Abstract
The right ventricular (RV) apex has been the standard pacing site since the development of implantable pacemaker technology. Although RV pacing was initially only utilized for the treatment of severe bradyarrhythmias usually due to complete heart block, today the indications for and implantation of RV pacing devices is dramatically larger. Recently, the adverse effects of chronic RV apical pacing have been described including an increased risk of heart failure and death. This review details the detrimental effects of RV apical pacing and their shared hemodynamic pathophysiology. In particular, the role of RV apical pacing induced ventricular dyssynchrony is highlighted with a specific focus on differential outcome based upon QRS morphology at implant.
Keywords: right ventricular pacing, down side
Introduction
The development of implantable pacemaker technology in the mid 20th century proved life saving for many patients with bradyarrhythmias [1]. In the intervening decades the indications for and volumes of permanent pacemaker placement have expanded [2-4] concurrent with a geographically dependent aging population. Right ventricular (RV) apical lead placement rapidly became standard practice secondary to the ease of site accessibility and lead stability. In most situations, RV apical pacing is effective and well tolerated.
However, an increasing amount of data has recently raised questions about the safety of RV apical pacing [5-14]. These safety concerns arise from the suggestion that RV apical pacing may have detrimental effects on cardiac structure and left ventricular (LV) function [15-17]. This is likely the result of the pathologic abnormal electrical and directly related abnormal mechanical activation of the ventricles seen as a consequence of RV apical pacing. An understanding of this pathophysiology has driven the development of pacing technology to limit the need for right ventricular apical pacing and the search for improved methods of ventricular pacing.
This review details the detrimental effects of RV apical pacing and its associated hemodynamic pathophysiology. In particular, the role of RV apical pacing induced ventricular dyssynchrony is highlighted with specific focus on differential outcome based upon QRS morphology at implant. Alternative and developing pacing strategies for patients with a permanent pacing indication including cardiac resynchronization therapy (CRT), alternative pacing sites, leads, programming configurations and energy sources are also discussed.
RV Apical Pacing and Outcome: Clinical Data
For decades, RV apical pacing proved to be an effective therapy for sinus node disease [11], atrio-ventricular (AV) block [10], drug-refractory atrial fibrillation [18] and some forms of LV dysfunction [19]. A number of randomized studies have focused on the optimal pacing configuration and mode dependent on the pacing indication [6,10-14]. In addition to answering their intended questions, these studies have also shed light, although often indirectly, on the association between RV apical pacing and adverse cardiovascular outcome.
Deleterious effects of RV apical pacing: Pacemaker Studies
Studies that have shown the deleterious effects of RV apical pacing can be categorized by the indication of pacemaker implantation and by the mode of pacing. Studies enrolling patients with sinus node dysfunction compared VVI with AAI pacing or DDD with AAI pacing whereas those enrolling patients with AV block compared the effects of DDD with VVI pacing.
Andersen et al [5] investigated 225 patients with sinus node dysfunction (SND), through a comparison of VVI to AAI pacing and found significantly higher cardiovascular mortality, incidence of heart failure (HF) and NYHA functional class in the ventricular pacemaker group.
Several clinical studies [14,20-22] in SND patients displayed that DDD pacing compared to AAI pacing induces left atrial dilation and, in the case of a high proportion of RV pacing, also reduces LV function, myocardial relaxation and myocardial blood flow. The DANPACE (Danish Multicenter Randomized Study on AAI Versus DDD Pacing in SND) study [13,14] was designed to prospectively determine if atrial pacing is superior to dual chamber pacing. DANPACE randomized 177 patients with isolated SND (without any significant atrio-ventricular conduction disturbance) to one of three pacing modes: AAIR, DDDR with a short atrio-ventricular (AV) delay (110-150 ms) and DDDR with a long atrio-ventricular delay (>250 ms). As expected, there was a significant difference in the frequency of ventricular pacing between modes: AAIR (0%), DDDR long AV (17%) and DDDR-short AV (90%). This programming dependent increase in frequency of RV pacing was associated with an unadjusted increased risk of atrial fibrillation (AF) and stroke. The risk for AF was lowest with atrial pacing: AAIR (3% per year), followed by DDDR-long (8.2% per year) and DDDR-short (11.7% per year), and there was a trend for fewer thrombo-embolic events with atrial pacing: AAIR (1.9% per year), followed by DDDR-long (2.2% per year) and DDDR-short (4.0% per year) [14].
Providing a possible mechanism for the increase in both endpoints, Nielsen et al. demonstrated that the use of dual-chamber pacing (i.e. high RV pacing) is associated with left atrial enlargement when compared to atrial pacing alone [14]. This suggests that one of the deleterious effects of right ventricular pacing may be increased atrial pressure, resulting in structural atrial remodeling and increased risk of AF.
Several studies of DDD vs. VVI only pacing in patients with AV block [10,11], or both AV block and SND [12], generated the hypothesis that RV apical pacing may be detrimental (Table 1). The expectation was that DDD pacing would be beneficial over RV apical only (VVI) pacing secondary to maintenance of AV synchrony, resulting in a reduction in heart failure (HF), cardiovascular mortality, AF and stroke. Within these studies, only AF was significantly reduced with DDD pacing leaving questions regarding the importance of maintaining AV synchrony on heart failure and mortality.
Table 1.
Pacing and ICD studies examining RV pacing and Outcome
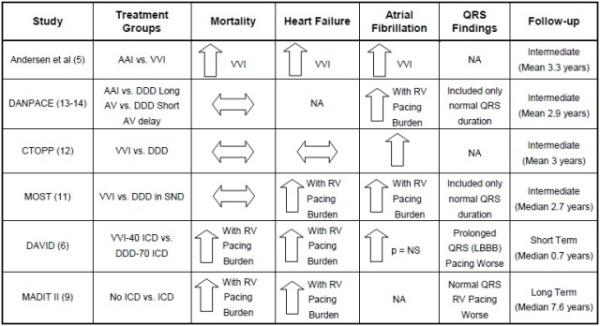
A subsequent subgroup analysis of MOST (MOde Selection Trial) among patients with QRS < 120 msec shed light on a possible underlying reason for the negative outcomes of these studies [11]. It demonstrated a strong association between RV pacing and the risk of HF events and AF in both DDD (mean 90% RV pacing burden, n = 707) and VVI pacing (mean 50% RV pacing burden, n = 632) groups. More specifically, patients with greater than 40% of ventricular pacing burden in the DDD group and > 80% of ventricular pacing in the VVI pacing group had more than two fold increased risk for HF events (DDD adjusted hazard ratio (HR): 2.60; 95% confidence interval (CI): 1.05 to 6.47; p < 0.05; VVI HR: 2.50; 95% CI: 1.44 to 4.36; p < 0.05). Similarly, each 25% increase in RV pacing burden was associated with an approximate mean increase of 28% (adjusted 36% for DDD and 21% for VVI only) in AF. The similar increase in HF and AF with DDD and VVI only pacing supports the notion that maintenance of AV synchrony does not convey a risk reduction in either. Rather, the RV apical pacing burden outweighed any benefit of AV synchrony in the DDD group and was the primary driver behind the negative trial result. Freudenberger et al, [23] examining more than 11,000 patients who underwent pacemaker implantation, found that permanent dual chamber or ventricular pacing in patients who did not have HF before implantation, significantly increased their risk for HF hospitalizations or HF-related deaths compared with matched control group.
Deleterious effects of RV apical pacing: Defibrillator Studies
The DAVID (Dual Chamber and Implantable Defibrillator) trial further substantiated the association between RV pacing and adverse cardiovascular outcome [6]. Operating with the hypothesis that DDD pacing will convey a reduction in HF, 506 patients with a standard indication for ICD implant and no indication for pacing were randomized to "physiologic pacing" ICD with DDD backup heart rate 70 (DDD -70) vs. single chamber ICD with VVIR backup heart rate 40 (VVI - 40). After one year follow up, the combined endpoint of hospitalization for HF or death was significantly higher for the DDD - 70 group (26.7%) compared to the VVI - 40 group (16.1%) with an adjusted HR of 1.61 (95% CI 1.06-2.44, p = 0.03). The difference in the backup rate between the two groups resulted in a marked difference in the burden of RV pacing with DDD - 70 patients paced 60% of the time and VVI - 40 patients just 3% of the time. Congruent with findings from MOST, an RV pacing dose dependent positive relationship with adverse cardiovascular events was noted.
The MADIT II (Multicenter Automated Defibrillator Implantation Trial II) enrolled 1,232 patients with ischemic cardiomyopathy randomized to ICD vs. medical therapy in a 3:2 ratio. ICD configurations within the study included single chamber programmed VVI-40 (44%) and dual chamber programmed DDD-70 (56%). Steinberg et al reported the short term (median 1.5 years) follow up of RV pacing in the ICD arm dichotomized by a pacing burden of greater (high RV pacing) and less than 50% (low RV pacing) [8]. Patients with high RV pacing were older, had higher blood urea nitrogen levels, and were more likely to have wide QRS and LBBB compared with non-ICD patients or patients with low RV pacing. After multivariate adjustment, high RV pacing patients were at significantly increased risk of new or worsened HF (HR 1.93, p = 0.002) and appropriate ICD therapy for VT/VF (HR 1.50, p = 0.02). However, the mortality rates were similar for high (13%) and low (10%) RV pacing groups (adjusted HR 1.07, p = 0.78) [8].
Most recently, we have analyzed the association between percent RV pacing and long term mortality in MADIT II during an 8-year follow-up [9]. Patients were categorized into three subgroups: low RV pacing (< 50% pacing, n = 369), high RV pacing (≥ 50% pacing, n = 198), and no ICD (n = 490). During the first 3 years after enrollment, the benefit of the ICD was prominent both in patients with low RV pacing (adjusted 65% reduction in risk of death, P<0.001) and in those with high RV pacing (adjusted 62% reduction in risk of death, P<0.001); In contrast, during the late phase of the extended follow-up period (4-8 years) ICD therapy was associated with a significant survival benefit among patients in the low RV pacing subgroup (adjusted 40% reduction in mortality risk, P = 0.001) but not in the high RV pacing subgroup (adjusted HR = 0.89, P = 0.45). In addition, during the total 8 year follow up, high RV pacing was shown to be associated with a significant adjusted 40% (P = 0.01) increase in the risk of death compared with low RV pacing (Figure 1). Thus, the long-term benefit of an ICD in reducing mortality is prominent in patients with low RV pacing but attenuated in patients with high RV pacing, and patients with high versus low RV pacing have increased long term mortality risk. A reasonable interpretation of these findings is that frequent RV pacing resulted in ventricular dyssynchrony, development and deterioration of HF which takes several years to be translated into increased mortality.
Figure 1.
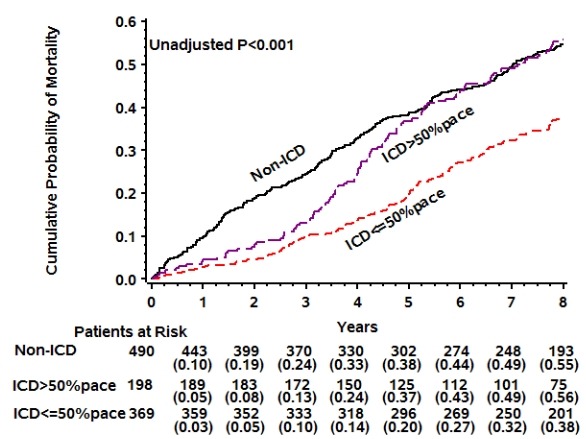
Probability of long-term mortality by percent of right ventricular pacing and ICD implantation in the MADIT II trial with extended follow up. (reproduced with permission of the publisher [9])
The Pathophysiology of RV Apical Pacing
The deleterious effects of RV apical pacing have been attributed to the abnormal electrical and mechanical activation induced secondary to this form of pacing. During RV apical pacing, the electrical wave front propagates through the myocardium, rather than through the His-Purkinje conduction system. As a result, the electrical wave front propagates more slowly and induces heterogeneity in electrical activation of the myocardium, comparable but not identical to left bundle branch block. This is characterized by wave front breakthrough at the interventricular septum and latest activation at the infero-posterior base of the left ventricle [24-26].
Similar to the changes in electrical activation of the ventricles, the mechanical activation pattern is altered during RV apical pacing. Importantly, not only does the anatomic onset of mechanical contraction differ, but also the resulting pattern of mechanical contraction [27]. Badke et al [28] detailed how apical pacing is associated with a diminished rate of change in left ventricular pressure (dP/dt) and an abnormal dyssynchronous contraction pattern. The paced region contract early at a time of low load, but then is stretched later in systole as the lateral wall finally contracts [28-29]. Hemodynamically, asynchronous myocardial contraction significantly decreases the stroke volume and right-shifts the left ventricular end-systolic pressure - volume relationship. Mismatch between the relaxation of early- and late-contracting regions leads to a decrease in left ventricular filling time. Thus, RV apical pacing leads to ventricular dyssynchronization, systolic and diastolic ventricular dysfunction, increase in wall stress and energetic inefficiency [29].
Beyond the hemodynamic effects of ventricular dyssynchrony, it has become clear that long-term RV pacing may also result in structural changes and adverse LV remodeling. Originally reported in1986 in an RV pacing dog complete heart block model [30], three months of RV pacing resulted in myofibrillar disarray in 75% of these dogs. In cardiomyopathies induced by high-rate right ventricular apical pacing, they observed significant differences in the expression of proteins involved in myocyte contraction, which were not seen in high-rate atrial-pacing-induced cardiomyopathies with preserved ventricular synchrony [31]. The lateral left ventricular free wall (late-activated) displayed the most pronounced cellular derangements, such as down-regulation of protein kinases, proteins involved in calcium homeostasis and intercellular connections [31]. In addition, changes in LV wall thickness (the early activated wall becomes thinner whereas the late activated wall becomes thicker) [17], LV remodeling [32], left atrial remodeling [33], functional mitral regurgitation [34], and perfusion abnormalities [35-36] all appear to play a role in the pathophysiology of RV apical pacing; predominately as downstream consequences of iatrogenic ventricular dyssynchrony.
RV Pacing and QRS morphology at implant
It has long been known that native LBBB can have profound hemodynamic effects due to ventricular dyssynchrony, particularly among patients with HF [37]. As detailed above, ventricular pacing contributes to the development or exacerbation of HF by producing an iatrogenic form of LBBB and ventricular dyssynchrony reducing systolic and diastolic ventricular function. Mechanical activation in patients with chronic RV pacing has been compared to those with native LBBB, with patients from both groups having intraventricular dyssynchrony, but RV pacing patients displaying greater interventricular dyssynchrony and more often had sites of earliest activation from the apex and inferior septum [38].
We explored the effects of RV pacing on ICD benefit according to LBBB at enrollment in MADIT-II during an extended long term follow-up of 8 years [9]. We found that high RV pacing was associated with increased long term mortality only in patients who did not have LBBB at baseline (Figure 2). Among patients without LBBB, high RV pacing was associated with an adjusted 63% (P = 0.002) increase in mortality risk compared with the low RV pacing subgroup, whereas among patients with LBBB there was no significant difference in mortality during long-term follow-up between high and low RV pacing patients (High vs. low RV pacing adjusted HR = 0.74, p=0.343; P value for interaction [QRS morphology by RV pacing burden] = 0.024). Consistent with our findings, Saad et al [39] showed in a small study (including 44 HF patients, 12 with LBBB) that high RV pacing is associated with poor outcome only in the absence of LBBB. These findings may suggest that dyssynchrony induced by RV apical pacing is somewhat worse than naturally occurring LBBB dyssynchrony. Alternatively, these findings may only suggest that RV pacing may not be harmful to patients with systolic HF and LBBB, as the incremental dyssynchrony induced by RV pacing is less significant.
Figure 2.
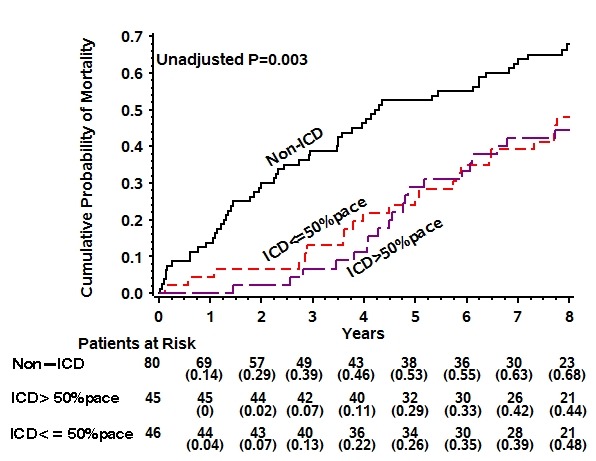
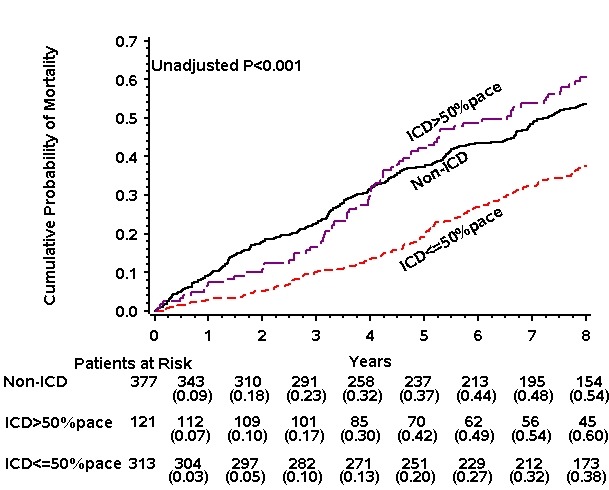
Probability of long-term mortality by percent of right ventricular pacing in patients with LBBB (A) and without LBBB (B) in the MADIT II trial with extended follow up. (reproduced with permission of the publisher [9])
In contrast to these findings, Hayes et al [40] in a substudy of the DAVID trial, found that patients programmed to high pacing volume (DDD-70) with an abnormal QRS duration (≥ 110 msec) had an unadjusted 25% increased risk of death or hospitalization for HF (p = 0.01). Breaking down the abnormal QRS duration group into BBB morphologies, the adverse outcome for the group as a whole appeared to be driven by the LBBB patients (30%) that experienced an unadjusted 40% increase in HF or death (p = 0.03).
Reconciling the stark differences among these studies remains difficult. The primary differences between the MADIT-II substudy and the DAVID substudy include different study endpoints (death in MADIT II vs. HF or death in DAVID), duration of follow up and indication for pacing. DAVID specifically randomized patients based upon pacing programming where the focus of MADIT II was the efficacy of primary prevention ICD therapy in ischemic cardiomyopathy. Within the MADIT II sub-analysis the primary limitation was the clinical differences between high and low RV pacing subgroups. We cannot completely exclude the possibility that sicker patients required more pacing and had poorer outcomes as a result. Furthermore, data on percent RV pacing were collected only among 79% of the 720 patients who received an ICD in MADIT II. Patients who were not included in this data analysis due to missing pacing information appeared to be sicker with an elevated in-trial mortality rate of 42% compared with 12% in patients analyzed in our study. The DAVID analysis was limited by size and follow up duration.
Minimizing the Detrimental Effects of RV Pacing
Methods to avoid the detrimental effects of RV pacing include device programming to minimize RV pacing, alternative pacing sites, and biventricular pacing. The current mainstay of programming based methods to avoid RV pacing includes AV search or MVP (managed ventricular pacing) mode. Such programming was evaluated in the SAVE PACe (Search AV Extension and Managed Ventricular Pacing for Promoting Atrioventricular Conduction) trial, where 1,065 patients with sinus node disease and intact AV conduction were randomized between conventional dual-chamber pacing and dual-chamber minimal ventricular pacing [41]. RV pacing burden was significantly reduced with MVP, compared with conventional dual-chamber ventricular pacing (9.1% vs. 99.0%, p < 0.001). After a mean follow-up of 1.7 years, the unadjusted development of persistent atrial fibrillation was significantly reduced with MVP (7.9% in minimal RV pacing vs. 12.7% in conventional dual-chamber pacing, p = 0.004). No significant difference in HF or mortality was noted with MVP programming.
When RV pacing is inevitable, the His bundle [42,43], right ventricular outflow tract [44,45] and right ventricular septum [46,47] have been studied as alternate pacing sites. Selectively pacing these sites may reduce pacing induced ventricular dyssynchrony [48-50] and potentially result in a reduction in ventricular volumes with improved LV function compared to RV apical pacing. However, with the small size, short term follow up and the absence of a reduction in HF or death within the randomized prospective studies, the benefit of alternative site RV pacing remains controversial.
For RV apical pacing dependent patients with abnormal LV function [51-56] or RV apical pacing associated HF [57,58], biventricular pacing has emerged as a viable option to minimize the detrimental effects of RV pacing. Although some studies have demonstrated a clear long-term benefit of CRT over RV pacing with regard to peak VO2 or functional class [51,53], others have demonstrated only modest [54,55,57,58] or minimal benefit [56].
The PACE trial [59] directly examined the efficacy of biventricular pacing within this population. Patients post biventricular pacemakers (177) were prospectively enrolled and randomized to receive biventricular pacing (89 patients) or RV apical pacing (88 patients). No significant differences in LV ejection fraction (61.5 ± 6.6% for RV pacing, 61.9 ± 6.7 for biventricular pacing, p = 0.86) or LV end systolic volume (28.6 ± 10.7ml for RV pacing, 28.6 ± 9.4ml for biventricular pacing, p = 0.71) were present at baseline. At 12 months, the mean LV ejection fraction was significantly lower in the RV pacing group than in the biventricular-pacing group (54.8 ± 9.1% vs. 62.2 ± 7.0%, p < 0.001). Similarly, the LV end-systolic volume was significantly higher in the RV pacing group than in the biventricular-pacing group (35.7 ± 16.3 ml vs. 27.6 ± 10.4 ml, p < 0.001), with a relative change from baseline of 25% (p < 0.001). These results support the detrimental effect of RV apical pacing manifest through adverse LV remodeling and deterioration in LV function with such effects prevented by biventricular pacing.
Conclusions
Right ventricular apical pacing is an integral part of the treatment of brady-arrhythmias for the majority of patients receiving pacemakers. Right ventricular apical pacing is, however, an often pathologic substitute for intrinsic ventricular activation over the His-Purkinje system. Several reports indicate that this form of pacing is detrimental potentially increasing the risk of heart failure episodes and death, particularly in patients with abnormal LV function. Further studies are needed to clarify the mechanisms underlying the deleterious effects of RV pacing, RV apical pacing burden to be avoided, and the specific risk factors for poor outcome among patients with high RV pacing burden. In the meantime, alternative pacemaker programming and configurations are available that can minimize the frequency and detrimental nature of ventricular pacing in many pacemaker patients. Additional research will determine if different forms of ventricular pacing, such as right ventricular outflow tract, RV septal pacing, or biventricular pacing will improve outcomes in patients who require ventricular stimulation.
References
- Zoll PM, et al. Use of external electric pacemaker in cardiac arrest. J Am Med Assoc . 1955;159:1428. doi: 10.1001/jama.1955.02960320004002. [DOI] [PubMed] [Google Scholar]
- Vardas PE, et al. Guidelines for cardiac pacing and cardiac resynchronization therapy. The Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Developed in collaboration with the European Heart Rhythm Association. Europace. 2007;9:959. doi: 10.1093/europace/eum189. [DOI] [PubMed] [Google Scholar]
- Mond HG, et al. The world survey of cardiac pacing and cardioverter defibrillators: calendar year 2001. Pacing Clin Electrophysiol . 2004;27:955. doi: 10.1111/j.1540-8159.2004.00565.x. [DOI] [PubMed] [Google Scholar]
- Uslan DZ, et al. Temporal trends in permanent pacemaker implantation: a population-based study. Am Heart J . 2008;155:896. doi: 10.1016/j.ahj.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen HR, et al. Long-term follow-up of patients from a randomised trial of atrial versus ventricular pacing for sick-sinus syndrome. Lancet . 1997;350:1210. doi: 10.1016/S0140-6736(97)03425-9. [DOI] [PubMed] [Google Scholar]
- Wilkoff BL, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA . 2002;288:3115. doi: 10.1001/jama.288.24.3115. [DOI] [PubMed] [Google Scholar]
- Lamas GA, et al. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med . 2002;346:1854. doi: 10.1056/NEJMoa013040. [DOI] [PubMed] [Google Scholar]
- Steinberg JS, et al. The clinical implications of cumulative right ventricular pacing in the multicenter automatic defibrillator trial II. J Cardiovasc Electrophysiol . 2005;16:359. doi: 10.1046/j.1540-8167.2005.50038.x. [DOI] [PubMed] [Google Scholar]
- Barsheshet A, et al. Long-term implications of cumulative right ventricular pacing among patients with an implantable cardioverter-defibrillator. Heart Rhythm . 2011;8:212. doi: 10.1016/j.hrthm.2010.10.035. [DOI] [PubMed] [Google Scholar]
- Toff WD, et al. Single-chamber versus dual-chamber pacing for high-grade atrioventricular block. N Engl J Med . 2005;353:145. doi: 10.1056/NEJMoa042283. [DOI] [PubMed] [Google Scholar]
- Sweeney MO, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation . 2003;107:2932. doi: 10.1161/01.CIR.0000072769.17295.B1. [DOI] [PubMed] [Google Scholar]
- Connolly SJ, et al. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. Canadian Trial of Physiologic Pacing Investigators. N Engl J Med. 2000;342:1385. doi: 10.1056/NEJM200005113421902. [DOI] [PubMed] [Google Scholar]
- Kristensen L, et al. Incidence of atrial fibrillation and thromboembolism in a randomised trial of atrial versus dual chamber pacing in 177 patients with sick sinus syndrome. Heart . 2004;90:661. doi: 10.1136/hrt.2003.016063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JC, et al. A randomized comparison of atrial and dual-chamber pacing in 177 consecutive patients with sick sinus syndrome: echocardiographic and clinical outcome. J Am Coll Cardiol . 2003;42:614. doi: 10.1016/s0735-1097(03)00757-5. [DOI] [PubMed] [Google Scholar]
- Lieberman R, et al. Ventricular pacing lead location alters systemic hemodynamics and left ventricular function in patients with and without reduced ejection fraction. J Am Coll Cardiol . 2006;48:1634. doi: 10.1016/j.jacc.2006.04.099. [DOI] [PubMed] [Google Scholar]
- Karpawich PP, et al. Altered cardiac histology following apical right ventricular pacing in patients with congenital atrioventricular block. Pacing Clin Electrophysiol . 1999;22:1372. doi: 10.1111/j.1540-8159.1999.tb00631.x. [DOI] [PubMed] [Google Scholar]
- van Oosterhout MF , et al. Asynchronous electrical activation induces asymmetrical hypertrophy of the left ventricular wall. Circulation . 1998;98:588. doi: 10.1161/01.cir.98.6.588. [DOI] [PubMed] [Google Scholar]
- Brignole M, et al. Assessment of atrioventricular junction ablation and DDDR mode-switching pacemaker versus pharmacological treatment in patients with severely symptomatic paroxysmal atrial fibrillation: a randomized controlled study. Circulation . 1997;96:2617. doi: 10.1161/01.cir.96.8.2617. [DOI] [PubMed] [Google Scholar]
- Brecker SJ, et al. Effects of dual-chamber pacing with short atrioventricular delay in dilated cardiomyopathy. Lancet . 1992;340:1308. doi: 10.1016/0140-6736(92)92492-x. [DOI] [PubMed] [Google Scholar]
- Nielsen JC, et al. Regional myocardial blood flow in patients with sick sinus syndrome randomized to long-term single chamber atrial or dual chamber pacing - effect of pacing mode and rate. J Am Coll Cardiol . 2000;35:1453. doi: 10.1016/s0735-1097(00)00593-3. [DOI] [PubMed] [Google Scholar]
- Leclercq C, et al. Hemodynamic importance of preserving the normal sequence of ventricular activation in permanent cardiac pacing. Am Heart J . 1995;129:1133. doi: 10.1016/0002-8703(95)90394-1. [DOI] [PubMed] [Google Scholar]
- Rosenqvist M, et al. Relative importance of activation sequence compared to atrioventricular synchrony in left ventricular function. Am J Cardiol . 1991;67:148. doi: 10.1016/0002-9149(91)90437-p. [DOI] [PubMed] [Google Scholar]
- Freudenberger RS, et al. Permanent pacing is a risk factor for the development of heart failure. Am J Cardiol . 2005;95:671. doi: 10.1016/j.amjcard.2004.10.049. [DOI] [PubMed] [Google Scholar]
- Vassallo JA, et al. Left ventricular endocardial activation during right ventricular pacing: effect of underlying heart disease. J Am Coll Cardiol . 1986;7:1228. doi: 10.1016/s0735-1097(86)80140-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez LM, et al. Variable patterns of septal activation in patients with left bundle branch block and heart failure. J Cardiovasc Electrophysiol . 2003;14:135. doi: 10.1046/j.1540-8167.2003.02421.x. [DOI] [PubMed] [Google Scholar]
- Auricchio A, et al. Characterization of left ventricular activation in patients with heart failure and left bundle-branch block. Circulation . 2004;109:1133. doi: 10.1161/01.CIR.0000118502.91105.F6. [DOI] [PubMed] [Google Scholar]
- Prinzen FW, et al. Relation between the pacing induced sequence of activation and left ventricular pump function in animals. Pacing Clin Electrophysiol . 2002;25:484. doi: 10.1046/j.1460-9592.2002.00484.x. [DOI] [PubMed] [Google Scholar]
- Badke FR, et al. Effects of ventricular pacing on regional left ventricular performance in the dog. Am J Physiol. 1980;238:H858. doi: 10.1152/ajpheart.1980.238.6.H858. [DOI] [PubMed] [Google Scholar]
- Prinzen FW, et al. Mapping of regional myocardial strain and work during ventricular pacing: experimental study using magnetic resonance imaging tagging. J Am Coll Cardiol . 1999;33:1735. doi: 10.1016/s0735-1097(99)00068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adomian GE, et al. Myofibrillar disarray produced in normal hearts by chronic electrical pacing. Am Heart J . 1986;112:79. doi: 10.1016/0002-8703(86)90682-4. [DOI] [PubMed] [Google Scholar]
- Park RC, et al. Effect of alteration of left ventricular activation sequence on the left ventricular end-systolic pressure-volume relation in closed-chest dogs. Circ Res . 1985;57:706. doi: 10.1161/01.res.57.5.706. [DOI] [PubMed] [Google Scholar]
- Vernooy K, et al. Ventricular remodeling during long-term right ventricular pacing following His bundle ablation. Am J Cardiol . 2006;97:1223. doi: 10.1016/j.amjcard.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Maurer G, et al. Two-dimensional echocardiographic contrast assessment of pacing-induced mitral regurgitation: relation to altered regional left ventricular function. J Am Coll Cardiol . 1984;3:986. doi: 10.1016/s0735-1097(84)80357-5. [DOI] [PubMed] [Google Scholar]
- Barold SS, et al. Pacemaker-induced mitral regurgitation. Pacing Clin Electrophysiol . 2005;28:357. doi: 10.1111/j.1540-8159.2005.09486.x. [DOI] [PubMed] [Google Scholar]
- Tse HF, et al. Long-term effect of right ventricular pacing on myocardial perfusion and function. J Am Coll Cardiol . 1997;29:744. doi: 10.1016/s0735-1097(96)00586-4. [DOI] [PubMed] [Google Scholar]
- Prinzen FW, et al. Redistribution of myocardial fiber strain and blood flow by asynchronous activation. Am J Physiol. 1990;259:H300. doi: 10.1152/ajpheart.1990.259.2.H300. [DOI] [PubMed] [Google Scholar]
- Manolis AS, et al. The deleterious consequences of right ventricular apical pacing: time to seek alternate site pacing. Pacing Clin Electrophysiol. 2006;29:298. doi: 10.1111/j.1540-8159.2006.00338.x. [DOI] [PubMed] [Google Scholar]
- Tanaka H, et al. Comparative mechanical activation mapping of RV pacing to LBBB by 2D and 3D speckle tracking and association with response to resynchronization therapy. JACC Cardiovasc Imaging . 2010;3:461. doi: 10.1016/j.jcmg.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Saad EB, et al. Frequency and associations of symptomatic deterioration after dual-chamber defibrillator implantation in patients with ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol . 2002;90:79. doi: 10.1016/s0002-9149(02)02396-2. [DOI] [PubMed] [Google Scholar]
- Hayes JJ, et al. Abnormal conduction increases risk of adverse outcomes from right ventricular pacing. J Am Coll Cardiol . 2006;48:1628. doi: 10.1016/j.jacc.2006.05.071. [DOI] [PubMed] [Google Scholar]
- Sweeney MO, et al. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med . 2007;357:1000. doi: 10.1056/NEJMoa071880. [DOI] [PubMed] [Google Scholar]
- Mera F, et al. A comparison of ventricular function during high right ventricular septal and apical pacing after his-bundle ablation for refractory atrial fibrillation. Pacing Clin Electrophysiol . 1999;22:1234. doi: 10.1111/j.1540-8159.1999.tb00606.x. [DOI] [PubMed] [Google Scholar]
- Deshmukh P, et al. Permanent, direct His-bundle pacing: a novel approach to cardiac pacing in patients with normal His-Purkinje activation. Circulation . 2000;101:869. doi: 10.1161/01.cir.101.8.869. [DOI] [PubMed] [Google Scholar]
- de Cock CC , et al. Comparison of the haemodynamic effects of right ventricular outflow-tract pacing with right ventricular apex pacing: a quantitative review. Europace . 2003;5:275. doi: 10.1016/s1099-5129(03)00031-x. [DOI] [PubMed] [Google Scholar]
- Vanerio G, et al. Medium- and long-term survival after pacemaker implant: Improved survival with right ventricular outflow tract pacing. J Interv Card Electrophysiol . 2008;21:195. doi: 10.1007/s10840-008-9238-x. [DOI] [PubMed] [Google Scholar]
- Victor F, et al. A randomized comparison of permanent septal versus apical right ventricular pacing: short-term results. J Cardiovasc Electrophysiol . 2006;17:238. doi: 10.1111/j.1540-8167.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- Kypta A, et al. Long-term outcomes in patients with atrioventricular block undergoing septal ventricular lead implantation compared with standard apical pacing. Europace . 2008;10:574. doi: 10.1093/europace/eun085. [DOI] [PubMed] [Google Scholar]
- Occhetta E, et al. Prevention of ventricular desynchronization by permanent para-Hisian pacing after atrioventricular node ablation in chronic atrial fibrillation: a crossover, blinded, randomized study versus apical right ventricular pacing. J Am Coll Cardiol . 2006;47:1938. doi: 10.1016/j.jacc.2006.01.056. [DOI] [PubMed] [Google Scholar]
- Yu CC, et al. Septal pacing preserving better left ventricular mechanical performance and contractile synchronism than apical pacing in patients implanted with an atrioventricular sequential dual chamber pacemaker. Int J Cardiol . 2007;118:97. doi: 10.1016/j.ijcard.2006.03.087. [DOI] [PubMed] [Google Scholar]
- Zanon F, et al. Direct His bundle pacing preserves coronary perfusion compared with right ventricular apical pacing: a prospective, cross-over mid-term study. Europace . 2008;10:580. doi: 10.1093/europace/eun089. [DOI] [PubMed] [Google Scholar]
- Doshi RN, et al. Left ventricular-based cardiac stimulation post AV nodal ablation evaluation (the PAVE study) J Cardiovasc Electrophysiol . 2005;16:1160. doi: 10.1111/j.1540-8167.2005.50062.x. [DOI] [PubMed] [Google Scholar]
- Horwich T, et al. Effects of resynchronization therapy on cardiac function in pacemaker patients "upgraded" to biventricular devices. J Cardiovasc Electrophysiol . 2004;15:1284. doi: 10.1046/j.1540-8167.2004.04279.x. [DOI] [PubMed] [Google Scholar]
- Kindermann M, et al. Biventricular versus conventional right ventricular stimulation for patients with standard pacing indication and left ventricular dysfunction: the Homburg Biventricular Pacing Evaluation (HOBIPACE) J Am Coll Cardiol . 2006;47:1927. doi: 10.1016/j.jacc.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Brignole M, et al. Comparative assessment of right, left, and biventricular pacing in patients with permanent atrial fibrillation. Eur Heart J . 2005;26:712. doi: 10.1093/eurheartj/ehi069. [DOI] [PubMed] [Google Scholar]
- Leclercq C, et al. Comparative effects of permanent biventricular and right-univentricular pacing in heart failure patients with chronic atrial fibrillation. Eur Heart J . 2002;23:1780. doi: 10.1053/euhj.2002.3232. [DOI] [PubMed] [Google Scholar]
- Albertsen AE, et al. Biventricular pacing preserves left ventricular performance in patients with high-grade atrio-ventricular block: a randomized comparison with DDD(R) pacing in 50 consecutive patients. Europace . 2008;10:314. doi: 10.1093/europace/eun023. [DOI] [PubMed] [Google Scholar]
- Leclercq C, et al. Comparative effects of permanent biventricular pacing for refractory heart failure in patients with stable sinus rhythm or chronic atrial fibrillation. Am J Cardiol . 2000;85:1154. doi: 10.1016/s0002-9149(00)00716-5. [DOI] [PubMed] [Google Scholar]
- Baker CM, et al. Addition of a left ventricular lead to conventional pacing systems in patients with congestive heart failure: feasibility, safety, and early results in 60 consecutive patients. Pacing Clin Electrophysiol . 2002;25:1166. doi: 10.1046/j.1460-9592.2002.01166.x. [DOI] [PubMed] [Google Scholar]
- Yu CM, et al. Biventricular pacing in patients with bradycardia and normal ejection fraction. N Engl J Med . 2009;361:2123. doi: 10.1056/NEJMoa0907555. [DOI] [PubMed] [Google Scholar]


