Abstract
Many insects are associated with obligate symbiotic bacteria, which are localized in specialized cells called bacteriocytes, vertically transmitted through host generations via ovarial passage, and essential for growth and reproduction of their hosts. Although vertical transmission is pivotal for maintenance of such intimate host–symbiont associations, molecular and cellular mechanisms underlying the process are largely unknown. Here we report a cellular mechanism for vertical transmission of the obligate symbiont Buchnera in the pea aphid Acyrthosiphon pisum. In the aphid body, Buchnera cells are transmitted from maternal bacteriocytes to adjacent blastulae at the ovariole tips in a highly coordinated manner. By making use of symbiont-manipulated strains of A. pisum, we demonstrated that the facultative symbiont Serratia is, unlike Buchnera, not transmitted from maternal bacteriocytes to blastulae, suggesting a specific mechanism for Buchnera transmission. EM observations revealed a series of exo-/endocytotic processes operating at the bacteriocyte–blastula interface: Buchnera cells are exocytosed from the maternal bacteriocyte, temporarily released to the extracellular space, and endocytosed by the posterior syncytial cytoplasm of the blastula. These results suggest that the selective Buchnera transmission is likely attributable to Buchnera-specific exocytosis by the maternal bacteriocyte, whereas both Buchnera and Serratia are nonselectively incorporated by the endocytotic activity of the posterior region of the blastula. The sophisticated cellular mechanism for vertical transmission of Buchnera must have evolved to ensure the obligate host–symbiont association, whereas facultative symbionts like Serratia may coopt the endocytotic component of the mechanism for their entry into the host embryos.
Keywords: Buchnera aphidicola, endocytosis, Serratia symbiotica
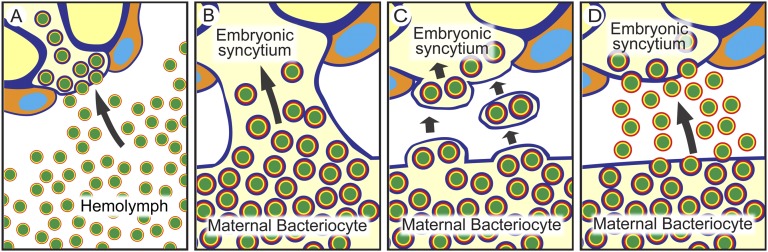
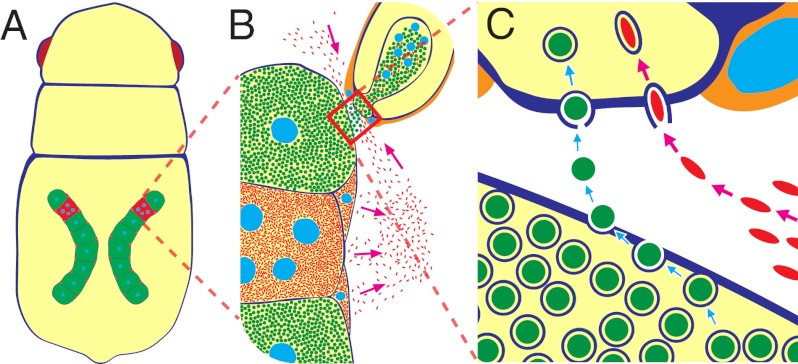
Many insects harbor endosymbiotic bacteria in their cells and tissues (1). Facultative symbionts like Wolbachia pipientis in diverse insects are of parasitic or conditionally beneficial nature, tend to cause negative effects on their hosts, and exhibit a broad cellular/tissue tropism (2, 3). Meanwhile, obligate symbionts like Buchnera aphidicola in aphids are of mutualistic nature, contribute to the fitness of their hosts, and are localized in specialized cells called bacteriocytes (4, 5). In general, these insect symbionts are stably maintained through host generations by vertical transmission from mothers to their offspring (1, 6). Vertical transmission is pivotal for maintenance of such host–symbiont associations, but our understanding of molecular and cellular mechanisms underlying the process is quite limited. In the well-studied facultative symbiotic association of the Drosophila–Wolbachia endosymbiosis (2), it has been shown that recognition of stem cell niches and association with dynein/kinesin/microtubule are important for symbiont transmission to host germline and symbiont segregation to host daughter cells (7–9). On the contrary, in the aphid–Buchnera endosymbiosis as the model obligate symbiotic association with host and symbiont genomic data available (10, 11), symbiont transmission mechanisms have been poorly understood except for some morphological/cytological aspects. There are a number of microscopic descriptions of the symbiont transmission process in various aphids: some have reported that symbionts circulating in hemolymph are transmitted to a posterior region of the blastula with enlarged follicle cells called “follicle pegs,” whereas others described that symbionts are transmitted directly from a neighboring bacteriocyte to the follicular region (reviewed in ref. 1). Recent studies that used specific molecular markers and modern microscopy have provided clearer pictures of the symbiont transmission process in the pea aphid Acyrthosiphon pisum (12–14). Based on immunohistochemistry against a symbiont protein, Wilkinson et al. (12) described “a stream of bacteria passing from a single maternal bacteriocyte to the recipient embryo, possibly via a membranous conduit.” Meanwhile, based on sophisticated confocal imaging, Miura et al. (13) stated that “the transfer of the bacteria appears to first involve the fusion of a membrane-bound maternal bacterial package with the follicular epithelium in the region of the enlarged posterior follicle cells. A channel between these enlarged follicle cells then appears, and the bacteria flow into the posterior of the embryo.” These previous results are summarized into the following hypotheses: the “free symbiont infection” hypothesis (Fig. 1A) (1), the “membranous conduit formation” hypothesis (Fig. 1B) (1, 12), and the “symbiont packet fusion” hypothesis (Fig. 1C) (13).
Fig. 1.
Hypotheses on the vertical transmission mechanism of the obligate aphid symbiont Buchnera from a maternal bacteriocyte to an early embryo: (A) free symbiont infection, (B) membranous conduit formation, (C) symbiont packet fusion, and (D) exo-/endocytotic transport.
In addition to the obligate symbiont Buchnera, A. pisum may be associated with an array of facultative symbionts such as Serratia symbiotica, Hamiltonella defensa, and Regiella insecticola, which are not essential but conditionally beneficial for the host depending on ecological contexts (3, 15). For vertical transmission processes of these facultative symbionts, previous histological descriptions are fragmentary, providing no coherent picture (1, 3).
Here, by making use of symbiont-manipulated strains of A. pisum whose infections with Buchnera and Serratia were experimentally manipulated under specific host genotypes, we demonstrate that a cellular mechanism is operating at the bacteriocyte–embryo interface. The mechanism selectively transports the obligate symbiont Buchnera, but not the facultative symbiont Serratia, from a maternal bacteriocyte to an adjacent blastula. We also describe the intricate segregation processes of the obligate symbiont and the facultative symbiont during bacteriocyte differentiation in aphid embryogenesis. We suggest that these cellular mechanisms might have evolved to ensure the obligate host–symbiont association, and propose an “exo-/endocytotic transport” hypothesis (Fig. 1D) for vertical transmission of Buchnera in A. pisum.
Results and Discussion
Aphid Strains with Different Symbiont Infections.
In this study, we used the following A. pisum strains: (i) the naturally disymbiotic strain IS, wherein localizations of Buchnera and Serratia are under normal control, the former in primary bacteriocytes and the latter in secondary bacteriocytes, sheath cells, and hemolymph (Fig. S1A); (ii) the naturally monosymbiotic strain AIST (named for the National Institute of Advanced Industrial Science and Technology) with Buchnera only (Fig. S1B); (iii) the artificial disymbiotic strain AISTIS generated by microinjection of IS hemolymph into an AIST insect, wherein Serratia exhibits a disordered localization, massively proliferating in hemolymph, often invading primary bacteriocytes and coexisting with Buchnera therein (Fig. S1C); and (iv) an artificial monosymbiotic strain AISTIS/rif with Serratia only, wherein only Serratia is present in bacteriocytes and hemolymph (Fig. S1D). In the absence of the obligate symbiont Buchnera, AISTIS/rif insects show smaller body size, retarded growth, and reduced fecundity, but manage to survive and reproduce in the presence of the facultative symbiont Serratia (16). Table 1 summarizes the attributes of aphid strains used in this study. Here we note that the ordered symbiont localization in the naturally disymbiotic strain, the disordered symbiont localization in the artificial disymbiotic strain, and the bacteriocyte localization of Serratia in the artificial monosymbiotic strain are commonly observed in A. pisum strains of different geographic origins and genetic backgrounds (16, 17).
Table 1.
Aphid strains used in this study
| Strain | Origin | Symbionts | Symbiont localization |
| IS | Natural | Buchnera, Serratia | Ordered and segregated; Buchnera in primary bacteriocytes; Serratia in secondary bacteriocytes, sheath cells and hemolymph |
| AIST | Natural | Buchnera only | Ordered; Buchnera in primary bacteriocytes |
| AISTIS | Manipulated | Buchnera, Serratia | Disordered and mixed; not only Buchnera but also Serratia in primary bacteriocytes |
| AISTIS/rif | Manipulated | Serratia only | Disordered; Serratia in primary bacteriocytes and hemolymph; fitness severely damaged |
Infection Dynamics of Buchnera and Serratia During Host Development.
Miura et al. (13) and Braendle et al. (14) provided detailed histological descriptions of developmental staging, endosymbiont localization, and bacteriocyte formation during parthenogenetic embryogenesis in an American monosymbiotic strain of A. pisum infected with Buchnera only. By using whole-mount in situ hybridization and confocal imaging (18), we examined a Japanese monosymbiotic strain AIST infected with Buchnera only, and confirmed the previous reports. Furthermore, we performed a similar detailed histological inspection of parthenogenetic embryogenesis in a naturally disymbiotic strain IS infected with Buchnera and Serratia. In the following sections, we adopt the developmental staging reported by Miura et al. (13).
Symbiont Cotransmission.
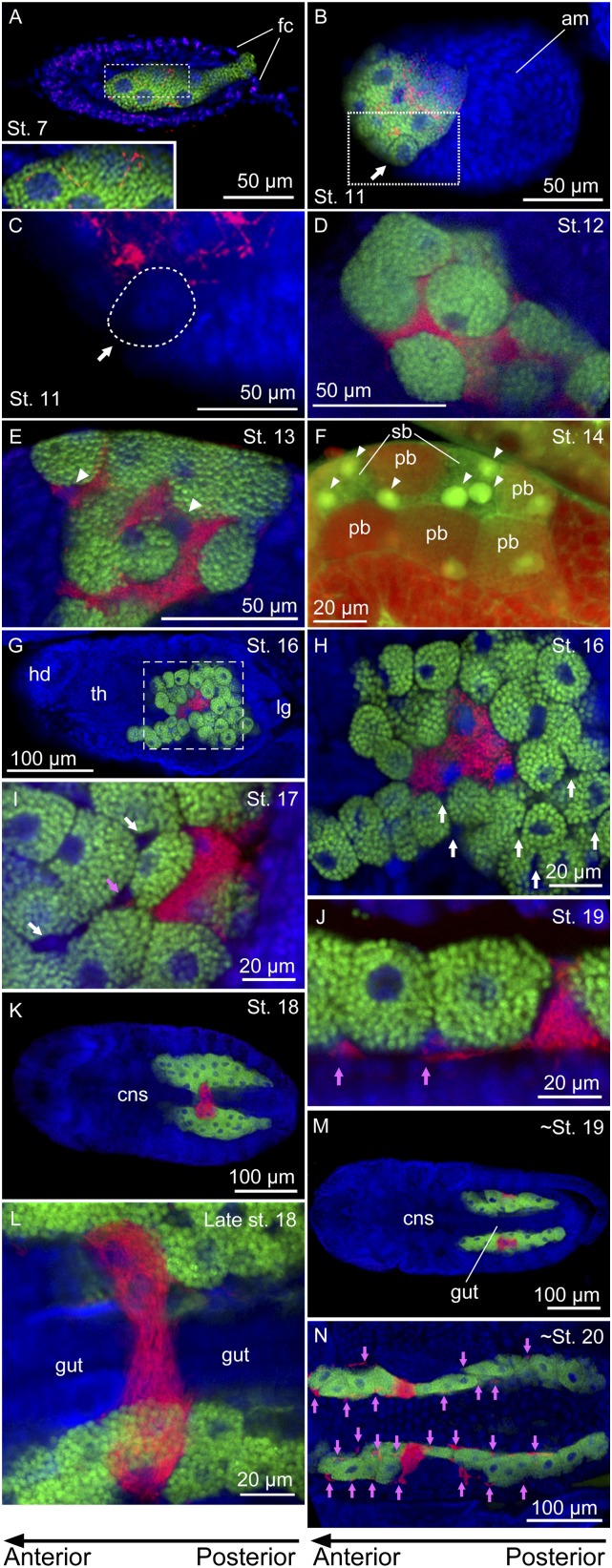
As reported previously (13, 14), symbiont transmission occurred in each blastula at the stage 7, wherein a population of Buchnera cells flowed into the embryo via a posterior passage and colonized a syncytium with several large nuclei at the center of the blastula (Fig. 2A). In addition to the majority of Buchnera cells, a much smaller number of Serratia cells also gained entry into the central syncytium (Fig. 2A, Inset).
Fig. 2.
Infection dynamics of Buchnera and Serratia during embryonic development in the strain IS of A. pisum. In the fluorescent microscopic images, green, red, and blue signals indicate Buchnera cells, Serratia cells, and host insect nuclei, respectively, unless otherwise indicated. Left and right of each panel are anterior and posterior sides, respectively. (A) A stage 7 embryo, to which Buchnera and Serratia are infecting via a posterior passage. Inset: Enlarged image of the dotted rectangle, wherein Serratia cells are clearly seen in addition to Buchnera cells in the central syncytium. (B) A stage 11 embryo in which the symbiont-infected syncytium is cellularizing. Arrow indicates a bacteriocyte that has almost completed the cellularization. (C) An enlarged image of Serratia distribution in the dotted rectangle of B, from which Buchnera signals are removed. Note that no Serratia signals are detected in the completed bacteriocyte (dotted area indicated by an arrow). (D) A late stage 12 embryo in which bacteriocyte formation and symbiont segregation have completed. (E) A stage 13 embryo in which multiple nuclei of the secondary bacteriocyte become evident (arrowheads). (F) A stage 13 embryo stained with green TOTO-3 and red Alexa Fluor 488 phalloidin in which the multiple nuclei (arrowheads) are clearly seen in a large and pleomorphic cytoplasm of the secondary bacteriocyte. (G) A stage 16 embryo in which uninucleate primary bacteriocytes harboring Buchnera and a syncytial secondary bacteriocyte harboring Serratia constitute a large bacteriome. (H) An enlarged image of the bacteriome in the dotted rectangle of G in which a number of small nuclei of sheath cells (white arrows) are seen. (I) The bacteriome of a stage 17 embryo in which Serratia signals appear in the sheath cells (pink arrow). (J) The bacteriome of a stage 19 embryo in which the sheath cells are densely populated by Serratia cells (pink arrows). (K) A stage 18 embryo, whose bacteriome is laterally separating at the region of the secondary bacteriocyte. (L) The bacteriome bridge of a late stage 18 embryo about to be torn apart. (M) A stage 19 embryo in which a pair of syncytial secondary bacteriocytes are established in the bacteriomes. (N) The bacteriomes of a stage 20 embryo. Buchnera is localized in a number of primary bacteriocytes, whereas Serratia is found in a pair of secondary bacteriocytes and many tiny sheath cells (pink arrows). am, amnion; cns, central nervous system; emb, embryo; fc, follicle cell; hd, head; pb, primary bacteriocyte; sb, secondary bacteriocyte; th, thorax.
Bacteriocyte Cellularization and Symbiont Segregation.
During stages 8 to 11, the germ band invaginated and extended into the embryo from the posterior side, pushing the symbiont-containing syncytium toward an anterior region (Fig. 2B). From late stage 10 into stage 11, cell membranes appeared in the syncytium, forming uninucleate bacteriocytes. Notably, although Buchnera cells and Serratia cells had coexisted in the same syncytial cytoplasm by these stages, symbiont segregation occurred in parallel with the bacteriocyte formation: as the cellularization proceeded, Serratia signals disappeared in the cellularized areas (Fig. 2C). By late stage 12 of embryonic segmentation, bacteriocyte formation as well as symbiont segregation completed: Buchnera cells were restricted in uninucleate, so-called “primary” bacteriocytes, whereas Serratia cells remained in interstitial areas between the primary bacteriocytes (Fig. 2D). The mechanism of the symbiont segregation is unknown, but, although speculative, bacteriocyte-specific lysozyme/lysosomal activities might be involved in the symbiont sorting (19, 20).
Establishment of Primary and Secondary Bacteriocytes.
By stage 13 of embryonic limb bud initiation, several large nuclei became evident in the Serratia-infected cytoplasmic areas (Fig. 2E). Careful confocal imaging revealed continuity of the Serratia-harboring cytoplasm, constituting a syncytial “secondary” bacteriocyte located between the primary bacteriocytes (Fig. 2F). After the germ band extension (stage 14) and the embryonic flip (or katatrepsis; stage 15), the postflip embryo (stage 16) was positioned with the head anteriorly and with the posterior germ band folded dorsally. Within the folded germ band, the uninucleate primary bacteriocytes and the syncytial secondary bacteriocyte formed a conspicuous symbiotic organ, or “bacteriome,” which was nestled on the dorsal side of the embryo (Fig. 2G).
Appearance of Sheath Cells.
At stage 16, a number of small cell nuclei became recognizable between the bacteriocytes within the bacteriome (Fig. 2H, white arrows). By stage 17 of germ band retraction, Serratia signals appeared in these small cells (Fig. 2I, pink arrow). Judging from cytologic findings and Serratia localization, these cells are “sheath cells” specialized for harboring facultative bacterial symbionts in aphids (15, 16, 21). By stage 18 of eye differentiation, the sheath cells were densely populated by Serratia cells (Fig. 2J, pink arrows). By using anti–Distal-less (Dll) antibody as molecular marker of bacteriocyte differentiation, Braendle et al. (14) identified a second population of bacteriocytes in the aphid embryogenesis: at stage 13, 40 to 60 Dll-expressing cells appear near the posterior end of the dorsal germ band region; during stages 14 and 15, these cells migrate to the region of the original bacteriocytes; and at stage 16, the smaller cells intercalate between the larger original bacteriocytes (14). We suggest that the second bacteriocyte population might correspond to the sheath cells, although this hypothesis should be verified by using a molecular marker that can specifically label the sheath cells.
Dynamic Rearrangement of Bacteriome and Fission of Secondary Bacteriocytes.
In late embryogenesis during stages 16 to 19, dynamic topological rearrangement of the bacteriome occurred. At the stage 16, the bacteriome was coherent, located between the embryonic dorsum and the folded posterior germ band (Fig. 2G and Figs. S2A and S3 A–F). At stage 17, as germ band retraction and dorsal closure of the embryonic body proceeded, the gut tube was located dorsally on the bacteriome while both sides of the bacteriome moved upward in a rotating manner (Figs. S2B and S3 G–J). At stage 18, the lateral upward rotation of the bacteriome further proceeded, so the bacteriome was torn off at the ventral side, where the syncytial secondary bacteriocyte formed a “bacteriome bridge” connecting the separating halves of the bacteriome (Fig. 2 K and L and Figs. S2C and S3 K–Q). At stage 19, the left and right halves of the bacteriome reassociated on the dorsal side, and a pair of syncytial secondary bacteriocytes were formed on the ventral side (Fig. 2 M and N and Fig. S2D). In this way, all the cellular components of the bacteriome, namely the primary bacteriocytes harboring Buchnera, and the secondary bacteriocytes and the sheath cells harboring Serratia (and potentially other facultative symbionts; ref. 15), were established and completed in the parthenogenetic embryogenesis of A. pisum (Movie S1).
Association of Blastulae with Maternal Bacteriocytes for Symbiont Transmission.
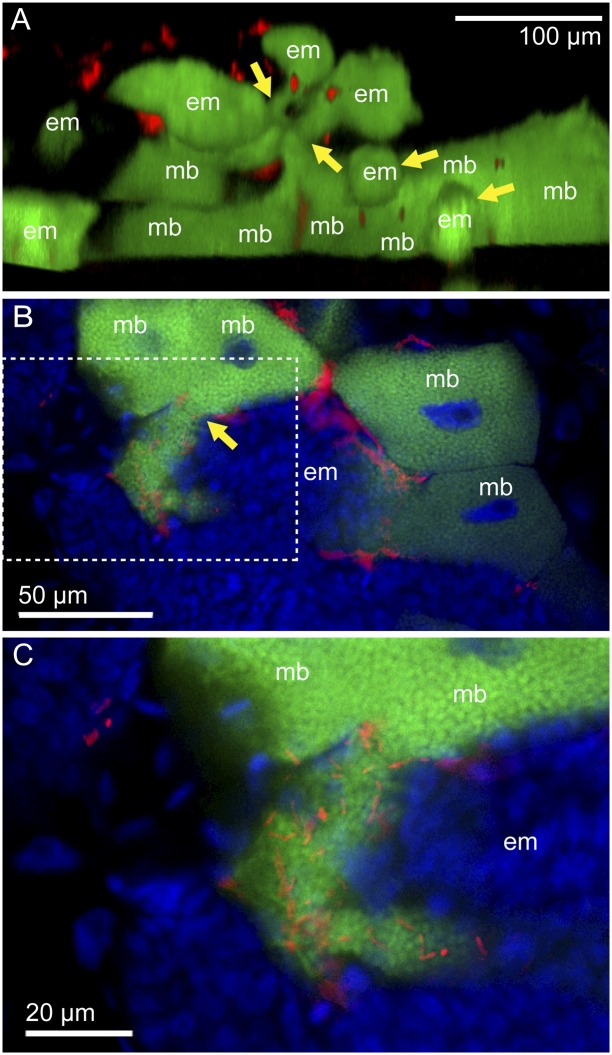
Among the developmental processes described here, the onset of the symbiotic association at the blastula stage (or stage 7; Fig. 2A) was of particular interest. Hence, we quenched autofluorescence of whole nymphal insects by a hydrogen peroxide treatment (18), performed whole-body in situ hybridization to preserve tissue integrity, and observed their embryos and bacteriocytes through transparent cuticles under a confocal microscope. The blastulae were located in the tip region of the ovarioles, and the majority of them were found adjacent to maternal bacteriocytes. Typically, the posterior side of each blastula was closely associated with a maternal bacteriocyte. At the attachment site, Buchnera signals in the embryos looked continuous with Buchnera signals in the associated maternal bacteriocytes (Fig. 3 A–C).
Fig. 3.
Vertical transmission of Buchnera and Serratia to blastulae in the strain IS of A. pisum. (A) Projection image of the bacteriome of a 3-d-old nymph constructed by serial confocal optical sections. Many embryos (em) and maternal bacteriocytes (mb) are seen. Several blastulae are associated with maternal bacteriocytes in the process of symbiont transmission. Yellow arrows indicate the junction sites between blastulae and maternal bacteriocytes. (B) A confocal section of a blastula and maternal bacteriocytes. (C) An enlarged image of the dotted box in B. Green, red, and blue signals indicate Buchnera, Serratia, and host nuclei, respectively. Note that blue signals of host nuclei are not shown in A.
Selective Transmission of Buchnera at Bacteriocyte–Blastula Interface.
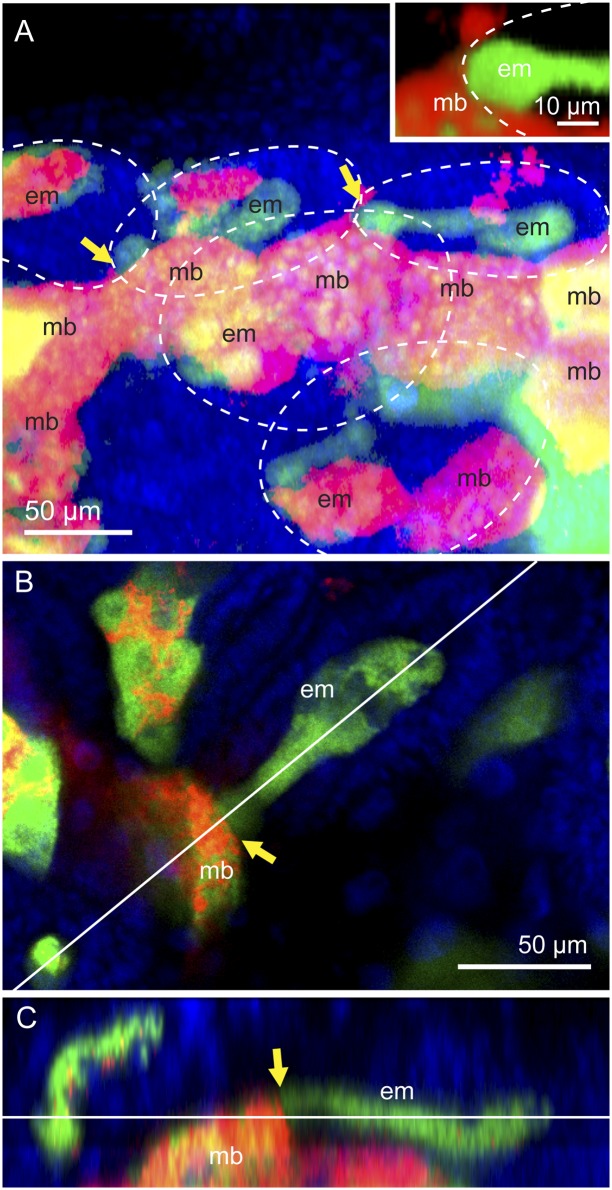
Similar histological inspection of the artificial disymbiotic strain AISTIS unveiled an unexpected phenomenon involved in Buchnera transmission at the bacteriocyte–blastula interface. In strain AISTIS, localization of Serratia is often strikingly disordered, massively proliferating in the hemolymph and invading the primary bacteriocytes that normally harbor Buchnera only, which may reflect uncontrolled or virulent behavior of the transfected facultative symbiont (16, 17). In the AISTIS insects, the blastulae were also found in the tip region of the ovarioles, and most of them were associated with maternal bacteriocytes. Interestingly, whereas the maternal bacteriocytes were coinfected with Buchnera and Serratia, all the blastulae were preferentially infected with Buchnera, with few Serratia signals therein (Fig. 4A). At the attachment site, a striking discontinuity was observed: although both Buchnera and Serratia signals were present in the maternal bacteriocyte, almost exclusively Buchnera was found in the blastulae (Fig. 4 A–C). These observations clearly refuted the presence of membrane conduit between the maternal bacteriocyte and the blastula, and suggested an unknown mechanism for selective Buchnera transmission at the bacteriocyte–blastula interface.
Fig. 4.
Selective Buchnera transmission at the bacteriocyte–blastula interface observed in the strain AISTIS of A. pisum. (A) Projection image of the bacteriome of a 3-d-old nymph constructed by serial confocal optical sections. All maternal bacteriocytes (mb) are coinfected with Buchnera (green) and Serratia (red). Several blastulae are associated with maternal bacteriocytes in the process of symbiont transmission (yellow arrows). Note that the embryo contains substantially Buchnera signals only, whereas the maternal bacteriocyte exhibits strong Serratia signals in addition to Buchnera signals. Inset: Highlighted blastula–bacteriocyte junction. Contour of embryos is shown by white dotted circles. (B) A confocal image of another blastula in the process of symbiont transmission. This section corresponds to the plane indicated by a horizontal line in C. (C) A z-axis image constructed from serial confocal sections of the area shown in B. This section corresponds to the plane indicated by a diagonal line in B. Green, red, and blue signals indicate Buchnera, Serratia, and host nuclei, respectively. Yellow arrows show blastula–bacteriocyte junctions.
Buchnera-Independent Serratia Transmission to Blastulae.
In the artificial disymbiotic strain AISTIS, Serratia cells in the maternal bacteriocytes were not transferred to the blastulae (Fig. 4 A–C). In the naturally disymbiotic strain IS, although the maternal bacteriocytes were Serratia-free, Serratia signals were found in the blastulae (Fig. 3 B and C). These observations suggested that not the maternal bacteriocyte but the surrounding hemolymph should be the source of Serratia inoculum to blastulae. During the embryogenesis of the artificial monosymbiotic strain AISTIS/rif infected with Serratia only, the facultative symbiont Serratia behaved as if it replaced the symbiotic niche of the obligate symbiont Buchnera, infecting the uninucleate primary bacteriocytes and forming a Serratia-occupied bacteriome (Fig. 5 A–D). In the AISTIS/rif insects, few blastulae were found associated with the Serratia-infected maternal bacteriocytes, but Serratia transmission via the posterior passage was consistently observed (Fig. 5A), reinforcing the hypothesis that not the maternal bacteriocyte but the surrounding hemolymph is the source of Serratia inoculum. These observations indicated that Serratia transmission can proceed independently of Buchnera transmission, suggesting different transmission mechanisms for the obligate symbiont and the facultative symbiont.
Fig. 5.
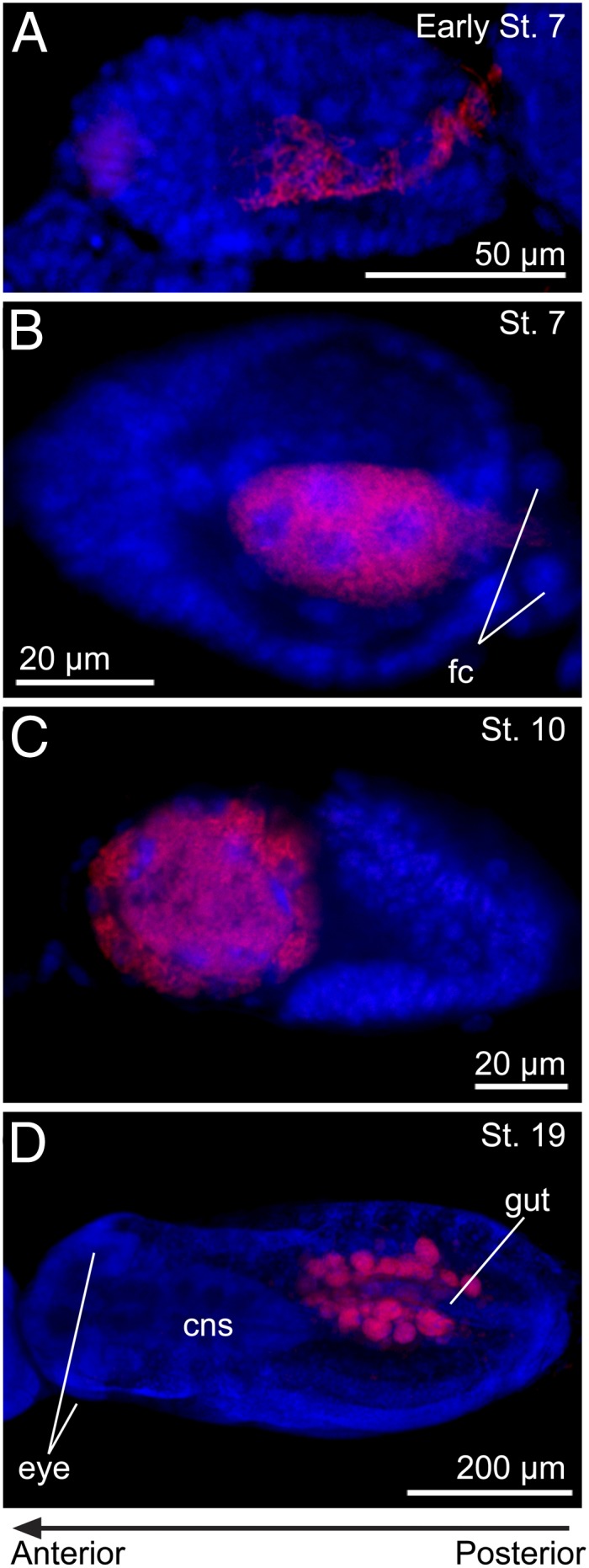
Vertical transmission and localization of Serratia in the Buchnera-eliminated strain AISTIS/rif of A. pisum. (A) An early stage 7 blastula in the process of Serratia transmission via the posterior passage. (B) A stage 7 blastula whose central syncytium is filled with Serratia cells. (C) A stage 10 embryo in which the Serratia-infected syncytium is located anteriorly. (D) A stage 19 embryo in which a pair of bacteriome lobes harboring Serratia are located in the abdomen. Red and blue signals indicate Serratia and host nuclei, respectively. cns, central nervous system; fc, follicle cell.
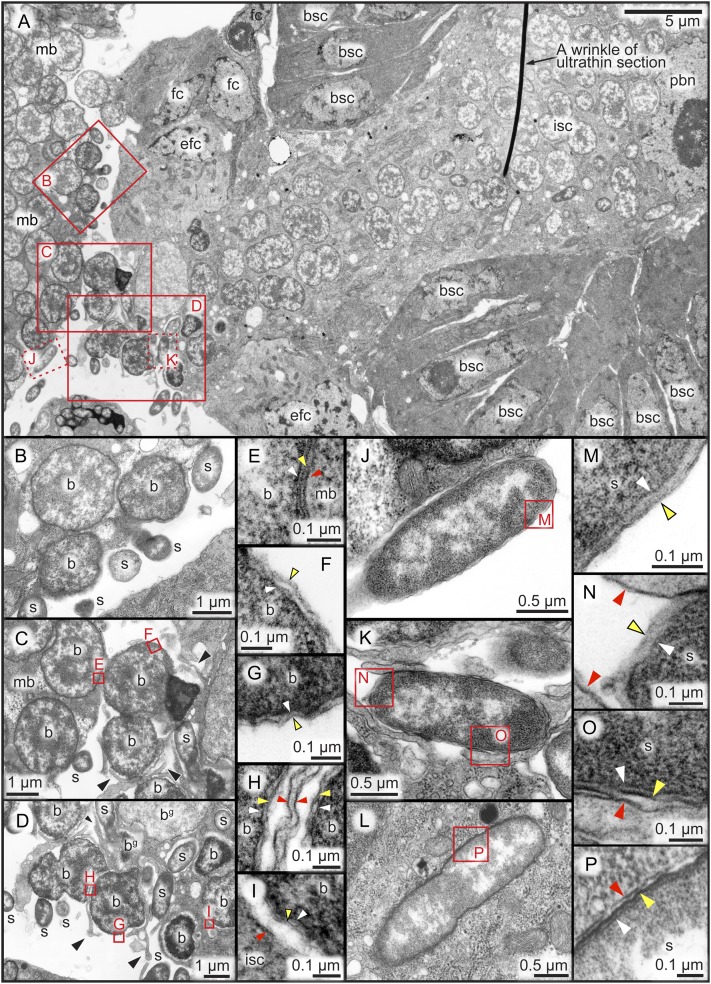
EM of Bacteriocyte–Blastula Interface.
What transmission processes are observed at the interface between the maternal bacteriocytes and the blastulae in A. pisum? Our detailed histological inspections revealed that mature embryos at stage 20 within maternal overioles already contained a few blastulae (i.e., the “telescoping aphid generations”), and vertical symbiont transmission was often taking place in these granddaughter embryos. We carefully dissected and collected mature embryos from naturally disymbiotic IS insects, cautiously fixed and embedded them, and processed the samples into serial ultrathin sections for transmission EM. After exhaustive trials and surveys, we finally obtained good preparations of blastulae wherein the symbiont transmission was taking place and the bacteriocyte–embryo interface was preserved without damage.
Junction Structure Between Maternal Bacteriocyte and Blastula.
Fig. 6A shows the posterior region of a blastula in close association with maternal bacteriocytes, where the symbiont transmission is ongoing. Despite the close spatial proximity, no cytoplasmic connection was present between the bacteriocyte and the embryo. No structure reminiscent of a cytoplasmic packet was observed. Between the bacteriocyte and the embryo, a narrow but distinct extracellular space did always exist. The main body of the blastula consisted of a surface cell layer and an inner syncytial cytoplasm that contained several polyploid presumptive bacteriocyte nuclei and many Buchnera and Serratia cells. Notably, a specialized cytological configuration was observed at the posterior embryonic region: highly enlarged polyploid follicle cells were located at the posterior pole of the blastula, surrounding the symbiont passage and forming a transmission apparatus. This structure probably corresponds to a follicle peg (1) or “enlarged follicle cells” (13) identified in previous light microscopic studies. Strikingly, specifically in the space between the transmission apparatus and the maternal bacteriocyte, many Buchnera and Serratia cells were freely present extracellularly (Fig. 6A). In the other regions around the maternal bacteriocytes and the blastulae, no Buchnera cells and only a few Serratia cells were found extracellularly. These cytological traits were consistently observed in multiple blastulae in the process of symbiont transmission. Hereafter, we designate the area between the transmission apparatus and the maternal bacteriocyte as the transmission center, and describe its fine structure and cytological details.
Fig. 6.
Transmission EM images of symbiont transmission processes at the bacteriocyte–blastula interface in the IS strain of A. pisum. (A) A low-magnification image of the posterior region of a blastula adjacent to a maternal bacteriocyte, where the symbiont transmission is ongoing. Red rectangles correspond to the locations of the following magnified images. (B) Buchnera cells protruding from the surface of a maternal bacteriocyte at the transmission center. (C) Extracellular Buchnera cells trapped by thin cytoplasmic extensions (black arrowheads) protruding from the posterior pole of the blastula. (D) Buchnera cells endocytosed into the inner syncytial cytoplasm of the blastulae. Serratia cells are also endocytosed into the syncytial cytoplasm. Some of the Buchnera cells look degenerative in the host cytoplasm. (E) Three-layered membrane structure of a Buchnera cell within the maternal bacteriocyte. White, yellow, and red arrowheads indicate bacterial inner membrane, bacterial outer membrane, and host cell membrane, respectively. (F) Two-layered membrane structure of an extracellular Buchnera cell. (G) Two-layered membrane structure of the extracellular side of a Buchnera cell in the process of endocytosis. (H) Three-layered membrane structure of the engulfed side of the same Buchnera cell. (I) Three-layered membrane structure of an endocellular Buchnera cell in the embryonic syncytial cytoplasm. (J) An extracellular Serratia cell in the transmission center. (K) A Serratia cell in the process of endocytosis. (L) A Serratia cell within the syncytial cytoplasm of the blastula. (M) Two-layered membrane structure of an extracellular Serratia cell. (N) Two-layered membrane structure of the extracellular side of a Serratia cell in the process of endocytosis. (O) Three-layered membrane structure of the engulfed side of the same Serratia cell. (P) Three-layered membrane structure of an endocellular Serratia cell in the embryonic syncytial cytoplasm. b, Buchnera cell; bg, presumably degenerating Buchnera cell; bsc, blastula surface cell; efc, enlarged follicle cell; fc, follicle cell; isc, inner syncytial cytoplasm; mb, maternal bacteriocyte; pbn, presumptive bacteriocyte nucleus; s, Serratia cell.
Transmission Process of Buchnera.
Specifically on the surface of the maternal bacteriocyte at the transmission center, many Buchnera cells were extruding to the extracellular space, presumably representing the process of exocytotic release (Fig. 6B). No other location on the surface of the maternal bacteriocytes exhibited such a peculiar cytological trait. On the surface of the transmission center of the embryonic side, strikingly, a number of thin cytoplasmic extensions were protruding to the extracellular space, by which free Buchnera cells were trapped (Fig. 6C) and engulfed into the cytoplasmic passage toward the inner syncytial cytoplasm of the blastula (Fig. 6D). Close examination of the membrane structures surrounding the Buchnera cells confirmed that exocytotic and endocytotic processes were involved in the symbiont transmission from the bacteriocyte to the embryo: endocellular Buchnera cells in the maternal bacteriocyte exhibited a three-layered membrane structure (Fig. 6E); extracellular Buchnera cells showed a two-layered membrane structure, losing the outermost host-derived membrane (Fig. 6 F and G); and endocellular Buchnera cells engulfed by the transmission apparatus toward the blastula showed a restored three-layered membrane structure (Fig. 6 H and I).
Exocytotic and Endocytotic Mechanisms Involved in Selective Buchnera Transmission at Bacteriocyte–Blastula Interface.
These results indicate that (i) vertical transmission of Buchnera occurs specifically at the bacteriocyte–blastula interface, or the transmission center, in a highly regulated manner; (ii) at the transmission center, Buchnera cells are exocytosed from the maternal bacteriocyte, temporarily released to the extracellular space, and endocytosed by the cytoplasm of the embryonic syncytium surrounded by enlarged follicle cells; and (iii) no cytoplasmic connection is formed between the maternal bacteriocyte and the blastula. Considering that both Buchnera and Serratia cells are endocytosed by the embryonic syncytium (Fig. 6A), the selective Buchnera transmission in AISTIS insects (Fig. 4 A–C) is likely attributable to Buchnera-specific exocytosis by the maternal bacteriocyte at the transmission center.
Contrasting Hypotheses on Buchnera Transmission Mechanism.
Based on these results, we propose the exo-/endocytotic transport hypothesis (Fig. 1D) for vertical transmission mechanism of Buchnera in A. pisum. Certainly, we obtained microscopic images that looked like showing bacteriocyte–blastula conduits or packet-fused–like blastulae at the resolution of confocal optics (e.g., Figs. 3 and 4), but our EM observations clearly rejected the membranous conduit formation hypothesis (Fig. 1B) (1, 12) and the symbiont packet fusion hypothesis (Fig. 1C) (13). At present, we cannot reject the free symbiont infection hypothesis (Fig. 1A). Although we did not observe such instances in the mature embryos, disintegrated bacteriocytes and released Buchnera cells in the hemocoel are often observed in adult aphids (1), and it is conceivable that such Buchnera cells might be incorporated into the blastulae. It should be also kept in mind that we examined only A. pisum, and cannot exclude the possibility that different mechanisms for symbiont transmission might be found in different aphid lineages.
Endocytotic Serratia Transmission to Blastula.
A number of free Serratia cells were observed in the extracellular space of the transmission center, whereas no Serratia cells were harbored in and exocytosed from the maternal bacteriocytes (Fig. 6A). At the embryonic side of the transmission center, free Serratia cells were trapped by cytoplasmic extensions and endocytosed into the transmission passage (Fig. 6 J–P), as Buchnera cells were incorporated into the blastula (Fig. 6 C–I). These results indicate that (i) vertical transmission of Serratia also occurs at the bacteriocyte–blastula interface; (ii) extracellular Serratia cells are endocytotically incorporated into the blastula; and (iii) unlike Buchnera transmission, no spatially regulated exocytotic process is involved in Serratia transmission. It seems plausible that the endocytotic mechanism for Serratia transmission might be the same as that for Buchnera transmission. On account of the facultative nature of the symbiosis, although speculative, Serratia might use the preexisting endocytotic mechanism of the blastula whose original role is for vertical transmission of the obligate symbiont Buchnera.
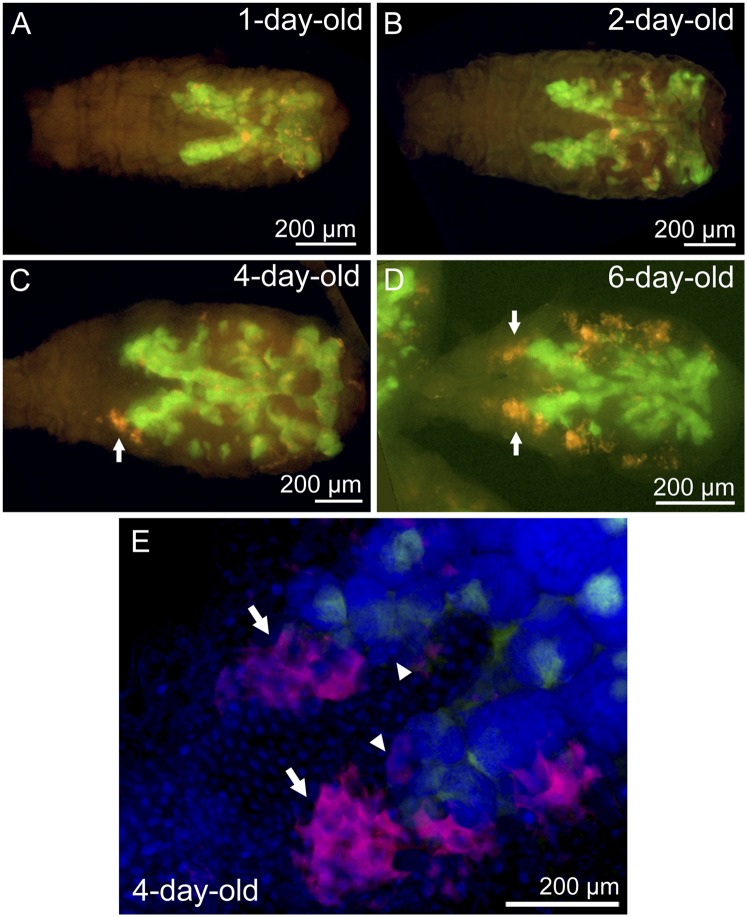
Aggregation of Serratia Around Ovariole Tips.
We identified an interesting in vivo localization of Serratia that might be relevant to its targeting to host embryos for vertical transmission. Fig. 7 shows whole-body in situ hybridization of Buchnera and Serratia in nymphal A. pisum. In newborn nymphs, Serratia signals were found in the secondary bacteriocytes and the sheath cells in association with Buchnera signals in the bacteriome (Fig. 7 A and B). In older nymphs, extracellular proliferation of Serratia became prominent, with particularly intense localization around the ovariole tips (Fig. 7 C and D, arrows). Confocal imaging of the ovariole tips confirmed aggregation of Serratia cells on and around the ovariole tips, the location of blastulae (Fig. 7E). Although speculative, these localization patterns might be facilitating encounter and vertical transmission of Serratia to blastulae of the host insect.
Fig. 7.
Localization of Serratia and Buchnera visualized by whole-body in situ hybridization in the IS strain of A. pisum: (A) 1-d-old, first instar nymph; (B) 2-d-old, second instar nymph; (C) 4-d-old, third instar nymph; and (D) 6-d-old, fourth instar nymph. (E) Ovariole tips of a 4-d-old, third instar nymph. Red, green, and blue signals indicate Serratia, Buchnera, and host nuclei, respectively. Note that blue signals of host nuclei are not shown in A–D. Arrows show the ovariole tips where Serratia cells aggregate, whereas arrowheads indicate blastulae in which vertical symbiont transmission occurs.
Possible Cellular and Molecular Mechanisms for Symbiont Transmission.
The spatially restricted formation of the transmission center and its peculiar cytological traits are suggestive of intricate molecular and cellular interactions between the maternal bacteriocyte and the blastula. The up-regulated endocytotic activity at the posterior pole of the blastula must be an important mechanism for vertical transmission of the obligate symbiont Buchnera and the facultative symbiont Serratia. It seems likely that the blastula might somehow induce exocytosis of Buchnera cells at an adjacent area of the neighboring maternal bacteriocyte, although it is totally unknown what signals mediate the interactions. Alternatively, it is also conceivable that the neighboring maternal bacteriocyte might be involved in the activated endocytosis at the posterior pole of the blastula. Buchnera is allied to the gammaproteobacterial family Enterobacteriaceae, which includes well studied pathogens like Salmonella, Shigella, and Yersinia (22). These pathogens are known for their sophisticated molecular mechanisms for invasion into eukaryotic host cells by delivering effector molecules into the host cytoplasm via the type III secretion system, manipulating the cytoskeletal machinery of the host cell, and facilitating lamellipodia formation, endocytotic trapping, and internalization of bacterial cells (23, 24). In this context, it seems meaningful that, although the Buchnera genome lacks orthologues of the type III secretion system genes (10, 25), numerous flagellar basal bodies, which are evolutionarily homologous to the type III secretion system (26), are present on the cell membrane of Buchnera, which might mediate molecular transports from and to the host cytoplasm (27).
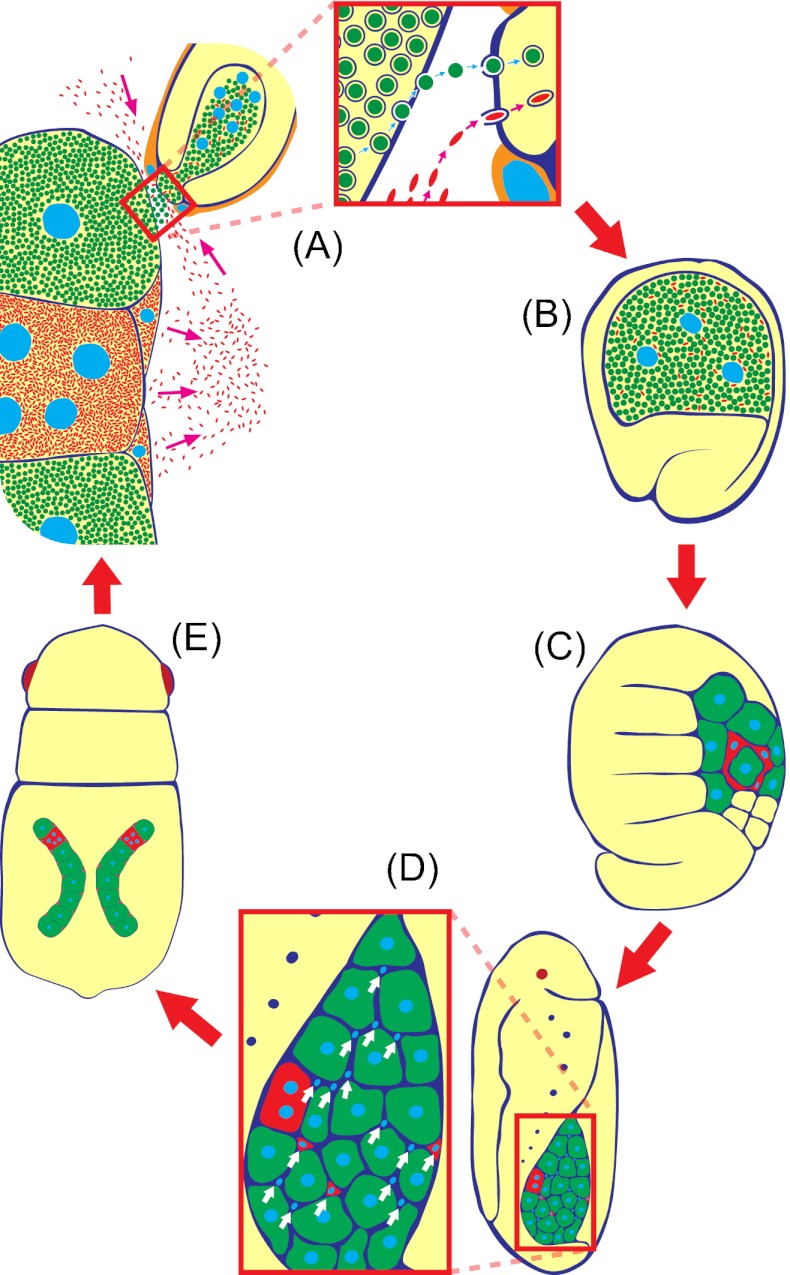
Conclusion and Perspective.
Fig. 8 summarizes the infection cycle of the obligate symbiont Buchnera and the facultative symbiont Serratia in the parthenogenetic phase of A. pisum. In conclusion, we describe a cellular mechanism for vertical transmission of Buchnera at the bacteriocyte–embryo interface, which must have evolved to ensure the mutualistic association with the obligate symbiont. Also, we suggest the possibility that facultative symbionts like Serratia are taking a “free ride” on the preexisting transmission mechanism to gain entry into the host embryos. Bacteriocytes for harboring obligate symbiotic bacteria are found in diverse insect taxa, including the Hemiptera, Coleoptera, Diptera, Phthiraptera, and others, and those symbiotic cells are likely to be of independent evolutionary origins (1, 5). Within the Hemiptera, meanwhile, most of the major taxa such as aphids, coccids, whiteflies, psyllids, leafhoppers, cicadas, and others possess the bacteriocytes in common, but their bacterial symbionts are often quite divergent phylogenetically (4, 5). The commonality and diversity of the symbiotic cells and associated transmission mechanisms in the Hemiptera are of evolutionary interest and to be established in future studies.
Fig. 8.
Infection dynamics of Buchnera and Serratia in the parthenogenetic life cycle of A. pisum. (A) Symbiont transmission from maternal bacteriocyte to blastula. (B) Formation of symbiont-harboring syncytial cytoplasm. (C) Bacteriocyte cellularization and symbiont sorting. (D) Appearance of sheath cells. (E) Establishment of paired bacteriomes. Green, orange, and yellow indicate Buchnera, Serratia, and host embryo, respectively.
Materials and Methods
Insects.
Aphid strains IS, AIST, AISTIS, and AISTIS/rif were used in this study (Table 1 and Fig. S1) (16, 17, 21). The insects were reared on seedlings of the broad bean Vicia faba at 20 °C in a long-day regimen of 16 h light and 8 h dark.
Cytological Staining.
Fixation and staining of dissected ovarioles were performed essentially as described (13). Ovarioles were dissected from second, third, and forth instar nymphs in ice-cold Dulbecco PBS solution (DPBS; Sigma) and fixed in ice-cold 4% (wt/vol) formaldehyde/DPBS for approximately 30 min. DNA was stained with 1 μM TOTO-3 (Molecular Probes), and filamentous actin was visualized with Alexa Fluor 488 phalloidin (Molecular Probes).
In Situ Hybridization.
Whole-mount in situ hybridization was performed as described previously (18). Ovarioles were dissected and fixed in Carnoy solution [ethanol:chloroform:acetic acid at 6:3:1 (vol/vol) ratio] overnight. Whole nymphs were fixed in Carnoy solution overnight; their head, legs, antennae, and cornicles were removed by forceps in 80% (vol/vol) ethanol; and their cuticle was pricked throughout the body with a thin needle. Tissue samples were then treated with 6% (wt/vol) hydrogen peroxide in 80% ethanol for several days for quenching autofluorescence of the tissues, thoroughly washed with 100% ethanol, and stored at −20 °C until use. The following oligonucleotide probes were used for in situ hybridization: Cy5-ApisP2a (5′-Cy5-CCT CTT TTG GGT AGA TCC-3′) targeting 16S rRNA of Buchnera and Cy3-PASSisR (5′-Cy3-CCC GAC TTT ATC GCT GGC-3′) targeting 16S rRNA of Serratia. The tissue samples were hydrated with DPBS containing 0.3% Triton X-100, incubated with hybridization buffer [20 mM Tris-HCl (pH 8.0), 0.9 M NaCl, 0.01% SDS, 30% (vol/vol) formamide] containing 100 nM each of the probes and 0.5 μM SYTOX Green (Molecular Probes) overnight, washed thoroughly with DPBS containing 0.3% Triton X-100, mounted in SlowFade antifade solution (Molecular Probes), and observed under an epifluorescent microscope (Axiophot; Carl Zeiss) and/or a laser scanning microscope (PASCAL5; Carl Zeiss).
EM.
Dissected mature embryos were fixed and embedded in Spurr resin as described previously (21). Initially, semi-ultrathin sections were made with an ultramicrotome (Ultracat-N; Leichert-Nissei), mounted on glass slides, stained with toluidine blue, and observed under a light microscope. A sample was scraped and observed little by little, and when a blastula in the process of symbiont transmission was identified, the sample was processed into serial ultrathin sections, mounted on copper meshes, stained with uranyl acetate and lead citrate, and observed with a transmission electron microscope (model H-7000; Hitachi).
Supplementary Material
Acknowledgments
The authors thank S. Koike, J. Makino, and W. Kikuchi for technical and secretarial assistance and Y. Kamagata for logistic support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 7597 (volume 109, number 20).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119212109/-/DCSupplemental.
References
- 1.Buchner P. Endosymbiosis of Animals with Plant Microorganisms. New York: Interscience; 1965. [Google Scholar]
- 2.Werren JH, Baldo L, Clark ME. Wolbachia: Master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 3.Oliver KM, Degnan PH, Burke GR, Moran NA. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol. 2010;55:247–266. doi: 10.1146/annurev-ento-112408-085305. [DOI] [PubMed] [Google Scholar]
- 4.Baumann P. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu Rev Microbiol. 2005;59:155–189. doi: 10.1146/annurev.micro.59.030804.121041. [DOI] [PubMed] [Google Scholar]
- 5.Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet. 2008;42:165–190. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- 6.Bright M, Bulgheresi S. A complex journey: Transmission of microbial symbionts. Nat Rev Microbiol. 2010;8:218–230. doi: 10.1038/nrmicro2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frydman HM, Li JM, Robson DN, Wieschaus E. Somatic stem cell niche tropism in Wolbachia. Nature. 2006;441:509–512. doi: 10.1038/nature04756. [DOI] [PubMed] [Google Scholar]
- 8.Serbus LR, Casper-Lindley C, Landmann F, Sullivan W. The genetics and cell biology of Wolbachia-host interactions. Annu Rev Genet. 2008;42:683–707. doi: 10.1146/annurev.genet.41.110306.130354. [DOI] [PubMed] [Google Scholar]
- 9.Fast EM, et al. Wolbachia enhance Drosophila stem cell proliferation and target the germline stem cell niche. Science. 2011;334:990–992. doi: 10.1126/science.1209609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa H. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature. 2000;407:81–86. doi: 10.1038/35024074. [DOI] [PubMed] [Google Scholar]
- 11.International Aphid Genomics Consortium Genome sequence of the pea aphid Acyrthosiphon pisum. PLoS Biol. 2010;8:e1000313. doi: 10.1371/journal.pbio.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkinson TL, Fukatsu T, Ishikawa H. Transmission of symbiotic bacteria Buchnera to parthenogenetic embryos in the aphid Acyrthosiphon pisum (Hemiptera: Aphidoidea) Arthropod Struct Dev. 2003;32:241–245. doi: 10.1016/S1467-8039(03)00036-7. [DOI] [PubMed] [Google Scholar]
- 13.Miura T, et al. A comparison of parthenogenetic and sexual embryogenesis of the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidoidea) J Exp Zoolog B Mol Dev Evol. 2003;295:59–81. doi: 10.1002/jez.b.3. [DOI] [PubMed] [Google Scholar]
- 14.Braendle C, et al. Developmental origin and evolution of bacteriocytes in the aphid-Buchnera symbiosis. PLoS Biol. 2003;1:E21. doi: 10.1371/journal.pbio.0000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moran NA, Russell JA, Koga R, Fukatsu T. Evolutionary relationships of three new species of Enterobacteriaceae living as symbionts of aphids and other insects. Appl Environ Microbiol. 2005;71:3302–3310. doi: 10.1128/AEM.71.6.3302-3310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koga R, Tsuchida T, Fukatsu T. Changing partners in an obligate symbiosis: A facultative endosymbiont can compensate for loss of the essential endosymbiont Buchnera in an aphid. Proc Biol Sci. 2003;270:2543–2550. doi: 10.1098/rspb.2003.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koga R, Tsuchida T, Sakurai M, Fukatsu T. Selective elimination of aphid endosymbionts: Effects of antibiotic dose and host genotype, and fitness consequences. FEMS Microbiol Ecol. 2007;60:229–239. doi: 10.1111/j.1574-6941.2007.00284.x. [DOI] [PubMed] [Google Scholar]
- 18.Koga R, Tsuchida T, Fukatsu T. Quenching autofluorescence of insect tissues for in situ detection of endosymbionts. Appl Entomol Zool (Jpn) 2009;44:281–291. [Google Scholar]
- 19.Nakabachi A, et al. Transcriptome analysis of the aphid bacteriocyte, the symbiotic host cell that harbors an endocellular mutualistic bacterium, Buchnera. Proc Natl Acad Sci USA. 2005;102:5477–5482. doi: 10.1073/pnas.0409034102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishikori K, Morioka K, Kubo T, Morioka M. Age- and morph-dependent activation of the lysosomal system and Buchnera degradation in aphid endosymbiosis. J Insect Physiol. 2009;55:351–357. doi: 10.1016/j.jinsphys.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Fukatsu T, Nikoh N, Kawai R, Koga R. The secondary endosymbiotic bacterium of the pea aphid Acyrthosiphon pisum (Insecta: Homoptera) Appl Environ Microbiol. 2000;66:2748–2758. doi: 10.1128/aem.66.7.2748-2758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumann P, et al. Genetics, physiology, and evolutionary relationships of the genus Buchnera: Intracellular symbionts of aphids. Annu Rev Microbiol. 1995;49:55–94. doi: 10.1146/annurev.mi.49.100195.000415. [DOI] [PubMed] [Google Scholar]
- 23.Galán JE, Zhou D. Striking a balance: Modulation of the actin cytoskeleton by Salmonella. Proc Natl Acad Sci USA. 2000;97:8754–8761. doi: 10.1073/pnas.97.16.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel JC, Galán JE. Manipulation of the host actin cytoskeleton by Salmonella—all in the name of entry. Curr Opin Microbiol. 2005;8:10–15. doi: 10.1016/j.mib.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Dale C, Plague GR, Wang B, Ochman H, Moran NA. Type III secretion systems and the evolution of mutualistic endosymbiosis. Proc Natl Acad Sci USA. 2002;99:12397–12402. doi: 10.1073/pnas.182213299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aizawa SI. Bacterial flagella and type III secretion systems. FEMS Microbiol Lett. 2001;202:157–164. doi: 10.1111/j.1574-6968.2001.tb10797.x. [DOI] [PubMed] [Google Scholar]
- 27.Maezawa K, et al. Hundreds of flagellar basal bodies cover the cell surface of the endosymbiotic bacterium Buchnera aphidicola sp. strain APS. J Bacteriol. 2006;188:6539–6543. doi: 10.1128/JB.00561-06. [DOI] [PMC free article] [PubMed] [Google Scholar]