Abstract
We present a unique perspective on the role of historical processes in community assembly by synthesizing analyses of species turnover among communities with environmental data and independent, population genetic-derived estimates of among-community dispersal. We sampled floodplain and terra firme communities of the diverse tree genus Inga (Fabaceae) across a 250-km transect in Amazonian Peru and found patterns of distance-decay in compositional similarity in both habitat types. However, conventional analyses of distance-decay masked a zone of increased species turnover present in the middle of the transect. We estimated past seed dispersal among the same communities by examining geographic plastid DNA variation for eight widespread Inga species and uncovered a population genetic break in the majority of species that is geographically coincident with the zone of increased species turnover. Analyses of these and 12 additional Inga species shared between two communities located on opposite sides of the zone showed that the populations experienced divergence 42,000–612,000 y ago. Our results suggest that the observed distance decay is the result not of environmental gradients or dispersal limitation coupled with ecological drift—as conventionally interpreted under neutral ecological theory—but rather of secondary contact between historically separated communities. Thus, even at this small spatial scale, historical processes seem to significantly impact species’ distributions and community assembly. Other documented zones of increased species turnover found in the western Amazon basin or elsewhere may be related to similar historical processes.
Keywords: historical biogeography, phylogeography, tropical trees
Studies of beta diversity, the turnover in species composition among communities, have provided major insights into the processes governing community assembly (1–3). Recent studies have principally focused on two explanations for beta diversity: species responding to environmental gradients (4, 5) and dispersal limitation coupled with demographic stochasticity within the context of neutral ecological theory (often termed ecological drift) (3, 6). Many authors have recognized the potential importance of historical and biogeographic processes in generating beta diversity (1–10), but to date no empirical study has detailed the historical biogeographies of individual species and their potential role in driving community-level patterns of beta diversity. Here we use population genetics to elucidate the historical biogeographies of the species that compose the communities in which we analyze beta diversity. This approach enables us to demonstrate that historical processes have limited the distribution of species and can directly influence present-day patterns of community assembly and species turnover among communities.
Our focal system is communities of the tropical tree genus Inga (Fabaceae) (11) in the lowland Amazon region of Madre de Dios, Peru. Tropical tree communities have become a model system for exploring patterns of beta diversity (3–5, 10, 12). In a landmark study, Condit et al. (3) used neutral ecological theory to analyze distance-decay in neotropical tree communities, the decline in compositional similarity of communities with geographic distance (2). They interpreted the observed distance-decay in terms of a balance between ecological drift and spatially limited dispersal. Other studies of tropical tree communities have emphasized environmental differences when examining species turnover among habitat types (4, 5). Nevertheless, when distance-decay has been found within habitat types, many investigators have attributed it to neutral ecological processes (4, 5, 13). None of these studies assessed the potential role of historical processes in driving distance-decay (although see ref. 14).
The relative importance of neutral ecological processes and historical biogeographic processes in generating distance-decay may depend on spatial scale. At small spatial scales within range boundaries, spatial autocorrelation in the presence/absence of species driven by neutral processes may be responsible for distance-decay. At large spatial scales, species’ range boundaries may be more significant. Here we focus on patterns within Madre de Dios, Peru, which for Amazonian trees is of a smaller spatial scale than the scale at which historical biogeographic processes have been considered significant.
Population genetic data can provide a means of inferring the dispersal and historical biogeography of species. For example, Dick and colleagues (14–16) found low genetic differentiation among populations of two tree species in the Amazon basin, which they interpreted to signify low dispersal limitation in the Amazon for the two species. If such population genetic studies were extended across more species and explicitly combined with analyses of species composition and turnover between communities, this could provide a powerful means of assessing the role of historical processes in shaping tropical tree communities.
In this study, we simultaneously examined spatial turnover in species composition among Amazonian tree communities, pertinent environmental data, and genetically estimated rates of seed dispersal among communities. We surveyed Inga communities at 13 sites arrayed along a roughly linear 250-km transect. We sampled communities in upland terra firme and bottomland floodplain separately at each site, because 76% of Inga species in Madre de Dios are specialists of either habitat (17). (Note: In the upper Amazon, floodplain forest is inundated only several days per year or less.) Previous work based on extensive morphological analyses and phylogenetic analysis of nuclear and plastid markers has confirmed the species identity of all sampled individuals, which is important to studies of community similarity given the difficulty of identifying tropical trees in the field (17). For 20 Inga species representing both terra firme and floodplain environments, we estimated plastid and nuclear gene flow between the two most thoroughly sampled communities on the transect, which lie 140 km apart. For further population sampling, we selected eight Inga species that were abundant along the transect and that demonstrated plastid genetic variation in our initial survey. Finally, we sampled these species in northern Peru and central Bolivia, >600 km from our transect, to place our local phylogeographic results within a broader geographic framework.
Remarkably, both terra firme and floodplain Inga communities showed significant distance-decay despite the small spatial scale and the fact that many of the Inga species in these communities have ranges extending far beyond our transect (11). Furthermore, the pattern of distance decay was the same in each habitat. Using a sliding-window approach, we found that the apparently smooth distance-decay masks a zone of increased species turnover in the middle of our transect. This zone corresponds to the region of a phylogeographic break that is geographically coincident across many Inga species. Our results suggest that historical processes can exert a profound influence on the modern distribution of tree species, species turnover, and community assembly in the lowland Amazon basin, even at the small scale of a 250-km transect.
Results
Community Similarity Analyses.
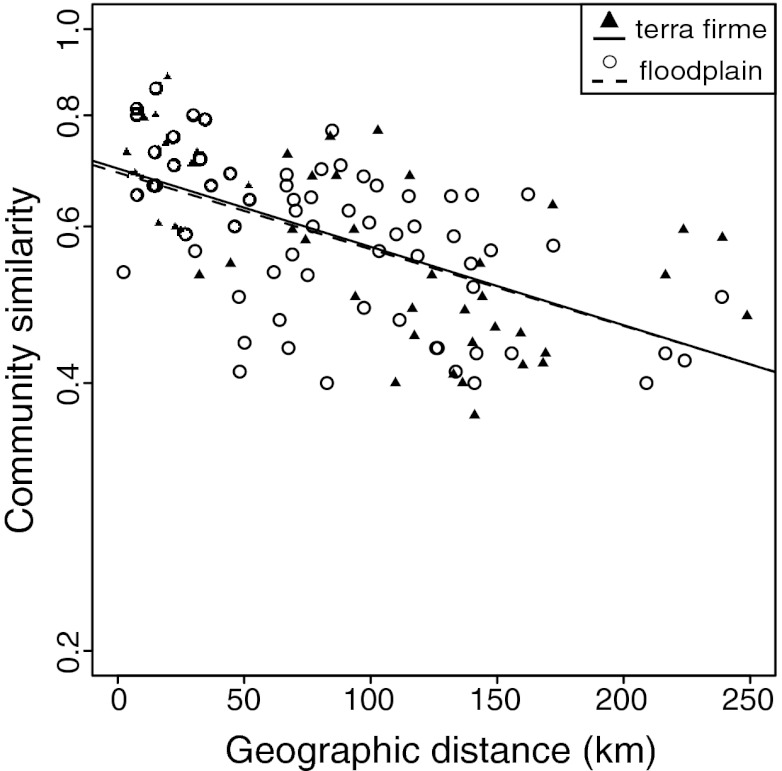
When considering all communities lying along the transect together, we found a weak, although significant, relationship between geographic distance and compositional similarity (Mantel r = −0.30, P = 0.003, y = 0.52e−0.0016×) (Fig. S1). Floodplain and terra firme habitats have strongly contrasting soils (Fig. S2), which affects Inga species distribution (17), and stronger patterns of distance-decay may be evident within habitat types (4, 5). Indeed, we found greater distance-decay when restricting our analyses to terra firme or floodplain communities (terra firme: Mantel r = −0.60, P = 0.007, y = 0.70e−0.0020×; floodplain: Mantel r = −0.55, P = 0.001; y = 0.68e−0.0019×) (Fig. 1). The intercepts of the distance-decay relationships were notably <1, as would be expected at a distance of 0 km. This is likely due to sampling effects; that is, our community surveys likely failed to capture all rare species present in a given community.
Fig. 1.
Distance-decay in the compositional similarity (measured as log of Sorensen index) of Inga communities in Madre de Dios for terra firme and floodplain. Best-fit exponential relationships are shown (linear on the log-linear plot). The Mantel test was used to test the strength and significance of correlations (Results).
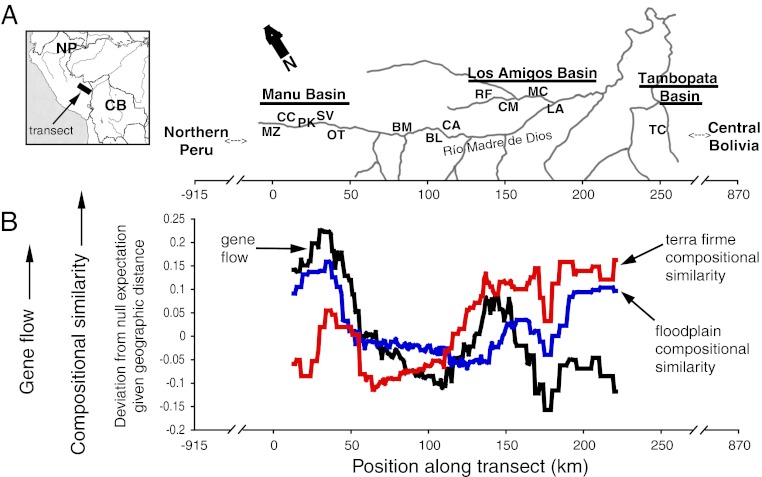
Remarkably, even though floodplain and terra firme share few species, both habitats showed nearly the same slope and intercept for the distance-decay relationship. The smooth distance-decay observed would seem to indicate regular species turnover across space, but this apparent regularity disappeared when we applied a geographically explicit approach (Fig. 2B). We used a spatially sliding window, which revealed significant variation in turnover along the transect. Furthermore, turnover was strikingly correlated between floodplain and terra firme (r = 0.44, P < 0.001) (Fig. 2B). In both habitats, there was notably high turnover (i.e., low compositional similarity) between the geographically proximate communities of MC and LA, as well as an extended zone of high turnover between the Manu and Los Amigos basins (Fig. 2B).
Fig. 2.
(A) Map of transect in southern Amazonian Peru with locations of community surveys and population genetic sampling. (B) Variation in compositional similarity (Sorensen index) and average plastid gene flow (1 − Fst) between communities along the transect. Values shown represent deviation from expected values given the geographic distance between sampling points. A sliding-window approach was taken, in which values were averaged over 26-km intervals (SI Materials and Methods).
Estimates of Gene Flow Between Communities.
Using a sliding-window approach as above, we found that plastid gene flow between communities, averaged across eight Inga species, varied substantially along the transect (Fig. 2B) and was significantly correlated with species turnover in both habitats (terra firme: r = 0.36, P = 0.002; floodplain: r = 0.26, P = 0.004).
We selected 12 additional species for population genetic sampling at two sites, Cocha Cashu (CC) and Los Amigos (LA; one site within the Los Amigos basin), which are found on either side of the zone of increased species turnover. For the plastid genetic marker, 7 of the 20 total sampled species showed no genetic variation between CC and LA, 9 showed significant genetic differentiation (including all 8 species sampled along the entire transect), and an additional 3 showed high (although statistically nonsignificant, likely due to low sample size) differentiation (Table S1). For a nuclear marker, the internal transcribed spacer (ITS), two species showed no genetic variation, whereas 11 showed significant genetic differentiation between CC and LA (Table S1). Genetic differentiation values (as measured by the fixation index Fst) were, on average, much higher for the plastid marker (mean Fst, 0.45 ± 0.10) than for the nuclear marker (mean Fst, 0.16 ± 0.04).
We also performed coalescent analyses of isolation with migration (18) to assess whether migration, or dispersal, occurred since the divergence between CC and LA populations, considering the two markers separately. When sample size was low (fewer than eight individuals per population) or when a species lacked genetic variation for a given marker, convergence could not be obtained for parameter estimates, presumably due to insufficient information in the data. Of the nine species for which convergence was obtained for the plastid marker, four showed significant migration since divergence between CC and LA (Table S1). Of the 14 species for which convergence was obtained for the nuclear marker, 13 showed significant migration since divergence (Table S1).
We used a community-level approach (19) to estimate the divergence time between CC and LA populations. The mean divergence time across all 20 species was at least 42 kya [lower 95% confidence interval using the upper substitution rate estimated for noncoding plastid DNA (20)] and perhaps as long as 433 kya [upper 95% confidence interval using a rate estimated for the genus Inga (21)]. The initial analysis of all species did not provide unambiguous support for one divergence event, although this was the most probable scenario. When we excluded species exhibiting no plastid genetic variation (n = 7), we found strong support for a single divergence event across the remaining species (n = 13). This divergence was estimated to have occurred at least 68 kya and perhaps as long as 612 kya (Table S2).
Analyses of Individual Species’ Distributions.
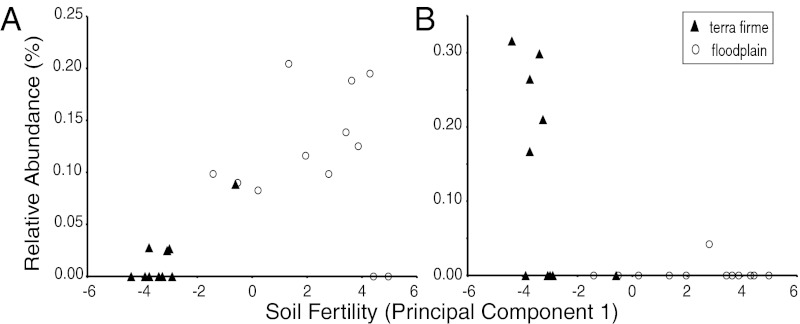
We found that soil conditions exert a strong influence on the distribution of Inga species and their relative abundance in communities. For example, I. bourgonii appears to be a floodplain specialist (Fig. 3A); its abundance was positively correlated with soil fertility, and it was most abundant on the most fertile floodplain soils. It was often absent from terra firme community surveys, which could be due either to genuine absence or to very low abundance that resulted in nondetection. In contrast, I. auristellae was most abundant on infertile soils and was nearly always absent from floodplain surveys, and thus it appears to be a terra firme specialist (Fig. 3B).
Fig. 3.
Relationship between relative abundance and soil fertility, represented by the first principal component axis of measured soil variables, for I. bourgonii (A) and I. auristellae (B).
Soil conditions cannot completely explain the distribution of these species, however, given that the species were sometimes absent from community surveys at sites with apparently favorable conditions. For example, I. bourgonii was absent from two neighboring floodplain surveys, OT and SV (Fig. 4A), even though these sites have the high soil fertility that I. bourgonii appears to favor (Fig. 3A). I. auristellae was absent from all community surveys west of RF, even though most terra firme sites there (CC, PK, SV, and BM) appear to have perfectly suitable soils (Fig. 3B and Fig. S2). Community surveys consisted of 50 × 50 m plots plus >1-km-long transects (17). Although we cannot exclude the possibility that these species were present in forest outside the plots and transects, it is notable that the sites where these species were enigmatically absent are neighboring and, in the case of I. bourgonii, fall geographically between the two diverged plastid clades (Fig. 4A).
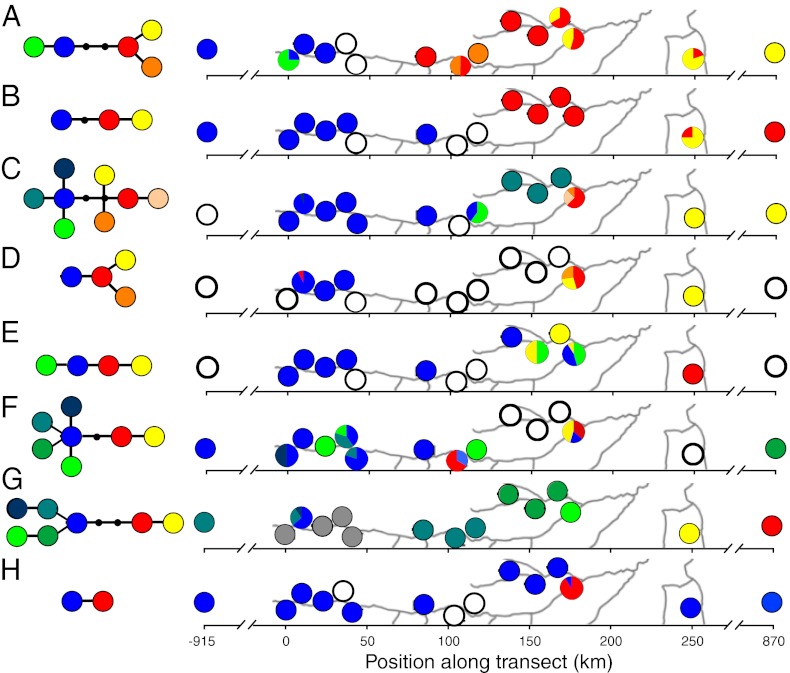
Fig. 4.
Plastid haplotype networks and distribution of those haplotypes along the transect for I. bourgonii (A), I. alata (B), I. ruiziana (C), I. alba (D), I. umbellifera (E), I. marginata (F), I. sapindoides (G), and I. edulis (H). In the networks, each circle represents a unique haplotype, and each line represents a single mutation (insertion-deletion or substitution). Black circles represent unsampled haplotypes. Haplotypes are not shared between species and are color-coded separately for each species. On the maps, a hollow circle indicates that the species was not found at a given site, and a gray circle indicates the species was present at that site, but DNA sequences were not obtained.
Analyses of Individual Species’ Phylogeographies.
There was a significant geographic component to plastid genetic variation for all eight species sampled along the entire transect. Populations from the western end of the transect were found to carry haplotypes that are diverged from haplotypes in the eastern end by at least one mutation, and sometimes as many as five mutations (Fig. 4). Haplotypes from the western populations rarely appeared in the eastern populations and vice versa. Multiple species showed deep genetic divergence over very short distances, such as I. ruiziana, which exhibited a divergence of three substitutions between populations separated by <10 km (Fig. 4C). Even more surprising, in multiple species, haplotypes from the western end of the transect were shared with populations in northern Peru, >900 km to the north, whereas haplotypes from the eastern end were shared with populations in central Bolivia, >600 km to the southeast (Fig. 4). These results are consistent with secondary contact between previously isolated populations.
Discussion
Our analysis of Inga community composition along a 250-km region of uninterrupted Amazonian forest in southern Peru identified a decline in compositional similarity in both floodplain and upland terra firme habitats (Fig. 1). Even more remarkable, the slope and intercept of distance decay were nearly the same in both habitats, despite few Inga species (24%) being generalists across both habitats and also despite the presumably different dispersal patterns in the largely one-dimensional floodplain and two-dimensional terra firme habitats. At first glance, this decline in compositional similarity resembles the equilibrium between dispersal and ecological drift described by previous investigators who discovered similar patterns of distance decay (2, 4–6, 13).
Closer inspection of the compositional data—and inclusion of population genetic data—reveals a very different story, however, one of secondary contact between historically isolated floras that experienced vicariance 42–612 kya. Our geographically explicit sliding-window analysis of compositional similarity revealed not uniform turnover, but rather sharp and simultaneous dips in compositional similarity in both floodplain and terra firme habitats, particularly within a zone of increased turnover between the Manu and Los Amigos basins (Fig. 2). This pattern is driven by the absence of individual species within or on either side of the zone of increased species turnover (Figs. 3 and 4). These enigmatic absences cannot be explained by soil conditions, but are entirely consistent with a hypothesis of secondary contact between floras. Moreover, the population genetic data show a sharp genetic discontinuity in plastid genes for eight species across the zone of increased species turnover, with relative homogeneity over larger distances toward northern Peru and central Bolivia (Figs. 2 and 4). The observed geographic correspondence of genetic divergences provides strong support for a zone of secondary contact between isolated floras.
Mechanisms Underlying Distance-Decay.
Distance-decay in the compositional similarity of communities may be related to underlying spatial environmental gradients to which species respond. Although habitat type (i.e., environment) clearly acts as a filter on Inga species distributions in Madre de Dios (17), environmental conditions do not appear to be responsible for the observed distance-decay within habitat types, because soil variation shows little evidence of spatial gradients (Fig. S2). In addition, the close correspondence of the distance-decay relationships in floodplain and terra firme habitats, which are unlikely to have identically spatially structured environmental variation, suggests that some other process may be responsible.
An alternative explanation for distance-decay is ecological drift coupled with dispersal limitation (sensu neutral ecological theory). Indeed, several authors have suggested that although habitat may determine where species are found, neutral processes predominate in structuring communities within single habitat types (4, 5, 13). However, this argument depends on ecological drift being the principal determinant of the relative abundances of species in communities, and our examination of the relative abundances of Inga species in Madre de Dios argues against this. Most species are either floodplain or terra firme specialists (17), achieving high abundance in their preferred habitat type and being relegated to low abundance or absence in their nonpreferred habitat type. For example, I. bourgonii (Fig. 3A), I. marginata, and I. nobilis (Fig. S3) are floodplain specialists in Madre de Dios (17), and their low abundance when present in terra firme suggests that terra firme soils are unsuitable, not that the species have consistently drifted to low abundance in that habitat. Likewise, even within habitat types, the relative abundances of species appear to be related to soil fertility (Fig. 3 and Fig. S2), although our sample size was insufficient to allow us to adequately test this. In general, it seems illogical to conclude that environmental conditions can determine where species are found, yet have no effect on relative abundances within a single habitat type.
We suggest that rather than environment or equilibrium-neutral processes, historical processes of population subdivision and colonization are driving distance-decay patterns in both habitats. Compositional similarity between communities and its inverse, species turnover, closely track the level of plastid gene flow (Fig. 2), suggesting that historical seed dispersal patterns impact species composition.
Further evidence for the processes of subdivision and colonization can be found when we consider the distributions of individual species. The two sites where I. bourgonii was enigmatically absent (SV and OT) fall precisely between the two geographically segregated, divergent plastid clades found in the species (Fig. 4A). I. auristellae is found across the Amazon basin, including in the eastern part of our transect, where it is the most abundant Inga species in terra firme (composing 30% of Inga stems in diverse communities of 25–30 Inga species), yet it was not found in the western part of the transect, where terra firme soils appear to be perfectly suitable. It seems likely that I. bourgonii and I. auristellae would do just fine in the sites where they are absent, but that historical population subdivision and subsequent, incomplete colonization have resulted in their patchy distributions. The distribution patterns of other Inga species reflect those of I. bourgonii and I. auristellae, suggesting that historical processes underlie the patterns of distance-decay that we have observed for Inga communities.
Interpretation of Population Genetic Results.
Introgression can be problematic for phylogeographic analyses (22), and several of the species in this study share alleles with other Inga species (17). However, Inga species are not known to hybridize (11, 23). Rather, shared alleles among Inga species are likely related to incomplete lineage sorting, which should not affect phylogeographic analyses and is expected given Inga's rapid rate of speciation (21).
We found strongly contrasting results for plastid and nuclear markers. Seven of 20 species showed no genetic variation across the two populations (CC and LA) for the plastid marker, and only 2 species lacked variation for the nuclear marker. This could indicate that species without plastid variability experienced recent migration of the plastid marker between the two populations (i.e., recent seed dispersal). Alternatively, these populations might have been isolated for a substantial period of time with no plastid substitutions. Plastid intergenic spacers, such as the trnD-T spacer used here, appear to have a slower rate of substitution than the nuclear ITS marker in Inga (21). Indeed, the finding of significant genetic differentiation for ITS in five of seven species with no plastid genetic variation (Table S1) suggests that these species might have experienced historical subdivision, as have the species showing plastid genetic divergence.
Fst values are difficult to interpret, being affected by both time since isolation and the level of migration between populations; thus, we also performed coalescent analyses that simultaneously examined isolation and migration. The majority of species that could be analyzed for the plastid marker showed no significant plastid migration since isolation between CC and LA, whereas all but one species showed significant migration for the nuclear marker. Given that plastid markers generally disperse only in seeds and nuclear markers disperse in seeds and pollen (24), this implies that seed dispersal between CC and LA has been limited, whereas pollen dispersal has been prevalent.
Our assessment of plastid gene flow between additional Inga populations shows that seed dispersal has been far from constant through time and space across our study region (Figs. 2 and 4). There are no evident extant barriers to seed dispersal in Madre de Dios except perhaps rivers, but the observed phylogeographic breaks are not associated with rivers (Fig. 4). Thus, extant spatial variation in seed dispersal does not seem to underlie the phylogeographic patterns. Instead, our results imply that some unknown historical barrier or process restricted seed dispersal among populations. Indeed, our finding of an abrupt genetic break between large, genetically homogeneous areas (shared alleles between either end of the transect and distant locations in northern Peru and Bolivia; Fig. 4) suggests that Inga species are colonizing this landscape from two or more source communities that were historically subdivided.
Our finding of highly limited seed dispersal across short spatial scales (10–250 km) is in contrast to the results of several recent studies suggesting that Amazonian trees have extensive dispersal ability (14–16, 25, 26). Those results are not necessarily in conflict with ours, however. Many of the Inga species in the present study have widespread distributions, extending across the Amazon basin and even as far north as Mexico. Likewise, we find common alleles at distances of 900 km. Clearly, Inga species can disperse far and wide; however, in at least one corner of the Amazon basin, historical events seem to have greatly impacted the distribution of Inga species and their haplotypes.
Potential Historical Scenarios.
The concordance of phylogeographic patterns of Inga species in Madre de Dios indicates that some climatic or geological process affected them in similar ways. The common divergence event that most species experienced seems to have occurred 42–612 kya, a time period extending from the middle to late Pleistocene. Thus, Pleistocene climate cycles, perhaps even the most recent glacial cycle, may be responsible for the observed divergence event. Rainforest was absent in areas of northern Bolivia during the last glacial maximum and has been expanding through the Holocene (27); a similar history may be underway in Madre de Dios.
An alternative explanation for the observed divergence is the Fitzcarrald Arch (28), which underlies the western half of our transect and began uplifting less than 4 mya. Other geologic arches in the Amazon are associated with zones of increased species turnover and/or phylogeographic breaks (29–32), although the mechanism by which geologic arches may generate these patterns is not clear (33). Although we are uncertain of the exact historical scenario underlying the observed phylogeographic and distribution patterns, it does seem clear that historical processes have impacted species turnover and community composition of Inga in southern Peru. Other biogeographic transition zones in the Amazon (29–32) or elsewhere may be due to similar processes.
Conclusions
Our comprehensive analysis of spatial variation in the species composition of communities in combination with a thorough, population genetic-based assessment of among-community dispersal provides insight into the processes structuring present-day ecological communities. Our results indicate that historical processes can exert a profound influence on species turnover and community assembly, even at the spatial scale of 250 km. This finding has significant implications for interpreting studies of Amazonian biodiversity and for determining the types of investigations needed to understand and preserve this biodiversity. Distance-decay in the compositional similarity of communities does not necessarily support neutral ecological theory, and the apparent determinism of relative abundances by the environment argues against it (34, 35). Future studies should use sliding-window approaches, as used in the present study and in the study of Pitman et al. (31), to determine whether species turnover is in fact uniform across seemingly homogeneous landscapes. Then, if biogeographic transition zones are documented, population genetic and environmental studies are needed to determine the underlying causes. Regarding conservation, if our aim is to preserve the maximal possible number of species, then protecting large areas and multiple habitat types might not be enough; we may also need to identify cryptic biogeographic transition zones to design protected areas that cover a multiplicity of communities with unique species compositions.
Materials and Methods
We performed Inga community surveys in both floodplain and terra firme habitats at all sites along the transect, except for OT, BL, and CA, where surveys in terra firme are lacking, and MZ, where a comprehensive survey in floodplain is lacking. Soil samples were collected at all survey sites and analyzed for 21 soil variables generally considered relevant to plant survival and growth (e.g., nutrient concentrations, soil texture). We conducted a standard principal component analysis on all soil variables across all sites (Fig. S2). Details of community surveys and soil analyses have been reported previously (17). We evaluated the compositional similarity of communities using the Sorensen index, and assessed the correlation of the natural logarithm of the Sorensen index with geographic distance using Mantel tests. We used a sliding-window approach to assess the variation of compositional similarity along the transect, controlling for the effect of geographic distance between communities (SI Materials and Methods). We used the first principal component from the soil data analysis as an index of soil fertility, and examined how the relative abundance of individual species varied with this index.
For eight Inga species, we obtained sequences of the plastid trnD-T intergenic spacer from 2–20 individuals (mean, 5.5) per population from each community along the transect in which the species were present. These 8 species were selected from the 20 species sampled at CC and LA (see below) because they demonstrated genetic variation and were found in high abundance along the length of the transect. Details on laboratory and sequence editing protocols and voucher and GenBank accession numbers for Madre de Dios samples are available elsewhere (17); corresponding information for northern Peru and Bolivia specimens is provided in Table S3. We calculated intraspecific Fst between populations and consider 1 – Fst to be representative of gene flow. We then used a sliding-window approach, analogous to that described above, to assess the variation in average plastid gene flow along the transect (SI Materials and Methods).
For 20 Inga species, we obtained plastid trnD-T and nuclear ITS sequences from 2–20 (mean, 7.6) individuals per population from CC and LA. ITS sequences often had multiple ambiguous sites, so we used PHASE v2.1.1 (36) to reconstruct ITS haplotypes for individuals. We excluded individuals for which we could not reconstruct haplotypes with high confidence (>0.8 probability) from the ITS population genetic analyses. We calculated Fst between the two populations for the trnD-T and ITS markers. We explicitly estimated migration after divergence between the two populations using a coalescent approach with the IMa program (18). Details of priors and preliminary runs are provided in SI Materials and Methods. We estimated divergence time, in number of substitutions, between CC and LA using the msBayes program, which takes a community-level approach, integrating data from multiple species (19). Details of priors and preliminary runs are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the Instituto Nacional de Recursos Naturales of Peru for permission to conduct fieldwork; Kew Botanic Garden for extractions of specimens from central Bolivia; R. Ricklefs and C. Dick for constructive reviews; and G. Asner, P. Baker, J. Chave, J. Clark, M. Higgins, E. Losos, P. Manos, M. Noor, and R. T. Pennington for discussion and comments on earlier versions of the manuscript. This work was funded by National Science Foundation Grant DDIG-0608368 and grants from the Society of Systematic Biologists, Amazon Conservation Association, Explorer's Club, American Philosophical Society, Sigma Xi, Duke University, and Organization for Tropical Studies.
Footnotes
The authors declare no conflict of interest.
Data deposition: Genbank accession numbers for sequences used in this paper are available in ref. 17 and Table S3.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203523109/-/DCSupplemental.
References
- 1.Whittaker RH. Vegetation of the Siskiyou mountains, Oregon and California. Ecol Monogr. 2006;30:279–338. [Google Scholar]
- 2.Condit R, et al. Beta-diversity in tropical forest trees. Science. 2002;295:666–669. doi: 10.1126/science.1066854. [DOI] [PubMed] [Google Scholar]
- 3.Nekola JC, White PS. The distance decay of similarity in biogeography and ecology. J Biogeogr. 1999;26:867–878. [Google Scholar]
- 4.Phillips O, et al. Habitat association among Amazonian tree species: A landscape-scale approach. J Ecol. 2003;91:757–775. [Google Scholar]
- 5.Tuomisto H, Ruokolainen K, Yli-Halla M. Dispersal, environment, and floristic variation of western Amazonian forests. Science. 2003;299:241–244. doi: 10.1126/science.1078037. [DOI] [PubMed] [Google Scholar]
- 6.Hubbell SP. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton: Princeton Univ Press; 2001. [DOI] [PubMed] [Google Scholar]
- 7.Ricklefs RE, Schluter D. Species Diversity in Ecological Communities. Chicago: Univ of Chicago Press; 1993. [Google Scholar]
- 8.Qian H, Ricklefs RE, White PS. Beta diversity in angiosperms in temperate floras of eastern Asia and eastern North America. Ecol Lett. 2005;8:15–22. [Google Scholar]
- 9.Chase JM, Myers JA. Disentangling the importance of ecological niches from stochastic processes across scales. Philos Trans R Soc Lond B Biol Sci. 2011;366:2351–2363. doi: 10.1098/rstb.2011.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraft NJB, et al. Disentangling the drivers of β diversity along latitudinal and elevational gradients. Science. 2011;333:1755–1758. doi: 10.1126/science.1208584. [DOI] [PubMed] [Google Scholar]
- 11.Pennington TD. The Genus Inga: Botany. London: Royal Botanic Gardens Kew; 1997. [Google Scholar]
- 12.Morlon H, et al. A general framework for the distance-decay of similarity in ecological communities. Ecol Lett. 2008;11:904–917. doi: 10.1111/j.1461-0248.2008.01202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chave J. Neutral theory and community ecology. Ecol Lett. 2004;7:241–253. [Google Scholar]
- 14.Dick CW, Abdul-Salim K, Bermingham E. Molecular systematic analysis reveals cryptic tertiary diversification of a widespread tropical rain forest tree. Am Nat. 2003;162:691–703. doi: 10.1086/379795. [DOI] [PubMed] [Google Scholar]
- 15.Dick CW, Bermingham E, Lemes MR, Gribel R. Extreme long-distance dispersal of the lowland tropical rainforest tree Ceiba pentandra L. (Malvaceae) in Africa and the Neotropics. Mol Ecol. 2007;16:3039–3049. doi: 10.1111/j.1365-294X.2007.03341.x. [DOI] [PubMed] [Google Scholar]
- 16.Dick CW, Heuertz M. The complex biogeographic history of a widespread tropical tree species. Evolution. 2008;62:2760–2774. doi: 10.1111/j.1558-5646.2008.00506.x. [DOI] [PubMed] [Google Scholar]
- 17.Dexter KG, Pennington TD, Cunningham CW. Using DNA to assess errors in tropical tree identifications: How often are ecologists wrong, and when does it matter? Ecol Monogr. 2010;80:267–286. [Google Scholar]
- 18.Hey J, Nielsen R. Integration within the Felsenstein equation for improved Markov chain Monte Carlo methods in population genetics. Proc Natl Acad Sci USA. 2007;104:2785–2790. doi: 10.1073/pnas.0611164104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hickerson MJ, Stahl E, Takebayashi N. msBayes: Pipeline for testing comparative phylogeographic histories using hierarchical approximate Bayesian computation. BMC Bioinformatics. 2007;8:268. doi: 10.1186/1471-2105-8-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolfe KH, Li WH, Sharp PM. Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast, and nuclear DNAs. Proc Natl Acad Sci USA. 1987;84:9054–9058. doi: 10.1073/pnas.84.24.9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson JE, Pennington RT, Pennington TD, Hollingsworth PM. Rapid diversification of a species-rich genus of neotropical rain forest trees. Science. 2001;293:2242–2245. doi: 10.1126/science.1061421. [DOI] [PubMed] [Google Scholar]
- 22.Saeki I, Dick CW, Barnes BV, Murakami N. Comparative phylogeography of red maple (Acer rubrum L.) and silver maple (Acer saccharinum L.): Impacts of habitat specialization, hybridization, and glacial history. J Biogeogr. 2011;38:992–1005. [Google Scholar]
- 23.Koptur S. Outcrossing and pollinator limitation of fruit set: Breeding systems of neotropical Inga trees (Fabaceae: Mimosoideae) Evolution. 1984;38:1130–1143. doi: 10.1111/j.1558-5646.1984.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 24.Latta RG. Integrating patterns across multiple genetic markers to infer spatial processes. Landscape Ecol. 2006;21:809–820. [Google Scholar]
- 25.Lavin M. In: Neotropical Savannas and Seasonally Dry Forests: Plant Biodiversity, Biogeographic Patterns, and Conservation. Pennington RT, Ratter JA, Lewis GP, editors. Boca Raton, FL: CRC; 2006. pp. 433–447. [Google Scholar]
- 26.Pennington RT, Lavin M, Oliveira A. Woody plant diversity, evolution, and ecology in the tropics: Perspectives from seasonally dry tropical forests. Annu Rev Ecol Evol Syst. 2009;40:437–457. [Google Scholar]
- 27.Mayle FE, Langstroth RP, Fisher RA, Meir P. Long-term forest-savannah dynamics in the Bolivian Amazon: Implications for conservation. Philos Trans R Soc Lond B Biol Sci. 2007;362:291–307. doi: 10.1098/rstb.2006.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Espurt N, et al. How does the Nazca Ridge subduction influence the modern Amazonian foreland basin? Geology. 2007;35:515–518. [Google Scholar]
- 29.da Silva MNF, Patton JL. Molecular phylogeography and the evolution and conservation of Amazonian mammals. Mol Ecol. 1998;7:475–486. doi: 10.1046/j.1365-294x.1998.00276.x. [DOI] [PubMed] [Google Scholar]
- 30.Gascon C, et al. Riverine barriers and the geographic distribution of Amazonian species. Proc Natl Acad Sci USA. 2000;97:13672–13677. doi: 10.1073/pnas.230136397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitman NCA, et al. Tree community change across 700 km of lowland Amazonian forest from the Andean foothills to Brazil. Biotropica. 2008;40:525–535. [Google Scholar]
- 32.Higgins MA, et al. Geological control of floristic composition in Amazonian forests. J Biogeogr. 2011;38:2136–2149. doi: 10.1111/j.1365-2699.2011.02585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossetti DF, de Toledo PM, Góes AM. New geological framework for Western Amazonia (Brazil) and implications for biogeography and evolution. Quat Res. 2005;63:78–89. [Google Scholar]
- 34.Terborgh J, Foster RB, Nuñez P. Tropical tree communities: A test of the nonequilibrium hypothesis. Ecology. 1996;77:561–567. [Google Scholar]
- 35.Pitman NCA, et al. Dominance and distribution of tree species in upper Amazonian terra firme forests. Ecology. 2001;82:2101–2117. [Google Scholar]
- 36.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.