Abstract
Stress-related variation in the intrauterine milieu may impact brain development and emergent function, with long-term implications in terms of susceptibility for affective disorders. Studies in animals suggest limbic regions in the developing brain are particularly sensitive to exposure to the stress hormone cortisol. However, the nature, magnitude, and time course of these effects have not yet been adequately characterized in humans. A prospective, longitudinal study was conducted in 65 normal, healthy mother–child dyads to examine the association of maternal cortisol in early, mid-, and late gestation with subsequent measures at approximately 7 y age of child amygdala and hippocampus volume and affective problems. After accounting for the effects of potential confounding pre- and postnatal factors, higher maternal cortisol levels in earlier but not later gestation was associated with a larger right amygdala volume in girls (a 1 SD increase in cortisol was associated with a 6.4% increase in right amygdala volume), but not in boys. Moreover, higher maternal cortisol levels in early gestation was associated with more affective problems in girls, and this association was mediated, in part, by amygdala volume. No association between maternal cortisol in pregnancy and child hippocampus volume was observed in either sex. The current findings represent, to the best of our knowledge, the first report linking maternal stress hormone levels in human pregnancy with subsequent child amygdala volume and affect. The results underscore the importance of the intrauterine environment and suggest the origins of neuropsychiatric disorders may have their foundations early in life.
Keywords: developmental programming, fetal origins, hypothalamus-pituitary-adrenal axis, depression, emotion regulation
Many common complex neuropsychiatric and neurodevelopmental disorders that confer the major burden of mental illness in society are characterized by altered brain anatomy (1, 2). Growing evidence suggests that the origins of many of these anatomic alterations can be traced back to the intrauterine period of life, when the developing embryo/fetus is influenced by environmental conditions during sensitive periods of cellular proliferation, differentiation, and maturation to produce structural and functional changes in the brain and peripheral systems [i.e., the concept of fetal programming (3)]. One key tenet of the programming hypothesis is that biological systems undergoing rapid developmental changes are particularly vulnerable to organizing and disorganizing influences (3). Human brain development begins early in gestation, occurs over a protracted period, and follows a rapid, orchestrated chain of partially overlapping ontogenic events, including neuronal proliferation, migration, differentiation, synaptogenesis, myelination, and pruning. A very recent landmark study of the whole-genome transcriptome of tissues from key human brain regions from embryonic and fetal life through birth, childhood, adolescence, and adulthood demonstrated that genes associated with neurodevelopmental processes are robustly expressed starting in the embryonic period, and that expression continues at a very high rate during the fetal and infant periods of life (4). Therefore, it is likely that the intrauterine milieu can influence neurodevelopmental trajectories. These effects may independently or in conjunction with other factors (genotypic variation, postnatal conditions) impact subsequent long-term susceptibility for various neuropsychiatric disorders (5).
The amygdala and hippocampus have received particular attention in the context of developmental programming because they develop at an early embryonic stage (6, 7), they are associated with a range of neurodevelopmental and psychopathological disorders (1, 2), and they are believed to be particularly sensitive during development to elevated levels of glucocorticoids (8). Intrauterine conditions that affect these structures and also are associated with increased risk for neuropsychiatric disorders include infection (9), maternal undernutrition (10, 11), unhealthy maternal behaviors such as smoking or alcohol or drug use (12–14), and excessive maternal stress exposure (15). We and others have suggested that glucocorticoids (cortisol in humans) may play a salient role in this process (16–18). First, cortisol, the end-product of the hypothalamic–pituitary–adrenal (HPA) axis, is known to exert a wide array of metabolic, endocrine, and immune effects on most if not all cells (19, 20), and is known to play a key role in events underlying the development of the brain and other organ systems (16). Second, cortisol is one of the primary biomarkers of the physiologically stressed state because its production, bioavailability, and activity is altered by all adverse conditions that have been shown to program the developing brain (17). Thus, although glucocorticoids play an essential role in normal brain development, abnormal or inappropriate levels, particularly during sensitive periods, may induce neurotoxicity with detrimental long-term consequences (21). Interestingly, a very recent study showed that elevated maternal cortisol concentrations during pregnancy were associated with reduced fetal brain growth, as measured by ultrasound (22).
Evidence in humans and animals indicates that intrauterine exposure to elevated glucocorticoids increases risk for affective disorders (23). In humans, prenatal exposure to elevated levels of endogenous maternal glucocorticoids (24–26) and the administration of synthetic glucocorticoids (27–30) is associated with HPA axis dysregulation, internalizing behavior, and social anxiety. Exposure to elevated glucocorticoids during gestation may increase vulnerability to affective disorders by altering the development of the hippocampus and amygdala, regions that play a role in the development of affective disorders (1, 31) and are especially susceptible to glucocorticoids (32). Although not directly tested in humans, there is substantial evidence in rodents and nonhuman primates that maternal stress exposure during gestation has adverse consequences for the developing hippocampus (33, 34) and amygdala (35). These effects on the nervous system appear to be mediated by stress-induced increases in maternal glucocorticoids (36, 37).
The objective of the present study was to prospectively examine the association between maternal cortisol concentrations over the course of gestation with subsequent measures of child hippocampus and amygdala volumes and affective function. More specifically, we tested the hypotheses that (i) maternal cortisol concentrations especially in early gestation would be associated with child hippocampus and amygdala volume and affect, and (ii) the association between maternal cortisol and child affect would be mediated, in part, by its effect on limbic structure anatomy.
Results
Maternal Cortisol over the Course of Gestation.
Maternal cortisol increased progressively in a linear manner over gestation [F(2.2, 59.9) = 53.4, P < 0.001]. There was no difference in maternal cortisol level at any stage in gestation between mothers with female vs. male fetuses (all P > 0.10).
Volumes of Child Hippocampus and Amygdala.
Mean volumes of the child hippocampus and amygdala are depicted in Table 1. Because manual segmentation of these brain regions was performed in standard stereotaxic space, these volumes take overall differences in brain size into account. Volumes of these structures were not different between boys and girls (all P > 0.2).
Table 1.
Mean volumes of hippocampus and amygdala in boys and girls
| Location | Boys (n = 30) | Girls (n = 35) | P value |
| Right amygdala, cm3 | 1.15 ± 0.19 | 1.12 ± 0.13 | 0.43 |
| Left amygdala, cm3 | 1.10 ± 0.19 | 1.07 ± 0.15 | 0.50 |
| Right hippocampus, cm3 | 3.91 ± 0.43 | 4.02 ± 0.29 | 0.20 |
| Left hippocampus, cm3 | 3.85 ± 0.40 | 3.88 ± 0.29 | 0.71 |
Values presented as mean ± SD.
Child Affective Problems.
The T-scores on the child affective symptoms subscale of the Child Behavior Checklist (CBCL) ranged between 50 and 70 (mean ± SD, 52.7 ± 4.9). Girls and boys did not differ in affective symptom scores (P = 0.72).
Associations Between Maternal Cortisol in Pregnancy and Subsequent Measures of Child Brain Volumes.
Maternal cortisol in early gestation and child brain volumes.
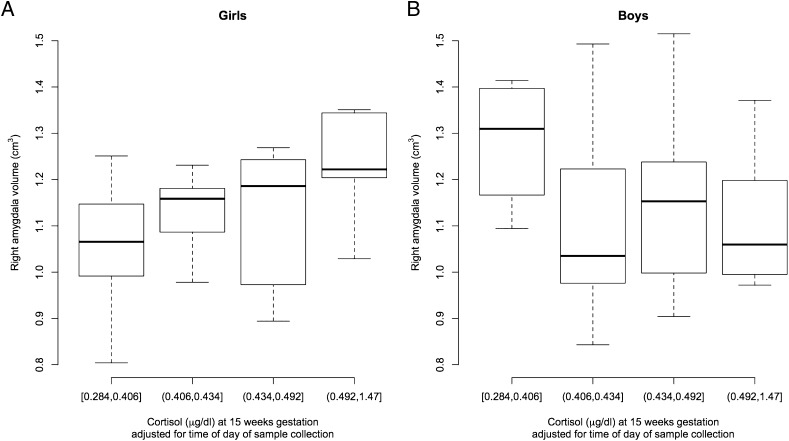
The results of the multivariate regression analyses depicting associations between maternal cortisol in early gestation and child brain volumes are provided in Table 2. Although the point estimates, SEs, and corresponding P values for the cortisol effects are presented separately for girls and boys, these estimates were obtained in the same model using the main effect of cortisol along with its interaction effect with sex, as described in Materials and Methods section and SI Materials and Methods. Higher maternal cortisol levels at 15 wk gestation was associated with larger right amygdala volumes among girls (1.06 ± 0.46; P = 0.02, q = 0.025; Fig. 1, Table 2). A one-unit increase in cortisol was associated with a 1.06-cm3 increase in right amygdala volume, which translates to a 0.11-cm3 (6.4%) increase in right amygdala volume with a 1-SD (0.06 μg/dL) increase in maternal cortisol at this gestational stage. The same regression analysis performed without the imputed missing cortisol values procedure revealed similar results (1.34 ± 0.55; P = 0.02), supporting the validity of the multiple imputation method. The interaction effect between maternal cortisol at 15 wk gestation and sex also was significant (P = 0.027; not shown in Table 2). Maternal cortisol at 15 wk gestation was not associated with left amygdala volume or right or left hippocampus volume in girls. In boys, maternal cortisol concentrations at 15 wk gestation were not associated with left or right amygdala volume (Fig. 1), but there was a trend for higher maternal cortisol levels at 15 wk being associated with smaller left (−1.18 ± 0.61, P = 0.06) and right hippocampal volume (−1.13 ± 0.66, P = 0.09).
Table 2.
Regression analyses of maternal cortisol in early (15 wk) gestation and child hippocampus and amygdala volumes
| Group | Right amygdala | Left amygdala | Right hippocampus | Left hippocampus |
| Girls | 1.06 ± 0.46 | 0.15 ± 0.48 | 0.47 ± 1.11 | −0.34 ± 0.97 |
| P value | 0.02 (q = 0.025) | 0.76 | 0.68 | 0.72 |
| Boys | −0.12 ± 0.28 | −0.24 ± 0.30 | −1.13 ± 0.66 | −1.18 ± 0.61 |
| P value | 0.66 | 0.41 | 0.09 | 0.06 |
Values presented as β ± SEM. Sex-specific associations between maternal cortisol concentrations and child brain volumes were assessed by including an interaction term between maternal cortisol in pregnancy and sex of the child. All regression analyses were adjusted for obstetric risk, length of gestation, birth weight percentile, child age, sex, handedness, and maternal depression at child follow-up. Significant P values were adjusted for multiple testing and q-values are presented that represent the minimum false-discovery rate that is incurred when calling a test significant. The following regression model was applied. Child brain volume = β0 + β1 * maternal cortisol at 15 wk gestation + β2 * sex + β12 * (maternal cortisol at 15 wk gestation * sex) + β3 * obstetric risk + β4 * birth weight percentiles + β5 * length of gestation + β6 * maternal depression at child assessment + β7 * child age at MRI assessment + β8 * child handedness. Note that, by setting sex = 0 for girls, the estimate of β1 provides the expected change in brain volume corresponding to one unit increase in maternal cortisol among girls. By setting sex = 1 for boys, the estimate of β1 + β12 provides the expected change in brain volume corresponding to one unit increase in maternal cortisol among boys.
Fig. 1.
Box plots show maternal cortisol concentrations at 15 wk gestation and children’s right amygdala volumes at 7 y of age. For illustrative purposes, right amygdala volumes are depicted based on quartiles of mothers’ cortisol concentrations (lowest and highest cortisol concentration for each of the four cortisol categories are provided). (A) In girls (n = 35), higher maternal cortisol concentrations at 15 wk gestation were associated with larger right amygdala volumes. (B) In boys (n = 30), there was no association between maternal cortisol concentrations at 15 wk gestation and right amygdala volumes.
Exploration of time of gestation-specific cortisol associations with child brain volumes.
The results for the estimates of the regression coefficients for area under the curve 1 (AUC1; controlling for the effect of cortisol at 15 wk gestation) and AUC2 (controlling for the effect of AUC1) are presented in Table 3. These measures of cortisol, representing estimates of effects of average maternal cortisol concentrations during the second and third trimesters of gestation adjusted for earlier cortisol levels, were not significantly associated with child brain volumes.
Table 3.
Explained variance in child amygdala and hippocampus volumes by maternal cortisol concentrations in mid and late gestation over and above variation explained by cortisol concentrations in early gestation
| Right amygdala | Left amygdala | Right hippocampus | Left hippocampus | |
| Model 1: Maternal cortisol in midgestation (AUC1) | ||||
| Girls | 0.008 ± 0.04 | 0.02 ± 0.03 | −0.03 ± 0.08 | 0.02 ± 0.07 |
| P value | 0.83 | 0.48 | 0.70 | 0.78 |
| Boys | −0.009 ± 0.02 | 0 ± 0.02 | 0 ± 0.05 | 0.02 ± 0.05 |
| P value | 0.67 | 0.89 | 0.92 | 0.68 |
| Model 2: Maternal cortisol in late gestation (AUC2) | ||||
| Girls | 0 ± 0.03 | 0 ± 0.03 | 0.01 ± 0.09 | 0.01 ± 0.09 |
| P value | 0.99 | 0.91 | 0.83 | 0.89 |
| Boys | −0.014 ± 0.04 | 0 ± 0.04 | −0.07 ± 0.13 | −0.04 ± 0.12 |
| P value | 0.73 | 0.99 | 0.59 | 0.76 |
Values presented as β ± SEM. Sex-specific associations between maternal cortisol concentrations and child brain volumes were assessed by including an interaction term between maternal cortisol in pregnancy and sex of the child. All regression analyses were adjusted for obstetric risk, length of gestation, birth weight percentile, child age, sex, handedness, and maternal depression at child follow-up. The following regression model was applied. Child brain volume = β0 + β1 * maternal cortisol1 + β2 * sex + β12 * (maternal cortisol1 * sex) + β3 * obstetric risk + β4 * birth weight percentiles + β5 * length of gestation + β6 * maternal depression at child assessment + β7 * child age at MRI assessment + β8 * child handedness” + β9 * maternal cortisol earlier in gestation2. (1AUC1 in model 1 and AUC2 in model 2; 2Maternal cortisol concentrations at 15 wk gestation in model 1 and AUC1 in model 2.) Note that, by setting sex = 0 for girls, the estimate of β1 provides the expected change in brain volume corresponding to one unit increase in maternal cortisol among girls. By setting sex = 1 for boys, the estimate of β1 + β12 provides the expected change in brain volume corresponding to one unit increase in maternal cortisol among boys.
Associations Between Maternal Cortisol in Pregnancy and Subsequent Measures of Child Affective Problems.
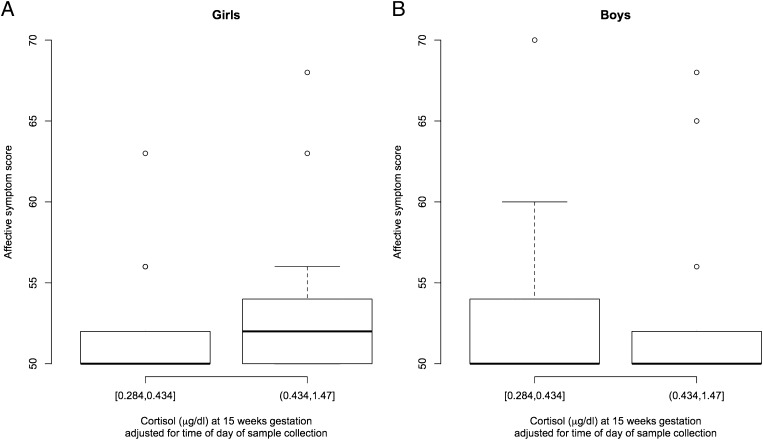
The association between maternal cortisol at 15 wk gestation and subsequent measures of child affective problems was assessed adjusting for the pregnancy, maternal, and child characteristics listed below. Higher maternal cortisol concentrations at 15 wk gestation were associated with more affective problems among girls (26.6 ± 13.0; P = 0.04; Fig. 2). Maternal cortisol in early gestation was not related to affective problems among boys (0.14 ± 7.9; P = 0.98; Fig. 2), and maternal cortisol in mid- and late gestation was not related to affective problems in girls or boys after accounting for the variance in maternal cortisol in early gestation (all P > 0.9).
Fig. 2.
Box plots show maternal cortisol concentrations at 15 wk gestation and children’s scores on the affective problem scale of the CBCL at 7 y of age. For illustrative purposes, right amygdala volumes are depicted based on median of mothers’ cortisol concentrations (lowest and highest cortisol concentration for each of the two cortisol categories are provided). (A) In girls (n = 35), higher maternal cortisol concentrations at 15 wk gestation were associated with more affective problems. (B) In boys (n = 30), there was no association between maternal cortisol concentrations at 15 wk gestation and affective problems.
Given these associations of maternal cortisol in early gestation with right amygdala volume and with affective symptoms in girls, we assessed the association between right amygdala volume and affective symptoms in girls. In girls, right amygdala volume was significantly and positively associated with affective symptoms (r = 0.39, P = 0.02). We next assessed whether the association between maternal cortisol in pregnancy and child affective symptoms was mediated by right amygdala volume. After adjusting for the effect of right amygdala volume, the association between maternal cortisol in early gestation and child affective problems in girls was no longer statistically significant (23.0 ± 13.7; P = 0.1). Because it is also possible that presence of affective problems can influence brain anatomy, we examined the association between maternal cortisol in early gestation and right amygdala volume in girls, adjusting for the effect of maternal cortisol on affective symptoms. This analysis demonstrated there was no change in the observed relationship (between maternal cortisol and amygdala volume, 0.97 ± 0.48; P = 0.04). Thus, taken together, these analyses support our hypothesis that the pathway linking maternal cortisol with child affect may be mediated, in part, by the effect of maternal cortisol on amygdala volume.
Discussion
The present findings represent, to the best of our knowledge, the first report linking maternal stress hormone levels in human pregnancy with subsequent volume of the amygdala and affective problems in childhood. Specifically, higher maternal cortisol concentrations in early gestation were associated with larger right amygdala volume in girls at 7 y of age. The effect was significant after controlling for the potential confounding effects of other pregnancy, birth, and concurrent child and maternal characteristics, including maternal depression. The magnitude of the effect is substantial, with a 1-SD increase in maternal cortisol being associated with an approximate 6.4% increase in the size of the right amygdala. The potential clinical significance of our finding is underscored by previous observations that the difference in amygdala volume between clinically depressed patients and healthy comparison volunteers is approximately 13% (38). Our results also demonstrate an effect of maternal cortisol level in early gestation on affective problems in girls, and suggest that this association is mediated, in part, by their cortisol-associated larger right amygdala volume. Last, our results suggest this effect is specific to maternal cortisol in early but not later gestation, and that it is moderated by fetal/child sex.
Implicit in this study is the premise that, during pregnancy, cortisol levels in the maternal compartment are an indicator of fetal glucocorticoid exposure. This premise is supported by several plausible pathways. Direct fetal exposure to maternal cortisol is regulated by the placental enzyme, 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), which oxidizes cortisol to its inactive form cortisone (39, 40). Because placental 11β-HSD2 serves only as a partial barrier, some proportion of active maternal cortisol does pass through the placenta into the fetal compartment (41). Interestingly, many adverse intrauterine conditions that have been associated with impaired fetal brain development also have been associated with a down-regulation of placental 11β-HSD2 activity, including high maternal anxiety (42), severe infection (43), high levels of proinflammatory cytokines (44), and alcohol exposure (45). Another plausible pathway by which maternal cortisol can produce elevations in fetal cortisol level is by stimulating and thereby increasing the production of placental corticotrophin-releasing hormone (46–48), which is known to act on the fetal HPA axis and stimulate adrenal steroid biosynthesis. Finally, a small number of human studies that have obtained paired maternal and fetal plasma samples during gestation (49) or paired maternal and cord blood samples at delivery (50) have reported significant correlations between maternal and fetal/newborn concentrations of cortisol.
The amygdala regulates a variety of emotions, including fear, depression, and anxiety, and plays a major role in emotional memory processing and reactivity of the HPA axis to stress (31). Exposure to high levels of stress in early postnatal life has been associated with altered development and function of the amygdala. For example, orphanage rearing is associated with an atypically large amygdala and increased incidence of anxiety disorders (51, 52), and maternal depression in the postnatal period is associated with larger amygdala volumes in preadolescent children (53). The findings of the present study extend the developmental window of susceptibility to stress-related environmental conditions on the developing amygdala from the postnatal to the early prenatal period of life.
We found a significant association between maternal cortisol levels in gestation and the volume of the child’s right but not left amygdala. This is consistent with previous evidence for an association between high anxiety and larger right amygdala volume (54) and right amygdala hyperactivity (55). A “valence” model has been discussed in this context (56), which suggests that negative emotions are predominantly processed in the right but not left amygdala, thereby supporting a specific role of the right amygdala in affective disorders.
Concentrations of maternal cortisol during pregnancy were not related to subsequent measures of hippocampal volume in the 7-y-old children in the present study. This pattern of enlarged amygdala but unaltered hippocampus volume is consistent with studies of orphanage rearing and exposure to maternal postnatal depression (51–53). The similarity between the pattern of our findings and those from previous studies raises the question of whether the amygdala may be more susceptible than the hippocampus to stress and elevated glucocorticoid exposure. Although evidence in animals indicates that exposure to prenatal stress or excess glucocorticoids in gestation impairs the development of the hippocampus (34, 57), it is possible that the consequences of in utero stress exposure on the hippocampus may emerge only at later stages (e.g., during puberty or aging), or upon additional exposure to postnatal stress (58–60). Furthermore, it has been suggested that, after exposure to insults, the hippocampus, but not the amygdala, has a high capability for regeneration (61). Also, we note that, in boys, a statistical trend was observed for higher maternal cortisol concentrations at 15 wk gestation being associated with smaller hippocampal volumes. This trend needs to be further explored in larger samples.
Higher maternal cortisol concentration in pregnancy was associated with larger amygdala volumes and more affective problems in girls but not boys. The distribution of maternal cortisol, amygdala volume, and affective problems did not differ across girls and boys. The fact that a similar concentration of cortisol is associated with different consequences in boys and girls suggests a sex-specific programming effect, i.e., the same environmental cue is not associated with the same outcome in boys and girls. There are several examples in the animal and human literature to suggest many prenatal insults produce outcome-specific sexually dimorphic developmental consequences (33, 60, 62–64). Mechanisms that have been discussed in this context include the presence of sex-specific placental adaptation to stress exposure (65) and the notion of increased susceptibility of the female brain to its milieu given the more rapid neurodevelopmental trajectory in females compared with males (66, 67).
We found that maternal cortisol concentrations in early but not later gestation were associated with the size of the child’s amygdala. This observation is consistent with the concept that exposure to potentially adverse conditions in earlier periods of brain development may produce more pronounced consequences than identical or equivalent degree of exposures in later periods (68). In terms of the development of the amygdala structures, it is established that, in humans, all major nuclear masses within the amygdaloid complex are already formed by 15 wk gestation, and that cytoarchitectonic parcellation of the lateral amygdaloid nucleus is occurring at this time (69). Furthermore, the expression level of genes supporting cell proliferation and neuron differentiation is higher during early fetal developmental stage than at any other period in prenatal or postnatal life, and genes sustaining gliogenesis are also being robustly expressed in the amygdala (4). Thus, excess levels of glucocorticoid exposure during this developmental period may have stable and permanent consequences by decreasing cell proliferation and neuronal differentiation and increasing gliogenesis (70), which, in turn, may account for the larger amygdala volumes observed in the context of excess prenatal glucocorticoid exposure (35). These findings are consistent with extensive evidence of chronic stress being associated with larger adult amygdala volumes as a result of hypertrophy of nerve cells in the amygdala (71).
In terms of the association between maternal and fetal cortisol concentrations across gestation, it is possible that, in humans, measures of maternal cortisol in early gestation may more closely reflect fetal levels than measures of maternal cortisol in later gestation. Cortisol levels in the fetal compartment represent the sum of that proportion of maternal cortisol that has crossed the placenta and the cortisol produced by the fetal adrenal gland. Although the human fetal adrenal gland develops and is active from very early gestation onward, it is believed that it is only after approximately 22 wk gestation that fetal adrenal cortisol is produced in appreciable quantities (72). Thus, in early gestation, cortisol of maternal origin may represent the primary source of cortisol in the fetal compartment. The implication of this important feature of maternal–placental–fetal endocrine physiology is not only that measures of maternal cortisol in early gestation may serve as a better marker of fetal cortisol concentrations than measures of maternal cortisol in mid- or late gestation, but more importantly that variations in maternal cortisol in early gestation may produce larger variations in fetal cortisol then the same degree of variation in maternal cortisol in later gestation, when the fetal adrenal is active in terms of de novo cortisol production and feedback regulation.
The present findings are highly relevant because they provide evidence for a brain structure–function relationship, because larger right amygdala volume was associated with more affective problems, and the association between high maternal cortisol in gestation and subsequent affective problems in the child was mediated by a larger amygdala volume. A causal inference cannot be made from the present finding because the temporal sequence of events is unknown (i.e., whether the larger amygdala volume preceded emergence of affective problems) and will require the conduct of longitudinal studies that include serial assessments of brain structure and function from birth onward. We are currently conducting such studies. Additional strengths of our present study include the prospective design with repeated measures of maternal cortisol over gestation and manual segmentation of all child brain magnetic resonance images by a highly reliable rater. This may be especially important for the assessment of amygdala volumes because it has been suggested that automatic segmentation protocols are less reliable for smaller subcortical structures (73).
The present findings are consistent with evidence from human studies indicating that exposure to elevated levels of maternal cortisol concentration during gestation is associated with risk factors for affective disorders, including anxious behavior and exaggerated stress reactivity (24–26). Our present study relied on naturally occurring variations in maternal biological stress signals and psychological states, rather than experimental manipulations. It is difficult, therefore, to separate the effects of maternal psychological state during pregnancy from the consequences of other factors that might contribute to this association, including genetic factors. Genetically informed study designs involving children conceived by in vitro fertilization who were not genetically related to their mothers provide strong evidence that the environment contributes to poor child mental health (74, 75). Studies evaluating the consequences of random exposure to extreme stressors during gestation further suggest that prenatal exposure to stress exerts a lasting influence on child development (76–79).
We note that fetal exposure to high levels of cortisol during gestation is a nonspecific marker of subsequent risk for mental and physical health problems. Thus, it is likely that, for any given individual, the risk of developing specific psychopathologic conditions in the context of elevated prenatal cortisol exposure may be influenced by additional factors such as genetic vulnerability, epigenetic alterations, and other environmental exposures (60, 80). However, we emphasize that the effects of maternal cortisol in pregnancy on child amygdala volume and affective function persisted after controlling for maternal depressive symptoms at the time of assessment of child outcomes.
In conclusion, findings from the present study provide support for the premise that susceptibility for affective disorders may, in part, be programmed in utero, and that this effect may be mediated through changes in anatomy of the amygdala. These results suggest potential clinical significance because they are in accordance with the higher prevalence of affective disorders in women than in men (81) and they point to a plausible etiological pathway. Thus, the results of the present study add to the growing awareness of the importance of the intrauterine environment and suggest the origins of neurodevelopmental disorders may have their foundations very early in life.
Materials and Methods
Participants.
Study participants comprised a cohort of mother–child dyads followed prospectively from gestation through childhood. Pregnant mothers were recruited in early gestation from obstetric care provider clinics at two major university medical centers in Southern California. Exclusionary criteria for pregnant mothers included tobacco, alcohol, or other drug use during pregnancy; uterine or cervical abnormalities; or presence of conditions associated with dysregulated neuroendocrine or immune function. Exclusion criteria for children were delivery less than 34 wk gestation, occurrence of any perinatal complications associated with neurological consequences (e.g., hypoxia), and congenital, genetic, or neurologic disorders. The mean age at child assessment was 7.5 ± 0.9 y (SD; range, 6–9 y), and the final sample consisted of 65 mother–child dyads. The study was approved by the institutional review boards of the participating institutions, informed written consent was obtained from all mothers, and informed assent was obtained from all children. The sociodemographic, pregnancy, birth, and maternal and child characteristics of the study population are presented in Table 4.
Table 4.
Sociodemographic characteristics, maternal cortisol concentrations and birth outcomes in mother–child dyads (N = 65)
| Outcome | Value |
| Maternal age, y | 31.1 ± 6.5 |
| Race/ethnicity, % | |
| Non-Hispanic white | 37 |
| Hispanic white | 22 |
| Black | 6 |
| Asian | 20 |
| Other | 15 |
| Annual household income, % | |
| $0 to $30,000 | 34.5 |
| $30,001 to $60,000 | 13.8 |
| $60,001 to $100,000 | 32.7 |
| Over $100,000 | 19 |
| Maternal education (highest level completed), % | |
| At least high school degree | 15.3 |
| Some college education | 22.0 |
| Bachelor degree | 38.9 |
| Graduate degree | 10.2 |
| Other degree | 12.6 |
| Depression (BDI) postpartum | 0.30 ± 0.34 |
| Parity (primiparous), % | 52 |
| Child sex (%) | |
| Female | 35 (54) |
| Male | 30 (46) |
| Length of gestation, wk | 38.9 ± 1.7 |
| 34.4–36.7 wk, % | 11 |
| Birth weight, g | 3,490.7 ± 665.8 |
| <2,500 g, % | 4.6 |
| Birth weight percentile | 57.7 ± 29.7 |
| <10th percentile, % | 9 |
Values presented as mean ± SD where appropriate.
Maternal Assessments During Index Pregnancy: Obstetric Risk, Duration of Gestation, and Birth Weight.
Obstetric risk was defined as the presence of medical complications in the index pregnancy. Risk conditions were ascertained by extensive medical chart review and coded as a binary variable as previously described (82). The study population was predominantly (72%) at low risk with respect to adverse birth outcomes. Gestational age was determined by best obstetric estimate with a combination of last menstrual period and early uterine size, and was confirmed by obstetric ultrasonographic biometry before 20 wk using standard clinical criteria (83). Birth outcomes were abstracted from medical charts after delivery, and birth weight percentiles were computed using national norms (84). All children included in the study had a stable neonatal course (Apgar scores at 5 min ≥ 8) and had normal neurologic findings at the time of assessment, as determined by MRI.
Maternal Cortisol Concentrations During Pregnancy.
Maternal saliva samples were obtained serially using a Salivette sampling device (Sarstedt) at 15 ± 1.0 wk (SD; n = 36), 19 ± 0.8 wk (n = 62), 25 ± 0.9 wk (n = 62), 31 ± 0.9 wk (n = 63), and 37 ± 0.7 wk (n = 50) gestation for measures of maternal cortisol over the course of the index pregnancy. Salivary cortisol levels were determined by a competitive luminescence immunoassay (IBL America). The intra- and interassay coefficients of variance were 5.5% and 7.6%, respectively.
The time of day of saliva sample collection across the different assessments over gestation varied between 9:10 AM to 6:02 PM, with the average time ranging around 2:00 PM. We note, however, that the majority of samples (∼80%) across all gestational time points were, in fact, collected in a fairly narrow afternoon time range. Sample collection before noon took place in only 17%, 10%, 22%, 22%, and 20% of the women at the 15-wk, 19-wk, 25-wk, 31-wk, and 37-wk gestation visits, respectively. It is important to note that the distribution of the collection time at the five different gestational time points was not significantly different [F(4,272) = 0.767, P = 0.55; Fig. S1]. As expected, there was a significant correlation between time of day of sample collection and cortisol concentration (all P < 0.05). For this association, at each of the five time points, the linear model was the best fit compared with higher-order polynomial regression, which included nonlinear terms (all P > 0.10 for nonlinear terms). Therefore, cortisol values at the average time of sample collection (approximately 2:00 PM) across subjects were estimated by applying linear regression models, which is an appropriate way of statistically accounting for the variation in time of day of sample collection. All statistical analyses were performed with these predicted cortisol concentrations (adjusted for time of day of sample collection).
Child Assessments.
MRI acquisition.
Each child received a 3-T Philips Achieva MRI brain scan. A 1-mm3 isotropic T1 anatomical scan was acquired in the sagittal plane. An inversion-recovery spoiled gradient recalled acquisition sequence with the following parameters was applied: repetition time, 11 ms; echo time, 3.3 ms; inversion time, 100 ms; turbo field echo factor, 192; 150 slices; sensitivity encoding for fast MRI acceleration; and flip angle, 18°.
Processing of MRI data.
The native anatomic images were processed before manual volume segmentation to first correct for intensity nonuniformity (85). All images were then registered into stereotaxic space by using the ICBM 152 template (86).
Volume analyses of the hippocampus and amygdala were performed by using the interactive software package DISPLAY developed at the Brain Imaging Center of the Montreal Neurological Institute, and a standardized segmentation protocol was applied to outline the anatomical boundaries of the hippocampus and amygdala (87). All brains were segmented by the same rater with demonstrated interrater reliability (intraclass correlation coefficient > 0.90) and intrarater reliability (>0.92).
Child handedness.
Child handedness was assessed with a modified version of the Edinburgh Handedness Inventory (88). The majority (85%) of children were right-handed.
Child affective problems.
Child affective problems were assessed by using the CBCL (89) subscale that reflects maternal report of affective problems in the child (in accordance with the Diagnostic and Statistical Manual of Mental Disorders IV). The raw sum scores were transferred to T-scores based on the sex-specific reference tables provided in the manual (89). The Diagnostic and Statistical Manual of Mental Disorders IV-oriented scales of the CBCL are known to correspond well with clinical diagnoses (90, 91), suggesting it is a reliable screening instrument.
Maternal Assessments at the Time of Child Follow-Up: Maternal Depressive Symptoms.
Maternal depressive symptoms at the time of the child assessment were measured by using the Beck Depression Inventory (92). Because maternal depression may influence her rating of her child on the CBCL (93) as well as her child’s amygdala volumes (53), all statistical analyses between cortisol in pregnancy and child outcomes controlled for concurrent maternal depression. Furthermore, by controlling for maternal depression, we address hereditary transmission of affective problems and focus on the environmental (i.e., maternal cortisol) contribution.
Statistical Analysis.
Separate regression models were run for the left and right hippocampus and amygdala and for child affective problems.
Cortisol.
To account for the diurnal variation of cortisol secretion, all statistical analyses were performed with cortisol concentration adjusted for time of day of sample collection.
Maternal cortisol at the first pregnancy assessment at 15 wk gestation was used to represent the cortisol milieu in early gestation. In additional exploratory analyses, the following cortisol measures were used to test time of gestation-specific cortisol associations with child brain volumes: (i) AUC1 for maternal cortisol from 15 to 28 wk gestation adjusting for cortisol at15 wk, to capture the possible relation between maternal cortisol in mid gestation with child brain volume over and above those of early gestation; and (ii) AUC2 for maternal cortisol from 28 to 37 wk gestation after adjusting for AUC1, to capture a possible association between maternal cortisol in late gestation over and above those of early and mid-gestation.
Sex.
Because many animal and human studies suggest that fetal/child sex may moderate the effects of pre- or postnatal exposures on developmental outcomes (62, 63, 94), we included an interaction term between maternal prenatal cortisol and sex of the child in all our statistical models (SI Materials and Methods provides further details).
Covariates.
The statistical analyses to test the association between maternal cortisol in pregnancy and child brain volumes were adjusted for the possible effects of several pregnancy-related and birth as well as concurrent maternal and child characteristics, including presence of obstetric complications, duration of gestation, birth weight percentile, maternal depression at child follow-up, and child sex, age at testing, and handedness. The same covariates, with the exception of child’s handedness, were included in the analyses testing the association between maternal cortisol in pregnancy and child affective symptoms. Further information is provided in SI Materials and Methods.
Multiple imputation.
Missing cortisol data were estimated by using the multiple-imputation method (95). Further details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank our research laboratory staff for their contributions and for data collection, especially Christina Canino, Natalie Hernandez, and Cheryl Crippen. We also thank the mothers and children who participated in this study. This research was primarily supported by US Public Health Service, National Institutes of Health Grant HD-51852 (to C.A.S.) and supported in part by US Public Health Service, National Institutes of Health Grants NS-41298 (to C.A.S.), HD-28413 (to C.A.S.), and MH-091351 (to C.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 7613 (volume 109, number 20).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201295109/-/DCSupplemental.
References
- 1.Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Mol Psychiatry. 2005;10:160–184. doi: 10.1038/sj.mp.4001579. [DOI] [PubMed] [Google Scholar]
- 2.Bellani M, Baiano M, Brambilla P. Brain anatomy of major depression II. Focus on amygdala. Epidemiol Psychiatr Sci. 2011;20:33–36. doi: 10.1017/s2045796011000096. [DOI] [PubMed] [Google Scholar]
- 3.Gluckman PD, Hanson MA. Living with the past: Evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 4.Kang HJ, et al. Spatio-temporal transcriptome of the human brain. Nature. 2011;478:483–489. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 6.Humphrey T. Some observations on the development of the human hippocampal formation. Trans Am Neurol Assoc. 1964;89:207–209. [PubMed] [Google Scholar]
- 7.Humphrey T. The development of the human amygdala during early embryonic life. J Comp Neurol. 1968;132:135–165. doi: 10.1002/cne.901320108. [DOI] [PubMed] [Google Scholar]
- 8.Teicher MH, et al. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 9.Brown AS, et al. Prenatal infection and cavum septum pellucidum in adult schizophrenia. Schizophr Res. 2009;108:285–287. doi: 10.1016/j.schres.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hulshoff Pol HE, et al. Prenatal exposure to famine and brain morphology in schizophrenia. Am J Psychiatry. 2000;157:1170–1172. doi: 10.1176/appi.ajp.157.7.1170. [DOI] [PubMed] [Google Scholar]
- 11.Antonow-Schlorke I, et al. Vulnerability of the fetal primate brain to moderate reduction in maternal global nutrient availability. Proc Natl Acad Sci USA. 2011;108:3011–3016. doi: 10.1073/pnas.1009838108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sowell ER, et al. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb Cortex. 2008;18:136–144. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson BL, Levitt P, Stanwood GD. Prenatal exposure to drugs: Effects on brain development and implications for policy and education. Nat Rev Neurosci. 2009;10:303–312. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lotfipour S, et al. Orbitofrontal cortex and drug use during adolescence: Role of prenatal exposure to maternal smoking and BDNF genotype. Arch Gen Psychiatry. 2009;66:1244–1252. doi: 10.1001/archgenpsychiatry.2009.124. [DOI] [PubMed] [Google Scholar]
- 15.Khashan AS, et al. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 2008;65:146–152. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- 16.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Entringer S, Buss C, Wadhwa PD. Prenatal stress and developmental programming of human health and disease risk: concepts and integration of empirical findings. Curr Opin Endocrinol Diabetes Obes. 2010;17:507–516. doi: 10.1097/MED.0b013e3283405921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandman CA, Davis EP, Buss C, Glynn LM. Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus. Neuroendocrinology. 2011;95:8–21. doi: 10.1159/000327017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chrousos GP, Kino T. Glucocorticoid signaling in the cell. Expanding clinical implications to complex human behavioral and somatic disorders. Ann N Y Acad Sci. 2009;1179:153–166. doi: 10.1111/j.1749-6632.2009.04988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 21.Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81:131–148. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, et al. Late gestational maternal serum cortisol is inversely associated with fetal brain growth. Neurosci Biobehav Rev. 2011;36:1085–1092. doi: 10.1016/j.neubiorev.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Wyrwoll CS, Holmes MC. Prenatal excess glucocorticoid exposure and adult affective disorders: A role for serotonergic and catecholamine pathways. Neuroendocrinology. 2011;95:47–55. doi: 10.1159/000331345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. J Child Psychol Psychiatry. 2011;52:119–129. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis EP, et al. Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry. 2007;46:737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- 26.de Weerth C, van Hees Y, Buitelaar JK. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Hum Dev. 2003;74:139–151. doi: 10.1016/s0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 27.Davis EP, Waffarn F, Sandman CA. Prenatal treatment with glucocorticoids sensitizes the HPA axis response to stress among full-term infants. Dev Psychobiol. 2011;53:175–183. doi: 10.1002/dev.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis EP, et al. Antenatal betamethasone treatment has a persisting influence on infant HPA axis regulation. J Perinatol. 2006;26:147–153. doi: 10.1038/sj.jp.7211447. [DOI] [PubMed] [Google Scholar]
- 29.Lajic S, Nordenström A, Hirvikoski T. Long-term outcome of prenatal dexamethasone treatment of 21-hydroxylase deficiency. Endocr Dev. 2011;20:96–105. doi: 10.1159/000321228. [DOI] [PubMed] [Google Scholar]
- 30.Trautman PD, Meyer-Bahlburg HF, Postelnek J, New MI. Effects of early prenatal dexamethasone on the cognitive and behavioral development of young children: Results of a pilot study. Psychoneuroendocrinology. 1995;20:439–449. doi: 10.1016/0306-4530(94)00070-0. [DOI] [PubMed] [Google Scholar]
- 31.Whalen PJ, Phelps EA. The Human Amygdala. New York: Guilford; 2009. [Google Scholar]
- 32.Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- 33.Behan AT, et al. Evidence of female-specific glial deficits in the hippocampus in a mouse model of prenatal stress. Eur Neuropsychopharmacol. 2011;21:71–79. doi: 10.1016/j.euroneuro.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Coe CL, et al. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry. 2003;54:1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- 35.Salm AK, et al. Lateral amygdaloid nucleus expansion in adult rats is associated with exposure to prenatal stress. Brain Res Dev Brain Res. 2004;148:159–167. doi: 10.1016/j.devbrainres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Barbazanges A, Piazza PV, Le Moal M, Maccari S. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci. 1996;16:3943–3949. doi: 10.1523/JNEUROSCI.16-12-03943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Setiawan E, Jackson MF, MacDonald JF, Matthews SG. Effects of repeated prenatal glucocorticoid exposure on long-term potentiation in the juvenile guinea-pig hippocampus. J Physiol. 2007;581:1033–104. doi: 10.1113/jphysiol.2006.127381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lange C, Irle E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychol Med. 2004;34:1059–1064. doi: 10.1017/s0033291703001806. [DOI] [PubMed] [Google Scholar]
- 39.Beitins IZ, Bayard F, Ances IG, Kowarski A, Migeon CJ. The metabolic clearance rate, blood production, interconversion and transplacental passage of cortisol and cortisone in pregnancy near term. Pediatr Res. 1973;7:509–519. doi: 10.1203/00006450-197305000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Brown RW, et al. The ontogeny of 11 beta-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology. 1996;137:794–797. doi: 10.1210/endo.137.2.8593833. [DOI] [PubMed] [Google Scholar]
- 41.Benediktsson R, Calder AA, Edwards CR, Seckl JR. Placental 11 beta-hydroxysteroid dehydrogenase: A key regulator of fetal glucocorticoid exposure. Clin Endocrinol (Oxf) 1997;46:161–166. doi: 10.1046/j.1365-2265.1997.1230939.x. [DOI] [PubMed] [Google Scholar]
- 42.O’Donnell KJ, et al. Maternal prenatal anxiety and downregulation of placental 11beta-HSD2. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 43.Johnstone JF, Bocking AD, Unlugedik E, Challis JR. The effects of chorioamnionitis and betamethasone on 11beta hydroxysteroid dehydrogenase types 1 and 2 and the glucocorticoid receptor in preterm human placenta. J Soc Gynecol Investig. 2005;12:238–245. doi: 10.1016/j.jsgi.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 44.Kossintseva I, et al. Proinflammatory cytokines inhibit human placental 11beta-hydroxysteroid dehydrogenase type 2 activity through Ca2+ and cAMP pathways. Am J Physiol Endocrinol Metab. 2006;290:E282–E288. doi: 10.1152/ajpendo.00328.2005. [DOI] [PubMed] [Google Scholar]
- 45.Liang G, Chen M, Pan XL, Zheng J, Wang H. Ethanol-induced inhibition of fetal hypothalamic-pituitary-adrenal axis due to prenatal overexposure to maternal glucocorticoid in mice. Exp Toxicol Pathol. 2011;63:607–611. doi: 10.1016/j.etp.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 46.Cheng YH, et al. Glucocorticoid stimulation of corticotropin-releasing hormone gene expression requires a cyclic adenosine 3′,5′-monophosphate regulatory element in human primary placental cytotrophoblast cells. J Clin Endocrinol Metab. 2000;85:1937–1945. doi: 10.1210/jcem.85.5.6552. [DOI] [PubMed] [Google Scholar]
- 47.Rehman KS, Sirianni R, Parker CR, Jr, Rainey WE, Carr BR. The regulation of adrenocorticotrophic hormone receptor by corticotropin-releasing hormone in human fetal adrenal definitive/transitional zone cells. Reprod Sci. 2007;14:578–587. doi: 10.1177/1933719107307908. [DOI] [PubMed] [Google Scholar]
- 48.Sandman CA, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27:1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Gitau R, Fisk NM, Glover V. Human fetal and maternal corticotrophin releasing hormone responses to acute stress. Arch Dis Child Fetal Neonatal Ed. 2004;89:F29–F32. doi: 10.1136/fn.89.1.F29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith AK, et al. Predictors of neonatal hypothalamic-pituitary-adrenal axis activity at delivery. Clin Endocrinol (Oxf) 2011 doi: 10.1111/j.1365-2265.2011.03998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tottenham N, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mehta MA, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian Adoptees study pilot. J Child Psychol Psychiatry. 2009;50:943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 53.Lupien SJ, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci USA. 2011;108:14324–14329. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Bellis MD, et al. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol Psychiatry. 2000;48:51–57. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- 55.Juranek J, et al. Association between amygdala volume and anxiety level: Magnetic resonance imaging (MRI) study in autistic children. J Child Neurol. 2006;21:1051–1058. doi: 10.1177/7010.2006.00237. [DOI] [PubMed] [Google Scholar]
- 56.Demaree HA, Everhart DE, Youngstrom EA, Harrison DW. Brain lateralization of emotional processing: Historical roots and a future incorporating “dominance”. Behav Cogn Neurosci Rev. 2005;4:3–20. doi: 10.1177/1534582305276837. [DOI] [PubMed] [Google Scholar]
- 57.Jia N, et al. Prenatal stress causes dendritic atrophy of pyramidal neurons in hippocampal CA3 region by glutamate in offspring rats. Dev Neurobiol. 2010;70:114–125. doi: 10.1002/dneu.20766. [DOI] [PubMed] [Google Scholar]
- 58.Meaney MJ, Aitken DH, Bhatnagar S, Sapolsky RM. Postnatal handling attenuates certain neuroendocrine, anatomical, and cognitive dysfunctions associated with aging in female rats. Neurobiol Aging. 1991;12:31–38. doi: 10.1016/0197-4580(91)90036-j. [DOI] [PubMed] [Google Scholar]
- 59.Koehl M, Lemaire V, Le Moal M, Abrous DN. Age-dependent effect of prenatal stress on hippocampal cell proliferation in female rats. Eur J Neurosci. 2009;29:635–640. doi: 10.1111/j.1460-9568.2009.06608.x. [DOI] [PubMed] [Google Scholar]
- 60.Buss C, et al. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J Neurosci. 2007;27:2592–2595. doi: 10.1523/JNEUROSCI.3252-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 62.Buss C, Davis EP, Hobel CJ, Sandman CA. Maternal pregnancy-specific anxiety is associated with child executive function at 6-9 years age. Stress. 2011;14:665–676. doi: 10.3109/10253890.2011.623250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zohar I, Weinstock M. Differential effect of prenatal stress on the expression of corticotrophin-releasing hormone and its receptors in the hypothalamus and amygdala in male and female rats. J Neuroendocrinol. 2011;23:320–328. doi: 10.1111/j.1365-2826.2011.02117.x. [DOI] [PubMed] [Google Scholar]
- 64.Bale TL. Sex differences in prenatal epigenetic programming of stress pathways. Stress. 2011;14:348–356. doi: 10.3109/10253890.2011.586447. [DOI] [PubMed] [Google Scholar]
- 65.Clifton VL. Review: Sex and the human placenta: Mediating differential strategies of fetal growth and survival. Placenta. 2010;31(suppl):S33–S39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 66.Buss C, et al. Maturation of the human fetal startle response: Evidence for sex-specific maturation of the human fetus. Early Hum Dev. 2009;85:633–638. doi: 10.1016/j.earlhumdev.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nathanielsz PW, et al. Life before birth: Effects of cortisol on future cardiovascular and metabolic function. Acta Paediatr. 2003;92:766–772. [PubMed] [Google Scholar]
- 68.Stern E, et al. Educational Research and Neurosciences—Expectations, Evidence, Research Prospects. Berlin: Federal Ministry of Education and Research; 2005. [Google Scholar]
- 69.Nikolić I, Kostović I. Development of the lateral amygdaloid nucleus in the human fetus: Transient presence of discrete cytoarchitectonic units. Anat Embryol (Berl) 1986;174:355–360. doi: 10.1007/BF00698785. [DOI] [PubMed] [Google Scholar]
- 70.Moors M, et al. Dickkopf 1 mediates glucocorticoid-induced changes in human neural progenitor cell proliferation and differentiation. Toxicol Sci. 2012;125:488–495. doi: 10.1093/toxsci/kfr304. [DOI] [PubMed] [Google Scholar]
- 71.McEwen BS. Stress, sex, and neural adaptation to a changing environment: Mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(suppl):E38–E59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. 1997;18:378–403. doi: 10.1210/edrv.18.3.0304. [DOI] [PubMed] [Google Scholar]
- 73.Luft AR, Skalej M, Welte D, Kolb R, Klose U. Reliability and exactness of MRI-based volumetry: A phantom study. J Magn Reson Imaging. 1996;6:700–704. doi: 10.1002/jmri.1880060421. [DOI] [PubMed] [Google Scholar]
- 74.Rice F, et al. The links between prenatal stress and offspring development and psychopathology: Disentangling environmental and inherited influences. Psychol Med. 2010;40:335–345. doi: 10.1017/S0033291709005911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lewis G, et al. Investigating environmental links between parent depression and child depressive/anxiety symptoms using an assisted conception design. J Am Acad Child Adolesc Psychiatry. 2011;50:451.e1. doi: 10.1016/j.jaac.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Entringer S, et al. Prenatal psychosocial stress exposure is associated with subsequent working memory performance in young women. Behav Neurosci. 2009;123:886–893. doi: 10.1037/a0016265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.King S, Laplante DP. The effects of prenatal maternal stress on children’s cognitive development: Project Ice Storm. Stress. 2005;8:35–45. doi: 10.1080/10253890500108391. [DOI] [PubMed] [Google Scholar]
- 78.Huizink AC, et al. Chernobyl exposure as stressor during pregnancy and behaviour in adolescent offspring. Acta Psychiatr Scand. 2007;116:438–446. doi: 10.1111/j.1600-0447.2007.01050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J, Olsen J, Vestergaard M, Obel C. Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: A nationwide follow-up study in Denmark. Eur Child Adolesc Psychiatry. 2010;19:747–753. doi: 10.1007/s00787-010-0113-9. [DOI] [PubMed] [Google Scholar]
- 80.Bergman K, Sarkar P, Glover V, O’Connor TG. Quality of child-parent attachment moderates the impact of antenatal stress on child fearfulness. J Child Psychol Psychiatry. 2008;49:1089–1098. doi: 10.1111/j.1469-7610.2008.01987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weissman MM, Olfson M. Depression in women: Implications for health care research. Science. 1995;269:799–801. doi: 10.1126/science.7638596. [DOI] [PubMed] [Google Scholar]
- 82.Wadhwa PD, et al. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: A prospective investigation. Am J Obstet Gynecol. 2004;191:1063–1069. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- 83.O’Brien GD, Queenan JT, Campbell S. Assessment of gestational age in the second trimester by real-time ultrasound measurement of the femur length. Am J Obstet Gynecol. 1981;139:540–545. doi: 10.1016/0002-9378(81)90514-7. [DOI] [PubMed] [Google Scholar]
- 84.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 86.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 87.Pruessner JC, et al. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: Minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- 88.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 89.Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: Univ Vermont Press; 2001. [Google Scholar]
- 90.Ebesutani C, et al. The Research Network on Youth Mental Health Concurrent Validity of the Child Behavior Checklist DSM-Oriented Scales: Correspondence with DSM diagnoses and comparison to syndrome scales. J Psychopathol Behav Assess. 2010;32:373–384. doi: 10.1007/s10862-009-9174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferdinand RF. Validity of the CBCL/YSR DSM-IV scales anxiety problems and affective problems. J Anxiety Disord. 2008;22:126–134. doi: 10.1016/j.janxdis.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 92.Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory II (BDI-II) San Antonio: Psychology Corporation; 1996. [Google Scholar]
- 93.van der Toorn SL, et al. Maternal depressive symptoms, and not anxiety symptoms, are associated with positive mother-child reporting discrepancies of internalizing problems in children: A report on the TRAILS study. Eur Child Adolesc Psychiatry. 2010;19:379–388. doi: 10.1007/s00787-009-0062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sandman CA, Davis EP, Buss C, Glynn LM. Prenatal programming of human neurological function. Int J Pept. 2011;2011:837596. doi: 10.1155/2011/837596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harrell FE. Regression Modeling Strategies, With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer-Verlag; 2001. [Google Scholar]