Abstract
The intracellular pathogen Mycobacterium tuberculosis (Mtb) causes tuberculosis. Enhanced intracellular survival (Eis) protein, secreted by Mtb, enhances survival of Mycobacterium smegmatis (Msm) in macrophages. Mtb Eis was shown to suppress host immune defenses by negatively modulating autophagy, inflammation, and cell death through JNK-dependent inhibition of reactive oxygen species (ROS) generation. Mtb Eis was recently demonstrated to contribute to drug resistance by acetylating multiple amines of aminoglycosides. However, the mechanism of enhanced intracellular survival by Mtb Eis remains unanswered. Therefore, we have characterized both Mtb and Msm Eis proteins biochemically and structurally. We have discovered that Mtb Eis is an efficient Nɛ-acetyltransferase, rapidly acetylating Lys55 of dual-specificity protein phosphatase 16 (DUSP16)/mitogen-activated protein kinase phosphatase-7 (MKP-7), a JNK-specific phosphatase. In contrast, Msm Eis is more efficient as an Nα-acetyltransferase. We also show that Msm Eis acetylates aminoglycosides as readily as Mtb Eis. Furthermore, Mtb Eis, but not Msm Eis, inhibits LPS-induced JNK phosphorylation. This functional difference against DUSP16/MKP-7 can be understood by comparing the structures of two Eis proteins. The active site of Mtb Eis with a narrow channel seems more suitable for sequence-specific recognition of the protein substrate than the pocket-shaped active site of Msm Eis. We propose that Mtb Eis initiates the inhibition of JNK-dependent autophagy, phagosome maturation, and ROS generation by acetylating DUSP16/MKP-7. Our work thus provides insight into the mechanism of suppressing host immune responses and enhancing mycobacterial survival within macrophages by Mtb Eis.
Keywords: Rv2416c, lysine acetylation, antituberculosis drug
Nearly one-third of the world’s population is infected with Mycobacterium tuberculosis (Mtb). This pathogenic bacterium causes tuberculosis, which claims the lives of millions of people every year (1). Tuberculosis has also become a global health issue owing to the increased incidences of multidrug-resistant and extensively drug-resistant strains of Mtb (2). This makes a search for targets of new antituberculosis drugs urgent. Mtb is a highly successful human pathogen, surviving and multiplying within the human macrophage cells of the infected people (3). Therefore, treatment of tuberculosis is difficult, requiring many months of taking a combination of antibiotics. Mtb has the ability to persist in the form of a long-term asymptomatic infection, referred to as latent tuberculosis (4). Latent tuberculosis becomes activated when the body’s immune system is weakened. As a result, tuberculosis is the major cause of death among immuno-compromised AIDS patients (5).
In mycobacterial infection, host innate immune responses may play a crucial role in early protection against Mtb infection, leading to establishment of effective adaptive immunity to tuberculosis (6). Additionally, MAPK pathways are activated by Mtb or its components and play an essential role in the regulation of innate immune signaling during mycobacterial infection (6). Because intracellular survival of Mtb plays a central role in its pathogenesis (7), it is important to understand the survival strategies of this bacterium within macrophages. Mtb has evolved a number of highly effective survival strategies inside the macrophage (8). The best-characterized survival mechanism of Mtb is the inhibition of phagosomal maturation and autophagy, between which a functional overlap was suggested (8–11). Both processes involve several steps, including fusion with lysosomes, and a number of protein factors, such as Beclin 1 and vacuolar sorting protein 34 (VPS34), the class III phosphatidylinositol 3-kinase (12). The identification and characterization of mycobacterial proteins that play a role in facilitating intracellular survival remain a priority for the development of new antituberculosis drugs.
The Rv2416c gene of Mtb H37Rv strain was found to enhance intracellular survival of Mycobacterium smegmatis (Msm) in the human macrophage-like cell line U-937, and thus it was designated as eis (enhanced intracellular survival) (7). The expression of its protein product directly correlated with the enhanced mycobacterial survival in U-937 cells (7). The Mtb Eis protein is produced during human tuberculosis infection and is released into the culture medium (3). The sigma factor SigA was shown to bind to the eis promoter in the W-Beijing strain of Mtb, and the activation of the Mtb eis gene correlated with increased SigA levels and enhanced intracellular survival (13). Treatment of T cells with Mtb Eis inhibited ERK1/2, JAK pathway, and subsequent production of TNF-α and IL-4 (14). Mtb Eis negatively regulated the secretion of TNF-α and IL-10 by primary human monocytes in response to infection with the pathogen (15).
Recently Mtb Eis was shown to suppress host innate immune defenses by negatively modulating inflammation, autophagy, and cell death in a redox-dependent manner (16). The reported data indicate that Mtb Eis plays an essential role in regulating both the early generation of reactive oxygen species (ROS) and inflammatory responses in macrophages (16). It was also found that abrogated production of both ROS and proinflammatory cytokines by Mtb Eis depends on its N-acetyltransferase domain in the N terminus (16). Enhanced macrophage survival by Mtb Eis was found to occur through the regulation of ROS signaling, which was JNK-dependent but was not p38- or ERK1/2-dependent (16). Forced expression of dual-specificity protein phosphatase 16 (DUSP16), also called MAPK phosphatase-7(MKP-7), suppressed activation of MAPKs in COS-7 cells in the order of selectivity, JNK >> p38 > ERK, suggesting that DUSP16/MKP-7 works as a JNK-specific phosphatase (17).
A bioinformatic analysis predicted that the Mtb Eis protein contains a single acetyltransferase domain of the GCN5-related N-acetyltransferase (GNAT) superfamily in the amino terminus (15). The acetyltransferase domain is predicted to cover residues 9−160 of the 408-residue protein and contains a variant of the characteristic sequence motif (V/I-x-x-x-x-Q/R-x-x-G-x-G/A) for acetyltransferases at positions between 93 and 103 (93VAPTHRRRGLL103) (Fig. S1) (15, 18). Our sequence numbering of Mtb Eis follows the current EXPASY UniProtKB/Swiss-Prot database; six residues 1MPQSDS6 at the amino terminus are missing from other databases, owing to a different translation initiation at Val7. Increased expression of Mtb Eis due to a mutation in the promoter region of the eis gene conferred resistance to aminoglycoside kanamycin, a second-line antituberculosis drug (19). More recently, Mtb Eis was demonstrated to have an unprecedented ability to acetylate multiple amines of many aminoglycosides, and its reaction mechanism was proposed on the basis of structural and mutational studies (20). However, the fundamental question about the mechanism of enhanced survival of the Msm clone, which contains the extra Mtb eis gene in addition to its own eis gene, still remains unanswered. Nonpathogenic Msm contains a homologous eis gene (MSMEG_3513) that encodes a homolog of Mtb Eis (58% amino acid sequence identity).
To better understand how Mtb Eis enhances mycobacterial survival in macrophages, we have carried out functional and structural characterization of Eis proteins from both Mtb and Msm. We find that both Eis proteins can acetylate aminoglycosides efficiently. We have discovered that Lys55 within the docking domain (also called the kinase interaction motif) of DUSP16/MKP-7 is readily acetylated by Mtb Eis, but not by Msm Eis. Furthermore, we show that Mtb Eis, but not Msm Eis, suppresses LPS-induced JNK phosphorylation and proinflammatory cytokine production in macrophages. The observed functional difference between these Eis proteins against DUSP16/MKP-7, a JNK-specific phosphatase, can be understood by comparing their active site features. The overall monomeric and oligomeric structures of both Mtb Eis and Msm Eis are highly similar to each other. The most notable structural difference between them is the presence of a narrow channel for potential sequence-specific binding of the acetylation target peptide in the active site of Mtb Eis but not in Msm Eis. On the basis of these findings, we propose that Mtb Eis initiates the inhibition of autophagy and phagosome maturation in infected macrophages by Nɛ-acetylating Lys55 of DUSP16/MKP-7 to suppress host immune responses for intracellular survival of mycobacteria.
Results
Eis Proteins from both Mtb and Msm Acetylate Aminoglycosides Efficiently and Have Similar Overall Structures.
Recently Mtb Eis was shown to acetylate multiple amines of many aminoglycosides, including the second-line injectable antituberculosis drugs kanamycin and amikacin (19, 20). It was also argued that kanamycin may not be a natural substrate of Mtb Eis because of a high Km value with it (19). To examine whether there is a functional difference between Eis proteins from Mtb and Msm in aminoglycoside acetylation, we compared acetylation activities of these two Eis proteins against amikacin, kanamycin A, tobramycin, and paromomycin. Interestingly, Msm Eis acetylated the tested aminoglycosides as quickly as, or more rapidly than, Mtb Eis (Fig. S2). Steady-state kinetic parameters, as measured by Km and kcat values (Table S1), indicate that the aminoglycoside acetyltransferase activity of Msm Eis is comparable to or higher than that of Mtb Eis. This result cannot explain the enhanced intracellular survival of mycobacteria by Mtb Eis.
To understand the observed catalytic properties, we have determined and compared the crystal structures of both Mtb and Msm Eis proteins (Table S2 and SI Results and Discussion). The crystal structure of selenomethionine-substituted Mtb Eis in the acetyl CoA-bound form was determined by de novo phasing using the single anomalous diffraction data to 2.80 Å. This model was used to solve the structures of Mtb Eis in the apo form at 2.46 Å and Msm Eis in the CoA-bound form at 2.03 Å by molecular replacement. The overall monomeric and hexameric structures of Mtb and Msm Eis proteins are similar to each other (Fig. S3). That is, each monomer of both Eis proteins comprises three “structural” domains, and six subunits are associated to form a hexamer of 32 symmetry. “Structural” domain 1 adopts the GNAT fold, as predicted. Unexpectedly, “structural” domain 2 is also folded into the GNAT structure (Fig. S4) despite an apparent lack of sequence similarity to other GNAT enzymes, including the Eis domain 1. “Structural” domain 3, containing a putative peroxisome targeting signal type 2 (Fig. S5), resembles sterol carrier protein-2. Either acetyl CoA or CoA is observed to be bound to “structural” domain 1 only, but not to “structural” domain 2, in the ligand-bound structures of both Eis proteins (Fig. S3). The active sites of Mtb and Msm Eis proteins are large and deep enough to accommodate aminoglycosides. However, they display distinct structural features that may explain the observed functional difference against peptide substrates (as discussed below). While we were preparing this article, the structure of Mtb Eis bound with acetyl CoA was reported by another group (20) [Protein Data Bank (PDB) code 3R1K]. Our structure of acetyl CoA-bound Mtb Eis is highly similar to the reported one, with the rmsds being 0.34 Å for 396 Cα atoms in a monomer (for chains A) and 0.67–0.75 Å for 2,376 Cα atoms in a hexamer. A minor difference is in the modeled residues (residues 8–161 and 167–408 in our structure; residues 9–57 and 62–408 in the reported structure).
Identification of DUSP16/MKP-7 as the Nɛ-Acetylation Target of Mtb Eis.
Besides aminoglycosides, Mtb Eis was previously shown to acetylate free histone proteins, but not the histone proteins in a nucleosomal complex (21). None of these acetylation targets can explain the enhanced intracellular survival of mycobacteria by Mtb Eis; Mtb Eis likely has other unidentified protein targets for acetylation. Therefore, we were interested in identifying physiologically more important protein acetylation targets of Mtb Eis. Mtb Eis enhances macrophage survival through the regulation of JNK-dependent ROS signaling (16), whereas DUSP16/MKP-7 works as a JNK-specific phosphatase in vivo (17). Acetylation at Lys57 of DUSP1/MKP-1, a nuclear-localized phosphatase that inactivates MAPK members by dephosphorylation, promoted the interaction of DUSP1/MKP-1 with its substrate p38 MAPK and inhibited innate immune signaling (22, 23). Three major subfamilies of MAPKs are ERKs, p38 MAPKs, and JNKs. These MAPKs are activated by MAPK kinases, which are in turn activated by a set of MAPK kinase kinases. The MAPK pathways that mediate innate immune signaling include MAPK kinases 3/4/6, p38, and JNKs (ref. 22 and references therein).
On the basis of these reports, we speculated that Mtb Eis may acetylate component(s) of the MAPK signaling pathways, such as MAPK phosphatases, to negatively control autophagy, phagosome maturation, and ROS generation, ultimately leading to the suppression of host immune responses. Therefore, we examined whether Mtb Eis may acetylate DUSP16/MKP-7, which is known to be a JNK-specific phosphatase (17). We tested a peptide within the MAPK-docking domain of DUSP16/MKP-7: 53LMKRRLQQDKVLIT66 [MKP-7(53−66)] (22). The underlined Lys62 was predicted to be the acetylation site in the DUSP16/MKP-7 docking domain (22). We also tested a peptide within the MAPK-docking domain of DUSP1/MKP-1: 50TIVRRRAKGAMGLE63 [MKP-1(50−63)] (22). The underlined lysine was established as the acetylation site in the DUSP1/MKP-1 docking domain (22). With these peptides as possible substrates, we performed in vitro acetylation assays using either Mtb Eis or Msm Eis.
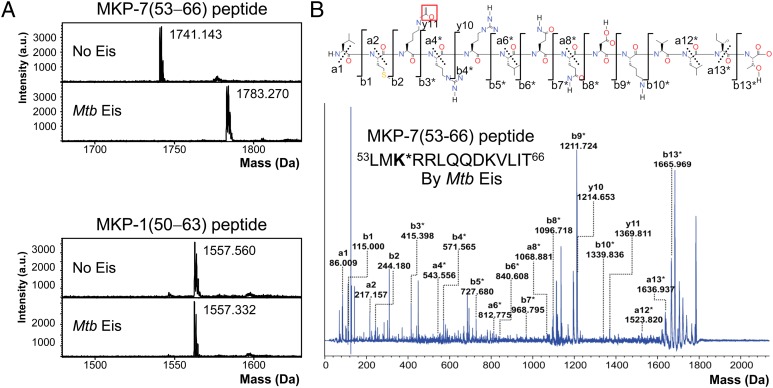
The MALDI-TOF mass spectra of the MKP-7(53−66) peptide show that the unmodified peptide peak at 1,741 Da is shifted to 1,783 Da after in vitro acetylation for 5 min by Mtb Eis (Fig. 1A). The observed increase of 42 Da in the peptide mass corresponds to covalent attachment of an acetyl group (CH3CO−). On the other hand, the mass (1,558 Da) of the MKP-1(50−63) peptide before and after in vitro acetylation for 30 min by Mtb Eis agrees with the predicted mass of the unmodified peptide (Fig. 1A). These results indicate that Mtb Eis quickly attaches a single acetyl group to the MKP-7(53−66) peptide, whereas it does not readily acetylate the MKP-1(50−63) peptide. The MKP-7(53−66) peptide has three potential N-acetylation sites: one Nα-acetylation site at the amino terminus and two Nɛ-acetylation sites at Lys55 and Lys62. To identify which of the three possible acetylation sites was actually modified, we performed de novo sequencing of the acetylated MKP-7(53−66) peptide by MALDI tandem mass spectrometry (Fig. 1B). Peaks of unacetylated a1, a2, y10, and y11 ions are present at 86.009, 217.157, 1,214.653, and 1,369.811 Da, respectively, whereas peaks of acetylated b-ion series b3*, b4*, b5*, and b8* are present at 415.398, 571.565, 727.680, and 1,096.718 Da, respectively. This result identifies Lys55 of the MKP-7(53−66) peptide as the Nɛ-acetylation site. By analogy with DUSP1/MKP-1 (22), we suggest that Nɛ-acetylation of DUSP16/MKP-7 at Lys55 by Mtb Eis may increase the interactions between DUSP16/MKP-7 and JNK by neutralizing the positive charge within the docking domain of DUSP16/MKP-7. Msm Eis acetylated both MKP-7(53−66) and MKP-1(50−63) peptides very quickly in approximately 5 min, resulting in a mass increase by 42 Da (Fig. S6). We have confirmed by MALDI tandem mass spectrometry that these peptides are Nα-acetylated at the amino terminus by Msm Eis (Fig. S6). Our data thus establish that Eis proteins from both Mtb and Msm are catalytically active as aminoglycoside N-acetyltransferases, but only Mtb Eis acts as an efficient Nɛ-acetyltransferase that can acetylate DUSP16/MKP-7.
Fig. 1.
Acetyltransferase assay of Mtb Eis using MKP-7(53−66) and MKP-1(50−63) peptides by mass spectrometry. (A) Mass spectra of the MKP-7(53−66) peptide before and after acetylation reaction by Mtb Eis (Upper). The observed increase in the peptide mass by 42 Da indicates that the peptide is acetylated at a single site. Mass spectra of the MKP-1(50−63) peptide before and after acetylation reaction by Mtb Eis (Lower) indicate that this peptide is not acetylated by Mtb Eis. (B) MALDI MS/MS spectrum of the MKP-7(53−66) peptide acetylated by Mtb Eis. The fragments marked with an asterisk (*) are +42 Da-shifted ions, compared with the counterparts that would be generated from the unmodified peptide. The acetylated MKP-7(53−66) fragmentation notation using the scheme of Roepstorff and Fohlman (45) is given above the spectrum. The acetyl group of modified Lys55 is highlighted by enclosing in a red box.
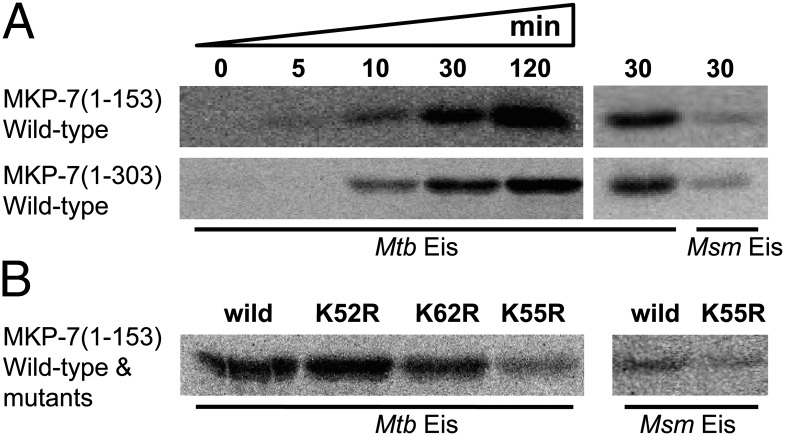
We further tested the protein N-acetyltransferase activity using recombinant human DUSP16/MKP-7. We could express DUSP/MKP-7(1−153), which covers the rhodanese-like domain and encompasses the docking domain, and DUSP/MKP-7(1−303), which additionally contains the phosphatase domain, whereas the full-length DUSP16/MKP-7 was not expressed in Escherichia coli. When DUSP16/MKP-7(1−153) and DUSP/MKP-7(1−303) were incubated with the wild-type Mtb Eis in the presence of [14C]-acetyl CoA, they were efficiently acetylated by Mtb Eis (Fig. 2A). However, their acetylation by Msm Eis was much less efficient (Fig. 2A). We next performed mutation experiments to confirm the acetylation site(s) in DUSP16/MKP-7(1−153). Each of Lys52, Lys55, and Lys62 in DUSP16/MKP-7(1−153) was replaced by arginine, and the acetylation reaction was carried out. Mutation of Lys55 drastically reduced the level of acetylation by Mtb Eis, whereas mutations of Lys52 and Lys62 showed no such drastic reduction (Fig. 2B). With Msm Eis, mutation of Lys55 had little effect on the level of acetylation, suggesting that acetylation by Msm Eis likely occurred at the amino terminus, but not at Lys55 (Fig. 2B). These results strongly suggest that Mtb Eis functions as a protein Nɛ-acetyltransferase toward human DUSP/MKP-7.
Fig. 2.
Acetyltransferase activity assay of Mtb Eis and Msm Eis using the recombinant human DUSP/MKP-7(1−153) and DUSP/MKP-7(1−303) proteins as potential substrates. (A) Time-course acetyltransferase activity assay of Mtb Eis and a comparison of Mtb Eis and Msm Eis activities. The wild-type DUSP16/MKP-7(1−153) and DUSP/MKP-7(1−303) were incubated with [14C]-labeled acetyl CoA and Eis for the indicated duration at 37 °C. The reaction products were separated by 15% (wt/vol) SDS/PAGE, and the acetylated protein bands were visualized using a Bioimage analyzer. (B) Acetyltransferase activity of Mtb Eis and Msm Eis toward the wild-type and mutants of DUSP16/MKP-7(1−153). All reactions were carried out at 37 °C for 30 min.
Mtb Eis Structure Reveals a Narrow Channel for Peptide Recognition.
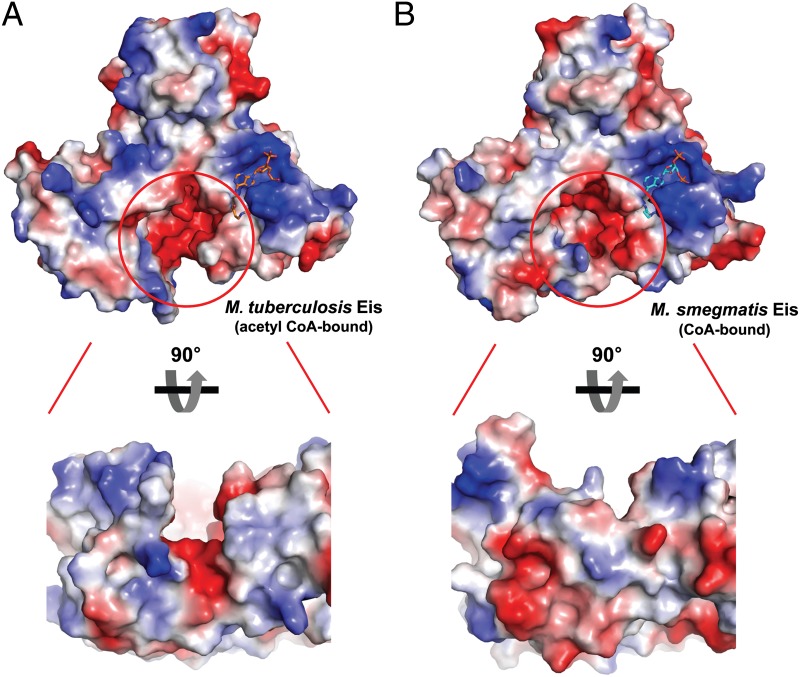
Our biochemical studies indicated that both Mtb and Msm Eis proteins could readily acetylate aminoglycosides, with Msm Eis being a marginally better catalyst as an aminoglycoside N-acetyltransferase (Fig. S2). In contrast, their substrate preferences against MKP-7(53−66) and MKP-1(50−63) peptides were different. Mtb Eis acetylated an internal lysine (Lys55) of the MKP-7(53−66) peptide rapidly, whereas Msm Eis preferentially acetylated the terminal amino group of these peptides (Fig. 1 and Fig. S6). To understand the observed difference in peptide acetylation site preferences, we have compared the active site features of these Eis proteins. Both Eis active sites show negative electrostatic potential surfaces (Fig. 3), and key residues around the thiol group of acetyl CoA (or CoA) are identical (Asp32/Tyr132/Phe408 in Mtb; Asp28/Tyr126/Phe402 in Msm). Interestingly, we noticed a difference in the shape of the predicted substrate binding sites adjacent to the bound ligand in their active sites. Mtb Eis has a deep and narrow channel, whereas Msm Eis has a deep and round pocket (Fig. 3). This structural difference in the substrate binding sites is mainly due to different arrangements of helix α2 (Gly35–Leu45 in Mtb Eis; Glu32–Met41 in Msm), part of “structural” domain 1, and helix α6 (Gln200–Cys210 in Mtb Eis; Asp190–Ala198 in Msm) and the following loop, which are part of “structural” domain 2 (Fig. S7A). The narrow channel of Mtb Eis is formed by limited hydrophobic interactions between these helices. In comparison, the round pocket-shaped substrate binding site of Msm Eis is formed by extensive interactions between helix α2 in “structural” domain 1 and helix α6 followed by an additional 310 helix α6’ in “structural” domain 2 (Figs. S1 and S7A). The 310 helix α6’ is present in Msm Eis only (Figs. S1 and S7A). The close contact between α2 and α6 helices in Msm Eis is stabilized by hydrogen bondings of Gln33-Arg200 and Thr34-Asp195 pairs (Fig. S7A). In addition, the side chain of Trp42 in Mtb Eis is located between the side chains of Trp19 (on α1) and Phe90 (on β4) in “structural” domain 1, whereas the corresponding residue in Msm Eis, Trp38, is located on the other side of Phe84 and makes van der Waals contacts with the side chains of Met41 (on α2), Leu192 (on α6), and Tyr400 (Fig. S7A). The elongated substrate-binding channel in Mtb Eis seems to be suitable not only for accommodating aminoglycosides but also for recognizing the polypeptide substrate in a sequence-specific manner. This channel is also present in the reported structure of Mtb Eis (20). The deep, round-shaped substrate-binding pocket in Msm Eis seems more suitable for accommodating aminoglycosides and the terminal amino group of peptides than sequence-specific recognition of polypeptides.
Fig. 3.
Comparison of Mtb and Msm Eis monomers. (A) Electrostatic potential at the surface of Mtb Eis monomer, with an enlarged view from an orthogonal angle. (B) Electrostatic potential at the surface of Msm Eis monomer, with an enlarged view from an orthogonal angle. Blue and red correspond to positive and negative potentials, respectively. Red circles indicate possible substrate binding sites of Mtb and Msm Eis.
Mtb Eis, but Not Msm Eis, Inhibits LPS-Induced JNK Phosphorylation and Proinflammatory Cytokine Production.
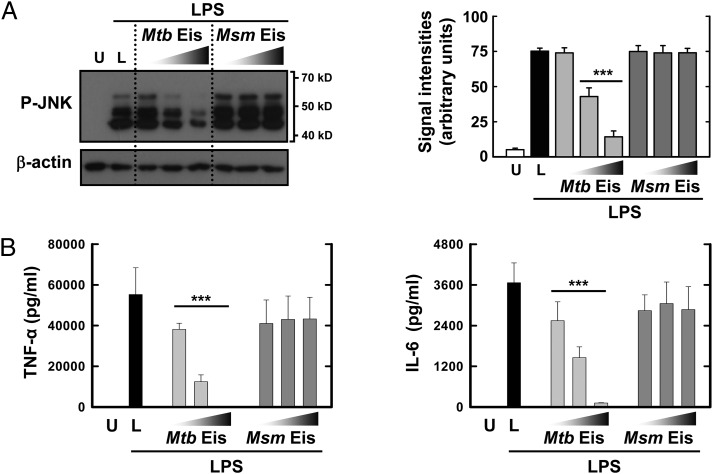
Because DUSP16/MKP-7 was reported to inhibit JNK activation in macrophages in response to LPS by dephosphorylating (24–28), we examined whether Mtb Eis or Msm Eis affects LPS-induced JNK phosphorylation in bone marrow-derived macrophages (BMDMs). Mtb Eis significantly reduced the level of phosphorylated JNK in BMDMs in response to LPS, whereas Msm Eis did not (Fig. 4). Furthermore, Mtb Eis significantly inhibited production of TNF-α and IL-6 in BMDMs in response to LPS, whereas Msm Eis did not (Fig. 4). Inhibition of JNK phosphorylation was reported to reduce production of these proinflammatory cytokines (29, 30). These data, together with our finding that Mtb Eis acetylates DUSP16/MKP-7 at Lys55, indicate that Mtb Eis suppresses host innate immune responses through inactivation of JNK via acetylation of DUSP16/MKP-7.
Fig. 4.
Effects of Mtb Eis on JNK activation and cytokine production in BMDM cells. (A) Mtb Eis, but not Msm Eis, suppresses JNK activation in BMDM cells upon LPS stimulation. Cells were treated with or without Mtb Eis (or Msm Eis) (5, 10, or 20 μg mL−1) for 1 h, followed by stimulation with LPS (100 ng mL−1) for 30 min. Cells were then harvested, lysed, and subjected to Western blot analysis using antibodies raised to phospho-JNK and β-actin. Data shown are representative of three independent experiments that all yielded similar results. Expression of phospho-JNK and β-actin in cytoplasmic extracts of BMDMs was quantified densitometrically (Right). Data represent the mean ± SD of three independent experiments. ***P < 0.001 vs. LPS-stimulated condition. U, LPS-untreated; L, LPS-treated condition without Mtb Eis or Msm Eis. (B) Mtb Eis, but not Msm Eis, suppresses proinflammatory cytokine production in BMDM cells upon LPS stimulation. Cells were treated with or without Mtb Eis (or Msm Eis) (5, 10, or 20 μg mL−1) for 1 h, followed by stimulation with LPS (100 ng mL−1) for 18 h. Supernatants were harvested, and the levels of TNF-α and IL-6 were measured by ELISA.
Discussion
Survival of Mtb inside human macrophages is central to tuberculosis infection, latency, disease activation, and transmission (31). The most important survival strategies of the pathogen are the inhibition of phagosomal maturation and autophagy (32). Autophagy has been recognized as innate and adaptive immune defense mechanisms (32). A functional overlap was suggested between phagosome maturation and autophagy, both of which depend on Beclin 1 and VPS34 as key players (32). Beclin 1, a pro-autophagy BH3 domain-containing protein, plays a central role in autophagosome formation by interacting with several other factors to promote the formation of Beclin 1–VPS34–VPS15 core complexes (12, 33). In mammalian cells under nonstarvation conditions, the antiapoptotic protein Bcl-2 binds to Beclin 1 and inhibits its autophagy function (34). Starvation induces Bcl-2 dissociation from Beclin 1, via phosphorylation of Bcl-2, and autophagy activation. Furthermore, JNK1, but not JNK2, was found to mediate starvation-induced Bcl-2 phosphorylation (34). Recently, JNK signaling was shown to mediate amplification of ROS production during multiple stresses, including infection (35). Cellular stress altered mitochondria, causing JNK to translocate to the mitochondria and to amplify up to 80% of the ROS generated largely by complex I. ROS activates JNK via a sequence of events for JNK mitochondrial signaling (35). There exists a molecular cross-talk between autophagy and apoptosis, because Beclin 1 can be cleaved by caspases, and its proautophagic activity is lost (36). Moreover, the resulting C-terminal fragment of Beclin 1 could amplify mitochondrion-mediated apoptosis (36, 37).
The kinase activity of MAPKs such as JNK is negatively regulated by DUSPs (also called MKPs). DUSP16/MKP-7 was found to work as a JNK-specific phosphatase in vivo, because forced expression of DUSP16/MKP-7 suppressed activation of MAPKs in the order of selectivity, JNK >> p38 > ERK (17). When expressed in mammalian cells, DUSP16/MKP-7 was localized exclusively in the cytoplasm (17). Acetylation of the components of MAPK pathways on serine/threonine and lysine residues was previously reported to serve as a regulatory mechanism in biological signaling. Yersinia YopJ was shown to act as an acetyltransferase to modify serine and threonine residues in the activation loop of MAPK kinase-6, thereby blocking phosphorylation and subsequent activation of the kinase activity (38, 39). A nuclear-localized DUSP1/MKP-1 is acetylated by p300 on Lys57 within its docking domain, resulting in deactivation of Toll-like receptor inflammatory signaling and inhibition of innate immune signaling (22). p300 contains a histone acetyltransferase domain. Acetylation of DUSP1/MKP-1 enhanced its interaction with p38, thereby increased its phosphatase activity, and interrupted MAPK signaling cascade (22, 23). In this study, we have discovered that Mtb Eis acts as an Nɛ-acetyltransferase to acetylate Lys55 within the docking domain of DUSP16/MKP-7. We have also shown that Mtb Eis, but not Msm Eis, significantly down-regulated the LPS-induced JNK phosphorylation. On the basis of these findings, we propose that acetylation of DUSP16/MKP-7 by Mtb Eis is the key initial event in the JNK-dependent inhibition of autophagy, phagosome maturation, and ROS generation, which ultimately contributes to enhanced survival of Mtb within the macrophage cells. Definitely establishing the proposed mechanism of enhanced intracellular survival would require functional studies with an engineered pathogen in a suitable host cell.
It has been well established that protein lysine acetylation critically regulates gene transcription by targeting histones as well as a variety of transcription factors in the nucleus (40). Numerous proteins located outside the nucleus have also been demonstrated to be acetylated (40). Indeed, protein lysine acetylation is emerging as a major mechanism by which key proteins are regulated in many physiological processes (40). Recent reports also link lysine acetylation to heterochromatin assembly, sister chromatid cohesion, cytoskeleton dynamics, autophagy, receptor signaling, RNA processing, and metabolic control (41). Proteomics studies indicate that the complexity of the acetylome potentially rivals that of the phosphoproteome (42). Therefore, it is not surprising to find that Mtb uses Eis to acetylate the host signaling protein DUSP16/MKP-7 in suppressing immune responses for its survival in macrophages. Mtb might have evolved in such as way that its eis gene product has retained much of the aminoglycoside N-acetyltransferase activity, whereas it has gained a significantly higher protein lysine Nɛ-acetyltransferase activity to disrupt the cellular signaling pathway for intracellular survival.
Similarly to Mtb Eis, protein kinase G (PknG) of Mtb inhibits phagosome–lysosome fusion and mediates intracellular survival of mycobacteria by disrupting the host cellular signaling (43). PknG is one of the 11 eukaryotic-like Ser/Thr protein kinases encoded by the Mtb genome and is secreted within macrophages. Mtb Eis, like Mtb PknG, could be an excellent target for the development of drugs that induce mycobacterial death inside macrophages. An advantage of targeting Eis or PknG is that it does not kill the bacteria per se but instead facilitates the macrophage to carry out its natural antibacterial activity, delivering intracellularly surviving mycobacteria to lysosomes for destruction (44). Another potential advantage of targeting a secreted protein such as Eis or PknG is that its inhibitors are not required to be transported through the extremely impermeable mycobacterial cell wall. This may greatly improve the bactericidal activity of the compounds (44). Mtb Eis has an extra advantage, because existing aminoglycoside drugs, such as kanamycin and amikacin, can be made effective by inhibiting its aminoglycoside N-acetyltransferase activity. Our structural information would be useful in structure-based discovery of peptidomimetic or small-molecule inhibitors that target the active site of Mtb Eis.
Materials and Methods
Detailed methods of protein expression/purification, in vitro acetylation assay, mass spectrometry, crystallization, X-ray data collection, structure determination, cell culture, Western blotting, and ELISA are provided in SI Materials and Methods. Briefly, the genes encoding Mtb and Msm Eis proteins were cloned into pET-28b(+) and were expressed in E. coli Rosetta II(DE3)pLysS cells. The recombinant Eis proteins with an N-terminal fusion tag were purified, crystallized, and characterized by in vitro acetylation assays. The acetylation site of the in vitro acetylated peptides was identified by MALDI MS/MS analysis.
Supplementary Material
Acknowledgments
We thank the beamline staff at Photon Factory, Japan (BL-5A, BL-17A, NW12), SPring-8, Japan (BL38B1), and Pohang Light Source, Korea (BL-4A) for assistance during X-ray diffraction experiments; Prof. Seung Bum Park for the use of the Synergy HT Multi-Mode Microplate Reader (BioTek Instruments); and Dong-Min Shin and Jin Kyung Kim (both at Chungnam National University) for excellent technical assistance. This work was funded by the Korea Ministry of Education, Science, and Technology (MEST), National Research Foundation (NRF) of Korea, Korea-New Zealand Cooperative Research Grant, Basic Science Outstanding Scholars Program (2008-0093867), World-Class University Program (Grant R31-10032), Grant R11-2007-107-00000-0 from the Innovative Drug Research Center for Metabolic and Inflammatory Disease, and by Grant A092006 from the Korea Ministry of Health, Welfare and Family Affairs, Korea Healthcare Technology R&D Project (S.W.S.); and by Korea MEST NRF Grant 2012-0005763 through the Infection Signaling Network Research Center at Chungnam National University (to E.-K.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The crystallography, atomic coordinates, and structure factors reported in this paper have been deposited in the Protein Data Bank, www.pdb.org [PDB ID code 3RYO (M. tuberculosis Eis, in complex with acetyl CoA), 3UY5 (M. tuberculosis Eis, apo), and 3SXN (M. smegmatis Eis, in complex with CoA)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120251109/-/DCSupplemental.
References
- 1.Dye C, Williams BG. The population dynamics and control of tuberculosis. Science. 2010;328:856–861. doi: 10.1126/science.1185449. [DOI] [PubMed] [Google Scholar]
- 2.Chiang CY, Centis R, Migliori GB. Drug-resistant tuberculosis: Past, present, future. Respirology. 2010;15:413–432. doi: 10.1111/j.1440-1843.2010.01738.x. [DOI] [PubMed] [Google Scholar]
- 3.Dahl JL, Wei J, Moulder JW, Laal S, Friedman RL. Subcellular localization of the Iitracellular survival-enhancing Eis protein of Mycobacterium tuberculosis. Infect Immun. 2001;69:4295–4302. doi: 10.1128/IAI.69.7.4295-4302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin PL, Flynn JL. Understanding latent tuberculosis: A moving target. J Immunol. 2010;185:15–22. doi: 10.4049/jimmunol.0903856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: The epidemiology and the response. Clin Infect Dis. 2010;50(Suppl 3):S201–S207. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 6.Jo EK, Yang CS, Choi CH, Harding CV. Intracellular signalling cascades regulating innate immune responses to Mycobacteria: Branching out from Toll-like receptors. Cell Microbiol. 2007;9:1087–1098. doi: 10.1111/j.1462-5822.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- 7.Wei J, et al. Identification of a Mycobacterium tuberculosis gene that enhances mycobacterial survival in macrophages. J Bacteriol. 2000;182:377–384. doi: 10.1128/jb.182.2.377-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meena LS, Rajni Survival mechanisms of pathogenic Mycobacterium tuberculosis H37Rv. FEBS J. 2010;277:2416–2427. doi: 10.1111/j.1742-4658.2010.07666.x. [DOI] [PubMed] [Google Scholar]
- 9.Ehrt S, Schnappinger D. Mycobacterial survival strategies in the phagosome: Defence against host stresses. Cell Microbiol. 2009;11:1170–1178. doi: 10.1111/j.1462-5822.2009.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vergne I, Chua J, Singh SB, Deretic V. Cell biology of Mycobacterium tuberculosis phagosome. Annu Rev Cell Dev Biol. 2004;20:367–394. doi: 10.1146/annurev.cellbio.20.010403.114015. [DOI] [PubMed] [Google Scholar]
- 11.Vergne I, et al. Autophagy in immune defense against Mycobacterium tuberculosis. Autophagy. 2006;2:175–178. doi: 10.4161/auto.2830. [DOI] [PubMed] [Google Scholar]
- 12.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu S, et al. Activation of the eis gene in a W-Beijing strain of Mycobacterium tuberculosis correlates with increased SigA levels and enhanced intracellular growth. Microbiology. 2009;155:1272–1281. doi: 10.1099/mic.0.024638-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lella RK, Sharma C. Eis (enhanced intracellular survival) protein of Mycobacterium tuberculosis disturbs the cross regulation of T-cells. J Biol Chem. 2007;282:18671–18675. doi: 10.1074/jbc.C600280200. [DOI] [PubMed] [Google Scholar]
- 15.Samuel LP, et al. Expression, production and release of the Eis protein by Mycobacterium tuberculosis during infection of macrophages and its effect on cytokine secretion. Microbiology. 2007;153:529–540. doi: 10.1099/mic.0.2006/002642-0. [DOI] [PubMed] [Google Scholar]
- 16.Shin DM, et al. Mycobacterium tuberculosis eis regulates autophagy, inflammation, and cell death through redox-dependent signaling. PLoS Pathog. 2010;6:e1001230. doi: 10.1371/journal.ppat.1001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masuda K, Shima H, Watanabe M, Kikuchi K. MKP-7, a novel mitogen-activated protein kinase phosphatase, functions as a shuttle protein. J Biol Chem. 2001;276:39002–39011. doi: 10.1074/jbc.M104600200. [DOI] [PubMed] [Google Scholar]
- 18.Neuwald AF, Landsman D. GCN5-related histone N-acetyltransferases belong to a diverse superfamily that includes the yeast SPT10 protein. Trends Biochem Sci. 1997;22:154–155. doi: 10.1016/s0968-0004(97)01034-7. [DOI] [PubMed] [Google Scholar]
- 19.Zaunbrecher MA, Sikes RD, Jr, Metchock B, Shinnick TM, Posey JE. Overexpression of the chromosomally encoded aminoglycoside acetyltransferase eis confers kanamycin resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2009;106:20004–20009. doi: 10.1073/pnas.0907925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen W, Biswas T, Porter VR, Tsodikov OV, Garneau-Tsodikova S. Unusual regioversatility of acetyltransferase Eis, a cause of drug resistance in XDR-TB. Proc Natl Acad Sci USA. 2011;108:9804–9808. doi: 10.1073/pnas.1105379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crossman DK. Tuscaloosa, AL: Univ of Alabama; 2007. Characterization of a novel acetylatransferase found only in pathogenic strains of Mycobacterium tuberculosis. PhD thesis. [Google Scholar]
- 22.Cao W, Bao C, Padalko E, Lowenstein CJ. Acetylation of mitogen-activated protein kinase phosphatase-1 inhibits Toll-like receptor signaling. J Exp Med. 2008;205:1491–1503. doi: 10.1084/jem.20071728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chi H, Flavell RA. Acetylation of MKP-1 and the control of inflammation. Sci Signal. 2008;1:pe44. doi: 10.1126/scisignal.141pe44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanoue T, Yamamoto T, Maeda R, Nishida E. A Novel MAPK phosphatase MKP-7 acts preferentially on JNK/SAPK and p38 alpha and beta MAPKs. J Biol Chem. 2001;276:26629–26639. doi: 10.1074/jbc.M101981200. [DOI] [PubMed] [Google Scholar]
- 25.Han SY, Kim SH, Heasley LE. Differential gene regulation by specific gain-of-function JNK1 proteins expressed in Swiss 3T3 fibroblasts. J Biol Chem. 2002;277:47167–47174. doi: 10.1074/jbc.M204270200. [DOI] [PubMed] [Google Scholar]
- 26.Matsuguchi T, Musikacharoen T, Johnson TR, Kraft AS, Yoshikai Y. A novel mitogen-activated protein kinase phosphatase is an important negative regulator of lipopolysaccharide-mediated c-Jun N-terminal kinase activation in mouse macrophage cell lines. Mol Cell Biol. 2001;21:6999–7009. doi: 10.1128/MCB.21.20.6999-7009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Kondoh K, Nishida E. Regulation of MAP kinases by MAP kinase phosphatases. Biochim Biophys Acta. 2007;1773:1227–1237. doi: 10.1016/j.bbamcr.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Assi K, Pillai R, Gómez-Muñoz A, Owen D, Salh B. The specific JNK inhibitor SP600125 targets tumour necrosis factor-α production and epithelial cell apoptosis in acute murine colitis. Immunology. 2006;118:112–121. doi: 10.1111/j.1365-2567.2006.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jang S, Kelley KW, Johnson RW. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci USA. 2008;105:7534–7539. doi: 10.1073/pnas.0802865105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell DG. Mycobacterium tuberculosis: Here today, and here tomorrow. Nat Rev Mol Cell Biol. 2001;2:569–577. doi: 10.1038/35085034. [DOI] [PubMed] [Google Scholar]
- 32.Gutierrez MG, et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 33.Funderburk SF, Wang QJ, Yue Z. The Beclin 1-VPS34 complex—at the crossroads of autophagy and beyond. Trends Cell Biol. 2010;20:355–362. doi: 10.1016/j.tcb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chambers JW, LoGrasso PV. Mitochondrial c-Jun N-terminal kinase (JNK) signaling initiates physiological changes resulting in amplification of reactive oxygen species generation. J Biol Chem. 2011;286:16052–16062. doi: 10.1074/jbc.M111.223602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Djavaheri-Mergny M, Maiuri MC, Kroemer G. Cross talk between apoptosis and autophagy by caspase-mediated cleavage of Beclin 1. Oncogene. 2010;29:1717–1719. doi: 10.1038/onc.2009.519. [DOI] [PubMed] [Google Scholar]
- 37.Wirawan E, et al. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis. 2010;1:e18. doi: 10.1038/cddis.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee S, et al. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science. 2006;312:1211–1214. doi: 10.1126/science.1126867. [DOI] [PubMed] [Google Scholar]
- 39.Mukherjee S, Hao YH, Orth K. A newly discovered post-translational modification—the acetylation of serine and threonine residues. Trends Biochem Sci. 2007;32:210–216. doi: 10.1016/j.tibs.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Close P, et al. The emerging role of lysine acetylation of non-nuclear proteins. Cell Mol Life Sci. 2010;67:1255–1264. doi: 10.1007/s00018-009-0252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim GW, Yang XJ. Comprehensive lysine acetylomes emerging from bacteria to humans. Trends Biochem Sci. 2011;36:211–220. doi: 10.1016/j.tibs.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Norris KL, Lee JY, Yao TP. Acetylation goes global: The emergence of acetylation biology. Sci Signal. 2009;2:pe76. doi: 10.1126/scisignal.297pe76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walburger A, et al. Protein kinase G from pathogenic mycobacteria promotes survival within macrophages. Science. 2004;304:1800–1804. doi: 10.1126/science.1099384. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen L, Pieters J. Mycobacterial subversion of chemotherapeutic reagents and host defense tactics: Challenges in tuberculosis drug development. Annu Rev Pharmacol Toxicol. 2009;49:427–453. doi: 10.1146/annurev-pharmtox-061008-103123. [DOI] [PubMed] [Google Scholar]
- 45.Roepstorff P, Fohlman J. Proposal for a common nomenclature for sequence ions in mass spectra of peptides. Biomed Mass Spectrom. 1984;11:601. doi: 10.1002/bms.1200111109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.