunol. 2008 Nov 15;181(10):7205–13. doi: 10.4049/jimmunol.181.10.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Locksley RM. Nine lives: plasticity among T helper cell subsets. J Exp Med. 2009 Aug 3;206(8):1643–6. doi: 10.1084/jem.20091442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009 May;30(5):646–55. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Shi G, Ramaswamy M, Vistica BP, Cox CA, Tan C, Wawrousek EF, Siegel RM, Gery I. Unlike Th1, Th17 cells mediate sustained autoimmune inflammation and are highly resistant to restimulation-induced cell death. J Immunol. 2009 Dec 1;183(11):7547–56. doi: 10.4049/jimmunol.0900519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He SH, Liu ZQ, Chen X, Song CH, Zhou LF, Ma WJ, Cheng L, Ou Y, Tang SO, Yang PC. IL-9+ IL-1 0+T cells link immediate allergic response to late phase reaction. Clin Exp. Immunol. 2011 Jul 1;165(1):29–37. doi: 10.1111/j.1365-2249.2011.04394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]