Abstract
The human brain undergoes protracted development, with dramatic changes in expression and regulation of emotion from childhood to adulthood. The amygdala is a brain structure that plays a pivotal role in emotion-related functions. Investigating developmental characteristics of the amygdala and associated functional circuits in children is important for understanding how emotion processing matures in the developing brain. The basolateral amygdala (BLA) and centromedial amygdala (CMA) are two major amygdalar nuclei that contribute to distinct functions via their unique pattern of interactions with cortical and subcortical regions. Almost nothing is currently known about the maturation of functional circuits associated with these amygdala nuclei in the developing brain. Using intrinsic connectivity analysis of functional magnetic resonance imaging data, we investigated developmental changes in functional connectivity of the BLA and CMA in twenty-four 7- to 9-y-old typically developing children compared with twenty-four 19- to 22-y-old healthy adults. Children showed significantly weaker intrinsic functional connectivity of the amygdala with subcortical, paralimbic, and limbic structures, polymodal association, and ventromedial prefrontal cortex. Importantly, target networks associated with the BLA and CMA exhibited greater overlap and weaker dissociation in children. In line with this finding, children showed greater intraamygdala connectivity between the BLA and CMA. Critically, these developmental differences were reproducibly identified in a second independent cohort of adults and children. Taken together, our findings point toward weak integration and segregation of amygdala circuits in young children. These immature patterns of amygdala connectivity have important implications for understanding typical and atypical development of emotion-related brain circuitry.
From childhood to adulthood, the development of functional brain networks underlies the maturation of increasingly nuanced cognitive and affective abilities (1, 2). In particular, human capabilities for a wide range of affective functions change dramatically from childhood to adulthood, suggesting remarkable maturation of the brain’s affective systems. The amygdala lies at the core of brain’s affective processing systems (3, 4) and multiple lines of research in human adults have implicated amygdala-centered networks in emotion (5–7). The amygdalar complex encompasses multiple anatomical subregions with distinct connectivity profiles that support distinct affective functions (3, 8). However, almost nothing is known about how networks associated with the amygdala mature with development. Knowledge of how major amygdalar circuits mature in children is important not only for gaining insights into typical development of affective functions, but also for understanding the ontogeny of affective dysfunction in disorders such as anxiety and depression (9, 10).
The basolateral amygdala (BLA) and centromedial amygdala (CMA) are two major groups of amygdalar nuclei that form dedicated networks for distinct functions via their unique pattern of interactions with other cortical and subcortical structures (3, 4). The CMA is essential for controlling the expression of fear responses, such as freezing behaviors, through projections to subcortical structures including thalamus, hypothalamus, striatum, brainstem, and cerebellum. The BLA, in contrast, plays a critical role in perception, evaluation, and regulation of emotionally salient stimuli via its abundant projections to widely distributed cortical regions (3, 4). Most of our knowledge of BLA and CMA functions and connectivity is based on animal models, thus the role of subregions of the amygdala in humans is still poorly understood. In humans, these major amygdala nuclei have only recently been anatomically delineated using cytoarchitectonic mapping studies on postmortem brains (11). Importantly, observer-independent cytoarchitectonically defined probabilistic maps of the amygdala subregions have been established in standard stereotaxic space (12), allowing delineation of nonoverlapping amygdala subregions in a quantitatively rigorous manner (13, 14). Using novel in vivo intrinsic connectivity analysis we recently demonstrated dissociable patterns of functional connectivity of BLA and CMA in adults (14), consistent with the wealth of observations in rodents and monkeys (3, 8). Almost nothing is currently known about the nature of functional circuits associated with the BLA and CMA in children and how they mature from childhood to adulthood.
There is now increasing evidence to suggest that brain maturation is characterized by increased integration and differentiation within functional circuits, a process mediated by a complex interplay of strengthening of long-range wiring, increasing of regional neuronal specialization, and experience-dependent plasticity (15, 16). These processes have been validated by other empirical observations, including widespread increases in myelination of white matter, and the initial increase in thickness and then slower thinning of gray matter, which occurs from childhood through adolescence to adulthood (2, 17). Intrinsic functional connectivity is a powerful tool for investigating how functional brain circuits mature with age (18, 19). One key finding that has emerged from this literature is the demonstration of strengthening links among cortical regions and weakening connections between subcortical and cortical regions between childhood and adulthood (20). Very little is known, however, about the maturation of cortical and subcortical connections related to the brain’s core emotion processing systems. Our study addresses this important gap by examining developmental changes in overall amygdala connectivity as well as the segregation of functional circuits associated with the BLA and CMA, its two major subdivisions.
We used resting-state functional magnetic resonance imaging (fMRI) to examine developmental changes in the intrinsic connectivity of amygdalar nuclei in 7- to 9-y-old typically developing children compared with 19- to 22-y-old healthy adults. This method has been widely used to identify spontaneously coupled and connected networks of brain regions (21, 22), and provides a unique way to examine differential patterns of amygdala connectivity over development. Intrinsic functional connectivity of the amygdala was examined by using observer-independent cytoarchitectonically determined probabilistic maps of the BLA and CMA (14). Functional imaging data from a second independent cohort of young children and adults were used in replication analyses to confirm the stability and robustness of the observed developmental effects. We hypothesized that amygdala connectivity would be weaker and less well differentiated in children compared with adults.
Results
Weaker Amygdala Connectivity in Children Compared with Adults.
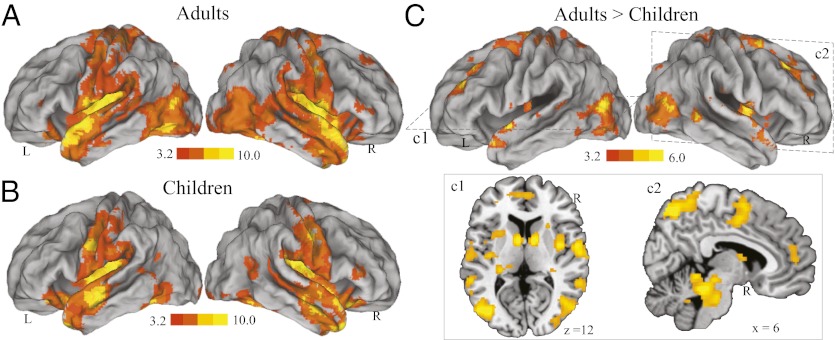
We first examined overall developmental changes in intrinsic functional connectivity of the amygdala by combining all voxels in BLA and CMA. In adults and children, we found that intrinsic activity of the amygdala was positively correlated with activity in a distributed set of cortical, subcortical, paralimbic, and limbic structures, as well as the cerebellum (Fig. 1 A and B). A direct group comparison collapsing across BLA and CMA revealed weaker intrinsic functional connectivity of the amygdala with multiple distributed cortical, subcortical, paralimbic, and limbic structures in children compared with adults (Fig. 1C). Neocortical regions included unimodal and polymodal association areas, the middle frontal gyrus, and ventromedial prefrontal cortex; limbic and paralimbic structures included the cingulate gyrus, insula, and parahippocampal gyrus; and subcortical structures included the caudate, putamen, midbrain/brainstem, and cerebellum (SI Appendix, Tables S1 and S2). No brain areas showed greater connectivity in children, compared with adults. These results indicate that intrinsic connectivity of the amygdala with a widely distributed set of cortical and subcortical structures is weaker in children compared with adults.
Fig. 1.
Immature functional connectivity of the amygdala in children compared with adults. Brain regions showing significant intrinsic functional connectivity with the amygdala in (A) adults and (B) children. (C) Brain regions showing significantly weaker amygdala connectivity in children, compared with adults. Representative axial and sagittal slices are depicted in panels c1 and c2. These results were replicated in a second cohort of adults and children (SI Appendix, Fig. S3). Connectivity maps are overlaid on either an inflated brain surface or high-resolution sections in Montreal Neurological Institute (MNI) space. Notes: L, Left; R, Right.
Weaker Segregation of BLA and CMA Connectivity in Children Compared with Adults.
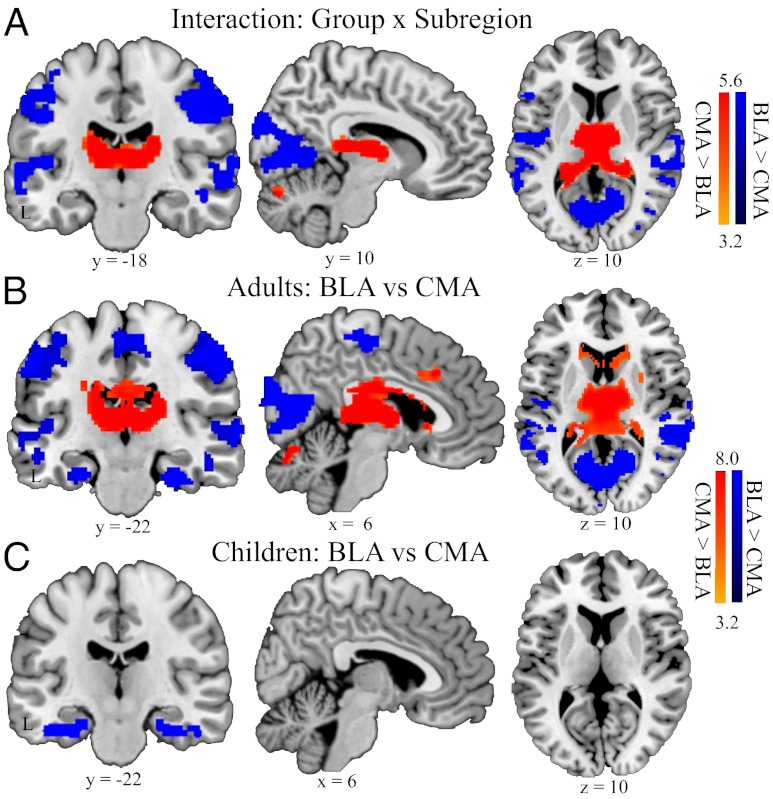
We next examined the maturation of amygdala subregional connectivity segregation, by comparing functional connectivity of the BLA and CMA in children and adults in a repeated-measure analysis of variance (ANOVA), with subregions (BLA vs. CMA) and hemisphere (left vs. right) as within-subject factors, and group (adults vs. children) as a between-subject factor. We found significant interactions between group and subregions in several subcortical structures including thalamus, hippocampus, caudate, and cerebellum, as well as widely distributed neocortical areas that included somatosensory cortex, superior and middle temporal gyri, and posterior visual and inferior temporal cortices (Fig. 2A) (SI Appendix, Table S3).
Fig. 2.
Immature differentiation between BLA and CMA functional connectivity in children compared with adults. Brain regions exhibiting significant interaction (A), and stronger functional connectivity with BLA, compared with CMA (shown in blue) and CMA, compared with BLA (shown in red) in (B) adults, and (C) children. This pattern of results was replicated in a second cohort of adults and children (SI Appendix, Fig. S4). Other details are as in Fig. 1.
Further analyses revealed that in adults, the BLA had stronger connectivity with widely distributed unimodal and polymodal association areas, including perirhinal cortex, temporal pole, superior and middle temporal gyri, motor and somatosensory areas, and primary visual cortex, whereas the CMA specifically showed stronger connectivity with multiple subcortical structures, including brainstem, striatum, thalamus, and cerebellar declive/vermis (Fig. 2B). This dissociated pattern of connectivity between the BLA and CMA is in line with previous findings from studies conducted in adults (13, 14). Unlike adults, however, children showed weaker dissociation of functional connectivity between BLA and CMA, with differences in connectivity only in perirhinal cortex and temporal pole (Fig. 2C) (SI Appendix, Table S4). To verify the specificity of our findings with respect to amygdala subregional connectivity, additional control analyses were conducted using ventral visual cortical subregions. Although there was a dissociation of connectivity patterns between these two visual cortical regions, we found no significant group-by-subregion interactions (SI Appendix, Figs. S1 and S2). Together, these results indicate that children, compared with adults, showed generally weaker and less pronounced differentiation of functional connectivity between BLA and CMA subregions. Notably, we found similar results in a second cohort of adults and children, including a main effect of group and group-by-subregion interaction effects (SI Appendix, Figs. S3 and S4).
Decreased Similarity of BLA and CMA Connectivity with Development.
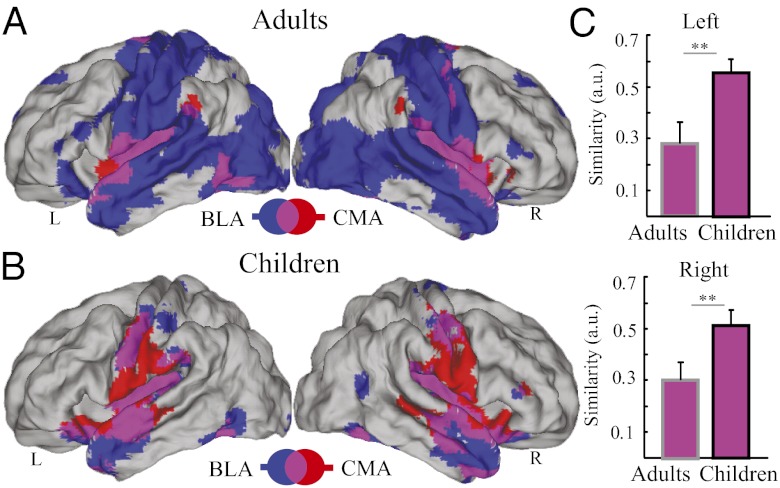
We then examined the similarity of BLA and CMA functional connectivity in adults and children. We found that the BLA and CMA target networks exhibited less overlapping, and distinct connectivity patterns in adults (Fig. 3A). In sharp contrast to adults, we found a more strongly overlapping pattern of functional connectivity between the BLA and CMA target networks in young children (Fig. 3B). Prominent overlap was observed in the brainstem, striatum, mid- and dorsal cingulate cortex, ventromedial prefrontal cortex, and cerebellum. To quantify developmental differences in the similarity between BLA and CMA target networks, we computed a similarity metric between BLA and CMA connectivity maps in each individual and then compared this similarity metric between adults and children. We found that compared with adults, children showed significantly higher similarity between BLA and CMA target networks (t(46) > 2.90, P < 0.01; Fig. 3C). These patterns of results were replicated in a second cohort of children and adults (SI Appendix, Fig. S5).
Fig. 3.
Overlap between BLA and CMA target networks in adults and children. Brain regions showing CMA target network (shown in red) and BLA target network (shown in blue) in (A) adults and (B) children. Overlap between CMA and BLA target networks is shown in pink. (C) Similarity between BLA and CMA target networks in adults and children. Target networks for the BLA and CMA showed greater overlap in children, compared with adults, in both the Left (Upper) and Right (Lower) hemispheres. Other details are as in Fig. 1. Notes: L, Left; R, Right; **P < 0.01.
Increased Differentiation Between BLA and CMA Target Networks with Development.
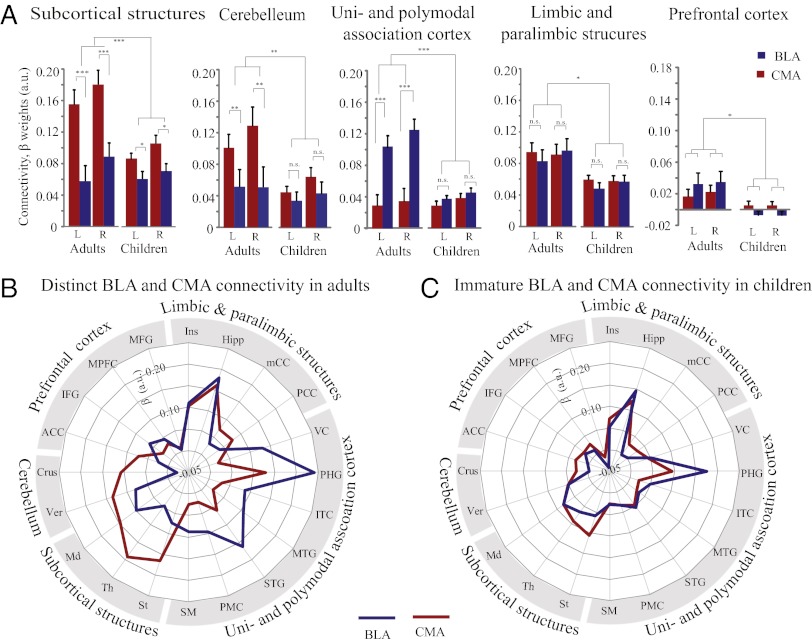
We used a systems neuroscience approach to further characterize differential patterns of connectivity for BLA and CMA in adults and children. We examined five major functional subsystems known to play important roles in affective information processing (3, 7, 8), as described in SI Appendix, Text S1, and Fig. S6. These subsystems encompassed subcortical structures, cerebellum, unimodal and polymodal association cortex, limbic and paralimbic structures, and prefrontal cortex. We conducted separate repeated-measures ANOVAs on connectivity measures extracted from each network. ANOVAs on connectivity β-weights, with amygdala subregion (BLA vs. CMA) and hemisphere (left vs. right) as within-subject factors and group (adults vs. children) as a between-subject factor, revealed a main effect of group for subcortical structures (F(1,46) = 5.72, P = 0.02), the cerebellum (F(1,46) = 4.23, P = 0.04), and paralimbic and limbic structures (F(1,46) = 6.86, P = 0.01), unimodal and polymodal association cortex (F(1, 46) = 7.46, P < 0.01), and prefrontal cortex (F(1,46) = 5.96, P = 0.02), with stronger connectivity in adults in general. These results indicate that children, compared with adults, show weaker connectivity of the amygdala with widely distributed subcortical and cortical structures (Fig. 4A).
Fig. 4.
Differential patterns of BLA and CMA functional connectivity in adults and children. (A) Parameter estimates represent the strength of functional connectivity between the BLA (shown in blue) and CMA (shown in red) with five target networks of interest – subcortical structures, cerebellum, uni- and polymodal association cortex, limbic and paralimbic structures, and prefrontal cortex. (B and C) Schematic polar plots illustrating weaker integration and differentiation of BLA and CMA connectivity in children compared to adults. (B) In adults, the BLA has stronger functional connectivity with unimodal and polymodal association cortex, whereas the CMA showed stronger functional connectivity with subcortical structures and cerebellum. (C) In children, these differential patterns are significantly less pronounced. See SI Appendix, Fig. S6 for abbreviations and additional details on anatomically-defined target networks and replication in the second cohort of children. n.s., not significant; *P < 0.05; **P < 0.01; ***P < 0.001.
Moreover, we found a group-by-amygdala subregion interaction for subcortical structures (F(1, 46) = 10.71, P < 0.01), unimodal and polymodal association cortex (F(1, 46) = 19.34; P < 0.001), and the cerebellum (F(1, 46) = 4.30, P = 0.04), but not for limbic and paralimbic structures (F < 1) and prefrontal cortex (F < 1). Additional analyses revealed that in adults, the BLA showed stronger intrinsic connectivity with unimodal and polymodal association cortex (both left and right hemispheres of seed-based connectivity analyses or “bilateral” herewith: t (1, 23) > 4.23, P < 0.001), whereas the CMA showed stronger connectivity to subcortical structures (bilateral: t (1, 23) > 4.15, P < 0.001) and in the cerebellum (bilateral t (23) > 2.55, P < 0.02). In children, we observed weaker yet significant (bilateral t (1, 46) > 3.20, P < 0.01) dissociation of CMA versus BLA connectivity only with subcortical structures (bilateral: t (1, 23) > 3.05, P < 0.01). There was no dissociation between BLA and CMA connectivity in other networks in children (t (1, 23) < 1.80, P > 0.05). Together, these results indicate that compared with adults, children show significantly less pronounced dissociation of functional connectivity between the BLA and CMA subregions (Fig. 4 A and B). Crucially, similar patterns of results were found in the second cohort of children (SI Appendix, Fig. S6), indicating high reliability of our findings.
Increased Differentiation of Intraamygdala Coupling with Development.
To directly examine differentiation of intrinsic activity within the amygdala subregions, we computed interregional correlations between time series representing mean BLA and CMA spontaneous activity in each hemisphere. These analyses revealed that adults, compared with children, showed weaker correlation between BLA and CMA activity (SI Appendix, Fig. S7), reflecting increased differentiation of these intraamygdala nuclei with development. Differences were observed both in ipsilateral connectivity within the left and right hemispheres (both: t (1, 46) > 2.42, P < 0.01) as well as across-hemisphere contralateral connectivity (BLA and CMA: t (1, 46) > 2.50, P < 0.01). Interestingly, the degree of intraamygdala coupling averaged across two hemispheres was positively correlated with the similarity of BLA and CMA target networks in both adults (r = 0.50, P = 0.014) and children (r = 0.68, P < 0.001). These results indicate that compared with adults, children show stronger intraamygdala coupling and greater similarity or weaker functional differentiation between the BLA and CMA target networks.
Discussion
In this study, we investigated developmental changes in functional connectivity of the BLA and CMA, two major, cytoarchitectonically distinct, nuclei of the amygdala. Compared with adults, the amygdala in children showed significantly weaker connectivity with multiple distributed cortical and subcortical regions. Critically, networks associated with each of the two individual amygdala nuclei showed stronger overlap and weaker dissociation of connectivity patterns in children than in adults. Furthermore, children also showed greater intraamygdala connectivity between the BLA and CMA. Because of potential developmental differences in amygdala structure, we sought to replicate our findings in a second cohort of children and adults. Critically, reproducible differences in large-scale amygdalar connectivity were identified in an independent cohort of children and adults. Our findings demonstrate that amygdala circuits in children are characterized by weaker overall integration and less segregated functional networks. Below we discuss the implications of these findings for understanding the maturation of emotion-related circuits in the developing brain.
We found that amygdala connectivity with multiple widely distributed cortical and subcortical regions is weaker in children than in adults. Cortical regions included the insula, cingulate gyrus, and parahippocampal gyrus, as well as unimodal and polymodal association cortex and ventromedial prefrontal cortex. The insula and cingulate gyrus are considered key nodes of the “salience network,” involved in detection and processing of novel and salient events (23). The ventromedial prefrontal cortex is known to play a critical role in emotion appraisal, as well as regulation (24, 25). The weaker connectivity of the amygdala with these regions in children may account for the poorer performance in tasks requiring rapid and accurate recognition and discrimination of different types of emotions (26, 27). Our findings are also consistent with previous accounts of maturation at the whole-brain level, reporting overall increases in long-range connectivity from childhood through adolescence to early adulthood (1, 19, 20). The observed increase in amygdala connectivity in adults compared with children stands in contrast to weakening connectivity, with age, of subcortical structures including the basal ganglia and thalamus. These subcortical areas have been found to be more strongly connected with primary sensory, association, and paralimbic areas in children (20), pointing to heterogonous patterns of functional maturation within different subcortical systems (28). Our findings in amygdala connectivity are more consistent with patterns of stronger corticocortical connectivity between paralimbic, limbic, and association areas observed in adults, supporting the notion that strengthening of specific long-range connections with age promotes faster communication and connection efficiency (1, 29).
A second major finding of our study is that 7- to 9 –y-old children show stronger similarity and less distinct BLA and CMA connectivity networks. In contrast, adults showed a clearly dissociable pattern of connectivity between BLA and CMA networks. The distinct connectivity profile observed in adults is reminiscent of established animal models of amygdala circuits (3, 8) and replicates previous findings from two neuroimaging studies of amygdala connectivity in adults (13, 14). Specifically, the BLA showed stronger connectivity with regions in widely distributed unimodal and polymodal association cortex whereas the CMA showed stronger connectivity with several subcortical structures including brainstem, thalamus, striatum, and cerebellum. These findings are in line with animal models in which the BLA, coordinating with sensory and polymodal association areas, plays critical roles in detecting and perceiving a stimulus associated with fear. On the other hand, the CMA and its interactions with subcortical regions are essential for regulating reflexive and defensive responses to fear (3, 8). To our knowledge no previous study has characterized the emergence of such a pattern of differentiated functional circuits from childhood to adulthood. We suggest that this development-related reconfiguration of intrinsic functional networks likely underlies the maturation of increasingly complex affective functions that typically occur during adolescence.
A third important finding of our study is that children showed greater intraamygdala connectivity. Specifically, intrinsic functional coupling between the BLA and CMA was higher in children in both hemispheres. This pattern is consistent with our finding of overlapping target networks associated with the BLA and CMA and suggests a possible mechanism by which distal target networks become more differentiated with development. Previous studies in nonhuman primates have suggested that differentiation of multiple cortical regions and networks is related to local synaptic pruning, deletion of unnecessary synapses, and an increase in processing efficiency and regional specialization (15, 16). Human data in support of regional anatomical changes comes from developmental neuroimaging studies showing an initial increase in thickness and then slower thinning of gray matter from childhood to adulthood (2, 17, 28). We suggest that similar neurobiological processes during childhood may lead to increased functional differentiation between the BLA and CMA and consequently to more distinct functional circuits involving these nuclei. Converging evidence from studies in rodents and humans suggests that experience-dependent development also plays a critical role in modulating development of neural wiring and regional specialization processes (1, 2, 16). For instance, studies have shown that behavioral training shapes intrinsic characteristics, such as volume, morphometry, myelination, and local circuits, in task-relevant brain structures (30, 31). Similar principles are likely to operate in the BLA and CMA, leading to increased segregation of function within these regions. This in turn contributes to the development of distinct connectivity with distributed brain regions that mediate more mature affective functions involving perception, detection, regulation, and appraisal of salient emotional stimuli.
To our knowledge, no studies to date have attempted to disentangle developmental changes in functional contributions of the BLA and CMA as they develop from childhood to adulthood. Previous structural neurodevelopmental studies of the amygdala have focused on changes in its volume and morphometry (28, 32), whereas functional studies have examined its overall functional reactivity and connectivity during emotion processing in children (27, 33) and adolescents (34). Notably, even in adults, only a limited set of studies has focused on dissociating the contributions of these distinct subregions. Recent studies in patients with anxiety disorders, however, have provided strong evidence that distinct connectivity patterns can be reliably identified for the BLA and CMA of the human amygdala (14). Furthermore, in patients with generalized anxiety disorder, BLA and CMA connectivity patterns were significantly less distinct (14). These findings raise important questions as to whether protracted immaturity in these circuits might be a risk factor for affective disorders for some children as they mature.
Further research is needed to clarify and extend our findings. First, we used cytoarchitectonic probabilistic maps of the BLA and CMA from adult postmortem brains to examine both children and adults, as similar maps are not currently available in children. However, findings from one recent postmortem case study of a 10-y-old boy suggests that the relative subregional positions and proportions of anatomical boundaries are similar in children and adults (35). Crucially, these cytoarchitectonic probabilistic maps have been shown to have high reliability and accuracy for guiding anatomical segmentation of amygdala subregions in children as young as 6- to 7-y old (35). In addition, evidence from our control analyses in visual cortical subregions also confirmed the feasibility and validity of our approach. Second, future studies, using high-resolution fMRI techniques, are required to better determine structural and functional amygdala connectivity, and how their interrelations evolve with development. Finally, analysis of a wider age group is needed to delineate changes during adolescence, a period important for development of affective and social behaviors (36–38).
In conclusion, the present study demonstrates that amygdalar nuclei in children show generally weaker large-scale connectivity with distributed cortical and subcortical regions. Importantly, intrinsic functional circuits associated with individual amygdalar nuclei are less differentiated in children. This lack of differentiation in large-scale functional circuits can be traced to higher intraamygdala connectivity between the BLA and CMA in children. Taken together, our findings point to weak integration and segregation of amygdala subregional networks in young children and provide unique insights into the nature of immature emotion-related circuitry in young children. The current work represents an important step toward characterizing the developmental maturation of functional brain systems underlying affective processing. Examining potentially aberrant maturation of amygdala functional circuits identified in our study may be important for understanding development psychopathology and affective disorders in children and adolescents.
Materials and Methods
Participants.
Two cohorts of children and adults (n = 87 in total) participated in this study after giving written, informed consent. Details regarding participant demographics are described in SI Appendix, Text S1.
Data Acquisition and Preprocessing.
For the resting-state fMRI scan, participants were instructed to keep their eyes closed and remain still for the duration of an 8-min scan. Whole brain functional images were acquired on a 3T GE Signa scanner. Details are provided in SI Appendix, Text S1.
Regions of Interest (ROIs) Definition.
Two ROIs encompassing the BLA and CMA were created using cytoarchitectonically defined probabilistic maps of the amygdala. Maximum probability maps were used to create nonoverlapping amygdala subregions using the Anatomy Toolbox (12). Voxels were included in the maximum probability maps only if the probability of their assignment to the BLA or CMA was higher than any other nearby structures with greater than 40% likelihood. Each voxel was exclusively assigned to only one region. Overlapping voxels were assigned to the region that had the greatest probability, resulting in four nonoverlapping ROIs representing CMA and BLA subregions in left and right hemispheres. To mitigate potential confounds related to differences in the size of the amygdala, we also analyzed the data using the first eigenvalue, rather than mean, of the ROI time series. The results were nearly identical to our original analyses (SI Appendix, Fig. S8).
Validity of using these cytoarchitectonic probabilistic maps in children and additional control analyses were summarized in SI Appendix, Text S2.
Functional Connectivity Analysis.
Regional time series within each seed ROI were extracted from data filtered with a bandpass temporal filter (0.008–0.10 Hz). Subsequently, each time series was submitted into an individual level fixed-effects analysis under the framework of the general linear model. A global signal regressor and six motion parameters for each participant were included as covariates of no interest in the model to account for physiological noise and movement-related artifacts. For each participant, four separate functional connectivity analyses were performed for both BLA and CMA in left and right hemispheres.
The contrast parameter images for each of the four seed regions from the individual level analyses were submitted to a second-level group analysis that treated participants as a random variable in a 2-by-2-by-2 ANOVA as described in Results. Significant clusters were estimated using a height threshold of P < 0.001 uncorrected, and familywise error corrections for multiple spatial comparisons using an extent threshold of P < 0.05 in terms of nonstationary suprathreshold cluster-size distributions based on Monte Carlo simulations (39).
To characterize differential patterns of BLA and CMA target networks in children and adults, complementary ROI analyses were conducted for five target networks of interest. Details are provided in SI Appendix, Text S1 and Fig. S6. Mean parameter estimates, representing the strength of functional connectivity of each seed with corresponding target masks, were extracted from BLA- and CMA-seeded functional connectivity analyses.
Spatial correlations, reflecting similarity of BLA and CMA connectivity, were computed between each participant’s BLA- and CMA-seeded functional connectivity maps. Moreover, temporal correlations between BLA and CMA seeds were calculated on the basis of their bandpass-filtered time series in ipsilateral and contralateral hemispheres to specifically investigate developmental differences in interregional coupling of BLA and CMA. Corresponding correlation coefficients were then Fisher’s Z transformed for further statistical testing. More details are described in SI Appendix, Text S1.
Supplementary Material
Acknowledgments
This work was supported by grants from National Institutes of Health HD047520, HD059205, and K01MH092288 and Netherlands Organization for Scientific Research NWO 446.10.010.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120408109/-/DCSupplemental.
References
- 1.Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 3.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 4.Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 5.Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- 6.Dolan RJ, Vuilleumier P. Amygdala automaticity in emotional processing. Ann N Y Acad Sci. 2003;985:348–355. doi: 10.1111/j.1749-6632.2003.tb07093.x. [DOI] [PubMed] [Google Scholar]
- 7.Pessoa L, Adolphs R. Emotion processing and the amygdala: From a 'low road' to 'many roads' of evaluating biological significance. Nat Rev Neurosci. 2010;11:773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeDoux J. The amygdala. Curr Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Thomas KM, et al. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 10.Menon V. Functional Connectivity, Neurocognitive Networks and Brain Dynamics. Cambridge, MA: MIT Press; 2011. [Google Scholar]
- 11.Amunts K, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 12.Eickhoff SB, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 13.Roy AK, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66:1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rakic P, Bourgeois JP, Goldman-Rakic PS. Synaptic development of the cerebral cortex: Implications for learning, memory, and mental illness. Prog Brain Res. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- 17.Shaw P, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uddin LQ, Supekar K, Menon V. Typical and atypical development of functional human brain networks: Insights from resting-state FMRI. Front Syst Neurosci. 2010;4:21. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dosenbach NU, et al. 2010 Prediction of individual brain maturity using fMRI. Science 329(5997):1358–1361. [Google Scholar]
- 20.Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 23.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dougherty DD, et al. Ventromedial prefrontal cortex and amygdala dysfunction during an anger induction positron emission tomography study in patients with major depressive disorder with anger attacks. Arch Gen Psychiatry. 2004;61:795–804. doi: 10.1001/archpsyc.61.8.795. [DOI] [PubMed] [Google Scholar]
- 25.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross AL, Ballif B. Children's understanding of emotion from facial expressions and situations: A review. Dev Rev. 1991;11:368–398. [Google Scholar]
- 27.Thomas KM, et al. Amygdala response to facial expressions in children and adults. Biol Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- 28.Ostby Y, et al. Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagmann P, et al. White matter maturation reshapes structural connectivity in the late developing human brain. Proc Natl Acad Sci USA. 2010;107:19067–19072. doi: 10.1073/pnas.1009073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woollett K, Spiers HJ, Maguire EA. Talent in the taxi: A model system for exploring expertise. Philos Trans R Soc Lond B Biol Sci. 2009;364:1407–1416. doi: 10.1098/rstb.2008.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. J Neurosci. 2003;23:9240–9245. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giedd JN, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 33.Perlman SB, Pelphrey KA. Developing connections for affective regulation: Age-related changes in emotional brain connectivity. J Exp Child Psychol. 2011;108:607–620. doi: 10.1016/j.jecp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guyer AE, et al. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JE, et al. Laterobasal amygdalar enlargement in 6- to 7-year-old children with autism spectrum disorder. Arch Gen Psychiatry. 2010;67:1187–1197. doi: 10.1001/archgenpsychiatry.2010.148. [DOI] [PubMed] [Google Scholar]
- 36.Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- 37.Crone EA, Güroğlu TE, editors. 2012 Development of Emotion and Social Reasoning in Adolescence (Oxford Press, New York) [Google Scholar]
- 38.Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Nichols T, Hayasaka S. Controlling the familywise error rate in functional neuroimaging: A comparative review. Stat Methods Med Res. 2003;12:419–446. doi: 10.1191/0962280203sm341ra. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.