Abstract
We have previously demonstrated an increased DNA copy number and expression of IGF1R to be associated with poor outcome in Wilms tumors. We have now tested whether inhibiting this receptor may be a useful therapeutic strategy by using a panel of Wilms tumor cell lines. Both genetic and pharmacological targeting resulted in inhibition of downstream signaling through PI3 and MAP kinases, G1 cell cycle arrest, and cell death, with drug efficacy dependent on the levels of phosphorylated IGF1R. These effects were further associated with specific gene expression signatures reflecting pathway inhibition, and conferred synergistic chemosensitisation to doxorubicin and topotecan. In the in vivo setting, s.c. xenografts of WiT49 cells resembled malignant rhabdoid tumors rather than Wilms tumors. Treatment with an IGF1R inhibitor (NVP-AEW541) showed no discernable antitumor activity and no downstream pathway inactivation. By contrast, Wilms tumor cells established orthotopically within the kidney were histologically accurate and exhibited significantly elevated insulin-like growth factor–mediated signaling, and growth was significantly reduced on treatment with NVP-AEW541 in parallel with signaling pathway ablation. As a result of the paracrine effects of enhanced IGF2 expression in Wilms tumor, this disease may be acutely dependent on signaling through the IGF1 receptor, and thus treatment strategies aimed at its inhibition may be useful in the clinic. Such efficacy may be missed if only standard ectopic models are considered as a result of an imperfect recapitulation of the specific tumor microenvironment.
Keywords: nephroblastoma, MAPK, PI3K
Wilms tumor is an embryonal renal tumor accounting for nearly 6% of all pediatric cancers and more than 90% of kidney tumors in children (1). It is the fourth most common type of solid tumor in children, usually presenting between the ages of 2 y and 4 y. During the past 40 y, improvement of treatment protocols has raised survival rates from 30% to more than 90%, and treatment of this tumor has become a paradigm for successful cancer therapy (1). However, approximately 15% of patients with favorable histology, and 50% of patients with anaplastic Wilms tumor, will experience recurrence, and, for these patients, the survival rate is closer to 60% (2).
Work in our laboratory using microarray-based comparative genomic hybridization revealed a significant correlation between increased copy number at the IGF1R locus at chromosome 15q26.3 and Wilms tumor relapse (3). Approximately 10% of Wilms tumors exhibited a low-level gain corresponding to three or four copies of the gene by microarray-based comparative genomic hybridization analysis, with tumors exhibiting increased copies and mRNA/protein overexpression having a significantly worse outcome (3). This was independent of specific histological subtype, although an increased prevalence of IGF1R aberrations were noted in tumors with anaplastic vs. favorable histology.
We hypothesized that paracrine activation of IGF1R by IGF2, produced in large amounts by the tumor as a result of loss of heterozygosity or loss of imprinting (4, 5), may result in an increased mitogenic/antiapoptotic action through PI3-kinase/Akt/S6K and/or Ras/MAP kinase signaling pathways. Novel therapeutic strategies targeting the IGF1 receptor might therefore be beneficial in patients with anaplastic/relapsed Wilms tumor, alone or in combination with existing chemotherapeutic agents.
Insulin-like growth factor (IGF) signaling has become an attractive target for novel cancer therapeutic strategies because of its crucial role in regulating cancer cell proliferation and survival (6). Numerous experimental approaches have been used to inhibit IGF1R signaling, including dominant-negative mutants, antisense oligonucleotides, soluble IGFBPs, antagonistic and/or neutralizing antibodies, and small-molecule kinase inhibitors (7). The most advanced strategies are those involving anti-IGF1R antibodies and small-molecule inhibitors, with both in vitro and in vivo efficacy demonstrated in a range of tumor types, including numerous childhood cancer models (8).
A major hindrance to the development of novel therapeutic strategies in Wilms tumor has been the lack of appropriate model systems, both in vitro and in vivo, for mechanistic and preclinical studies, with few well-characterized cell lines available. Most recently, the Pediatric Preclinical Testing Program has developed a small number of Wilms tumor cell lines (one anaplastic, three nonanaplastic) as s.c. xenografts for the screening of a variety of chemotherapeutic and targeted agents in the wider context of childhood cancer (9).
The present study sought to assess the efficacy of strategies targeting the IGF1 receptor in a panel of Wilms tumor cell lines, and to determine the major determinants of response to genetic and pharmacologic inhibition. In particular, we have addressed the lack of clinically relevant models available by xenografting Wilms tumor cells in the kidney of immunocompromised mice, and investigated the differential signaling activation, and thus therapeutic response, compared with standard ectopic (i.e., s.c.) models.
Results
In Vitro Sensitivity of Wilms Tumor Cells to NVP-AEW541 Is IGF1R-Dependent.
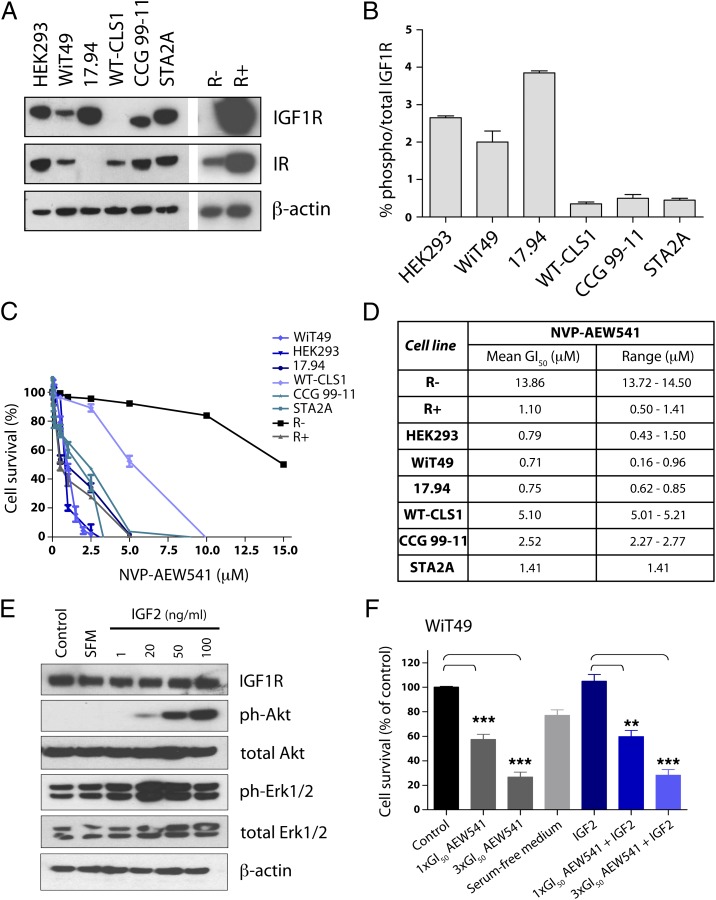
To screen the in vitro efficacy of the small molecule IGF1R inhibitor NVP-AEW541 in Wilms tumor cells, we assembled a panel of cell lines (17.94, WT-CLS1, CCG 99–11, STA2A, and WiT49), as well as human embryonic kidney HEK293 cells, and fibroblast lines engineered to have high (R+) or no (R−) IGF1R expression for comparison. Differential IGF1R protein expression was observed across the lines, with particularly high levels noted in 17.94, and an absence of receptor in WT-CLS1 as determined by Western blot (Fig. 1A). By contrast, WT-CLS1 cells expressed the closely related insulin receptor, common to all lines with the exception of 17.94 (Fig. 1A). Higher levels of phosphorylated IGF1R were detected in WiT49 and 17.94 cells compared with the very low levels found in the other Wilms tumor lines, as assessed by the electrochemiluminescent Meso Scale Discovery (MSD) System assay (Fig. 1B).
Fig. 1.
IGF1R as a therapeutic target in Wilms tumor cell lines. (A) Western blot for IGF1R protein expression in a panel of Wilms tumor cell lines in vitro. Also included for comparison are the IGF1R-null (R−) and -overexpressing (R+) fibroblast cell lines, as well as blots for the homologous insulin receptor (IR). β-Actin is used as a loading control. (B) Quantitative measure of phosphorylated IGF1R in Wilms tumor cell lines assessed by the MSD assay. (C) Effects on cell survival of treatment with Wilms tumor cells with the IGF1R inhibitor NVP-AEW541, in comparison with the resistant R− and sensitive R+ cells. (D) GI50 values for cell lines treated with NVP-AEW541 as assessed by the MTS assay. Mean and range (in μM) for triplicate experiments are given. (E) Increased downstream signaling although PI3 and MAP kinase pathways in WiT9 cells in the presence of IGF2 assessed by Western blot analysis of phospho-Akt and phospho-Erk1/2. (F) NVP-AEW541 induces a concentration-dependent reduction in cell survival in WiT49 cells in the absence and presence of IGF2. Cells were treated with 1× and 3× GI50 concentrations of NVP-AEW541 in the absence of IGF2, and in serum-free medium including 50 ng/mL IGF2. (***P < 0.001 and **P < 0.01, t test).
In vitro sensitivity to NVP-AEW541 appeared to correlate with the levels of IGF1R/phospho-IGF1R, with the most sensitive lines (WiT49 and 17.94) having the highest levels of constitutive expression and concentration needed to reduce the growth of treated cells to 50% of that of untreated cells (GI50) of 0.71 μM and 0.75 μM, respectively, even lower than the R+ cells (1.10 μM; Fig. 1C). The least sensitive line was WT-CLS1 (5.10 μM), in which IGF1R expression was undetectable by Western blot (Fig. 1C). GI50s for CCG 99–11 (2.52 μM) and STA2A (1.41 μM) cells were between these values (Fig. 1D). Culturing WiT49 cells in the presence of the IGF1R ligand IGF2 resulted in an increase of phospho-Akt and phospho-Erk1/2 levels, indicating activation of PI3 and MAP kinase pathways (Fig. 1E). Cell survival was diminished in a concentration-dependent manner by NVP-AEW541 both in the absence and presence of IGF2 (Fig. 1F).
IGF1R Inhibition Results in Cell Cycle Arrest, Apoptosis, and Chemosensitization via Down-Regulation of PI3 and MAP Kinase Pathways in Wilms Tumor Cells in Vitro.
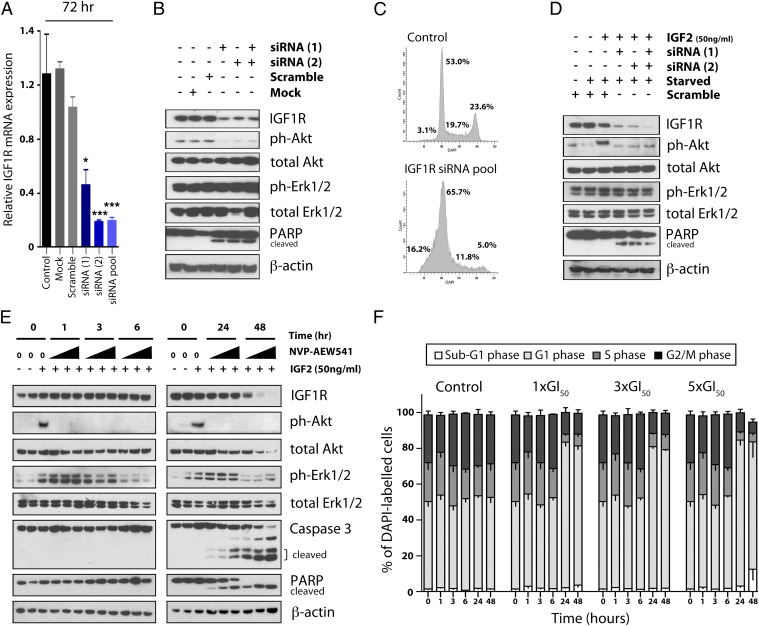
To assess the effects on downstream signaling of IGF1R abrogation in Wilms tumor cells, we compared genetic knockdown by specific siRNA with pharmacological inhibition by NVP-AEW541. siRNA directed against IGF1R in WiT49 cells resulted in efficient reduction of IGF1R (Fig. 2A) along with inhibition of phospho-Akt and induction of apoptosis as measured by PARP cleavage (Fig. 2B); no effect on phospho-Erk1/2 levels were observed. A profound cell cycle arrest at G1 was seen by FACS analysis after 48 h (Fig. 2C). These observations were replicated in the absence and presence of IGF2 (Fig. 2D). Such effects on protein expression (Fig. 2E) and cell cycle (Fig. 2F) were mimicked by NVP-AEW541 in WiT49 cells in a concentration- and time-dependent manner, with the additional inhibition of phospho-Erk1/2 levels. NVP-AEW541 blocked IGF2-induced PI3-kinase activation as detected by loss of phospho-Akt after 1 h of treatment and induced apoptosis as seen by PARP and caspase 3 cleavage after 24 h in WiT49 cells. Levels of phospho-Erk1/2 were reduced after 3 h drug exposure. After 48 h, there was significant cell death and evidence of receptor degradation.
Fig. 2.
Effects of genetic and pharmacological targeting of IGF1R on downstream signaling in Wilms tumor cells. (A) Relative IGF1R mRNA expression in WiT49 cells transfected with siRNA targeting the gene, as determined by quantitative RT-PCR (***P < 0.001, t test). (B) Western blots demonstrate efficient knockdown of IGF1R protein in association with diminished phospho-Akt and induced PARP cleavage in WiT49 cells transfected with IGF1R siRNA. (C) FACS analysis of siRNA-transfected WiT49 cells vs. scrambled control oligos demonstrate an accumulation of cells in G1 and sub-G1 phases in contrast to a reduction of S and G2 phases. (D) Western blots confirming the knockdown of IGF1R, reduction in phospho-Akt and phospho-Erk1/2, and induction of PARP cleavage after treatment of WiT49 cells with IGF1R siRNA in the presence of the ligand IGF2. (E) Effects on downstream signaling in WiT49 cells after treatment with NVP-AEW541 in the presence of IGF2. Cells were treated for 1, 3, 6, 24, or 48 h with 1×, 3×, or 5× GI50 compound (increase in triangle). Treatment with IGF1R inhibitor decreased phospho-Akt and phospho-Erk1/2 and induced PARP and caspase-3 cleavage in a time- and concentration-dependent manner. At 48 h, the highest concentration of compound results in a significant cell death with little protein recoverable. β-Actin is used as a loading control. (F) Effects on cell cycle in WiT49 cells treated with NVP-AEW541. An accumulation of cells in G1 and sub-G1 phases is induced by NVP-AEW541 in a time- and concentration-dependent manner. At 48 h, the highest concentration of compound results in significant cell death.
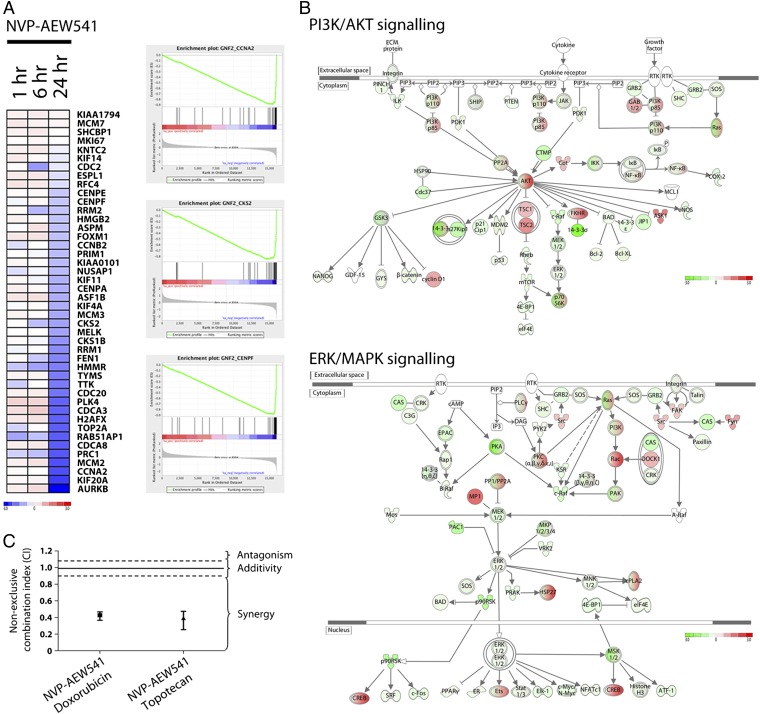
To better understand the mechanism of action of NVP-AEW541 in Wilms tumor cells, global gene expression profiling and pathway analysis on samples was performed after a time-course exposure to 5× GI50 concentration. Genes dysregulated by IGF1R inhibition in a time-dependent manner relative to vehicle control included numerous genes associated with cell cycle progression and DNA replication, including CCNA2, CCNB1, CKS2, CDC20, E2F2, FEN1, MCM2, and CENPF (Fig. 3A). Examining effects on canonical pathway signaling using the Ingenuity Pathway Analysis tool, the two highest scoring pathways with dysregulated gene expression upon treatment with 5× GI50 NVP-AEW541 for 24 h were those associated with PI3K/AKT (P < 0.0001) and ERK/MAPK signaling (P < 0.0001; Fig. 3B).
Fig. 3.
Treatment of WiT49 cells with NVP-AEW541 induces gene expression changes associated with cell cycle arrest and PI3K/MAPK down-regulation, and sensitizes the cells to chemotherapy. (A) Heat map demonstrates expression of genes associated with cell cycle progression in WiT49 cells treated with 5× GI50 of NVP-AEW541 for 1, 6, and 24 h. Genes are colored according to global, not relative, expression values (blue, down-regulated; red, up-regulated). Top-ranking gene set enrichment analysis scores for GNF2_CCNA2 [enrichment score = −0.89, P < 0.00001, false discovery rate (FDR) q < 0.00001] GNF2_CKS2 (enrichment score= −0.88, P < 0.00001, FDR q < 0.00001), and GNF2_CENPF (enrichment score = −0.85, P < 0.00001, FDR q < 0.00001) highlights coordinate down-regulation of genes in the expression neighborhood of these cell cycle control genes. (B) Top-ranking canonical pathways identified by Ingenuity Pathway Analysis after treatment of WiT49 cells with NVP-AEW541 were those associated with PI3K/AKT (P < 0.0001) and ERK/MAPK signaling (P < 0.0001). Pathway components are colored by their global gene expression levels (green, down-regulated; red, up-regulated). (C) Median effects analysis of combining NVP-AEW541 with chemotherapeutic agents in WiT49 cells. Highly synergistic interactions are seen for doxorubicin (combination index = 0.43) and topotecan (combination index = 0.39).
Given the known chemosensitizing effects of IGF1R inhibition in other cell systems showing similar effects on cell signaling and gene expression, we investigated the use of NVP-AEW541 in combination with clinically relevant chemotherapeutics in Wilms tumor cells. Combined treatment of NVP-AEW541 with doxorubicin or topotecan gave rise to highly synergistic interactions in vitro, with combination indices of 0.43 and 0.39, respectively, using median effects analysis (Fig. 3C).
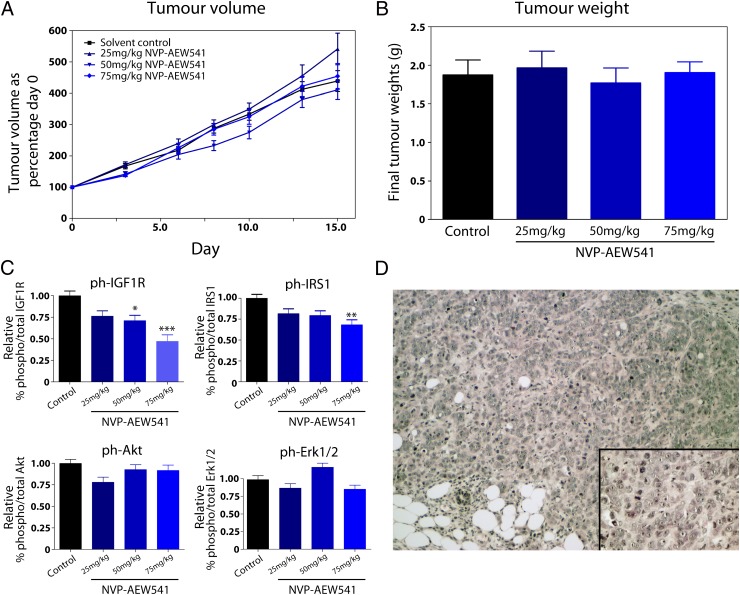
NVP-AEW541 Inhibits Tumor Growth in an Orthotopic, but Not s.c., Wilms Tumor Xenograft Model.
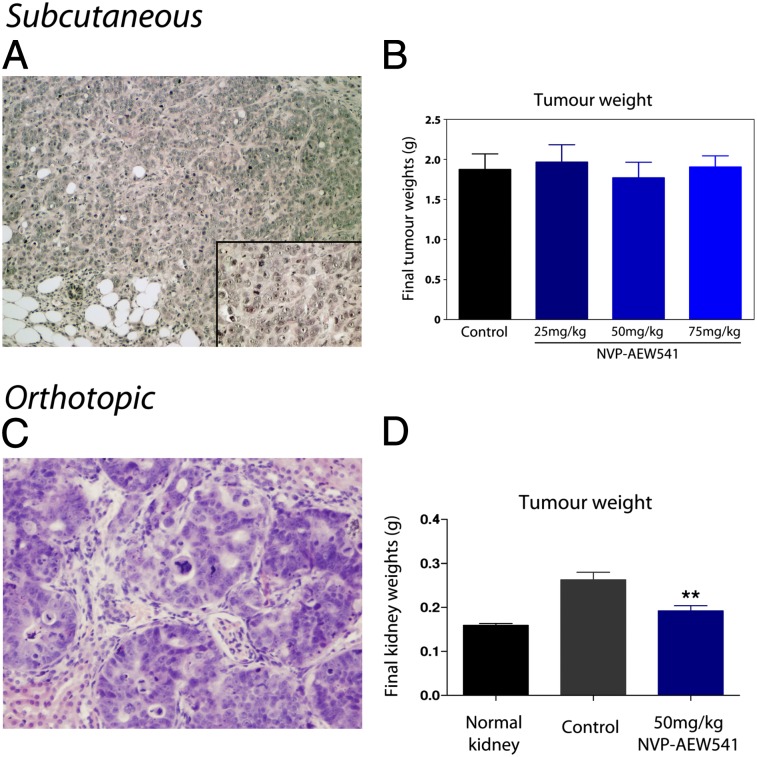
Initially, WiT49 cells were inoculated s.c. in female athymic nude mice. Palpable tumors developed within 7 d with a 100% incidence. At this point, animals were treated with 25 mg/kg, 50 mg/kg, or 75 mg/kg NVP-AEW541, and their tumor growth rate compared with a vehicle-treated control group. No significant differences were observed in tumor volume (Fig. 4A) or final tumor weight (Fig. 4B) upon treatment with the IGF1R inhibitor. This was despite pharmacokinetic assessment of drug concentration revealing the presence of 3.66 μM, 16.79 μM, and 210.59 μM NVP-AEW541 within the tumors of animals treated with 25 mg/kg, 50 mg/kg, and 75 mg/kg, respectively, amounting to fivefold, 24-fold, and 30-fold higher than the values of the in vitro GI50 for WiT49 cells. Assessment of pharmacodynamic endpoints by MSD demonstrated a significant inhibition of phospho-IGF1R (P < 0.001, t test) and phospho-IRS1 (P = 0.006) at the highest doses of compound, but no effect on phospho-Akt or phospho-Erk1/2 (Fig. 4C), the latter of which was found at particularly high constitutive levels. Histopathological assessment of the tumors revealed them to be composed of islands and sheets of medium and large undifferentiated cells surrounded by delicate fibrovascular stroma. The cells had prominent nucleoli and abundant slightly eosinophilic cytoplasm, more typical of malignant rhabdoid tumors of the kidney than classic Wilms tumors (Fig. 4D). A misidentification of the cell line was ruled out by screening for SMARCB1 (INI1) mutation/deletion, which was found to be wild-type.
Fig. 4.
Treatment with NVP-AEW541 shows no efficacy in an s.c. xenograft Wilms tumor model. (A) Tumor volumes in mice implanted with WiT49 cells s.c. and treated with 25 mg/kg, 50 mg/kg, or 75 mg/kg for 15 d. Tumor volume is plotted as percentage of day 0 for all groups, with no statistically significant differences compared with vehicle-treated controls. (B) Final tumor weights for the same experiment also show no differences between treated and untreated groups. (C) Pharmacodynamic biomarkers assessed in the WiT49 s.c. model after treatment with NVP-EW541. Although a dose-dependent decrease in phospho-IGF1R and, to a lesser extent, phospho-IRS1 were observed, no significant inhibition of the downstream PI3 and MAP kinase pathways was seen. (D) Histology of the s.c. WiT49 model. The cells had prominent nucleoli and abundant slightly eosinophilic cytoplasm, with a morphological appearance more closely resembling malignant rhabdoid tumors of the kidney than classic Wilms tumors. [Original magnification of ×100 (inset, magnification of ×400).]
We hypothesized that the specific microenvironment of the tumor cells may be playing a significant role in the phenotype demonstrated by WiT49 cells grown s.c., and also their response to targeted therapy. We therefore established an orthotopic model of these Wilms tumor cells by implantation into the renal subcapsule of the left kidney in female athymic nude mice. Tumors were detected by using ultrasound within 14 d, with a 100% take rate. Tumor cells grew deep into the renal parenchyma in a penetrating fashion reflecting a highly invasive phenotype (Fig. 5A). Large areas with epithelial differentiation and prominent tubular formation were seen, and in some places a thin fibrotic pseudocapsule was detected. Cells mostly demonstrated a cohesive growth pattern, often arranged in trabeculae. Moreover, cells exhibited anaplastic features such as large hyperchromatic nuclei and atypical mitotic figures (Fig. 5B). In total, WiT49 xenografts established within the kidney closely recapitulated the histology of anaplastic Wilms tumors. Similar morphological differences between orthotopic and s.c. xenografts were observed for 17.94 and CCG 99–11 cells (Fig. S1); however, neither model was readily suitable for in vivo efficacy assays. Orthotopically implanted CCG 99–11 cells formed large, rapidly growing, and predominantly extrarenal tumors, thus precluding study within the specific kidney microenvironment. Attempts to implant cells deeper within the renal parenchyma resulted in a significant lowering of the take rate (four of 24, 17%). The 17.94 cells were considerably slower-growing, and only four of eight (50%) s.c. implants formed tumors, even after enhancement, at more than 100 d. Orthotopically, small tumors developed within the kidney after extensive optimization of conditions with a similar efficiency (12 of 39, 31%) albeit within the shorter timeframe of 31 d. Because of the difficulty in establishing these models efficiently in both sites, we thus focused our efforts on WiT49 cells.
Fig. 5.
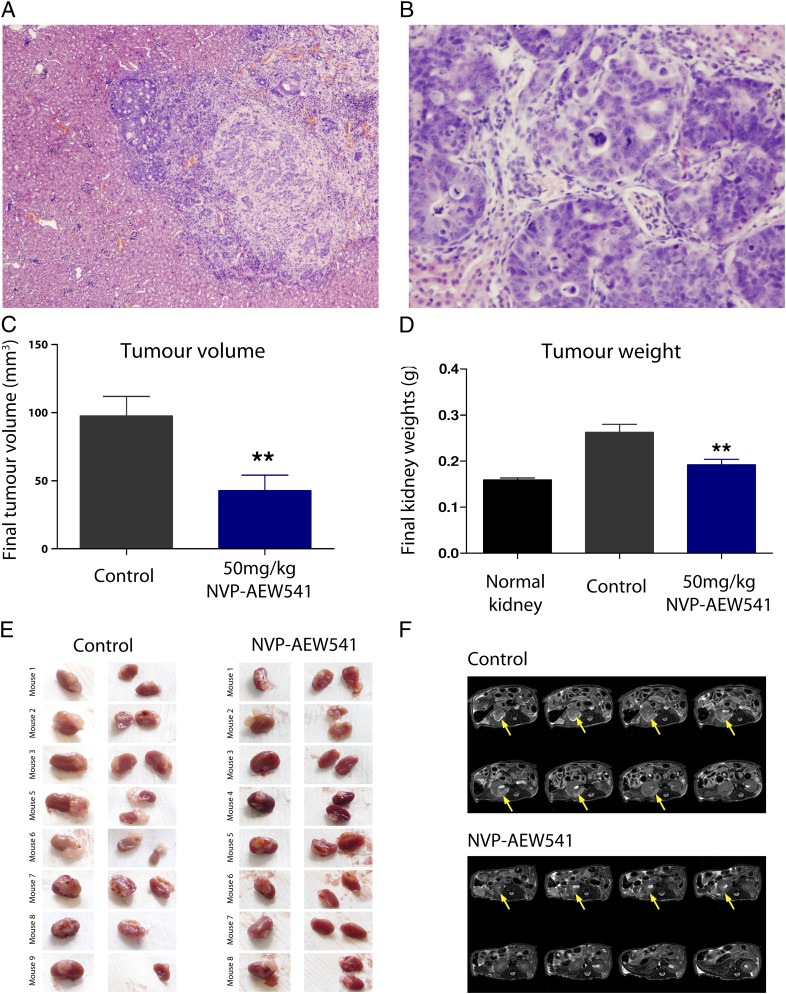
Antitumor activity of NVP-AEW541 in an orthotopic Wilms tumor xenograft model. (A) Low-power histological section of WiT49 cells implanted in the kidney. Tumor cells demonstrated a highly invasive phenotype, growing deep into the renal parenchyma. (Original magnification of ×100.) (B) High-power histological section of the orthotopic WiT49 tumor. Large areas with epithelial differentiation and prominent tubular formation were seen, with some cells exhibiting anaplastic features such as large cells, hyperchromatic nuclei, and atypical mitotic figures. (Original magnification of ×400.) (C) Significant reduction in tumor volume of the orthotopic WiT49 model after treatment with NVP-AEW541 (**P < 0.01, t test). (D) Significant reduction in final kidney weights of the orthotopic WiT49 model after treatment with NVP-AEW541. Tumor weights could not be directly assessed as a result of the infiltrative growth patterns observed (**P < 0.01, t test). (E) Images of the kidney in vehicle-treated control and NVP-AEW541–treated mice with orthotopic WiT49 xenografts. Extensive growth of tumor cells is observed extrarenally and deep within the kidney in the control animals, with considerably less observed in mice treated with the IGF1R inhibitor. (F) Hydrogen 1 MRI of a representative vehicle control- and NVP-AEW541–treated mouse implanted orthotopically with WiT49 cells. Tumors appear hyperintense on T2-weighted images. Considerably less tumor burden is evident in animals treated with IGF1R inhibitor compared with vehicle controls, as indicated by the yellow arrow.
Upon treatment with 50 mg/kg NVP-AEW541 for 14 d, orthotopic WiT49 tumors showed a significantly reduced final tumor volume, as measured by MRI (P = 0.006, t test; Fig. 5C). Because of the infiltrative growth within the kidney, final organ weights of vehicle- or drug-treated animals were compared with normal (i.e., tumor-free) kidneys in age-matched mice. There was a significant reduction in final kidney weights with the NVP-AEW541–treated mice compared with vehicle controls (P = 0.002, t test), with mean kidney weights approaching those without established tumors (Fig. 5D). In some cases, almost-complete regression of tumor was noted on macroscopic examination (Fig. 5E) and by MRI (Fig. 5F).
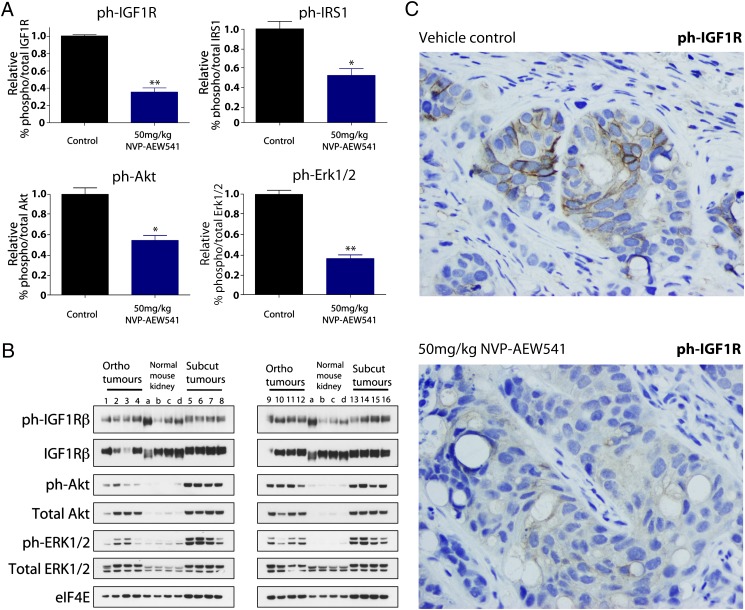
The pharmacokinetics of the orthotopic and s.c. models were similar, with a drug concentration of 24.86 μM within the WiT49 cells grown in the kidney. Pharmacodynamically, however, major differences were observed in response to equivalent doses of NVP-AEW541. In the kidney, tumor cells showed a reduction of phosphorylated IGF1R levels of nearly 70% (Fig. 6A) compared with less than 30% when implanted s.c. (Fig. 4C), with attendant significant reductions in downstream signaling activation not observed in the heterotopic transplants. Significant reductions in phospho-IGF1R (P = 0.0059, t test), phospho-IRS1 (P = 0.045, t test), phospho-Akt (P = 0.031, t test), and phospho-Erk1/2 (P = 0.0077, t test) were all seen on treatment with 50 mg/kg NVP-AEW541 in the orthotopic model. This is despite any confounding cross-reactivity between mouse and human protein in the infiltrative orthotopic models (Fig. 6B), as significant reduction of activated IGF1R on treatment with NVP-AEW541 could be visualized by immunohistochemistry (Fig. 6C). Thus, the specific orthotopic microenvironment in which the cells were grown clearly exerts a significant effect on tumor response, mediated by enhanced abrogation of downstream signaling, rendering the cells more susceptible to targeted therapeutic inhibition.
Fig. 6.
Pharmacodynamic biomarkers of response of an orthotopic Wilms tumor model treated with IGF1R inhibitor. (A) MSD assay analysis of relative phospho-IGF1R, phospho-IRS1, phospho-Akt, and phospho-Erk1/2 in WiT49 cells implanted in the kidney. All markers were significantly reduced on treatment with 50 mg/kg NVP-AEW541 (**P < 0.01 and *P < 0.05, t test). (B) Western blot of constitutive levels of phospho-/total protein in WiT49 cells grown in the kidney (“ortho”) compared with the equivalent s.c. (“subcut”) model and normal mouse kidneys. (C) Representative immunohistochemistry for phospho-IGF1R in the orthotopically grown WiT49 cells demonstrates high levels in the vehicle-treated controls and significant reduction on exposure to NVP-AEW541.
Discussion
Despite a clear link between Wilms tumorigenesis and dysregulation of the IGF network, specific inhibition of this pathway has not been extensively assessed as a molecularly targeted therapeutic strategy in this malignancy. This has in part been because of a lack of well validated experimental Wilms tumor models. For reasons that remain unclear, Wilms tumors appear very resistant to establishment as long-term cultures, and several early models such as SK-NEP-1 and G401 have later been proven to be tumors other than Wilms (10, 11). In the present study, we have used a small panel of more recently described Wilms tumor cell lines derived from primary in vitro culture [17.94 (12), WT-CLS1 (13), CCG 99–11 (14), STA2A (15)] or s.c. mouse xenografts of human Wilms tumor metastasis [WiT49 (16)].
We addressed the lack of in vitro preclinical data by studying the effect of targeting the IGF1R in these models by genetic and pharmacological means. We demonstrated that the small-molecule inhibitor NVP-AEW541 had a potent cytotoxic effect against Wilms tumor cells in vitro through down-regulation of PI3K and MAPK pathways, as well as cell cycle control genes such as CCNA2 and CCNB1, leading to a marked G1 arrest and subsequent induction of apoptosis in a caspase-dependent manner. These responses appeared well correlated with constitutive IGF1R activation; although significant off-target effects cannot be ruled out, they are not suggested by cell signaling and gene expression assays. It thus seems likely that, in these relatively cytogenetically stable tumors, a critical dependence is forced on IGF1R mediation of downstream signaling cascades, with the result that monotherapy may effectively block multiple pathways.
Given that we have previously shown copy number gain of IGF1R to be associated with treatment failure and relapse in Wilms tumor (3), coupled with paracrine activation of the receptor by IGF2, also overexpressed via loss of heterozygosity or loss of imprinting (4, 5), IGF1R appears to represent a useful therapeutic target whose inhibition may be beneficial, especially in patients with relapsed and/or anaplastic Wilms tumor. Additionally, we provide evidence for a significant synergistic chemopotentiation of the clinically relevant chemotherapeutic agents doxorubicin (2) and topotecan (17). Thus, for the broadest applicability, IGF1R-targeting compounds may be combined with conventional chemotherapy and used in an upfront setting, potentially allowing for reduced long-term toxicity through lower cytotoxic drug doses (18).
To assess whether the in vitro observations of IGF1R inhibition could be replicated in the in vivo setting, we established Wilms tumor cells both s.c. as well as within the kidney. A previous study using the anti-IGF1R monoclonal antibody IMC-A12 showed little efficacy against three s.c. Wilms tumor models (i.e., favorable histology) as part of the Pediatric Preclinical Testing Program initiative (19). No information regarding IGF1R status in these models was provided. Similarly a more recent study that used the small molecule BMS-754807 showed modest activity in the same models, but again without correlative molecular data (20). Our data provide clear evidence that cells grown in the appropriate kidney environment not only more accurately recapitulate the histology of human Wilms tumors, but also have considerably enhanced response to abrogated IGF-mediated signaling than when implanted s.c.
Ectopic models are widely used as they provide a simple, rapid and reproducible means to evaluate emerging therapies. However, they do not imitate the natural tumor environment, omitting organ-specific host–tumor interactions that may influence response to therapy (21–24). This has previously been demonstrated in the kidney, in which levels of basic fibroblast growth factor were found to be 10 to 20 times higher in renal cell carcinoma cells implanted orthotopically compared with s.c. (25). In the context of Wilms tumor, the predictive value of the IGF2-stimulated in vitro models, in which critical dependence on IGF1R-mediated signaling was also evident, was only recapitulated in the orthotopic setting, calling into question the value of studies focusing only on ectopic models, in which IGF2 levels are low (26–28). Although our efficacy data were based, for practical reasons, on only a single cell line model, the phenotypic effects of the differing microenvironments were recapitulated in two additional cell lines, suggesting a generalizable conclusion. Of note, these cell lines were originally harvested from extrarenal metastatic sites, which is clinically relevant, as recurrences tend not to occur in the kidney, as the patients have undergone nephrectomy. Specific intracellular signaling dependencies associated with, e.g., cell growth in the lung may also be considered as useful models better reflective of treatments aimed at the recurrent setting.
A recently developed genetically engineered mouse model combined loss of Wt1 function and Igf2 up-regulation to drive tumor initiation and proliferation through IGF1R signaling transduced via phospho-IRS1 and phospho-ERK1/2, thus potentially providing a biologically relevant model for testing the efficacy of agents targeting the receptor (29). Although impressive clinical results have been observed in pediatric sarcomas with anti-IGF1R monotherapy, in most tumor types, blockade of the receptor is being considered in combination with additional rationally designed agents (30). Although Wilms tumors may be particularly dependent on IGF1R because of the relative lack of genomic complexity (31) and the high levels of constitutive MAP kinase activation observed in xenograft and transgenic models, and with a significant fraction of human Wilms tumors also displaying increased phospho-IRS1 and phospho-ERK1/2 (29), strategies aimed at coinhibition of MEK or mTOR may open up further yet-unexplored combination strategies in this disease.
Materials and Methods
Cell Lines and Reagents.
R− (i.e., IGF1R-null) and R+ (i.e., IGF1R-overexpressing) mouse fibroblasts were a gift from Renato Baserga (Thomas Jefferson University, Philadelphia, PA). The human embryonic kidney HEK293 cell line was obtained from the American Type Culture Collection, and the Wilms tumor WT-CLS1 cells were obtained from Cell Lines Service. The Wilms tumor cell line WiT49 was donated by Herman Yeger (Hospital for Sick Children, Toronto, ON, Canada); STA2A by Peter Ambros (St. Anna Children’s Hospital, Vienna, Austria); 17.94 by Keith W. Brown (University of Bristol, Bristol, United Kingdom); and CCG99-11 by Jonathan D. Licht (Robert H. Lurie Comprehensive Cancer Center, Chicago, IL). The IGF1R inhibitor NVP-AEW541 was provided by Novartis Pharma. Doxorubicin and topotecan were purchased from Sigma-Aldrich and Eurasia, respectively. IGF2 was purchased from Abcam.
Tumor Cell Growth Inhibition in Vitro.
Growth inhibition following drug exposure was determined using the MTS assay as previously described (32). For the assessment of combination effects, cells were treated with increasing concentrations of drugs alone or concurrently at their equipotent molar ratio, and combination indices were calculated by the method of Chou and Talaly (33). All values are given as mean ± SD of at least three independent experiments.
siRNA.
Predesigned siRNA duplexes directed against IGF1R (catalog no. SI02624552 and SI00017521) were purchased from Qiagen. Cells were transfected with 5 nM IGF1R siRNAs as well as with a scrambled sequence control duplex by using HiPerFect (Qiagen) transfection reagent.
Western Blot Analysis.
Immunodetection was performed as previously described (34) by using antibodies against IGF1R (no. 3027), phospho-Aktser473 (no. 9271), Akt (no. 9271), phospo-Erk1/2 (no. 9101), Erk1/2 (no. 9102), PARP (no. 9542), and caspase-3 (no. 9662), all at 1:1,000 dilution (Cell Signaling Technology).
Electrochemiluminescent Immunoassay.
MSD 96-well multispot assays for total/phospho-IGF1R/IR/IRS-1, total/phospho-Akt, and total/phospho-Erk1/2 were carried out per the manufacturer’s protocol. Briefly, plates were blocked for 1 h, and then 20 to 25μg of protein was added to the plate in duplicate wells and incubated overnight at 4 °C. Plates were washed and incubated with detection antibody for 2 h with shaking. Plates were washed four times, read buffer was added, and the plates were analyzed on a SECTOR 6000 instrument (i.e., MSD).
mRNA Expression Profiling Analysis.
Expression profiling after treatment of WiT49 cells with NVP-AEW541 at 5× GI50 by Illumina HT-12 BeadChips was carried out according to the manufacturer’s instructions and normalized using the lumi package in R software (version 2.11). All data has been deposited in the Minimum Information About a Microarray Experiment-compliant ArrayExpress database (accession no. E-TABM-891). Supervised analysis was performed by using an absolute signal to noise metric of greater than 1.5 in GenePattern software. Time-course analysis was carried out by using a Pearson correlation coefficient metric. To determine pathways and networks that were significantly dysregulated upon treatment with NVP-AEW541, pathway analysis was performed by using the Ingenuity Pathway Analysis program, and coordinate gene regulation was identified by using Gene Set Enrichment Analysis.
In Vivo Antitumor Activity of NVP-AEW541.
Six- to 8-week-old female athymic nude mice (CrTac:NCr-Foxn1nu; Taconic) were used to establish s.c. and orthotopic Wilms tumor xenograft models. Experiments were conducted in accordance with United Kingdom Home Office regulations under the Animals (Scientific Procedures) Act 1986 and National Council of Rural Institutes guidelines for the welfare and use of animals in cancer research (35). WiT49 cells were implanted s.c., bilaterally, at 5 × 106 per site. Mice were randomly assigned to three groups (n = 6), and treated with NVP-AEW541 (25, 50, or 75 mg/kg orally twice per day) or vehicle comprising 25 mM L-(+)-tartaric acid. Body weight and tumor volume were measured every 3 to 4 d until the animals were euthanized, with final tumor weights measured following dissection. For the orthotopic Wilms tumor model, mice were anesthetized with isoflurane, a small incision between the spleen and the spinal column was made to the skin and abdominal wall, and the left kidney was carefully exteriorized. A total of 5 × 105 WiT49 cells in 10 μL Matrigel (BD Biosciences) was injected into the subcapsule of the kidney. The abdominal wall was closed with sterile 4–0 Mersilk suture (Ethicon) and the skin closed with surgical clips. Postoperative analgesia was administered according to the manufacturer’s instructions. Animals were treated with 50 mg/kg NVP-AEW541 or vehicle control by mouth twice per day for 15 d (n = 9). Tumor size was monitored by ultrasound (Vevo 770; VisualSonics) and final tumor volume measured by MRI (as described later). Tumor samples were homogenized and extracts used for the MSD assay described earlier. Tumor drug levels were measured by LC/tandem MS by using a Synergi Polar-RP analytical column (80 Å, 4 μeq, 50 × 2 mm; 00B-4336-B0; Phenomenex) with a gradient mobile phase of methanol and 0.1% formic acid, at a flow rate of 0.2 mL/min, in positive ionization mode by multiple reaction monitoring. Expression of phospho-IGF1R was assessed by immunohistochemistry using a rabbit monoclonal antibody against IGF1R pY950 (clone 7/4; Novartis) at 1:10,000 dilution using an epitope retrieval solution at 100 °C (Vision Biosystems).
MRI.
Tumor volume after NVP-AEW541 treatment of the orthotopic Wilms tumor model was measured by 1H MRI performed on a 7T Bruker horizontal-bore microimaging system by using a 3-cm birdcage coil. Anesthesia was induced by an i.p. injection of a combination of fentanyl citrate (0.315 mg/mL) plus fluanisone (10 mg/mL; Hypnorm; Janssen), midazolam (5 mg/mL; Roche), and water (1:1:2). Anatomical T2-weighted images were acquired from 20 contiguous 1-mm-thick transverse slices through the mouse abdomen with an in-plane resolution of 120 μm [rapid acquisition with relaxation enhancement (RARE) sequence; number of excitations (NEX), 4; effective echo time, 36 ms, repetition time, 4,500 ms; turbo factor, 8; 1-mm-thick contiguous; matrix, 256 × 256]. Tumor volume was measured by using segmentation from regions of interest drawn on every slice containing tumor using in-house software (ImageView; written under Interactive Data Language programming platform; ITT).
Statistical Analysis.
All statistical tests were performed in GraphPad Prism 4 (GraphPad). Associations between continuous and categorical variables were analyzed using ANOVA, with the posttest Bonferroni multiple comparison method applied. Student t test was applied for the other statistical analyses. All tests were two-tailed, with a confidence interval of 95%. P values less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
Expression profiling was carried out by University College London Genomics, and we thank Dr. Anita Grigoriadis (Breakthrough Breast Cancer Unit, King’s College London) for bioinformatic assistance. SMARCB1 (INI1) mutation screening was carried out by the Paediatric Malignancy Unit, Great Ormond Street Hospital, London. We also thank Dr. David MacVicar (Royal Marsden Hospital) for the assessment of ultrasound images. This work was supported by Cancer Research United Kingdom Grants C13468/A6718 and C309/A8274; Cancer Research United Kingdom and Engineering and Physical Sciences Research Council Cancer Imaging Centre Grant C1060/A10334 in association with the Medical Research Council and Department of Health (England); and National Health Service funding to the National Institute for Health ResearchBiomedical Research Centre.
Footnotes
Conflict of interest statement: V.R., S.J., and F.H. are employees of Novartis Pharma.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the ArrayExpress database, www.ebi.ac.uk/arrayexpress/ (accession no. E-TABM-891).
See Author Summary on page 7604 (volume 109, number 20).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105034109/-/DCSupplemental.
References
- 1.Pastore G, et al. Malignant renal tumours incidence and survival in European children (1978-1997): Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2103–2114. doi: 10.1016/j.ejca.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Kalapurakal JA, et al. Management of Wilms’ tumour: Current practice and future goals. Lancet Oncol. 2004;5:37–46. doi: 10.1016/s1470-2045(03)01322-6. [DOI] [PubMed] [Google Scholar]
- 3.Natrajan R, et al. Blastemal expression of type I insulin-like growth factor receptor in Wilms’ tumors is driven by increased copy number and correlates with relapse. Cancer Res. 2006;66:11148–11155. doi: 10.1158/0008-5472.CAN-06-1931. [DOI] [PubMed] [Google Scholar]
- 4.Bjornsson HT, et al. Epigenetic specificity of loss of imprinting of the IGF2 gene in Wilms tumors. J Natl Cancer Inst. 2007;99:1270–1273. doi: 10.1093/jnci/djm069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rainier S, et al. Relaxation of imprinted genes in human cancer. Nature. 1993;362:747–749. doi: 10.1038/362747a0. [DOI] [PubMed] [Google Scholar]
- 6.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 7.Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14:6364–6370. doi: 10.1158/1078-0432.CCR-07-4879. [DOI] [PubMed] [Google Scholar]
- 8.Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther. 2007;6:1–12. doi: 10.1158/1535-7163.MCT-06-0080. [DOI] [PubMed] [Google Scholar]
- 9.Houghton PJ, et al. The pediatric preclinical testing program: Description of models and early testing results. Pediatr Blood Cancer. 2007;49:928–940. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 10.Smith MA, et al. SK-NEP-1 and Rh1 are Ewing family tumor lines. Pediatr Blood Cancer. 2008;50:703–706. doi: 10.1002/pbc.21099. [DOI] [PubMed] [Google Scholar]
- 11.Garvin AJ, Re GG, Tarnowski BI, Hazen-Martin DJ, Sens DA. The G401 cell line, utilized for studies of chromosomal changes in Wilms’ tumor, is derived from a rhabdoid tumor of the kidney. Am J Pathol. 1993;142:375–380. [PMC free article] [PubMed] [Google Scholar]
- 12.Dallosso AR, et al. Alternately spliced WT1 antisense transcripts interact with WT1 sense RNA and show epigenetic and splicing defects in cancer. RNA. 2007;13:2287–2299. doi: 10.1261/rna.562907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Royer-Pokora B, et al. Wilms tumor cells with WT1 mutations have characteristic features of mesenchymal stem cells and express molecular markers of paraxial mesoderm. Hum Mol Genet. 2010;19:1651–1668. doi: 10.1093/hmg/ddq042. [DOI] [PubMed] [Google Scholar]
- 14.Kim MK, et al. A pathologic link between Wilms tumor suppressor gene, WT1, and IFI16. Neoplasia. 2008;10:69–78. doi: 10.1593/neo.07869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stock C, et al. Genetic changes of two Wilms tumors with anaplasia and a review of the literature suggesting a marker profile for therapy resistance. Cancer Genet Cytogenet. 2002;135:128–138. doi: 10.1016/s0165-4608(01)00647-1. [DOI] [PubMed] [Google Scholar]
- 16.Alami J, Williams BR, Yeger H. Derivation and characterization of a Wilms’ tumour cell line, WiT 49. Int J Cancer. 2003;107:365–374. doi: 10.1002/ijc.11429. [DOI] [PubMed] [Google Scholar]
- 17.Metzger ML, et al. Topotecan is active against Wilms’ tumor: Results of a multi-institutional phase II study. J Clin Oncol. 2007;25:3130–3136. doi: 10.1200/JCO.2007.10.9298. [DOI] [PubMed] [Google Scholar]
- 18.Casa AJ, Dearth RK, Litzenburger BC, Lee AV, Cui X. The type I insulin-like growth factor receptor pathway: A key player in cancer therapeutic resistance. Front Biosci. 2008;13:3273–3287. doi: 10.2741/2925. [DOI] [PubMed] [Google Scholar]
- 19.Houghton PJ, et al. Initial testing of a monoclonal antibody (IMC-A12) against IGF-1R by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2010;54:921–926. doi: 10.1002/pbc.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolb EA, et al. Initial testing (stage 1) of the IGF-1 receptor inhibitor BMS-754807 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2010 doi: 10.1002/pbc.22741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li MH, Yamase H, Ferrer F. Characterization of a WiT49 cell line derived orthotopic model of Wilms tumor. Pediatr Blood Cancer. 2010;54:316–318. doi: 10.1002/pbc.22205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Box C, Rogers SJ, Mendiola M, Eccles SA. Tumour-microenvironmental interactions: Paths to progression and targets for treatment. Semin Cancer Biol. 2010;20:128–138. doi: 10.1016/j.semcancer.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Talmadge JE, Singh RK, Fidler IJ, Raz A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol. 2007;170:793–804. doi: 10.2353/ajpath.2007.060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived-but they can be improved. Cancer Biol Ther. 2003;2(4 suppl 1):S134–S139. [PubMed] [Google Scholar]
- 25.Singh RK, et al. Organ site-dependent expression of basic fibroblast growth factor in human renal cell carcinoma cells. Am J Pathol. 1994;145:365–374. [PMC free article] [PubMed] [Google Scholar]
- 26.Van Dyke T. Finding the tumor copycat: Approximating a human cancer. Nat Med. 2010;16:976–977. doi: 10.1038/nm0910-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis LM, Fidler IJ. Finding the tumor copycat. Therapy fails, patients don’t. Nat Med. 2010;16:974–975. doi: 10.1038/nm0910-974. [DOI] [PubMed] [Google Scholar]
- 28.Hazen-Martin DJ, Re GG, Garvin AJ, Sens DA. Distinctive properties of an anaplastic Wilms’ tumor and its associated epithelial cell line. Am J Pathol. 1994;144:1023–1034. [PMC free article] [PubMed] [Google Scholar]
- 29.Hu Q, et al. Wt1 ablation and Igf2 upregulation in mice result in Wilms tumors with elevated ERK1/2 phosphorylation. J Clin Invest. 2011;121:174–183. doi: 10.1172/JCI43772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olmos D, Basu B, de Bono JS. Targeting insulin-like growth factor signaling: Rational combination strategies. Mol Cancer Ther. 2010;9:2447–2449. doi: 10.1158/1535-7163.MCT-10-0719. [DOI] [PubMed] [Google Scholar]
- 31.Natrajan R, et al. Array CGH profiling of favourable histology Wilms tumours reveals novel gains and losses associated with relapse. J Pathol. 2006;210:49–58. doi: 10.1002/path.2021. [DOI] [PubMed] [Google Scholar]
- 32.Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207–212. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 33.Chou TC, Talaly P. A simple generalized equation for the analysis of multiple inhibitions of Michaelis-Menten kinetic systems. J Biol Chem. 1977;252:6438–6442. [PubMed] [Google Scholar]
- 34.Bax DA, et al. Molecular and phenotypic characterisation of paediatric glioma cell lines as models for preclinical drug development. PLoS ONE. 2009;4:e5209. doi: 10.1371/journal.pone.0005209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Workman P, et al. Committee of the National Cancer Research Institute Guidelines for the welfare and use of animals in cancer research. Br J Cancer. 2010;102:1555–1577. doi: 10.1038/sj.bjc.6605642. [DOI] [PMC free article] [PubMed] [Google Scholar]